Abstract
Background
Anesthetics administered to immature brains may cause histopathological changes and long-term behavioral abnormalities. The association between perinatal exposure to anesthetics during Cesarean delivery (CD) and development of learning disabilities (LD) was determined in a population-based birth cohort.
Methods
The educational and medical records of all children born to mothers residing in five townships of Olmsted County, MN from 1976-1982 and remaining in the community at age 5 were reviewed to identify those with LDs. Cox proportional hazards regression was used to compare rates of LD between children delivered vaginally and via CD (with general or regional anesthesia).
Results
Of the 5,320 children in this cohort, 497 were delivered via CD (under general anesthesia N=193, and regional anesthesia N=304). The incidence of LD depended on mode of delivery (P = 0.050, adjusted for sex, birth weight, gestational age, exposure to anesthesia prior to age 4, and maternal education). LD risk was similar in children delivered by vagina or CD with general anesthesia, but was reduced in children receiving CD with regional anesthesia (hazard ratio = 0.64, 95% confidence interval 0.44 to 0.92; P=0.017 for comparison of CD under regional anesthesia compared to vaginal delivery).
Conclusion
Children exposed to general or regional anesthesia during CD are not more likely to develop LD compared to children delivered vaginally, suggesting that brief perinatal exposure to anesthetic drugs does not adversely affect long-term neurodevelopmental outcomes. The risk of LD may be lower in children delivered by CD whose mothers received regional anesthesia.
Introduction
The short- and long-term effects of obstetric anesthetic techniques on behavior and development of the neonate, infant and child have been of long-standing interest. It is clear that these techniques may at least transiently affect some aspects of newborn behavior.1-7 However, the impact of obstetric analgesia and anesthesia on long-term outcomes in the absence of concurrent events such as fetal asphyxia is not known. Studies evaluating the association between perinatal and environmental characteristics and childhood behavioral outcomes have suggested that operative or instrumented deliveries per se are not linked to childhood behavioral disorders or abnormalities in cognitive, verbal, or reading functioning,8-12 but these studies do not specifically evaluate the impact of anesthesia and analgesia.
In the peripartum period, children may be exposed to anesthetic and analgesic drugs that are administered to the mother. Exposure of fetal or neonatal animals (including primates) to anesthetics can cause histopathological changes in their brains, even following single, relatively brief administration.13-19 These changes may be associated with a diminished capacity to retain learned behavior20 and abnormal social behaviors resembling disorders in the autism spectrum.21 However, the significance of these findings to humans is not clear. We recently demonstrated that repeated, but not single, exposure to anesthesia prior to the age of 4 is associated with an approximately 2-fold increase in the incidence of learning disabilities (LD).22 Our data could not distinguish whether exposure to anesthesia itself increases the risk of LD, or whether the need for anesthesia represents a marker for unidentified confounders that promote LD. Although the lack of effect of a single anesthetic exposure on the incidence of LD in infants and young children is reassuring,22 it is still possible that even brief exposure of the less mature neonatal brain to anesthesia might have neurotoxic effects.23
Using the same unique population-based birth cohort described in our recent study22 we sought to determine if an association exists between fetal exposure to anesthesia during Cesarean delivery (CD) and the subsequent development of LD in a birth cohort of children. Based on our recent report22 we hypothesized that brief fetal exposure to anesthetics during CD would not increase the risk for LD.
Materials and Methods
The Mayo Clinic and Olmsted Medical Center Institutional Review Boards (both Rochester, Minnesota) approved this study. A birth cohort of children born in Rochester, MN identified in prior work by the authors24-28 formed the basis of the present study. All children born between January 1, 1976 and December 31, 1982 to mothers residing at the time of delivery in the five Olmsted County, Minnesota, townships comprising Minnesota Independent School District No. 535 (the Rochester public school system) were identified through computerized birth certificate information obtained from the Minnesota Department of Health, Division of Vital Statistics (N = 8,548). To ascertain vital status (still living in Rochester, moved, or deceased), for each member of the birth cohort during the 1995–1996 school year, resources available from the Rochester Epidemiology Project,29 Minnesota Independent School District No. 535, the Reading Center/Dyslexia Institute of Minnesota, and the Minnesota Department of Health were utilized. Children who left Olmstead County before age 5 (i.e., moved or died, N = 2,830) were not included in the final study cohort.24 Through the Rochester Epidemiology Project, all diagnoses and surgical procedures recorded at all Rochester medical facilities are indexed for automated retrieval. This index expedites retrieval of the unit (or dossier) medical record, which includes the history of all encounters in the hospital, community and ambulatory medical and social services, emergency department, outpatient clinics, and home visits as well as laboratory and psychological test results from birth until patients no longer reside in the community. Through a contractual research agreement, all public (19 primary, 3 junior high, 3 high schools) and nonpublic (12 primary, 10 junior high, 4 high schools) schools gave permission to access their richly documented cumulative educational records for every child from this birth cohort. Under a second research agreement permission was obtained to access the resources of the privately owned Reading Center/Dyslexia Institute of Minnesota, the only private tutoring agency in the community during the years relevant to this study. The Reading Center/Dyslexia Institute of Minnesota files included a pool of some 3,000 evaluations and outcomes of tutorial instruction that spanned nearly 50 years. Thus, the overall strategy in identifying all children in this cohort with LD employed multiple sources of information (school, medical and Reading Center/Dyslexia Institute of Minnesota records) and relied on a richly documented history of any learning/behavior concerns, information of educational intervention and individually administered test results.
Identification of Learning Disabilities
The details of LD ascertainment have been previously described in prior reports examining the epidemiology of LD.25,26,28 To summarize, all school, medical and Reading Center/Dyslexia Institute records were reviewed by trained personnel who used detailed data abstraction protocols, seeking evidence for reported learning difficulties. Based on the initial review, potential LD was identified in 1,510 children (26% of all 5,718 the birth cohort). The results of individually administered intelligence quotient (IQ, primarily age-appropriate Wechsler scales) and achievement (primarily Woodcock-Johnson tests) tests, medical, educational, and socioeconomic information were abstracted. Research criteria utilizing an average of two individually administered IQ and three individually administered achievement tests were then applied to these children to diagnose reading, written language, and math LD. Children were classified as having LD if they met criteria according to at least one of three standard formulas. In each of the following formulas, X is equal to the study subject's IQ score, and Y represents the predicted standard score from the achievement test. The regression formula–Minnesota, Y<17.40+ 0.62X, is issued by the Minnesota Department of Education.30 Children classified as having LD by this formula had standard scores in academic achievement that were more than 1.75 SD below their predicted standard score from an individually administered measure of cognitive ability (IQ). The value 0.62 represents the correlation between IQ and achievement used in the formula from the state of Minnesota. The discrepancy nonregression method was used in Minnesota Independent School District No. 535 before 1989 and included the school years of the children in the birth cohort. By using this approach, differences between standard scores on measures of intelligence and aptitude and measures of test achievement that were believed to be important varied by grade as follows: (1) kindergarten-3rd grade, 15 or more standard score points difference, with achievement lower; (2) 4th-6 th grade, 19 or more points difference, achievement lower; and (3) 7th -12 th grade, 23 or more points difference. Finally, the low-achievement method (X≥80 (aptitude) and Y≤90 (achievement) represents a recent concept in identifying LD independent of measured cognitive ability, assuming that cognitive ability is at least in the low average range.31 Children meeting the criteria prior to age 19 for at least one of the three LDs (reading, written language, and math disorders) using IQ and achievement scores obtained within the same calendar year were identified as LD cases regardless of presence or absence of any comorbid conditions.25,26,28
Other variables
We identified all children who were delivered via CD under general or regional anesthesia using the Mayo Clinic and Olmsted County Medical Center Surgical Information Retrieval Systems. For mothers who delivered with CD the following information was abstracted: American Society of Anesthesiologists physical status classification, urgency of CD (elective, emergent), type of anesthesia (general or regional, defined as epidural or spinal anesthesia), agents used (inhalational, intravenous, local anesthetics) and duration of anesthesia. Duration of anesthesia was defined as follows: 1) general anesthesia as the time from administration of induction agent to delivery of the child; 2) spinal anesthesia as the time from injection of neuraxial local anesthetic until the delivery, and; 3) epidural anesthesia as the time from the first epidural administration of drug (which could be a labor epidural) until delivery of child. If a failed regional anesthetic required conversion to general anesthesia, for the purpose of analyses the anesthetic was classified as general anesthesia, and the duration was recorded as time from administration of induction agent to delivery.
Age and education (<12 years (some high school education), 12 years (high school graduate), and >12 years (any post-secondary education)) for both mother and father were recorded from both school records and birth certificates. Pregnancy complications were abstracted from birth certificates, including pregnancy induced hypertension, preeclampsia, and eclampsia, hemorrhage during pregnancy, premature rupture of membranes, abnormalities of placenta or fetus. Information obtained for each child included sex, the number of births (multiple or single), gestational age at birth, birth weight and complications of labor and delivery (need for induced labor and method of induction, hemorrhage during delivery, feto-pelvic disproportion, dystocic position of the fetus, prolonged labor, and umbilical cord compression). APGAR scores (Appearance, Pulse, Grimace, Activity, Respiration) at 1 and 5 min were available only for children born 1980-1982 (N = 1,635).
Statistical analysis
The primary outcome for the current analysis was LD based on individually administered IQ and academic achievement test scores using any of the 3 standard formulas for determining the presence of reading written language or math LDs. The primary risk factor of interest for this investigation was the association of LD and exposure to CD under either general or regional anesthesia. Analyses were performed to compare demographic, pregnancy and delivery complications, and parental characteristics across delivery modes (vaginal, CD with general anesthesia, CD with regional anesthesia) using analysis of variance (ANOVA) for continuous variables and the chi-square test (or Fisher's exact test) for categorical variables. Individuals were followed from birth until the date they first met the LD criteria using any of the 3 standard formulas. Cumulative incidence rates of LD were calculated according to the method of Kaplan and Meier with data censored at the initial occurrence of emigration, death, last follow-up date, or the age of 19 years. Proportional hazards regression was used to assess whether anesthetic exposure at birth was a risk factor for LD. Both unadjusted and adjusted analyses were performed. For the adjusted analyses the covariates were selected a priori based on previous work24-27 and included gestational age (≤31 weeks, 32-36 weeks, ≥37 weeks), sex (male, female), birth weight (<2500 g, ≥2500 g), maternal education (some high school, high school graduate, any college) and number of anesthesia exposures before the age of 4 (0, 1, 2 or more).22 To supplement these planned analyses, additional post hoc analyses were performed eliminating children who were exposed to anesthesia after birth and prior to age 4 (a factor shown in prior work to increase the risk of LD in children receiving multiple anesthetics),22 and also eliminating all children who were delivered by emergency CD. An additional post hoc adjusted analysis included additional covariates for all characteristics which were found to differ significantly across delivery groups. These additional covariates included maternal age (≤19 years, 20-34 years, ≥35 years), paternal education (some high school, high school graduate, any post-secondary education), complications of pregnancy (none vs. any), induction of labor (no vs. yes), and complications of labor and delivery (none vs. any). Although APGAR scores were found to differ significantly across delivery modes, these scores were not used as covariates in this analysis because they were only available for neonates born between 1980 and 1982, and are therefore missing for 69% of the cohort. In all adjusted analyses only those individuals for whom complete covariate information was available were included. Results were summarized using hazard ratio estimates and corresponding 95% confidence intervals. In all cases, two-tailed P values less than 0.05 were considered to be statistically significant. Analyses were performed using SAS statistical software (Version 9.1, SAS Institute, Inc., Cary, NC).
Results
Between 1976 and 1982 there were 8,548 children born in the five Olmsted County, Minnesota townships comprising Independent School District No. 535, and 5,718 of these children still resided in the community at 5 years of age. Of these, 19 children were diagnosed with severe mental retardation and were excluded, as were 341 children who denied research authorization for the use of their medical records. An additional 37 individuals were excluded because the mother denied research authorization for use of their medical records or the charts were missing. Therefore, the cohort consisted of 5,320 children. Of these, 497 children were delivered via CD, 193 with general anesthesia and 304 with regional anesthesia. Most general anesthetics included sodium thiopental, nitrous oxide, and potent inhalational anesthetics (Table 1).
Table 1.
Anesthetic Agents used for Cesarean Delivery during General Anesthesia among Children in the 1976-1982 Rochester, Minnesota Birth Cohort (N=497).
| Intravenous agents* | N | (%) |
|---|---|---|
| Sodium Thiopental | 189 | (96%) |
| Ketamine | 2 | (1%) |
| Inhalational agents | ||
| Enflurane | 15 | (8%) |
| Halothane | 105 | (53%) |
| Isoflurane | 6 | (3%) |
| Methoxyflurane | 42 | (21%) |
| Nitrous Oxide | 191 | (97%) |
Data were missing for 1 patient. N, number, % percentage
Several demographic and birth characteristics differed among groups (Table 2 and 3). Notably, children delivered via CD under general anesthesia had lower mean birth weight, lower gestational age and lower APGAR scores at 1 and 5 minutes, were more likely to weigh <2500g, and were more likely to have a gestational age < 37 weeks (Table 2). Their mothers were also more likely to experience complications of pregnancy and delivery such as hemorrhage and eclampsia/preeclampsia (Table 3). For children delivered by CD, an emergency indication was more common among those whose mothers received general anesthesia (67%, N = 137)] compared to CD under regional anesthesia (30%, N = 87) (P < 0.001). Median duration of epidural analgesia/anesthesia (see methods for definitions) was 63.5 min (interquartile range 52, 82), spinal anesthesia was 21 min (interquartile range 15.5, 28), and general anesthesia was 14 min (interquartile range 8, 23).
Table 2. Sex and Birth Characteristics of Children in the 1976-1982 Rochester, Minnesota Birth Cohort (N=5320*).
| Variable | Vaginal Delivery (N=4,823) | Cesarean delivery with general (N=193) | Cesarean delivery with regional (N=304) | P-value |
|---|---|---|---|---|
| Sex | 0.15 | |||
| Female | 2,321 (48%) | 99 (51%) | 131 (43%) | |
| Male | 2,502 (52%) | 94 (49%) | 173 (57%) | |
| Number at birth | < 0.001 | |||
| Single | 4,752 (99%) | 181 (94%) | 287 (94%) | |
| Twin | 71 (1%) | 12 (6%) | 17 (6%) | |
| Birth weight (grams) | 3,477 ± 528.0 | 3,248 ± 779 | 3,453 ± 609 | <0.001 |
| Birth weight | < 0.001 | |||
| < 2500 | 178 (4%) | 31 (16%) | 16 (5%) | |
| 2500+ | 4,635 (96%) | 162 (84%) | 288 (95%) | |
| Gestational age | 40.1 ± 2.0 | 39.3 ± 3.2 | 39.5 ± 2.2 | <0.001 |
| Gestational age (weeks) | <0.001 | |||
| Less than 31.9 | 30 (1%) | 8 (4%) | 3 (1%) | |
| 32 to 36.9 | 241 (5%) | 27 (15%) | 23 (8%) | |
| 37+ | 4,250 (94%) | 145 (81%) | 264 (91%) | |
| APGAR score (1 minute) | 8.0 ± 1.3 | 6.8 ± 1.7 | 7.4 ± 1.8 | < 0.001 |
| APGAR score (5 minutes) | 9.2 ± 0.7 | 8.6 ± 1.0 | 8.8 ± 0.9 | < 0.001 |
APGAR, Appearance, Pulse, Grimace, Activity, Respiration score
Birth weight was missing for 10 (0.2%) and gestational age was missing for 329 (6.2%). APGAR scores were only available children born in 1980-1982 (N=1360).
Table 3. Maternal and Paternal Characteristics, Complications of Pregnancy and Labor among Children in the 1976-1982 Rochester, Minnesota birth cohort (N=5320*).
| Variable | Vaginal Delivery (N=4,823) | CD with general (N=193) | CD with regional (N=304) | P-value |
|---|---|---|---|---|
| Age of mom at birth | 26.4± 4.7 | 26.7± 5.2 | 27.8± 4.8 | <0.001 |
| Mother's education | 0.003 | |||
| Some-high school | 292 (7%) | 10 (6%) | 10 (4%) | |
| High-school graduate | 1,511 (34%) | 74 (43%) | 75 (28%) | |
| Any college education | 2,609 (59%) | 90 (52%) | 185 (69%) | |
| Father's education | 0.007 | |||
| Some-high school | 192 (5%) | 11 (7%) | 3 (1%) | |
| High-school graduate | 1,282 (31%) | 49 (30%) | 66 (25%) | |
| Any college education | 2685 (65%) | 101 (63%) | 192 (74%) | |
| Complications of pregnancy | ||||
| Hemorrhage | 22 (0%) | 20 (10%) | 6 (2%) | <0.001 |
| Premature rupture of membranes | 16 (0%) | 0 (0%) | 0 (0%) | 0.44 |
| Abnormalities of placenta | 13 (0%) | 3 (2%) | 4 (1%) | <0.001 |
| Preeclampsia/eclampsia | 108 (2%) | 22 (11%) | 11 (4%) | <0.001 |
| Urgency status | <0.001 | |||
| Elective | 62 (32%) | 211 (69%) | ||
| Emergency | 131 (68%) | 93 (31%) | ||
| Induction of labor (0=none,1-9) | <0.001 | |||
| None | 3,734 (77%) | 152 (79%) | 263 (87%) | |
| Method not indicated | 50 (1%) | 2 (1%) | 2 (1%) | |
| Amniotomy or rupture of membrane | 85 (2%) | 0 (0%) | 0 (0%) | |
| Drug induction | 792 (16%) | 37 (19%) | 35 (12%) | |
| Amniotomy plus drug induction | 132 (3%) | 1 (1%) | 4 (1%) | |
| Other methods | 30 (1%) | 1 (1%) | 0 (0%) | |
| Complications of labor and delivery | ||||
| Hemorrhage | 23 (0%) | 13 (7%) | 1 (0%) | <0.001 |
| Fetopelvic disproportion | 45 (1%) | 26 (13%) | 32 (11%) | <0.001 |
| Dystocic position of fetus | 328 (7%) | 49 (25%) | 82 (27%) | <0.001 |
| Prolonged labor of other origin | 383 (8%) | 32 (17%) | 37 (12%) | <0.001 |
| Umbilical cord complication | 48 (1%) | 5 (3%) | 9 (3%) | 0.001 |
| Birth trauma | 25 (1%) | 1 (1%) | 1 (0%) | 0.90 |
| Intrauterine hypoxia | 2 (0%) | 0 (0%) | 0 (0%) | 0.90 |
Mother's education was missing for 464 (8.7%) and father's education was missing for 739 (13.9%). CD= Cesarean delivery.
A Priori Analyses
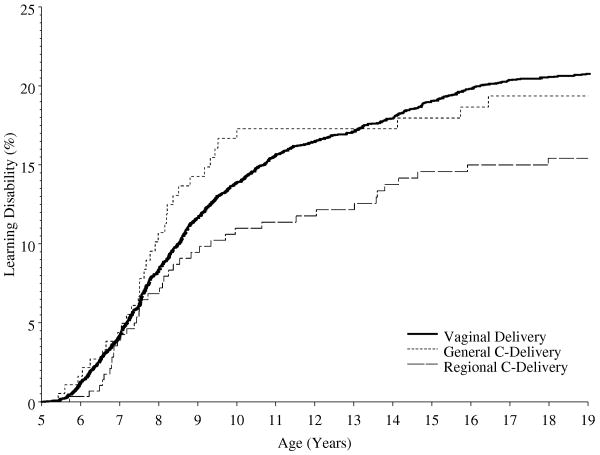
Within the cohort 921 children were diagnosed with LD prior to age 19 years. The cumulative incidence of LD among those who were delivered by vagina was 20.8% (95% confidence interval 19.5% to 22.0%) compared with 19.4% (95% confidence interval 13.2% to 25 %) for those whose mothers received a general anesthesia for a CD, and 15.4% (95% confidence interval 11.0% to 19.7%) for those whose mothers received a regional anesthetic for CD. With proportional hazard regression, not adjusting for covariates, the incidence of LD did not differ significantly when compared across the three modes of delivery (overall P = 0.135). However, the confidence interval for the hazard ratio comparing those delivered via CD with regional anesthesia with those delivered vaginally did not include unity (hazard ratio = 0.73, 95% confidence interval 0.53 to 0.99; P = 0.046 for pair-wise comparison of CD with regional anesthesia compared to vaginal delivery) (Figure 1 and Table 4), suggesting a lower risk of LD in the former. After adjusting for sex, birth weight, gestational age, exposure to anesthesia between ages 0 and 4 and maternal education (factors determined a priori) a similar pattern of results was observed (overall comparison across the 3 delivery modes P = 0.050; hazard ratio = 0.64, 95% confidence interval 0.44 to 0.92; P = 0.017 for pair-wise comparison of CD under regional anesthesia compared to vaginal delivery).
Figure 1.
Cumulative percentage of learning disabilities diagnosis (Kaplan-Meier estimates) shown separately for those that had vaginal delivery, and via Cesarean delivery under general or regional anesthesia in the 1976-1982 Rochester, Minnesota birth cohort.
Table 4.
Effects of Mode of Delivery and Anesthetic Exposure on Risk for Developing Learning Disabilities among Children in the 1976-1982 Rochester, Minnesota Birth Cohort*.
| Unadjusted | Adjusted s | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Type of Delivery | 0.135 | 0.050 | ||||
| Vaginal | 1.00 | 1.00 | ||||
| CD (general anesthetic) | 0.97 | 0.68 to 1.37 | 0.88 | 0.59 to 1.31 | ||
| CD (regional anesthetic) | 0.73 | 0.53 to 0.99 | 0.64 | 0.44 to 0.92 | ||
| Gestational Age | 0.314 | |||||
| ≥ 37 weeks | 1.00 | |||||
| 32-36 weeks | 0.86 | 0.62 to 1.20 | ||||
| ≤ 31 weeks | 0.50 | 0.18 to 1.39 | ||||
| Birth Weight (g) | 0.269 | |||||
| ≥ 2500 | 1.00 | |||||
| < 2500 | 1.24 | 0.85 to 1.81 | ||||
| Gender | <0.001 | |||||
| Female | 1.00 | |||||
| Male | 1.66 | 1.44 to 1.93 | ||||
| Anesthesia Exposure < 4 yrs† | 0.008 | |||||
| 0 | 1.00 | |||||
| 1 | 1.04 | 0.82 to 1.34 | ||||
| 2 or more | 1.80 | 1.25 to 2.61 | ||||
| Mother's Education | <0.001 | |||||
| Any college education | 1.00 | |||||
| High-school graduate | 1.64 | 1.41 to 1.91 | ||||
| Some high school | 3.09 | 2.46 to 3.89 | ||||
Analyses were performed using proportional hazards regression, with findings presented as hazard ratio (HR) and corresponding 95% confidence interval (CI). Mode of delivery was available for all 5,320 individuals (unadjusted analysis). Due to missing covariate information only 4,553 individuals were included in the adjusted analysis. CD= Cesarean delivery.
Number of exposures to anesthesia under the age of 4 (excluding exposure during labor and delivery).
Post-hoc Analyses
The analysis was repeated after eliminating children who were exposed to anesthesia after birth and prior to age 4, with similar findings (overall comparison across the 3 delivery modes P = 0.076; hazard ratio = 0.63, 95% confidence interval 0.42 to 0.94; P = 0.024 for pair-wise comparison of CD under regional anesthesia compared to vaginal delivery). A similar pattern was also observed after eliminating all children who were delivered by emergency CD (overall comparison across the 3 delivery modes P = 0.171; hazard ratio = 0.68, 95% confidence interval 0.44 to 1.06; P = 0.086 for pair-wise comparison of CD under regional anesthesia compared to vaginal delivery) and from a multivariable analysis which included additional covariates for other characteristics found to differ across delivery modes (overall comparison across the 3 delivery modes P = 0.152; hazard ratio = 0.68, 95% confidence interval 0.46 to 1.00; P = 0.052 for pair-wise comparison of CD under regional anesthesia compared to vaginal delivery).
Discussion
The main finding of this study is that children exposed to general or regional anesthesia for CD are not more likely to develop LD compared to children delivered vaginally. An unexpected finding of this study was evidence suggesting that the adjusted risk of LD was lower in children delivered via CD under regional anesthesia compared with children delivered vaginally.
The potential long-term effects of anesthesia on central nervous system structure and function, especially exposure at the extremes of age, have attracted considerable recent interest based on in vitro and animal data showing that these drugs can cause apoptosis and other degenerative changes when applied to the young (or aging) brain.13-19 We recently published the first evidence that repeated, but not single, exposure to anesthesia and surgery in children (prior to age 4) is associated with an increased risk of LD, using population-based birth cohort in Olmsted County, Minnesota.22 This birth cohort provides several unique advantages. All these children resided in the same community, all attended public and private schools, and received health care at one of two local facilities (Mayo Clinic and Olmsted County Medical Center), making it possible to review all available medical and educational records. These records, combined with rigorous definitions of LD,25,26,32 made it possible to perform a population assessment of a clinically-significant outcome that plausibly reflects the learning abnormalities observed in animal model post anesthesia.13-19 Availability of nearly complete data from birth records made it possible to control for several important confounders known to affect the frequency of LD (sex, gestational age, birth weight, repeated exposure to anesthesia, and maternal education).
It is clear that the newborn may be at least transiently affected by anesthetics and analgesics administered during labor and delivery.3,33-35 For example, the use of opioids for labor analgesia is associated with neonatal respiratory depression. In contrast to these short-term effects, there are only few studies attempting to assess the effect of mode of delivery on long-term child neurodevelopment. The Collaborative Perinatal Project8 found that school achievement of 26,760 children, measured by standardized test of intelligence, did not differ between children delivered vaginally or via CD; however this study did not examine the method used to provide anesthesia and used standardized rather than individually administered tests of achievement. McGee et al.9 compared ‘normal spontaneous delivery’ vs. ‘other than normal spontaneous delivery’ (rotation/CD/forceps etc.) and found no effect on behavioral maturation at age 7 as defined by standardized test of intelligence and other behavioral domains. McBride et al.11 did not find any deleterious effect of mode of delivery (various types of forceps delivery vs elective CD vs spontaneous delivery) on developmental outcomes in children at age 5 (compared by standardized IQ tests). Pasamanick et al.10 found no difference in behavioral disorders between children delivered by ‘serious operative procedures’ (CD, forceps, breech extraction, internal version and extraction) compared to children delivered vaginally, although there were only a few CD in this study.10 In all these studies, the type of anesthesia used for CD was not reported.
There are very few animal studies that could provide insight into potential effects of obstetric anesthesia and analgesia on neonatal outcome. Rizzi et al.19 found that four hours of maternal exposure to isoflurane caused neuroapoptosis of the fetal guinea pig brain; however, this is a much longer exposure than during typical CD. Therefore, this may not be applicable model to a term human fetus. Golub et al.36 examined the role of epidural anesthesia administered to primates on long-term infant behavior. Monkeys at term received either epidural bupivacaine or saline during induced vaginal delivery. At one year, infant monkeys born of mothers receiving bupivacaine did not exhibit abnormalities or specific cognitive deficits in learning, memory or attention, although some earlier phases of behavioral development were affected.36 Again, this study likely has limited applicability to our findings, other than suggesting a lack of deleterious long-term effects attributable to absorbed bupivacaine on the term fetus.
The fact that CD in our study was not associated with an increased risk of LD is consistent with these prior findings, and is reassuring regarding any concern that the brief exposure to general anesthesia during CD could adversely affect learning. This lack of adverse effect occurred despite several potential risk factors present in the CD deliveries with general anesthesia, including an increased frequency of prematurity and emergent delivery. What was not expected was the apparent decrease in risk associated with regional anesthesia for CD, which persisted after adjustment for known risk factors for LD.
Given the lack of relevant animal data to suggest a potential mechanism, we can only speculate. One potential factor that could be relevant is the stress response to labor and delivery. The perinatal period is critical to subsequent behavioral development and stress responses during that period play a significant factor in developmental outcomes.37 Perinatal stress has been associated with synaptic loss resulting in learning abnormalities in animals38 and maladaptive responses in humans and animals.37,39-44 Labor and vaginal delivery in humans is associated with a significant increase in the levels of stress hormones such as epinephrine, norepinephrine and cortisol.40 Evidence is accumulating that exposure of the developing human brain to stress can produce lasting organizational changes that can lead to a variety of abnormal behaviors in later life (hyperactivity, hyperreactivity, decreased cognition, etc).37 For example, an increased frequency of attention deficit hyperactivity disorder has been linked to the presence of peripartum stressors.45 Anesthesia and analgesia during labor and delivery may reduce the level of stress. CD performed under epidural anesthesia decreases stress hormone levels in both the mother and fetus compared with vaginal delivery (both with and without labor epidural analgesia),40,46 and significantly decreases these levels compared with elective CD performed under general anesthesia.47 It is not known whether the stress response accompanying vaginal delivery and CD with general anesthesia is of sufficient intensity and duration to affect subsequent neurodegeneration. If so, CD with regional anesthesia could inhibit the stress response sufficiently to potentially affect long-term outcome. We emphasize that this is a speculation that depends on several assumptions; this result should be regarded as generating hypotheses that can be tested in future studies.
Limitations
Our study has several limitations beyond those inherent in any study that employs a retrospective approach. We have previously argued that LD is a relevant outcome to search for evidence of anesthetic-induced neurodegeneration in population-based studies,22 but we cannot exclude that injury may occur that does not result in LD, or conversely that LD is not a consequence of any injury that may occur. Study of the relationship between perinatal factors such as delivery mode and subsequent behavioral development is complicated by the multiple confounding factors that can affect development. For example, Pasamanick and Knobloch48 introduced a theory of ‘continuum of reproductive casualty’ to describe the spectrum of factors that can affect fetal and neonatal brain and cause behavioral abnormalities, including perinatal factors (such as complications of pregnancy) and various socioeconomic and environmental conditions during early childhood. Although we included adjustor variables in our a priori analysis for those factors known to affect the risk of LD (such as prematurity), we cannot exclude the possibility that other unrecognized factors may be responsible for the observed differences, such as those related to the decision to perform elective CD with regional anesthesia. Data regarding many characteristics of those experiencing vaginal deliveries was not abstracted, such as the use of labor epidural analgesia, which could be relevant. We did perform additional post hoc adjusted analyses which included covariates for several peripartum characteristics found to differ significantly across groups. The findings from these models remained consistent (albeit with varying levels of statistical significance) and revealed comparable point estimates, suggesting a decreased likelihood of LD with CD under regional anesthesia vs. vaginal delivery. However, it should be noted that all of these models were performed using the same dataset, therefore the consistency in results should not be interpreted as an independent validation of the study findings.
This birth cohort has been the subject of prior reports describing the epidemiology of LD, and exhaustive efforts were made as a part of this work to obtain as complete case ascertainment of LD as possible, as previously discussed.25,26,28 Information on all children in this cohort was gathered from three independent sources: school folders, medical records, and records at a local tutoring/reading center. All school records were examined page by page for each of the 5,718 children for even subtle signs of learning or behavior problems, including any kind of notation indicating that teachers, parents, or anyone else had concerns about the child's school or learning performance. Because some of the children in the cohort might have had learning or performance difficulties that were not recorded in the school record, the medical and Reading Center/Dyslexia Institute of Minnesota records of all children were searched for any indication of concerns about a learning or behavior problem. Comparison of our cumulative rates of reading disability is consistent with published prevalence estimates by Shaywitz et al,49 suggesting the validity of our ascertainment methods.
Another limitation relates to the birth cohort study design, which can be biased due to migration from the community. However, in a prior analysis of this cohort, comparison of children who left the community before age 5 and those who stayed after age 5 indicated that the children included in the study are representative of the entire birth cohort.24 Also, this cohort was comprised of almost exclusively Caucasian children, which may limit the generalizability of these results to other populations.29 The incidence of CD in this series is comparable with other reports from this era50,51 but considerably less than in contemporary practice,52 and it is not known how these potential changes in the indications for CD may affect these results. Finally, although potent inhalational agents were used in many of the mothers receiving general anesthesia for CD, the most commonly-used agents (halothane and methoxyflurane) are no longer in widespread clinical use.
Summary
Children exposed to general or regional anesthesia during CD are not more likely to develop LD compared to children delivered vaginally, suggesting that brief perinatal exposure to anesthetic drugs does not adversely affect long-term neurodevelopmental outcomes. Rather, the risk of LD appears to be lower in children delivered by CD whose mothers received regional anesthesia. We caution that such a result from an observational study can only be viewed as hypothesis-generating and needs to be confirmed (or refuted), especially considering the possibility that undergoing CD with regional analgesia is simply a marker for unidentified confounders that may explain the differential risk for LD between groups. We propose the hypothesis that regional anesthesia for CD attenuates the neonatal stress response to vaginal delivery that in turn has significant effects on later neural development. Future studies are needed to evaluate this hypothesis.
Acknowledgments
We acknowledge the late Leonard T. Kurland, M.D. (Epidemiologist, Mayo Clinic, Rochester, Minnesota) for his vision in initiating the Rochester Epidemiology Project, and we thank Dr. Robert Colligan, PhD (Professor of Psychology, Mayo Clinic, Rochester, Minnesota) for sharing his knowledge and experience in the science of learning disability. We also thank Ms. Candice Klein, B.S. (Clinical Research Coordinator, Mayo Clinic, Rochester, Minnesota), Ms. Peg Farrell, R.N. (Data abstractor) and other members of the Learning Disability team for data collection; Independent School District #535; and the Reading Center/Dyslexia Institute of Minnesota for their cooperation and collaboration. We would also like to thank data analyst, Mr. Andrew Hanson, B.A. (Statistical Program Analyst, Mayo Clinic Rochester, Minnesota), and Anthony Santamaria, M.D. (Consultant Anesthesiologist) for assistance in obtaining medical records from Olmsted County Medical Center, Rochester, Minnesota.
Funding Sources: Support was provided from the Department of Anesthesiology, College of Medicine, Mayo Clinic, Rochester, MN, 55905 and from research grants HD29745 and AR30582 from the National Institutes of Health, Bethesda, Maryland, USA.
References
- 1.Lester BM, Als H, Brazelton TB. Regional obstetric anesthesia and newborn behavior: A reanalysis toward synergistic effects. Child Dev. 1982;53:687–92. [PubMed] [Google Scholar]
- 2.Brackbill Y, Kane J, Manniello RL, Abramson D. Obstetric meperidine usage and assessment of neonatal status. Anesthesiology. 1974;40:116–20. doi: 10.1097/00000542-197402000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Kraemer HC, Korner A, Anders T, Jacklin CN, Dimiceli S. Obstetric drugs and infant behavior: A reevaluation. J Pediatr Psychol. 1985;10:345–53. doi: 10.1093/jpepsy/10.3.345. [DOI] [PubMed] [Google Scholar]
- 4.Scanlon JW, Brown WU, Jr, Weiss JB, Alper MH. Neurobehavioral responses of newborn infants after maternal epidural anesthesia. Anesthesiology. 1974;40:121–8. doi: 10.1097/00000542-197402000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Scanlon JW, Ostheimer GW, Lurie AO, Brown wu JR, Weiss JB, Alper MH. Neurobehavioral responses and drug concentrations in newborns after maternal epidural anesthesia with bupivacaine. Anesthesiology. 1976;45:400–5. doi: 10.1097/00000542-197610000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Friedman SL, Brackbill Y, Caron AJ, Caron AF. Obstetric medication and visual processing in 4- and 5-month old infants. Merrill-Palmer Quarterly. 1978;24:111–28. [Google Scholar]
- 7.Kron RE, Stein M, Goddard KE. Newborn sucking behavior affected by obstetric sedation. Pediatrics. 1966;37:1012–6. [PubMed] [Google Scholar]
- 8.Broman SH, Nichols PL, Kennedy WA. In: Preschool IQ: Prenatal and early developmental correlates. Erlbaum, editor. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc., John Wiley & Sons; 1975. [Google Scholar]
- 9.McGee R, Silva PA, Williams S. Perinatal, neurological, environmental and developmental characteristics of seven-year-old children with stable behaviour problems. J Child Psychol Psychiatry. 1984;25:573–86. doi: 10.1111/j.1469-7610.1984.tb00173.x. [DOI] [PubMed] [Google Scholar]
- 10.Pasamanick B, Rogers ME, Lilienfeld AM. Pregnancy experience and the development of behavior disorders in children. Am J Psychiatry. 1956;112:613–8. doi: 10.1176/ajp.112.8.613. [DOI] [PubMed] [Google Scholar]
- 11.McBride WG, Black BP, Brown CJ, Dolby RM, Murray AD, Thomas DB. Method of delivery and developmental outcome at five years of age. Med J Aust. 1979;1:301–4. doi: 10.5694/j.1326-5377.1979.tb112116.x. [DOI] [PubMed] [Google Scholar]
- 12.Wesley BD, van den Berg BJ, Reece EA. The effect of forceps delivery on cognitive development. Am J Obstet Gynecol. 1993;169:1091–5. doi: 10.1016/0002-9378(93)90261-g. [DOI] [PubMed] [Google Scholar]
- 13.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–4. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 14.Olney JW, Wozniak DF, Jevtovic-Todorovic V, Farber NB, Bittigau P, Ikonomidou C. Drug-induced apoptotic neurodegeneration in the developing brain. Brain Pathol. 2002;12:488–98. doi: 10.1111/j.1750-3639.2002.tb00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jevtovic-Todorovic V, Benshoff N, Olney JW. Ketamine potentiates cerebrocortical damage induced by the common anaesthetic agent nitrous oxide in adult rats. Br J Pharmacol. 2000;130:1692–8. doi: 10.1038/sj.bjp.0703479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olney JW, Young C, Wozniak DF, Jevtovic-Todorovic V, Ikonomidou C. Do pediatric drugs cause developing neurons to commit suicide? Trends Pharmacol Sci. 2004;25:135–9. doi: 10.1016/j.tips.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Mellon RD, Simone AF, Rappaport BA. Use of anesthetic agents in neonates and young children. Anesth Analg. 2007;104:509–20. doi: 10.1213/01.ane.0000255729.96438.b0. [DOI] [PubMed] [Google Scholar]
- 18.Wang C, Sadovova N, Fu X, Schmued L, Scallet A, Hanig J, Slikker W. The role of the N-methyl-D-aspartate receptor in ketamine-induced apoptosis in rat forebrain culture. Neuroscience. 2005;132:967–77. doi: 10.1016/j.neuroscience.2005.01.053. [DOI] [PubMed] [Google Scholar]
- 19.Rizzi S, Carter LB, Ori C, Jevtovic-Todorovic V. Clinical anesthesia causes permanent damage to the fetal guinea pig brain. Brain Pathol. 2008;18:198–210. doi: 10.1111/j.1750-3639.2007.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–37. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 22.Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson M, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early Exposure to Anesthesia and Learning Disabilities in a Population-Based Birth Cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108:511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katusic SK, Colligan RC, Barbaresi WJ, Schaid DJ, Jacobsen SJ. Potential influence of migration bias in birth cohort studies. Mayo Clin Proc. 1998;73:1053–61. doi: 10.4065/73.11.1053. [DOI] [PubMed] [Google Scholar]
- 25.Katusic SK, Colligan RC, Barbaresi WJ, Schaid DJ, Jacobsen SJ. Incidence of reading disability in a population-based birth cohort, 1976-1982. Rochester, Minn Mayo Clin Proc. 2001;76:1081–92. doi: 10.4065/76.11.1081. [DOI] [PubMed] [Google Scholar]
- 26.Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Jacobsen SJ. Math learning disorder: Incidence in a population-based birth cohort, 1976-82. Rochester, Minn Ambulatory Pediatrics. 2005;5:281–9. doi: 10.1367/A04-209R.1. [DOI] [PubMed] [Google Scholar]
- 27.Katusic SK, Barbaresi WJ, Colligan RC, Weaver AL, Leibson CL, Jacobsen SJ. Case definition in epidemiologic studies of AD/HD. Ann Epidemiol. 2005;15:430–7. doi: 10.1016/j.annepidem.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Katusic SK, Colligan RC, Weaver AL, Barbaresi WJ. The forgotten learning disability: epidemiology of written-language disorder in a population-based birth cohort (1976-1982) Rochester, Minnesota Pediatrics. 2009;123:1306–13. doi: 10.1542/peds.2008-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 30.SLD Companion Manual, Revision Edition. Vol. 5. Minnesota Department of Children, Families, and Learning, Division of Special Education; Roseville, Minnesota: 1998. pp. 1–11. (Educational). [Google Scholar]
- 31.Fletcher J, Shaywitz S, Shankweiler D, Katz L, Liberman I, Stuebing K, Francis D, Fowler A, Shaywitz B. Cognitive profiles of reading disability: comparisons of discrepancy and low achievement definitions. J Educ Psychol. 1994;86:6–23. [Google Scholar]
- 32.St Sauver JL, Katusic SK, Barbaresi WJ, Colligan RC, Jacobsen SJ. Boy/girl differences in risk for reading disability: potential clues? Am J Epidemiol. 2001;154:787–94. doi: 10.1093/aje/154.9.787. [DOI] [PubMed] [Google Scholar]
- 33.Rosenblatt DB, Belsey EM, Lieberman BA, Redshaw M, Caldwell J, Notarianni L, Smith RL, Beard RW. The influence of maternal analgesia on neonatal behaviour: II. Epidural bupivacaine. Br J Obstet Gynaecol. 1981;88:407–13. doi: 10.1111/j.1471-0528.1981.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 34.Hodgkinson R, Bhatt M, Grewal G, Marx GF. Neonatal neurobehavior in the first 48 hours of life: Effect of the administration of meperidine with and without naloxone in the mother. Pediatrics. 1978;62:294–8. [PubMed] [Google Scholar]
- 35.Lieberman BA, Rosenblatt DB, Belsey E, Packer M, Redshaw M, Mills M, Caldwell J, Notarianni L, Smith RL, Williams M, Beard RW. The effects of maternally administered pethidine or epidural bupivacaine on the fetus and newborn. Br J Obstet Gynaecol. 1979;86:598–606. doi: 10.1111/j.1471-0528.1979.tb10820.x. [DOI] [PubMed] [Google Scholar]
- 36.Golub MS, Germann SL. Perinatal bupivacaine and infant behavior in rhesus monkeys. Neurotoxicol Teratol. 1998;20:29–41. doi: 10.1016/s0892-0362(97)00068-8. [DOI] [PubMed] [Google Scholar]
- 37.Charmandari E, Kino T, Souvatzoglou E, Chrousos GP. Pediatric stress: Hormonal mediators and human development. Horm Res. 2003;59:161–79. doi: 10.1159/000069325. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi A, Nagaoka M, Yamada K, Ichitani Y, Miake Y, Okado N. Maternal stress induces synaptic loss and developmental disabilities of offspring. Int J Dev Neurosci. 1998;16:209–16. doi: 10.1016/s0736-5748(98)00028-8. [DOI] [PubMed] [Google Scholar]
- 39.King S, Laplante DP. The effects of prenatal maternal stress on children's cognitive development: Project Ice Storm. Stress. 2005;8:35–45. doi: 10.1080/10253890500108391. [DOI] [PubMed] [Google Scholar]
- 40.Vogl SE, Worda C, Egarter C, Bieglmayer C, Szekeres T, Huber J, Husslein P. Mode of delivery is associated with maternal and fetal endocrine stress response. BJOG. 2006;113:441–5. doi: 10.1111/j.1471-0528.2006.00865.x. [DOI] [PubMed] [Google Scholar]
- 41.Kapoor A, Matthews SG. Short periods of prenatal stress affect growth, behaviour and hypothalamo-pituitary-adrenal axis activity in male guinea pig offspring. J Physiol. 2005;566:967–77. doi: 10.1113/jphysiol.2005.090191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider ML, Roughton EC, Koehler AJ, Lubach GR. Growth and development following prenatal stress exposure in primates: An examination of ontogenetic vulnerability. Child Dev. 1999;70:263–74. doi: 10.1111/1467-8624.00020. [DOI] [PubMed] [Google Scholar]
- 43.O'Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children's behavioural/emotional problems at 4 years. Report from the Avon longitudinal study of parents and children. Br J Psychiatry. 2002;180:502–8. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- 44.Griffin WC, 3rd, Skinner HD, Salm AK, Birkle DL. Mild prenatal stress in rats is associated with enhanced conditioned fear. Physiol Behav. 2003;79:209–15. doi: 10.1016/s0031-9384(03)00097-0. [DOI] [PubMed] [Google Scholar]
- 45.Zappitelli M, Pinto T, Grizenko N. Pre-, peri-, and postnatal trauma in subjects with attention-deficit hyperactivity disorder. Can J Psychiatry. 2001;46:542–8. doi: 10.1177/070674370104600609. [DOI] [PubMed] [Google Scholar]
- 46.Taylor A, Fisk NM, Glover V. Mode of delivery and subsequent stress response (Research Letter) Lancet. 2000;355:120. doi: 10.1016/S0140-6736(99)02549-0. [DOI] [PubMed] [Google Scholar]
- 47.Loughran PG, Moore J, Dundee JW. Maternal stress response associated with caesarean delivery under general and epidural anaesthesia. Br J Obstet Gynaecol. 1986;93:943–9. doi: 10.1111/j.1471-0528.1986.tb08013.x. [DOI] [PubMed] [Google Scholar]
- 48.Pasamanick B, Knobloch H. Retrospective studies on the epidemiology of reproductive casualty: Old and new. Merill Palmer Quart. 1966;12:7–26. [Google Scholar]
- 49.Shaywitz SE, Shaywitz BA, Fletcher JM, Escobar MD. Prevalence of reading disability in boys and girls. Results of the Connecticut Longitudinal Study. JAMA. 1990;264:998–1002. [PubMed] [Google Scholar]
- 50.Rooks JP, Weatherby NL, Ernst EK, Stapleton S, Rosen D, Rosenfield A. Outcomes of care in birth centers. The National Birth Center Study. N Engl J Med. 1989;321:1804–11. doi: 10.1056/NEJM198912283212606. [DOI] [PubMed] [Google Scholar]
- 51.Taffel SM, Placek PJ, Liss T. Trends in the United States cesarean section rate and reasons for the 1980-85 rise. Am J Public Health. 1987;77:955–9. doi: 10.2105/ajph.77.8.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Healthy People 2010. 2nd. 2000. U.S. Department of Health and Human Services: Maternal, infant and child health; pp. 15–16.pp. 28 [Google Scholar]