Abstract
There are multiple mechanisms by which cells evade TGF-β-mediated growth inhibitory effects. In this report, we describe a novel mechanism by which cells become resistant to TGF-β-mediated growth suppression. While having all the components of the TGF-β signaling pathway, different cell lines, RL, HaCaT, and BJAB, have different sensitivities towards TGF-β-induced growth suppression. The TGF-β resistance of RL, a B cell lymphoma cell line, was due to ligand-induced down-regulation of TGF-β receptor II (TβRII) and only transient TGF-β-induced nuclear translocation of Smad2 and Smad3. With low dose PMA or anti-IgM treatment, TGF-β sensitivity was restored by stabilizing TβRII expression and sustaining TGF-β signaling. The MEK inhibitor U0126 blocked both PMA- and anti-IgM-induced up-regulation of TβRII. In HaCaT and BJAB, two TGF-β-sensitive cell lines, which had higher basal levels of phospho-MEK and TβRII compared to RL, U0126 induced down-regulation of TβRII and blocked subsequent TGF-β signaling. Similar results were also obtained with normal B cells, where MEK1 inhibitor down regulated TβRII and subsequent TGF-β signaling. Constitutively active MEK1, but not constitutively active ERK2, induced up-regulation of TβRII. Furthermore, TβRII physically interacted with the constitutively active MEK1, but not with wild type MEK1, indicating involvement of active MEK1 in stabilizing TβRII. Collectively, our data suggest a novel mechanism for MEK1 in regulating the sensitivity to TGF-β signaling by stabilizing TβRII.
Keywords: Signaling, ERK, Lymphoma, Smad, MEK
INTRODUCTION
TGF-β signaling affects numerous cellular processes, including proliferation, differentiation, migration and apoptosis, depending on the cell type and its stage in malignant progression (1, 2). TGF-β initiates signaling by binding to and bringing together type I (TβRI) and type II (TβRII) receptor serine/threonine kinases on the cell surface (3). This allows receptor II to phosphorylate the receptor I kinase domain, which then propagates the signal through phosphorylation of the receptor-regulated Smad protein (Smad2/3), which is directly phosphorylated and activated by the type I receptor kinase. It then undergoes homotrimerization and formation of heteromeric complexes with a common partner, Smad4. The activated Smad complexes translocate into the nucleus and, in conjunction with other nuclear cofactors, regulate the transcription of target genes. The Smad pathway is negatively regulated by Inhibitory Smad (Smad6/7) (4, 5) and PPM1A phosphatase (6).
Low expression levels of TβRII were found in human B cell lymphoma cell lines (7) and breast cancers (8), and were thought to be responsible for the development of resistance to TGF-β-induced growth arrest. Mutations in the TβRII were also found in colon and gastric cancer with microsatellite instability (MSI) (9, 10). TβRII frameshift mutations have been found in human gliomas (11). TβRII missense mutations have been observed in two head and neck carcinoma cell lines (12). TGF-β receptor expression may also be reduced in tumor cells through hypermethylation of CpG islands in the TβRII gene promoters (13), or from mutations in the TβRII promoter that interfere with transcription factor binding (14). The oncogene EWSR1 can repress TβRII expression by blocking TβRII promoter activity and may account for decreased responsiveness to TGF-β in some cancer cells (15).
TβRs can interact with the pro-apoptotic adaptor protein Daxx (16), TGF-β-activated kinase 1 (TAK1) (17) and Rho GTPase (18), which lead to the induction of apoptosis or Epithelial/endothelial-Mesenchymal Transitions (EMT). In addition, several intracellular proteins have been shown to interact with the TGF-β receptor complex, including SARA (19), FKBP12 (20), STRAP (21), TRIP-1 (22) and chimeric tyrosine kinase ETV6-NTRK3 (23), to facilitate or suppress TGF-β signaling.
Although Smads are the most well-characterized target proteins, activated TβRs can also lead to the activation of MAPKs, such as the ERK (24), c-Jun N-terminal kinase (25) and p38 MAP kinase (26), the extent and kinetics of which differ among different cell lines and types. The canonical mitogen activated protein kinase (MAPK) cascade, including the Ras-Raf-MEK-ERK module, is critically involved in the regulation of normal cell proliferation, survival and differentiation. Aberrant regulation of MAPK cascades contribute to cancer and other human diseases (27). MAPKs may also modulate TGF-β signaling. ERK has been shown to phosphorylate the linker region of Smad2/3, which results in blocking the nuclear translocation of activated Smad2/3 (28). ERK MAP kinase pathway has also been shown to downregulate TGF-β signaling by activating TACE-mediated ectodomain shedding of TβRI (29). Moreover, ERK, JNK and TAK1/p38 pathways were shown to regulate TGF-β signaling via Smad7 expression, depending on the cell type (30, 31).
While having all the components of the TGF-β signaling pathway, RL, a germinal center-derived lymphoma cell line was resistant to TGF-β-induced growth arrest. We wanted to determine the mechanism of TGF-β resistance in RL cells, and examined the involvement of that underlying mechanism in TGF-β sensitive cell lines. In the present study we have demonstrated that the TGF-β resistance of RL was due to ligand-induced down-regulation of TGF-β receptor II (TβRII). Activated MEK1 stabilized TβRII, and a MEK1 inhibitor, U0126, induced down-regulation of TβRII in two TGF-β responsive cells lines and in normal human peripheral blood B cells with TGF- β resistance developing as a consequence. Moreover, we have shown that constitutively active, but not wild type MEK1 physically interacted with TβRII, indicating a role for active MEK1 in stabilizing TβRII. Our findings identify a novel mechanism by which tumor cells may evade TGF-β-induced growth suppression by controlling the stability of the TβRII.
MATERIALS AND METHODS
Reagents
For Western blot analysis and immunoprecipitation, rabbit polyclonal anti-TβRI antibody (sc-398), anti-TβRII (sc-220 and sc-400), phospho-MEK1/2 (sc-7995), MEK (sc-6250), phospho-ERK1/2 (sc-16982R), ERK1 (sc-93) and ERK2 (sc-154) were obtained from Santa Cruz Biotechnology Inc (Santa Cruz, CA, USA); rabbit polyclonal phospho-Smad2 antibody, phospho-Smad3 were purchased from Cell Signaling Technology Inc. (Beverly, MA, USA); Rabbit polyclonal anti-Smad2 antibody (51–1300) and anti-Smad3 (51–1500) were obtained from Zymed Laboratories Inc. (South San Francisco, CA, USA); anti-Nucleoporin p62 and anti-BiP/GRP78 were from BD Transduction Laboratories (San Jose, CA, USA); mouse monoclonal anti-FLAG (F1804) was purchased from Sigma (St. Louis, MO, USA); mouse monoclonal anti-HA (clone 12CA5) was purchased from Roche Applied Science (Indianapolis, IN, USA); β-Actin was purchased from Abcam (Cambridge, United Kingdom). All HRP conjugated secondary antibodies were purchased from GE Healthcare. Recombinant TGF-β1 (240-B) and anti-human TGF-β RII-Phycoerythrin were purchased from R&D Systems (Minneapolis, MN, USA); phorbol 12-myristate 13-acetate (PMA), and Phorbol 12,13-dibutyrate (PDBu) were from Sigma-Aldrich (St. Louis, MO, USA); U0126 was from EMD Biosciences. DSP (dithiobis[succinimidylpropionate]), Sulfo-NHS-LC-Biotin and NeutrAvidin™ Agarose Resins were from Pierce (Rockford, IL, USA).
Cell culture
BJAB is an EBV-negative Burkitt’s lymphoma cell line (Kindly provided by Dr. Jeffery Sample, St. Jude Children’s Research Hospital). RL and BJAB cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 2mM L-glutamine, 1,000 U of penicillin/mL, 100µg of streptomycin/mL. HEK293A and HaCaT cells were cultured in DMEM medium supplemented with 10% FBS, 2mM L-glutamine, 1,000U of penicillin/mL, 100µg of streptomycin/mL. No exogenous growth factors were added. Cells were grown at 37°C in 5% CO2. Fresh growth medium was added to cells every 3 to 4 days. For growth observation, RL or RL/pWPI/HA-TβRII cells were stimulated with either 0.15ng/mL PMA or 2ng/mL recombinant TGF-β1 and/or PMA in RPMI 1640 with 5% FBS. Human B cells were purified from peripheral blood mononuclear cells (PBMCs) isolated from normal donors’ blood by negative selection using the B Cell Isolation Kit II (Miltenyi Biotec, Germany). Donors provided written informed consent. Freshly isolated B cells were cultured in RPMI 1640 medium supplemented with 1% fetal bovine serum (FBS), 2mM L-glutamine, 1,000 U of penicillin/ml, 100µg of streptomycin/ml. The routine purity of the isolated B cells was over 95%.
Cell surface Biotinylation
Biotinylation and Immunoprecipitation of cell surface TGF-β1 receptors were performed according to the procedure reported by Liu et al.(29). Briefly, cells were washed twice with ice-cold PBS, and incubated with EZ-link Sulfo-NHS-LC-Biotin (0.5mg/ml) at room temperature for 30 minutes. Biotinylation was quenched with 0.1 M glycine and remove excess biotin reagent. Cells were lysed in lysis buffer (10mM Tris-Cl (pH 8.0), 150mM NaCl, 1mM MgCl2, 5µg/mL E-64, 1mM PMSF, 2.5 mM Sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM NaOV4, 1mM NaF and 1% NP-40). Biotinylated proteins were precipitated with NeutrAvidin™ Agarose Resins and analyzed by immunoblotting using anti-TβRI (V-22) antibody and anti-TβRII (L-21) antibody.
Flow cytometry
Cells were washed with cold PBS and blocked with 0.2% Human IgG for 30 minutes at 4°C. Cells were then incubated with polyclonal anti-human TBRII-Phycoerythrin for 30 minutes at 4°C, washed with PBS, and fixed in 2% paraformaldehude before analyzed in FACScan.
Cell lysis and Western Blot Analysis
Cytoplasmic and nuclear extracts were prepared according to the procedure reported earlier (32). Membrane preparation was carried out according to the previous procedure (13). Whole cell lysates were prepared according to the following procedure: harvested cells were resuspended in lysis buffer [10mM Tris-Cl (pH 8.0), 150mM NaCl, 1mM MgCl2, 5µg/mL E-64, 1mM PMSF, 2.5 mM Sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM NaOV4, 1mM NaF and 1% Triton X-100] and were incubated on ice for 30 min. Samples were then homogenized, centrifuged, and the supernatants were collected. Protein concentrations were measured with Bio-Rad Protein Assay Dye reagent (Bio-Rad Laboratories, Hercules, California). Gel-electrophoresis was carried out using 4~12% SDS-PAGE under reducing conditions. After membrane transfer, bound antibodies were detected using chemiluminescent detection system (GE Healthcare).
Immunoprecipitation
Lysates were precleared with protein A/G plus-agarose (sc-2003, Santa Cruz Biotechnology Inc., Santa Cruz, California) for 30 min at 4 °C, and the pre-cleared lysates were incubated overnight with either anti-MEK1 or anti-Flag antibody and A/G plus-agarose beads. The agarose beads were washed three times with extraction buffer containing 25 mM MOPS (pH 7.2), 15mM MgCl2, 137mM NaCl, 1mM PMSF, 15mM EGTA, 5µg/mL E-64, 1 mM sodium vanadate and 1mM NaF and 0.1% Triton X-100. The immune complexes were dissociated with loading buffer containing LDS and boiled for 5 min. Electrophoresis was carried out by 4~12% SDS-PAGE under reducing conditions.
Protein Cross-linking
Cross-linking using DSP was performed according to the manufacturer’s protocol. Briefly, DSP was dissolved in dimethyl sulfoxide as a stock solution of 250 mg/ml. Samples were rinsed twice with cold PBS and incubated with PBS containing 1mg/ml DSP for 6 minutes at room temperature. The cross-linking reaction was quenched by adding 1M Tris-Cl pH8.0 to a final concentration of 100mM followed by 1 minute incubation in room temperature. Cells were rinsed once with cold PBS before lysates were prepared as described above.
Quantitative Real-time PCR
Total RNA was isolated with Rneasy Mini Kit (Qiagen, Valencia, CA). The complementary DNA was generated from 2µg of RNA by using the SuperScript VILO cDNA synthesis kit and random hexamers (Invitrogen). One hundredth of the samples were used in a real-time PCR reaction containing a 0.2µM concentration of both forward (tcactgacaacaacggtgc) and reverse (tgcactttggagaagcagc) primers of TβRII and SYBR green PCR master mix (Applied Biosystems, Foster City, CA). Quantitations of fold induction were normalized by β-actin and analyzed by the method described by Schmittigen et al (33).
Statistical analysis
Values were obtained from three independent experiments and were expressed as means ± SD. Statistical analysis was performed using Student's t-test and analyzed by two tailed test of paired samples. Values were considered significant (*) if ‘p’ values were <0.05.
DNA constructs and transfection
To generate Flag-tagged constructs, sequences corresponding to the wild type TβRI and TβRII were amplified from DNA constructs expressing HA-tagged TβRI or TβRII (generous gifts from Drs. Carl-Henrik Heldin and Joan Massague, respectively), and were subcloned into pENTRY/SD/D-TOPO vector (Invitrogen). The pENTRY/SD/D/Flag-TβRII was then used in recombination with pcDNA-DEST40 according to the manufacturer’s protocol. Wild type and constitutively active form of MEK1 constructs were obtained from Dr. Natalie Ahn (University of Colorado at Boulder). Wild type and constitutively active form of ERK2 constructs were obtained from Dr. Melanie Cobb (University of Texas Southwestern Medical Center). Wild type and constitutively active form of ERK1 constructs were obtained from Dr. David Engelberg (Hebrew University of Jerusalem, Israel). In HEK293A cells, transient transfections were performed with Lipofectamine 2000 reagent according to the protocol provided by the manufacturer (Invitrogen). RL cells were transfected by electroporation as follows: Exponentially growing RL cells were resuspended in 100µl Kit V solution (Lonza, Gaithersburg, Maryland) containing 3µg plasmid. Cells were then exposed to electroporation (program S-18) using Nucleofector Device (Lonza, Gaithersburg, Maryland). After a12-hour incubation, cells were transferred to fresh medium, and were further cultured for 24 hours.
Virus production and transduction
A DNA fragment corresponding to the wild type TβRII containing HA sequence was subcloned into lentiviral vector pWPI (kindly provided by Dr. Didier Trono, Global Health Institute, Lausanne, Switzerland). To introduce the human Coxsackie Adenovirus Receptor (hCAR) to the B cell lymphoma cell line, pHRCMV/hCAR-EGFP (generously provide by Dr. Mikko Mättö, University of Kuopio, Finland) was used. Lentiviral production with pWPI/HA-TβRII and pHRCMV/hCAR-EGFP, using the envelope vector pMD.G and the packaging vector pCMVR8.91 (kindly provided by Dr. Didier Trono) were carried out as described before (34). RL cells were seeded in a 12 well plate at 1×106 cells per well and virus was added with a multiplicity of infection (MOI) equal to 10. EGFP positive cells were isolated by limited dilution and checked by FACScan (BD Biosciences). Recombination between Flag-TβRII from pENTRY/SD/D and pAD/CMV/V5-DEST vector was done by LR Clonase II Enzyme Mix (Invitrogen) and preparation of adenoviruses bearing Flag-TβRII were done according to the manufacturer’s instructions. RL/hCAR-EGFP cells were infected with adenovirus harboring Flag-TβRII with an MOI equal to 200. The culture medium was replaced with fresh medium the next day, and incubated for another 24h. Cells were then treated with PMA (10ng/ml) and U0126 (20µM), with or without TGF-β1 (2ng/ml).
RESULTS
Rapid loss of TGF-β signaling accounts for the resistance of RL cells to TGF-β-mediated growth suppression
We have shown previously that a B cell lymphoma cell line RL is unresponsive to TGF-β-mediated growth suppression, whereas in the presence of a low dose of PMA, RL cells can be rendered responsive to TGF-β by up-regulating p21CIP/WAF1 and down-regulating c-Myc (Fig. 1A & B) (13, 35). RL cells can also be rendered responsive to TGF-β with regards to the growth suppression and up-regulation of p21CIP/WAF1 by biologically relevant stimulation delivered by anti-IgM treatment (Fig. 1C & D). To understand the mechanism behind the lack of TGF-β responsiveness, RL cells were treated with TGF-β in the presence or absence of PMA for various time periods, and the TGF-β signaling was monitored by the nuclear translocation of phospho-Smad2. As shown in Fig. 1E, although RL cells had all the components of the TGF-β pathway, the TGF- β signaling was short lived. The levels of nuclear phospho-Smad2 were decreased by 12 hours and were further down-regulated by 24 hours after TGF-β treatment. By contrast, in the presence of low dose PMA, TGF-β signaling was sustained at 24 hours and growth inhibition was observed. These data suggested that the transient TGF-β signaling might be responsible for the unresponsiveness of RL cells to TGF-β.
Figure 1.
Effect of TGF-β1 on RL cells in the presence or absence of low dose PMA. (A) RL cells were plated at 0.125×106 cells/mL and treated with either medium alone, TGF-β1 (2ng/mL), PMA (0.15 ng/mL), or PMA plus TGF-β1 for various time periods. At the end of each time point, cell counts were performed. Results are representative of triplicate experiments. * indicates the inhibition is statistically significant (p<0.01). (B) RL cells were treated with either medium alone, TGF-β1, PMA, or PMA plus TGF-β1for various time periods. Equal amounts of whole cell lysates were analyzed by western blot analysis. (C) Effect of TGF-β1 on RL cells in the presence or absence of αIgM. RL cells were treated as in (A) except αIgM (1 µg/ml) was used instead of PMA, and cell counts were performed at the end of each time point. * indicates the inhibition is statistically significant (p<0.01). (D) RL cells were treated with either medium alone, TGF-β1, αIgM, or αIgM plus TGF-β1for 24 hours. Equal amounts of whole cell lysates were analyzed by western blot analysis. (E) Time course of nuclear translocation of phospho-Smad2 induced by TGF-β1 (2ng/mL) with or without PMA (0.15ng/mL) treatment. Equal amounts of nuclear lysates for the time points indicated were analyzed by western blot analysis. The membranes were probed and reprobed with different antisera after stripping, as indicated above. Bar graphs represent the quantification of the phospho-Smad2 and total Smad2 blots normalized with the nucleoporin bands.
TGF-β1–induced down-regulation of TβRII was responsible for transient TGF-β1 signaling in RL cells
To explore whether ligand-induced down-regulation of TGF-β receptors (TβRs) accounts for the transient signaling, we examined the status of TβRI and TβRII upon treatment with TGF-β1 in the presence or absence of PMA for various time periods. As shown in Fig. 2A (Upper panel), TGF-β1 alone induced down-regulation of TβRII at 24 hours, whereas in the presence of PMA, TβRII levels were sustained for at least 48 hours. In contrast, the levels of TβRI were unchanged throughout the time points tested. To determine the status of the cell surface TβRI and TβRII, protein biotinylation was used according to the procedure described by Liu et al. (29). As shown in Fig. 2A (lower panel), cell surface expression of TβRII was also down-regulated upon TGF-β1 stimulation and the down-regulation was prevented by the PMA treatment. Like the total TβRI level, cell surface TβRI was unaffected by the TGF-β treatment in the presence or absence of PMA. Cell surface status of TβRII was also examined by fluorochrome-conjugated anti-TβRII antibody and analyzed by fluorescence activated cell sorter (FACS) (Supplemental Fig. 1A). TGF-β1-induced down-regulation of TβRII, both total and cell surface, was also blocked by the proteasome inhibitor MG132 (Fig. 2B, and Supplemental Fig. 1B). This stability of TβRII along with the stability of Smad2 by MG132 (data not shown; (36)) resulted in the sustained phosphorylation of Smad2 upon TGF-β1 treatment (Fig. 2C). To verify further whether ligand-induced down-regulation of TβRII was responsible for transient TGF-β1 signaling, we generated RL cells stably over-expressing HA-tagged TβRII (Supplemental Fig. 2). As shown in Fig. 2D, RL cells over-expressing TβRII were rendered responsive to TGF-β1-mediated growth suppression, and the growth suppression was correlated with higher surface expression of TβRII, sustained activation of Smad2, and expression of p21CIP/WAF1 (Fig. 2E and Supplemental Fig. 3). These data suggested that ligand-induced down-regulation of TβRII was responsible for transient TGF-β1 signaling, and this down regulation was prevented by a proteasome inhibitor and PMA treatment.
Figure 2.
Restoration of TβRII renders RL cells responsive to TGF-β1 mediated growth arrest. (A) Upper panel: RL cells were treated with either medium alone or with TGF-β1 (2ng/mL) in the presence or absence of PMA (0.15ng/mL) for various time periods. Equal amounts of membrane proteins were analyzed by western blot analysis. Lower panel: RL cells were treated with either medium alone, TGF-β1, PMA, or PMA plus TGF-β1 for 24 hours. Cell surface TGF-β receptors were detected by biotinylation, neutravidin bead adsorption and western blot analysis according to the procedure described in Methods and Materials. (B) RL cells were pretreated with PDBU (Phorbol 12,13-dibytyrate, a washable phorbol ester, 1.2ng/mL) for 24hrs, washed and resuspended in fresh medium before treatment with either medium alone or TGF-β1 in the presence or absence of either MG132 (20µM) or PMA (0.15ng/mL) for 8 hrs. Equal amounts of membrane proteins were analyzed by western blot analysis. (C) RL cells were treated with either medium alone or TGF-β1 in the presence or absence of MG132 for the indicated time points. Equal amounts of whole cell lysates were analyzed by western blot analysis. (D) RL/pWPI/HA-TβRII cells were plated at 0.125×106 cells/mL and treated with either medium alone or TGF-β1 for various time periods. At the end of each time point, cell counts were performed. Results are representative of triplicate experiments. * indicates the inhibition is statistically significant (p<0.01). (E) RL or TβRII over-expressing stable RL cell line (RL/pWPI/HA- TβRII) was treated with TGF-β1 for different time periods. Equal amounts of whole cell lysates were analyzed for phospho- and total Smad2, p21Cip1/WAF1, β-actin by western blot analysis.
MEK activation is involved in the stabilization of TβRII
To determine the kinase responsible for PMA-induced TβRII stabilization, we investigated the phosphorylation status of MEK1/2 and ERK1/2. As shown in Fig. 3A, dose-dependent increases in phosphorylation of both MEK1/2 and ERK1/2 were observed upon PMA treatment of RL cells. We did not observe any phosphorylation of PKC, as determined by the phospho-specific (pan) PKC antibody, at the low PMA concentration used here (data not shown). Low dose PMA-induced phosphorylation of MEK1/2 and ERK1/2 correlated with increased total and cell surface TβRII levels in RL cells (Fig. 3B and Supplemental Fig. 4A). To evaluate whether MEK activation was the cause of the TβRII stabilization induced by PMA, the MEK inhibitor, U0126, was used. As shown in Fig. 3B, although U0126 had no effect on the phosphorylation status of MEK1/2 (since U0126 only affects MEK1/2 kinase activity), it blocked the phosphorylation of its downstream target ERK1/2. U0126 treatment resulted in the blockage of PMA-induced increase in TβRII level, indicating the possible involvement of MEK1/2 kinase activity in PMA-induced TβRII stabilization in RL cells (Fig. 3B). U0126 treatment also inhibited PMA-induced increase in cell surface TβRII (Supplemental Fig. 4A). The activated MEK-induced up-regulation of cell surface TβRII level was also observed in case of anti-IgM treated RL cells, and the up-regulation was sensitive to U0126 (Supplemental Fig. 4B). Down-regulation of TβRII by U0126 resulted in a decrease in PMA/TGF-β1-induced phosphorylation of Smad2 (Fig. 3C). Interestingly, treatment with U0126 also caused down-regulation of the TβRII levels and subsequent TGF-β1 signaling in two TGF-β1-responsive cell lines, HaCaT and BJAB (Fig. 3B, D, Supplemental Fig. 4C, & data not shown). The basal level of phospho-MEK1/2 was significantly higher in HaCaT and BJAB in comparison to RL cells, which correlated with higher basal levels of TβRII in these cell lines (Fig. 3B, Supplemental Fig. 4, & data not shown).
Figure 3.
TβRII expression was correlated with MEK activation. (A) RL cells were treated with either medium alone or PMA (0.15ng/mL and 10ng/mL) for 6 hrs. Equal amounts of whole cell lysates were analyzed by western blot analysis. (B) RL, BJAB, and HaCaT cells were treated with either medium alone or PMA in the presence or absence of U0126 (20µM) for indicated time periods, and equal amounts of whole cell lysates were analyzed by western blot analysis. (C) RL cells were treated with medium alone or PMA plus TGF-β1 in the presence or absence of U0126 for indicated time periods, and equal amounts of whole cell lysates were analyzed by western blot analysis. (D) HaCaT and BJAB cells were treated with TGF-β1 in the presence or absence of U0126 for 6 hours, and equal amounts of whole cell lysates were analyzed for phospho- and total Smad3 by western blot analysis. (E) Human peripheral blood B cells were pretreated with U0126 for 6 hours before treatment with TGF- β1 for 1 hour. An equal amount of whole cell lysates were analyzed by western blot analysis.
To determine whether the relation between the level of active MEK and TβRII status was unique to cell lines, freshly isolated normal human peripheral blood B cells were pre-treated with U0126 followed by the treatment with TGF-β, and the total and cell surface TβRII levels were analyzed (Fig. 3E and Supplemental Fig. 4D). The pre-treatment with U0126 caused down regulation of total TβRII levels and subsequent blockage in the phosphorylation of Smad2. Surprisingly, the levels of cell surface TβRII was increased upon U0126 treatment in the presence or absence of TGF-β1, indicating the involvement of MEK1 in TβRII levels in normal B cells is different than the immortalized cell lines (Supplemental Fig. 4D).
To investigate the effect of TGF-β treatment on the levels of TβRII and subsequent TGF-β signaling in TGF-β sensitive and insensitive cell lines, RL, HaCaT and BJAB cells were treated with TGF-β for various time periods, and the levels of TβRII and phospho-Smad2 were determined by western blot analysis, and the cell surface TβRII was determined by FACS. Although TGF-β treatment caused down regulation of TβRII in RL cells in a time-dependent manner, the levels of TβRII were unaffected in TGF-β sensitive cell lines HaCaT and BJAB (Supplemental Fig. 5A, B, C, & data not shown). The TβRII status was correlated with the TGF-β signaling in these cell lines (Supplemental Fig.5A). These data suggested that active MEK1/2 plays an important role in TβRII stabilization.
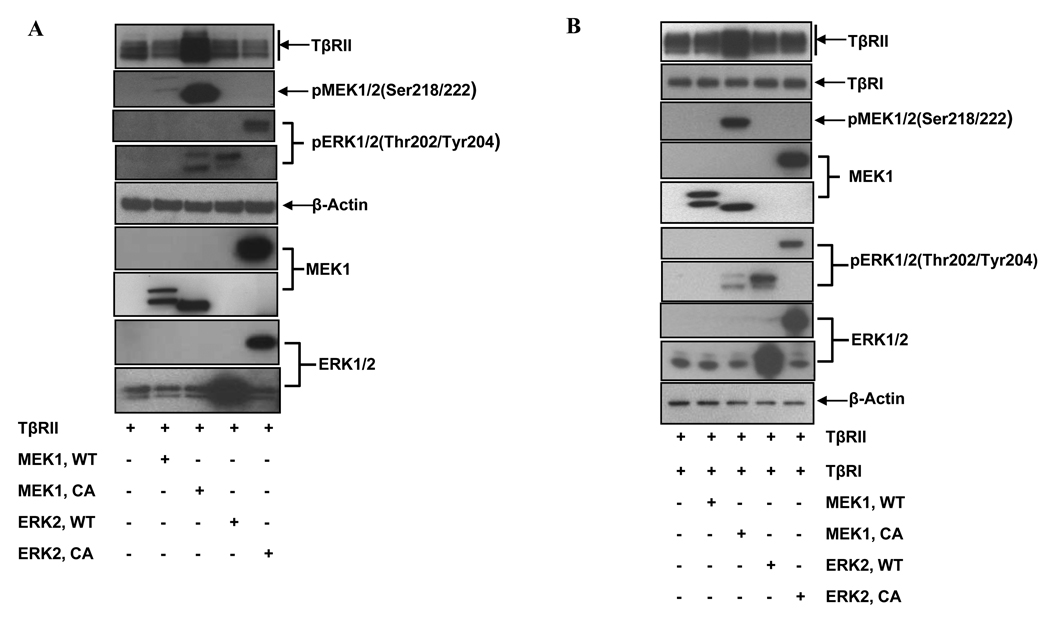
Constitutively active MEK1 up-regulates TβRII via protein stabilization
If the activation of MEK1/2 was responsible for the up-regulation of TβRII, ectopically expressed active MEK1 should up-regulate TβRII and convey TGF-β1-responsiveness to the RL cells. We co-transfected RL cells with TβRII and either a wild type (MEK1-WT/ERK-WT) or a constitutively active form of MEK1 or ERK2 (MEK1-CA/ERK2-CA), and then checked the expression of TβRII by western blot analysis and cell surface staining. As shown in Figure 4A, the constitutively active, but not the wild type MEK1, up-regulated both total as well as cell surface TβRII level (lane 3 vs. 1, and lane 2 vs. 1, and Supplemental Fig. 6A). Interestingly, neither wild type nor constitutively active ERK2 had any effect on TβRII levels (lanes 4 & 5 vs. 1). Similar results were also obtained with wild type and constitutively active ERK1 (Supplemental Fig. 7), suggesting that the up-regulation of TβRII was independent of ERK activation. To determine whether this phenomenon was unique to RL cells, the same experiment was performed in HEK293A cells. As shown in Figure 4B, only constitutively active, but not wild type MEK1, up-regulated both total as well as cell surface TβRII in HEK293A cells (lane 3 vs. 1, and lane 2 vs. 1, and Supplemental Fig. 6B). These data suggested that the activation of MEK1, but not ERK1/2, was mainly responsible for the up-regulation of TβRII.
Figure 4.
Constitutively active MEK1 up-regulates TβRII expression through protein stabilization. (A) RL and (B) HEK293A cells were co-transfected with TβRII and either wild type MEK1 (MEK1-WT), constitutively active MEK1 (MEK1-CA), wild type ERK2 (ERK2-WT) or constitutively active ERK2 (ERK2-CA) constructs. After 24 hours, whole cell lysates were prepared and analyzed by western blot analysis. Constitutively active form of ERK2 (ERK2-CA) is a chimeric construct in which ERK2 was coupled to its upstream activator MEK1 resulting in a larger protein (45). (C) Upper panel: HEK293A cells were transfected with either wild type MEK1 (MEK1-WT, lanes 1–5) or constitutively active MEK1 (MEK1-CA, lanes 6–10) constructs. After 24 hours, cycloheximide (CHX, 20µg/ml) was added to the culture, and cells were collected at different time points indicated post CHX treatment. Equal amounts of whole cell lysates were analyzed by western blot analysis. Lower panel: Graphical representation of the densitometric analysis of the western blot from three independent CHX experiments. * indicates the statistical significance (p< 0.01).
To determine whether the up-regulation of TβRII was due to a transcriptional or a post-transcriptional mechanism, we first tested the effect of PMA in the presence or absence of U0126 on the mRNA levels of TβRII by real-time PCR. Treatment with PMA in the absence or presence of U0126 did not have any effect on the TβRII mRNA levels throughout the time points tested, whereas these treatments affected the TβRII protein levels as observed in Fig. 3B (Supplemental Fig. 8). To investigate whether MEK1 activation was involved in the stabilization of TβRII protein, HEK293A cells were co-transfected with TβRII and either wild type or constitutively active MEK1. After 24 hours, cells were exposed to cycloheximide (CHX) for different periods of time as indicated. At each time point, cells were collected and the levels of TβRII were determined by western blot analysis. As shown in Figure 4C (upper panel), CHX treatment resulted in the rapid down-regulation of the basal TβRII levels (lanes 1–5), whereas constitutively active MEK1-induced TβRII levels were sustained for longer periods of time (lanes 6–10). To calculate the half lives (t1/2) of TβRII in the presence of either wild type or constitutively active MEK1, densitometric analysis of the autoradiographs within the linear range of the films were performed. As shown in Fig. 4D (lower panel), t1/2 for wild type MEK1 was 64±6.9 minutes, whereas t1/2 for constitutively active MEK1 was 136±27.7 minutes (p<0.01, n=3). These data indicate that the up-regulation of TβRII by active MEK1 was via stabilization of TβRII protein.
Constitutively active MEK1 physically interacts with TβRII
In order to determine whether MEK1 stabilizes TβRII via physical interaction, co-immunoprecipitation was performed using lysates prepared from HEK293A cells co-transfected with FLAG-tagged TβRII and either wild type or constitutively active form of MEK1. As shown in Fig. 5A, anti-MEK1 antibody was able to pull down TβRII only from the cells expressing the constitutively active form of MEK1, but not the wild type MEK1 (left panel). We were also able to demonstrate the interaction in reverse immunoprecipitation, where anti-FLAG antibody co-immunoprecipitated the constitutively active form of MEK1 (right panel). Although, ERK was found to be associated with active MEK1 during immunoprecipitation with anti-MEK1 antibody, immunoprecipitation with anti-FLAG antibody failed to pull down ERK, indicating ERK was not the effectors for MEK1-mediated TβRII stabilization. Also, we did not observe any interaction between TβRI and active MEK (data not shown). Next, we wanted to know whether the MEK inhibitor, U0126, had any effect on the interaction between TβRII and active MEK1. To evaluate that question, HEK293A cells were co-transfected with TβRII and the constitutively active form of MEK1, and after 24 hours, cells were treated with U1026 for different periods of time. As shown in Fig. 5B, U0126 blocked the interaction between TβRII and the constitutively active form of MEK1 in a time-dependent manner without affecting the TβRII levels (Fig. 5B). As a consequence of blocking the interaction of MEK1 and TβRII, U0126 prevented the up-regulation of TβRII induced by both exogenous and endogenous MEK1 in HEK293A and RL cells, respectively (Fig. 5C and Supplemental Fig. 6A & B).
Figure 5.
TβRII physically interacts with constitutively active, but not wild type MEK1. (A) HEK293A cells were co-transfected with TβRII and either wild type (MEK1-WT) or constitutively active MEK1 (MEK1-CA) constructs. After 24 hours, whole cell lysates were prepared, and equal amounts of whole cell lysates were used for immunoprecipitation with anti-TβRII or anti-MEK1 antibody, and the immunocomplexes were analyzed by western blot analysis. (B) HEK293A cells were co-transfected with TβRII and constitutively active MEK1 (MEK1-CA) constructs. After 24 hours, transfected cells were treated with either medium alone or U0126 for different times. Equal amounts of whole cell lysates were used for immunoprecipitation with anti-MEK1 antibody, and the immunocomplexes were analyzed by western blot analysis. (C) Left panel: HEK293A cells were co-transfected with TβRII and either wild type (MEK1-WT) or a constitutively active MEK1 (MEK1-CA) constructs. After 6 hours, transfected cells were treated with either medium alone or U0126 overnight. Equal amounts of whole cell lysates were analyzed by western blot analysis; Right panel: RL/hCAR-EGFP cells were infected with pAD/CMV/Flag-TβRII (MOI: 200). 48 hours later, cells were treated with either medium alone or PMA in the presence or absence of U0126 for 6 hours. Equal amounts of whole cell lysates were analyzed by western blot analysis. (D) RL cells were stimulated with either medium alone or PMA for 24 hours. Cells were then treated with U0126 for indicated time periods. Following cross-linking by DSP, whole cell lysates were prepared, and equal amounts of whole cell lysates were used for immunoprecipitation either with anti-TβRII or anti-MEK1 antibody, and immunocomplexes were analyzed by western blot analysis. (E) TβRII over-expressing stable RL cells were stimulated with either medium alone or IgM for 1 hour. Cells were then treated with U0126 for indicated time periods. Following cross-linking by DSP, whole cell lysates were prepared, and equal amounts of whole cell lysates were used for immunoprecipitation with anti-TβRII antibody, and immunocomplexes were analyzed by western blot analysis.
To determine whether interaction between TβRII and MEK occurs endogenously, RL cells were stimulated with either medium alone or PMA for 24 hours. Cells were then treated with U0126 for different time periods, after which cross-linker was used to monitor the interaction. As shown in Fig. 5D (left panel), anti-TβRII antibody was able to pull down endogenous phospho-MEK1 in absence of U0126. Treatment with U0126 affected the interaction in a time-dependent manner. Similarly in reverse immunoprecipitation analysis, anti-MEK1 antibody was also able to pull down endogenous TβRII in the absence of U0126 (right panel). We were unable to demonstrate the interaction between TβRII and MEK1 in the absence of any cross-linker indicating the fact that either the interaction is transient, or the lysis conditions are not optimal to preserve the interaction. Our data indicate that the endogenous TβRII comes in contact with phospho-MEK1 upon PMA treatment, and the MEK1 inhibitor U0126 prevents the interaction between TβRII and phospho-MEK1. Finally, to determine whether biologically relevant stimulation can induce interaction between TβRII and active MEK, TβRII over-expressing stable RL cells were stimulated with either medium alone or anti-IgM for 1 hour. Cells were then treated with U0126 for indicated time periods. Following cross-linking by DSP, whole cell lysates were used for immunoprecipitation. As shown in Fig. 5E, anti-TβRII antibody was able to pull down endogenous phospho-MEK1 in absence of U0126. Treatment with U0126 affected the interaction in a time-dependent manner. Collectively, these data strongly suggested that the interaction between TβRII and active MEK1 was essential for the up-regulation of TβRII.
DISCUSSION
Although the mechanism of activation of the TGF-β signaling pathway is well established, additional non-canonical components greatly influence the activity of this pathway. TβRII is responsible for the initiation of multiple TGF-β signaling pathways, and loss of its function is associated with many types of human cancers (9, 10). In this report, we describe a novel mechanism by which cells become resistant to TGF-β-mediated growth suppression. A B-cell lymphoma cell line, RL, which had intact components of the TGF-β signaling pathway, was resistant to TGF-β-induced growth arrest due to ligand-induced down-regulation of TGF-β receptor II (TβRII) and only transient TGF-β-induced nuclear translocation of Smad2, without affecting TβRI. The down-regulation of TβRII was blocked by the proteosome inhibitor MG132, and mitogenic stimulation like low dose PMA or αIgM treatment. We have found that active MEK1 was involved in the stabilization of TβRII based on the following observations: (1) the MEK1/2 inhibitor U0126 blocked PMA-induced up-regulation of TβRII and subsequent TGF-β signaling in RL cells; (2) two TGF-β-responsive cell lines, HaCaT and BJAB, which had higher basal levels of phospho-MEK and TβRII (Fig. 3B), had attenuated TGF-β signaling upon U0126 treatment, which was associated with TβRII down-regulation; (3) in normal B cells, the MEK1/2 inhibitor U0126 down regulated TβRII and subsequent TGF-β signaling; (4) ectopically expressed active MEK1, but not active ERK1 or ERK2, was able to up-regulate TβRII; (5) both exogenous and endogenous active MEK1 physically interacted with TβRII; and (6) interference in the interaction between TβRII and active MEK1 by U0126 resulted in the inhibition of TβRII up-regulation. These data suggest that the stabilization of TβRII by active MEK is a general phenomenon.
The number of cell surface TGF-β receptors is tightly controlled by internalization, recycling and ubiquitin-dependent down-regulation, which contribute to the regulation of cell surface expression of TGF-β receptors. In mink lung epithelium, the half-life of TβRII has been shown to be 60 minutes, which was further reduced to 45 minutes in the presence of exogenous ligand (37). TGF-β receptors can be internalized through both caveolin and EEA1-positive vesicles, and reside in both lipid raft and non-raft membrane domains (38). Clathrin-dependent internalization in the non-raft membrane domain, EE1-positive endosomes, which are enriched in Smad2 and SARA, activates TGF-β signaling, whereas internalization in caveolin-containing raft domains, which are enriched in Smad7 and Smurf2, stimulates receptor turn over through the ubiquitinylation pathway (38–40). Recycling occurs as receptors are internalized through clathrin-coated pits, and then returned to the plasma membrane via a rab11-dependent mechanism (41). Interestingly, internalization of TβRII is unaffected by ligand-receptor interaction (38, 41). Our findings indicate an important role that MEK1 plays in determining the fate of TβRII possibly by redirecting TβRII to recycling as opposed to the degradation pathway. Whether MEK1 does this either by directly associating with TβRII or by modifying TβRII through phosphorylation is currently under investigation. The role of MEK1 in TβRII stabilization is not unique in B cell lymphoma cell lines as we have shown this effect in multiple cell types: BJAB (B cell lymphoma); HaCaT (keratinocyte), HEK293 (epithelial), and normal human peripheral blood B cells.
Active MEK-mediated TβRII stabilization may have significant physiological relevance to the conversion from a tumor suppressor to a tumor promoter role for TGF-β. As a case in point, epidermal growth factor receptor (EGFR) is expressed in a wide variety of human tumors (42), and EGFR engagement triggers a number of downstream signaling pathways including Ras/Raf/MEK (43). In work that has similarity to ours, it has been shown that MEK1/2 was involved in the permissive role of epidermal growth factor in TGF-β signaling by stabilizing TβRII mRNA in prostate adenocarcinoma cells (44). Our data suggest that MEK-mediated modification may play an important role in maintaining TβRII level on the cell surface and prevent ligand-induced down-regulation of TβRII. These findings also suggest a mechanism by which tumor cells evade TGF-β-induced growth suppression by increasing the ligand-induced turnover of the TβRII. Furthermore, it is possible that an agent capable of activating MEK phosphorylation could restore TGF-β responsiveness to some lymphomas in vivo. Experiments to test this notion are underway.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported (in part) by the Intramural Research Program of the NIH, National Institute on Aging.
REFERENCES
- 1.Miyazono K, ten Dijke P, Heldin C. TGF-â signaling by Smad proteins. Adv Immunol. 2000;75:115–157. doi: 10.1016/s0065-2776(00)75003-6. [DOI] [PubMed] [Google Scholar]
- 2.Massagué J, Blain SW, Lo RS. TGF[beta] Signaling in Growth Control, Cancer, and Heritable Disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 3.Shi Y, Massagué J. Mechanisms of TGF-[beta] Signaling from Cell Membrane to the Nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 4.Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi H, Abdollah S, Qiu Y, Cai J, Xu Y-Y, Grinnell BW, et al. The MAD-Related Protein Smad7 Associates with the TGF[beta] Receptor and Functions as an Antagonist of TGF[beta] Signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 6.Lin X, Duan X, Liang Y-Y, Su Y, Wrighton KH, Long J, et al. PPM1A Functions as a Smad Phosphatase to Terminate TGF[beta] Signaling. Cell. 2006;125:915–928. doi: 10.1016/j.cell.2006.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inman GJ, Allday MJ. Resistance to TGF-{beta}1 correlates with a reduction of TGF-{beta} type II receptor expression in Burkitt's lymphoma and Epstein-Barr virus-transformed B lymphoblastoid cell lines. J Gen Virol. 2000;81:1567–1578. doi: 10.1099/0022-1317-81-6-1567. [DOI] [PubMed] [Google Scholar]
- 8.Gobbi H, Arteaga CL, Jensen RA, Simpson JF, Dupont WD, Olson SJ, et al. Loss of expression of transforming growth factor beta type II receptor correlates with high tumour grade in human breast in-situ and invasive carcinomas. Histopathology. 2000;36:168–177. doi: 10.1046/j.1365-2559.2000.00841.x. [DOI] [PubMed] [Google Scholar]
- 9.Grady WM, Myeroff LL, Swinler SE, Rajput A, Thiagalingam S, Lutterbaugh JD, et al. Mutational Inactivation of Transforming Growth Factor {beta} Receptor Type II in Microsatellite Stable Colon Cancers. Cancer Res. 1999;59:320–324. [PubMed] [Google Scholar]
- 10.Parsons R, Myeroff LL, Liu B, Willson JKV, Markowitz SD, Kinzler KW, et al. Microsatellite Instability and Mutations of the Transforming Growth Factor {beta} Type II Receptor Gene in Colorectal Cancer. Cancer Res. 1995;55:5548–5550. [PubMed] [Google Scholar]
- 11.Dams E, Van de Kelft EJZ, Martin J-J, Verlooy J, Willems PJ. Instability of Microsatellites in Human Gliomas. Cancer Res. 1995;55:1547–1549. [PubMed] [Google Scholar]
- 12.Garrigue-Antar L, Munoz-Antonia T, Antonia SJ, Gesmonde J, Vellucci VF, Reiss M. Missense Mutations of the Transforming Growth Factor {beta} Type II Receptor in Human Head and Neck Squamous Carcinoma Cells. Cancer Res. 1995;55:3982–3987. [PubMed] [Google Scholar]
- 13.Chen G, Ghosh P, Osawa H, Sasaki CY, Rezanka L, Yang J, et al. Resistance to TGF-beta 1 correlates with aberrant expression of TGF-beta receptor II in human B-cell lymphoma cell lines. Blood. 2007;109:5301–5307. doi: 10.1182/blood-2006-06-032128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amoroso SR, Huang N, Roberts AB, Potter M, Letterio JJ. Consistent loss of functional transforming growth factor beta receptor expression in murine plasmacytomas. Proc Natl Acad Sci U S A. 1998;95:189–194. doi: 10.1073/pnas.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahm KB, Cho K, Lee C, Im YH, Chang J, Choi SG, et al. Repression of the gene encoding the TGF-beta type II receptor is a major target of the EWS-FLI1 oncoprotein. Nat Genet. 1999;23:222–227. doi: 10.1038/13854. [DOI] [PubMed] [Google Scholar]
- 16.Perlman R, Schiemann WP, Brooks MW, Lodish HF, Weinberg RA. TGF-beta-induced apoptosis is mediated by the adapter protein Daxx that facilitates JNK activation. Nat Cell Biol. 2001;3:708–714. doi: 10.1038/35087019. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi K, Nagai S, Ninomiya-Tsuji J, Nishita M, Tamai K, Irie K, et al. XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1-TAK1 in the BMP signaling pathway. EMBO J. 1999;18:179–187. doi: 10.1093/emboj/18.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 2005;307:1603–1609. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- 19.Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL. SARA, a FYVE Domain Protein that Recruits Smad2 to the TGF[beta] Receptor. Cell. 1998;95:779–791. doi: 10.1016/s0092-8674(00)81701-8. [DOI] [PubMed] [Google Scholar]
- 20.Wang T, Li B-Y, Danielson PD, Shah PC, Rockwell S, Lechleider RJ, et al. The Immunophilin FKBP12 Functions as a Common Inhibitor of the TGF[beta] Family Type I Receptors. Cell. 1996;86:435–444. doi: 10.1016/s0092-8674(00)80116-6. [DOI] [PubMed] [Google Scholar]
- 21.Datta PK, Chytil A, Gorska AE, Moses HL. Identification of STRAP, a novel WD domain protein in transforming growth factor-beta signaling. J Biol Chem. 1998;273:34671–34674. doi: 10.1074/jbc.273.52.34671. [DOI] [PubMed] [Google Scholar]
- 22.Griswold-Prenner I, Kamibayashi C, Maruoka E, Mumby M, Derynck R. Physical and functional interactions between type I transforming growth factor-â receptors and Balpha, a WD-40 repeat subunit of phosphatase 2A. Mol Cell Biol. 1998;18:6595–6604. doi: 10.1128/mcb.18.11.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin W, Kim BC, Tognon C, Lee HJ, Patel S, Lannon CL, et al. The ETV6-NTRK3 chimeric tyrosine kinase suppresses TGF-beta signaling by inactivating the TGF-beta type II receptor. Proc Natl Acad Sci U S A. 2005;102:16239–16244. doi: 10.1073/pnas.0503137102. Epub 2005 Oct 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan Z, Winawer S, Friedman E. Two different signal transduction pathways can be activated by transforming growth factor beta 1 in epithelial cells. J Biol Chem. 1994;269:13231–13237. [PubMed] [Google Scholar]
- 25.Hocevar B, Brown T, Howe P. TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 1999;18:1345–1356. doi: 10.1093/emboj/18.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanafusa H, Ninomiya-Tsuji J, Masuyama N, Nishita M, Fujisawa J, Shibuya H, et al. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta-induced gene expression. J Biol Chem. 1999;274:27161–27167. doi: 10.1074/jbc.274.38.27161. [DOI] [PubMed] [Google Scholar]
- 27.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 28.Kretzschmar M, Doody J, Timokhina I, Massague J. A mechanism of repression of TGFbeta/ Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu C, Xu P, Lamouille S, Xu J, Derynck R. TACE-mediated ectodomain shedding of the type I TGF-beta receptor downregulates TGF-beta signaling. Mol Cell. 2009;35:26–36. doi: 10.1016/j.molcel.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uchida K, Suzuki H, Ohashi T, Nitta K, Yumura W, Nihei H. Involvement of MAP Kinase Cascades in Smad7 Transcriptional Regulation. Biochemical and Biophysical Research Communications. 2001;289:376–381. doi: 10.1006/bbrc.2001.5984. [DOI] [PubMed] [Google Scholar]
- 31.Dowdy SC, Mariani A, Janknecht R. HER2/Neu- and TAK1-mediated up-regulation of the transforming growth factor beta inhibitor Smad7 via the ETS protein ER81. J Biol Chem. 2003;278:44377–44384. doi: 10.1074/jbc.M307202200. [DOI] [PubMed] [Google Scholar]
- 32.Uzzo R, Rayman P, Kolenko V, Clark P, Cathcart M, Bloom T, et al. Renal cell carcinoma-derived gangliosides suppress nuclear factor-kappaB activation in T cells. J Clin Invest. 1999;104:769–776. doi: 10.1172/JCI6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative Reverse Transcription-Polymerase Chain Reaction to Study mRNA Decay: Comparison of Endpoint and Real-Time Methods. Analytical Biochemistry. 2000;285:194–204. doi: 10.1006/abio.2000.4753. [DOI] [PubMed] [Google Scholar]
- 34.Salmon P, Kindler V, Ducrey O, Chapuis B, Zubler RH, Trono D. High-level transgene expression in human hematopoietic progenitors and differentiated blood lineages after transduction with improved lentiviral vectors. Blood. 2000;96:3392–3398. [PubMed] [Google Scholar]
- 35.Sing G, Ruscetti F, Beckwith M, Keller J, Ellingsworth L, Urba W, et al. Growth inhibition of a human lymphoma cell line: induction of a transforming growth factor-beta-mediated autocrine negative loop by phorbol myristate acetate. Cell Growth Differ. 1990;1:549–557. [PubMed] [Google Scholar]
- 36.Izzi L, Attisano L. Regulation of the TGFbeta signalling pathway by ubiquitin-mediated degradation. Oncogene. 2004;23:2071–2078. doi: 10.1038/sj.onc.1207412. [DOI] [PubMed] [Google Scholar]
- 37.Koli KM, Arteaga CL. Processing of the transforming growth factor beta type I and II receptors. Biosynthesis and ligand-induced regulation. J Biol Chem. 1997;272:6423–6427. doi: 10.1074/jbc.272.10.6423. [DOI] [PubMed] [Google Scholar]
- 38.Di Guglielmo G, Le Roy C, Goodfellow A, Wrana J. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 39.Ebisawa T, Fukuchi M, Murakami G, Chiba T, Tanaka K, Imamura T, et al. Smurf1 interacts with transforming growth factor-beta type I receptor through Smad7 and induces receptor degradation. J Biol Chem. 2001;276:12477–12480. doi: 10.1074/jbc.C100008200. [DOI] [PubMed] [Google Scholar]
- 40.Tajima Y, Goto K, Yoshida M, Shinomiya K, Sekimoto T, Yoneda Y, et al. Chromosomal region maintenance 1 (CRM1)-dependent nuclear export of Smad ubiquitin regulatory factor 1 (Smurf1) is essential for negative regulation of transforming growth factor-beta signaling by Smad7. J Biol Chem. 2003;278:10716–10721. doi: 10.1074/jbc.M212663200. Epub 2003 Jan 7. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell H, Choudhury A, Pagano R, Leof E. Ligand-dependent and -independent transforming growth factor-beta receptor recycling regulated by clathrin-mediated endocytosis and Rab11. Mol Biol Cell. 2004;15:4166–4178. doi: 10.1091/mbc.E04-03-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitsudomi T, Yatabe Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 277:301–308. doi: 10.1111/j.1742-4658.2009.07448.x. [DOI] [PubMed] [Google Scholar]
- 43.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 44.Song K, Krebs TL, Danielpour D. Novel permissive role of epidermal growth factor in transforming growth factor beta (TGF-beta) signaling and growth suppression. Mediation by stabilization of TGF-beta receptor type II. J Biol Chem. 2006;281:7765–7774. doi: 10.1074/jbc.M511781200. Epub 2006 Jan 20. [DOI] [PubMed] [Google Scholar]
- 45.Robinson MJ, Stippec SA, Goldsmith E, White MA, Cobb MH. A constitutively active and nuclear form of the MAP kinase ERK2 is sufficient for neurite outgrowth and cell transformation. Curr Biol. 1998;8:1141–1150. doi: 10.1016/s0960-9822(07)00485-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.