Abstract
Lysophospatidic acid (LPA) is a bioactive lipid mediator implicated in tissue repair and wound healing. It mediates diverse functional effects in fibroblasts, including proliferation, migration and contraction, but less is known about its ability to evoke paracrine signaling to other cell types involved in wound healing. We hypothesized that human pulmonary fibroblasts stimulated by LPA would exhibit ectodomain shedding of EGFR ligands that signal to lung epithelial cells. To test this hypothesis, we used alkaline phosphatase (AP) -tagged EGF receptor (EGFR) ligand plasmids transfected into CCL-151 lung fibroblasts, and ELISAs to detect shedding of native ligands. LPA induced shedding of transfected AP-tagged HB-EGF, amphiregulin and TGF-alpha;non-transfected fibroblasts shed amphiregulin and HB-EGF under baseline conditions, and increased shedding of HB-EGF in response to LPA.. Treatment of fibroblasts with LPA (10 μM) resulted in elevated phosphorylation of ERK1/2 (3.3 ± 0.04 fold induction at 5 minutes), enhanced expression of mRNA for c-fos (59 ± 7.9-fold at 30 minutes), HB-EGF (28 ± 4.7-fold at 4 hours) and amphiregulin (5.7 ± 1.8-fold at 4 hours), and enhanced proliferation at 96 hours. However, none of these fibroblast responses to LPA required ectodomain shedding or EGFR activity. To test the ability of LPA to stimulate paracrine signaling from fibroblasts, we transferred conditioned medium from LPA stimulated- CCL-151 cells, and found enhanced EGFR and ERK1/2 phosphorylation in reporter A549 cells in excess of what could be accounted for by transferred LPA alone. About one-third of th response (37%, P < 0.05) was attributable to EGFR activation. These data demonstrate that LPA mediates EGF-family ectodomain shedding, resulting in enhanced paracrine signaling from lung fibroblasts to epithelial cells.
Keywords: epidermal growth factor receptor, LPA, A549 cells
INTRODUCTION
Lysophospatidic acid (LPA) is a lipid derived mediator that evokes growth factor – like responses through activation of G protein-coupled receptors (1). In fibroblasts LPA signaling is involved in a variety of physiological and pathological processes including cell proliferation, cytoskeletal rearrangement, migration and contraction (2–5). As a prominent component of serum and a product of activated platelets (6), LPA is thought to play a key role in tissue repair and wound healing (7). In the lung, LPA is detectable in human bronchoalveolar lavage fluid (8), and recent work has shown that increased LPA levels are present in idiopathic pulmonary fibrosis (IPF), and contribute to lung injury and fibrosis in a murine experimental model of fibrosis (9). The latter finding demonstrates LPA has important effects in the setting of the pulmonary microenvironment.
LPA transduces signals through interactions with seven-transmembrane domain G protein-coupled receptors (GPCRs), termed LPA1–6. These receptors activate heterotrimeric G proteins which are coupled to multiple intracellular signaling pathways via the four Gα subunit classes Gi, Gq, G12/13, and Gs leading to regulation of cell proliferation, migration and survival (10–13). In addition to LPA effects on GPCRs, cross-talk between LPA receptors and receptor tyrosine kinases (RTKs) contributes to LPA-induced intracellular signaling and cellular responses (14, 15). The EGF receptor (EGFR) has been recognized as a protein tyrosine kinase that can play a central role in mediating LPA-induced extracellular signal-regulated kinase 1/2 (ERK 1/2) activation (16). The importance of EGFR transactivation in direct fibroblast responses to LPA is uncertain, though microarray analysis indicates only a minor role in overall transcriptomic responses to LPA (17). However, the role of LPA-induced ectodomain shedding in paracrine signaling from fibroblasts to neighboring epithelial cells is largely uncharacterized. Here we demonstrate that shedding of EGFR ligands is stimulated by LPA in human lung fibroblasts. However, ligand shedding and autocrine EGFR activation are not necessary for fibroblast ERK1/2 activation and expression of EGF-family mRNA in response to LPA stimulation. Instead, soluble mediators shed into the conditioned media from LPA stimulated fibroblasts mediate a signaling response in lung epithelial A549 reporter cells, in part through EGFR activation, demonstrating a paracrine wound healing role for the ectodomain shedding response of lung fibroblasts to LPA stimulation.
MATERIALS AND METHODS
Materials
LPA (18:0 Lyso PA; 1-stearoyl-2-hydroxy-sn-glycero-3-phosphate, VPC 32183 and VPC 12249 (LPA receptor 1 and 3 antagonists) were purchased from Avanti Polar Lipids (Alabaster, AL). PMA, AG1478, GM6001 and Pertussis toxin (PTX) were purchased from Calbiochem (San Diego, CA). Human recombinant Heparin-Binding EGF like growth factor (HB-EGF) was purchased from R&D systems (Minneapolis, MN). Antibodies to ERK1/2 and horseradish peroxidase-conjugated goat anti-rabbit and phospho-specific antibody for ERK1/2 and EGFR (Tyr1068) were purchased from Cell Signaling Technology (Beverly, MA). Goat anti-βactin pAb and rabbit anti-goat IgG HRP-conjugated were from Santa Cruz Biotechnology (Santa Cruz, CA). Halt Protease and Phosophatase Inhibitor Cocktail and the enhanced chemiluminescence (ECL) kit for the detection of proteins by Western blotting were purchased from PIERCE (Rockford, IL). Laemmli Sample buffer and Tris-HCl precast gels were purchased from Bio-Rad (Hercules, CA). Nitrocellulose membranes were from Whatman (Dassel, Germany). Lipofectamine LTX reagent and Opti-MEM medium were purchased from Invitrogen (Carlsbad, CA). Alkaline phosphatase (AP) -tagged EGF receptor (EGFR) ligand (HB-EGF, amphiregulin, TGF-alpha) plasmids were prepared as described (18). F-12K nutrient mixture medium was purchased from American type Culture Collection (ATCC) (Rockville, MD). Penicillin (100 U/ml) and streptomycin sulfate (100 μg/ml) was purchased from Mediatech (Manassas, VA). Fetal bovine serum (FBS) was purchased from Lonza (Rockland, MD). All other reagents and chemicals were purchased from Sigma-Aldrich (St-Louis, MO). DuoSet Elisa kit Human HB-EGF and human amphiregulin were from R&D systems. Quantikine Human Immunoassay kit for detection of EGF and TGFα were purchased from R&D systems.
Cell culture
Human lung fibroblasts (CCL-151) and the human A549 alveolar epithelial cell line were purchased from ATCC and grown on tissue culture-treated plastic flasks in F-12K nutrient mixture medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin sulfate, and 10% FBS in a humidified incubator at 37 °C and an atmosphere of 5% CO2. Cells were grown to ~80% confluence in flasks, and subsequently propagated in 6-well plates or 12-well plates.
RNA isolation, reverse transcription PCR
Total RNA was purified from CCL-151 cell lysates with a commercially available kit (RNeasy; Qiagen, Valencia, CA). Equal amounts of RNA (2 μg) were reverse-transcribed using random hexamer primers and MultiScribe reverse transcriptase from Applied Biosystems (Foster city, CA), following the manufacturer’s protocol. The mRNAs for the subtypes of LPA receptors were amplified by RT-PCR using specific primers for each receptor subtype (LPA1–6). Gene-specific primers were designed with the Primer 3 software (Whitehead Institute for Biomedical Research, Cambridge, MA) (LPA1, sense 5′-ATCTTTGGCTATGTTCGCCA-3′, anti-sense 5′-TTGCTGTGAACTCCAGCCA- 3′; LPA2, sense 5′-TTGTCTTCCTGCTCATGGTG-3′, anti-sense 5′-AAGGGTGGAGTCCATCAGTG-3′; LPA3, sense 5′-TGCTCATTTTGCTTGTCTGG-3′, anti-sense 5′-GCCATACATGTCCTCGTCCT-3′; LPA4, sense 5′-TTCCTGGCCATTGTCTATCC-3′, anti-sense 5′-GTTCAGAGTTGCAAGGCA-CA-3′; LPA5, sense 5′-CAACAGCTCCTCAACCAACA-3′, anti-sense 5′-TGAGCATCAGGAAG- ATGCAG-3′; LPA6, sense 5′-TGGTGTTTGTGCTTGGGTTA-3′, anti-sense 5′-CCATGTGGCTTCTGGAAAAT-3′). The PCR products were electrophoretically separated on 1.5% agarose gel, stained with ethidium bromide, and analyzed by the ChemiGenius bioimaging system (Syngene, Federick, MD).
Preparation of cell lysates and Western blotting
After the indicated treatments, CCL-151 or A549 cells were lysed on ice in PBS lysis buffer containing 1% Triton X-100, phosphatase inhibitors and protease inhibitors. Lysates were precleared by centrifugation for 10 minutes at 14,000 x g at 4 °C. Supernatants were mixed with Laemmli Sample buffer containing 50 mM dithiothreitol and were boiled for 5 minutes and then cooled rapidly on ice. The samples were loaded on 7.5% or 10% SDS Tris-HCl precast gels and were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20 (TBS-T) for 3 hours at room temperature. The blots were probed with rabbit polyclonal anti-human phospho-ERK1/2 antibody or anti-human ERK1/2 antibody using a 1:1,000 dilution for both in TBS-T overnight at 4 °C. For EGF receptor phosphorylation, blots were probed with phospho-EGF receptor (Tyr1068) polyclonal antibody at 1:200 dilution. Blots were washed in TBS-T and subsequently incubated for 1 hour at room temperature in TBS-T with 5% nonfat dry milk containing horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2,000 dilution). The proteins were visualized by chemiluminescence. The bands were quantified by standard densitometry and normalized using the nonphosphorylated protein levels for ERK measurement or β-actin for p-EGFR.
Cell proliferation assay
Proliferation assays were performed in 96-well tissue culture treated plastic plates in quadruplicate. CCL-151 cells were plated at a density of 2×103 cells/well in 200 μl of F-12K nutrient mixture medium containing 10% FBS and incubated for 48 hours at 37°C. Media was removed and replaced with media containing 1% FBS along with various stimuli. Cells were incubated for an additional 96 hours. Cell number was measured using CyQUANT cell proliferation assay kit (Molecular Probes, Eugene, OR). Fluorescence measurements were made using a microplate reader with excitation at 485 nm and emission detection at 530 nm. The readings of the quadruplicate samples were averaged together for each experimental condition.
Transient transfection of AP-tagged EGFR ligands
Cells were seeded at 3×105 cells/well in 6 well plates and grown overnight. Five μl of Lipofectamine LTX reagent was added to prediluted 2 μg AP-tagged EGFR ligand (HB-EGF, amphiregulin, TGFα) in 500 μl Opti-MEM medium and incubated for 30 minutes. Spent growth medium was removed and replaced with 2 ml of fresh medium and 500 μl of the plasmid/lipofectamine mixture for 5 hours at 37 °C. The transfection mix was removed, the growth medium replaced and the mixture incubated for 24 hours before the shedding assays were performed.
AP tagged ligand shedding assay
The shedding assay was performed the day after transfection as described by Sahin et al. (19). Cells transfected with AP-tagged EGFR ligands were exposed to PMA (25 ng/ml) or LPA (10 μM). After stimulation, cell media (1ml per well) from non-stimulated and stimulated cells were collected and centrifuged at 15,000 x g for 30 minutes. Supernatants were saved at −80 °C until further use. Cells were lysed by adding 0.5 ml per well of lysis buffer containing 1x PBS, 1% Triton X-100 and protease inhibitor cocktail. Lysates were centrifuged at 15,000 x g for 30 minutes and supernatants were saved at −80 °C until further use. Analysis and quantification for the shedding of AP-tagged EGFR ligand was performed by colorimetry or by running the concentrated supernatants on SDS-polyacrylamide gels and staining the gels for alkaline phosphatase (AP) activity (termed the gel detection method) (19). Fresh Opti-MEM was added for 1 hour to the cells transfected with AP-tagged EGFR ligands. After 1 hour supernatants were collected and Opti-MEM with PMA (25 ng/ml) or LPA (10 μM) was added for 1 hour to the cells. The ratio between the AP activity in the supernatants collected from first 1 hour in the absence of stimuli (basal level) and those from next 1 hour in the presence of stimuli was calculated.
Native ligand shedding assays
Native EGFR ligands were measured in CCL-151 cell culture supernatants collected after 3 hours exposure to LPA (25 μM) or control media by sandwich ELISA technique. EGF and TGFα were quantified with pre-coated Elisa plates and reagents from Quantikine Human Immunoassay kit according to manufacturer’s instructions. For detection of HB-EGF and amphiregulin, capture antibody and biotinylated detection antibody were purchased from R&D Systems. 10 μg/mL capture antibody diluted in PBS solution was applied to clear 96 well microplates overnight. 1% bovine serum albumin (BSA) solution diluted in PBS was then applied as blocking solution for 1 hour. EGFR ligand standards were diluted serially in PBS and added alongside cell culture supernatants for 2 hours. Detection antibody at 2 μg/mL diluted in 1% BSA in PBS was added for 2 hours. To visualize results, HRP-Streptavidin enzyme diluted in PBS was added for 20 minutes. Optical density readouts were generated with Bio-Rad microplate reader 680 (Bio-Rad, Hercules, CA).
Quantitative PCR
To evaluate the expression level of HB-EGF, amphiregulin and c-fos mRNA, real-time PCR was performed using SYBR Green master Mix (Applied Biosystems) in a 7300 Real-Time PCR system (Applied Biosystems). For this purpose, total RNA was isolated from CCL-151 cell lysates after exposure to LPA (10 μM) or control medium using the RNeasy kit. Fold changes in transcript levels were calculated using the “delta delta Ct” method (20). The expression of housekeeping gene GAPDH was used as the reference standard. The target primers for HB-EGF, amphiregulin and GAPDH were previously described (21). The specific primers for c-fos (sense 5′-CGTGCCAGACATGGACCTATC-3′ and anti-sense 5′-GGCTCCCAGTCTGCTGCATA-3′) were selected from the c-fos nucleotide sequences by using Primer Express 3.0 (Applied Biosystems). c-fos primer has been tested against GAPDH over a range of concentrations to ensure similar PCR efficiency.
Paracrine Signaling Bioassay
To test effect of LPA-evoked paracrine signal from CCL-151 cells we used A549 lung epithelial cells as a reporter. Culture media was prepared from appropriately stimulated (LPA or no treatment) CCL-151 cells, or from control wells incubated under similar conditions in the absence of CCL-151 cells. Conditioned media samples were collected, centrifuged at 14,000 x g for 30 minutes and applied to A549 cells. To evaluate the role of EGFR ligands in paracrine signaling, A549 cells were incubated with AG1478 (500 nM) or vehicle for 1 hour before applying the supernatants from conditioned media.
Statistical Analysis
Comparisons between multiple groups were made by one-way analysis of variance; when significant differences were found, further comparisons were made by Bonferroni post hoc analysis. Student’s -t test was performed for experiments with one variable only. Data are expressed as means ± SEM from at least three or four independent experiments and statistical significance was accepted at P < 0.05.
RESULTS
LPA activates CCL-151 lung fibroblasts
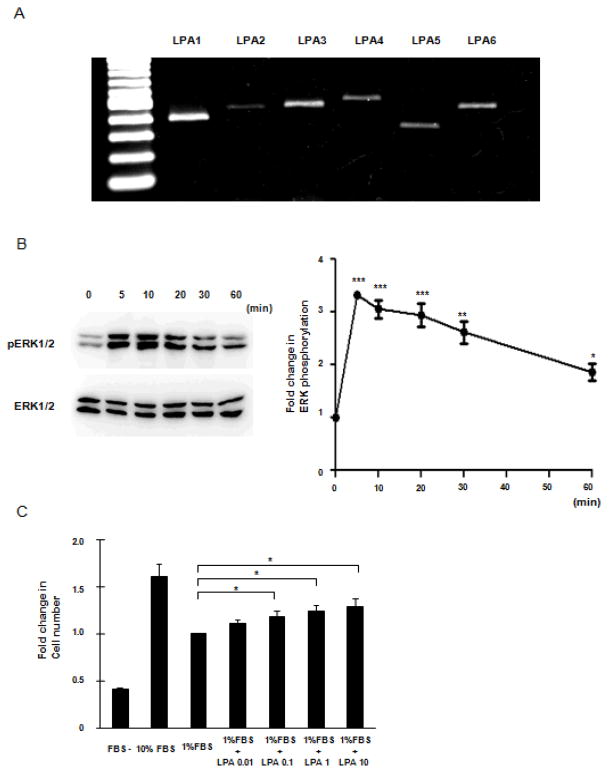
To investigate the expression of LPA receptors in CCL-151 normal lung fibroblast cell line we performed rt-PCR. A representative experiment (of five) shown in Figure 1A demonstrated the presence of mRNA encoding each of six known LPA subclass receptors on CCL-151 cells. While LPA1 was strongly expressed, in agreement with previous experiments in lung fibroblasts (9), we observed detectable message for all LPA receptors tested including the presence of the recently identified LPA6 (13).
Figure 1.
LPA receptor expression and responsiveness in CCL-151 cells. A: LPA receptor mRNA expression in CCL-151 cells assessed by PCR performed using primers to amplify either LPA1 (lane2), LPA2 (lane3), LPA3 (lane4), LPA4 (lane5), LPA5 (lane6) or LPA6 (lane7). Lane1 is a DNA ladder. B: Left side. Immunoblot showing phosphorylated ERK1/2 (p-ERK1/2) after stimulation with LPA; representative blots are shown from 3 independent experiments. Right side: Time course data are shown for 5, 10, 20, 30, 40, 50, and 60 minutes. Data were normalized by nonphosphorylated ERK and expressed relative to unstimulated controls. Results are expressed as mean ± SEM, n=3; ANOVA analysis showed significant differences between time course (P < 0.0001). Significant differences are indicated by ***P < 0.001, **P < 0.01 and * P < 0.05 compared with baseline. C: LPA induced proliferation of CCL-151 cells. Significant differences are indicated by *P < 0.05 compared with 1% FBS sample. Data are expressed as mean ± SEM of 3 independent experiments.
To confirm the functional activity of LPA receptors expressed on CCL-151 cells, we measured ERK activation in response to LPA stimulation, a well known response characterized in many cell types (22). To examine the response of CCL-151 cells to LPA, ERK phosphorylation was assessed using Western blot analysis with specific antibodies to ERK1/2 and phosphorylated (Thr202/Tyr204) ERK1/2. Treatment with LPA (10 μM) resulted in elevated phosphorylation of ERK1/2 (Fig. 1B) with the maximal response, an approximately threefold induction, noted at the first time point examined (5 minutes, 3.3 ± 0.04-fold induction, P < 0.001). The response waned over the following 60 minutes but ERK1/2 phosphorylation was still elevated above baseline at that time (1.8 ± 0.16-fold, P < 0.05).
Because it is well known LPA can induce cell proliferation for a variety of fibroblasts (2, 3, 23), we also assessed the effect of LPA on CCL-151 proliferation. Cell number was quantified after exposure to varying concentrations of LPA for 96 hours. We observed that LPA increased the number of CCL-151 cells in a dose-dependent manner, with the largest effect recorded at 10 μM (1.28 ± 0.09-fold, P < 0.05), resulting in an increase in cell number approximately half that observed with 10% FBS.
LPA induces ectodomain shedding of EGFR ligands
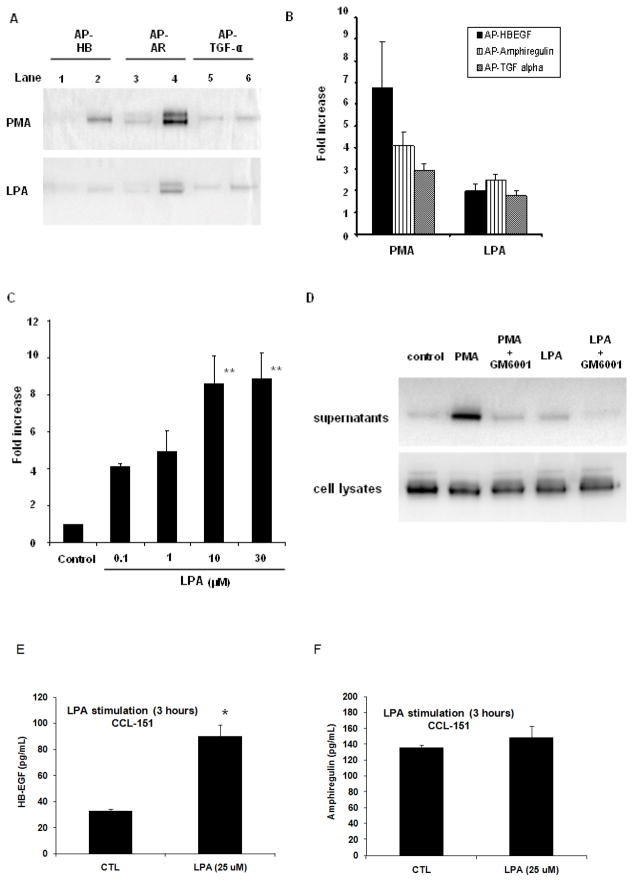
It is known that activation of GPCRs can stimulate ectodomain shedding of EGFR ligands (15, 24–26). Since LPA activates such receptors, we used a shedding assay (19) to determine if LPA would lead to ectodomain shedding of EGF-family ligands in CCL-151 fibroblasts. Our positive control, PMA exposure, resulted in an increase in AP activity of 6.7 ± 2.1-fold for AP-HB-EGF, 4.1 ± 0.6-fold for AP-amphiregulin and 2.9 ± 0.3-fold for AP-TGF alpha (all P < 0.05 compared with basal level). In response to LPA (10 μM), AP activity increased 2.0 ± 0.3-fold for AP-HB-EGF, 2.5 ± 0.3-fold for AP-amphiregulin and 1.8 ± 0.2-fold for AP-TGF alpha (P < 0.05 compared with basal level, Fig. 2A, B) The dose response for EGFR ligand release following LPA was examined in separate experiments using the release of AP-tagged amphiregulin as the outcome indicator. Increasing LPA concentrations (0.1 to 30 μM) were added to CCL-151 cells transfected with AP-tagged amphiregulin, with a plateau in response noted at 10μM (Fig. 2C). We tested whether pre-incubation of CCL-151 cells with a broad spectrum matrix metalloprotease inhibitor, GM6001 (10 μM) for 1 hour followed by stimulation with PMA (25 ng/ml) or LPA (10 μM) would modify AP-tagged ligand release. Treatment with GM6001 prominently decreased the PMA and LPA-induced increase in AP release (Fig. 2D). These data demonstrate that LPA induces increased release of AP-tagged EGFR ligands into the supernatant through ectodomain shedding.
Figure 2.
Shedding of EGFR ligands in CCL-151 cells. Shown here is the recovery of alkaline phosphatase-tagged HB-EGF (AP-HB), amphiregulin (AP-AR) or TGF-alpha (AP-TGFα). Cells were stimulated with PMA (20 ng/ml) or LPA (10 μM). AP-tagged EGFR ligands were detected by SDS-PAGE (A) or through spectrophotometry (B). A; lanes 1, 3, 5 show the AP-tagged EGFR ligands released into the supernatant from transfected CCL-151 cells under resting conditions (basal level). Lanes 2, 4, 6 show the AP-tagged EGFR ligands released 1 hour after addition of PMA or LPA. Data are representative from four independent experiments. B: AP activity of each EGFR ligand significantly increased after treatment with PMA (P < 0.05) or LPA (P < 0.05) compared with basal level. Data are expressed as mean ± SEM of 4 independent experiments. C: CCL-151 cells were transfected with alkaline phosphatase-tagged amphiregulin (AP-AR); AP activity was calculated by spectrophotometry. ANOVA analysis showed significant differences over the dose response (P = 0.0016). Significant differences are indicated by **P < 0.01 compared with control. Data are expressed as mean ± SEM of 3 independent experiments. D: CCL-151 cells were transfected with alkaline phosphatase-tagged HB-EGF (AP-HB) and stimulated with PMA (20 ng/ml) or LPA (10 μM) for 1 hour. Some samples were preincubated with the broad spectrum matrix metalloprotease inhibitor GM6001 (10 μM) for 1 hour before stimulation with PMA or LPA. GM6001 blocked release of AP-HB-EGF from the cells stimulated with PMA or LPA. Control indicates constitutively released AP-HB-EGF from transiently transfected CCL-151 cells. Data are representative from 3 independent experiments. E: LPA stimulation induces significant increase in HB-EGF shedding from CCL-151 cells (P= 0.006). Serum-starved CCL-151 cells were stimulated with LPA (25 μM) for 3 hours. After treatment HB-EGF release was examined by ELISA. Results are mean ± SD from 3 samples per condition, and are representative of 3 independent experiments. F: CCL-151 cells shed amphiregulin, but LPA did not significantly increase amphiregulin levels (P=0.24). Serum- starved CCL-151 cells were stimulated with LPA (25 μM) for 3 hours. After treatment amphiregulin release was examined by ELISA. Results are mean ± SD from 3 samples per condition, and are representative of 3 independent experiments.
To test whether LPA induces shedding of native ligands expressed by CCL-151 fibroblasts, we collected supernatants from fibroblasts stimulated for 3 hours with LPA (25 μM), or time matched controls. EGF and TGF-α were undetectable in supernatants, with detection limits of 3.9 pg/ml and 15.6 pg/ml respectively. However, both HB-EGF and amphiregulin were detectable in the supernatants under baseline culture conditions, and HB-EGF levels were significantly enhanced by LPA treatment, consistent with LPA induced shedding of HB-EGF (Figure 2E–F).
LPA stimulation of ERK1/2 in CCL-151 cells is independent of shedding
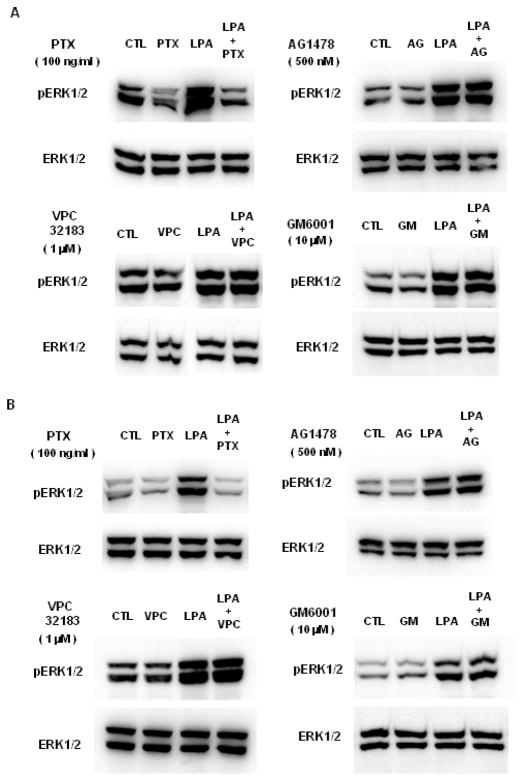
We previously demonstrated that CCL-151 fibroblasts express functional EGF receptors and respond to exogenous EGF (27). Therefore, to explore whether ectodomain shedding plays any role in the phosphorylation of ERK1/2 induced by LPA in CCL-151 cells we examined the effects of a panel of inhibitors. Cells were pretreated before LPA stimulation with pertussis toxin (PTX) (100 ng/ml), as a Gi/o inhibitor, or VPC32183 (1 μM), as a LPA1 and LPA3 receptor antagonist. Because EGFR transactivation has been previously recognized as contributing to LPA-induced ERK1/2 activation (16), we tested AG1478 (500 nM) as an EGFR inhibitor, and GM6001 (10 μM), as a broad spectrum matrix metalloprotease inhibitor. We first examined the effect of each inhibitor on ERK1/2 phosphorylation in response to 5 minutes of stimulation with LPA. We found that among the inhibitors tested only PTX had an effect on this response (Fig 3A). To see if there were different contributions to sustained ERK1/2 activation beyond 5 minutes, we also performed experiments using the same inhibitors following 60 minutes of stimulation and again found only PTX inhibited LPA-induced ERK1/2 phosphorylation (Fig 3.B). We confirmed that AG1478 blocked HB-EGF-induced phosphorylation of ERK1/2 in parallel experiments (data not shown), confirming both the functionality of AG1478 at the concentration used here, and the presence of functional EGFR on CCL-151 fibroblasts. Similarly, the data shown in Figure 2D established the functional effectiveness of GM6001 in blocking ectodomain shedding at the concentration used here. These results indicate that in CCL-151 cells LPA-induced ERK1/2 phosphorylation depends on Gi/o receptor signaling, but not EGFR signaling. When CCL-151 cells were directly stimulated with LPA (10 μM) or LPA plus EGF (100 ng/ml) for 5 minutes, the ERK phosphorylation responses were indistinguishable (data not shown). This result strongly suggests that the ERK response to LPA is saturated by non-autocrine signaling, and that the ligands shed from fibroblasts in response to LPA are therefore ineffective in further increasing the state of ERK activation.
Figure 3.
LPA-induced ERK1/2 phosphorylation is independent of EGFR. Serum-starved CCL-151 cells were stimulated with serum-free medium (control, CTL) or medium containing LPA (10 μM) in the absence or presence of inhibitors. Total cell lysates were immunoblotted with antibodies against phospho-ERK1/2 and total ERK1/2. A: CCL-151 cells were pretreated with Pertussis toxin (PTX) (100 ng/ml) for 18 hours, AG1478 (AG; 500 nM) for 1 hour, VPC32183 (VPC; 1 μM) for 30 minutes, GM6001 (GM; 10 μM) for 1 hour or vehicle (H2O for PTX; DMSO for AG1478 and GM6001; BSA for VPC32183) for the same period of time as each inhibitor, then stimulated with serum-free medium or LPA for 5 minutes (A) or for 1 hour (B). Results are representative of 3 independent experiments.
We do not have confirmatory evidence of the functionality of VPC32183 in fibroblasts, though we used it at a concentration that has been shown to be more than adequate to prevent LPA1 and LPA3 activation in other experiments (28, 29). In preliminary studies we found that VPC32183 did inhibit ERK phosphorylation in LPA-stimulated primary human bronchial epithelial cells, providing evidence of efficacy and indicating different receptor dependency in different lung cell types (data not shown). In a further effort to confirm the independence of the fibroblast ERK1/2 response from LPA1 and LPA3 receptors, we also used VPC12249, another combined LPA1/3 receptor antagonist, which yielded identical results of no attenuation of ERK1/2 activation (data not shown). These results indicate that receptors other than LPA1 and LPA3 are likely sufficient for LPA-mediated ERK1/2 activation in CCL-151 fibroblasts.
LPA induces c-fos, HB-EGF and amphiregulin expression in CCL-151 cells
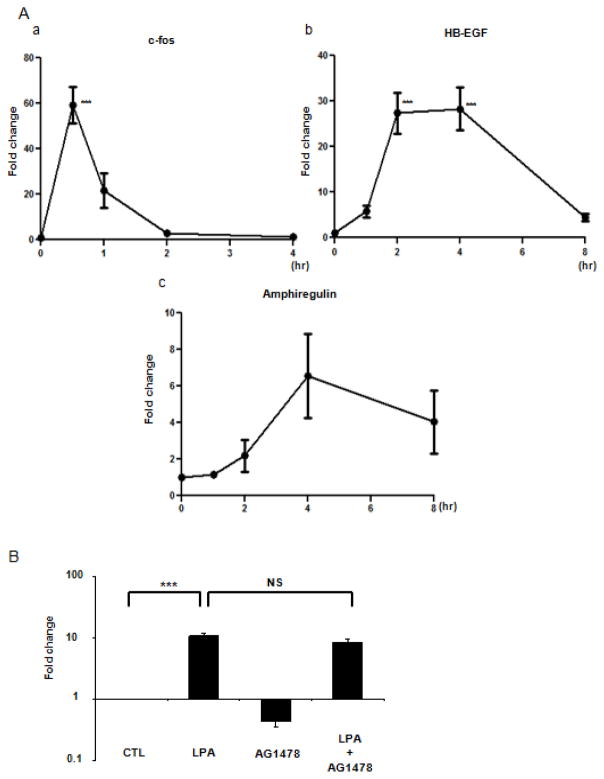
It has been previously shown that LPA exposure can induce c-fos, HB-EGF and amphiregulin expression in murine embryonic fibroblasts (17); we therefore examined gene expression for these entities in CCL-151 cells. We stimulated cells with LPA (10 μM) for 0, 0.5, 1, 2, or 4 hours; real time PCR was performed to assess the expression of c-fos, HB-EGF and amphiregulin. Expression of c-fos mRNA reached a maximum at 30 minutes (59 ± 7.9-fold, P < 0.001); RNA levels returned to baseline by 4 hours after treatment (Fig. 4A.a). LPA induced expression of HB-EGF mRNA that reached a maximum by 4 hours (28 ± 4.7-fold, P < 0.001). At 8 hours the expression of HB-EGF remained elevated and returned to baseline by 24 hours of treatment (data not shown) (Fig. 4A.b). Transcripts levels for amphiregulin were also upregulated in response to LPA stimulation, but with somewhat more modest level of enhanced expression (Fig. 4A.c). The increased expression of EGF-family ligands clearly lags behind the original shedding response and therefore does not play a direct role in the proximal signaling response to LPA. However, we have previously found that EGF-family ligand gene expression is strongly induced downstream of EGFR activation (21). To test whether EGFR transactivation plays a role in LPA-mediated expression of HB-EGF, we blocked EGF receptor activity using the selective EGF receptor tyrosine kinase inhibitor AG1478 (500 nM). This inhibitor had no effect on LPA-induced HB-EGF expression indicating that EGFR transactivation does not contribute to LPA-induced expression of HB-EGF (Fig.4B). Similar results were found using GM6001 (data not shown), indicating that ectodomain shedding in response to LPA stimulation is not required for induction of HB-EGF mRNA in CCL-151 fibroblasts. Because HB-EGF expression is known to be downstream of ERK activation (30), these results are consistent with LPA directly saturating the ERK pathway with no additional contribution from shed ligands.
Figure 4.
LPA induces c-fos, HB-EGF, and amphiregulin gene expression. A: Serum-starved CCL-151 cells were stimulated with LPA (10 μM) for 0.5, 1, 2 and 4 hours and probed for c-fos (a), or for 1, 2, 4 and 8 hours for HB-EGF (b), and amphiregulin (c) gene expression. Data are expressed as mean ± SEM of 3 independent experiments. ANOVA analysis showed significant differences in c-fos and HB-EGF gene expression (P < 0.0001). Significant differences after Bonferroni correction are indicated by ***P < 0.001 compared with time 0. B: Serum-starved CCL-151 cells were pretreated with vehicle (0.05% DMSO) or the EGFR inhibitor AG1478 (500 nM) for 1 hour and then stimulated with LPA (10 μM) for 4 hours. After treatments HB-EGF gene expression was examined. Data are expressed as mean ± SEM of 3 independent experiments; ANOVA analysis showed significant differences between groups (P < 0.0001). ***P < 0.001 CTL versus LPA; NS (not significant) LPA versus LPA + AG1478.
Activation of A549 cells by conditioned medium from LPA stimulated CCL-151 cells
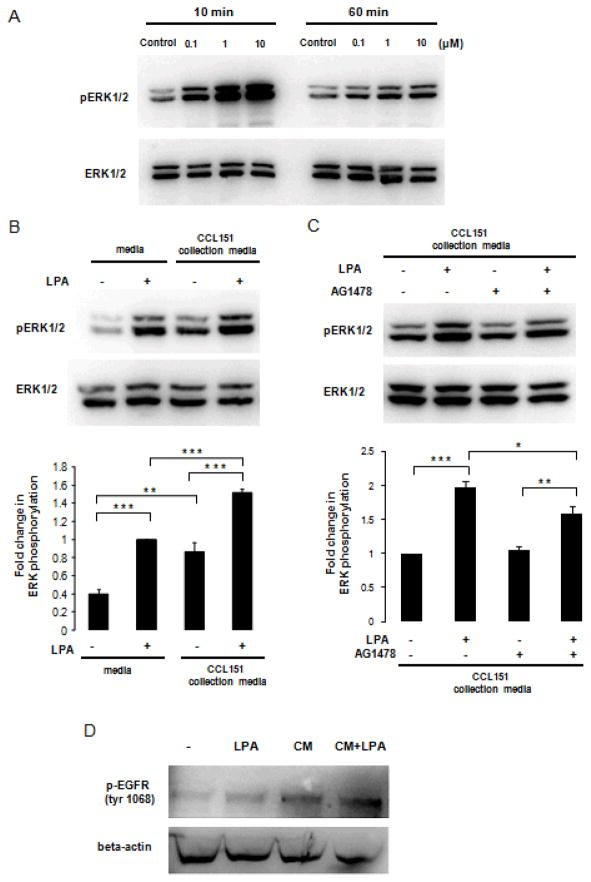
Based on our observation that LPA can induce EGF-family ligand shedding and enhances expression of EGF-family member mRNA, we tested whether soluble signals evoked in response to LPA play a paracrine role in cell-cell signaling. In these experiments we applied conditioned medium from CCL-151 cells stimulated with LPA to reporter A549 cells; phosphorylation of EGFR and ERK1/2 in the A549 cells were used as the outcome indicators. Since it is difficult to selectively and completely remove the LPA used to stimulate the CCL-151 cells, we first determined the extent to which LPA stimulates ERK1/2 in A549 cells directly. Stimulation of A549 cells with LPA (0.1, 1.0, or 10 μM) for 10 or 60 minutes resulted in a dose-dependent phosphorylation of ERK1/2 (Fig. 5A) in a manner similar to that observed in fibroblasts.
Figure 5.
LPA-stimulated fibroblasts activate A549 cells through paracrine signaling. A: Serum-starved A549 cells were stimulated with LPA 0.1–10 μM for 10 minutes or 60 minutes. Antibodies specific for phospho-ERK1/2 and total ERK1/2 were used for immunoblotting. B: Serum-starved A549 cells were treated with serum-free medium, serum-free medium containing 10 μM LPA, or conditioned media from CCL-151 cells with or without LPA. Results are representative of 3 independent experiments. Data are expressed as mean ± SEM of 3 independent experiments; ANOVA analysis showed significant differences between groups (P < 0.0001). Significant differences are indicated by **P < 0.01, ***P < 0.001. C: Serum-starved A549 cells were treated with AG1478 (500 nM) or vehicle for 1 hour. After treatments A549 cells were incubated with conditioned media from CCL-151 cells with or without LPA. Results are representative of 3 independent experiments. Data are expressed as mean ± SEM of 5 independent experiments; ANOVA analysis showed significant differences between groups (P < 0.0001). Significant differences are indicated by *P < 0.05, **P < 0.01, ***P < 0.001 D: Serum-starved A549 cells were treated with serum-free medium (-), serum-free medium containing 25 μM LPA (LPA), or conditioned media from CCL-151 cells treated for 3 hours with (CM+LPA) or without (CM) 25 μM LPA. EGFR phosphorylation at tyrosine 1068 was measured by Western blotting, and equal loading of proteins assessed byβ-actin level. Blots are representative of 2 independent experiments.
To directly measure the contribution of fibroblast shed ligands to paracrine A549 activation, we next stimulated CCL-151 cells with LPA (10 μM) for 1 hour, collected conditioned media and applied to A549 cells for 1 hour and measured resulting ERK phosphorylation. Because of the background effect of LPA on A549 cells we studied 4 conditioned media groups: serum-free medium collected from CCL-151 cells treated with and without LPA (10 μM), and serum-free medium that was incubated in empty wells (not exposed to CCL-151 cells) with and without LPA (10 μM). Conditioned media from CCL-151 cells incubated with 10 μM LPA, induced ERK1/2 phosphorylation in A549 cells by 1.5 ± 0.05-fold (P < 0.001) compared to serum-free medium containing 10 μM LPA in the absence of conditioning (Fig. 5B). Thus media collected from CCL-151 cells stimulated with LPA resulted in enhanced paracrine signal transduction due to soluble products released from the CCL-151 cells. To test the role EGFR activation plays in this paracrine ERK activation, the conditioned media experiments were repeated with AG1478 added to a subset of A549 cells prior to and during exposure to conditioned media. Blockade of EGFR using AG1478 diminished the ERK phosphorylation response elicited using media from LPA-stimulated fibroblasts by ~37% (P < 0.05) (Fig. 5C). Finally, to directly confirm that LPA stimulated fibroblasts release factors that activate the EGFR, we compared A549 cells responses to LPA (25 μM) alone, or to conditioned media from fibroblasts that had been stimulated with or without LPA (25 μM) for 3 hours. The conditioned media from LPA stimulated fibroblasts markedly enhanced EGFR phosphorylation in A549 cells (Fig. 5B), demonstrating that fibroblast products directly activate the EGFR. Together these findings indicate that LPA-stimulated fibroblasts emit soluble effectors that enhance ERK activation in lung epithelial cells, and that a statistically significant portion of this response can be attributed to shedding of EGF-family ligands.
DISCUSSION
LPA is present at high concentrations in serum, and is synthesized by activated platelets, rendering it a prominent mediator of tissue injury and wound healing responses (6, 7). Recent studies indicate that LPA is found in the airways in response to allergen challenge (8), and in patients with idiopathic pulmonary fibrosis (9). In the case of experimental fibrosis, LPA appears to play a prominent role in the injury and fibrotic response to bleomycin (9). Thus studying fibroblast responses to LPA could promote better understanding of lung repair and remodeling processes. At a molecular level LPA is known to exert powerful effects on cell proliferation, migration and contraction through activation of GPCRs LPA1–6. LPA is also known to induce transactivation of the EGFR through ectodomain shedding of EGF-family ligands in a variety of cell types (15, 16, 24–26, 31). We set out here to test whether ectodomain shedding of EGF-family ligands occurs in lung fibroblasts, and assess what role it plays in autocrine fibroblast signaling and paracrine activation of lung epithelium. Our data show that LPA induces ectodomain shedding of EGF-family ligands in human lung fibroblasts cells, but that this shedding process is not necessary for LPA-induced activation of ERK and early gene expression of HB-EGF, consistent with findings in murine embryonic fibroblasts (17). However, the LPA-induced activation of fibroblasts stimulated soluble factor release, a portion of which acted through the EGFR, to stimulate lung epithelial cells, thus amplifying the local fibroblast response.
Our data confirm prior work by showing that lung fibroblasts express high levels of message for the LPA1 receptor (9), and extend this work by demonstrating measurable expression of LPA2–6 receptor subtypes. While lung fibroblasts from patients with idiopathic pulmonary fibrosis express increased levels of LPA1 (9), and genetic deletion of LPA1 is protective in the bleomycin model of pulmonary fibrosis, we found that the selective LPA1 and LPA3 antagonists, VPC32183 and VPC12249, did not block LPA-induced ERK1/2 phosphorylation. Our results indicate that other LPA receptors present on lung fibroblasts are capable of transducing LPA stimulation into ERK phosphorylation. Further study will be needed to assign selective roles of LPA receptor subtypes to the ERK response to LPA stimulation documented here.
All ligands of the EGF family are produced as membrane bound forms and released by ectodomain shedding (19), and more broadly ectodomain shedding has emerged as an important posttranslational mechanism to regulate the functions of a variety of membrane proteins (32). Based on evidence for involvement of LPA in lung disease (8, 9) and the fact that LPA acts through G protein-coupled receptors that are known to stimulate ectodomain shedding, we hypothesized LPA would stimulate the shedding of EGF-family ligands in lung fibroblasts. Using an ectodomain shedding assay (19) and measurement of native ligand shedding, our data demonstrate for the first time that LPA stimulation induces EGF-family shedding in human lung fibroblasts.
We observed that LPA elicited the phosphorylation of ERK1/2 with peak activation occurring on the order of 5 to 10 minutes after stimulation in lung fibroblasts; this is consistent with the findings of others (33). The observed LPA-induced ERK1/2 phosphorylation was sensitive to Gi/o protein inhibition by PTX, a finding common to many though not all cell types (26, 34, 35). However, LPA-induced ERK1/2 phosphorylation was insensitive to the EGFR inhibitor AG1478 and the metalloprotease inhibitor GM6001. These findings demonstrate that ERK1/2 activation in lung fibroblasts is downstream of LPA-GPCR interaction, but not dependent on transactivation of EGFR or ectodomain shedding of EGFR ligands. Our findings concur with previous observations in other fibroblast systems that LPA-induced MAP kinase activation does not require EGFR transactivation (36), and suggest that LPA saturates ERK activation independently of ligand shedding effects. In contrast, another lung cell type, bronchial epithelial cells (37), have been shown to require EGF-family ectodomain shedding and EGFR transactivation to link LPA stimulation to ERK activation. Our observation that AG1478 did not inhibit LPA-induced expression of HB-EGF concurs with the recent report that LPA-induced gene expression is largely independent of EGF receptor activity in mouse embryonic fibroblasts (17). Taken together, these data indicate that LPA is capable of inducing ectodomain shedding in lung fibroblasts, but that in these cells shedding is not necessary for ERK activation and early gene expression responses to LPA.
Since LPA-induced shedding was not critical to the fibroblast responses studied here, we expanded our study to examine paracrine effects on the lung epithelial cell line A549. We found that LPA induced changes in the conditioned medium harvested from CCL-151 cells that led to enhanced EGFR and ERK1/2 phosphorylation in A549 reporter cells. A portion of this response was inhibited by AG1478 blockade of EGFR signaling, consistent with a paracrine role for the ligands shed from fibroblasts in response to LPA stimulation. Coupled with the prominent induction of EGF-family members HB-EGF and amphiregulin in fibroblasts at later time points in response to LPA, the paracrine signaling mechanism documented here has the potential to both prolong the response to LPA and expand its reach to neighboring cells. Similar paracrine activity of shed ligands has been previously observed in mammary ductal morphogenesis, but interestingly in this scenario the ligands are shed from the epithelium and stimulate neighboring stromal cells (38). Because EGFR activation is implicated in migration and wound healing in epithelium (39, 40), the paracrine signaling from LPA-stimulated fibroblasts may contribute to the overall wound healing response in neighboring epithelium. The remaining soluble factors evoked in response to LPA that signal from fibroblasts to epithelium remain to be determined, but given the short time course studied here shedding of additional non-EGF family peptides are attractive candidates (41).
In total, our data expand the role of LPA signaling in fibroblasts beyond direct effects on proliferation, migration and contraction to include stimulated release of soluble substances that can activate lung epithelial cells. We speculate that in the context of wound healing, such soluble factor production and subsequent paracrine signaling may constitute an important promoter of repair, and may help to spread and prolong the wound healing response, effectively amplifying the signal that is initiated by LPA binding to fibroblasts at the site of injury.
Acknowledgments
This work was supported by NIH grants RO1 HL088028 and HL092961.
References
- 1.Moolenaar WH. Bioactive lysophospholipids and their G protein-coupled receptors. Exp Cell Res. 1999;253:230–8. doi: 10.1006/excr.1999.4702. [DOI] [PubMed] [Google Scholar]
- 2.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–99. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 3.van Corven EJ, van Rijswijk A, Jalink K, van der Bend RL, van Blitterswijk WJ, Moolenaar WH. Mitogenic action of lysophosphatidic acid and phosphatidic acid on fibroblasts. Dependence on acyl-chain length and inhibition by suramin. Biochem J. 1992;281 ( Pt 1):163–9. doi: 10.1042/bj2810163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolodney MS, Elson EL. Correlation of myosin light chain phosphorylation with isometric contraction of fibroblasts. J Biol Chem. 1993;268:23850–5. [PubMed] [Google Scholar]
- 5.van Corven EJ, Groenink A, Jalink K, Eichholtz T, Moolenaar WH. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989;59:45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- 6.Eichholtz T, Jalink K, Fahrenfort I, Moolenaar WH. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J. 1993;291 ( Pt 3):677–80. doi: 10.1042/bj2910677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watterson KR, Lanning DA, Diegelmann RF, Spiegel S. Regulation of fibroblast functions by lysophospholipid mediators: potential roles in wound healing. Wound Repair Regen. 2007;15:607–16. doi: 10.1111/j.1524-475X.2007.00292.x. [DOI] [PubMed] [Google Scholar]
- 8.Georas SN, Berdyshev E, Hubbard W, Gorshkova IA, Usatyuk PV, Saatian B, Myers AC, Williams MA, Xiao HQ, Liu M, Natarajan V. Lysophosphatidic acid is detectable in human bronchoalveolar lavage fluids at baseline and increased after segmental allergen challenge. Clin Exp Allergy. 2007;37:311–22. doi: 10.1111/j.1365-2222.2006.02626.x. [DOI] [PubMed] [Google Scholar]
- 9.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, Hart WK, Pardo A, Blackwell TS, Xu Y, Chun J, Luster AD. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 10.Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278:25600–6. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 11.Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem. 2006;281:23589–97. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- 12.Tabata K, Baba K, Shiraishi A, Ito M, Fujita N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem Biophys Res Commun. 2007;363:861–6. doi: 10.1016/j.bbrc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 13.Yanagida K, Masago K, Nakanishi H, Kihara Y, Hamano F, Tajima Y, Taguchi R, Shimizu T, Ishii S. Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6. J Biol Chem. 2009;284:17731–41. doi: 10.1074/jbc.M808506200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Natarajan V. Lysophosphatidic acid signaling in airway epithelium: role in airway inflammation and remodeling. Cell Signal. 2009;21:367–77. doi: 10.1016/j.cellsig.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–8. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 16.Wu J, Cunnick JM. Trans-regulation of epidermal growth factor receptor by lysophosphatidic acid and G protein-coupled receptors. Biochim Biophys Acta. 2002;1582:100–6. doi: 10.1016/s1388-1981(02)00143-9. [DOI] [PubMed] [Google Scholar]
- 17.Stortelers C, Kerkhoven R, Moolenaar WH. Multiple actions of lysophosphatidic acid on fibroblasts revealed by transcriptional profiling. BMC Genomics. 2008;9:387. doi: 10.1186/1471-2164-9-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tokumaru S, Higashiyama S, Endo T, Nakagawa T, Miyagawa JI, Yamamori K, Hanakawa Y, Ohmoto H, Yoshino K, Shirakata Y, Matsuzawa Y, Hashimoto K, Taniguchi N. Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. J Cell Biol. 2000;151:209–20. doi: 10.1083/jcb.151.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahin U, Weskamp G, Zheng Y, Chesneau V, Horiuchi K, Blobel CP. A sensitive method to monitor ectodomain shedding of ligands of the epidermal growth factor receptor. Methods Mol Biol. 2006;327:99–113. doi: 10.1385/1-59745-012-X:99. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Chu EK, Foley JS, Cheng J, Patel AS, Drazen JM, Tschumperlin DJ. Bronchial epithelial compression regulates epidermal growth factor receptor family ligand expression in an autocrine manner. Am J Respir Cell Mol Biol. 2005;32:373–80. doi: 10.1165/rcmb.2004-0266OC. [DOI] [PubMed] [Google Scholar]
- 22.Moolenaar WH. Lysophosphatidic acid signalling. Curr Opin Cell Biol. 1995;7:203–10. doi: 10.1016/0955-0674(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Han Y, Zhu W, Ma R, Han B, Cong X, Hu S, Chen X. Specific receptor subtype mediation of LPA-induced dual effects in cardiac fibroblasts. FEBS Lett. 2006;580:4737–45. doi: 10.1016/j.febslet.2006.07.061. [DOI] [PubMed] [Google Scholar]
- 24.Takenobu H, Yamazaki A, Hirata M, Umata T, Mekada E. The stress- and inflammatory cytokine-induced ectodomain shedding of heparin-binding epidermal growth factor-like growth factor is mediated by p38 MAPK, distinct from the 12-O-tetradecanoylphorbol-13-acetate- and lysophosphatidic acid-induced signaling cascades. J Biol Chem. 2003;278:17255–62. doi: 10.1074/jbc.M211835200. [DOI] [PubMed] [Google Scholar]
- 25.Herrlich A, Klinman E, Fu J, Sadegh C, Lodish H. Ectodomain cleavage of the EGF ligands HB-EGF, neuregulin1-beta, and TGF-alpha is specifically triggered by different stimuli and involves different PKC isoenzymes. Faseb J. 2008;22:4281–95. doi: 10.1096/fj.08-113852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirata M, Umata T, Takahashi T, Ohnuma M, Miura Y, Iwamoto R, Mekada E. Identification of serum factor inducing ectodomain shedding of proHB-EGF and sStudies of noncleavable mutants of proHB-EGF. Biochem Biophys Res Commun. 2001;283:915–22. doi: 10.1006/bbrc.2001.4879. [DOI] [PubMed] [Google Scholar]
- 27.Boudreault F, Tschumperlin DJ. Stretch-induced mitogen-activated protein kinase activation in lung fibroblasts is independent of receptor tyrosine kinases. Am J Respir Cell Mol Biol. 2010;43:64–73. doi: 10.1165/rcmb.2009-0092OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heise CE, Santos WL, Schreihofer AM, Heasley BH, Mukhin YV, Macdonald TL, Lynch KR. Activity of 2-substituted lysophosphatidic acid (LPA) analogs at LPA receptors: discovery of a LPA1/LPA3 receptor antagonist. Mol Pharmacol. 2001;60:1173–80. doi: 10.1124/mol.60.6.1173. [DOI] [PubMed] [Google Scholar]
- 29.Hurst-Kennedy J, Boyan BD, Schwartz Z. Lysophosphatidic acid signaling promotes proliferation, differentiation, and cell survival in rat growth plate chondrocytes. Biochim Biophys Acta. 2009;1793:836–46. doi: 10.1016/j.bbamcr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Tschumperlin DJ, Shively JD, Swartz MA, Silverman ES, Haley KJ, Raab G, Drazen JM. Bronchial epithelial compression regulates MAP kinase signaling and HB-EGF-like growth factor expression. Am J Physiol Lung Cell Mol Physiol. 2002;282:L904–11. doi: 10.1152/ajplung.00270.2001. [DOI] [PubMed] [Google Scholar]
- 31.Gschwind A, Hart S, Fischer OM, Ullrich A. TACE cleavage of proamphiregulin regulates GPCR-induced proliferation and motility of cancer cells. Embo J. 2003;22:2411–21. doi: 10.1093/emboj/cdg231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlondorff J, Blobel CP. Metalloprotease-disintegrins: modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J Cell Sci. 1999;112 ( Pt 21):3603–17. doi: 10.1242/jcs.112.21.3603. [DOI] [PubMed] [Google Scholar]
- 33.Wallert MA, Thronson HL, Korpi NL, Olmschenk SM, McCoy AC, Funfar MR, Provost JJ. Two G protein-coupled receptors activate Na+/H+ exchanger isoform 1 in Chinese hamster lung fibroblasts through an ERK-dependent pathway. Cell Signal. 2005;17:231–42. doi: 10.1016/j.cellsig.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Fang X, Yu S, LaPushin R, Lu Y, Furui T, Penn LZ, Stokoe D, Erickson JR, Bast RC, Jr, Mills GB. Lysophosphatidic acid prevents apoptosis in fibroblasts via G(i)-protein-mediated activation of mitogen-activated protein kinase. Biochem J. 2000;352(Pt 1):135–43. [PMC free article] [PubMed] [Google Scholar]
- 35.Kranenburg O, Moolenaar WH. Ras-MAP kinase signaling by lysophosphatidic acid and other G protein-coupled receptor agonists. Oncogene. 2001;20:1540–6. doi: 10.1038/sj.onc.1204187. [DOI] [PubMed] [Google Scholar]
- 36.Andreev J, Galisteo ML, Kranenburg O, Logan SK, Chiu ES, Okigaki M, Cary LA, Moolenaar WH, Schlessinger J. Src and Pyk2 mediate G-protein-coupled receptor activation of epidermal growth factor receptor (EGFR) but are not required for coupling to the mitogen-activated protein (MAP) kinase signaling cascade. J Biol Chem. 2001;276:20130–5. doi: 10.1074/jbc.M102307200. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, He D, Saatian B, Watkins T, Spannhake EW, Pyne NJ, Natarajan V. Regulation of lysophosphatidic acid-induced epidermal growth factor receptor transactivation and interleukin-8 secretion in human bronchial epithelial cells by protein kinase Cdelta, Lyn kinase, and matrix metalloproteinases. J Biol Chem. 2006;281:19501–11. doi: 10.1074/jbc.M511224200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sternlicht MD, Sunnarborg SW, Kouros-Mehr H, Yu Y, Lee DC, Werb Z. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development. 2005;132:3923–33. doi: 10.1242/dev.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazie AR, Spix JK, Block ER, Achebe HB, Klarlund JK. Epithelial cell motility is triggered by activation of the EGF receptor through phosphatidic acid signaling. J Cell Sci. 2006;119:1645–54. doi: 10.1242/jcs.02858. [DOI] [PubMed] [Google Scholar]
- 40.Puddicombe SM, Polosa R, Richter A, Krishna MT, Howarth PH, Holgate ST, Davies DE. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. Faseb J. 2000;14:1362–74. doi: 10.1096/fj.14.10.1362. [DOI] [PubMed] [Google Scholar]
- 41.Overall CM, Blobel CP. In search of partners: linking extracellular proteases to substrates. Nat Rev Mol Cell Biol. 2007;8:245–57. doi: 10.1038/nrm2120. [DOI] [PubMed] [Google Scholar]