Abstract
Purpose
Advanced-stage cancers are extremely difficult to treat and rarely result in a cure. The application of oncolytic viruses is a potential strategy for controlling advanced-stage cancer because intratumoral (i.t.) injection of an oncolytic virus, such as vaccinia virus, results in tumor cell lysis and subsequent release of tumor antigens into the microenvironment. Furthermore, the viruses can serve as a vehicle for delivering genes of interest to cancer cells.
Experimental Design
In the current study, we hypothesize that in tumor-bearing mice primed with DNA encoding an immunogenic foreign antigen, ovalbumin (OVA) followed by a boost with i.t. administration of vaccinia virus encoding the same foreign antigen, OVA, can generate enhanced antitumor effects through the combination of viral oncolysis and tumor-specific immunity.
Results
We observed that tumor-bearing mice primed with OVA DNA and boosted with vaccinia encoding OVA (Vac-OVA) generated significant therapeutic antitumor effects as well as induced significant levels of OVA-specific CD8+ T cells in two different tumor models. Furthermore, treatment with Vac-OVA not only kills the tumor and stromal cells directly but also renders the tumor cells and surrounding stromal cells susceptible to OVA-specific CD8+ T-cell killing, resulting in enhanced antitumor therapeutic effects.
Conclusions
Thus, the current study may provide a novel therapeutic strategy for the control of advanced-stage cancers.
Cancers in an advanced state are difficult to treat and very rarely result in a cure. Efforts to improve early detection and treatment of advanced-stage cancers have been relatively unsuccessful. Existing therapies for advanced disease, such as chemotherapy and radiation therapy, have not improved the overall survival of patients with locally advanced or metastatic disease (1–3). Therefore, there is a strong need to develop innovative therapeutic approaches for the control of advanced-stage cancer.
Several groups have investigated various immunotherapeutic strategies to enhance the immune response against tumor-associated antigens (TAA). Recombinant viruses have been studied as vehicles of delivering genes of interest to cancer cells due to their transduction efficiency and potential oncolytic properties. Several oncolytic viruses, such as adenovirus, herpes simplex virus, poxvirus, vesicular stomatitis virus, measles virus, Newcastle disease virus, influenza virus, and reovirus, have been used as promising anticancer agents (for reviews, see refs. 4, 5). Furthermore, oncolytic vaccinia virus has been shown to preferentially infect tumor cells over surrounding normal tissues in several cancer models (6–8). Hung et al. recently showed that vaccinia virus administered to mice i.p. can preferentially infect ovarian tumor cells but not normal tissue and generates significant antitumor responses based on a noninvasive luminescence imaging system, which facilitates monitoring of cancer cell proliferation and growth (9). Vaccinia has also been widely used as a vehicle for vaccine delivery and has been shown to be highly effective in generating antigen-specific immune responses in DNA vaccine prime followed by vaccinia boost regimens (10). Thus, vaccinia represents a promising agent for viral-mediated tumor oncolysis as well as immunomodulatory gene delivery to advanced cancer cells.
Intratumoral (i.t.) injection of vaccinia also represents a potentially promising approach to generate tumor-specific immunity. Vaccinia may directly cause tumor cell lysis, which can lead to the release of tumor antigens into the microenvironment. This may result in tumor antigen epitope spreading, thus generating cross-priming to induce tumor-specific immunity. In addition, vaccinia may also be engineered to carry specific genes that can facilitate the induction of tumor-specific immunity. The ability to generate antigenic epitope spreading is particularly important for a vast majority of tumors that do not have well-defined TAAs. Furthermore, these TAAs may vary among patients. Thus, the identification of a specific gene that can be incorporated into the vaccinia vector for enhancing tumor-specific immunity is an important endeavor for the therapeutic strategy using i.t. injection of vaccinia.
In this study, we hypothesize that tumor-bearing mice initially primed with DNA encoding a highly immunogenic foreign antigen, such as ovalbumin (OVA), followed by an i.t. vaccinia booster, encoding the same foreign antigen, OVA, can generate enhanced antitumor effects through the combination of viral oncolysis and tumor-specific immunity. The OVA antigen expressed by the vaccinia-infected tumor cells will serve as a suitable target antigen for OVA-specific CD8+ T cell–mediated cytotoxic activity without raising concerns for immune tolerance. In addition, i.t. infection of vaccinia encoding OVA can generate i.t. inflammation and “danger signals,” which can recruit immune cells, such as OVA-specific T cells generated by the initial DNA vaccination, to the tumor site. The tumor cell death caused by oncolytic vaccinia as well as the OVA-specific CD8+ T cell–mediated killing can lead to the release of further TAAs, which may be processed and presented by dendritic cells to T cells, resulting in de novo activation of tumor-specific immunity. This would lead to systemic therapeutic antitumor effects against tumor cells not infected by vaccinia (epitope spreading). This strategy may prove more effective and broadly applicable for the generation of systemic tumor-specific immunity.
In the current study, we tested our hypothesis by first priming tumor-bearing mice with intradermal vaccination of DNA encoding OVA (11) followed by i.t. injection of vaccinia encoding OVA (Vac-OVA; ref. 12). We observed that tumor-bearing mice primed with OVA DNA and boosted with Vac-OVA generated significantly better therapeutic antitumor effects in two different tumor models, B16 as well as TC-1 tumors. Tumor-bearing mice treated with the prime-boost regimen was also found to induce significant levels of activated OVA-specific CD8+ T cells. Furthermore, tumors treated with the DNA prime and vaccinia boost regimen showed the best control of tumor growth and improved survival. Furthermore, treatment with Vac-OVA rendered the tumor cells as well as surrounding stromal cells susceptible to viral oncolysis and OVA-specific CD8+ T cell killing, resulting in enhanced antitumor therapeutic effects. Thus, the current study may provide a novel therapeutic strategy for the control of advanced-stage cancers.
Materials and Methods
Mice
Female C57BL/6 mice (H-2Kb and I-Ab), 5 to 6 wk of age, were purchased from the National Cancer Institute. Transgenic mice, OT-1, that express TCR specific for ovalbumin peptide, SIINFEKL, were purchased from The Jackson Laboratory. All of the mice were maintained under specific pathogen-free conditions in the animal facility at Johns Hopkins Hospital. Animals were used in compliance with institutional animal health care regulations, and all animal experimental procedures were approved by the Johns Hopkins Institutional Animal Care and Use Committee.
Cell lines
The production and maintenance of TC-1 cells or TC-1–luciferase transduced (TC-1 luc) cells have been described previously (13, 14). Mouse melanoma cell B16/F10 and thymoma cells EL4 (H-2b) were purchased from American Type Culture Collection. For the generation of CTLs specific for H-2Kb-OVA, 1 × 107 EG7 cells (EL4 cells transfected with ovalbumin cDNA) were irradiated (10,000 rad) and cultured for 6 d in complete RPMI 1640 with 1 × 107 spleen cells from OT-1 mice. All cell lines were grown in RPMI 1640, supplemented with 10% (v/v) fetal bovine serum, 50 units/mL penicillin/streptomycin, 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, 2 mmol/L nonessential amino acids, and 0.4 mg/mL G418 at 37°C with 5% CO2.
Plasmid DNA constructs
The generation of recombinant plasmid pcDNA3 encoding CRT/E7 (p-CRT/E7) or recombinant pcDNA3 encoding ovalbumin (p-OVA) has been described previously (11, 15). The accuracy of the DNA construct was confirmed by DNA sequencing. For the gene gun–mediated intradermal vaccination, 2 μg/mouse of recombinant plasmid DNA were delivered to the shaved abdominal region of C57BL/6 mice using a helium-driven gene gun (Bio-Rad) with a discharge pressure of 400 p.s.i., according to a previously described protocol (16).
Recombinant vaccinia viruses
The wild-type vaccinia virus (Vac-WT) was prepared as described previously (17). The luciferase-expressing vaccinia virus (Vac-luc) was generated using a previously described protocol. It contains two reporter genes (luc and lacZ) inserted into the thymidine kinase region of VV (tk-) as described (18). The vaccinia virus expressing the full-length chicken OVA (Vac-OVA) was generated using a previously described protocol (12). The generation of recombinant vaccinia virus encoding calreticulin (CRT) linked to a model tumor antigen HPV-16 E7 (Vac-CRT/E7) was done using a protocol similar to what has been described earlier (19). The generation of recombinant vaccinia virus expressing green fluorescent protein (Vac-GFP) was done using a protocol similar to what has been described earlier (20).
In vivo bioluminescence imaging
We quantitatively compared levels of viral replication within the tumor administered through different routes of injection. TC-1 tumor-bearing mice (tumor size = 8–10 mm) were administered by either i.p. or i.t. injection of 1 × 107 plaque-forming units (pfu)/mouse of vaccinia-luc in 200 μL phosphate-buffered saline. Bioluminescence imaging was conducted on days 1, 3, and 7 after virus injection on a cryogenically cooled IVIS system (Xenogen/Caliper Life Sciences). The region of interest was manually drawn over tumor areas by using Living Image software 2.5 (Xenogen/Caliper Life Sciences).
Characterization of CD31+ cells infected by vaccinia
We then characterized the frequency of CD31+ cells infected by vaccinia virus after TC-1 tumor-bearing mice (tumor size = 8–10 mm) were administered by either i.p. or i.t. injection of 1 × 107 pfu vaccinia-GFP in 200 μL PBS. Tumor cells were harvested 24 h after viral injection, made into single-cell suspensions, and subjected to CD31 staining.
To evaluate for killing of CD31+ cells, TC-1 tumors were grown in C57BL/6 mice and harvested as tumor size reached 8 to 10 mm. Tumors were dissociated into single-cell suspensions and seeded (3 × 105/well) into a 24-well microtiter plate in complete medium. At 24 h, Vac-WT or Vac-OVA at 0.5 multiplicity of infection (MOI) was added to each well, and at 48 hours, the complete medium was changed and activated OT-1 T cells were added to each well at an effector-to-target ratio (E/T) of 1:1. Cells were then harvested 4 h later and stained with phycoerythrin (PE) anti-mouse CD31 monoclonal antibody and 7-Amino-actinomycin D (7-AAD), and analyzed by flow cytometry using the FACSCalibur flow cytometer and CellQuest software. Data are presented as absolute numbers of CD31+7-AAD− cells per 3 × 105 cells.
Heterologous prime-boost immunization
Groups of mice (five per group) were inoculated with either B16/F10 cells or TC-1 cells (5 × 104/mouse) at day 0. Mice were then primed with 2 μg of either control pcDNA3, p-OVA, or p-CRT/E7 DNA by a gene gun at day 5 and were boosted with i.t. injection (1 × 107 pfu/mouse, in 200 μL PBS) of Vac-WT, Vac-OVA, or Vac-CRT/E7 at day 12.
Evaluation of frequency of E7-specific CD8+ T cells by intracellular cytokine staining and flow cytometry analysis
For characterization of E7-specific CD8+ T cells, both splenocytes and tumor xenografts were harvested 1 wk after last immunization. Before intracellular cytokine staining, 2 × 106 pooled splenocytes and pooled tumors from each treatment group were separately incubated for 16 h with either an H-2Kb–restricted peptide (SIINFEKL; 1.0 μmol/L) or an I-Ab–restricted peptide (LSQAVHAAHAEINEAGR; 1.0 μmol/L). In addition, 2 × 106 pooled splenocytes and pooled tumors from each treatment group were incubated for 16 h with 1 μg/mL of E7 peptide (aa 49–57) containing an MHC class I epitope for detecting E7-specific CD8+ T-cell precursors (21). Cells were then harvested and stained for CD8 and IFN-γ using previously described standard protocols (22). Samples were analyzed on a FACSCalibur flow cytometer using CellQuest software. All of the analyses shown were carried out on a gated lymphocyte population.
In vitro cytotoxicity assay
Luciferase-expressing TC-1 tumor cells were added to 96-well plates at a dose of 2 × 104 per well. After 24 h, Vac-WT or Vac-OVA (MOI = 0.5) was added to each well. At 48 h, the complete medium was changed and activated OT-1 T cells at an E/T ratio of 1:1 were added to each well. Bioluminescence imaging was done 4 h later. The degree of CTL-mediated killing of the tumor cells was indicated by the decrease of luminescence activity using the IVIS luminescence imaging system series 200. Bioluminescence signals were acquired for 10 s.
Statistical analysis
Statistical analysis was done using Prism 3.0 software (GraphPad). All data are expressed as means ± SD and are representative of at least two independent experiments. Comparisons between individual data points were made using a Student’s t test or repeated-measure ANOVA (analysis of variance) test, as appropriate. Tumor size was measured during the treatment, twice a week by digital calipers, and tumor volume (mm3) was calculated using the following equation: (tumor length × width × height)/2. Death of mouse was arbitrarily defined as tumor diameter >2 cm. Differences in survival between experimental groups were analyzed using the log-rank test. A P value of ≤0.05 was set for the significance of difference among groups.
Results
Tumor-bearing mice primed with DNA encoding a foreign antigen and treated with i.t. injection of vaccinia virus encoding the same foreign antigen leads to significant therapeutic antitumor effects
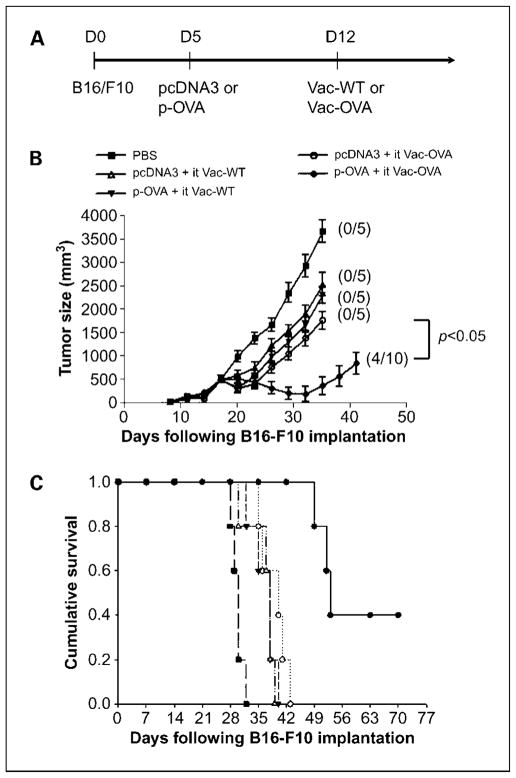
We recently showed that i.t. injection of vaccinia encoding a marker gene, such as luciferase, can result in significant expression of luciferase within the tumor, indicating that i.t. injection of vaccinia can lead to significant viral infection of the tumor cells (Supplementary Fig. S1). Thus, to determine the antitumor effects generated in tumor-bearing mice primed with DNA encoding a foreign antigen, such as OVA, and treated with i.t. injection of vaccinia virus encoding the same foreign antigen, we first challenged groups of C57BL/6 mice (five per group) with B16 tumor cells and then primed them with control pcDNA3 alone or pcDNA3 encoding ovalbumin (p-OVA). One week later, mice were treated with i.t. injections of either wild-type vaccinia (Vac-WT) or vaccinia encoding OVA (Vac-OVA). Tumor-bearing mice treated with 1× PBS were used as negative controls. A graphical representation of the treatment regimen is depicted in Fig. 1A. As shown in Fig. 1B, tumor-bearing mice primed with the p-OVA followed by i.t. Vac-OVA injection showed the best therapeutic antitumor effects compared with treatment with the other prime-boost regimens. Furthermore, tumor-bearing mice primed with the p-OVA prime followed by i.t. Vac-OVA injection showed improved survival compared with treatment with the other therapeutic regimens (P < 0.01; Fig. 1C). Thus, our data indicate that the treatment with p-OVA followed by i.t. Vac-OVA injection produces significant therapeutic antitumor effects and long-term survival in B16 tumor-bearing mice.
Fig. 1.
In vivo tumor treatment experiments with B16 tumors. A, diagrammatic representation of the prime-boost treatment regimen. Groups of C57BL/6 mice (five per group) were s.c. challenged with 5 × 104 per mouse of B16/F10 tumor cells. Five days after tumor challenge, mice were immunized with either 2 μg/mouse of pcDNA3 DNA or pcDNA3-expressing ovalbumin (p-OVA) by gene gun. On day 12, mice were boosted by i.t. injection of 1 ×107 pfu/mouse of either wild-type vaccinia (Vac-WT) or vaccinia encoding ovalbumin (Vac-OVA). B16 tumor-bearing mice treated with 1×PBS were used as a control. B, line graph depicting the tumor volume in B16 tumor-bearing mice treated with the different prime-boost regimens. Numbers in parentheses indicate complete tumor rejection rates. C, Kaplan-Meier survival analysis of B16 tumor-bearing mice treated with the different treatment regimens. Data shown are representative of two experiments. Points, mean; bars, SD.
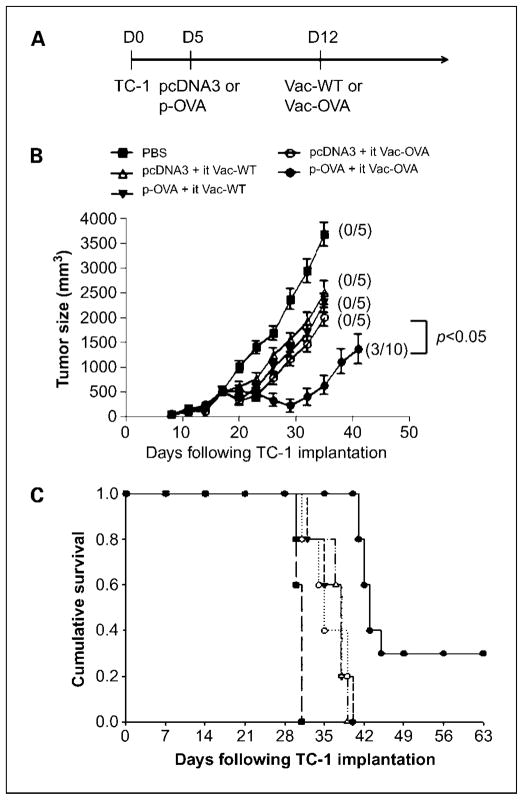
We further tested the same therapeutic approach using another tumor model, TC-1. We first challenged groups of C57BL/6 mice (five per group) with TC-1 tumor cells and then primed them with control pcDNA3 or p-OVA. One week later, mice were treated with either Vac-WT or Vac-OVA by i.t. injection. Tumor-bearing mice treated with PBS were used as negative controls. A graphical representation of the treatment regimen is depicted in Fig. 2A. As shown in Fig. 2B, tumor-bearing mice treated with p-OVA followed by i.t. Vac-OVA injection showed the best therapeutic antitumor effects compared with treatment with the other prime-boost regimens. Furthermore, tumor-bearing mice treated with the p-OVA followed by i.t. Vac-OVA injection showed improved survival compared with treatment with the other therapeutic regimens(P < 0.01; Fig. 2C). Thus, our data indicate that the treatment with p-OVA followed by i.t. Vac-OVA injection produces significant therapeutic antitumor effects and long-term survival in TC-1 tumor-bearing mice. We also tested the therapeutic approach using an antigenic system specific to TC-1 tumor cells, specifically E7. We found that vaccination with CRT/E7 DNA vaccine intradermally followed by i.t. injection of vaccinia encoding CRT/E7 also generated significant therapeutic anti-tumor effects and long-term survival in TC-1 tumor-bearing mice (see Supplementary Fig. S2). Taken together, our data show that the treatment with a foreign antigen-specific DNA vaccine followed by i.t. injection of vaccinia encoding the same foreign antigen produces significant therapeutic antitumor effects and long-term survival in tumor-bearing mice in two different tumor models.
Fig. 2.
In vivo tumor treatment experiments withTC-1tumors. A, diagrammatic representation of the prime-boost treatment regimen. Groups of C57BL/6 mice (five per group) were s.c. challenged with 5 × 104/mouse ofTC-1tumor cells. Five days after tumor challenge, mice were immunized with either 2 μg/mouse of pcDNA3 DNA or pcDNA3-expressing ovalbumin (p-OVA) by gene gun. On day12, mice were boosted by i.t. injection of 1 ×107 pfu/mouse of either wild-type vaccinia (Vac-WT) or vaccinia encoding ovalbumin (Vac-OVA). TC-1tumor-bearing mice treated with 1×PBS were used as a control. B, line graph depicting the tumor volume inTC-1tumor-bearing mice treated with the different prime-boost regimens. Numbers in parentheses indicate complete tumor rejection rates. C, Kaplan-Meier survival analysis ofTC-1tumor-bearing mice treated with the different treatment regimens. Data shown are representative of two experiments. Points, mean; bars, SD.
Tumor-bearing mice primed with DNA encoding foreign antigen and treated with i.t. injection of vaccinia encoding the same foreign antigen leads to significant number of foreign antigen-specific CD8+ T cells
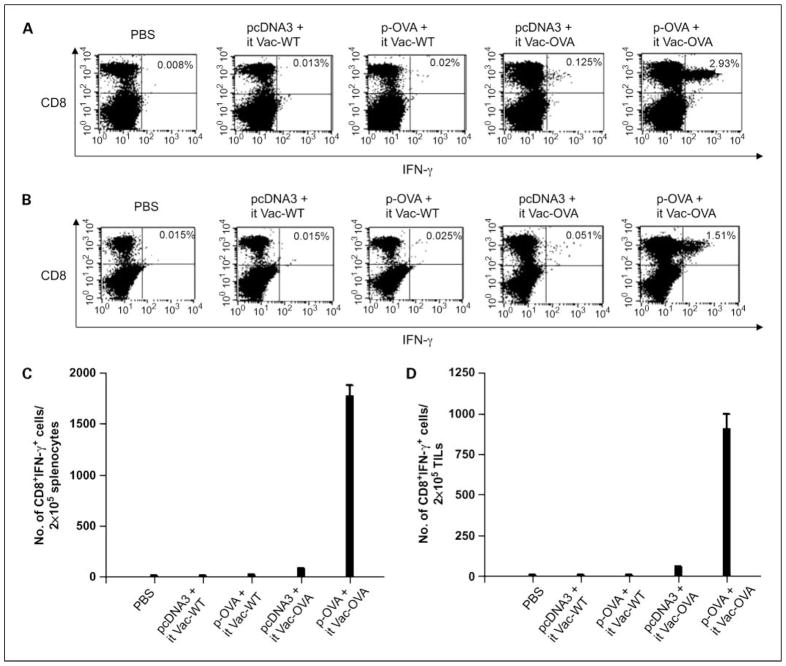
To determine the antigen-specific CD8+ T-cell immune response against OVA in tumor-bearing mice using the DNA prime and i.t. viral boost model, we first challenged groups of C57BL/6 mice (five per group) with B16 tumor cells and treated them with either pcDNA3 or p-OVA followed by i.t. injection with either Vac-WT or Vac-OVA as previously described in Fig. 1. Tumor-bearing mice treated with 1× PBS were used as negative controls. Cells were harvested from the spleens and tumors of vaccinated mice 7 days after vaccinia injection and were characterized for the presence of OVA-specific CD8+ T cells using intracellular cytokine staining for IFN-γ followed by flow cytometry analysis. As shown in Fig. 3, tumor-bearing mice that were treated with p-OVA followed by i.t. Vac-OVA injection generated significantly higher numbers/percentages of OVA-specific CD8+ T cells both in the spleens as well as tumors compared with tumor-bearing mice treated with the other regimens. We also determined the antigen-specific immune responses elicited in another tumor model, TC-1, which uses a different antigenic system, E7. We first challenged groups of C57BL/6 mice (five per group) with TC-1 tumor cells and then primed them with either pcDNA3 or p-CRT/E7 DNA vaccine intradermally. One week later, mice were treated with either Vac-WT or Vac-CRT/E7 by either i.p. or i.t. injection. Tumor-bearing mice treated with PBS were used as negative controls. We observed that tumor-bearing mice that were treated with p-CRT/E7 DNA followed by i.t. Vac-CRT/E7 injection generated a significantly higher number of E7-specific CD8+ T cells both in the spleens as well as tumors compared with tumor-bearing mice treated with the other regimens (See Supplementary Fig. S3). Taken together, our data indicate that treatment of tumor-bearing mice with a foreign antigen-specific DNA vaccine followed by i.t. injection of vaccinia encoding the same foreign antigen leads to the strongest antigen-specific CD8+ T-cell immune responses in the spleens and tumors.
Fig. 3.
Intracellular cytokine staining followed by flow cytometry analysis to determine the number of OVA-specific CD8+ T cells in tumor-bearing mice treated with the different prime-boost regimens. Groups of C57BL/6 mice (five per group) were challenged s.c. with 5 × 104 per mouse of B16/F10 tumor cells. Five days after tumor challenge, mice were immunized with either pcDNA3 or p-OVA DNA by gene gun and boosted by i.t. injection of either Vac-WT or Vac-OVA as shown in Fig. 1. TC-1 tumor-bearing mice treated with PBS were used as a control. Seven days after vaccinia infection, cells from the spleens (A and C) and tumors (B and D) of mice were harvested, incubated overnight with the OVA peptide, and stained for CD8 and intracellular IFN-γ and then characterized for OVA-specific CD8+ T cells using intracellular IFN-γ staining followed by flow cytometry analysis. Representative flow cytometry data showing the percentage of OVA-specific IFN-γ+ CD8+ T cells in the spleens (A) and tumors (B) of mice treated with the different prime boost regimens. Numbers of OVA-specific IFN-γ – secreting CD8+ T cells per 2 × 105 pooled cells in the spleens (C) and tumors (D) of treated mice. Data shown are representative of two experiments. Columns, mean; bars, SD.
We also determined the OVA-specific CD4+ T-cell immune responses in tumor-bearing mice treated with p-OVA followed by i.t. Vac-OVA injection. We found that whereas the OVA-specific CD4+ T-cell immune responses in the spleens of treated mice were not significantly different from those in tumor-bearing mice treated with the other regimens, the OVA-specific CD4+ T-cell immune responses within the tumors of treated mice were significantly higher compared with those in tumor-bearing mice treated with the other regimens (Supplementary Fig. S4). Thus, our data indicate that treatment with p-OVA followed by i.t. Vac-OVA injection leads to increased OVA-specific CD4+ T-cell immune responses in the tumors but not in the spleens of tumor-bearing mice.
To determine the subset of immune cells that are important for the observed antitumor effects, we performed in vivo antibody depletion experiments in tumor-bearing mice treated with the p-OVA followed by i.t. Vac-OVA injection. We found that mice depleted of CD8+ T cells showed a significant reduction in survival compared with treated mice without depletion in both tumor models (see Supplementary Fig. S5). Furthermore, depletion of CD4+ T cells showed a slight reduction in survival, although not as significant as CD8+ T-cell depletion. Taken together, our data indicate that CD8+ T cells, as well as CD4+ T cells, play an important role in the antitumor effects observed in mice treated with p-OVA followed by i.t. Vac-OVA injection.
Treatment with OVA expressing vaccinia not only kills the tumor cells directly but also renders tumor cells more susceptible to killing by OVA-specific T cells
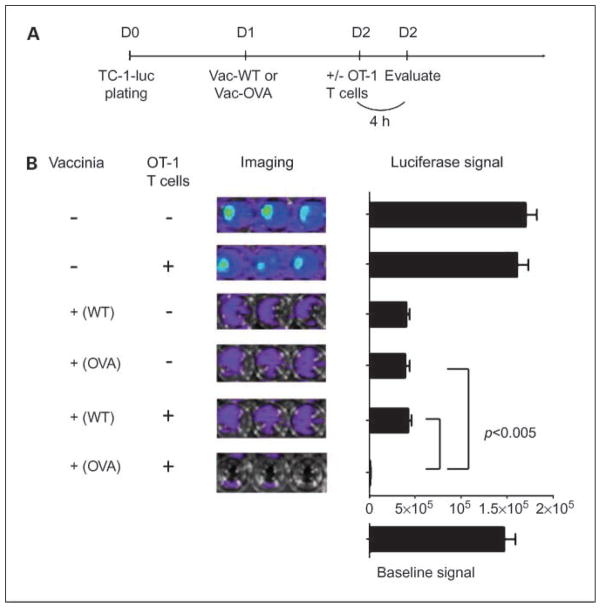
To determine if treatment of tumor cells with Vac-OVA will render the tumor cell more susceptible to viral oncolysis as well as OVA-specific T cell–mediated killing, we performed a cytotoxicity assay using luciferase-expressing TC-1 tumor cells. TC-1/luc tumor cells were plated on day 0 and treated with either Vac-OVA or Vac-WT on day 1. The cells were then treated with or without OVA-specific CD8+ T cells (OT-1 T cells) on day 2 as shown in Fig. 4A. Four hours later, the CTL-mediated killing of the TC-1 tumor cells in each well was monitored using bioluminescent imaging system. The degree of CTL-mediated killing of the tumor cells was indicated by the decrease of luminescence activity. As shown in Fig. 4B, we observed that tumor cells incubated with Vac-WT or Vac-OVA alone showed a significant reduction in luciferase activity, indicating that tumor killing was contributed by viral oncolysis. Furthermore, the lowest luciferase activity was observed in TC-1 cells treated with Vac-OVA in conjunction with OT-1 T cells but not in cells treated with Vac-WT. These data suggest that the increased tumor lysis is contributed by OVA-specific cytotoxic T cell–mediated killing. Taken together, our data suggest that the treatment of tumor cells with Vac-OVA and OT-1 cells can lead to tumor lysis by a combination of viral oncolysis and OVA-specific cytotoxic T cell–mediated killing.
Fig. 4.
In vitro cytotoxicity assay. A, schematic diagram of the experimental design for the cytotoxicity assay. Luciferase-expressing TC-1 tumor cells (2 × 104/well) were added to 96-well plates. Twenty-four hours later, Vac-WT or Vac-OVA (MOI = 0.5) was added to each well. Forty-eight hours later, the complete medium was changed and activated OT-1Tcells were added to each well at an E/T ratio of 1:1. Bioluminescence imaging was done 4 h later. The degree of CTL-mediated killing of the tumor cells was indicated by the decrease of luminescence activity using the IVIS luminescence imaging system series 200. Bioluminescence signals were acquired for 10 s. B, representative luminescence images of 96-well plates and bar graphs depicting the luminescence intensity in each well containing tumor cells with different treatments. Columns, mean; bars, SD.
Intratumoral injection of vaccinia leads to infection of CD31+ nontumor cells by vaccinia
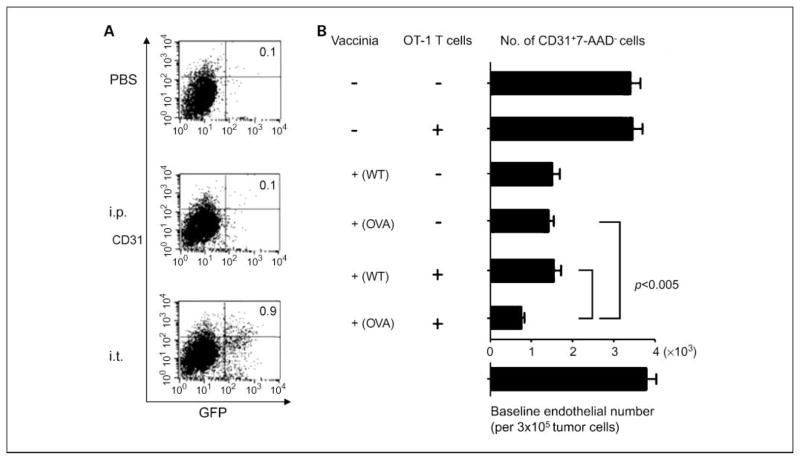
We further investigated whether Vac-OVA treatment could exert cytotoxic effects on the surrounding nontumor cells, including CD31+ endothelial and stromal cells. To determine the number of CD31+ nontumor cells infected by vaccinia in tumor-bearing mice, groups of C57BL/6 mice (five per group) were s.c. challenged with TC-1 tumor cells and treated with either i.t. or i.p. injection with Vac-GFP. Tumor cells were harvested 24 hours after vaccinia virus injection, stained for CD31, and characterized by flow cytometry analysis. As shown in Fig. 5A, the percentage of CD31+ nontumor cells infected with Vac-GFP was significantly higher in tumor-bearing mice injected i.t. with Vac-GFP compared with mice injected i.p. or mice treated with PBS. Thus, our data indicate that i.t. injection of vaccinia leads to increased infection of CD31+ nontumor cells by vaccinia compared with i.p. injection.
Fig. 5.
Characterization of vaccinia infectivity of CD31+ cells in tumor. A, flow cytometry data demonstrating the percentage of CD31+ cells in the tumor infected with vaccinia. Groups of C57BL/6 mice (five per group) were s.c. challenged with 5 × 104 per mouse ofTC-1tumor cells. When tumor size reached about 8 to 10 mm, mice were treated with either i.t. or i.p. with Vac-GFP at 1 ×107 pfu/mouse. Tumors were harvested 24 h after virus injection, stained for CD31, and characterized by flow cytometry analysis. B, representative bar graphs depicting the number of CD31+AAD- cells per 3 × 105 cells derived from the tumors in the different treatment groups. Columns, mean; bars, SD. Cells derived from explanted tumors (2 × 104/well) were added to 96-well plates. Twenty-four hours later, Vac-WT or Vac-OVA (MOI = 0.5) were added to each well and 48 h later, activated OT-1Tcells (E/Tratio1:1) were added to each well. Four hours later, the cells were stained with PE-labeled anti-mouse CD31monoclonal antibody and FITC labeled 7-Amino-actinomycin (7-AAD) and analyzed by flow cytometry analysis.
Treatment with vaccinia-OVA not only kills the surrounding CD31+ stromal cells in the tumor microenvironment directly but also renders them more susceptible to killing by OVA-specific T cells
We then determined if treatment of explanted tumor injected with Vac-OVA would render the CD31+ nontumor cells derived from the surrounding tumor stroma more susceptible to viral oncolysis and to OVA-specific CD8+ T cell–mediated killing. Therefore, we plated explanted TC-1 tumor cells in 96-well plates on day 0 and treated them with Vac-OVA or Vac-WT on day 1. The cells were then treated with or without OVA-specific CD8+ T cells (OT-1 T cells) on day 2. Four hours later, the cells were analyzed by flow cytometry analysis for expression of CD31 and 7-AAD. As shown in Fig. 5B, we observed that CD31+ cells incubated with Vac-WT or Vac-OVA alone showed a significant reduction in luciferase activity, indicating that killing was contributed by viral oncolysis. Furthermore, the lowest luciferase activity was observed in CD31+ cells treated with Vac-OVA and OT-1 T cells but not in cells treated with Vac-WT, suggesting that the increased tumor lysis is contributed by OVA-specific cytotoxic T cell–mediated killing. Taken together, our data suggest that the treatment of CD31+ cells with Vac-OVA and OT-1 cells can lead to lysis by a combination of viral oncolysis and OVA-specific cytotoxic T cell–mediated killing.
Discussion
In the current study, we investigated an innovative strategy to enhance therapeutic antitumor effects through viral oncolysis and tumor-specific immunity. We observed that tumor-bearing mice primed with OVA DNA followed by i.t. Vac-OVA injection generated significant therapeutic antitumor effects against B16, a melanoma tumor model, and TC-1, an HPV tumor model, as well as generated significant levels of OVA-specific CD8+ T cells. Treatment with Vac-OVA was also shown to render tumor cells as well as surrounding nontumor stromal cells more susceptible to viral oncolysis as well as OVA-specific T cell–mediated killing.
Because our approach has proved to be successful in inducing therapeutic antitumor effects in two different tumor models, TC-1 and B16 (Figs. 1 and 2), our results suggest that this prime-boost strategy is not restricted to a certain type of tumor but may also be used for the treatment of various cancers. Furthermore, this strategy is not restricted to a specific foreign antigen but may also be applied to other immunogenic foreign antigens, thus avoiding the concern for immunotolerance. Our data suggest that such a heterologous prime-boost immunotherapeutic strategy may potentially be universally applied to any cancer system for the treatment of established tumors.
In our study, we observed that i.t. injection of vaccinia led to infection of surrounding nontumor stromal cells (Fig. 5A). Furthermore, treatment with Vac-OVA has a cytolytic effect on the CD31+ nontumor cells, presumably endothelial cells and other types of cells in the tumor stroma (Fig. 5B). The destruction of tumor stroma can contribute to the observed antitumor effects. Previous studies have suggested that the manipulation of the stromal microenvironment of tumors may induce immune recognition of the tumor, leading to tumor regression (for review, see ref. 23). For example, a recent study showed that sensitization of the tumor stromal cells for destruction by CTLs leads to eradication of the tumor (24). Another study suggested that the cross-presentation of cancer-specific antigens by stromal cells can lead to the activation of CTLs, thus causing the rapid destruction of the tumor microenvironment (25). Thus, it may be important to target the surrounding nontumor cells present in the tumor microenvironment, such as stromal and endothelial cells, in addition to the tumor cells to effectively control tumor growth.
Oncolytic virotherapy has been extensively explored in a variety of murine tumor models with several oncolytic viruses. For example, systemic cellular delivery of oncolytic vesicular stomatitis virus has been shown to generate antitumor immune responses in a murine model of melanoma lymph node metastases (26). Furthermore, i.t. injection of vesicular stomatitis virus into B16 melanomas led to the enhancement of antitumor T-cell responses (27). Similarly, oncolytic herpes simplex virus strains have been shown to induce systemic antitumor immune responses in several tumor models (28–31). Thus, oncolytic virotherapy has significant potential in the control of several tumors.
A potential drawback of oncolytic virotherapy is that it most likely requires repeated treatment because the virus may infect only a portion of tumor cells in vivo. The development of neutralizing antibodies specific to the vaccinia viral vector can dampen any further booster effect of the recombinant vaccinia viral vector and prevent successful tumor infection with vaccinia (32). Furthermore, previously vaccinated individuals may possess preexisting immunity against vaccinia and may not be suitable candidates for the treatment with the same kind of vaccinia vector (9). Therefore, it is important to develop a strategy to allow for repeated vaccination with vaccinia, such as the employment of different types of viral vectors for serial treatment.
In summary, our study shows that priming with DNA encoding foreign antigen followed by i.t. injection of vaccinia encoding the same foreign antigen leads to significant therapeutic antitumor effects through the combination of viral oncolysis as well as antigen-specific T cell–mediated killing. Such a strategy may potentially be applied to a wide range of cancer models. Our data serve as an important foundation for further clinical translation of this new therapeutic approach for the control of advanced cancer.
Supplementary Material
Acknowledgments
Grant support: Ovarian cancer grants from the National Cooperative Drug Discovery Groups (1U19 CA113341-01), American Cancer Society, National Cancer Institute Specialized Programs of Research Excellence in Cervical Cancer P50 CA098252, and grant 1 RO1 CA114425-01.
We thank Dr. T-C. Wu for his helpful input and critical review of the manuscript.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet. 1992;339:1–15. [PubMed] [Google Scholar]
- 2.Baum M, Ebb S, Brooks M. Biological fall out from trials of adjuvant tamoxifen in early ovarian cancer. In: Salmon SE, editor. Adjuvant therapy of cancer V1. Philadelphia: WB. Saunders; 1990. pp. 269–74. [Google Scholar]
- 3.Swain SM. Selection of therapy for stage III breast cancer. Surg Clin North Am. 1990;70:1061–80. doi: 10.1016/s0039-6109(16)45230-8. [DOI] [PubMed] [Google Scholar]
- 4.Liu TC, Hwang TH, Bell JC, Kirn DH. Development of targeted oncolytic virotherapeutics through translational research. Expert Opin Biol Ther. 2008;8:1381–91. doi: 10.1517/14712598.8.9.1381. [DOI] [PubMed] [Google Scholar]
- 5.Guo ZS, Thorne SH, Bartlett DL. Oncolytic virotherapy: molecular targets in tumor-selective replication and carrier cell-mediated delivery of oncolytic viruses. Biochim Biophys Acta. 2008;1785:217–31. doi: 10.1016/j.bbcan.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gnant MF, Noll LA, Irvine KR, et al. Tumor-specific gene delivery using recombinant vaccinia virus in a rabbit model of liver metastases. J Natl Cancer Inst. 1999;91:1744–50. doi: 10.1093/jnci/91.20.1744. [DOI] [PubMed] [Google Scholar]
- 7.Yu YA, Shabahang S, Timiryasova TM, et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol. 2004;22:313–20. doi: 10.1038/nbt937. [DOI] [PubMed] [Google Scholar]
- 8.Puhlmann M, Brown CK, Gnant M, et al. Vaccinia as a vector for tumor-directed gene therapy: biodistribution of a thymidine kinase-deleted mutant. Cancer Gene Ther. 2000;7:66–73. doi: 10.1038/sj.cgt.7700075. [DOI] [PubMed] [Google Scholar]
- 9.Hung CF, Tsai YC, He L, et al. Vaccinia virus preferentially infects and controls human and murine ovarian tumors in mice. Gene Ther. 2007;14:20–9. doi: 10.1038/sj.gt.3302840. [DOI] [PubMed] [Google Scholar]
- 10.Chen CH, Wang TL, Hung CF, Pardoll DM, Wu TC. Boosting with recombinant vaccinia increases HPV-16 E7-specificT cell precursor frequencies of HPV-16 E7-expressing DNA vaccines. Vaccine. 2000;18:2015–22. doi: 10.1016/s0264-410x(99)00528-9. [DOI] [PubMed] [Google Scholar]
- 11.Kim TW, Hung CF, Ling M, et al. Enhancing DNA vaccine potency by coadministration of DNA encoding antiapoptotic proteins. J Clin Invest. 2003;112:109–17. doi: 10.1172/JCI17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norbury CC, Princiotta MF, Bacik I, et al. Multiple antigen-specific processing pathways for activating naive CD8+ T cells in vivo. J Immunol. 2001;166:4355–62. doi: 10.4049/jimmunol.166.7.4355. [DOI] [PubMed] [Google Scholar]
- 13.Lin KY, Guarnieri FG, Staveley-O’Carroll KF, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–6. [PubMed] [Google Scholar]
- 14.Kim D, Hung CF, Wu TC. Monitoring the trafficking of adoptively transferred antigen-specific CD8-positiveT cells in vivo, using noninvasive luminescence imaging. Hum Gene Ther. 2007;18:575–88. doi: 10.1089/hum.2007.038. [DOI] [PubMed] [Google Scholar]
- 15.Peng S, Trimble C, Ji H, et al. Characterization of HPV-16 E6 DNA vaccines employing intracellular targeting and intercellular spreading strategies. J Biomed Sci. 2005;12:689–700. doi: 10.1007/s11373-005-9012-3. [DOI] [PubMed] [Google Scholar]
- 16.Chen CH, Wang TL, Hung CF, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60:1035–42. [PubMed] [Google Scholar]
- 17.Wu TC, Guarnieri FG, Staveley-O’Carroll KF, et al. Engineering an intracellular pathway for major histocompatibility complex class II presentation of antigens. Proc Natl Acad Sci U S A. 1995;92:11671–5. doi: 10.1073/pnas.92.25.11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen B, Timiryasova TM, Haghighat P, et al. Low-dose vaccinia virus-mediated cytokine gene therapy of glioma. J Immunother. 2001;24:46–57. doi: 10.1097/00002371-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Earl PL, Moss B. Mutational analysis of the assembly domain of the HIV-1envelope glycoprotein. AIDS Res Hum Retroviruses. 1993;9:589–94. doi: 10.1089/aid.1993.9.589. [DOI] [PubMed] [Google Scholar]
- 20.Ward BM. Pox, dyes, and videotape: making movies of GFP-labeled vaccinia virus. Methods Mol Biol. 2004;269:205–18. doi: 10.1385/1-59259-789-0:205. [DOI] [PubMed] [Google Scholar]
- 21.Feltkamp MC, Smits HL, Vierboom MP, et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23:2242–9. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 22.Cheng WF, Hung CF, Chai CY, et al. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J Clin Invest. 2001;108:669–78. doi: 10.1172/JCI12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu P, Rowley DA, Fu YX, Schreiber H. The role of stroma in immune recognition and destruction of well-established solid tumors. Curr Opin Immunol. 2006;18:226–31. doi: 10.1016/j.coi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiotto MT, Schreiber H. Rapid destruction of the tumor microenvironment by CTLs recognizing cancer-specific antigens cross-presented by stromal cells. Cancer Immun. 2005;5:8. [PubMed] [Google Scholar]
- 26.Qiao J, Kottke T, Willmon C, et al. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat Med. 2008;14:37–44. doi: 10.1038/nm1681. [DOI] [PubMed] [Google Scholar]
- 27.Diaz RM, Galivo F, Kottke T, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–8. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- 28.Miller CG, Fraser NW. Requirement of an integrated immune response for successful neuroattenuated HSV-1therapy in an intracranial metastatic melanoma model. Mol Ther. 2003;7:741–7. doi: 10.1016/S1525-0016(03)00120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Dutuor A, Tao L, Fu X, Zhang X. Virotherapy with a type 2 herpes simplex virus-derived oncolytic virus induces potent antitumor immunity against neuroblastoma. Clin Cancer Res. 2007;13:316–22. doi: 10.1158/1078-0432.CCR-06-1625. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Dutuor A, Fu X, Zhang X. Induction of strong antitumor immunity by an HSV-2-based oncolytic virus in a murine mammary tumor model. J Gene Med. 2007;9:161–9. doi: 10.1002/jgm.1005. [DOI] [PubMed] [Google Scholar]
- 31.Toda M, Rabkin SD, Kojima H, Martuza RL. Herpes simplex virus as an in situ cancer vaccine for the induction of specific anti-tumor immunity. Hum Gene Ther. 1999;10:385–93. doi: 10.1089/10430349950018832. [DOI] [PubMed] [Google Scholar]
- 32.Ikeda K, Ichikawa T, Wakimoto H, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5:881–7. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.