Abstract
The nucleolus is a highly dynamic nuclear substructure that was originally described as the site of ribosome biogenesis. The advent of proteomic analysis has now allowed the identification of over 4500 nucleolus associated proteins with only about 30% of them associated with ribogenesis (1). The great number of nucleolar proteins not associated with traditionally accepted nucleolar functions indicates a role for the nucleolus in other cellular functions such as mitosis, cell-cycle progression, cell proliferation and many forms of stress response including DNA repair (2). A number of recent reviews have addressed the pivotal role of the nucleolus in the cellular stress response (1, 3, 4). Here, we will focus on the role of Nucleolin and Nucleophosmin, two major components of the nucleolus, in response to genotoxic stress. Due to space constraint only a limited number of studies are cited. We thus apologize to all our colleagues whose works are not referenced here.
Keywords: Nucleolus, Nucleolin, Nucleophosmin, Genotoxic stress
A role for the nucleolus in ribosome biogenesis was first proposed in the 1960’s after the identification of RNA and ribosomal genes in these subnuclear compartments (5,6). These early studies pointed to the potential regulatory functions of the nucleolus for protein synthesis in response to a variety of cellular demands. At about the same time, modification of protein synthesis patterns was identified as one of the first phenomena occurring following cellular stress (7). Typically, an immediate arrest of protein synthesis followed by an increased rate of protein synthesis was observed after UV radiation (7). Down regulation of protein synthesis in response to stress is thought to be an adaptive response triggered to protect the cells and conserve the resources required to survive (8). On the other hand, induction of specific ribosomal proteins in response to stress may indicate the involvement of the translational machinery in sensing, responding and recovering from cellular stress (9). The association of several ribosomal proteins with the oxidative stress response (10) is additional evidence that translation regulation is a significant component of the cellular stress response. Several types of stress, including heat shock stress and several chemical compounds, can induce the synthesis of stress regulated proteins while inhibiting the rate of overall protein synthesis (11).
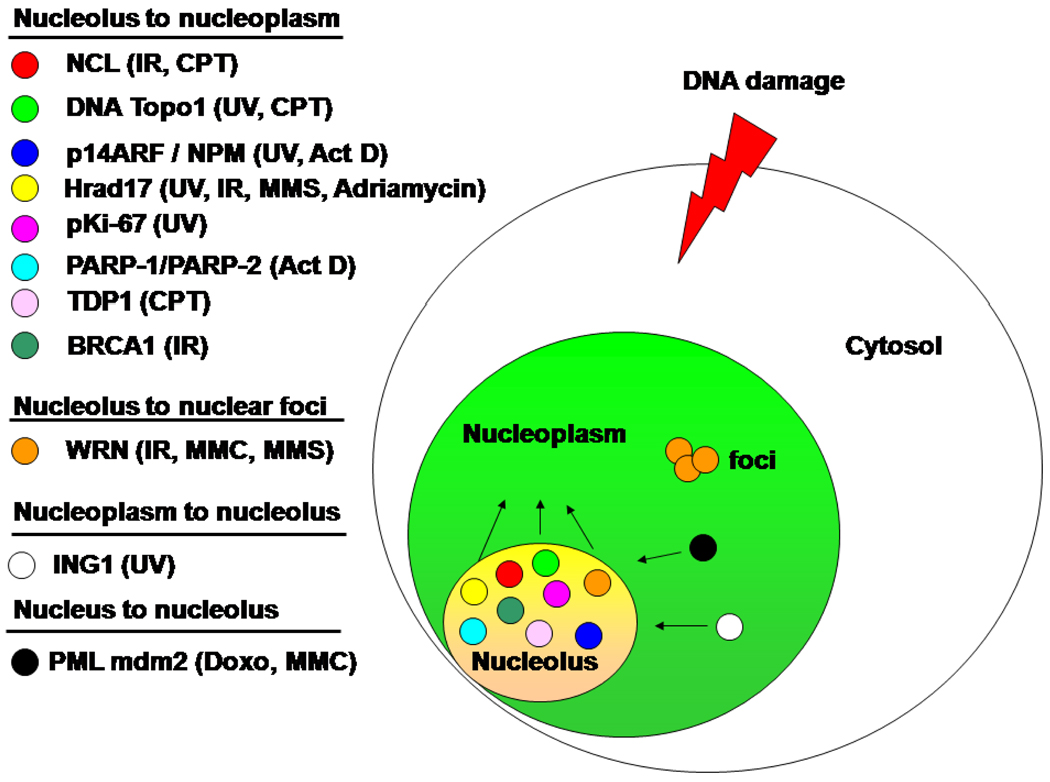
While the nucleolus can directly impact the rate of protein synthesis by regulating the levels of ribosome biogenesis it can also sense and respond to cellular stress by sequestering and releasing a variety of proteins affecting cell cycle and DNA repair. The nucleolus is thus in constant flux, assembling at the end of mitosis and disassembling at prophase while modifying its size and content in response to cellular demands throughout interphase. Thus, large nucleoli are a hallmark of active proliferating cells while terminal stages of differentiation such as lymphocytes are characterized by limited nucleolar size. In fact, silver-staining of the nucleolar organizer regions (AgNORs) is used as a marker for cancer progression in several tumors. This staining method selectively stains the major protein components of the nucleolus- Nucleolin (NCL), Nucleophosmin (NPM) and the upstream binding factor (UBF), the largest RNA polymerase 1 (RP1) subunit. Nucleolin is over-expressed in malignant tumors and its synthesis is associated with increased rates of cell division (12). NPM also known as B23, NO38 and numatrin (13), is 20 times higher in Novikoff hepatoma and 5 times higher in hypertrophic rat liver compared to normal rat liver (14). Both NCL and NPM are considered “hub” proteins in that they can interact with multiple proteins or nucleic acids and serve as scaffold proteins (15). This capacity to interact with multiple partners is mainly due to NCL and NPM disordered domains founds in large part of the protein and corresponding to 55% and 47% respectively of the overall protein structure. In contrast to NPM, NCL does not have a canonical nucleolar localization sequence (NoLS) but its association with NPM warrants its nucleolar localization (16). There are several classes of NoLS but unlike the nuclear localization signal there is no consensus on a particular NoLS. Interaction with a nucleolar protein or RNA or rDNA seems to be the driving force behind nucleolar localization (17). Proteins can either associate transiently with nucleoli or accumulate only under specific metabolic conditions. The protein composition of the nucleolus is therefore not static and can be altered significantly in response to different metabolic status or stress. In fact, the nucleolus is depopulated of proteins due to a sharp migration of nucleolar proteins toward the nucleoplasm in response to cellular stress (Figure. 1). We have previously suggested that the nucleolus serves as a convenient depot for many proteins involved in DNA damage response (18). In response to stress, the nucleolus becomes a major traffic controller that allows key players to uncouple from their quiescent hub and quickly reach the scene of damaged DNA. Interactions with NPM and NCL are thus at the center stage of this critical response. Tables 1 and 2 list nuclear and nucleolar proteins interacting with NCL and NPM respectively.
Figure 1. Nucleoli protein trafficking in response to DNA damage.
The nucleolus is depopulated of its protein content in response to a variety of cellular stress. Proteins are grouped according to the direction they are moving. Arrows indicate the direction of the proteins movement. The Werner protein helicase accumulates in intranuclear repair foci in response to camptothecin (CPT). NCL: Nucleolin, IR: Ionizing Radiation, TDP1: Tyrosyl DNA phosphodiesterase 1, PML: Promyelocytic Leukemia protein, MMC: Mitomycin C, PARP: poly(ADPribose) polymerase (4, 52) (53).
Table 1.
Nucleolin interacting proteins in the nucleus and nucleolus
| Functions | Proteins | References |
|---|---|---|
| Ribosomal proteins | L3, L4, L5, L6, L7, L8, L9, L13a, L18, L18a, L28, L35a, L37a, S3a S8, S9, S11 | (54) |
| DNA replication, recombination and repair proteins | hRPA, SWAP-70, topoisomerase I (Top1), hTERT, p53-inducible and death domain-containing PIDD/LRDD, PCNA, and NPM | (55) (56) (57) (58) (59) (16) (19) |
| Cell cycle and cell differentiation regulatory proteins | pRB, P53, CDC2 kinase, GDNF-inducible zinc finger protein 1(GZF1), casein kinase II (CKII), Histone H1, H2B and H3, interferon regulatory factor-2 (IRF-2), Hdm2, brefeldin A-inhibited guanine nucleotide-exchange protein (BIG1), A-Myb, C-Myb, glucocorticoid receptor (GR), RNA methyl transferase (NSUN2) | (60) (24) (61) (62) (63) (64) (65) (66) (67) (68) (69) (70) |
| Proteases | Granzyme A | (71) |
Table 2.
Nucleophosmin interacting proteins in the nucleus and nucleolus
| Functions | Proteins | References |
|---|---|---|
| Ribosomal proteins | S9, L23 | (72) (73) |
| DNA replication, recombination and repair proteins | ATR, BRCA1 and BRD1, APE1, Chk1, H2AX | (33) (74) (75) (76) |
| Cell cycle and cell differentiation regulatory proteins | NCL, NSUN2, ARF, p53, SENP3 and SENP5, p21WAF1/CIP1, Hdm2, YY1, PKR (eIF2 kinase), HEXIM1, Ebp1, Polo-like kinase 1 (Plk1), YB1, Nucleostemin, p120, USP36, CTCF, c-Jun, H2B, H3 and H4 | (16) (70) (77) (78) (79) (80) (81) (82) (83) (84) (85) (86) (87) (88) (89) (90) (91) (92) (93) |
| Proteases | Granzyme M | (94) |
Modulating repair enzymes activity
NCL and NPM can not only affect the nucleolar trafficking but can also influence the cellular stress response by modulating the activity of stress response proteins. We have recently shown that NCL interacts with PCNA and inhibits Nucleotide Excision Repair (NER) (19). There are two subpathways of NER: global genomic repair (GGR) and transcription coupled repair (TCR). Nucleolin helicase activity and its capacity to bind to a hairpin RNA structure (20) support its possible role in TCR. Nucleolin can also accelerate the annealing of oligonucleotides, including those containing mismatches (21) and thus might be involved in recombinational DNA repair. The multifunctional protein NCL can also inhibit replication by binding to the Replication Protein A (RPA) under stress conditions (22). Heat-shock for example triggers nucleolin translocation to the nucleoplasm, where it binds RPA (23), and consequently inhibits DNA replication initiation (24). Like many helicases, the Werner Syndrome protein (WRNp) is localized to the nucleoli under normal cellular growth but translocates to intranuclear repair foci in response to the Topoisomerase inhibitor camptothecin (CPT) (25) (Figure.1). In response to the genotoxic agent 4-nitroquinoline-1-oxide or serum starvation, it translocates to the nucleoplasm (26,27). WRNp is one of the best characterized RecQ helicases and is known to have roles in DNA replication and repair, transcription, and telomere maintenance. WRNp owes its nucleolar location to its nucleolar targeting sequence (28) but may also interact with nucleolar proteins such as nucleolin. It has been proposed that tyrosine phosphorylation, either by direct modification of WRNp or of a putative “WRN-nucleolar carrier” may modulate the nucleolar trafficking of WRNp (26). In fact nucleolin is also translocated to the nucleoplasm in response to CPT (24). Thus, certain kinds of damage (CPT-induced DNA breaks, for example), prompt the release from the nucleolus of many proteins such as Topo1 (29), the AAA ATPase p97/VCP (18), WRNp and NCL that may be involved in DNA repair. The rapid dispersal of nucleolar DNA damage response proteins such as WRNp and nucleolin to the nucleoplasm enables an immediate and effective accumulation of these proteins at the damage sites where they can assemble into specific repair foci or be used as scaffold proteins for critical DNA repair enzymes.
In addition to its direct interaction with repair proteins, NCL could also modify repair efficiency indirectly by regulating transcripts affecting repair. In that respect we have identified the ribosomal protein S3a (RPS3a) mRNA as of one of nucleolin’s putative targets (30). RPS3a has an Apurinic/Apyrimidinic (AP) endonuclease activity and can also cleave phosphodiester bonds within cyclobutane pyrimidine dimers (31). This activity is unusual since AP endonucleases are supposed to repair AP sites. It has thus been suggested that, at least in mammalian cells, the cleavage of AP sites by a β-lyase activity is not a DNA repair event in the classical sense but may rather represent a novel function such as forestalling of DNA repair (31). The cleavage of a phosphodiester bond is also a new function for a ribosomal protein. This activity could help relax DNA distortions brought about by the dimers and possibly help the replication process to pass over the dimers (32).
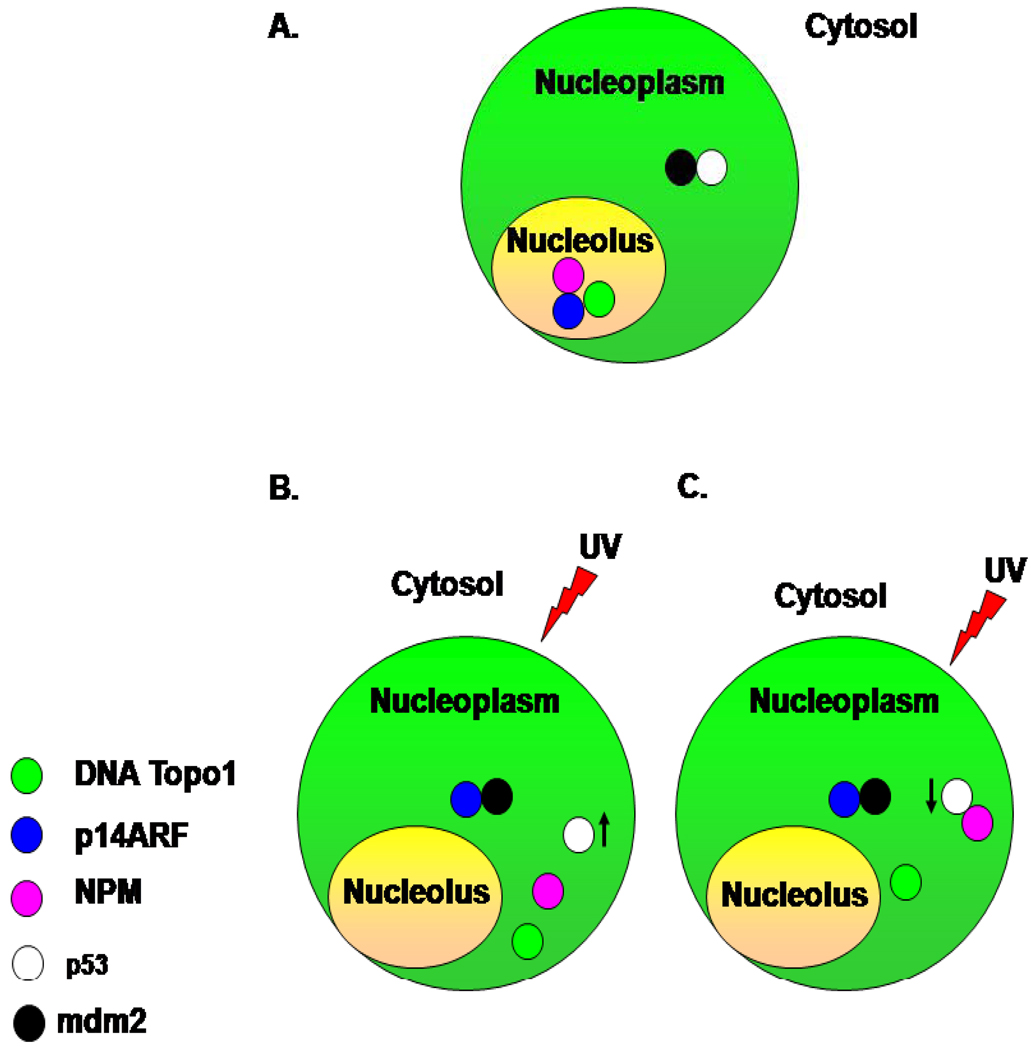
Our data indicate that NPM levels can set a threshold for p53 activation by ATR in response to UV radiation (33). NPM mediates the down regulation of the cyclin dependent kinase inhibitor p21 usually observed in response to low doses (10 Jm−2) of UV radiation (33, 34). This is probably due to the inhibitory effect of high levels of NPM on p53 phosphorylation at Ser15. Phosphorylation is usually not observed until 14 Jm−2 of UV radiation but when NPM levels are lowered, p53 phosphorylation occurs at 10 Jm−2. NPM is also known to indirectly stabilize p53 by releasing ARF from the nucleolus in response to UV radiation. Once in the nucleoplasm, ARF binds to and inactivates mdm2, which leads to p53 stabilization (reviewed in (4)). These apparently contradictory results may actually be part of what are now becoming classical p53 regulatory loops, in which mechanisms involved in p53 up regulation also participate in its down regulation in a sequential manner. In this case it is very likely that the release of ARF from NPM also allow NPM to subsequently bind to p53 in order to prevent over activation (Figure.2). This is reminiscent of what has been described for mdm2 and S100B interaction with p53 where in both cases p53 can up regulate the proteins at the transcriptional levels and over expression of the proteins represses p53 expression (35, 36).
Figure 2. Schematic representation of potential NPM regulation of p53 in response to UV radiation.
(A) Under normal conditions, ARF is retained in the nucleolus by its association with NPM and Topo1. (B) In response to UV radiation ARF dissociates from NPM and is release into the nucleoplasm where it associates with mdm2 and consequently stabilizes p53. (C) The dissociation of ARF from NPM frees NPM to interact with other proteins including p53 which represses its activation. ARF: Alternative Reading Frame, mdm2: murine double minute, NPM: nucleophosmin.
Functional significance of NPM and NCL phosphorylation
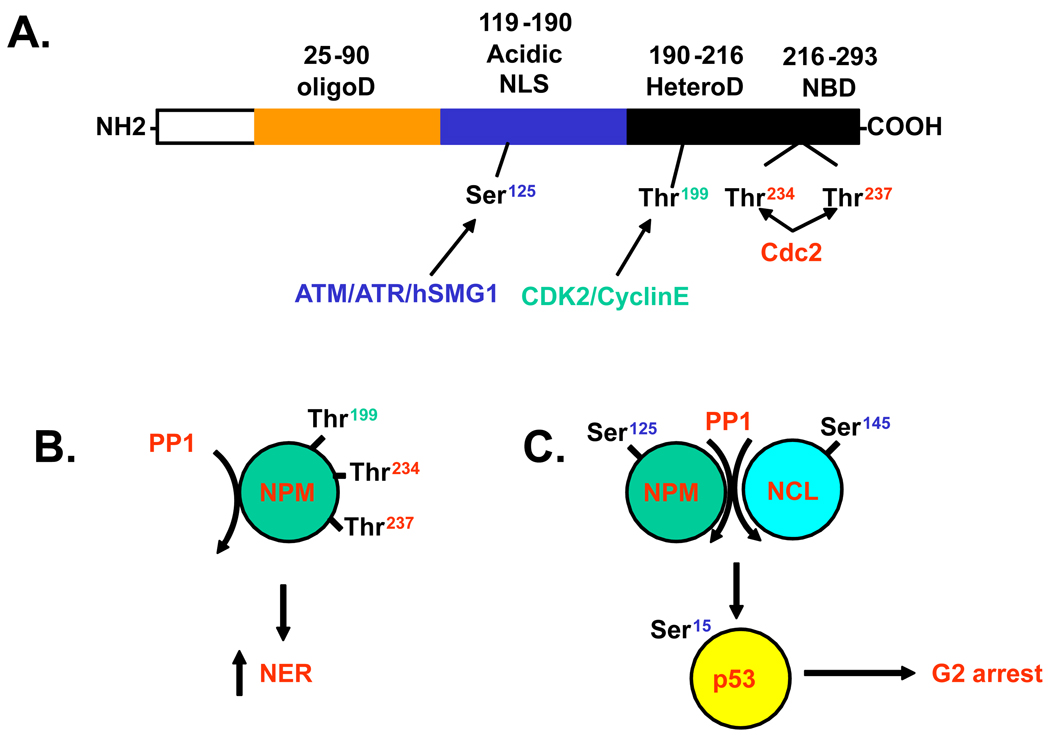
A recent report indicates that dephosphorylation of NPM at Thr 199, 234 and 237 by the protein phosphatase 1 (PP1) facilitates Nucleotide Excision Repair (37). PP1 accumulates in nucleoli during interphase but diffuses into the cytoplasm at mitosis and associates with the kinetochore, while it relocalizes to chromosomes at the onset of anaphase and accumulates again in nucleoli (reviewed in (38)). PP1 does not have a NoLS but is sequestered to the nucleolus by its interaction with the nucleolar protein NOM1(39). PP1 is activated by Ionizing Radiation (IR) in an ATM–dependent manner (40). ATM regulates PP1 activity by phosphorylating its inhibitor (I-2) on Serine 43 which leads to I-2 dissociation from PP1 (41). Activation of PP1 by ATM leads to a G2/M checkpoint through inhibition of Aurora-B kinase and down regulation of histone H3 phosphorylation at Serine 10. Our data (42) indicate that NPM is hyperphosphorylated at Ser125 in AT cells and that overexpression of a functional ATM in AT cells reduces NPM phosphorylation to the levels of normal cells. Moreover down regulating PP1 in ATM corrected AT cells results in NPM hyperphosphorylation, suggesting that the inability to activate PP1 in AT cells is responsible for NPM hypherphorylation, which could interfere with DNA repair (37). Nonetheless, phosphorylation of a small pool of NPM at Thr199 has been shown to be required for NPM recruitment to nuclear DNA damage foci induced by IR (43). Replacement of endogenous NPM with its nonphosphorylable T199A mutant prolonged persistence of IR-induced RAD51 foci and correlates with unrepaired DNA damage (43). Therefore, phosphorylation of NPM could prevent or increase DNA repair depending on the source of DNA damage and the NPM phosphorylation sites involved. Nonetheless, it has also been suggested that NPM has a role in reducing the susceptibility of chromosomal DNA to damage rather than promoting DNA damage repair (44).
NPM phosphorylation at Ser125 could impact DNA repair indirectly by competing out with p53 for remaining active kinases in ATM deficient cells (42). In fact we have shown that NPM is a substrate for the ATM related kinase ATR and can compete, both in vitro and in vivo, with p53 for ATR phosphorylation (33).
Nucleolin phoshorylation for its part can either decrease (45) or increase (30) its RNA binding activity. We have already determined that phosphorylation of nucleolin by MAPK p38 increases nucleolin RNA binding activity (30). However, because SB203580, a specific inhibitor of MAPK p38, reduced the UV-induced RNA binding activity of nucleolin by only 50%, it is possible that other stress responsive kinases such as DNA-PK, ATM, ATR and human SMG-1 (hSMG-1) are involved in nucleolin activation. DNA-PK, ATM, ATR and hSMG-1 belong to a family of protein serine-threonine kinases whose catalytic domains share an evolutionary relationship with mammalian and yeast phosphoinositide-3 kinases (PI-3K) (46). DNA-PK activation requires free DNA ends and is thus considered a DNA damage sensor. ATM is activated by IR and is one of the primary sensors that can activate p53 and the cell cycle checkpoints in response to stress (46). ATR is an ATM and Rad 3-related kinase that was identified during a search of an EST database for gene products containing the catalytic domain of one of the phosphoinositide kinases. ATR is also activated by DNA damage including UV radiation and is important for the S-phase checkpoint where it is used as a damage sensor and scaffolding protein (46). hSMG-1 is involved in nonsense-mediated mRNA decay, and like ATM, plays a role in the recognition and repair of damaged DNA (47). The consensus sequence for phosphorylation by ATM/ATR overlaps extensively with the PI-3K site. Generally the sequence Ser/Thr-Gln-Glu is targeted. In the case of ATM, hydrophobic or acidic residues surrounding the Ser-Gln motif is favorable for phosphorylation, while positively charged amino acids are inhibitory (46). NPM and NCL do not contain perfectly matched ATM/ATR consensus sites but both are phosphorylated in vitro by ATM/ATR/hSMG-1 and are indirectly regulated by ATM through PP1 (42). Non consensus ATM/ATR sites have been identified in bona fide ATM substrates such as BRCA1 (48) and ATM itself (49), and proximity to the ATM/ATR kinases, rather than sequence context is believed to play a pivotal role in the selection of the substrates in vivo (46). Therefore, NCL and NPM abundance and their proximity to ATM in response to cellular stress could favor some direct phosphorylation in vivo.
The nucleolus is thus a large repository of stress responsive proteins poised to provide critical assistance to damaged DNA. Phosphorylation provides a rapid and effective way to uncouple and regulate the activity of several nucleolar proteins. By sequestering phosphatases such as PP1 and Cdc14p to the nucleolus (38, 50, 51), the cells have evolved a mechanism to keep these proteins on call until their functions are urgently needed in the nucleoplasm or other cellular compartments. Given the diversity of possible post-translational modifications that can occur in response to genotoxic stress, it is likely that other modifications, either by themselves or in concert with phosphorylation, will prove to be important regulators of nucleolar proteins trafficking in response to cellular insults.
Figure 3.
(A) Schematic representation of NPM domains and phosphorylation sites. OligoD: Oligomerization domain, Acidic domain including nuclear localization signal (NLS), HeteroD: heterodimerization domain, NBD: nucleic acid binding domain. ATM/ATR, CDK2/CyclinE and Cdc2 phosphorylation sites are indicated. (B) Dephosphorylation of NPM at Thr 199, 234 and 237 by PP1 facilitates NER. (C) Dephosphorylation of NPM at Ser 125 and possibly NCL at Ser145 by PP1 allows p53 phosphorylation at Ser15 in AT cells and facilitates G2 checkpoint in these cells. NPM: Nucleophosmin, PP1: Protein Phosphatase 1, NCL: Nucleolin, NER: Nucleotide Excision Repair. See text for details.
Acknowledgements
This work was supported in part by the A–T Children's Project Foundation (FC) and the National Institutes of Health (1RO1GM57827:RO1CA116491 (FC)).
Footnotes
Conflicts of Interest
No potential conflicts of interest to disclose.
References
- 1.Boulon S, Westman BJ, Hutten S, Boisvert FM, Lamond AI. The nucleolus under stress. Mol Cell. 2010;40:216–227. doi: 10.1016/j.molcel.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen JS, Lyon CE, Fox AH, Leung AK, Lam YW, Steen H, Mann M, Lamond AI. Directed proteomic analysis of the human nucleolus. Curr Biol. 2002;12:1–11. doi: 10.1016/s0960-9822(01)00650-9. [DOI] [PubMed] [Google Scholar]
- 3.Mayer C, Bierhoff H, Grummt I. The nucleolus as a stress sensor: JNK2 inactivates the transcription factor TIF-IA and down-regulates rRNA synthesis. Genes Dev. 2005;19:933–941. doi: 10.1101/gad.333205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tembe V, Henderson BR. Protein trafficking in response to DNA damage. Cell Signal. 2007;19:1113–1120. doi: 10.1016/j.cellsig.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Perry RP. The Cellular Sites of Synthesis of Ribosomal and 4s Rna. Proc Natl Acad Sci U S A. 1962;48:2179–2186. doi: 10.1073/pnas.48.12.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritossa FM, Spiegelman S. Localization of DNA Complementary to Ribosomal Rna in the Nucleolus Organizer Region of Drosophila Melanogaster. Proc Natl Acad Sci U S A. 1965;53:737–745. doi: 10.1073/pnas.53.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden HP, Pearlman C. The Effect of Ultraviolet Light on Protein and Nucleic Acid Synthesis in the Epidermis. J Invest Dermatol. 1964;42:71–75. [PubMed] [Google Scholar]
- 8.Hinnebusch AG. The eIF-2 alpha kinases: regulators of protein synthesis in starvation and stress. Semin Cell Biol. 1994;5:417–426. doi: 10.1006/scel.1994.1049. [DOI] [PubMed] [Google Scholar]
- 9.Bertram J, Palfner K, Hiddemann W, Kneba M. Overexpression of ribosomal proteins L4 and L5 and the putative alternative elongation factor PTI-1 in the doxorubicin resistant human colon cancer cell line LoVoDxR. Eur J Cancer. 1998;34:731–736. doi: 10.1016/s0959-8049(97)10081-8. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka T, Kondo S, Iwasa Y, Hiai H, Toyokuni S. Expression of stress-response and cell proliferation genes in renal cell carcinoma induced by oxidative stress. Am J Pathol. 2000;156:2149–2157. doi: 10.1016/S0002-9440(10)65085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan RF, Hershey JW. Translational repression by chemical inducers of the stress response occurs by different pathways. Arch Biochem Biophys. 1987;256:651–661. doi: 10.1016/0003-9861(87)90622-9. [DOI] [PubMed] [Google Scholar]
- 12.Derenzini M, Sirri V, Trere D, Ochs RL. The quantity of nucleolar proteins nucleolin and protein B23 is related to cell doubling time in human cancer cells. Lab Invest. 1995;73:497–502. [PubMed] [Google Scholar]
- 13.Wu MH, Chang JH, Yung BY. Resistance to UV-induced cell-killing in nucleophosmin/B23 over-expressed NIH 3T3 fibroblasts: enhancement of DNA repair and up-regulation of PCNA in association with nucleophosmin/B23 overexpression. Carcinogenesis. 2002;23:93–100. doi: 10.1093/carcin/23.1.93. [DOI] [PubMed] [Google Scholar]
- 14.Chan WY, Liu QR, Borjigin J, Busch H, Rennert OM, Tease LA, Chan PK. Characterization of the cDNA encoding human nucleophosmin and studies of its role in normal and abnormal growth. Biochemistry. 1989;28:1033–1039. doi: 10.1021/bi00429a017. [DOI] [PubMed] [Google Scholar]
- 15.Emmott E, Hiscox JA. Nucleolar targeting: the hub of the matter. EMBO Rep. 2009;10:231–238. doi: 10.1038/embor.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li YP, Busch RK, Valdez BC, Busch H. C23 interacts with B23, a putative nucleolar-localization-signal-binding protein. Eur J Biochem. 1996;237:153–158. doi: 10.1111/j.1432-1033.1996.0153n.x. [DOI] [PubMed] [Google Scholar]
- 17.Carmo-Fonseca M, Mendes-Soares L, Campos I. To be or not to be in the nucleolus. Nat Cell Biol. 2000;2:E107–E112. doi: 10.1038/35014078. [DOI] [PubMed] [Google Scholar]
- 18.Partridge JJ, Lopreiato JO, Jr, Latterich M, Indig FE. DNA damage modulates nucleolar interaction of the Werner protein with the AAA ATPase p97/VCP. Mol Biol Cell. 2003;14:4221–4229. doi: 10.1091/mbc.E03-02-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang C, Kim MS, Chakravarty D, Indig FE, Carrier F. Nucleolin Binds to the Proliferating Cell Nuclear Antigen and Inhibits Nucleotide Excision Repair. Mol Cell Pharmacol. 2009;1:130–137. doi: 10.4255/mcpharmacol.09.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghisolfi-Nieto L, Joseph G, Puvion-Dutilleul F, Amalric F, Bouvet P. Nucleolin is a sequence-specific RNA-binding protein: characterization of targets on pre-ribosomal RNA. J Mol Biol. 1996;260:34–53. doi: 10.1006/jmbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- 21.Hanakahi LA, Bu Z, Maizels N. The C-terminal domain of nucleolin accelerates nucleic acid annealing. Biochemistry. 2000;39:15493–15499. doi: 10.1021/bi001683y. [DOI] [PubMed] [Google Scholar]
- 22.Kim K, Dimitrova DD, Carta KM, Saxena A, Daras M, Borowiec JA. Novel checkpoint response to genotoxic stress mediated by nucleolin-replication protein a complex formation. Mol Cell Biol. 2005;25:2463–2474. doi: 10.1128/MCB.25.6.2463-2474.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Guan J, Wang H, Wang Y, Leeper D, Iliakis G. Regulation of dna replication after heat shock by replication protein a-nucleolin interactions. J Biol Chem. 2001;276:20579–20588. doi: 10.1074/jbc.M100874200. [DOI] [PubMed] [Google Scholar]
- 24.Daniely Y, Dimitrova DD, Borowiec JA. Stress-dependent nucleolin mobilization mediated by p53-nucleolin complex formation. Mol Cell Biol. 2002;22:6014–6022. doi: 10.1128/MCB.22.16.6014-6022.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakamoto S, Nishikawa K, Heo SJ, Goto M, Furuichi Y, Shimamoto A. Werner helicase relocates into nuclear foci in response to DNA damaging agents and co-localizes with RPA and Rad51. Genes Cells. 2001;6:421–430. doi: 10.1046/j.1365-2443.2001.00433.x. [DOI] [PubMed] [Google Scholar]
- 26.Gray MD, Wang L, Youssoufian H, Martin GM, Oshima J. Werner helicase is localized to transcriptionally active nucleoli of cycling cells. Exp Cell Res. 1998;242:487–494. doi: 10.1006/excr.1998.4124. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki T, Shiratori M, Furuichi Y, Matsumoto T. Diverged nuclear localization of Werner helicase in human and mouse cells. Oncogene. 2001;20:2551–2558. doi: 10.1038/sj.onc.1204344. [DOI] [PubMed] [Google Scholar]
- 28.von Kobbe C, Bohr VA. A nucleolar targeting sequence in the Werner syndrome protein resides within residues 949–1092. J Cell Sci. 2002;115:3901–3907. doi: 10.1242/jcs.00076. [DOI] [PubMed] [Google Scholar]
- 29.Laine JP, Opresko PL, Indig FE, Harrigan JA, von Kobbe C, Bohr VA. Werner protein stimulates topoisomerase I DNA relaxation activity. Cancer Res. 2003;63:7136–7146. [PubMed] [Google Scholar]
- 30.Yang C, Maiguel DA, Carrier F. Identification of nucleolin and nucleophosmin as genotoxic stress-responsive RNA-binding proteins. Nucleic Acids Res. 2002;30:2251–2260. doi: 10.1093/nar/30.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J, Chubatsu LS, Admon A, Stahl J, Fellous R, Linn S. Implication of mammalian ribosomal protein S3 in the processing of DNA damage. J Biol Chem. 1995;270:13620–13629. doi: 10.1074/jbc.270.23.13620. [DOI] [PubMed] [Google Scholar]
- 32.Galloway AM, Liuzzi M, Paterson MC. Metabolic processing of cyclobutyl pyrimidine dimers and (6-4) photoproducts in UV-treated human cells. Evidence for distinct excision-repair pathways. J Biol Chem. 1994;269:974–980. [PubMed] [Google Scholar]
- 33.Maiguel DA, Jones L, Chakravarty D, Yang C, Carrier F. Nucleophosmin sets a threshold for p53 response to UV radiation. Mol Cell Biol. 2004;24:3703–3711. doi: 10.1128/MCB.24.9.3703-3711.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang JA, Fan S, Yuan RQ, Ma YX, Meng Q, Goldberg ID, Rosen EM. Ultraviolet radiation down-regulates expression of the cell-cycle inhibitor p21WAF1/CIP1 in human cancer cells independently of p53. Int J Radiat Biol. 1999;75:301–316. doi: 10.1080/095530099140483. [DOI] [PubMed] [Google Scholar]
- 35.Wade M, Wang YV, Wahl GM. The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol. 20:299–309. doi: 10.1016/j.tcb.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin J, Yang Q, Yan Z, Markowitz J, Wilder PT, Carrier F, Weber DJ. Inhibiting S100B restores p53 levels in primary malignant melanoma cancer cells. J Biol Chem. 2004;279:34071–34077. doi: 10.1074/jbc.M405419200. [DOI] [PubMed] [Google Scholar]
- 37.Lin CY, Tan BC, Liu H, Shih CJ, Chien KY, Lin CL, Yung BY. Dephosphorylation of Nucleophosmin by PP1(beta) Facilitates pRB Binding and Consequent E2F1-dependent DNA Repair. Mol Biol Cell. doi: 10.1091/mbc.E10-03-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boisvert FM, van Koningsbruggen S, Navascues J, Lamond AI. The multifunctional nucleolus. Nat Rev Mol Cell Biol. 2007;8:574–585. doi: 10.1038/nrm2184. [DOI] [PubMed] [Google Scholar]
- 39.Gunawardena SR, Ruis BL, Meyer JA, Kapoor M, Conklin KF. NOM1 targets protein phosphatase I to the nucleolus. J Biol Chem. 2008;283:398–404. doi: 10.1074/jbc.M706708200. [DOI] [PubMed] [Google Scholar]
- 40.Guo CY, Brautigan DL, Larner JM. Ionizing radiation activates nuclear protein phosphatase-1 by ATM-dependent dephosphorylation. J Biol Chem. 2002;277:41756–41761. doi: 10.1074/jbc.M207519200. [DOI] [PubMed] [Google Scholar]
- 41.Tang X, Hui ZG, Cui XL, Garg R, Kastan MB, Xu B. A novel ATM-dependent pathway regulates protein phosphatase 1 in response to DNA damage. Mol Cell Biol. 2008;28:2559–2566. doi: 10.1128/MCB.01711-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nalabothula N, Chakravarty D, Pierce A, Carrier F. Over expression of Nucleophosmin and Nucleolin contributes to the suboptimal activation of a G2/M checkpoint in Ataxia Telangiectasia fibroblasts. MolCellPharmacol. 2010 In Press. [PMC free article] [PubMed] [Google Scholar]
- 43.Koike A, Nishikawa H, Wu W, Okada Y, Venkitaraman AR, Ohta T. Recruitment of phosphorylated NPM1 to sites of DNA damage through RNF8-dependent ubiquitin conjugates. Cancer Res. 70:6746–6756. doi: 10.1158/0008-5472.CAN-10-0382. [DOI] [PubMed] [Google Scholar]
- 44.Li J, Sejas DP, Rani R, Koretsky T, Bagby GC, Pang Q. Nucleophosmin regulates cell cycle progression and stress response in hematopoietic stem/progenitor cells. J Biol Chem. 2006;281:16536–16545. doi: 10.1074/jbc.M601386200. [DOI] [PubMed] [Google Scholar]
- 45.Spicer EK, Bandyopadhyay S, Sengupta TK, Fernandes DT. Taxol-induced bcl2 mRNA destabilization in HL-60 cells is associated withdecreased binding of nucleolin to a bcl-2 A+U rich element; American Association for Cancer Research 93rd Annual Meeting; San Francisco, California. 2002. p. 883. [Google Scholar]
- 46.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 47.Abraham RT. The ATM-related kinase, hSMG-1, bridges genome and RNA surveillance pathways. DNA Repair (Amst) 2004;3:919–925. doi: 10.1016/j.dnarep.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Cortez D, Wang Y, Qin J, Elledge SJ. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science. 1999;286:1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- 49.Kozlov SV, Graham ME, Peng C, Chen P, Robinson PJ, Lavin MF. Involvement of novel autophosphorylation sites in ATM activation. Embo J. 2006;25:3504–3514. doi: 10.1038/sj.emboj.7601231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Visintin R, Hwang ES, Amon A. Cfi1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature. 1999;398:818–823. doi: 10.1038/19775. [DOI] [PubMed] [Google Scholar]
- 51.Stegmeier F, Visintin R, Amon A. Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell. 2002;108:207–220. doi: 10.1016/s0092-8674(02)00618-9. [DOI] [PubMed] [Google Scholar]
- 52.Tulchin N, Chambon M, Juan G, Dikman S, Strauchen J, Ornstein L, Billack B, Woods NT, Monteiro AN. BRCA1 protein and nucleolin colocalize in breast carcinoma tissue and cancer cell lines. Am J Pathol. 2010;176:1203–1214. doi: 10.2353/ajpath.2010.081063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rubbi CP, Milner J. Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. Embo J. 2003;22:6068–6077. doi: 10.1093/emboj/cdg579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouvet P, Diaz JJ, Kindbeiter K, Madjar JJ, Amalric F. Nucleolin interacts with several ribosomal proteins through its RGG domain. J Biol Chem. 1998;273:19025–19029. doi: 10.1074/jbc.273.30.19025. [DOI] [PubMed] [Google Scholar]
- 55.Daniely Y, Borowiec JA. Formation of a complex between nucleolin and replication protein A after cell stress prevents initiation of DNA replication. J Cell Biol. 2000;149:799–810. doi: 10.1083/jcb.149.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jessberger R, Wabl M, Borggrefe T. Biochemical studies of class switch recombination. Curr Top Microbiol Immunol. 1996;217:191–202. doi: 10.1007/978-3-642-50140-1_13. [DOI] [PubMed] [Google Scholar]
- 57.Edwards TK, Saleem A, Shaman JA, Dennis T, Gerigk C, Oliveros E, Gartenberg MR, Rubin EH. Role for nucleolin/Nsr1 in the cellular localization of topoisomerase I. J Biol Chem. 2000;275:36181–36188. doi: 10.1074/jbc.M006628200. [DOI] [PubMed] [Google Scholar]
- 58.Khurts S, Masutomi K, Delgermaa L, Arai K, Oishi N, Mizuno H, Hayashi N, Hahn WC, Murakami S. Nucleolin interacts with telomerase. J Biol Chem. 2004;279:51508–51515. doi: 10.1074/jbc.M407643200. [DOI] [PubMed] [Google Scholar]
- 59.Pick R, Badura S, Bosser S, Zornig M. Upon intracellular processing, the C-terminal death domain-containing fragment of the p53-inducible PIDD/LRDD protein translocates to the nucleoli and interacts with nucleolin. Biochem Biophys Res Commun. 2006;349:1329–1338. doi: 10.1016/j.bbrc.2006.08.176. [DOI] [PubMed] [Google Scholar]
- 60.Grinstein E, Shan Y, Karawajew L, Snijders PJ, Meijer CJ, Royer HD, Wernet P. Cell cycle-controlled interaction of nucleolin with the retinoblastoma protein and cancerous cell transformation. J Biol Chem. 2006;281:22223–22235. doi: 10.1074/jbc.M513335200. [DOI] [PubMed] [Google Scholar]
- 61.Liu HT, Yung BY. In vivo interaction of nucleophosmin/B23 and protein C23 during cell cycle progression in HeLa cells. Cancer Lett. 1999;144:45–54. doi: 10.1016/s0304-3835(99)00184-6. [DOI] [PubMed] [Google Scholar]
- 62.Dambara A, Morinaga T, Fukuda N, Yamakawa Y, Kato T, Enomoto A, Asai N, Murakumo Y, Matsuo S, Takahashi M. Nucleolin modulates the subcellular localization of GDNF-inducible zinc finger protein 1 and its roles in transcription and cell proliferation. Exp Cell Res. 2007;313:3755–3766. doi: 10.1016/j.yexcr.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 63.Schneider HR, Issinger OG. Nucleolin (C23), a physiological substrate for casein kinase II. Biochem Biophys Res Commun. 1988;156:1390–1397. doi: 10.1016/s0006-291x(88)80786-1. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki T, Suzuki N, Hosoya T. Limited proteolysis of rat liver nucleolin by endogenous proteases: effects of polyamines and histones. Biochem J. 1993;289(Pt 1):109–115. doi: 10.1042/bj2890109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Masumi A, Fukazawa H, Shimazu T, Yoshida M, Ozato K, Komuro K, Yamaguchi K. Nucleolin is involved in interferon regulatory factor-2-dependent transcriptional activation. Oncogene. 2006;25:5113–5124. doi: 10.1038/sj.onc.1209522. [DOI] [PubMed] [Google Scholar]
- 66.Saxena A, Rorie CJ, Dimitrova D, Daniely Y, Borowiec JA. Nucleolin inhibits Hdm2 by multiple pathways leading to p53 stabilization. Oncogene. 2006;25:7274–7288. doi: 10.1038/sj.onc.1209714. [DOI] [PubMed] [Google Scholar]
- 67.Padilla PI, Uhart M, Pacheco-Rodriguez G, Peculis BA, Moss J, Vaughan M. Association of guanine nucleotideexchange protein BIG1 in HepG2 cell nuclei with nucleolin, U3 snoRNA, and fibrillarin. Proc Natl Acad Sci U S A. 2008;105:3357–3361. doi: 10.1073/pnas.0712387105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ying GG, Proost P, van Damme J, Bruschi M, Introna M, Golay J. Nucleolin, a novel partner for the Myb transcription factor family that regulates their activity. J Biol Chem. 2000;275:4152–4158. doi: 10.1074/jbc.275.6.4152. [DOI] [PubMed] [Google Scholar]
- 69.Schulz M, Schneider S, Lottspeich F, Renkawitz R, Eggert M. Identification of nucleolin as a glucocorticoid receptor interacting protein. Biochem Biophys Res Commun. 2001;280:476–480. doi: 10.1006/bbrc.2000.4141. [DOI] [PubMed] [Google Scholar]
- 70.Sakita-Suto S, Kanda A, Suzuki F, Sato S, Takata T, Tatsuka M. Aurora-B regulates RNA methyltransferase NSUN2. Mol Biol Cell. 2007;18:1107–1117. doi: 10.1091/mbc.E06-11-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pasternack MS, Bleier KJ, McInerney TN. Granzyme A binding to target cell proteins. Granzyme A binds to and cleaves nucleolin in vitro. J Biol Chem. 1991;266:14703–14708. [PubMed] [Google Scholar]
- 72.Lindstrom MS, Zhang Y. Ribosomal protein S9 is a novel B23/NPM-binding protein required for normal cell proliferation. J Biol Chem. 2008;283:15568–15576. doi: 10.1074/jbc.M801151200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wanzel M, Russ AC, Kleine-Kohlbrecher D, Colombo E, Pelicci PG, Eilers M. A ribosomal protein L23-nucleophosmin circuit coordinates Mizl function with cell growth. Nat Cell Biol. 2008;10:1051–1061. doi: 10.1038/ncb1764. [DOI] [PubMed] [Google Scholar]
- 74.Sato K, Hayami R, Wu W, Nishikawa T, Nishikawa H, Okuda Y, Ogata H, Fukuda M, Ohta T. Nucleophosmin/B23 is a candidate substrate for the BRCA1-BARD1 ubiquitin ligase. J Biol Chem. 2004;279:30919–30922. doi: 10.1074/jbc.C400169200. [DOI] [PubMed] [Google Scholar]
- 75.Vascotto C, Fantini D, Romanello M, Cesaratto L, Deganuto M, Leonardi A, Radicella JP, Kelley MR, D'Ambrosio C, Scaloni A, et al. APE1/Ref-1 interacts with NPM1 within nucleoli and plays a role in the rRNA quality control process. Mol Cell Biol. 2009;29:1834–1854. doi: 10.1128/MCB.01337-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Du YC, Gu S, Zhou J, Wang T, Cai H, Macinnes MA, Bradbury EM, Chen X. The dynamic alterations of H2AX complex during DNA repair detected by a proteomic approach reveal the critical roles of Ca(2+)/calmodulin in the ionizing radiation-induced cell cycle arrest. Mol Cell Proteomics. 2006;5:1033–1044. doi: 10.1074/mcp.M500327-MCP200. [DOI] [PubMed] [Google Scholar]
- 77.Itahana K, Bhat KP, Jin A, Itahana Y, Hawke D, Kobayashi R, Zhang Y. Tumor suppressor ARF degrades B23, a nucleolar protein involved in ribosome biogenesis and cell proliferation. Mol Cell. 2003;12:1151–1164. doi: 10.1016/s1097-2765(03)00431-3. [DOI] [PubMed] [Google Scholar]
- 78.Colombo E, Marine JC, Danovi D, Falini B, Pelicci PG. Nucleophosmin regulates the stability and transcriptional activity of p53. Nat Cell Biol. 2002;4:529–533. doi: 10.1038/ncb814. [DOI] [PubMed] [Google Scholar]
- 79.Yun C, Wang Y, Mukhopadhyay D, Backlund P, Kolli N, Yergey A, Wilkinson KD, Dasso M. Nucleolar protein B23/nucleophosmin regulates the vertebrate SUMO pathway through SENP3 and SENP5 proteases. J Cell Biol. 2008;183:589–595. doi: 10.1083/jcb.200807185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiao J, Zhang Z, Chen GG, Zhang M, Ding Y, Fu J, Li M, Yun JP. Nucleophosmin/B23 interacts with p21WAF1/CIP1 and contributes to its stability. Cell Cycle. 2009;8:889–895. doi: 10.4161/cc.8.6.7898. [DOI] [PubMed] [Google Scholar]
- 81.Kurki S, Peltonen K, Laiho M. Nucleophosmin, HDM2 and p53: players in UV damage incited nucleolar stress response. Cell Cycle. 2004;3:976–979. [PubMed] [Google Scholar]
- 82.Mai RT, Yeh TS, Kao CF, Sun SK, Huang HH, Wu Lee YH. Hepatitis C virus core protein recruits nucleolar phosphoprotein B23 and coactivator p300 to relieve the repression effect of transcriptional factor YY1 on B23 gene expression. Oncogene. 2006;25:448–462. doi: 10.1038/sj.onc.1209052. [DOI] [PubMed] [Google Scholar]
- 83.Pang Q, Christianson TA, Koretsky T, Carlson H, David L, Keeble W, Faulkner GR, Speckhart A, Bagby GC. Nucleophosmin interacts with and inhibits the catalytic function of eukaryotic initiation factor 2 kinase PKR. J Biol Chem. 2003;278:41709–41717. doi: 10.1074/jbc.M301392200. [DOI] [PubMed] [Google Scholar]
- 84.Gurumurthy M, Tan CH, Ng R, Zeiger L, Lau J, Lee J, Dey A, Philp R, Li Q, Lim TM, et al. Nucleophosmin interacts with HEXIM1 and regulates RNA polymerase II transcription. J Mol Biol. 2008;378:302–317. doi: 10.1016/j.jmb.2008.02.055. [DOI] [PubMed] [Google Scholar]
- 85.Okada M, Jang SW, Ye K. Ebp1 association with nucleophosmin/B23 is essential for regulating cell proliferation and suppressing apoptosis. J Biol Chem. 2007;282:36744–36754. doi: 10.1074/jbc.M706169200. [DOI] [PubMed] [Google Scholar]
- 86.Zhang H, Shi X, Paddon H, Hampong M, Dai W, Pelech S. B23/nucleophosmin serine 4 phosphorylation mediates mitotic functions of polo-like kinase 1. J Biol Chem. 2004;279:35726–35734. doi: 10.1074/jbc.M403264200. [DOI] [PubMed] [Google Scholar]
- 87.Gonda K, Wudel J, Nelson D, Katoku-Kikyo N, Reed P, Tamada H, Kikyo N. Requirement of the protein B23 for nucleolar disassembly induced by the FRGY2a family proteins. J Biol Chem. 2006;281:8153–8160. doi: 10.1074/jbc.M512890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma H, Pederson T. Nucleophosmin is a binding partner of nucleostemin in human osteosarcoma cells. Mol Biol Cell. 2008;19:2870–2875. doi: 10.1091/mbc.E08-02-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Valdez BC, Perlaky L, Henning D, Saijo Y, Chan PK, Busch H. Identification of the nuclear and nucleolar localization signals of the protein p120. Interaction with translocation protein B23. J Biol Chem. 1994;269:23776–23783. [PubMed] [Google Scholar]
- 90.Endo A, Kitamura N, Komada M. Nucleophosmin/B23 regulates ubiquitin dynamics in nucleoli by recruiting deubiquitylating enzyme USP36. J Biol Chem. 2009;284:27918–27923. doi: 10.1074/jbc.M109.037218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zlatanova J, Caiafa P. CTCF and its protein partners: divide and rule? J Cell Sci. 2009;122:1275–1284. doi: 10.1242/jcs.039990. [DOI] [PubMed] [Google Scholar]
- 92.Yogev O, Saadon K, Anzi S, Inoue K, Shaulian E. DNA damage-dependent translocation of B23 and p19 ARF is regulated by the Jun N-terminal kinase pathway. Cancer Res. 2008;68:1398–1406. doi: 10.1158/0008-5472.CAN-07-2865. [DOI] [PubMed] [Google Scholar]
- 93.Swaminathan V, Kishore AH, Febitha KK, Kundu TK. Human histone chaperone nucleophosmin enhances acetylation-dependent chromatin transcription. Mol Cell Biol. 2005;25:7534–7545. doi: 10.1128/MCB.25.17.7534-7545.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cullen SP, Afonina IS, Donadini R, Luthi AU, Medema JP, Bird PI, Martin SJ. Nucleophosmin is cleaved and inactivated by the cytotoxic granule protease granzyme M during natural killer cell-mediated killing. J Biol Chem. 2009;284:5137–5147. doi: 10.1074/jbc.M807913200. [DOI] [PubMed] [Google Scholar]