Abstract
Whole-body heating decreases pulmonary capillary wedge pressure and cerebral vascular conductance, and causes an inotropic shift in the Frank-Starling curve. Whole-body cooling increases pulmonary capillary wedge pressure and cerebral vascular conductance without changing systolic function. These and other data indicate factors affecting cardiac function may mechanistically contribute to syncope during heat stress and improvements in orthostatic tolerance during cold stress.
Keywords: hyperthermia, hypothermia, cardiac contractility, pressure-volume relations, orthostatic tolerance
INTRODUCTION
Heat and cold stress have direct impact on many of the fundamental control mechanisms of stroke volume such as preload, afterload, diastolic function or compliance, and systolic function or inotropy. The purpose of this review is to integrate a recent series of thermal physiology studies performed by the authors (2, 4, 10, 29, 32, 35) that identify the mechanisms by which heat and cold stress alter cardiac mechanics and function. These data and mechanisms will then be applied to our understanding of thermal-induced changes in orthostatic tolerance, where heat stress increases and cold stress decreases the likelihood of syncope during an orthostatic challenge in humans. The importance of thermal-induced changes in orthostatic tolerance is evident in occupations where workers are in hot ambient conditions while in the upright position and thus are at risk for heat syncope or where a countermeasure such as skin-surface cooling may need to be employed to increase orthostatic tolerance.
In this review, heat and cold stress are delimited, unless otherwise mentioned, to human passive-thermal stresses where human subjects are exposed to a warm or cool environment via a water-perfused garment (fitted suit that covers the body except the hands, feet, neck and head) rather than a stress that also induces a significant change in metabolism (e.g., occupational tasks, exercise, or shivering). This important distinction of passive-thermal stress versus exercise-thermal stress allows for the determination of direct thermal effects or physiological responses to the thermal stress independent of increases in cardiac output needed to perfuse working muscles or other changes associated with exercise-heat (28) or exercise-cold (25) stress. The water-perfused garment method of passive-thermal stress clamps skin temperature at an elevated temperature which then increases internal temperature during whole-body heating, while during whole-body (or skin-surface) cooling skin temperature is clamped at a lower temperature but does not change internal temperature. These passive-thermal stresses cause large redistributions of blood flow, especially in the skin, but these blood flow redistributions are different than those seen during exercise and some other passive-thermal stress models such as water immersion.
CONTROL AND REGULATION OF STROKE VOLUME
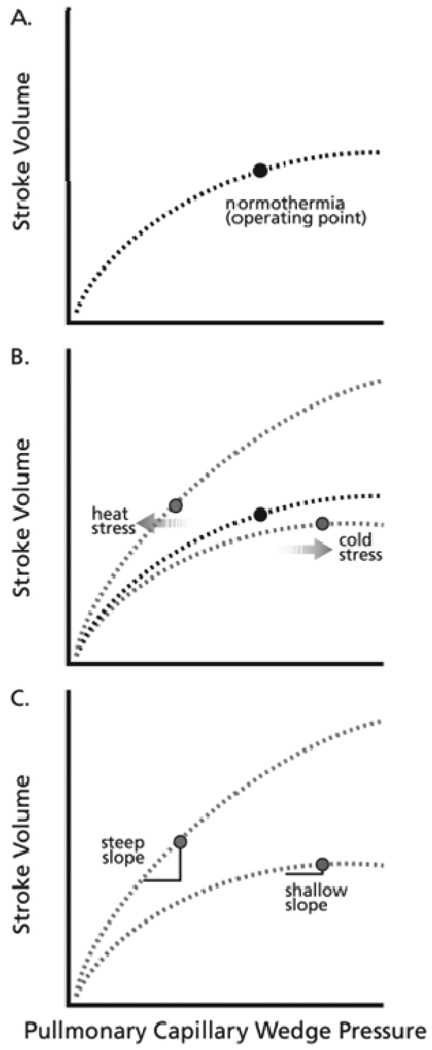
Cardiac output increases during whole-body heating but does not significantly change during whole-body cooling (17, 31, 34, 35). In contrast, stroke volume is maintained or slightly increased by both thermal stressors (17, 34, 35). Hyperbolic Frank-Starling relations (plotting stroke volume to left-ventricular filling pressure) can be used to explore the control and regulation of stroke volume during thermal stresses (Fig. 1, Panel A). In healthy humans, left-ventricular filling pressure can be indexed with pulmonary capillary wedge pressure obtained via a Swan-Ganz catheter. Data from our studies indicate that whole-body heating shifts the operating point to a steeper portion of a Frank-Starling curve, compared to the relatively plateaued location of the operating point on a normothermic Frank-Starling curve (Fig. 1, Panel B). This shift results in a larger reduction in stroke volume per reduction in left ventricular end-diastolic pressure indexed from pulmonary capillary wedge pressure ((29); Fig. 1, Panel C). In contrast, during whole-body cooling the operating point shifts upward on the plateau portion of a Frank-Starling curve compared to normothermia; this shift results in a relatively small reduction in stroke volume per reduction in left ventricular end-diastolic pressure during a subsequent orthostatic challenge ((29); Fig. 1, Panels B and C). The downward shift of the operating point to a steeper portion of a Frank-Starling curve during heat stress and the upward shift to a flatter portion of a Frank-Starling curve during cold stress demonstrate direct thermal effects on the control and regulation of stroke volume. However, other physiological parameters affect the Frank-Starling relation and are modified by thermal stress, such as preload, afterload, compliance, and inotropy.
Figure 1.
Schematic of the effect of thermal stress on the Frank-Starling relations. Panel A represents a normothermia Frank-Starling relation with the labeled point being the operating point in the supine position; Panel B represents associated changes in the curves related to heat stress and cold stress as well as the location of the supine operating points on those curves; Panel C highlights slope changes in the curves where a similar decrease in pulmonary capillary wedge pressure would cause a relatively large change in stroke volume during heat stress and a relatively small change during cold stress.
Preload
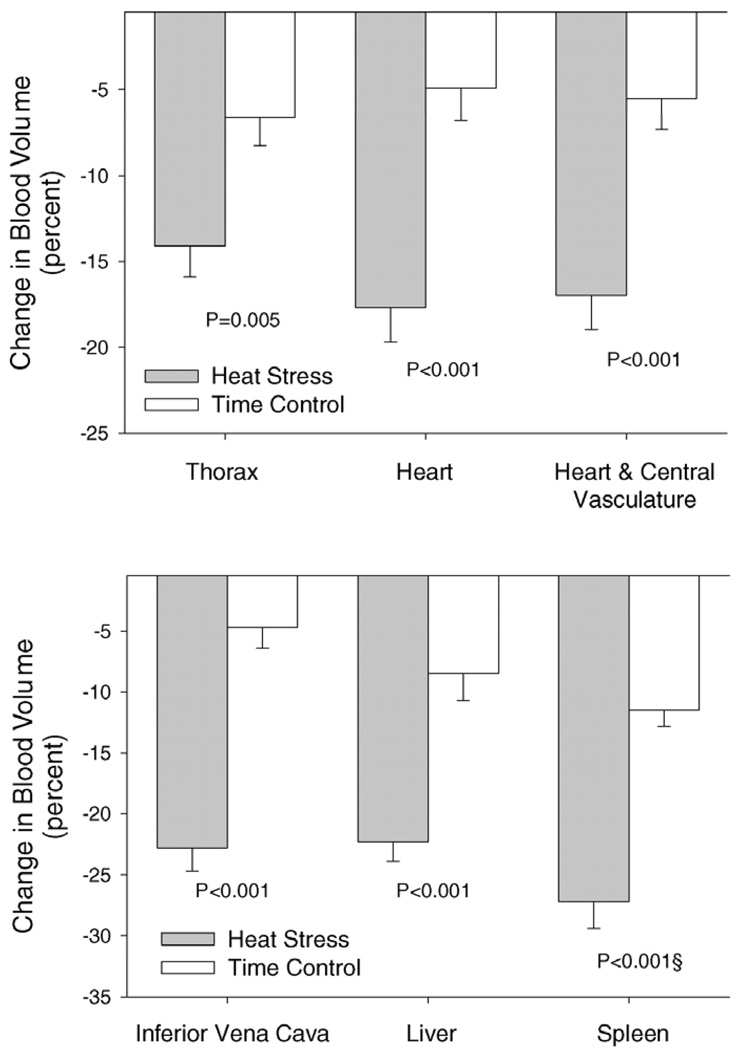
Whole-body heating decreases right-sided preload (right atrial pressure and central venous pressure; (8, 10, 18, 19, 21, 22, 35)), as well as left-sided preload (left-ventricular end diastolic volume and pulmonary capillary wedge pressure (35)). In healthy humans exposed to heat stress, right- and left-sided preload decrease by a similar magnitude (35). This indicates that central venous pressure can be used to predict changes in left-ventricular filling pressure during thermal stress. The reason for this decrease in preload during heat stress is likely multifactoral being related to blood volume distribution, cardiac output/venous return, and systemic vascular conductance. Heat stress increases cutaneous vascular volume (14) and translocates blood volume out of the splanchnic, renal, and central reservoirs ((10); Fig. 2). Decreases in central blood volume (10) along with decreases in left-ventricular end diastolic volume (29) lead to a scenario where preload is decreased initially and further changes in preload, such as that which occurs during an orthostatic challenge, could lead to large reductions in stroke volumes as predicted and identified in Figure 1.
Figure 2.
Percentage change in blood volume from the indicated regions between whole-body heating and associated normothermic time control subjects. (Reprinted from Crandall CG, Wilson TE, Marving J, et al. Effects of passive heating on central blood volume and ventricular dimensions in humans. J Physiol. 2008;586:293-301. Copyright © 2008 Wiley-Blackwell. Used with permission.)
Cold stress marginally increases both right (central venous pressure; (12, 35)) and left (pulmonary capillary wedge pressure; (35)) sided preload. In healthy humans, right- and left-sided preload increase by a similar magnitude and thus indicates that central venous pressure can be used to predict changes in left-ventricular filling pressure during cold stress (35). However, despite these changes in pressure, corresponding changes in left-ventricular end-diastolic volumes are often not observed in young healthy individuals, but are in older individuals (29, 32). Central blood volume assessments during whole-body cooling have only been indirectly measured via impedance, with mixed results (5, 12). Thus, in the supine/prone position, cold stress increases pulmonary capillary wedge pressure without altering stroke volume and subsequent reductions in stroke volume for a given reduction in pulmonary capillary wedge pressure during an orthostatic stress are attenuated. This is consistent with shifts in the operating point to a flatter portion of a Frank-Starling curve (Fig. 1).
Afterload
Heat stress does not change or slightly decreases mean arterial pressure despite large increases in cardiac output, while systemic vascular conductance increases substantially due primarily to large increases in cutaneous vascular conductance (17). In contrast to the cutaneous circulation, renal and splanchnic vascular conductance decreases by heat stress (18, 22, 27). Increases in muscle sympathetic nerve activity by this thermal condition (7, 13, 24) indicates a greater vasoconstrictor signal is sent to muscle blood vessels, but it is currently unclear if muscle vascular conductance is altered in these conditions. Mean pulmonary artery pressure decreases during heat stress and this, coupled with the increase in cardiac output, results in no significant alteration in pulmonary vascular conductance (35).
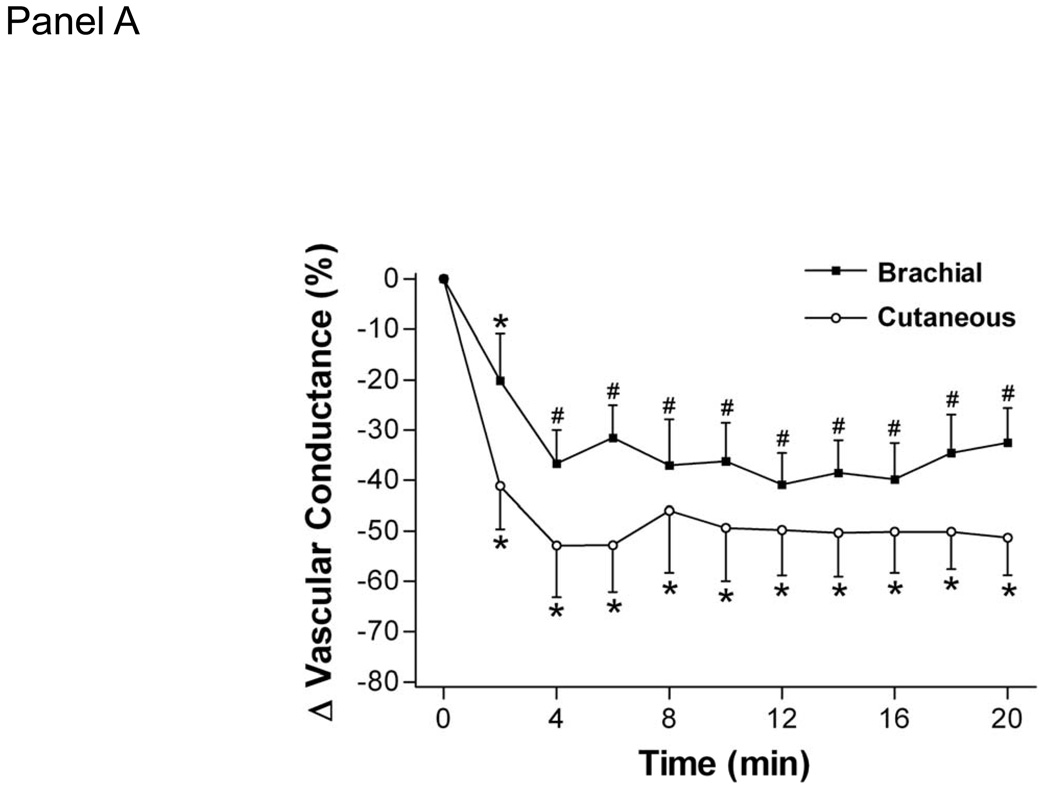
Cold stress increases mean arterial pressure and decreases systemic vascular conductance (11, 12, 15, 16, 31, 32, 35). This increase in mean arterial pressure is related to decreases in vascular conductance in both glabrous and non-glabrous cutaneous vasculature, and in the brachial, renal, celiac, and superior mesenteric arteries ((34); Fig. 3). Decreases in brachial vascular conductance are believed to be confined primarily to the skin because muscle sympathetic nerve activity does not appreciably change during whole-body cooling (11). The increase in mean arterial pressure in response to whole-body cooling is related to age and to central arterial stiffness, where individuals with stiffer arteries experience greater increases in arterial blood pressure (16). Pulmonary vascular conductance does not significantly change during whole-body cooling, likely because there is little to no change in cardiac output coupled with both mean pulmonary artery pressure and pulmonary capillary wedge pressure increasing by a similar magnitude (35).
Figure 3.
Effect of 20-min whole body cooling on the percent change (Δ) from baseline of brachial vascular conductance, cutaneous vascular conductance of the dorsal hand, celiac superior mesenteric, and renal vascular conductance indexes. Time 0 denotes normothermic baseline; baseline brachial vascular conductance =32 ± 15 cm·s−1mmHg−1, cutaneous vascular conductance =0.93 ± 0.68 flux units/mmHg, baseline celiac vascular conductance =85 ± 22 cm·s−1 mmHg−1, superior mesenteric vascular conductance = 55 ± 16 cm·s−1·mmHg−1, and renal vascular conductance = 74 ± 26 cm·s−1·mmHg−1. *Significant difference from time 0; P < 0.05. #Significant difference from both time 0 and minute 2, P < 0.05. (Reprinted from Wilson TE, Sauder CL, Kearney ML, et al. Skin-surface cooling elicits peripheral and visceral vasoconstriction in humans. J Appl Physiol. 2007;103:1257–1262. Copyright © 2007 The American Physiological Society. Used with permission.)
Compliance and Diastolic Function
No significant alteration in left-ventricular compliance is observed during acute thermal perturbations, as represented when we express pulmonary capillary wedge pressure to left-ventricular end-diastolic volume (29). During whole-body heating indices of diastolic function (e.g., ratio of blood velocity/mitral annular tissue velocity during the early phase of diastole and rate of untwisting) are preserved despite decreases in preload, thus indicating that subtle improvements in diastolic function may be occurring (2, 23). During whole-body cooling, variables associated with diastolic function are not significantly altered in young healthy individuals or in older individuals with already reduced diastolic function (32).
Inotropy
Heat stress decreases preload, yet stroke volume is maintained or slightly increased (17). Radionucleotide multi-gated acquisition and echocardiography data indicate increases in ejection fraction (10, 29) and isovolumic acceleration of the septal and lateral mitral annulus (2) with the imposition of a heat stress. Also identifying a positive inotropy effect during whole-body heating, Nelson et al. (23) observed increases in left-ventricular twist rates. During recent studies to more fully characterize the impact of heat stress on the Frank-Starling relation, we included colloid volume loading (12 ml/kg Voluven) during whole-body heating (4). The intent of the colloid volume loading was to increase preload in order to identify if there was a leftward shift in the Frank-Starling relation or if the volume loading just further extended the normothermic curve. Our data identify a leftward shift in the Frank-Starling relation and provide further support that positive inotropy is present during whole-body heating (Fig. 1, Panel B).
Whole-body cooling does not significantly change ejection fraction or tissue-Doppler derived values of systolic function (32). This finding was important to identify because it is possible that a rightward shift in the Frank-Starling relation due to afterload could have been masking positive inotropy changes (leftward shifts in the Frank-Starling relation). Because whole-body cooling increases mean arterial pressure and central venous pressure, it is possible that loading of arterial and cardiopulmonary baroreceptors is inhibiting a positive inotropy response to cold stress.
CONTROL AND REGULATION OF HEART RATE
Cardiac output increases to values often well above 10 L•min−1 during whole-body heating (17). Because stroke volume is maintained or only slightly increased during heat stress, the increase in heart rate is the primary driving force behind these increases in cardiac output. The control and regulation of heart rate during heat stress may be related to: 1) direct effects of temperature on the sinoatrial node, and 2) sympathetic and parasympathetic effects on the heart due to either the baroreflexes or a global hyperadrenergic state. During cold stress heart rate does not significantly change (29, 35).
The efferent arm of the arterial baroreflexes may participate in increasing heart rate during whole-body heating if mean arterial pressure trends downward during the heat stress (6). In contrast, during cooling baroreflexes could be acting to suppress heart rate as there is loading of carotid, aortic, and cardiopulmonary baroreceptors. The absence of a change in heart rate during whole-body cooling suggests other factors are counteracting this baroreflex suppression of heart rate. Heat stress can be described as a global hyperadrenergic state (26) resulting in both increased noradrenergic signaling and circulating catecholamines. It is unknown if cold stress results in a global hyperadrenergic state although increases in plasma norepinephrine are observed (15).
IMPLICATION FOR ORTHOSTATIC TOLERANCE
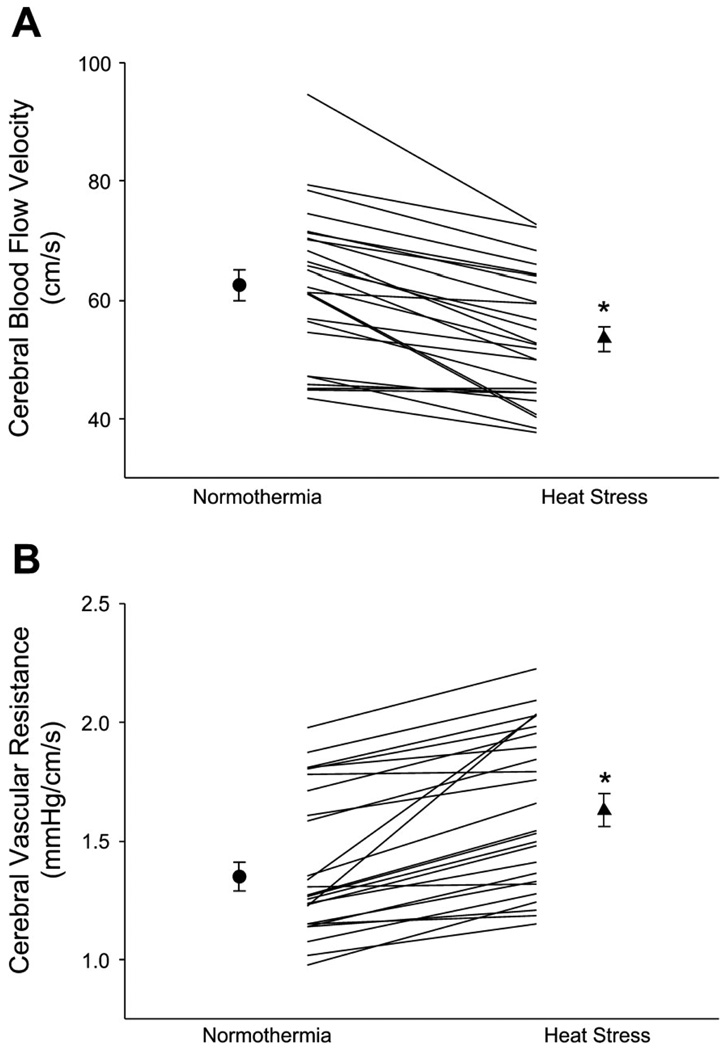
Thermal stress alters the cardiovascular response to postural changes, where heat stress decreases (1, 19, 20, 30, 31) and cold stress in the absence of shivering increases (15, 31) orthostatic tolerance. We have identified decreases in resting cerebral blood velocity during heat stress and even greater changes during gravitational perturbations such as lower body negative pressure (LBNP; (3, 30); Fig. 4). Slight increases in cerebral blood velocity during cold stress are observed, and these increases are maintained during heat-up tilt (15, 31). Alterations in orthostatic tolerance and cerebral blood flow due to thermal stress appear to be independent of the gain of other neural-induced postural reflexes (13, 33) but rather, in part, are related to previously alluded-to alterations in cardiac function and mechanics.
Figure 4.
Effects of whole body heating on cerebral blood velocity from the middle cerebral artery (A) and calculated cerebral vascular resistance (B). The symbols with error bars denote mean responses, while lines denote individual responses. *P < 0.001. (Reprinted from Wilson TE, Gao Z, Hess KL, Monahan KD. Effect of aging on cardiac function during cold stress in humans. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1627–1633. Copyright © 2010 The American Physiological Society. Used with permission.)
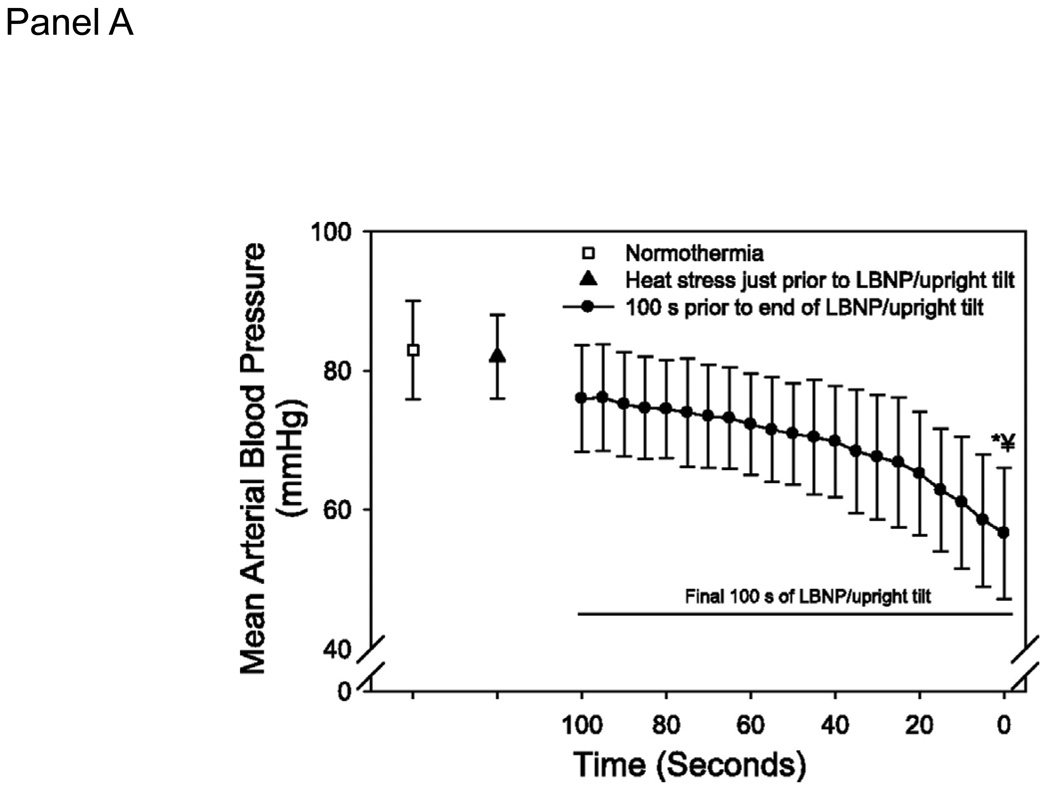
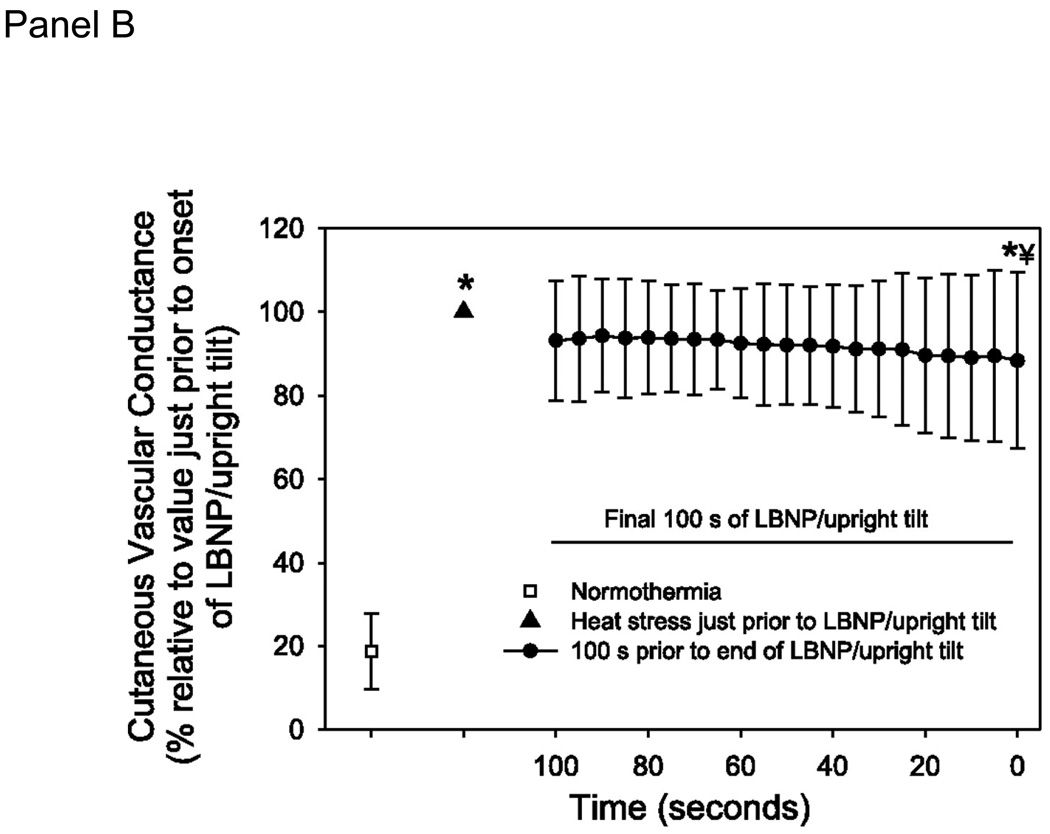
Heat stress requires an increase in cardiac output to perfuse the cutaneous vasculature for heat dissipation purposes. The combination of heat and orthostatic stress can lead to instances where cardiac output, albeit higher than normothermic values, is not adequate for the vascular conductance needs of the body. To prevent syncope in this condition, either cutaneous vascular conductance needs to be reduced or orthostatic induced decreases in cardiac output need to be attenuated. Leading up to the point of syncope the reduction in cutaneous vasculature is only ~15% from peak heat stress values and thus is not sufficient to prevent a fall in mean arterial pressure (Fig. 5; (9)). Highlighting this issue, if brief skin-cooling is performed on hyperthermic individuals, causing pronounced decreases in cutaneous vascular conductance, mean arterial pressure and cerebral blood velocity are maintained during head-up tilt despite a similar decrease cardiac output (31). An alternate method to prevent heat syncope is to attenuate orthostatic-induced decreases in cardiac output. In our experiments heat stress exacerbates the reduction in stroke volume during an orthostatic challenge (29). This inability to maintain adequate stroke volume, leading to compromised mean arterial pressure and cerebral blood velocity, is likely due to the decrease in preload because volume loading increases orthostatic tolerance time (20) and moves the operating point from a steep to a plateau portion of the Frank-Starling relation (Fig. 1, Panel C).
Figure 5.
A. Cutaneous vascular conductance (CVC) at normothermia, heat stress, and the final 100 seconds prior to the end of the orthostatic challenge due to syncopal symptoms. Data are normalized relative to CVC just prior to the onset of the orthostatic challenge. Absolute CVC values are: normothermia: 29±20; heat stress: 160±58; end of orthostatic stress; 138±61 CVC units. B. Mean arterial blood pressure at normothermia, heat stress, and the final 100 seconds prior to the end of the orthostatic challenge due to syncopal symptoms. LBNP: lower body negative pressure. * significantly different from normothermia; � significantly different from heat stress. (Reprinted from Crandall CG, Shibasaki M, Wilson TE. Insufficient Cutaneous Vasoconstriction Leading up to and during Syncopal Symptoms in the Heat Stressed Human. Am J Physiol Heart Circ Physiol., 2010; 299:H1168–H1173. Copyright © 2010 The American Physiological Society. Used with permission.)
The mechanisms by which cold stress improves orthostatic tolerance may be the opposite of what is problematic during heat stress. Whole-body cooling causes peripheral and visceral vasoconstriction to allow for adequate control of systemic vascular conductance during orthostatic stress. The resultant increase in mean arterial pressure by this thermal provocation insures adequate cerebral blood flow during orthostatic stress in otherwise heat stressed and normothermic individuals (15, 31). Whole-body cooling also increases preload and attenuates the reduction in stroke volume and cardiac output to orthostatic stress (12, 35). This response may be due to cold stress moving the operating point of a Frank-Starling curve to a flatter portion where decreases in preload result in relatively small reductions in stroke volume (Fig. 1, Panel C).
Summary
In summary, during heat stress preload and afterload decrease, while inotropy increases to maintain or increase stroke volume (Table). Heart rate increases through a direct effect of local heat on the sinoatrial node in combination with withdrawal of parasympathetic neural activity coupled with increases in cardiac sympathetic neural activity. These combined stroke volume and heart rate responses result in large increases in cardiac output during the heat stress. During cold stress, preload and afterload increase, with no quantifiable change in inotropy (Table). Heart rate does not change, possibly because of a baroreflex suppression, with the net result being no significant changes in cardiac output during cold stress. Changes in cardiac output and function are proposed to mechanistically contribute to decreases in orthostatic tolerance during heat stress and improvements in orthostatic tolerance during cold stress.
Table.
Summary of thermal-induced changes from normothermic conditions in the following cardiac parameters.
| Heat Stress | Cold Stress | |
|---|---|---|
| Cardiac Output | ↑↑ | ↔ |
| Heart Rate | ↑↑ | ↔ |
| Stroke Volume | ↔ | ↔ |
| Preload | ↓↓ | ↑ |
| Afterload | ↓ | ↑↑ |
| Diastolic Function / Compliance | ↔ | ↔ |
| Systolic Function / Inotropy | ↑ | ↔ |
ACKNOWLEDGEMENTS
The authors would like to express their appreciation to the many collaborators at Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital Dallas; Heart and Vascular Institute, Penn State College of Medicine; and Rigshospitalet, University of Copenhagen who contributed to the studies discussed in this review. The authors would also like to thank the Office of Communication at Ohio University College of Osteopathic Medicine for their aid in developing Figure 1. This research project was funded in part by grants from the National Heart, Lung, and Blood Institute (HL-10488, HL-61388, HL-67422, and HL-84072) and American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of funding received for this work: National Heart, Lung, and Blood Institute (HL-10488, HL-61388, HL-67422, and HL-84072) and the American Heart Association.
REFERENCES
- 1.Allan JR, Crossley RJ. Effect of controlled elevation of body temperature on human tolerance to +G z acceleration. J Appl Physiol. 1972;33:418–420. doi: 10.1152/jappl.1972.33.4.418. [DOI] [PubMed] [Google Scholar]
- 2.Brothers RM, Bhella PS, Shibata S, Wingo JE, Levine BD, Crandall CG. Cardiac systolic and diastolic function during whole body heat stress. Am J Physiol Heart Circ Physiol. 2009;296:H1150–1156. doi: 10.1152/ajpheart.01069.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brothers RM, Wingo JE, Hubing KA, Crandall CG. The effects of reduced end-tidal carbon dioxide tension on cerebral blood flow during heat stress. J Physiol. 2009;587:3921–3927. doi: 10.1113/jphysiol.2009.172023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bundgaard-Nielsen M, Wilson TE, Seifert T, Secher NH, Crandall CG. Effect of volume loading on the Frank-Starling relation during reductions in central blood volume in heat stressed humans. J Physiol. 2010;588:3333–3339. doi: 10.1113/jphysiol.2010.191981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai Y, Jenstrup M, Ide K, Perko M, Secher NH. Influence of temperature on the distribution of blood in humans as assessed by electrical impedance. Eur J Appl Physiol. 2000;81:443–448. doi: 10.1007/s004210050066. [DOI] [PubMed] [Google Scholar]
- 6.Crandall CG. Heat stress and baroreflex regulation of blood pressure. Med Sci Sports Exerc. 2008;40:2063–2070. doi: 10.1249/MSS.0b013e318180bc98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crandall CG, Etzel RA, Farr DB. Cardiopulmonary baroreceptor control of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol. 1999;277:H2348–H2352. doi: 10.1152/ajpheart.1999.277.6.h2348. [DOI] [PubMed] [Google Scholar]
- 8.Crandall CG, Levine BD, Etzel RA. Effect of increasing central venous pressure during passive heating on skin blood flow. J Appl Physiol. 1999;86:605–610. doi: 10.1152/jappl.1999.86.2.605. [DOI] [PubMed] [Google Scholar]
- 9.Crandall CG, Shibasaki M, Wilson TE. Insufficient Cutaneous Vasoconstriction Leading up to and during Syncopal Symptoms in the Heat Stressed Human. Am J Physiol Heart Circ Physiol. 2010;299:H1168–H1173. doi: 10.1152/ajpheart.00290.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crandall CG, Wilson TE, Marving J, et al. Effects of passive heating on central blood volume and ventricular dimensions in humans. J Physiol. 2008;586:293–301. doi: 10.1113/jphysiol.2007.143057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui J, Durand S, Crandall CG. Baroreflex control of muscle sympathetic nerve activity during skin surface cooling. J Appl Physiol. 2007;103:1284–1289. doi: 10.1152/japplphysiol.00115.2007. [DOI] [PubMed] [Google Scholar]
- 12.Cui J, Durand S, Levine BD, Crandall CG. Effect of skin surface cooling on central venous pressure during orthostatic challenge. Am J Physiol Heart Circ Physiol. 2005;289:H2429–H2433. doi: 10.1152/ajpheart.00383.2005. [DOI] [PubMed] [Google Scholar]
- 13.Cui J, Wilson TE, Crandall CG. Baroreflex modulation of sympathetic nerve activity to muscle in heat-stressed humans. Am J Physiol Regul Integr Comp Physiol. 2002;282:R252–R258. doi: 10.1152/ajpregu.00337.2001. [DOI] [PubMed] [Google Scholar]
- 14.Deschamps A, Magder S. Skin vascular bed is a potential blood reservoir during heat stress. Am J Physiol Heart Circ Physiol. 1990;259:H1796–H1802. doi: 10.1152/ajpheart.1990.259.6.H1796. [DOI] [PubMed] [Google Scholar]
- 15.Durand S, Cui J, Williams KD, Crandall CG. Skin surface cooling improves orthostatic tolerance in normothermic individuals. Am J Physiol Regul Integr Comp Physiol. 2004;286:R199–R205. doi: 10.1152/ajpregu.00394.2003. [DOI] [PubMed] [Google Scholar]
- 16.Hess KL, Wilson TE, Sauder CL, Gao Z, Ray CA, Monahan KD. Aging affects the cardiovascular responses to cold stress in humans. J Appl Physiol. 2009;107:1076–1082. doi: 10.1152/japplphysiol.00605.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Fregly MJ, Blatteis CM, editors. Handbook of physiology: Environmental physiology. Oxford University Press: New York; 1996. pp. 215–243. [Google Scholar]
- 18.Johnson JM, Rowell LB, Niederberger M, Eisman MM. Human splanchnic and forearm vasoconstrictor responses to reductions of right atrial and aortic pressures. Circ Res. 1974;34:515–524. doi: 10.1161/01.res.34.4.515. [DOI] [PubMed] [Google Scholar]
- 19.Keller DM, Low DA, Wingo JE, et al. Acute volume expansion preserves orthostatic tolerance during whole-body heat stress in humans. J Physiol. 2009;587:1131–1139. doi: 10.1113/jphysiol.2008.165118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lind AR, Leithead CS, McNicol GW. Cardiovascular changes during syncope induced by tilting men in the heat. J Appl Physiol. 1968;25:268–276. doi: 10.1152/jappl.1968.25.3.268. [DOI] [PubMed] [Google Scholar]
- 21.Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol. 1998;84:1323–1332. doi: 10.1152/jappl.1998.84.4.1323. [DOI] [PubMed] [Google Scholar]
- 22.Minson CT, Wladkowski SL, Pawelczyk JA, Kenney WL. Age, splanchnic vasoconstriction, and heat stress during tilting. Am J Physiol Regul Integr Comp Physiol. 1999;276:R203–R212. doi: 10.1152/ajpregu.1999.276.1.r203. [DOI] [PubMed] [Google Scholar]
- 23.Nelson MD, Haykowsky MJ, Petersen SR, DeLorey DS, Cheng-Baron J, Thompson RB. Increased left ventricular twist, untwisting rates, and suction maintain global diastolic function during passive heat stress in humans. Am J Physiol Heart Circ Physiol. 2010;298:H930–H937. doi: 10.1152/ajpheart.00987.2009. [DOI] [PubMed] [Google Scholar]
- 24.Niimi Y, Matsukawa T, Sugiyama Y, Shamsuzzaman AS, Ito H, Sobue G, Mano T. Effect of heat stress on muscle sympathetic nerve activity in humans. J Auton Nerv Syst. 1997;63:61–67. doi: 10.1016/s0165-1838(96)00134-8. [DOI] [PubMed] [Google Scholar]
- 25.Nimmo M. Exercise in the cold. J Sports Sci. 2004;22:898–915. doi: 10.1080/0264041400005883. [DOI] [PubMed] [Google Scholar]
- 26.Rowell LB. Hyperthermia: a hyperadrenergic state. Hypertension. 1990;15:505–507. doi: 10.1161/01.hyp.15.5.505. [DOI] [PubMed] [Google Scholar]
- 27.Rowell LB, Brengelmann GL, Blackmon JR, Twiss RD, Kusumi F. Splanchnic blood flow and metabolism in heat-stressed man. J Appl Physiol. 1968;24:475–484. doi: 10.1152/jappl.1968.24.4.475. [DOI] [PubMed] [Google Scholar]
- 28.Sawka MN, Wenger CB. Physiological responses to acute exercise-heat stress. In: Pandolf KB, Sawka MN, Gonzalez RR, Carmel IN, editors. Human performance physiology and environmental medicine at terrestrial extremes. Cooper Publishing: 1988. pp. 97–151. [Google Scholar]
- 29.Wilson TE, Brothers RM, Tollund C, et al. Effect of thermal stress on Frank-Starling relations in humans. J Physiol. 2009;587:3383–3392. doi: 10.1113/jphysiol.2009.170381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson TE, Cui J, Zhang R, Crandall CG. Heat stress reduces cerebral blood velocity and markedly impairs orthostatic tolerance in humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1443–R1448. doi: 10.1152/ajpregu.00712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson TE, Cui J, Zhang R, Witkowski S, Crandall CG. Skin cooling maintains cerebral blood flow velocity and orthostatic tolerance during tilting in heated humans. J Appl Physiol. 2002;93:85–91. doi: 10.1152/japplphysiol.01043.2001. [DOI] [PubMed] [Google Scholar]
- 32.Wilson TE, Gao Z, Hess KL, Monahan KD. Effect of aging on cardiac function during cold stress in humans. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1627–R1633. doi: 10.1152/ajpregu.00099.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson TE, Ray CA. Effect of thermal stress on the vestibulosympathetic reflexes in humans. J Appl Physiol. 2004;97:1367–1370. doi: 10.1152/japplphysiol.00403.2004. [DOI] [PubMed] [Google Scholar]
- 34.Wilson TE, Sauder CL, Kearney ML, et al. Skin-surface cooling elicits peripheral and visceral vasoconstriction in humans. J Appl Physiol. 2007;103:1257–1262. doi: 10.1152/japplphysiol.00401.2007. [DOI] [PubMed] [Google Scholar]
- 35.Wilson TE, Tollund C, Yoshiga CC, et al. Effects of heat and cold stress on central vascular pressure relationships during orthostasis in humans. J Physiol. 2007;585:279–285. doi: 10.1113/jphysiol.2007.137901. [DOI] [PMC free article] [PubMed] [Google Scholar]