Abstract
The butter flavorant diacetyl (2,3-butanedione) is implicated in causing obliterative bronchiolitis in microwave popcorn plant workers. Because diacetyl modifies arginine residues, an immunological basis for its toxicity is under investigation. Reaction products of diacetyl with N-α-acetylarginine (AcArg) were determined as a model for hapten formation, with characterization by mass spectrometry, NMR, and HPLC with UV detection and radiodetection. Four products were identified by LCMS, each with a positive ion of m/z 303 (diacetyl + AcArg); one pair displayed an additional ion at m/z 217 (AcArg), the other pair at m/z 285 (– H2O). Their 1H–13C NMR correlation spectra were consistent with the addition of one or two of the guanidine nitrogens to form aminols. Open-chain pairs interconverted at pH 2, as did the cyclized, but all four interconverted at neutral pH. This is the first structural characterization of the covalent adducts between diacetyl and an arginine moiety.
Keywords: Diacetyl; 2,3-butanedione; artificial butter flavoring; arginine adducts; toxicity
INTRODUCTION
Diacetyl (2,3-butanedione) is an α-diketone that is present naturally in butter and a variety of foods including dairy products and alcoholic beverages as a product of bacterial fermentation (1–4). Diacetyl is also a major component of artificial butter flavorings that are added to food products to impart a buttery flavor. In addition to its presence in foods, diacetyl has been detected in tobacco smoke and as an off-gas from carpet glue, thereby contributing to the wide range of volatile organic compounds present in indoor air (5,6). In industry, diacetyl is used as an analytical reagent (7), as an antimicrobial agent (8), and as a starting material for chemical synthesis (9). Therefore, there is significant potential for general and occupational human exposure to diacetyl.
The U.S. Food and Drug Administration granted diacetyl GRAS (generally recognized as safe) status as a direct food ingredient, and consumption of the low levels of diacetyl present in food has not been reported to present a human health risk. However, recent reports have implicated butter flavoring vapors, particularly diacetyl, the predominant volatile component, as the causative agent of obliterative bronchiolitis, a severe respiratory disease, in workers at microwave-popcorn packaging plants (10–12). In animal studies, acute inhalation exposure of male Sprague–Dawley rats to butter flavoring vapors normalized to 203–371 ppm diacetyl, concentrations similar to peak workplace levels, for 6 h resulted in necrosis of both nasal and pulmonary airway epithelium (13). Longer term exposure of male C57Bl/6 mice to 100 ppm diacetyl 6 h/day 5 days/week for 12 weeks also caused significant injury to the epithelium of the nasal cavity and upper airways, as well as an increased incidence of lymphocytic bronchiolitis (14). Although the significance of lymphocytic bronchiolitis in diacetyl-exposed mice is unknown, this lesion is a known risk factor for the development of obliterative bronchiolitis in transplant patients (15).
Obliterative bronchiolitis is a relatively rare lung disease most commonly associated with transplant patients undergoing chronic rejection. Immune-mediated injury to the respiratory epithelium has been proposed as an initiating event in transplant-related bronchiolitis obliterans (16). It is also possible that the injury to the respiratory epithelium caused by diacetyl exposure may lead to immune-mediated progression of lung injury to obliterative bronchiolitis. Diacetyl monomers, dimers, and trimers have been shown to specifically react with arginyl residues in proteins (17–21). Several modifications of peptidyl-arginines have been shown to result in highly specific autoantibody responses (22). An immunological response to these modified cell membrane proteins may be a contributing factor in the development of obliterative airway disease in workers exposed to diacetyl. Quantitative structure–activity relationships (QSARs) lend further support to this hypothesis, indicating the potential for diacetyl to act as a contact allergen via the formation of an antigenic complex as a result of covalent modification of proteins (23). The goal of the present study is to characterize the interaction of diacetyl with AcArg as a model of potential reactions with arginine-containing peptides and proteins as a prelude to determining the chemical basis for potential immunological effects.
MATERIALS AND METHODS
Chemicals
N-α-Acetyl-L-arginine (AcArg) and 2,3-butanedione (stated purity of ≥99.0%) were obtained from Sigma Chemical Co. (St. Louis, MO). [14C1-1,4]Butane-2,3-dione was obtained from Midwest Research Institute (Kansas City, MO). Radiochemical purity was assessed to be 92.0% by HPLC, using a Bio-Rad (Hercules, CA) Aminex HPX-87H, 7.8 × 300 mm, ion exclusion column heated to 35 °C and an isocratic mobile phase of 0.01 N H2SO4 at 0.8 mL/min. [14C]2,3-Butanedione was detected by measuring absorbance at 290 nm with a Waters 2487 absorbance detector (Milford, MA) and monitoring the column effluent with a β-Ram radioactivity detector (IN/US Systems, Inc., Tampa, FL) equipped with a 250 μL glass solid scintillant cell. The effluent was collected in fractions, and the radioactivity in each fraction was quantitated by liquid scintillation spectrometry (LSS).
Formation and Analysis of Reaction Products of Diacetyl/N-α-Acetyl-L-arginine
For characterization by LC-MS, an equimolar aqueous solution (10 mM each) of diacetyl and AcArg was allowed to react at 37 °C for 6 days before analysis by reversed phase HPLC using a Grace Vydac C4 column (4.6 × 250 mm, 5 μm particle size; Deerfield, IL). All HPLC systems for analysis of reaction products used mobile phases A [0.1% aqueous trifluoroacetic acid (TFA)] and B (0.1% TFA in acetonitrile). The reaction products were separated for LC-MS using a binary gradient system with a flow rate of 1 mL/min. The initial mobile phase composition of 100% A was maintained for 4 min post injection before changing linearly over the next 16 min to 20% solvent B and then over 8 min to 80% B. Detection was by a MDS Sciex (Toronto, Canada) API 150 single-quadrupole mass spectrometer. The source temperature was 400 °C; electrospray ionization was used, and detection was in the positive mode. Flow was split with 50% going to the mass spectrometer and 50% going to waste.
For HPLC-UV characterization, an aqueous solution of AcArg and diacetyl, each at 100 mM, was reacted at room temperature for 24 h prior to fractionation by HPLC. The HPLC system consisted of a Waters 600E solvent delivery module and an Applied Biosystems (Foster City, CA) 759a absorbance detector with data acquisition by a Waters Empower data collection system. Mobile phase A was delivered isocratically at a flow rate of 1.0 mL/min through a Grace Vydac C4 column, 25 cm × 4.6 mm. Eluent was monitored for UV absorbance at 210 nm with a Perkin-Elmer (Waltham, MA) 785-A detector. Eluting peaks were collected individually and reanalyzed 2 h later on this system.
For characterization by NMR, the diacetyl/AcArg reaction mixture prepared as described above was fractionated by semipreparative HPLC. The HPLC system was composed of a Waters 600 Multi Solvent Delivery System, a Rheodyne 725 manual Injector (Rohnert Park, CA), an Applied Biosystems 759a absorbance detector, and a Phenomenex (Torrance, CA) Aqua C18 semipreparative column (250 × 10 mm, 5 μm). The initial mobile phase composition of 100% A was held for 3 min before changing linearly over 5 min to 5% B, held for 4 min, then changed linearly over 10 min to 20% B and then to 50% B over 1 min, and held for 7 min; the flow rate was 5 mL/min. Eluent was monitored at 210 nm, and peaks were collected after injection of 200 μL of the solution. Eight iterations of this process were conducted, and fractions 2 and 6 and fractions 4 and 7 were combined. The fractions were dried under nitrogen and then lyophilized overnight. Dried fractions were reconstituted in DMSO-d6 for NMR analysis.
NMR spectra were acquired on a Varian Inova NMR spectrometer (Palo Alto, CA) operating at 500 MHz for protons. Spectra were acquired at 25 °C in DMSO-d6 using a 5 mm broadband probe with inverse detection. Proton spectra were acquired using a spectral width of 7200 Hz and 32 transients. Carbon spectra were acquired with a spectral width of 32000 Hz and 25000 transients. 1H–13C heteronuclear multiple bond correlation (HMBC) spectra were acquired as a 4K × 400 point data set with 128 transients for each increment. Linear prediction was used to produce a 4K × 1K data set prior to 2D Fourier transform.
The propensity for the isolated components present in fractions 2, 4, 6, and 7 (Figure 1A) to re-equilibrate upon standing in solution was addressed in experiments using radiolabeled butanedione. A 1 mL reaction mixture consisting of 100 mM AcArg and 93 mM butanedione (containing ca. 20 μCi of [14C]butanedione) was incubated for 3 h at 37 °C before analysis by HPLC; 100 μL of this solution was injected onto the column. The HPLC system consisted of a Waters 600E solvent delivery module, a Phenomenex Aqua (250 × 4.6 mm, 5 μm particle size) C18 column, a Perkin-Elmer 785A UV detector, and a β-Ram flow-through radioactivity detector (IN/US Systems Inc.). Data acquisition was conducted by a Waters Empower data collection system. The initial mobile phase composition, 100% A, was held for 3 min before changing linearly over the next 3 min to 5% B, held for 4 min, then changed linearly over the next 10 min to 20% B and then to 50% B over 1 min, and held for 7 min. The flow rate was 1 mL/min. Eluting products were monitored online for radioactivity and for absorbance at 210 nm.
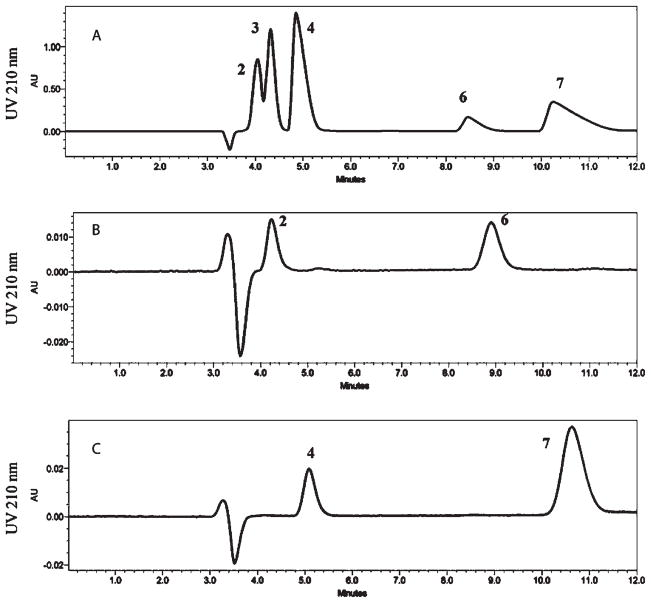
Figure 1.
HPLC-UV analysis of the reaction of diacetyl with N-α-acetylarginine (3) for 24 h at room temperature showing (A) the four reaction products 2, 4, 6, and 7, (B) the re-equilibration of isolated fraction 6 into fractions 2 and 6, 2 h following isolation, and (C) the re-equilibration of isolated fraction 7 into fractions 4 and 7, 2 h following isolation.
Fractions 2, 4, 6, and 7 were collected into vials containing 35 μL of a 0.85 M sodium bicarbonate solution. Fractions were collected in 1 mL portions, yielding a final pH between 7 and 7.5 per fraction. These solutions (100 μL) were reapplied to the column 0.5–2 h later, and the percentage of radioactivity associated with each fraction was determined by radiochemical peak area.
RESULTS
The products of the reaction of diacetyl with AcArg were characterized by NMR and mass spectrometry, and their equilibration between interconverting pairs of open-chain adducts and interconverting pairs of cyclized adducts was monitored by HPLC. HPLC analysis (Figure 1A) of the reaction products of diacetyl with N-α-acetylarginine (peak 3) shows the formation of four products (fractions 2, 4, 6, and 7). Fractions 2 and 6 were isolated separately and reanalyzed 2 h later. The HPLC-UV chromatogram (Figure 1B) demonstrates that these two products are in equilibrium and display identical chromatograms upon reanalysis (results for fraction 6 shown). Similarly, fractions 4 and 7 were found to be equilibrating pairs (Figure 1C; results for fraction 7 shown).
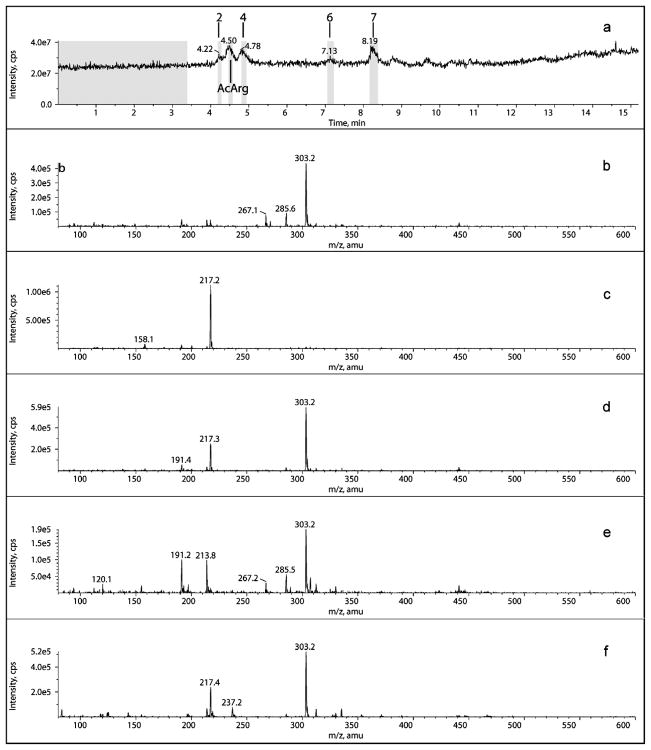
The positive ion mass spectra of fractions 2 and 6 (Figure 2, panels b and e, respectively) are virtually identical (except for unassigned ions of m/z 191 and 214 in Figure 2b), and display ions of m/z 303 (diacetyl + AcArg), 285 (– H2O), and 267 (– H2O). Similarly, the mass spectra of fractions 4 and 7 (Figure 2, panels d and f, respectively) are virtually identical and display ions of m/z 303 (diacetyl + AcArg) and 217 (AcArg).
Figure 2.
HPLC–mass spectral analysis (electrospray ionization, detection in positive mode) of the reaction products of diacetyl with AcArg (both at 10 mM) after 6 days at 37 °C: (a) total ion current; (b) mass spectrum of fraction 2 at 4.22 min; (c) mass spectrum of AcArg at 4.50min; (d) mass spectrum of fraction 4 at 4.78 min; (e) mass spectrum of fraction 6 at 7.13 min; (f) mass spectrum of fraction 7 at 8.19 min. The mass spectra of fractions 2 and 6 were virtually identical, as were those of fractions 4 and 7.
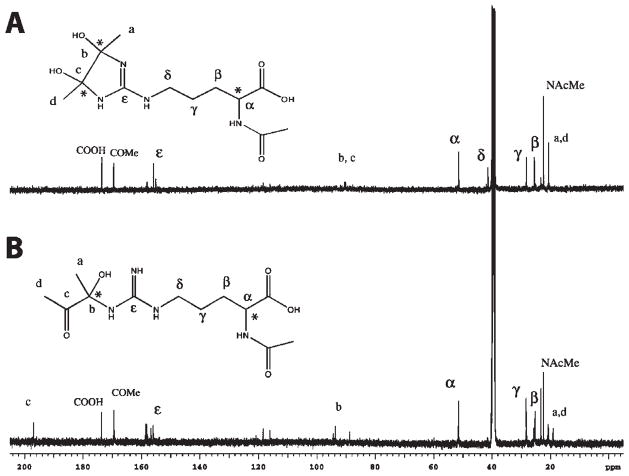
The 13C NMR spectrum for fraction 2/6 (A) and fraction 4/7 (B) are shown in Figure 3 along with the structural assignments of the resonances. The molecular weights of these components determined by mass spectrometry are in agreement with these structures. The main difference between fractions 2/6 and 4/7 is the carbonyl resonance at ~195 ppm in fraction 4/7, which is lost in the cyclization reaction.
Figure 3.
13C NMR spectra of the isolated products of the reaction of diacetyl with N-α-acetylarginine: (A) fraction 2/6; (B) fraction 4/7.
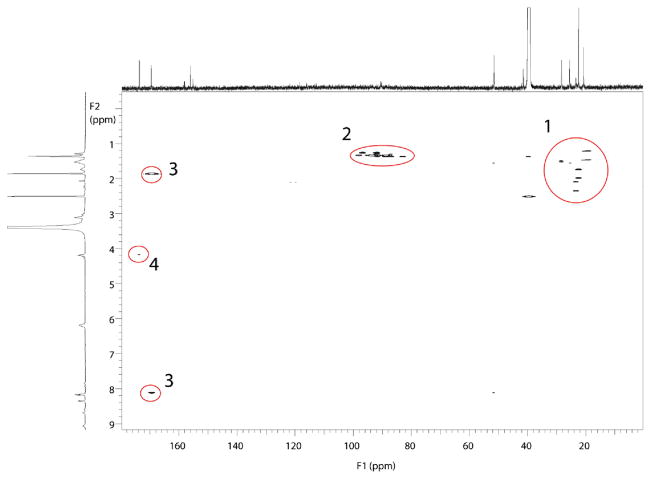
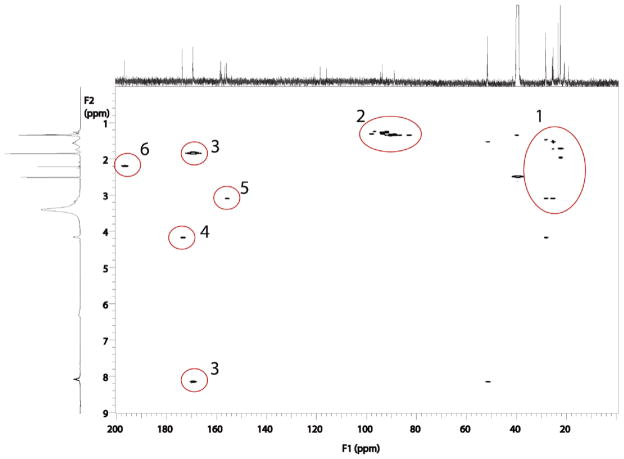
The 1H–13C HMBC NMR spectrum of fraction 2/6 with regions of interest highlighted is shown in Figure 4, and that of fraction 4/7 is shown in Figure 5. The HMBC spectrum produces correlations between carbons and protons that are separated by two or three bonds. These correlations observed in region 1 of the HMBC spectra are similar between the two fractions and are consistent with arginine side-chain interactions. Region 2 of the HMBC spectra shows long-range correlations between methyl proton resonances and resonances consistent with carbons bearing two heteronuclei. This region is attributed to correlations between the methyl protons of the diacetyl group with the carbon at the site of conjugation. This region is similar for both fractions as expected for the two structures postulated. Region 3 is also similar in both fractions and is assigned as correlations between the N-acetyl carbonyl with the acetyl methyl proton and the amide proton. Likewise, region 4 is assigned as the correlations between the α proton of arginine and the carbon of the carboxylic acid. Regions 1, 3, and 4 are consistent with an intact N-acetyl arginine group. Regions 5 and 6 differentiate the two fractions. Region 5 shows the correlations between the δ arginine protons and the guanidino carbon. The resonance in region 6 is due to the interaction between a methyl and a ketone. This resonance is attributed to an unconjugated carbonyl of diacetyl.
Figure 4.
1H–13C HMBC spectra of isolated products of the reaction of diacetyl with N-α-acetylarginine, fraction 2/6, with correlated regions of 1H–13C highlighted.
Figure 5.
1H–13C HMBC NMR spectrum of isolated products of the reaction of diacetyl with N-α-acetylarginine, fraction 4/7, with correlated regions of 1H–13C highlighted.
Using radiolabeled diacetyl, relative extinction coefficients for the UV absorbance of the pairs were estimated from the ratio of the integrated areas of the radioactivity and UV absorbance (210 nm) signals. The UV–visible spectra (200–500 nm) of the four pairs exhibited a maxima at 200 nm (bordering the charge transfer region), with no significant maxima at longer wavelengths (data not shown). Fractions 4 and 7 had 2.4 times the relative extinction coefficient of fractions 2 and 6, consistent with two imino/keto groups in the former and one for the latter.
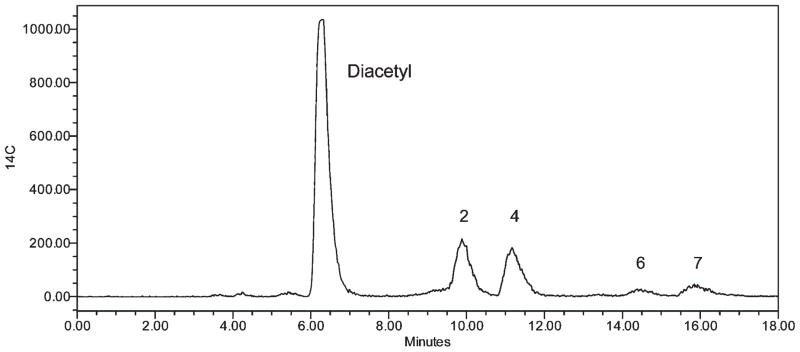
The HPLC radiochromatogram of the products of the reaction of [14C]diacetyl and AcArg (ca. 100 mM each) is shown in Figure 6. Interestingly, fractions 2 and 4, one cyclized and one open-chain product, respectively (Figure 7), predominate. The relative composition percentages were 46, 38, 5, and 11% for fractions 2, 4, 6, and 7, respectively. The re-equilibration of the forms in isolated, neutralized fractions is shown in Table 1. Notably, both fractions 6 and 7 readily revert to a mixture that is chiefly composed of fractions 2 and 4, with the same modest preference for fraction 2 over fraction 4.
Figure 6.
HPLC radiochromatogram of the products of the reaction of diacetyl and AcArg. A 1 mL reaction mixture containing a solution 100 mM in AcArg and 93 mM in diacetyl (containing ca. 20 μCi of [14C]diacetyl) was incubated for 3 h at 37 °C before analysis by HPLC (as described under Materials and Methods).
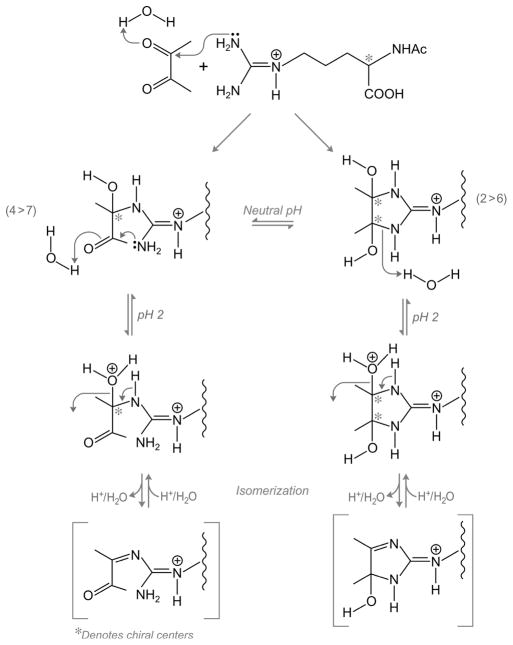
Figure 7.
Proposed mechanism for the formation and re-equilibration of diacetyl/AcArg adducts.
Table 1.
Re-equilibration of Isolated Diacetyl/AcArg Adducts at Neutral pH: Re-equilibration Composition (Relative Percent Composition)
See Figure 7 for chemical structures.
nd, not detected.
DISCUSSION
Reaction of diacetyl and AcArg produced four major products. LC-MS analysis of these products indicated that each had a molecular ion of m/z 303, consistent with the covalent bonding of diacetyl with AcArg. Taken together with NMR data, these products are consistent with the addition of one or two of the guanidine nitrogens of AcArg to form aminols. The data support the characterization of fractions 2 and 6 as the cyclized forms (Figure 3A) and fractions 4 and 7 as the open-chain components (Figure 3B) in that (1) the mass spectra of fractions 4 and 7 are consistent with amore facile elimination of AcArg (m/z 217) from a single point of attachment; (2) the mass spectra of fractions 2 and 6 exhibit no ions of m/z 217, but instead ions consistent with the loss of one and two water molecules, the latter consistent with a symmetrical, dehydrated ring product isolated previously following heating of fractions 2 and 6 (24); and (3) the ratio of UV absorbance to molar content for fractions 4 and 7 was 2.4 times that of fractions 2 and 6, consistent with two imino/keto groups for the former versus one for the latter.
The chiral centers contributed by AcArg, as well as those from aminol carbons, provide the potential for producing several diastereomeric products that could give rise to the four products observed. Consistent with that interpretation and the reversibility of aminol bond formation, isolated m/z 303 fractions 2 and 6 and fractions 4 and 7 were pairs found to re-equilibrate at pH 2 to produce their diastereotopic products. The reversibility of this reaction has been reported previously (19, 25).
Whereas the chromatographic resolution of peaks by HPLC benefited from inclusion of TFA, the re-equilibration reactions occurring at pH 2 were decidedly different than those in neutralized solutions. A proposed mechanism for the formation and re-equilibration of diacetyl/AcArg adducts is shown in Figure 7, with initial formation of predominately fraction 4 and subsequent cyclization to form predominately fraction 2. Fractions 2 and 6 may be cis/trans isomers, with fraction 2 the more thermodynamically favored form. At neutral pH the four forms should be able to readily interconvert by ring opening and closing at the aminol carbon. It is speculated that the acid-catalyzed isomerization occurs by protonation of the hydroxyl group to allow water as a leaving group, with intermediate formation of an imine that is part of a reactive Michael system that can readily rehydrate. If correct, this reaction, a vestige of nonphysiological pH, could conceivably alter the nature of protein binding to diacetyl during processing with TFA, a common method employed in the purification of adducted proteins, giving rise to artificial cross-linking with nucleophilic amino acid residues.
Although diacetyl has been used for over 40 years to modify arginine residues, this work provides the first report of the structural characterization of the complex formed between diacetyl and an arginine moiety. Riordan (19), while noting that there was no supporting structural data, did speculate on the nature of the adduct and postulated the same structure for the cyclized adducts identified here as one of the products of the addition of diacetyl to arginine. Accordingly, in that work borate stabilized the adduct in an interaction postulated to involve formation of a diester of the vicinal diols with boric acid. Those workers also found that periodate stabilized the adduct, although no mechanism was postulated. In retrospect, the expected product of the cleavage of the vicinal diol carbon–carbon bond would yield a stable diamide. Treatment with sodium borohydride did not stabilize the adduct, consistent with an aminol structure rather than one in which loss of waters would produce a reducible imine bond.
The synthesis and characterization of this diacetyl–arginine complex has important implications for investigating the mechanism(s) of diacetyl-mediated lung disease. Currently, the mechanism(s) of diacetyl toxicity is (are) unknown; however, the results of this study suggest that injury to the airway epithelium may involve alteration of cellular proteins containing arginine, including those on cell membranes. An immunological response to these modified cell membrane proteins may contribute to the development of obliterative bronchiolitis. Studies are underway to utilize this new information to investigate the immune response to membrane proteins and enzymes altered by diacetyl reaction with arginyl residues.
Acknowledgments
This work was performed for the National Toxicology program, National Institute of Environmental Health Sciences, National Institutes of Health, U.S. Department of Health and Human Services, under Contract N01-ES-75563 (HHSN29120077563). This paper may be the work product of an employee or group of employees of the National Institute of Environmental Health Sciences (NIEHS), National Institute of Health (NIH); however, the statements, opinions, or conclusions contained therein do not necessarily represent the statements, opinions, or conclusions of NIEHS, NIH, or the U.S. government.
We are grateful to Dr. Suramya Waidyanatha, Dr. J. Michael Sanders, and Sherry Black for their review of the manuscript and to Kathy Ancheta for her assistance in the preparation of the manuscript.
ABBREVIATIONS USED
- AcArg
N-α-acetylarginine
- LSS
liquid scintillation spectrometry
- TFA
trifluoroacetic acid
- HMBC
heteronuclear multiple bond correlation
- HPLC
high-performance liquid chromatography
- LC-MS
liquid chromatography–mass spectrometry
LITERATURE CITED
- 1.Yamaguchi M, Ishida J, Xuan-Xuan Z, Nakamura M, Yoshitake T. Determination of glyoxal, methylglyoxal, diacetyl, and 2,3-pentanedione in fermented foods by high-performance liquid chromatography with fluorescence detection. J Liq Chromatogr. 1994;17:203–211. [Google Scholar]
- 2.Marsili RT. Monitoring bacterial metabolites in cultured buttermilk by high performance liquid chromatography and headspace gas chromatography. J Chromatogr Sci. 1981;19(9):451. doi: 10.1093/chromsci/19.9.451. [DOI] [PubMed] [Google Scholar]
- 3.Shane BS, Troxclair AM, McMillin DJ, Henry CB. Comparative mutagenicity of nine brands of coffee to Salmonella typhimurium TA100, TA102, and TA104. Environ Mol Mutagen. 1988;11(2):195–206. doi: 10.1002/em.2850110205. [DOI] [PubMed] [Google Scholar]
- 4.Tucker JD, Taylor RT, Christensen ML, Strout CL, Hanna ML, Carrano AV. Cytogenetic response to 1,2-dicarbonyls and hydrogen peroxide in Chinese hamster ovary AUXB1 cells and human peripheral lymphocytes. Mutat Res. 1989;224(2):269–279. doi: 10.1016/0165-1218(89)90166-3. [DOI] [PubMed] [Google Scholar]
- 5.Crump D, Gardiner D. Sources and concentrations of aldehydes and ketones in indoor environments in the UK. Environ Int. 1989;15:455–462. [Google Scholar]
- 6.Rothweiler H, Waeger P, Schlatter C. Long term emissions from two glued carpets with different backings measured in indoor air. Environ Technol. 1992;13:891–896. [Google Scholar]
- 7.Poulose AJ, Kolattukudy PE. Enzymatic reduction of phenylglyoxal and 2,3-butanedione, two commonly used arginine-modifying reagents, by the ketoacyl reductase domain of fatty acid synthase. Int J Biochem. 1986;18(9):807–812. doi: 10.1016/0020-711x(86)90057-1. [DOI] [PubMed] [Google Scholar]
- 8.Jay JM. Antimicrobial properties of diacetyl. Appl Environ Microbiol. 1982;44(3):525–532. doi: 10.1128/aem.44.3.525-532.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho C, Hartman G. Formation of oxazolines and oxazoles in Strecker degradation of DL-alanine and L-cysteine with 2,3-butanedione. J Agric Food Chem. 1982;30:793–794. [Google Scholar]
- 10.Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N Engl J Med. 2002;347(5):330–338. doi: 10.1056/NEJMoa020300. [DOI] [PubMed] [Google Scholar]
- 11.Boylstein R, Piacitelli C, Grote A, Kanwal R, Kullman G, Kreiss K. Diacetyl emissions and airborne dust from butter flavorings used in microwave popcorn production. J Occup Environ Hyg. 2006;3(10):530–535. doi: 10.1080/15459620600909708. [DOI] [PubMed] [Google Scholar]
- 12.Fedan JS, Dowdy JA, Fedan KB, Hubbs AF. Popcorn worker’s lung: in vitro exposure to diacetyl, an ingredient in microwave popcorn butter flavoring, increases reactivity to methacholine. Toxicol Appl Pharmacol. 2006;215(1):17–22. doi: 10.1016/j.taap.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Hubbs AF, Battelli LA, Goldsmith WT, Porter DW, Frazer D, Friend S, Schwegler-Berry D, Mercer RR, Reynolds JS, Grote A, Castranova V, Kullman G, Fedan JS, Dowdy J, Jones WG. Necrosis of nasal and airway epithelium in rats inhaling vapors of artificial butter flavoring. Toxicol Appl Pharmacol. 2002;185(2):128–135. doi: 10.1006/taap.2002.9525. [DOI] [PubMed] [Google Scholar]
- 14.Morgan DL, Flake GP, Kirby PJ, Palmer SM. Respiratory toxicity of diacetyl in C57BL/6 mice. Toxicol Sci. 2008;103(1):169–180. doi: 10.1093/toxsci/kfn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharples LD, McNeil K, Stewart S, Wallwork J. Risk factors for bronchiolitis obliterans: a systematic review of recent publications. J Heart Lung Transplant. 2002;21(2):271–281. doi: 10.1016/s1053-2498(01)00360-6. [DOI] [PubMed] [Google Scholar]
- 16.Babu A, Nichols M. Critical pathways leading to obliterative bronchiolitis in lung allografts. Curr Opin Organ Transplant. 2006;11:483–489. [Google Scholar]
- 17.Yankeelov JA, Jr, Mitchell CD, Crawford TH. A simple trimerization of 2,3-butanedione yielding a selective reagent for the modification of arginine in proteins. J Am Chem Soc. 1968;90(6):1664–1666. doi: 10.1021/ja01008a056. [DOI] [PubMed] [Google Scholar]
- 18.Yankeelov JA., Jr Modification of arginine in proteins by oligomers of 2,3-butanedione. Biochemistry. 1970;9(12):2433–2439. doi: 10.1021/bi00814a007. [DOI] [PubMed] [Google Scholar]
- 19.Riordan JF. Functional arginyl residues in carboxypeptidase A. Modification with butanedione. Biochemistry. 1973;12(20):3915–3923. doi: 10.1021/bi00744a020. [DOI] [PubMed] [Google Scholar]
- 20.Fliss H, Tozer NM, Viswanatha T. The reaction of chymotrypsin with 2,3-butanedione trimer. Can J Biochem. 1975;53(3):275–283. doi: 10.1139/o75-039. [DOI] [PubMed] [Google Scholar]
- 21.Miller AG, Meade SJ, Gerrard JA. New insights into protein crosslinking via the Maillard reaction: structural requirements, the effect on enzyme function, and predicted efficacy of crosslinking inhibitors as anti-ageing therapeutics. Bioorg Med Chem. 2003;11(6):843–852. doi: 10.1016/s0968-0896(02)00565-5. [DOI] [PubMed] [Google Scholar]
- 22.Van Boekel M, van Benrooij W. Modifications of arginines and their role in autoimmunity. Autoimmunity Rev. 2003;2:57–62. doi: 10.1016/s1568-9972(02)00128-3. [DOI] [PubMed] [Google Scholar]
- 23.Patlewicz G, Roberts D, Walker J. QSARs for the skin sensitization potential of aldehydes and related compounds. QSAR Comb Sci. 2003;22:196. [Google Scholar]
- 24.Mathews JM, Burgess JP, Snyder RW, Etheridge AS, Burka LT. Reaction of the butter flavorant butanedione with N-α-acetylarginine. Drug Metab Rev. 2005;37(Suppl 2):273. [Google Scholar]
- 25.Yankeelov JJ. Modification of arginine by diketones. Methods Enzymol. 1972;25:566–576. doi: 10.1016/S0076-6879(72)25056-X. [DOI] [PubMed] [Google Scholar]