Abstract
Plasmodium falciparum-infected erythrocytes (pRBCs) adhere to the endothelium via receptors expressed on the surface of vascular endothelial cells (EC) and sequester in the microvasculature of several organs and block the blood circulation. The sequestration, which involves receptors, may be related to the severity of malaria. Here, we report that pRBCs bind to the membrane-bound form of Fractalkine/CX3CL1 (FKN), which is expressed on the surface of vascular EC in various organs. pRBCs adhered to FKN on the surface of FKN cDNA-transfected Chinese hamster ovary cells (CHO-FKN cells). Both the recombinant human FKN-chemokine domain (FKN-CD) and anti-FKN-CD antibody efficiently blocked adherence of pRBCs to CHO-FKN cells. Similar to binding between FKN and FKN receptor on blood mononuclear cells, two amino acid residues, Lys-7 and Arg-47 within FKN-CD, were critical for FKN-pRBC binding. Immunohistological analysis revealed the expression of FKN on EC at the site of sequestration in the brain of a patient with cerebral malaria. These results suggest that the membrane-bound form of FKN acts as a receptor for pRBCs, and this may contribute to furthering our present understanding of cytoadherence in the pathology of falciparum malaria.
Human cerebral malaria is caused by excessive adherence of Plasmodium falciparum-infected erythrocytes (pRBCs) to the microvasculature of several organs, including the brain. pRBCs containing mature parasites adhere to endothelial cells (EC) lining the lumens of postcapillary vessels, and this phenomenon is known as sequestration. After sequestration, cerebral malaria develops through a complex process involving multiple events, including obstruction of the microvasculature by pRBCs (1, 2). Several host receptors, including CD36, intercellular adhesion molecule 1 (ICAM-1), chondroitin sulfate A (CSA), platelet/endothelial cell adhesion molecule 1 (PECAM-1/CD31), vascular cell adhesion molecule 1 (VCAM-1), thrombospondin, and E-selectin, have been identified on EC as potential mediators for cytoadherence of pRBCs (3-8). Host and parasite factors are thought to participate in the pathogenesis of severe malaria, in which symptoms of cerebral malaria are the most fulminant. However, the mechanism underlying the cytoadherence of pRBCs in the pathology of severe malaria is not completely understood. It has been suggested by several investigators that other unidentified receptors that mediate the adherence of pRBCs to EC may be present (9-11).
Fractalkine/CX3CL1 (FKN) is a recently identified chemokine with a unique structure. The molecule possesses a single CXXXC CD at the N terminus, which precedes 17 mucin-like repeats (the central stalk region) and the transmembrane segment at the C terminus. FKN is expressed as a membrane-bound form on EC activated by proinflammatory cytokines such as tumor necrosis factor α, IFN-γ, and IL-1β (12, 13). FKN is constitutively expressed on cells in a variety of nonhematopoietic tissues, including the brain, heart, kidneys, and lungs (12). The membrane-bound form of FKN on EC mediates adherence of blood mononuclear cells with the FKN receptor (CX3CR-1) (14, 15). At the same time, the soluble form promotes chemotaxis of these cells to sites of acute inflammation (12, 13). Because the key cytokine produced during malaria infection induces expression of FKN on EC at sites of sequestration, it is possible that FKN contributes to disease manifestations in patients with severe malaria.
Here we report that the membrane-bound form of FKN on EC can mediate cytoadherence of pRBCs.
Materials and Methods
P. falciparum Culture. P. falciparum was cultured in vitro as described (16). The 21 clinical isolates of P. falciparum used in this study were obtained from uncomplicated, complicated, and cerebral malaria patients at the hospital of the International Medical Center of Japan (Tokyo), Tokyo Metropolitan Komagome Hospital, and Davao Regional Hospital (Davao, The Philippines). Informed consent was obtained from each patient before the blood sampling. Peripheral blood was collected into tubes coated with EDTA and centrifuged at 1,500 rpm for 5 min at room temperature to remove the plasma. The packed cells were washed three times with RPMI 1640 (-) (RPMI medium 1640 supplemented with 24 mM NaHCO3 and 25 mM Hepes, pH 7.4) and were suspended at 5-10% (vol/vol) in the same medium containing 10% human serum (from a healthy Japanese volunteer; blood type A) and 25 μg/ml gentamicin (Sigma-Aldrich) for the parasite culture.
Cell Culture. Human umbilical vein endothelial cells (HUVEC; Asahi Technoglass, Tokyo) were grown in MCDB-131 medium containing 0.1% gentamicin/amphotericin B, 10 ng/ml epidermal growth factor, 1 μg/ml hydrocortisone, 12 μg/ml bovine brain extracts, and 2% FCS. The human amelanotic melanoma cell line, C32, was maintained in minimum essential medium supplemented with 1% nonessential amino acids and 10% heat-inactivated FCS. Chinese hamster ovary (CHO) cells were grown in Ham's F-12 medium supplemented with 10% FCS.
Transfection. A CHO cell line that expresses FKN was generated by transfecting CHO cells with the entire coding sequence of FKN in a pcDNA3.1 expression vector system (Invitrogen). The coding sequence of FKN was amplified by RT-PCR from mRNA of HUVEC stimulated with tumor necrosis factor α (Strathmann Biotech, Hamburg, Germany) with primers 5′-CGC GGA TCC TCA GCC ATG GCT CCG ATA-3′ and 5′-CCA CTC GAG TTC ACA CGG GCA CCA GGA CAT-3′. The primers, which contained a BamHI or XhoI site (indicated in boldface) adjacent to the initiation or the termination codon (indicated in italics), respectively, were designed from the original sequence of the FKN gene (GenBank accession no. NM_002996). The PCR product was digested with BamHI and XhoI for directional ligation into pcDNA3.1. The recombinant plasmid (FKN/pcDNA3.1) was transfected into CHO cells with LipofectAMINE Plus (BRL). FKN-expressing CHO cells (CHO-FKN cells) and CHO-mock cells, which had been transfected with empty expression vector, were grown in Ham's F-12 medium containing 10% FCS and G418 (0.8 mg/ml; BRL). Expression of FKN on surface of transfectants was evaluated by flow cytometry scanning (FACScan, BD Biosciences). Cells were washed three times with PBS containing 1% BSA and 0.1% NaN3 and incubated with goat anti-human FKN-CD polyclonal antibody (anti-FKN-CD antibody; R & D Systems) for 1 h on ice. After washing, cells were incubated with fluorescein isothiocyanate-conjugated donkey anti-goat IgG antibody (Protos Immunoresearch, Burlingame, CA) at 0.2 mg/ml for 1 h on ice. Single-cell suspensions were prepared by passage through a 100-μm nylon cell strainer (Becton Dickinson). Events were acquired on a FACSCalibur cytometer and analyzed with cellquest software (BD Biosciences).
Cytoadherence and Its Inhibition Assays. The isolate JK-1120 of P. falciparum, which was initially used for the cytoadherence assay, was obtained from a Japanese patient with no complications at the hospital of the International Medical Center of Japan. Stock JK-1120FHS was generated from JK-1120 by selecting for binding of mixed-stage pRBCs to recombinant human FKN-CD polypeptide (rFKN-CD; R & D Systems) immobilized on plastic Petri dishes three times and then selecting for adherence to CHO-FKN cells five times. Similar strategies have often been used to enrich a subpopulation of pRBCs that bind to adhesion molecules on endothelial membrane (8, 17). Most experiments were conducted with mixed-stage pRBCs, except for the experiment analyzing parasite stage-specific adherence, in which pRBCs were synchronized by the sorbitol method (18). The CHO-FKN and C32 cells were plated onto 15-mm coverslips (Matsunami, Tokyo) and maintained in 24-well plates (Corning). Cells were seeded at a density of 10,000 cells per well and grown for 48 h before assays. All experiments were done with unfixed cells. The culture medium was removed and replaced with 1 ml of pRBC (2.5% hematocrit, 5-10% parasitemia) in modified binding medium (RPMI medium 1640 supplemented with 25 mM Hepes and 10% human serum, pH 7.4). The plates were incubated for 90 min at 37°C with gentle mixing every 15 min. After incubation, coverslips were removed from the wells, unbound erythrocytes were gently washed with RPMI medium 1640, and the cells were fixed with 1% glutaraldehyde in PBS for 30 min at room temperature. Cells on coverslips were stained with Giemsa, and the number of adherent pRBC per 300 cells was counted under a light microscope. For inhibition assays, pRBCs were incubated for 90 min at 37°C with CHO-FKN cells in the presence of rFKN-CD (0.05, 0.1, and 0.2 μg/ml), CSA (Sigma-Aldrich; 1 and 1,000 μg/ml), heparin (Takeda Pharmaceutical, Osaka; 10 and 100 units/ml), or anti-FKN-CD antibody (0.2 μg/ml). The same conditions were used for the inhibition assays with vMIP-2 (Genzyme; 0.2, 0.4, and 0.8 μg/ml) and anti-CX3CR-1 antibody (Abcam, Cambridge, U.K.; 0.2, 0.4, and 0.8 μg/ml).
Site-Directed Mutagenesis of FKN-CD. In vitro site-directed mutagenesis was carried out with the QuikChange Site-Directed Mutagenesis System (Stratagene) with oligonucleotide primers containing the desired mutation (indicated in italics). The primers used for the K7A mutation were 5′-G CAC CAC GGT GTG ACG GCA TGC AAC ATC ACG TGC-3′ and 5′-GCA CGT GAT GTT GCA TGC CGT CAC ACC GTG GTG C-3′. Those for the R47A mutation were 5′-G GAG ACG AGA CAG CAC GCG CTG TTC TGT GCC GAC-3′ and 5′-GTC GGC ACA GAA CAG CGC GTG CTG TCT CGT CTC C-3′. Each mutation was generated in the FKN/pcDNA3.1 and was confirmed by DNA sequencing. Mutated plasmids were transfected into CHO cells, and the transfectants were confirmed for surface expression of FKN by FACScan. Proper transfectants were then used for cytoadherence and cytoadherence inhibition assays.
Assay of Binding to Immobilized Proteins. Binding assays were done with JK-1120FHS pRBCs at 3-10% parasitemia. rFKN (0.2 μg/ml), vMIP-2 (5 μg/ml), and anti-CX3CR-1 antibody (10 μg/ml) were immobilized in plastic dishes as described (19) with slight modifications. Briefly, on the day of the assay, each protein were spotted as three dots (5 μl per dot) onto 4-cm plastic dishes and incubated for 30 min in a humidified incubator at 37°C. Two milliliters of pRBCs in modified binding medium were added to each dish and incubated for 90 min at 37°C with gentle mixing every 15 min. Binding of pRBCs to each protein was measured as described (19). Results were expressed as the number of pRBCs bound per mm2 of surface area.
Immunohistochemistry. Paraffin-embedded brain, kidneys, liver, and spleen specimens from a cerebral malaria patient were used. These specimens were sectioned at 5 μm, deparaffinized, soaked for 15 min at room temperature in 0.3% H2O2/methanol to block endogenous peroxidase activity, and hydrated before blocking with 10% normal rabbit serum. Sections were stained with goat-anti FKN-CD antibody at 4°C for 24 h. The primary antibody was visualized with the Histofine Simple Stain MAX-PO (G) kit (Nichirei, Osaka) according to the manufacturer's protocol. The sections were counterstained with hematoxylin.
Statistical Analysis. Differences were evaluated by using Welch's t test. P < 0.05 were considered statistically significant.
Results
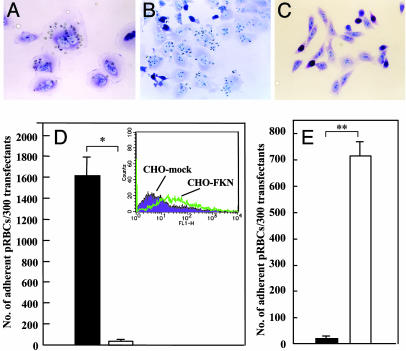
Adherence of pRBC to CHO-FKN. To determine whether expression of FKN on the surface of EC promotes adherence between EC and pRBCs, CHO cells were transfected with FKN cDNA. CHO cells only express CSA molecules that mediate cytoadherence of pRBCs (20). The parasite population in JK-1120 was selected for the binding to FKN molecule (see Materials and Methods), and it was used as JK-1120FHS. After 30 min of coculture, JK-1120 pRBCs (Fig. 1A) and JK-1120FHS pRBCs (Fig. 1B) readily adhered to CHO-FKN. In contrast, no adherence was observed if pRBCs were cocultured with CHO-mock cells (Fig. 1C). JK-1120FHS was used in the following experiments. The number of JK-1120FHS pRBCs that adhered to CHO-FKN cells was 1,614.3 ± 289.1 per 300 CHO cells (mean ± SE), which was significantly greater than the number that adhered to CHO-mock cells (22.7 ± 11.8 per 300 CHO cells; P = 0.011; Fig. 1D). We then examined the developmental stages of P. falciparum that can promote adherence of pRBCs to CHO-FKN cells. The number of pRBCs that adhered to CHO-FKN cells was significantly higher in pRBCs with the trophozoite/schizont (mature-stage parasites) (714.0 ± 53.3 per 300 CHO cells) than in those with ring-form parasites (16.3 ± 6.2 per 300 CHO cells; P = 0.002; Fig. 1E). To determine whether other clinical isolates could bind to FKN, 20 clinical isolates from eight Asian and African countries where falciparum malaria is endemic were examined for their ability to adhere to CHO-FKN cells. In this experiment, C32 amelanotic melanoma cells, which express CD36 and CSA, served as the positive control for the cytoadherence activity of the tested isolates. pRBCs of all isolates showed various degrees of adherence to CHO-FKN cells as well as to C32 cells (Table 1). pRBCs of the clinical isolates showed greater adherence to CHO-FKN cells (mean 173.5 ± 344.0 per 300 CHO cells) than to CHO-mock cells (mean 5.4 ± 12.5 per 300 CHO cells; P = 0.0001). The majority of isolates showed greater adherence to C32 cells than to CHO-FKN cells; however, some isolates (6 of 20 isolates H, N, O, P, R, and T) adhered better to CHO-FKN cells rather than to C32 cells.
Fig. 1.
Binding of pRBCs to the membrane-bound form of FKN. (A-C) Adherence of JK-1120 pRBCs to CHO-FKN (A), JK-1120 FHS to CHO-FKN (B), and JK-1120 FHS to CHO-mock (C). (D) Adherence of JK-1120FHS pRBCs (mixed-stages) to CHO-FKN (filled column) and CHO-mock (open column) cells. (Inset) Confirmation of FKN expression on CHO-FKN by FACScan analysis. (E) Adherence of either ring-form (filled bar) or trophozoite/schizont-infected (open bar) JK-1120FHS pRBCs to CHO-FKN cells. Results in D and E are the number of adherent pRBCs per 300 test cells (mean ± SE of three independent experiments). Welch's t test: *, P < 0.05; **, P < 0.01.
Table 1. Number of adherent P. falciparum-infected erythrocytes to target cells.
| Sample | C32 | CHO-FKN | CHO-mock | Origin |
|---|---|---|---|---|
| A | NT | 111 | 9 | The Philippines |
| B | 359 | 119 | 57 | The Philippines |
| C | 88 | 67 | 3 | The Philippines |
| D | 940 | 414 | NT | The Philippines |
| E | 1,069 | 116 | 9 | The Philippines |
| F | 127 | 50 | 9 | The Philippines |
| G | 106 | 103 | 1 | The Philippines |
| H | 62 | 96 | 1 | The Philippines |
| I | 428 | 52 | 3 | Malawi |
| J | 377 | 38 | 5 | Central Africa |
| K | 19 | 17 | 0 | NI |
| L | 107 | 70 | 2 | Botswana |
| M | 222 | 90 | 0 | Madagascar |
| N | 7 | 19 | 0 | Nigeria |
| O | 21 | 52 | 0 | Nigeria |
| P | 14 | 112 | 1 | The Philippines |
| Q | 130 | 71 | 3 | Indonesia |
| R | 14 | 73 | 1 | Nigeria |
| S | 212 | 141 | 3 | Cote d'Ivoire |
| T | 109 | 202 | 1 | NI |
| JK-1120FHS | 13 | 1,630 | 0 | JK-1120 (Central Africa) |
Parasite isolates were tested for binding to C32 cells, CHO-FKN cells, and CHO cells that had been transfected with empty expression vector (CHO-mock cells). Data show the number of adherent pRBCs per 300 experimental cells (mean of triplicate wells in a single experiment). NT, not tested; NI, not identified. JK-1120FHS was selected from JK-1120 for the binding to FKN molecule.
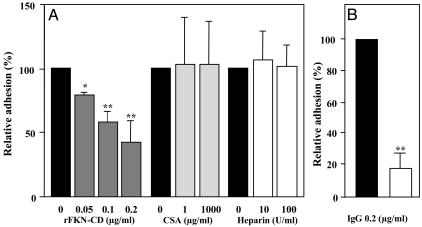
Inhibition of pRBC Adherence by rFKN-CD and Anti-FKN-CD Antibody. The specificity of the binding of pRBCs to FKN was assessed by competing the binding with rFKN-CD or other adherence factors. Adherence of pRBCs to CHO-FKN cells was inhibited by rFKN-CD in a concentration-dependent manner. Adherence was inhibited to 41.3 ± 18.9% (mean ± SE) of the control value when pRBCs were pretreated with 0.2 μg/ml rFKN-CD (Fig. 2A). In contrast, adherence was not inhibited when pRBCs were pretreated with either 1,000 μg/ml CSA (102.8 ± 36.0% of the control) or 100 units/ml heparin (101.4 ± 18.4% of the control) (Fig. 2A). Anti-FKN-CD antibody blocked adherence of pRBCs to CHO-FKN cells more efficiently. Adherence was inhibited to 18.5 ± 8.5% of the control assay when pRBCs were pretreated with the antibody (IgG) at a concentration of 0.2 μg/ml (Fig. 2B).
Fig. 2.
Inhibition of adherence of JK-1120FHS pRBCs to CHO-FKN cells. (A) Inhibition of adherence by rFKN-CD, CSA, and heparin. (B) Inhibition of adherence by goat anti-human FKN-CD IgG (open bar) or goat IgG (filled bar). Results (mean ± SE of three independent experiments) in A and B show adherence as a percentage of control (filled bar). Welch's t test: *, P < 0.05; **, P < 0.01.
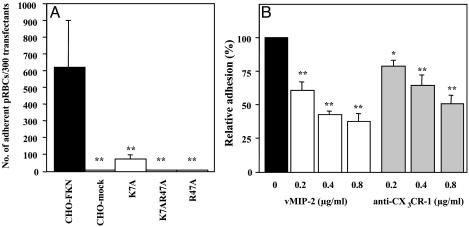
Adherence of pRBCs to Mutated FKN-CD and Inhibition of Adherence by Chemokine Antagonist or Anti-CX3CR-1 Antibody. Two amino acid residues (K7 and R47) in FKN-CD were reported to be critical for interactions between FKN and FKN receptor (15). To examine whether the interaction between FKN and its putative ligand(s) on pRBCs is similar to that between FKN and its authentic receptor on blood mononuclear cells, we replaced the K7 and R47 residues in FKN-CD with alanine and tested the abilities of these mutants to bind pRBC. The mutants FKN/K7A, FKN/R47A, and FKN/K7AR47A, which had an alanine at K7, R47, and both K7 and R47 in the CD region, respectively, were successfully expressed on CHO cells (data not shown). The expression levels of these mutated FKN were confirmed by FACScan at the same levels as wild type (data not shown). In the cytoadherence assay, all mutants displayed significantly reduced pRBC binding in comparison with the wild-type CHO-FKN cells (P < 0.01; Fig. 3A). Changes in the protein conformation due to these mutations in FKN-CD have been analyzed with a panel of monoclonal antibodies and do not appear to occur (15). Adherence between the wild-type FKN and pRBCs can be competed and blocked with chemokine antagonist (vMIP-2) and anti-CX3CR-1 antibody, respectively. Adherence with vMIP-2 (0.8 μg/ml) was 37.7 ± 7.6% (mean ± SE), and that with anti-CX3CR-1 antibody (0.8 μg/ml) was 50.85 ± 8.1% that of the control (Fig. 3B).
Fig. 3.
Adherence of JK-1120FHS pRBCs to FKN-CD mutants and block of the adherence to the wild type by chemokine antagonist or anti-CX3CR-1 antibody. (A) Adherence of pRBCs to CHO-FKN (wild type), CHO-mock, CHO-FKN/K7A, CHO-FKN/K7AR47A, and CHO-FKN/R47A cells. Results are number of adherent pRBCs per 300 experimental cells (mean ± SE of three independent experiments). Statistical analyses were made between CHO-FKN and other experimental cells. (B) Inhibition of the adherence to the wild type by vMIP-2 or anti-CX3CR-1 antibody. Results (mean ± SE of three independent experiments) show adherence as a percentage of control (filled bar). Welch's t test: *, P < 0.05; **, P < 0.01.
Binding of pRBCs to Immobilized Proteins. JK-1120FHS pRBCs bound to rFKN-CD, vMIP-2, and anti-CX3CR-1 antibody immobilized on plastic dishes (Table 2). Binding to BSA was not observed. The number of pRBCs bound to rFKN-CD was 36.6 ± 9.0 pRBCs per mm2 (mean ± SE). The number of pRBCs bound to vMIP-2 was 20.2 ± 3.4 pRBCs per mm2, which was significantly higher than the number bound to BSA (0.0 ± 0.0; P = 0.009). The number of pRBCs bound to anti-CX3CR-1 antibody was 15.5 ± 4.2 pRBCs per mm2, which was also significantly greater than the number bound to BSA (P = 0.02).
Table 2. Number P. falciparum-infected erythrocytes bound to immobilized proteins.
| Cells per mm2
|
||||
|---|---|---|---|---|
| rFKN-CD | vMIP-2 | anti-CX3CR-1 | BSA | |
| JK-1120FHS | 36.6 ± 9.0 | 20.2 ± 3.4* | 15.5 ± 4.2** | 0.0 ± 0.0 |
Data are mean ± SE of triplicate determinations in three separate experiments. Welch's t test: *, P < 0.01; **, P < 0.05.
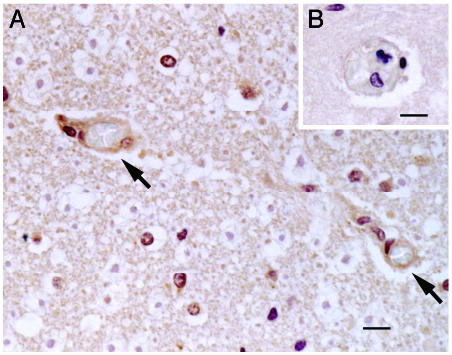
FKN Expression in Tissues of a Cerebral Malaria Patient. Paraffin-embedded tissues, including the brain, kidneys, liver, and spleen, from a cerebral malaria patient were subjected to immunohistochemical analysis. Staining with anti-FKN antibody revealed expression of FKN on EC of the microvasculature with sequestration in the cerebral cortex (Fig. 4A). Control staining of the same preparation with normal goat IgG as the primary antibody did not show any signal on EC (Fig. 4B). The positive staining in the brain tissue was blocked by preabsorption of anti-FKN antibody with rFKN-CD polypeptide (data not shown). Little or no signal was detected on EC at sites of sequestration in the kidneys, liver, and spleen (data not shown).
Fig. 4.
Immunohistochemical analysis of FKN expression in cerebral cortex of the patient with cerebral malaria. Excised tissues were stained with polyclonal anti-FKN-CD antibody (A) or goat IgG (B). Arrows indicate expression of FKN on EC of the microvasculature with sequestration. (Scale bar, 10 μM.)
Discussion
We have shown that the membrane-bound form of FKN acts as a receptor for the cytoadherence of pRBCs to the EC. All tested malaria isolates, including 21 patient isolates, showed binding to FKN molecule expressed on the surface of CHO cells. This adherence was observed only for pRBCs with mature parasites at physiological pH 7.4, and there was no adherence of uninfected RBCs or pRBCs with ring-stage parasites. These findings are consistent with the pathology observed in the malaria patient, where only pRBCs that contain trophozoites and schizonts adhered to vascular EC at the sites of sequestration (1, 2).
In our study of the clinical isolates, the preferences of the isolates for adherence to CHO-FKN cells and C32 cells were not completely identical. The majority of isolates showed preferential adherence to C32 cells rather than to CHO-FKN cells; however, some interacted preferentially with CHO-FKN cells. Although the present study was conducted with a limited number of isolates, our findings as shown in Table 1 suggest that receptor(s) for pRBCs cytoadherence differ between the isolates. Although the number of the binding cells to CHO-FKN cells were less than that of JK-1120FHS, cytoadherence of clinical isolates to CHO-FKN cells can be clinically relevant because the adhesion was statistically significant.
Proinflammatory cytokines such as tumor necrosis factor α, IFN-γ, and IL-1β stimulate EC to express adherence molecules including ICAM-1, VCAM-1, PECAM-1, E-selectin, and membrane-bound FKN (4, 6, 8, 12, 13). However, these proinflammatory cytokines are essential for protecting the host against malaria infection (21-24). In this sense, expression of these cytokines in a malaria patient may boost systemic immunity toward protection of the host from the disease, but at the same time, it may establish favorable conditions for sequestration in various organs in the patient.
In the present study, we found that FKN is expressed on EC in the microvasculature with sequestration in the brain of a cerebral malaria patient. Expression of FKN was lower or was not observed on microvascular EC at the sites of sequestration in several other organs, including the liver, kidneys, and spleen. This result suggests that the host molecules that act as the receptor for adherence of pRBCs differ between organs and that membrane-bound FKN mediates this adherence, at least in the brain of one patient with cerebral malaria.
Such variations in the interactions between pRBCs and adherence molecules on the endothelium and the organ or tissue distributions of such molecules may reflect differences in the pathogenesis of the isolates or the clinical manifestation of the disease in each patient.
The data from the inhibition studies with rFKN-CD and anti-FKN-CD antibody suggested that interaction between FKN and pRBCs was specific. The fact that adherence was not blocked by CSA or heparin excludes the possibilities that pRBC binding to CHO-FKN cells was due to these molecules.
Similar to the physiological binding of FKN and blood mononuclear cells with the specific FKN receptor CX3CR-1, two amino acid residues, K7 and R47, within FKN-CD were found to be important in the binding of FKN to pRBCs. Interestingly, binding between FKN and pRBCs was blocked by vMIP-2 (a broad-spectrum chemokine antagonist) and anti-CX3CR-1 antibody; however, binding was not blocked completely as it was with rFKN-CD.
To our knowledge, there have been no reports of CX3CR-1 expression on human RBCs, and therefore, the molecule(s) that bind to FKN on pRBCs are most likely parasite molecule(s). The lack of any significant findings with BLAST searches of the full-length CX3CR-1 sequence against the Apicomplexan databases suggests that lateral transfer of the gene from mammalian host cell to parasite is also unlikely. Our findings that pRBCs bind to vMIP-2 and anti-CX3CR-1 antibody suggest that the FKN binding molecule(s) on pRBCs are structurally similar to CX3CR-1. However, only a critical amino acid residue and not the FKN binding motif has been reported for CX3CR-1 (25). However, the fact that we observed no significant immunofluorescent signal against anti-CX3CR-1 antibody on pRBCs suggests low expression of the molecule(s) (data not shown). The present findings may be useful for future efforts to clone and characterize parasite molecule(s) that are expressed on pRBCs as ligand(s) for FKN on EC. Efforts to identify and characterize such molecule(s) are currently underway.
In summary, we have presented evidence supporting binding of pRBCs to membrane-bound FKN on EC. Expression of this molecule was observed on EC at the sites of sequestration in the brain of one cerebral malaria patient. Although it is difficult at present to characterize the significance of this adherence molecule in disease manifestation, such information may provide a novel way to investigate the relationship between cytoadherence and disease severity for falciparum malaria.
Acknowledgments
We thank Prof. Patrick Duffy (Seattle Biomedical Research Institute, Seattle) for critical review of the manuscript and Prof. Pilarita Rivera (College of Public Health, University of The Philippines, Manila, The Philippines), Dr. Romulo Busuego (Davao Regional Hospital, Davao, The Philippines), doctors in the Department of Internal Medicine, Davao Regional Hospital, and Dr. Gohta Masuda (Komagome Hospital) for their assistance and support in obtaining the clinical isolates. This work was supported by International Health Cooperation Research Grant 13C-5 from the Ministry of Health, Labor, and Welfare of Japan; Grants-in-Aid for Scientific Research on Priority Areas (C) (15019127) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; and a grant for Precursory Research for Embryonic Science and Technology from the Japan Science and Technology Agency.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CD, chemokine domain; CHO, Chinese hamster ovary; CSA, chondroitin sulfate A; CX3CR-1, receptor for FKN; EC, endothelial cells; FKN, fractalkine/CX3CL1; pRBCs, Plasmodium falciparum-infected erythrocytes.
References
- 1.Berendt, A. R., Turner, G. D. H. & Newbold, C. I. (1994) Parasitol. Today 10, 412-414. [DOI] [PubMed] [Google Scholar]
- 2.Pongponratn, E., Riganti, M., Punpoowong, B. & Aikawa, M. (1991) Am. J. Trop. Med. Hyg. 44, 168-175. [DOI] [PubMed] [Google Scholar]
- 3.Oquendo, P., Hundt, E., Lawler, J. & Seed, B. (1989) Cell 58, 95-101. [DOI] [PubMed] [Google Scholar]
- 4.Berendt, A. R., Simmons, D. L., Tansey, J., Newbold, C. I. & Marsh, K. (1989) Nature 341, 57-59. [DOI] [PubMed] [Google Scholar]
- 5.Rogerson, S. J., Chaiyaroj, S. C., Ng, K., Reeder, J. C. & Brown, G. V. (1995) J. Exp. Med. 182, 15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Treutiger, C. J., Heddini, A., Fernandez, V., Muller, W. A. & Wahlgren, M. (1997) Nat. Med. 3, 1405-1408. [DOI] [PubMed] [Google Scholar]
- 7.Rock, E. P., Roth, E. F., Jr., Rojas-Corona, R. R., Sherwood, J. A., Nagel, R. L., Howard, R. J. & Kaul, D. K. (1988) Blood 71, 71-75. [PubMed] [Google Scholar]
- 8.Ockenhouse, C. F., Tegoshi, T., Maeno, Y., Benjamin, C., Ho, M., Kan, K. E., Thway, Y., Win, K., Aikawa, M. & Lobb, R. R. (1992) J. Exp. Med. 176, 1183-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Udomsangpetch, R., Reinhardt, P. H., Schollaardt, T., Elliott, J. F., Kubes, P. & Ho, M. (1997) J. Immunol. 158, 4358-4364. [PubMed] [Google Scholar]
- 10.Turner, G. D. H., Morrison, H., Jones, M., Davis, T. M. E., Looareesuwan, S., Buley, I. D., Gatter, K. C., Newbold, C. I., Pukritayakamee, S., Nagachinta, B., et al. (1994) Am. J. Pathol. 145, 1057-1069. [PMC free article] [PubMed] [Google Scholar]
- 11.Smith, H., Nelson, J. A., Gahmberg, C. G., Crandall, I. & Sherman, I. W. (1992) Exp. Parasitol. 75, 269-280. [DOI] [PubMed] [Google Scholar]
- 12.Bazan, J. F., Bacon, K. B., Hardiman, G., Wang, W., Soo, K., Rossi, D., Geaves, D. R., Zlotnik, A. & Schall, T. J. (1997) Nature 385, 640-644. [DOI] [PubMed] [Google Scholar]
- 13.Yoshie, O., Imai, T. & Nomiyama, H. (2001) Adv. Immunol. 78, 57-110. [DOI] [PubMed] [Google Scholar]
- 14.Fong, A. M., Robinson, L. A., Steeber, D. A., Tedder, T. F., Yoshie, O., Imai, T. & Patel, D. D. (1998) J. Exp. Med. 188, 1413-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison, J. K., Fong, A. M., Swain, P. A. W., Chen, S., Yu, Y. R. A., Salafranca, M. N., Greenleaf, W. B., Imai, T. & Patel, D. D. (2001) J. Biol. Chem. 276, 21632-21641. [DOI] [PubMed] [Google Scholar]
- 16.Trager, W. & Jensen, J. B. (1976) Science 193, 673-675. [DOI] [PubMed] [Google Scholar]
- 17.Chaiyaroj, S. C., Coppel, R. L., Novakovic, S. & Brown, G. V. (1994) Proc. Natl. Acad. Sci. USA 91, 10805-10808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambros, C. & Vanderberg, J. P. (1979) J. Parasitol. 65, 418-420. [PubMed] [Google Scholar]
- 19.Gardner, J. P., Pinches, R. A., Roberts, D. J. & Newbold, C. I. (1996) Proc. Natl. Acad. Sci. USA 93, 3503-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pouvelle, B., Buffet, P. A., Lépolard, C., Scherf, A. & Gysin, J. (2000) Nat. Med. 6, 1264-1268. [DOI] [PubMed] [Google Scholar]
- 21.Deloron, P., Chougnet, C., Lepers, J. P., Tallet, S. & Coulanges, P. (1991) J. Clin. Microbiol. 29, 1757-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Artavanis-Tsakonas, K. & Riley, E. M. (2002) J. Immunol. 169, 2956-2963. [DOI] [PubMed] [Google Scholar]
- 23.Kremsner, P. G., Winkler, S., Brandts, C., Wildling, E., Jenne, L., Graninger, W., Prada, J., Bienzle, U., Juillard, P. & Grau, G. E. (1995) Am. J. Trop. Med. Hyg. 53, 532-538. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs, P., Radzioch, D. & Stevenson, M. M. (1996) Infect. Immun. 64, 535-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fong, A. M., Alam, S. M., Imai, T., Haribabu, B. & Patel, D. D. (2002) J. Biol. Chem. 277, 19418-19423. [DOI] [PubMed] [Google Scholar]