Abstract
Patients with diabetes mellitus (DM) are prone to a diffuse and rapidly progressive form of atherosclerosis, which increases their likelihood of requiring revascularization. However, the unique pathophysiology of atherosclerosis in patients with DM modifies the response to arterial injury, with profound clinical consequences for patients undergoing percutaneous coronary intervention (PCI). Multiple studies have shown that DM is a strong risk factor for restenosis following successful balloon angioplasty or coronary stenting, with greater need for repeat revascularization and inferior clinical outcomes. Early data suggest that drug eluting stents reduce restenosis rates and the need for repeat revascularization irrespective of the diabetic state and with no significant reduction in hard clinical endpoints such as myocardial infarction and mortality. For many patients with 1- or 2-vessel coronary artery disease, there is little prognostic benefit from any intervention over optimal medical therapy. PCI with drug-eluting or bare metal stents is appropriate for patients who remain symptomatic with medical therapy. However, selection of the optimal myocardial revascularization strategy for patients with DM and multivessel coronary artery disease is crucial. Randomized trials comparing multivessel PCI with balloon angioplasty or bare metal stents to coronary artery bypass grafting (CABG) consistently demonstrated the superiority of CABG in patients with treated DM. In the setting of diabetes CABG had greater survival, fewer recurrent infarctions or need for re-intervention. Limited data suggests that CABG is superior to multivessel PCI even when drug-eluting stents are used. Several ongoing randomized trials are evaluating the long-term comparative efficacy of PCI with drug-eluting stents and CABG in patients with DM. Only further study will continue to unravel the mechanisms at play and optimal therapy in the face of the profoundly virulent atherosclerotic potential that accompanies diabetes mellitus.
Keywords: Coronary artery bypass graft, Diabetes mellitus, Percutaneous coronary intervention, Revascularization, Stents
1 Introduction
Diabetes mellitus (DM) is associated with a 2 to 4-fold increased risk of coronary artery disease, and ischemic coronary artery disease is responsible for three-quarters of diabetes-related deaths [1]. The adverse macrovascular consequences of DM are well recognized, as is the accompanying accelerated rate of atherosclerosis that predisposes patients to occlusive coronary artery disease, myocardial infarction and death. Patients with DM are prone to a diffuse and rapidly progressive form of atherosclerosis, which increases their likelihood of requiring revascularization [1, 6]. In the United States, approximately one third of all percutaneous coronary intervention (PCI) procedures are performed on patients with DM. As the prevalence of DM increases, the number of patients with diabetes requiring revascularization for advanced coronary artery disease will escalate. The unique pathophysiology of atherosclerosis in DM modifies the response to arterial injury, with profound clinical consequences to patients with diabetes undergoing PCI. Although there has been considerable improvement in the management of patients with coronary artery disease, coronary event rates remain heightened [2, 3], and mortality greater among patients with diabetes [4, 5].
Selection of the optimal myocardial revascularization strategy for patients with diabetes and multivessel coronary artery disease (CAD) is crucial to improving the outcome of these high-risk patients. The following review summarizes current evidence regarding the effectiveness of various revascularization methods in patients with diabetes.
2 Balloon angioplasty and bare metal stents
2.1 Procedure-related and in-hospital outcomes
The patient with diabetes presents challenges to all revascularization procedures and the technology is itself slower to adapt and optimize. At least three epochs can be defined in interventional approaches to coronary disease—early, mid and modern. The earliest tracking of patient well-being soon after the advent of angioplasty revealed the unique biology of diabetic vascular disease [7–10]. Kip and associates described the short- and long-term outcomes of percutaneous transluminal coronary angioplasty (PTCA) in 2114 consecutive patients including 281 patients with diabetes from the National Heart Lung and Blood Institute (NHLBI) 1985 to 1986 Registry [7]. There was then no difference in rates of procedural angiographic success and completeness of revascularization. However, combined in-hospital complication including death, myocardial infarction, and need for emergency surgery were almost two-fold higher, and inhospital death was more than 6-fold more prevalent in patients with DM [7]. In the middle period of interventional approaches, the differences in patients with and without diabetes fell as the technology of interventional cardiology progressed even as sicker patients were intervened upon. Angiographic success was achieved more frequently in patients with diabetes in the 1997–2001 NHLBI Dynamic Registry, where stenting was used in nearly 90% of patients. Whereas procedures were successful in 77% of patients with diabetes in the 1985–1986 cohort, success increased to 96% in the four years that followed. Abrupt closure fell more than two-fold from 2.2% to 0.9%, and the hard end points were all similarly reduced, including inhospital mortality (1.9% vs. 4.3%), myocardial infarction (1.0% vs. 7.4%), and in-hospital CABG surgery (0.8% vs. 6.2%) [11].
The refinement of interventional technology has propelled us into a third epoch, the late or modern period. Methodological and technical advances have solved many of the hurdles that had limited initial success. Yet, even in the face of similar procedural success and procedural related complications, in-hospital mortality amongst patients with diabetes was far higher than all others undergoing coronary stenting [12]. Patients with diabetes tolerate multivessel intervention and acute ischemic complications of angioplasty poorly [13].
2.2 Repeat revascularization and long-term outcome
The incidence of restenosis, the need to reintervene after angioplasty, has dropped significantly with the progression of interventional methods and technology except in the face of DM. Numerous studies have shown that patients with diabetes experience a greater need for repeat revascularization after intervention, and much of the difference in the interventional risk associated with DM occurs within the first 6 months, corresponding to the period in which restenosis is most active. The initial report from the 1985 NHLBI Angioplasty Registry indicated that the angiographic restenosis rate in patients with diabetes was 47%, as compared to 32% in patients without diabetes [14]. In the ensuing 25 years restenosis rates have spanned to an even higher 71% when DM is present [15–18]. In a study of 485 consecutive patients with DM undergoing balloon angioplasty without stenting, at least one vessel with restenosis was found in 68% of patients [19]. Even with stents, patients with diabetes have higher restenosis rates compared with patients without diabetes [20–22], and greater need for repeat revascularization. Data from 5 studies with over 4800 patients document the increased angiographic restenosis rates in patients with diabetes with an absolute overall 10% difference between patients with and without the disease [23].
The restenotic process is more virulent in patients with diabetes—more often leading to total vessel occlusion after coronary stenting [24–26], and resulting in myocardial infarction, reduced left ventricular function, heart failure [19] and long-term mortality [7, 9, 23, 25]. In a consecutive series of patients with successful stent placement (715 with DM and 2,839 without DM), the 1-year cardiac mortality rate was almost twice as high in patients with diabetes (5.7 vs. 2.9%, p<0.001). The incidence of the composite endpoint of cardiac death and nonfatal myocardial infarction was significantly and commensurately higher as well (8.0 vs. 4.6%) [25].
Since the earliest reports of differences in the DM population question has been raised as to whether these differences reflect an extreme of the biologic processes that affect all patients or a different set of pathologic events all together. The clinical manifestations are different and may be part of the diabetic process. The defective anginal warning system (DAWS) the plagues patients with diabetes and ischemic heart disease may be evident here as well, as many of restenotic events were clinically silent, with no angina in 36% and stable angina in 33% of patients. Progression of native disease is far more prevalent in patients with diabetes as the majority of angioplasty procedures performed more than 1 year after the index intervention are at a site different from the initially treated lesion. Patients with diabetes require more frequent revascularization with either surgery or angioplasty after the first year of the initial angioplasty, indicating that patients with diabetes are more prone to progression of coronary disease [9].
3 Restenosis in patients with diabetes
A large number of clinical, anatomical, and procedural variables have been associated with restenosis in various studies. Patient-related variables include demographics, concomitant medical diseases, serologic markers, and genetic polymorphism. However, the most consistent patient-related risk factors associated with restenosis is DM [27]. In a pooled analysis of several major stent trials, Cutlip et al. found DM to be the strongest clinical predictor for restenosis, with an almost 50% increased risk for target lesion revascularization at one-year follow-up [28]. In a series of patients enrolled in 16 PCI studies with 6-months angiographic follow-up, 31.1% of patients with diabetes developed restenosis. Smaller vessel reference diameter before the procedure and greater stented length of the vessel were independent predictors of restenosis in patients with diabetes [29]. The higher risk for restenosis exists in patients with diabetes throughout the vessel size range [25].
3.1 Mechanisms of restenosis in diabetes mellitus
Restenosis is a characteristic response to mechanical vessel injury. Endothelial denudation and dysfunction enables and promotes local thrombosis, superficial and then deep inflammation, which trigger smooth muscle cell proliferation [30, 31], and eventually matrix remodeling and extracellular matrix deposition. Each of these events can be exacerbated by hyperglycemia, hyperinsulinemia, insulin [32, 33] or frank diabetes [34]. Hyperglycemia promotes inflammation [35, 36], induces the production of growth factors that promote restenosis [27] and increases matrix gene transcriptions [37]. Advanced glycosylation end products (AGEs) accumulate in vascular tissues with normal aging and at an accelerated rate in DM. AGE formation is accelerated in correlation with glucose concentration and time of exposure to hyperglycemia. AGEs interact with specific receptors (RAGE) present on all cells relevant to the restenosis process including inflammatory cells and smooth muscle cells. AGEs–RAGE interaction in vessel wall may lead to inflammation, smooth muscle cell proliferation, and extracellular matrix production, culminating in exaggerated intimal hyperplasia and restenosis [27, 34]. Enhanced intimal proliferation was found in patients with diabetes at the site of angioplasty-induced arterial injury, particularly in restenotic lesions [38]. Animal models of restenosis implicate RAGE in promoting the mononuclear phagocytes and smooth muscle cell response to arterial injury [39, 40].
Corpus et al. [41] assessed the effect of glycemic control on target vessel revascularization at the time of coronary intervention in 179 patients with diabetes as compared with 60 controls. Patients who had optimal diabetic control, defined as HbA1C≤7%, had a target vessel revascularization rate of 15%, compared with 34% among counterparts with an HbA1C >7%. By multivariate analysis, poor glycemic control, HbA1C >7%, was a major independent predictor for target vessel revascularization with an odds ratio of 2.87. Similar repeat revascularization rates were observed among patients with diabetes and optimal glycemic control and patients without diabetes.
Insulin has several biological actions, which may be related to the process of restenosis. Insulin can potentiate proliferation and migration of smooth muscle cells, most likely through the action of insulin-like growth factor [42] or other stimulatory factors. There are suggestive data in humans that insulin-resistance is associated with restenosis among patients without diabetes [43–45].
The risk of restenosis may also be dictated by the anti-diabetic drugs used. Thiazolidinediones (TZDs) are anti-diabetic agents that increase insulin sensitivity and are currently used to treat patients with type 2 DM. Beyond their metabolic action, TZDs exhibit anti-inflammatory and anti-atherogenic effects in vascular cells in vitro and limit lesion development in various animal models of arteriosclerosis. Several studies have shown that TZD treatment reduces restenosis and neointima formation after coronary stenting in patients with type 2 DM [46, 47] and in patients without diabetes [48], independent on the glucose-lowering properties of these agents.
4 Drug eluting stents
4.1 Angiographic outcome
Drug-eluting stents (DES) reduce angiographic restenosis and need for repeat revascularization procedures amongst all patients. The benefit in diabetes appears to be similar. Initial results from subgroup analysis of patients with diabetes from a number of randomized controlled trials (including the SIRIUS and TAXUS IV) have been encouraging, demonstrating significant reductions in rates of restenosis, TLR and/or MACE in patients receiving a DES [49, 50]. Sirolimus-eluting stents (SES) reduced the relative incidence of in-lesion angiographic restenosis from 59.5% to 17.6% in patients with DM (65% reduction) and from 30.7% to 6.1% in patients without DM (80% reduction) [51]. In both patients with and without DM, SES transformed restenosis from a diffuse to a focal pattern. However, the absolute late loss and restenosis remain higher in patients with DM receiving SES, and DM remained an independent predictor of TLR (OR 1.65, p=0.03). Furthermore, in insulin-requiring patients, the angiographic in-segment restenosis rate was 35% in the SES arm and 50% in the BMS arm [51]. Similarly, in the small (n=160) DIABETES Trial, target-lesion revascularization at 9-months was significantly lower in the SES group as compared with the BMS group (6.3% versus 31.3%) [52]. The TAXUS-IV randomized trial compared paclitaxel-eluting stents (PES), to their bare-metal counterpart. PES reduced the rate of 9-month binary angiographic restenosis (i.e. >50% diameter stenosis of treated segment at follow-up) by 81% (6.4% vs. 34.5%), and 12-month rates of target lesion revascularization by 65% (7.4% vs. 20.9%). Moreover, diffuse in-stent restenosis was reduced by more than 90% in patients with DM receiving the paclitaxel-eluting stent, such that when angiographic restenosis did occur, it was predominantly focal in nature [53].
As restenosis was the major limitation of BMS use in patients with diabetes, DES are considered by many to be the standard of care for patients with diabetes undergoing stent placement. However, the most complex patients with DM (e.g. those with multivessel and diffuse disease) were excluded from enrollment in the DES trials, and only future studies will confirm if the effects of DES are lasting in diabetes.
4.2 Clinical outcome with DES
Although the magnitude of restenosis reduction achieved with DES is impressive, it is important to recognize that these trials mandated an angiographic follow-up. Revascularization was therefore driven not only by clinical necessity but also by the angiographic appearance of narrowing within the treated segment even in patients who did not have documented ischemia [54]. Although DES are effective in reducing the need for repeat revascularization, most of the current information has been obtained by studying selected patient populations in selected medical centers. In real world practice, the benefit of DES in patients with DM might be less impressive [55]. For example, in the Swedish Coronary Angiography and Angioplasty Registry, the numbers needed to treat patients with diabetes with DES to avoid one additional restenosis per year with BMS ranged from 21 to 47 lesions in patients treated with one stent and 11 to 27 in patients with multiple stents [56].
Equally critical is to consider is whether a reduction in restenosis will result in improvement in the hard end points of myocardial infarction and death, which are considerably higher in patients with diabetes. As several randomized trials and meta-analyses [57–59] have shown no significant reduction in rates of death and myocardial infarction with DES, as compared with BMS, it seems unlikely that greater use of DES in the diabetic population can meaningfully alter the outcome of these patients.
A collaborative network meta-analysis of 35 trials including 3852 patients with DM and 10,947 patients without DM demonstrated that compared with BMS, TLR rates are strongly decreased by use of SES and PES in patients with DM. It was determined that only 6 patients with diabetes would need to be treated before one revascularization event would be circumvented in the four years after intervention, in contrast to the 8 needed in patients without DM [60]. However, in trials with dual anti-platelet therapy (DAT) with aspirin and a thienopyridine for less than six months, the risk of death associated with SES in patients with diabetes was more than twice the risk associated with BMS. These results imply that one death will arise over four years for every seven with intervention. Conversely, trials with dual anti-platelet therapy for six months or more showed no increase in risk from using SES compared with BMS [60]. The duration of DAT modified the safety profile of drug eluting stents mainly in patients with DM. In an analysis of patients with diabetes in the Swedish Coronary Angiography and Angioplasty Registry, DES significantly reduced restenosis to half the rate seen with BMS. However, there was no difference in the combined outcome of death or myocardial infarction in patients with diabetes treated with DES (n=4754) or BMS (n=4956) with up to 4 years of follow-up [56].
4.3 Comparison between different types of drug eluting stents
The ISAR-DIABETES trial compared SES and PES in patients with diabetes. PES were associated with a higher rate of in-segment late luminal loss as well as an increased risk of angiographic restenosis. However, the study was not powered to demonstrate clinically important differences between the two groups [61]. Jensen et al. performed a randomized multicentre IVUS study comparing neointimal hyperplasia formation and distribution within stents in patients with diabetes treated SES and PES [62]. The neointimal response differed between patients treated with SES and PES as the latter generated greater and more diffuse neointimal hyperplasia than the former. Although angiographic late loss might differ from one DES to another, it is still unknown whether this surrogate end point will translate into a clinical end point of efficacy such as restenosis [55].
4.4 Stent thrombosis after DES implantation
The possibility of increased rates of stent thrombosis (ST) after DES has been a matter of concern and can be particularly pertinent to patients with diabetes. ST is classified based on the time of the adverse event relative to the index procedure. Early ST refers to the first 30 days after stent implantation and is further stratified into acute (<24 hours) and subacute (24 h to 30 days). Late ST defines the time interval between 1 month and 1 year after stent implantation; very late ST includes any event beyond 1 year [63]. After DES implantation, late ST occurs at a relatively constant rate over time up to at least 4 years after stent implantation [64]. Delayed healing and impaired endothelialization (i.e., incomplete endothelial coverage of stent vascular segments associated with persistence of fibrin deposits) are common features of late and very late ST, and combine with chronic inflammation and hypersensitivity reactions to promote local clot [65]. Increased inflammation, hypersensitivity, and outward remodeling might prolong the window of vulnerability to stent thrombosis and at all times [63, 66]. The definitive diagnosis of ST requires either angiographic or postmortem evidence of thrombotic stent occlusion. Probable ST encompasses any unexplained death within 30 days of stent implantation or any myocardial infarction in the territory of the implanted stent regardless of time [67]. Though ST is multifactorial [63] several studies confirmed higher rates in patients with diabetes especially for patients on insulin therapy [68–70].
In the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial, insulin-requiring DM was a significant independent predictor of definite or probable ST occurring within 30 days (odds ratio, 2.35; 95% CI, 1.36 to 4.07) [71]. In the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel–Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38), patients with DM and acute coronary syndrome (n=3146) had a two fold higher rate of stent thrombosis than those without DM (2.8% versus 1.4%), with highest rates among subjects treated with insulin (3.7%) [72]. In a large 2-Institutional Cohort Study of 8,146 patients who underwent percutaneous coronary intervention with a SES (n=3,823) or PES (n=4,323) and were followed up to 4 years after stent implantation, DM was an independent predictor of overall, early, and late definite ST [73], and the only predictor of early ST. Similarly, in the Swedish Coronary Angiography and Angioplasty Registry (n=73 798 DES and BMS), insulin-treated DM was an independent predictor of ST (1.8-fold increase) [74]. Finally, in the e-Cypher registry (n=15157), insulin-dependent DM was an independent predictor of ST at 1-year (2.8-fold risk increase) [68].
The increased risk of patients with diabetes for ST might be related to the more diffuse nature of atherosclerosis, accompanied by longer lesion lengths, smaller vessel size, and greater plaque burden, which might impose less optimal procedural results. Previous studies have identified both vessel size and lesion length as predictors of stent thrombosis [64], which may explain in part the predisposition of people with DM to this adverse event in the absence of adequate anti-platelet therapy. Additionally, the detrimental effects of DM on endothelial function [75] and platelet function [76] may also promote the development of ST. Further analyses are needed to assess the optimal duration of dual anti-platelet therapy after implantation of a DES, particularly in the diabetic population. However, because patients with diabetes appear to be at higher risk for ST, longer use of dual anti-platelet therapy beyond 1 year may is reasonable [77], and any cessation of anti-platelet therapy should be avoided.
When other medical procedures requiring interruption of aspirin and clopidogrel are scheduled, stent thrombosis becomes a matter of concern. Until more data are available, physicians should be aware of the potentially high risk of stent thrombosis when interrupting anti-platelet agent regimens in patients with DM, particularly if other risk factors for ST are present (e.g. renal insufficiency, bifurcation lesions) [55]. Thus, given the importance of a 1-year course of dual anti-platelet therapy, it is recommended that elective surgery be postponed for 1 year [77], and among those patients for whom surgery cannot be deferred, aspirin therapy should be considered during the perioperative period.
5 Multivessel angioplasty vs. CABG
5.1 CABG versus PTCA
The Bypass Angioplasty Revascularization Investigation (BARI) study enrolled 1829 patients with angiographically-documented multivessel CAD and either clinically severe angina or objective evidence of marked myocardial ischemia requiring revascularization. At 5-years there was a near doubling of mortality among patients with diabetes on insulin or oral hypoglycemic therapy assigned to multi-vessel angioplasty compared with those assigned to surgery (35% vs. 19%). In contrast, mortality was an identical 9% in both revascularization strategies if no drugs were required to control the diabetic state [78]. The 5.4-year cardiac mortality was 3.5-fold higher in the PTCA group (20.6 % vs. 5.8%) [10]. The survival benefit of CABG was limited to the 81% of patients with diabetes receiving an internal mammary graft. Cardiac mortality was 2.9% when an internal mammary graft were used and rose 6-fold to 18.2% when only vein grafts were employed. The latter rate was similar to patients receiving PTCA (20.6%) [10]. The BARI investigators recently reported that the survival benefit of CABG persisted at 10 years [79]. The 10-year survival in the overall study population was 71.0% for PTCA and 73.5% for CABG. However, in the subgroup with treated DM, patients randomized to CABG had higher survival than those randomized to PTCA (PTCA 45.5% vs. CABG 57.8%, P=0.025). Today these results pose a virtual black-box warning pushing clinicians to propose CABG for patients with diabetes and multi-vessel disease.
The results of other randomized trials comparing multi-vessel angioplasty to CABG are consistent with the BARI findings. Long-term follow-up of patients participating in the Emory Angioplasty versus Surgery Trial (EAST) demonstrated an improved survival in patients with diabetes randomized to CABG (75.5% vs. 60.1% with PTCA at 8 years). The angioplasty patients with DM had a worse survival than patients without DM by eight years (without 82.6%, and with DM 60.1%) [80].
5.2 CABG versus PCI with stents
The BARI trial provided important insights that increased our understanding of the benefit of revascularization techniques in DM. However, the initial cardiac revascularization procedures in BARI were performed between 1988 and 1991, before the introduction of coronary stents and other technical refinements in angioplasty and surgery. The Arterial Revascularization Therapy Study (ARTS) compared stenting and CABG for the treatment of patients with multivessel coronary disease. Patients with diabetes treated with stenting had reduced event-free survival at 1 year as compared with those treated with CABG (63% vs. 84%, P<0.001) and compared with patients without diabetes treated with stents (76%, P=0.04); differences were driven primarily by an increased need for repeat revascularization [81]. At 5-years, the major adverse cardiac and cerebrovascular events (MACCE) in patients with diabetes treated with stents was 55%, versus 39% in those without diabetes, again largely attributable to the higher rate of repeat revascularization (43% vs. 28%). Conversely, there was no significant difference in the five-year MACCE rate between patients with and without diabetes treated with CABG (25% vs. 21%) [82].
The incidence of 5-year mortality in patients with DM assigned to multivessel stenting was 5.1% higher (13.4% compared with 8.3% in the surgical group), although the study was not powered to show mortality differences between patients with and without diabetes. Within the stent group, patients with diabetes had a significantly higher mortality rate than patients without diabetes (13.4% vs. 6.8%). The SYNTAX (SYNergy Between PCI With TAXus and Cardiac Surgery) study was the first to compare coronary artery bypass graft surgery (CABG) and the TAXUS Express PES in patients with and without diabetes and with complex left main and/or 3-vessel disease (452 with medically treated DM; 71% were treated for 3-vessel disease and 29% for left main disease). In patients with diabetes, the 1-year composite MACCE rate was significantly higher after PES treatment compared with CABG treatment (RR 1.83). The relative risk of repeat revascularization of PES over CABG was 3.18 in patients with diabetes compared with 1.94 in patients without diabetes [83]. Compared with CABG, mortality was higher after PES use for patients with diabetes with highly complex lesions (4.1% vs. 13.5%). Revascularization with PES resulted in higher repeat revascularization for both patients without diabetes (5.7% vs. 11.1%) and patients with diabetes (6.4% vs. 20.3%) [83]. Importantly, follow-up at 1 year may not yet reflect the true long-term differences between CABG and PES treatments of patients with diabetes because previous reports demonstrated reduced long-term mortality in CABG compared with PCI [79, 84].
Hlatky and colleagues recently analyzed pooled individual patient data from 10 randomized trials comparing the effectiveness of CABG with PCI (n=7812). During a median follow-up of 5.9 years, mortality in patients with diabetes (CABG, n=615; PCI, n=618) was 30% lower in the CABG group than in the PCI group. In contrast, mortality was similar between groups in patients without DM. The beneficial effect of CABG compared with PCI on survival did not differ between balloon angioplasty (n=6) and bare-metal stent (n=4) trials [84].
The CARDia (Coronary Artery Revascularization in Diabetes) trial enrolled patients with diabetes with either multivessel CAD or complex single-vessel CAD (ostial or proximal left anterior descending artery disease) in whom coronary revascularization was recommended on clinical grounds. Patients were randomized only if an experienced interventional cardiologist and cardiac surgeon agreed that there was reasonable equipoise in the risks and benefits of PCI and CABG. The primary end point was a composite of death, MI, and stroke assessed at 1 year after randomization with a major secondary end point of the composite of the primary outcome and repeat revascularization. The primary comparison used a noninferiority method with the upper boundary of the 95% CI not to exceed 1.3 to declare PCI noninferior. The study enrolled 510 patients with diabetes, with 69% of patients in the PCI arm receiving SES. The combined rate of death, MI, and stroke in the CABG group was 10.5% compared with 13.0% in the PCI group (HR: 1.25, 95% CI: 0.75–2.09). Thus, the noninferiority margin of 1.3 was exceeded by the upper limit of the CI for the primary end point, indicating that the study results could not demonstrate that PCI is noninferior to CABG. The rates of death, MI, stroke, or repeat revascularization were 11.3% and 19.3% in the CABG and PCI groups, respectively [85].
5.3 Revascularization versus medical therapy
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI-2D) trial tested the hypothesis that in patients with both DM and stable coronary disease, prompt revascularization, surgical or catheter-based, would reduce long-term rates of death and cardiovascular events as compared with medical therapy alone. A second hypothesis, that insulin sensitization would be superior to insulin provision will not be discussed here. Coronary artery disease was documented on angiography (≥50% stenosis of a major epicardial coronary artery associated with a positive stress test or ≥70% stenosis of a major epicardial coronary artery and classic angina) before randomization. All patients had to be appropriate candidates for both elective PCI or CABG. Patients were randomized to undergo either prompt coronary revascularization or medical therapy. The appropriate method of revascularization for each patient (PCI or CABG) was determined a priori by the responsible physician. Approximately one third of patients in the PCI stratum who were assigned to undergo revascularization received a drug eluting stent. The primary end point was death from any cause, and the secondary end point was a composite of death, myocardial infarction, or stroke.
The rates of death from any cause did not differ significantly between the revascularization group and the medical-therapy group. The 5-year survival rate was 88% among patients in the revascularization and medical-therapy groups, with no statistical difference in rates of major cardiovascular events or death. The initial selection process of patients for the CABG stratum resulted in a population of patients with a much greater atherosclerotic burden and more lesions than in the PCI stratum. These patients had more 3-vessel disease (52% versus 20%), more total occlusions (61% versus 32%), more proximal left anterior descending stenosis ≥50% (19% versus 10%), a greater number of nonobstructive and obstructive atherosclerotic and class C lesions [86]. Prompt revascularization significantly reduced major cardiovascular events, as compared with intensive medical therapy, among patients who were selected to undergo CABG largely because of a reduction in MI events, but not among those who were selected to undergo PCI (P=0.002 for the interaction between study-group assignment and intended method of revascularization) [86, 87]. Of the patients who were assigned to receive medical therapy, 42% had changes in their clinical course that led to coronary revascularization during the 5 years of follow up. The COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial also showed that percutaneous intervention with optimal medical therapy was no better than optimal medical therapy alone for patients with stable CAD in general and for a subgroup of patients with DM (34% of patients enrolled) [88].
5.4 Explaining the mortality benefit of CABG
The BARI investigators have identified two mechanisms for the protective effect of CABG in DM. First, there appears to be a strong protective effect with respect to survival in the group of patients with diabetes who sustained a myocardial infarction. The striking reduction in the risk of death after spontaneous Q-wave myocardial infarction in the patients with DM (relative risk, 0.09; 95 percent confidence interval, 0.03–0.29) accounted for about 50% of the overall reduction in mortality attributable to CABG. Second, there was a moderate but constant reduction in mortality throughout follow-up that occurred in the majority of patients with diabetes who remained free of myocardial infarction. These protective effects of CABG may be related to the increased restenosis rates following angioplasty in DM and incomplete revascularization associated with multivessel angioplasty [89, 90]. In the BARI study population, 3.1 grafts were placed per patient undergoing CABG, whereas the mean number of successfully treated lesions in the PTCA group was 2.0 [10]. Together with the high restenosis rate among patients with diabetes, it is likely that a higher proportion of the myocardium remains unprotected and unrevascularized in patients with DM, and a greater proportion of the myocardium becomes ischemic during an acute spontaneous myocardial infarction. The impact of incomplete revascularization may be even more severe owing to new arterial narrowings and coronary artery disease progression [91], more diffuse and distal coronary disease and micro-circulatory dysfunction in DM.
The amount of jeopardized myocardium decreases initially following revascularization and increases subsequently with target lesion restenosis, graft failure, or the development of new narrowings in native vessels. Follow-up angiographic analysis of the BARI patients at years 1 and 5 revealed that the total percentage of jeopardized myocardium, defined as the overall percentage of the coronary perfusion territory compromised by stenoses ≥50%, was higher in patients with diabetes [92]. The mean percentage increase in total jeopardized myocardium was significantly greater in patients with diabetes compared with patients without diabetes at 1-year protocol-directed angiography (42% versus 24%) and on the first clinically performed (unscheduled) angiogram within 30 months (63% versus 50%) but not at 5-year protocol-directed angiography (34% versus 26%). In contrast, among CABG patients, DM was not associated with an increase in jeopardized myocardium at any angiographic follow-up interval. In this context, DM does not seem to affect the patency of internal mammary grafts, or the accelerated atherosclerotic process that characterizes vein grafts [93, 94]. The lower rate of nonfatal myocardial infarction with surgical revascularization observed in BARI-2D [87] is consistent with the hypothesis that, bypass grafts to the mid-coronary vessel treat the culprit lesion and prophylaxes against new proximal disease, progression of proximal narrowing or plaque rupture occurring proximal to a patent graft insertion. Proximal coronary arterial stents, bare metal and drug-eluting, cannot protect against new disease.
6 Approach to coronary revascularization in patients with diabetes
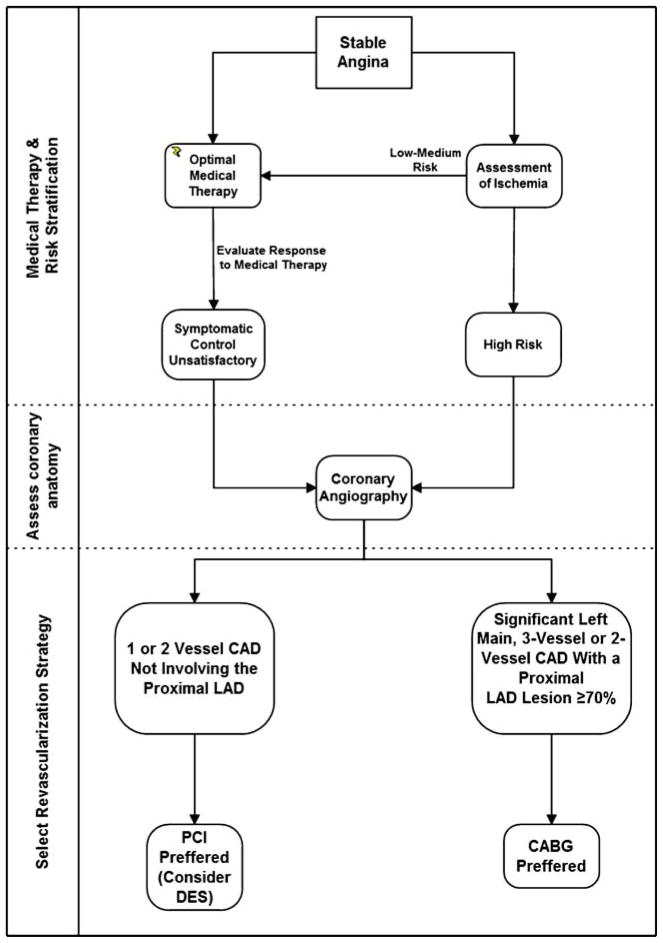
Efforts to prevent myocardial infarction and improve long-term survival in patients with diabetes with established CAD should focus primarily on reducing the incidence of acute thrombotic events and the development of ventricular dysfunction. The BARI 2D trial demonstrated that for many patients with DM and CAD, optimal medical therapy rather than any intervention is an excellent first-line strategy, particularly for those with less severe disease. Revascularization can be applied later if drug therapy does not adequately control symptoms without incurring an increased risk of MI or cardiac death [86, 95]. For patients with diabetes with less severe CAD, single-vessel or two-vessel CAD not involving the proximal left anterior descending artery and normal left ventricular function, there is little prognostic benefit from any intervention over optimum medical therapy. In such patients who require intervention after optimization of medical therapy there is no obvious survival advantage for either PCI or CABG, but there is a significantly higher risk of repeat revascularizations with PCI. Although DES reduce restenosis in comparison with bare-metal stents in patients with diabetes [60], DES studies have consistently shown higher repeat revascularization rates after PCI compared with surgical revascularization [83, 96]. Notwithstanding, PCI with DES or BMS is a reasonable approach in these patients (Fig. 1).
Fig. 1.
Revascularization strategy in patients with diabetes with stable angina
The effectiveness of PCI for treated patients with diabetes with asymptomatic ischemia or CCS class I or II angina who have 2- or 3-vessel CAD with significant proximal LAD who are otherwise eligible for CABG is not well established [97, 98]. CABG is superior in terms of survival, recurrent infarctions and freedom from re-intervention for patients with treated DM with moderate to severe symptoms and multivessel CAD in the setting of significant proximal left anterior descending artery involvement, and patients with diabetes with a significant stenosis (≥50%) of the left main coronary artery, [86, 95, 97] (Fig. 1). However, the clinician’s judgment on the revascularization strategy remains an important factor.
The clinical management of patients with diabetes and asymptomatic CAD in the face of silent ischemia and the value of coronary revascularization are not clear. Revascularization has not proven beneficial at reducing mortality or major cardiac events for patients with asymptomatic or stable CAD. Indeed, there can be no benefit from relief of angina in asymptomatic patients. Furthermore, in the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study, a prospective randomized trial evaluating outcomes after screening for asymptomatic CAD in type 2 patients with diabetes, cardiac event rates were not significantly reduced by myocardial perfusion imaging screening for myocardial ischemia over 4.8 years [99]. Even moderate or large defects had a cardiac event rate of 2.4% per year and a positive predictive value of just 12% for cardiac events. Finally, the results of on-going randomized trials including FREEDOM [100] and long-term results of the SYNTAX and CARDia trials are awaited to inform us on the long-term comparative efficacy of PCI with DES and CABG in patients with DM.
7 Summary
Insulin therapy converted the acute devastating process that is diabetes mellitus into a chronic disease. Vicissitudes of glucose levels and insulin exposure expose vascular tissues to extremes that exaggerate potent injurious signals and impede repair. The patient with diabetes and CAD is confronted with multiple risks, challenges, and issues as yet unresolved. Angioplasty is less well tolerated and less definitive in patients with diabetes, and full-blown bypass grafting is likely superior to balloon and stent intervention in the face of multi-vessel disease. The benefit and risk of DES in patients with diabetes is not fully defined but likely similarly presents evidence of a potential safety concern. Future studies will define more rigorously the interplay of diabetes mellitus, control of disease and choice of intervention and therapy. Time and scientific investigation will determine if diabetes represents an extreme form of the normal response to vascular intervention or a unique set of processes of its own. For now we must be satisfied in more assiduous control of the endocrinologic abnormalities and more precise vascular intervention.
Acknowledgments
Elazer R. Edelman is supported in part by a grant from the USA National Institutes of Health (GM 49039).
Contributor Information
Doron Aronson, Email: d_aronson@rambam.health.gov.il, Department of Cardiology, Rambam Medical Center and the Rappaport Research Institute, Technion, Israel Institute of Technology, Haifa, Israel.
Elazer R. Edelman, Email: ere@mit.edu, Harvard–MIT Division of Health Sciences and Technology, and Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Massachusetts Institute of Technology, Cambridge, MA, USA
References
- 1.Luscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part II. Circulation. 2003;108:1655–61. doi: 10.1161/01.CIR.0000089189.70578.E2. [DOI] [PubMed] [Google Scholar]
- 2.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 3.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aronson D, Rayfield EJ, Chesebro JH. Mechanisms determining course and outcome of diabetic patients who have had acute myocardial infarction. Ann Intern Med. 1997;126:296–306. doi: 10.7326/0003-4819-126-4-199702150-00006. [DOI] [PubMed] [Google Scholar]
- 5.Hasdai D, Granger CB, Srivatsa SS, Criger DA, Ellis SG, Califf RM, et al. Diabetes mellitus and outcome after primary coronary angioplasty for acute myocardial infarction: lessons from the GUSTO-IIb Angioplasty Substudy. Global Use of Strategies to Open Occluded Arteries in Acute Coronary Syndromes. J Am Coll Cardiol. 2000;35:1502–12. doi: 10.1016/s0735-1097(00)00591-x. [DOI] [PubMed] [Google Scholar]
- 6.Berry C, Tardif JC, Bourassa MG. Coronary heart disease in patients with diabetes: part I: recent advances in prevention and noninvasive management. J Am Coll Cardiol. 2007;49:631–42. doi: 10.1016/j.jacc.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 7.Kip KE, Faxon DP, Detre KM, Yeh W, Kelsey SF, Currier JW. Coronary angioplasty in diabetic patients. The National Heart, Lung, and Blood Institute Percutaneous Transluminal Coronary Angioplasty Registry. Circulation. 1996;94:1818–25. doi: 10.1161/01.cir.94.8.1818. [DOI] [PubMed] [Google Scholar]
- 8.Levine GN, Jacobs AK, Keeler GP, Whitlow PL, Berdan LG, Leya F, et al. Impact of diabetes mellitus on percutaneous revascularization (CAVEAT-I). CAVEAT-I Investigators. Coronary Angioplasty Versus Excisional Atherectomy Trial. Am J Cardiol. 1997;79:748–55. doi: 10.1016/s0002-9149(96)00862-4. [DOI] [PubMed] [Google Scholar]
- 9.Stein B, Weintraub WS, Gebhart SP, Cohen-Bernstein CL, Grosswald R, Liberman HA, et al. Influence of diabetes mellitus on early and late outcome after percutaneous transluminal coronary angioplasty. Circulation. 1995;91:979–89. doi: 10.1161/01.cir.91.4.979. [DOI] [PubMed] [Google Scholar]
- 10.The Bypass Angioplasty Revascularization Investigation (BARI) Influence of diabetes on 5-year mortality and morbidity in a randomized trial comparing CABG and PTCA in patients with multivessel disease. Circulation. 1997;96:1761–9. doi: 10.1161/01.cir.96.6.1761. [see comments] [DOI] [PubMed] [Google Scholar]
- 11.Freeman AM, Abbott JD, Jacobs AK, Vlachos HA, Selzer F, Laskey WK, et al. Marked improvements in outcomes of contemporary percutaneous coronary intervention in patients with diabetes mellitus. J Interv Cardiol. 2006;19:475–82. doi: 10.1111/j.1540-8183.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- 12.Abizaid A, Kornowski R, Mintz GS, Hong MK, Abizaid AS, Mehran R, et al. The influence of diabetes mellitus on acute and late clinical outcomes following coronary stent implantation. J Am Coll Cardiol. 1998;32:584–9. doi: 10.1016/s0735-1097(98)00286-1. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg S, Savage MP, Fischman DL. The interventional cardiologist and the diabetic patient. Have we pushed the envelope too far or not far enough? Circulation. 1996;94:1804–6. doi: 10.1161/01.cir.94.8.1804. [editorial; comment] [DOI] [PubMed] [Google Scholar]
- 14.Holmes DJ, Vietstra R, Smith H, et al. Restenosis after percutanous transluminal coronary angioplasty (PTCA): a report from the PTCA Registry of the National Heart, Lung, and Blood Institute. Am J Cardiol. 1984;53:77C–81C. doi: 10.1016/0002-9149(84)90752-5. [DOI] [PubMed] [Google Scholar]
- 15.Weintraub W, Kosinski A, Brown C, King S. Can restenosis after coronary angioplasty be predicted from clinical variables. J Am Coll Cardiol. 1993;21:6–14. doi: 10.1016/0735-1097(93)90711-9. [DOI] [PubMed] [Google Scholar]
- 16.Rensing BJ, Hermans WR, Vos J, Tijssen JG, Rutch W, Danchin N, et al. Luminal narrowing after percutaneous transluminal coronary angioplasty. A study of clinical, procedural, and lesional factors related to long-term angiographic outcome. Coronary Artery Restenosis Prevention on Repeated Thromboxane Antagonism (CARPORT) Study Group. Circulation. 1993;88:975–85. doi: 10.1161/01.cir.88.3.975. [DOI] [PubMed] [Google Scholar]
- 17.Vandormael MG, Deligonul U, Kern MJ, Harper M, Presant S, Gibson P, et al. Multilesion coronary angioplasty: clinical and angiographic follow-up. J Am Coll Cardiol. 1987;10:246–52. doi: 10.1016/s0735-1097(87)80003-7. [DOI] [PubMed] [Google Scholar]
- 18.Quigley PJ, Hlatky MA, Hinohara T, Rendall DS, Perez JA, Phillips HR, et al. Repeat percutaneous transluminal coronary angioplasty and predictors of recurrent restenosis. Am J Cardiol. 1989;63:409–13. doi: 10.1016/0002-9149(89)90309-3. [DOI] [PubMed] [Google Scholar]
- 19.Van Belle E, Abolmaali K, Bauters C, McFadden EP, Lablanche JM, Bertrand ME. Restenosis, late vessel occlusion and left ventricular function six months after balloon angioplasty in diabetic patients. J Am Coll Cardiol. 1999;34:476–85. doi: 10.1016/s0735-1097(99)00202-8. [DOI] [PubMed] [Google Scholar]
- 20.Carrozza JP, Jr, Kuntz RE, Fishman RF, Baim DS. Restenosis after arterial injury caused by coronary stenting in patients with diabetes mellitus. Ann Intern Med. 1993;118:344–9. doi: 10.7326/0003-4819-118-5-199303010-00004. [DOI] [PubMed] [Google Scholar]
- 21.Kastrati A, Schomig A, Elezi S, Schuhlen H, Dirschinger J, Hadamitzky M, et al. Predictive factors of restenosis after coronary stent placement. J Am Coll Cardiol. 1997;30:1428–36. doi: 10.1016/s0735-1097(97)00334-3. [DOI] [PubMed] [Google Scholar]
- 22.Kastrati A, Schomig A, Elezi S, Schuhlen H, Wilhelm M, Dirschinger J. Interlesion dependence of the risk for restenosis in patients with coronary stent placement in in multiple lesions. Circulation. 1998;97:2396–401. doi: 10.1161/01.cir.97.24.2396. [DOI] [PubMed] [Google Scholar]
- 23.Mak KH, Faxon DP. Clinical studies on coronary revascularization in patients with type 2 diabetes. Eur Heart J. 2003;24:1087–103. doi: 10.1016/s0195-668x(03)00111-8. [DOI] [PubMed] [Google Scholar]
- 24.Van Belle E, Bauters C, Hubert E, Bodart J, Abolmaali K, Meurice T, et al. Restenosis rates in diabetic patients: a comparison of coronary stenting and balloon angioplasty in native coronary vessels. Circulation. 1997;96:1454–60. doi: 10.1161/01.cir.96.5.1454. [DOI] [PubMed] [Google Scholar]
- 25.Elezi S, Kastrati A, Pache J, Wehinger A, Hadamitzky M, Dirschinger J, et al. Diabetes mellitus and the clinical and angiographic outcome after coronary stent placement. J Am Coll Cardiol. 1998;32:1866–73. doi: 10.1016/s0735-1097(98)00467-7. [DOI] [PubMed] [Google Scholar]
- 26.Schofer J, Schluter M, Rau T, Hammer F, Haag N, Mathey DG. Influence of treatment modality on angiographic outcome after coronary stenting in diabetic patients: a controlled study. J Am Coll Cardiol. 2000;35:1554–9. doi: 10.1016/s0735-1097(00)00574-x. [DOI] [PubMed] [Google Scholar]
- 27.Aronson D, Bloomgarden Z, Rayfield EJ. Potential mechanisms promoting restenosis in diabetic patients. J Am Coll Cardiol. 1996;27:528–35. doi: 10.1016/0735-1097(95)00496-3. [DOI] [PubMed] [Google Scholar]
- 28.Cutlip DE, Chauhan MS, Baim DS, Ho KK, Popma JJ, Carrozza JP, et al. Clinical restenosis after coronary stenting: perspectives from multicenter clinical trials. J Am Coll Cardiol. 2002;40:2082–9. doi: 10.1016/s0735-1097(02)02597-4. [DOI] [PubMed] [Google Scholar]
- 29.West NE, Ruygrok PN, Disco CM, Webster MW, Lindeboom WK, O’Neill WW, et al. Clinical and angiographic predictors of restenosis after stent deployment in diabetic patients. Circulation. 2004;109:867–73. doi: 10.1161/01.CIR.0000116750.63158.94. [DOI] [PubMed] [Google Scholar]
- 30.Welt FG, Edelman ER, Simon DI, Rogers C. Neutrophil, not macrophage, infiltration precedes neointimal thickening in balloon-injured arteries. Arterioscler Thromb Vasc Biol. 2000;20:2553–8. doi: 10.1161/01.atv.20.12.2553. [DOI] [PubMed] [Google Scholar]
- 31.Orford JL, Selwyn AP, Ganz P, Popma JJ, Rogers C. The comparative pathobiology of atherosclerosis and restenosis. Am J Cardiol. 2000;86:6H–11H. doi: 10.1016/s0002-9149(00)01094-8. [DOI] [PubMed] [Google Scholar]
- 32.Jonas M, Edelman ER, Groothuis A, Baker AB, Seifert P, Rogers C. Vascular neointimal formation and signaling pathway activation in response to stent injury in insulin-resistant and diabetic animals. Circ Res. 2005;97:725–33. doi: 10.1161/01.RES.0000183730.52908.C6. [DOI] [PubMed] [Google Scholar]
- 33.Morss AS, Edelman ER. Glucose modulates basement membrane fibroblast growth factor-2 via alterations in endothelial cell permeability. J Biol Chem. 2007;282:14635–44. doi: 10.1074/jbc.M608565200. [DOI] [PubMed] [Google Scholar]
- 34.Aronson D. Potential role of advanced glycosylation end products in promoting restenosis in diabetes and renal failure. Med Hypotheses. 2002;59:297–301. doi: 10.1016/s0306-9877(02)00172-x. [DOI] [PubMed] [Google Scholar]
- 35.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–72. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Zalewski A, Liu Y, Mazurek T, Cowan S, Martin JL, et al. Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation. 2003;108:472–8. doi: 10.1161/01.CIR.0000080378.96063.23. [DOI] [PubMed] [Google Scholar]
- 37.Roy S, Sala R, Cagliero E, Lorenzi M. Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc Natl Acad Sci USA. 1990;87:404–8. doi: 10.1073/pnas.87.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kornowski R, Mintz GS, Kent KM, Pichard AD, Satler LF, Bucher TA, et al. Increased restenosis in diabetes mellitus after coronary interventions is due to exaggerated intimal hyperplasia. A serial intravascular ultrasound study. Circulation. 1997;95:1366–9. doi: 10.1161/01.cir.95.6.1366. [DOI] [PubMed] [Google Scholar]
- 39.Sakaguchi T, Yan SF, Yan SD, Belov D, Rong LL, Sousa M, et al. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest. 2003;111:959–72. doi: 10.1172/JCI17115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takeda R, Suzuki E, Satonaka H, Oba S, Nishimatsu H, Omata M, et al. Blockade of endogenous cytokines mitigates neointimal formation in obese Zucker rats. Circulation. 2005;111:1398–406. doi: 10.1161/01.CIR.0000158482.83179.DB. [DOI] [PubMed] [Google Scholar]
- 41.Corpus RA, George PB, House JA, Dixon SR, Ajluni SC, Devlin WH, et al. Optimal glycemic control is associated with a lower rate of target vessel revascularization in treated type II diabetic patients undergoing elective percutaneous coronary intervention. J Am Coll Cardiol. 2004;43:8–14. doi: 10.1016/j.jacc.2003.06.019. [DOI] [PubMed] [Google Scholar]
- 42.Bayes-Genis A, Conover CA, Schwartz RS. The insulin-like growth factor axis: A review of atherosclerosis and restenosis. Circ Res. 2000;86:125–30. doi: 10.1161/01.res.86.2.125. [DOI] [PubMed] [Google Scholar]
- 43.Osanai H, Kanayama H, Miyazaki Y, Fukushima A, Shinoda M, Ito T. Usefulness of enhanced insulin secretion during an oral glucose tolerance test as a predictor of restenosis after direct percutaneous transluminal coronary angioplasty during acute myocardial infarction in patients without diabetes mellitus. Am J Cardiol. 1998;81:698–701. doi: 10.1016/s0002-9149(97)01021-7. [DOI] [PubMed] [Google Scholar]
- 44.Nishimoto Y, Miyazaki Y, Toki Y, Murakami R, Shinoda M, Fukushima A, et al. Enhanced secretion of insulin plays a role in the development of atherosclerosis and restenosis of coronary arteries: elective percutaneous transluminal coronary angioplasty in patients with effort angina. J Am Coll Cardiol. 1998;32:1624–9. doi: 10.1016/s0735-1097(98)00428-8. [DOI] [PubMed] [Google Scholar]
- 45.Piatti P, Di Mario C, Monti LD, Fragasso G, Sgura F, Caumo A, et al. Association of insulin resistance, hyperleptinemia, and impaired nitric oxide release with in-stent restenosis in patients undergoing coronary stenting. Circulation. 2003;108:2074–81. doi: 10.1161/01.CIR.0000095272.67948.17. [DOI] [PubMed] [Google Scholar]
- 46.Takagi T, Akasaka T, Yamamuro A, Honda Y, Hozumi T, Morioka S, et al. Troglitazone reduces neointimal tissue proliferation after coronary stent implantation in patients with non-insulin dependent diabetes mellitus: a serial intravascular ultrasound study. J Am Coll Cardiol. 2000;36:1529–35. doi: 10.1016/s0735-1097(00)00895-0. [DOI] [PubMed] [Google Scholar]
- 47.Choi D, Kim SK, Choi SH, Ko YG, Ahn CW, Jang Y, et al. Preventative effects of rosiglitazone on restenosis after coronary stent implantation in patients with type 2 diabetes. Diabetes Care. 2004;27:2654–60. doi: 10.2337/diacare.27.11.2654. [DOI] [PubMed] [Google Scholar]
- 48.Marx N, Wohrle J, Nusser T, Walcher D, Rinker A, Hombach V, et al. Pioglitazone reduces neointima volume after coronary stent implantation: a randomized, placebo-controlled, double-blind trial in nondiabetic patients. Circulation. 2005;112:2792–8. doi: 10.1161/CIRCULATIONAHA.105.535484. [DOI] [PubMed] [Google Scholar]
- 49.Seabra-Gomes R. Percutaneous coronary interventions with drug eluting stents for diabetic patients. Heart. 2006;92:410–9. doi: 10.1136/hrt.2005.062992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berry C, Tardif JC, Bourassa MG. Coronary heart disease in patients with diabetes: part II: recent advances in coronary revascularization. J Am Coll Cardiol. 2007;49:643–56. doi: 10.1016/j.jacc.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 51.Moussa I, Leon MB, Baim DS, O’Neill WW, Popma JJ, Buchbinder M, et al. Impact of sirolimus-eluting stents on outcome in diabetic patients: a SIRIUS (SIRolImUS-coated Bx Velocity balloon-expandable stent in the treatment of patients with de novo coronary artery lesions) substudy. Circulation. 2004;109:2273–8. doi: 10.1161/01.CIR.0000129767.45513.71. [DOI] [PubMed] [Google Scholar]
- 52.Sabate M, Jimenez-Quevedo P, Angiolillo DJ, Gomez-Hospital JA, Alfonso F, Hernandez-Antolin R, et al. Randomized comparison of sirolimus-eluting stent versus standard stent for percutaneous coronary revascularization in diabetic patients: the diabetes and sirolimus-eluting stent (DIABETES) trial. Circulation. 2005;112:2175–83. doi: 10.1161/CIRCULATIONAHA.105.562421. [DOI] [PubMed] [Google Scholar]
- 53.Hermiller JB, Raizner A, Cannon L, Gurbel PA, Kutcher MA, Wong SC, et al. Outcomes with the polymer-based paclitaxel-eluting TAXUS stent in patients with diabetes mellitus: the TAXUS-IV trial. J Am Coll Cardiol. 2005;45:1172–9. doi: 10.1016/j.jacc.2004.10.075. [DOI] [PubMed] [Google Scholar]
- 54.King SB., 3rd Is surgery preferred for the diabetic with multivessel disease?. Surgery is preferred for the diabetic with multivessel disease. Circulation. 2005;112:1500–7. doi: 10.1161/CIRCULATIONAHA.104.483339. discussion 14–5. [DOI] [PubMed] [Google Scholar]
- 55.Legrand V. Therapy insight: diabetes and drug-eluting stents. Nat Clin Pract Cardiovasc Med. 2007;4:143–50. doi: 10.1038/ncpcardio0804. [DOI] [PubMed] [Google Scholar]
- 56.Stenestrand U, James SK, Lindback J, Frobert O, Carlsson J, Schersten F, et al. Safety and efficacy of drug-eluting vs. bare metal stents in patients with diabetes mellitus: long-term of follow-up in the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) Eur Heart J. 2009 doi: 10.1093/eurheartj/ehp424. [DOI] [PubMed] [Google Scholar]
- 57.Babapulle MN, Joseph L, Belisle P, Brophy JM, Eisenberg MJ. A hierarchical Bayesian meta-analysis of randomised clinical trials of drug-eluting stents. Lancet. 2004;364:583–91. doi: 10.1016/S0140-6736(04)16850-5. [DOI] [PubMed] [Google Scholar]
- 58.Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schomig A, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937–48. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- 59.Trikalinos TA, Alsheikh-Ali AA, Tatsioni A, Nallamothu BK, Kent DM. Percutaneous coronary interventions for non-acute coronary artery disease: a quantitative 20-year synopsis and a network meta-analysis. Lancet. 2009;373:911–8. doi: 10.1016/S0140-6736(09)60319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stettler C, Allemann S, Wandel S, Kastrati A, Morice MC, Schomig A, et al. Drug eluting and bare metal stents in people with and without diabetes: collaborative network meta-analysis. BMJ. 2008;337:a1331. doi: 10.1136/bmj.a1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dibra A, Kastrati A, Mehilli J, Pache J, Schuhlen H, von Beckerath N, et al. Paclitaxel-eluting or sirolimus-eluting stents to prevent restenosis in diabetic patients. N Engl J Med. 2005;353:663–70. doi: 10.1056/NEJMoa044372. [DOI] [PubMed] [Google Scholar]
- 62.Jensen LO, Maeng M, Thayssen P, Christiansen EH, Hansen KN, Galloe A, et al. Neointimal hyperplasia after sirolimus-eluting and paclitaxel-eluting stent implantation in diabetic patients: the Randomized Diabetes and Drug-Eluting Stent (DiabeDES) Intravascular Ultrasound Trial. Eur Heart J. 2008;29:2733–41. doi: 10.1093/eurheartj/ehn434. [DOI] [PubMed] [Google Scholar]
- 63.Windecker S, Meier B. Late coronary stent thrombosis. Circulation. 2007;116:1952–65. doi: 10.1161/CIRCULATIONAHA.106.683995. [DOI] [PubMed] [Google Scholar]
- 64.Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369:667–78. doi: 10.1016/S0140-6736(07)60314-6. [DOI] [PubMed] [Google Scholar]
- 65.Pfisterer ME. Late stent thrombosis after drug-eluting stent implantation for acute myocardial infarction: a new red flag is raised. Circulation. 2008;118:1117–9. doi: 10.1161/CIRCULATIONAHA.108.803627. [DOI] [PubMed] [Google Scholar]
- 66.Luscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007;115:1051–8. doi: 10.1161/CIRCULATIONAHA.106.675934. [DOI] [PubMed] [Google Scholar]
- 67.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 68.Urban P, Gershlick AH, Guagliumi G, Guyon P, Lotan C, Schofer J, et al. Safety of coronary sirolimus-eluting stents in daily clinical practice: one-year follow-up of the e-Cypher registry. Circulation. 2006;113:1434–41. doi: 10.1161/CIRCULATIONAHA.104.532242. [DOI] [PubMed] [Google Scholar]
- 69.Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–30. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 70.Machecourt J, Danchin N, Lablanche JM, Fauvel JM, Bonnet JL, Marliere S, et al. Risk factors for stent thrombosis after implantation of sirolimus-eluting stents in diabetic and nondiabetic patients: the EVASTENT Matched-Cohort Registry. J Am Coll Cardiol. 2007;50:501–8. doi: 10.1016/j.jacc.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 71.Aoki J, Lansky AJ, Mehran R, Moses J, Bertrand ME, McLaurin BT, et al. Early stent thrombosis in patients with acute coronary syndromes treated with drug-eluting and bare metal stents: the Acute Catheterization and Urgent Intervention Triage Strategy trial. Circulation. 2009;119:687–98. doi: 10.1161/CIRCULATIONAHA.108.804203. [DOI] [PubMed] [Google Scholar]
- 72.Wiviott SD, Braunwald E, Angiolillo DJ, Meisel S, Dalby AJ, Verheugt FW, et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-Thrombolysis in Myocardial Infarction 38. Circulation. 2008;118:1626–36. doi: 10.1161/CIRCULATIONAHA.108.791061. [DOI] [PubMed] [Google Scholar]
- 73.Wenaweser P, Daemen J, Zwahlen M, van Domburg R, Juni P, Vaina S, et al. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. J Am Coll Cardiol. 2008;52:1134–40. doi: 10.1016/j.jacc.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 74.Lagerqvist B, Carlsson J, Frobert O, Lindback J, Schersten F, Stenestrand U, et al. Stent thrombosis in sweden: a report from the Swedish coronary angiography and angioplasty registry. Circ Cardiovasc Interv. 2009;2:401–8. doi: 10.1161/CIRCINTERVENTIONS.108.844985. [DOI] [PubMed] [Google Scholar]
- 75.Rask-Madsen C, King GL. Mechanisms of Disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:46–56. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- 76.Colwell JA, Nesto RW. The platelet in diabetes: focus on prevention of ischemic events. Diabetes Care. 2003;26:2181–8. doi: 10.2337/diacare.26.7.2181. [DOI] [PubMed] [Google Scholar]
- 77.King SB, 3rd, Smith SC, Jr, Hirshfeld JW, Jr, Jacobs AK, Morrison DA, Williams DO, et al. 2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee. Circulation. 2008;117:261–95. doi: 10.1161/CIRCULATIONAHA.107.188208. [DOI] [PubMed] [Google Scholar]
- 78.The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med. 1996;335:217–25. doi: 10.1056/NEJM199607253350401. [DOI] [PubMed] [Google Scholar]
- 79.The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol. 2007;49:1600–6. doi: 10.1016/j.jacc.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 80.King SB, 3rd, Kosinski AS, Guyton RA, Lembo NJ, Weintraub WS. Eight-year mortality in the Emory Angioplasty versus Surgery Trial (EAST) J Am Coll Cardiol. 2000;35:1116–21. doi: 10.1016/s0735-1097(00)00546-5. [see comments] [DOI] [PubMed] [Google Scholar]
- 81.Abizaid A, Costa MA, Centemero M, Abizaid AS, Legrand VM, Limet RV, et al. Clinical and economic impact of diabetes mellitus on percutaneous and surgical treatment of multivessel coronary disease patients: insights from the Arterial Revascularization Therapy Study (ARTS) trial. Circulation. 2001;104:533–8. doi: 10.1161/hc3101.093700. [DOI] [PubMed] [Google Scholar]
- 82.Serruys PW, Ong AT, van Herwerden LA, Sousa JE, Jatene A, Bonnier JJ, et al. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol. 2005;46:575–81. doi: 10.1016/j.jacc.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 83.Banning AP, Westaby S, Morice MC, Kappetein AP, Mohr FW, Berti S, et al. Diabetic and Nondiabetic Patients With Left Main and/or 3-Vessel Coronary Artery Disease Comparison of Outcomes With Cardiac Surgery and Paclitaxel-Eluting Stents. J Am Coll Cardiol. 2010 doi: 10.1016/j.jacc.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 84.Hlatky MA, Boothroyd DB, Bravata DM, Boersma E, Booth J, Brooks MM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet. 2009;373:1190–7. doi: 10.1016/S0140-6736(09)60552-3. [DOI] [PubMed] [Google Scholar]
- 85.Kapur A, Hall R, Malik I, Qureshi A, Butts J, de Belder M, et al. Randomized Comparison of Percutaneous Coronary Intervention With Coronary Artery Bypass Grafting in Diabetic Patients: 1-Year Results of the CARDia (Coronary Artery Revascularization in Diabetes) Trial. J Am Coll Cardiol. 2010;55:432–40. doi: 10.1016/j.jacc.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 86.Chaitman BR, Hardison RM, Adler D, Gebhart S, Grogan M, Ocampo S, et al. The bypass angioplasty revascularization investigation 2 diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation. 2009;120:2529–40. doi: 10.1161/CIRCULATIONAHA.109.913111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–15. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 89.Gum P, O’Keefe JJ, Borkon A, Spertus J, Bateman T, McGraw J, et al. Bypass surgery versus coronary angioplasty for revascularization of treated diabetic patients. Circulation. 1997;96:II–II710. [PubMed] [Google Scholar]
- 90.Detre KM, Lombardero MS, Brooks MM, Hardison RM, Holubkov R, Sopko G, et al. The effect of previous coronary-artery bypass surgery on the prognosis of patients with diabetes who have acute myocardial infarction. Bypass Angioplasty Revascularization Investigation Investigators. N Engl J Med. 2000;342:989–97. doi: 10.1056/NEJM200004063421401. [DOI] [PubMed] [Google Scholar]
- 91.Alderman EL, Corley SD, Fisher LD, Chaitman BR, Faxon DP, Foster ED, et al. Five-year angiographic follow-up of factors associated with progression of coronary artery disease in the Coronary Artery Surgery Study (CASS). CASS Participating Investigators and Staff. J Am Coll Cardiol. 1993;22:1141–54. doi: 10.1016/0735-1097(93)90429-5. [DOI] [PubMed] [Google Scholar]
- 92.Kip KE, Alderman EL, Bourassa MG, Brooks MM, Schwartz L, Holmes DR, Jr, et al. Differential influence of diabetes mellitus on increased jeopardized myocardium after initial angioplasty or bypass surgery: bypass angioplasty revascularization investigation. Circulation. 2002;105:1914–20. doi: 10.1161/01.cir.0000014967.78190.bb. [DOI] [PubMed] [Google Scholar]
- 93.Schwartz L, Kip KE, Frye RL, Alderman EL, Schaff HV, Detre KM. Coronary bypass graft patency in patients with diabetes in the Bypass Angioplasty Revascularization Investigation (BARI) Circulation. 2002;106:2652–8. doi: 10.1161/01.cir.0000038885.94771.43. [DOI] [PubMed] [Google Scholar]
- 94.Hoogwerf BJ, Waness A, Cressman M, Canner J, Campeau L, Domanski M, et al. Effects of aggressive cholesterol lowering and low-dose anticoagulation on clinical and angiographic outcomes in patients with diabetes: the Post Coronary Artery Bypass Graft Trial. Diabetes. 1999;48:1289–94. doi: 10.2337/diabetes.48.6.1289. [DOI] [PubMed] [Google Scholar]
- 95.Boden WE, Taggart DP. Diabetes with coronary disease—a moving target amid evolving therapies? N Engl J Med. 2009;360:2570–2. doi: 10.1056/NEJMe0904090. [DOI] [PubMed] [Google Scholar]
- 96.Daemen J, Kuck KH, Macaya C, LeGrand V, Vrolix M, Carrie D, et al. Multivessel coronary revascularization in patients with and without diabetes mellitus: 3-year follow-up of the ARTS-II (Arterial Revascularization Therapies Study-Part II) trial. J Am Coll Cardiol. 2008;52:1957–67. doi: 10.1016/j.jacc.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 97.Smith SC, Jr, Feldman TE, Hirshfeld JW, Jr, Jacobs AK, Kern MJ, King SB, 3rd, et al. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention–summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention) Circulation. 2006;113:156–75. doi: 10.1161/CIRCULATIONAHA.105.170815. [DOI] [PubMed] [Google Scholar]
- 98.Flaherty JD, Davidson CJ. Diabetes and coronary revascularization. JAMA. 2005;293:1501–8. doi: 10.1001/jama.293.12.1501. [DOI] [PubMed] [Google Scholar]
- 99.Young LH, Wackers FJ, Chyun DA, Davey JA, Barrett EJ, Taillefer R, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009;301:1547–55. doi: 10.1001/jama.2009.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Farkouh ME, Dangas G, Leon MB, Smith C, Nesto R, Buse JB, et al. Design of the Future REvascularization Evaluation in patients with Diabetes mellitus: Optimal management of Multi-vessel disease (FREEDOM) Trial. Am Heart J. 2008;155:215–23. doi: 10.1016/j.ahj.2007.10.012. [DOI] [PubMed] [Google Scholar]