Abstract
Myofibrillogenesis in striated muscles is a highly complex process that depends on the coordinated assembly and integration of a large number of contractile, cytoskeletal, and signaling proteins into regular arrays, the sarcomeres. It is also associated with the stereotypical assembly of the sarcoplasmic reticulum and the transverse tubules around each sarcomere. Three giant, muscle-specific proteins, titin (3–4 MDa), nebulin (600–800 kDa), and obscurin (~720–900 kDa), have been proposed to play important roles in the assembly and stabilization of sarcomeres. There is a large amount of data showing that each of these molecules interacts with several to many different protein ligands, regulating their activity and localizing them to particular sites within or surrounding sarcomeres. Consistent with this, mutations in each of these proteins have been linked to skeletal and cardiac myopathies or to muscular dystrophies. The evidence that any of them plays a role as a “molecular template,” “molecular blueprint,” or “molecular ruler” is less definitive, however. Here we review the structure and function of titin, nebulin, and obscurin, with the literature supporting a role for them as scaffolding molecules and the contradictory evidence regarding their roles as molecular guides in sarcomerogenesis.
I. INTRODUCTION
Myofibrillogenesis is a highly complex process that depends on the coordinated assembly and integration of a number of contractile, cytoskeletal, and signaling proteins into regular arrays, the sarcomeres (321–324). Three giant, muscle-specific proteins, titin (3–4 MDa), nebulin (600–800 kDa), and obscurin (~720–900 kDa) (76, 83, 209, 218, 296), play key roles in organizing sarcomeres.
Titin is the third most abundant muscle protein, after actin and myosin. Remarkably, a single titin molecule spans half the sarcomere, anchoring its NH2 and COOH termini in the Z-disk and M-band, respectively (99). Titin is modular in structure: ~90% of its mass consists of repeating immunoglobulin-C2 (Ig-C2) and fibronectin-III (Fn-III) domains that provide binding sites for diverse myofibrillar proteins, including myosin, actin, α-actinin, T-cap/telethonin, myomesin, myosin binding protein-C (MyBP-C), and obscurin (325). As ~10% of its mass consists of nonrepetitive sequences that include phosphorylation motifs, binding sites for muscle-specific calpain proteases and other proteins, and a COOH-terminal Ser/Thr kinase domain, titin may also be involved in signal transduction from the myofibrils to other compartments of the myoplasm, including the nucleus (309, 362). In addition to its role as a signaling molecule and a molecular scaffold, titin has been proposed to serve two major functions: as a “molecular blueprint” that specifies and coordinates the precise assembly of many of the structural, regulatory, and contractile proteins that compose the sarcomere, and as a “molecular spring” that gives striated muscle its distinct biomechanical properties and integrity during contraction, relaxation, and stretch (121–123, 209).
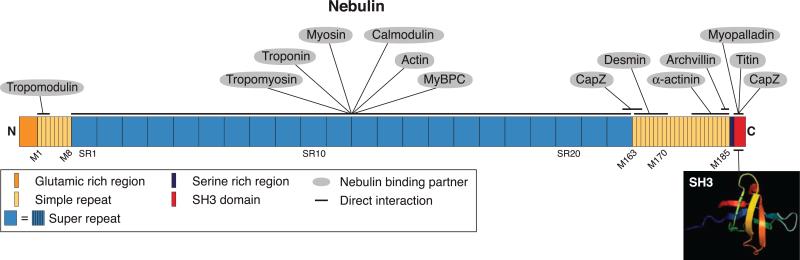
Nebulin is a giant actin-binding protein of vertebrate striated muscle. In situ, a single molecule is incorporated and is coextensive with the thin filaments of α-actin that form the I-band and interact with myosin to produce contraction (76, 149). Nebulin is ~1.0 μm in length. Its NH2 terminus extends to the pointed ends of thin filaments, whereas its COOH terminus is partially inserted into the Z-disks. Most of its mass (~97%) is composed of ~185 modular, 35-amino acid repeats (166). The central 154 modules are organized into 22 super-repeats of 7 modules each, the organization of which complements the periodicity of the actin filaments (245). Consistent with this, nebulin isoforms of different sizes, generated by alternative splicing of a single transcript, correspond to the various sizes of thin filaments present in developing and adult muscle fibers. The extreme COOH-terminal end of nebulin contains a Ser-rich region with multiple phosphorylation sites and an SH3 domain that binds to myopalladin, a Z-disk protein. In addition to its lateral interactions with actin, nebulin contains distinct sites at its NH2 terminus that interact with two actin-associated proteins, tropomyosin and troponin I/C/T, and the thin filament capping protein tropomodulin, providing a mechanism for terminating the growth of the actin filaments at precisely the length of nebulin (186, 239). Thus nebulin and titin associate with, and help to organize, the key structures required for contraction of striated muscle.
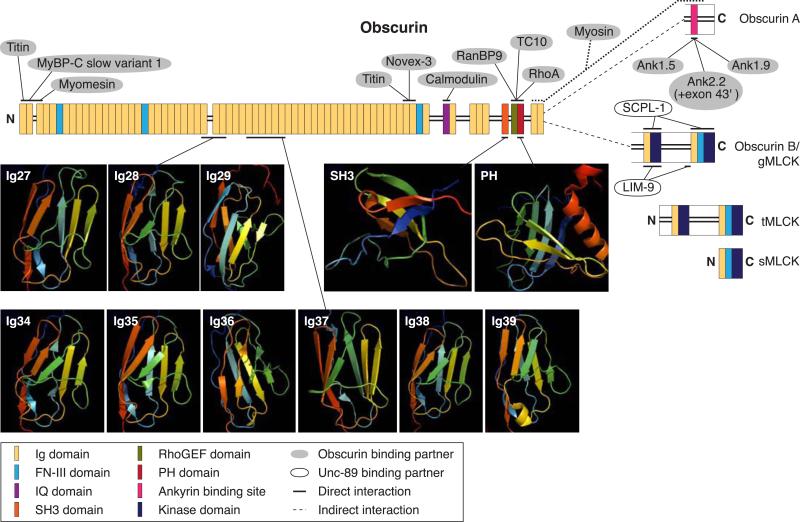
Obscurin is the third giant protein of the contractile apparatus identified in vertebrate striated muscle (19, 320, 421). Like titin, obscurin is a multidomain protein composed of adhesion modules and signaling domains arranged mostly in tandem. Specifically, its NH2 terminus contains 54 Ig-C2 and 2 FN-III domains, followed by an IQ motif and a conserved SH3 domain adjacent to Rho-guanine nucleotide exchange factor (Rho-GEF) and pleckstrin homology (PH) domains. The COOH-terminal end of the protein consists of two additional Ig domains followed by a nonmodular region of ~420 amino acid residues that contains several copies of a consensus phosphorylation motif for ERK kinases, similar to that found in the NH2-terminal region of titin. The obscurin gene, obscurin-MLCK, also encodes two Ser/Thr kinase domains (85, 320). Although these are found at the COOH-terminal region of a ~900-kDa form of obscurin, they are more commonly expressed as smaller, alternatively spliced products, mainly in heart. Unlike titin and nebulin, which are integral components of sarcomeres, obscurin is not present within sarcomeres but intimately surrounds them, primarily at the level of the Z-disk and M-band, where it is appropriately positioned to participate in their assembly and integration with other sarcoplasmic elements (181). Consistent with this, obscurin, too, interacts with diverse protein partners located in distinct compartments within the cell, including small ankyrin 1, an integral component of the sarcoplasmic reticulum (SR) membranes, as well as titin, sarcomeric myosin, and MyBP-C slow (176). Given its ability to associate tightly, selectively and periodically with the periphery of the myofibril and with thick filaments, obscurin is ideally suited to coordinate the assembly and organization of the SR with myofibrillar elements in the middle of the sarcomere.
Collectively, the unique structural properties and subcellular locations of titin, nebulin, and obscurin suggest that they may function as molecular scaffolds during myofibrillogenesis by facilitating the integration of actin and myosin filaments into sarcomeres, providing binding sites for a plethora of sarcomeric proteins and coordinating the sarcomeric alignment of nearby structures, like the SR. In addition, both titin and obscurin are likely to play important roles in signaling cascades that control homeostasis and muscle gene expression. Consistent with these central roles in muscle physiology and development, all three giant proteins have been linked, either directly or indirectly, to several forms of cardiomyopathies and muscular dystrophies (10, 103, 135, 142, 293, 382, 386).
II. TITIN
Titin (also known as connectin) represents the third filamentous system in striated muscle cells (191, 230–234, 390), after the thin and thick filaments, composed of actin and myosin, respectively. It is encoded by a single gene (TTN) that is localized to a 294-kb region on the long arm of chromosome 2 in both human and mouse and contains 363 exons, which undergo extensive alternative splicing (19, 190). Titin is a giant protein (~3–4 MDa) that extends from the Z-disk to the M-band within the sarcomere, which it helps to organize. It is highly modular: ~90% of its mass consists of repeating immunoglobulin (Ig) and fibronectin-III (FN-III) domains. Specifically, it contains 244 recognizable β-sheet domains of which 112 have been assigned to the immunoglobulin superfamily and 132 to the fibronectin type III superfamily (Table 1) (93, 190, 191). The Ig repeats were initially believed to belong to the C2 type, but later they were shown to share considerable similarities with the V type of Ig repeats present in telokin (363). Both Ig and FN-III domains provide binding sites for diverse proteins, including myofibrillar and membrane components, as well as enzymes and signaling molecules. The remaining ~10% of titin's mass consists of 17 unique, nonrepetitive sequence motifs situated between the Ig and FN-III modules, that contain several phosphorylation sites, 28–30 residue PEVK motifs, and a COOH-terminal Ser/Thr kinase domain (95, 98, 121, 126–128, 134, 173, 329). Titin filaments with opposite polarity overlap in both Z-disks and M-bands, forming a contiguous system within myofibrils.
TABLE 1.
Structures of domains of titin, nebulin, and obscurin
| Domain | Method of Structure Solution | Reference Nos. |
|---|---|---|
| Titin | ||
| Ig1-2 | X-ray diffraction | 229 |
| Ig1-2 bound to telethonin | X-ray diffraction | 303, 428 |
| Zr7 bound to α-actinin | NMR | 15 |
| I-Ig1 | X-ray diffraction | 237 |
| I-Ig27 | NMR | 155 |
| I-Ig27 | X-ray diffraction | http://www.rcsb.org/pdb/home/home.do |
| I-Ig67-Ig69 | X-ray diffraction | 380 |
| I-Ig65-Ig70 | X-ray diffraction | 380 |
| A-Ig71 | NMR | 109 |
| A-Ig168-Ig169 | X-ray diffraction | 250, 253 |
| A-Ig168-Ig170 | X-ray diffraction | 249 |
| Kinase domain | X-ray diffraction | 236 |
| M-Ig1 | X-ray diffraction | http://www.rcsb.org/pdb/home/home.do |
| M-Ig5 | NMR | 299, 300 |
| Nebulin | ||
| SH3 domain | NMR | 305 |
| Obscurin | ||
| Ig27 | NMR | http://www.rcsb.org/pdb/home/home.do |
| Ig28 | NMR | http://www.rcsb.org/pdb/home/home.do |
| Ig29 | NMR | http://www.rcsb.org/pdb/home/home.do |
| Ig34 | NMR | http://www.rcsb.org/pdb/home/home.do |
| Ig35 | NMR | http://www.rcsb.org/pdb/home/home.do |
| Ig36 | NMR | http://www.rcsb.org/pdb/home/home.do |
| Ig37 | NMR | http://www.rcsb.org/pdb/home/home.do |
| Ig38 | NMR | http://www.rcsb.org/pdb/home/home.do |
| Ig39 | NMR | http://www.rcsb.org/pdb/home/home.do |
| SH3 | NMR | http://www.rcsb.org/pdb/home/home.do |
| PH | NMR | 31 |
A. Titin at the Z-Disk: Ligands and Functional Implications
1. Molecular composition of the Z-disk portion of titin
The extreme NH2 terminus of titin contains the first ~90 kDa of the protein and includes amino acids 1–826 (Fig. 1) (96, 130, 132, 251, 339, 422). Detailed immunological studies have postulated that 1) the first 200 amino acids of titin reside at the periphery of the Z-disk (referred to as “Z-line titin edge residues”), where they mark the edge of the Z-line region; 2) amino acids 201–750 span the entire width of the Z-disk (referred to as “Z-line titin integrative residues”), consistent with the idea that titin filaments from neighboring sarcomeres fully overlap within the Z-disk lattice in an antiparallel manner; and 3) residues 751–826 are located at the junction of the Z-disk with the I-band (referred to as “Z/I junction residues”).
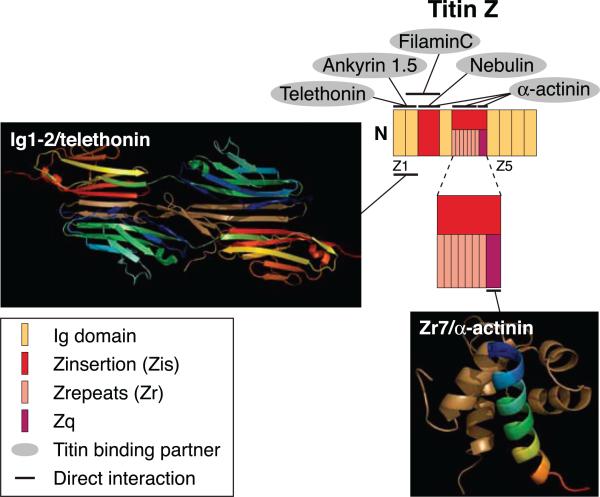
FIG. 1.
Schematic representation of titin at the level of the Z-disk, illustrating its domain orientation and the structure of some of its domains, as well as identifying its binding partners. Exons 1–27 of the TTN gene code for the Z-disk portion of the titin. This region is composed of seven Ig domains and two Z insertions (Zis) that are unique to titin and flank the third Ig domain. The second Z insertion is comprised of 7 Z repeats (Zr) that can be alternatively spliced, and a Zq region (see key for complete domain list and color coding). Proteins that bind to titin in this region are indicated at their sites of interaction. Structures of the complexes formed by two of the protein ligands, T-cap/telethonin with the two NH2-terminal Ig domains, and α-actinin with the Zq domain, are shown as ribbon diagrams, with tan representing the ligands and other colors representing their binding regions on titin.
At the molecular level, the Z-disk region of titin shows a complex pattern of Ig motifs and large interdomain insertions (Fig. 1). It is divided into three subdomains based on their molecular features and proposed functional activities. The extreme NH2 terminus (Z-line titin edge residues 1–200) encompasses the first two Ig repeats, ZIg1 and ZIg2, which are constitutively expressed in all titin isoforms identified to date. Each of these domains contains ~100 amino acid residues folded in a β-sheet “sandwich.” The first unique interdomain segment or Zinsertion-1 (Zis-1) follows ZIg1 and ZIg2. Zis-1 contains amino acids 201–430 and includes several copies of the SPXR phosphorylation motif as well as multiple Ser/Pro sites that can be phosphorylated by cell cycle-dependent proline-directed kinases, including cdc2 and ERK (17, 96, 330). Adjacent to Zis-1 is the third Ig domain of titin (ZIg3), which precedes the second interdomain segment or Zinsertion-2 (Zis-2).
Zis-2 is predicted to be structured as extended β-sheet and α-helical elements connected alternately in series (132). It encompasses amino acids 431–700 and shows a complex pattern of alternative splicing (96, 339). Zis2 contains a variable copy number of a 45-amino acid repeat, called the Z-repeat (Zr), which shows no homology to any other protein motif known to date (96, 132, 422, 423). Z-repeats extend longitudinally within the Z-disk lattice, are enriched in charged and hydrophobic residues arranged in clusters, and do not contain any aromatic amino acids. They form two subgroups: the first one contains the two invariant flanking repeats (Zr1 and Zr7), which are highly homologous to each other and are shared by all titin isoforms; the second subgroup includes the central repeats (Zr2-Zr6), which are more divergent in sequence and are differentially expressed (96, 132, 292, 415, 422). The copy number of the Z-repeats can vary from 2 to 7 depending on the type of striated muscle, the developmental stage, and the species studied. Consistent with this, the adult human heart contains seven copies of the Zr motif, whereas the fetal human heart contains a mixture of isoforms, with the most prominent carrying four Z-repeats (339). The adult chicken heart possesses only six Z-repeats, lacking Zr4 present in mammalian titins (17), while the simple Z-disk of avian fast-twitch muscle, which has a “zig-zag” appearance, contains only two Zrs (278, 279). In the fast-twitch psoas muscle of the rabbit, the three central repeats, Zr4 to Zr6, are excluded by exon skipping, whereas in the slow-twitch muscles of soleus and tongue, a mixture of isoforms carrying either four (Zr1, Zr2, Zr3, and Zr7) or six (Zr4 is excluded) Z-repeats has been identified (96, 292).
The structure of the Z-disk varies considerably in thickness among different striated muscles, indicating that the number of its constituent proteins is regulated in a way that depends on the fiber type. Fast muscle fibers typically have thin Z-disks, whereas slow muscle fibers have thick Z-disks. The complexity and compactness of the Z-disk have been recently attributed, at least in part, to titin (96, 132, 292). In particular, the differential expression of Z-repeats is an important determinant of the architecture, thickness, and biomechanical properties of the Z-disk, as fibers with narrow Z-disks contain titin isoforms with a small number of Z-repeats, and fibers with thick Z-disks contain titin isoforms with more Z-repeats.
COOH terminal to the Z-repeats lies the Zq region of titin, which is within the second interdomain of titin, or Zis-2, but is distinct from the Z-repeats. Zq is nonmodular in structure, contains amino acids 750–826, which are located at the junction of the Z-disk with the I-band, and is immediately adjacent to the fourth Ig domain of titin (ZIg4) that precedes ZIg5-ZIg7, which are arranged in tandem.
2. Ligands of titin at the Z-disk
A combination of molecular and biochemical experiments has demonstrated that the Z-disk portion of titin interacts with both myofibrillar and membrane-associated proteins (Fig. 1, Table 2). This suggests that it may play an essential role in sarcomeric stability and maintenance as well as contributing to the assembly of the contractile apparatus and associated systems of intracellular membranes.
Table 2.
Titin binding partners
| Protein | Region of Binding Partner | Region of Titin | Reference Nos. |
|---|---|---|---|
| Titin-Z region | |||
| T-Cap/telethonin | ZIg1-ZIg2 | 132, 251 | |
| Small ankyrin 1 | Residues 61-89 | ZIg1-ZIg2 | 177 |
| FilaminC | FLINIG23-FLINIG24 | ZIg2-Zis1 | 196 |
| α-Actinin | COOH-terminal 10 kDa | Zr1-Zr7 | 339, 422 |
| Rod domain (repeats slr2-slr3) | Zq sequence (residues 760–826) | 422 | |
| COOH-terminal 70 kDa | Zis1 | 196 | |
| Nebulin | SH3 domain | Zis1 (Pro-rich region) | 196 |
| Titin-I region | |||
| Actin | I-Ig domains | 213, 215, 261, 361 | |
| PEVK | |||
| Tropomyosin | N1 line (Z/I junction) | 313 | |
| PEVK/N2 line | |||
| PKA | N2B | 419 | |
| PKG | N2B and N2A | 183 | |
| Ca2+ | PEVK (E-rich motif) | 418 | |
| S100A1 | PEVK | 418 | |
| Nebulin | SH3 domain | PEVK | 94, 305 |
| Obscurin | Ig48-Ig49 | ZIg9-ZIg10 | 421 |
| αB-Crystallin | N2B region-IIg27 | 108 | |
| DRAL/FHL-2 | N2B | 197 | |
| FHL-1 | N2B | 332 | |
| Calpain-1 | ZIg8-IIg5 | 314 | |
| PEVK and flanking regions | |||
| Calpain3/p94 | Inserted domain 2 | Ig83 of N2A | 341 |
| MARP family (CARP, ankrd-2/Arpp, DARP) | Second ankyrin repeat | Sequence between IgI80 and IgI81 | 244 |
| Titin- A/M region | |||
| Myosin | LMM | FN-III domains of A band | 158 |
| S1 | |||
| MyBP-C | IgC8-IgC10 | Super repeat 2 (the first Ig domain of each repeat) | 90 |
| MuRF-1 | Middle 144 amino acids | IgA168-IgA169 | 49 |
| MuRF-2 | IgA164-IgA169 | 304 | |
| Calmodulin | Kinase domain | 94 | |
| Nbr1/p62 | NH2-terminal phox/bem1p (PB1) motif | Kinase domain | 198 |
| DRAL/FHL2 | Central 270 amino acids of N2B | 197 | |
| Is2 | |||
| Myomesin | FN-III4-FN-III6 | IgM4 | 274 |
| P94/calpain-3 | IgM9-Mis7 | 341 | |
| Obscurin | Ig1 | IgM10 | 86 |
| M-protein | 276 | ||
A) TITIN-CAP (T-CAP)/TELETHONIN
A yeast two-hybrid assay in which the extreme NH2-terminal ZIg1 and ZIg2 repeats of titin were used as “bait” identified Titin-cap or T-cap, also known as telethonin (370), as one of their binding partners (132, 251). T-cap/telethonin is a ~19-kDa protein that is specifically expressed in heart and skeletal muscle. It localizes at the periphery of the Z-disk in close proximity with the NH2-terminal ZIg1 and ZIg2 repeats of titin. No apparent structural motifs have been identified for the NH2-terminal 140 amino acids of T-cap, which contain the binding site for the titin ZIg1 and ZIg2 repeats. Its COOH terminus contains a domain of 27 amino acids that is rich in Ser/Pro and basic residues, with several consensus phosphorylation motifs for Ser/Pro-dependent kinases (132, 251, 370, 428). Although it is not clear how they associate, the COOH terminus of T-cap/telethonin can be phosphorylated in vitro by the serine/threonine kinase domain of titin, located at the M-band (236).
Recent crystallographic studies demonstrated a (2: 1)2 molar ratio for the titin/T-cap complex (429). In particular, they showed that the NH2-terminal 90 amino acids of T-cap/telethonin can cross-link two titin molecules in antiparallel orientation through two sets of palindromic sequences organized as β-sheets, which interact with the first two Ig domains of adjacent titin molecules. This results in an unusual, asymmetric structure in which the four Ig domains of titin in the complex are linked by two equivalent β-sheets, but only one of the two gaps between them is occupied by a globular portion of T-cap/telethonin. The structure of the COOH-terminal 77 residues of T-cap/telethonin cannot be detected in crystals but appears to promote the formation of dimeric complexes of T-cap and the two terminal Ig domains of titin (303). As it does not target specifically to the Z-disk, the COOH-terminal region of T-cap/telethonin is not likely to have a high affinity for the domains of titin found in this structure, so its role in promoting additional oligomerization in vivo is unclear. In contrast, the NH2-terminal 90 residues of T-cap/telethonin associate avidly with Z-disks in situ, consistent with their ability to bind in a tight complex with the Z-disk region of titin (429). The orientation of the pair of titin molecules that complex with T-cap/telethonin in situ cannot be determined directly. Nevertheless, Zou et al. (429) argue that the close alignment of titin filaments in other regions of the sarcomere suggests that they arise from the same sarcomere and overlap at the boundary of the Z-disk, rather than from neighboring sarcomeres (429). In this case, it is difficult to envision how the Ig domains of titin, located at the Z-disk, that interact with α-actinin (see below) can partition to the interior of the Z-disk. This model is also inconsistent with the idea that T-cap/telethonin acts as a molecular “bolt” to align the NH2-terminal domains of titin with respect to the Z-disk and to reinforce the links between adjacent sarcomeres (132).
Although the functional significance of the interaction of T-cap with titin is still elusive, overexpression studies of the binding domains of the two proteins in primary cultures of cardiomyocytes have indicated that it may be essential for sarcomeric assembly and integrity and have further suggested that T-cap may help to anchor the NH2 terminus of the titin filament to the Z-disk (132). As T-cap/telethonin is only expressed at later stages of myofibril formation, after primitive Z-bodies have been transformed to mature, regularly spaced Z-disks, its binding to titin probably does not play a role in early sarcomerogenesis, but instead is more likely to contribute to the structural integrity and maintenance of Z-disks after they have formed.
B) SMALL ANKYRIN 1
In addition to binding T-cap, the two NH2-terminal Ig domains of titin interact specifically and directly with small ankyrin 1 (sAnk1) (177). sAnk1 is an integral component of the network compartment of the SR that is organized in register with Z-disks and M-bands (426). Detailed molecular and biochemical studies demonstrated that a 29-amino acid-long peptide (residues 61–89) in the cytoplasmic, hydrophilic tail of sAnk1 contains the minimal sequence that mediates binding to titin ZIg1/ZIg2. Similar to T-cap, both ZIg1 and ZIg2 domains of titin are required for binding to sAnk1 (177). The direct and specific association of the extreme NH2 terminus of titin with sAnk1 suggested a role for these proteins in coordinating the assembly of the contractile apparatus with the network SR that surrounds the myofibrillar Z-disk. T-cap and sAnk1 can simultaneously bind to titin ZIg1/ZIg2 in vitro (177). Thus titin, T-cap, and sAnk1 may form a three-way complex at the periphery of the Z-disk. T-cap has also been shown to bind to MinK, the β-subunit of the potassium channel of the transverse tubular membranes (t-tubules) (91). Consequently, just as sAnk1 may link titin at the periphery of the Z-disk to the network compartment of the SR membranes, T-cap may link titin in this same region to the t-tubules of cardiac muscle. Thus titin may serve as a scaffold for the coordinated assembly of the sarcomere, the SR, and the t-tubules, through its ability to interact simultaneously with sAnk1 and T-cap.
C) α-ACTININ
α-Actinin is the major component of the Z-disk and titin's most prominent ligand there. α-Actinin is a member of the spectrin gene superfamily and contains three major structural domains: an NH2-terminal actin-binding motif, a central rod domain composed of four spectrin-like repeats (slr), and a COOH-terminal domain with EF-hand structures (25). α-Actinin forms homodimers through the antiparallel association of the rod domains, which can then cross-link actin and titin filaments from adjacent sarcomeres, via their NH2 and COOH termini, respectively.
A number of groups have characterized the α-actinin binding site(s) on titin, although not always with the same results (14, 96, 165, 279, 292, 339, 422). Taken together, however, the data indicate that titin binds to α-actinin in three distinct ways. The first involves the titin Z-repeats, Zr1-Zr7, which provide binding sites for the extreme COOH-terminal 10 kDa of α-actinin. The different Zr motifs show distinct binding affinities for α-actinin. In particular, the highly homologous flanking Zr1 and Zr7 repeats interact more strongly with the COOH terminus of α-actinin and may bind independently (339), whereas the central repeats (Zr2-Zr6), which are differentially expressed, act together in groups of two or more Zrs, to provide additional, although significantly weaker, binding sites (422).
A second α-actinin binding site is immediately downstream of the Z-repeats, in the nonmodular Zq sequence of titin and involves amino acids 760–826 (422). This site binds to the rod domain of α-actinin, specifically to its two central spectrin repeats (slr 2 and slr 3). Binding requires both spectrin repeats, suggesting that each contributes to the binding site. A third binding site for the COOH-terminal 70-kDa portion of α-actinin was recently identified in titin's Zis domain in a yeast two-hybrid assay (196). A detailed biochemical characterization of this interaction has not yet been completed, so little is known about the specificity of this binding and its potential regulation.
Several studies have proposed that the variable number of Z-repeats and their distinct binding affinities for the COOH terminus of α-actinin may influence the structural properties of Z-disks from different muscle types (96, 223, 224, 279, 339). This in turn may control the ability of Z-disks to respond to mechanical stress, through dynamic alterations of their lattice, and to withstand and transmit different levels of tension. Moreover, the fact that all Z-repeats are able to bind α-actinin, albeit with different affinities, suggests that they may function in a cooperative manner. If this is true, then the number of Z-repeats may determine the number and spacing of α-actinin cross-links within the Z-disk lattice. This idea was challenged by ultrastructural and biochemical studies that indicated that a one-to-one correspondence of Z-repeats and α-actinin cross-links is not possible (225, 422). Consequently, Young et al. (422) proposed that the maximal number of cross links would be the highest integer of half the number of Z-repeats, and Luther and Squire (225) postulated that two Z-repeats per cross-link is a more likely arrangement. Additional experimentation is needed to assess the quantitative relationship between the number of Z-repeats and α-actinin cross-links.
The interaction between α-actinin and the Z-repeat modules of titin is conformationally regulated (423). Downstream of its actin-binding domain (ABD), α-actinin contains a 30-residue peptide that is highly homologous to the Z-repeats of titin. When an α-actinin homodimer is in a “closed” or “inactive” conformation, this 30-residue pseudoligand of one α-actinin molecule binds to the COOH-terminus of a neighboring α-actinin with apparent nanomolar affinity, preventing binding to titin. This inhibition is relieved upon binding of phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2], an anionic phospholipid present at Z-disks, that binds to the ABD of α-actinin (82, 269). Binding of PI(4,5)P2 to the ABD induces a conformational change in the α-actinin homodimer that renders the COOH termini of the two α-actinin subunits available to bind to titin's Z-repeats (423). This autoinhibition may provide a regulatory mechanism that controls the local association of α-actinin and titin during the assembly and turnover of myofibrils. Contrary to the binding of the Z-repeats to the COOH terminus of α-actinin, the interaction between Zq and the rod domain of α-actinin is constitutive and independent of phospholipids.
Two obvious questions that emerge from these studies are whether titin's three binding sites for α-actinin function cooperatively or competitively, and which are required for the formation of Z-disks during sarcomerogenesis. Further experimentation will be needed to answer these questions and to decipher the role of each one of the binding sites in the assembly and maintenance of Z-disks.
D) OBSCURIN
The peripheral Z-disk region of titin contains a binding site for obscurin (19, 421). Obscurin is a giant myofibrillar protein that contains adhesion (mainly Ig and FN-III domains) and signaling (IQ, Src-homology-3 or SH3, guanine nucleotide exchange factor or GEF, and pleckstrin homology or PH motifs) modules arranged in tandem (421) (see below). Titin Ig motifs 9 and 10 bind specifically to obscurin Ig repeats 48 and 49 with nanomolar affinity (421). Additionally, a unique stretch of 198 amino acid residues located NH2-terminally to Ig repeat 21 of the I-band portion of Novex-3, a truncated ~700-kDa splice variant of titin that extends from the Z-disk to the I-band, also binds to obscurin Ig domains 48 and 49 (19). The functional significance of the interaction between titin and obscurin is speculative at this time. One possibility is that their binding may directly link signaling pathways regulated by Ca2+, SH3 domains, and small GTPases with the formation of new myofibrils. Alternatively, their association may modulate sarcomeric restructuring linked to stress or contractile activity during normal development and adulthood as well as in disease states.
E) FILAMIN C AND NEBULIN
The actin binding proteins, filamin C and nebulin, also bind titin's ZIg2-Zis1 region in yeast two-hybrid screens, as well as in vitro (196). The last two Ig domains of filamin C, FLNIG23 and FLNIG24, are necessary and sufficient for binding to titin's ZIg2-Zis1 fragment, whereas the COOH-terminal SH3 domain of nebulin contains the binding activity for a 26-residue, proline-rich sequence in titin's Zis1 domain (196, 404).
Similarly to titin, filamin C is expressed during early stages of myofibrillar assembly and is targeted to distinct compartments within the cell, including the Z-disk and the sarcolemma (357, 377). Thus titin and filamin C may coassemble early in myogenesis, and their interaction may specify sites where membrane-myofibrillar connections are established. Since titin also interacts with sAnk1 and the T-cap/Mink complex, it may coordinate the alignment of sarcomeric structures with internal membranes as well as with specialized sarcolemmal domains in developing muscle.
The interaction of titin and nebulin at the level of the Z-disk is also intriguing. Recent in vivo studies of nebulindeficient mice have suggested that nebulin may contribute to the architecture of the Z-disk (21, 404). Given the extensive diversity of alternative splicing of the Z-disk regions of nebulin and titin (see below), which appears to correlate with the thickness of the Z-disk, as well as their ability to associate directly at the Z-disk, these two giant proteins may act together to specify the width of the Z-disk (404).
3. Role of the Z-disk region of titin in myofibrillogenesis
The importance of the Z-disk region of titin in myofibril assembly, stabilization, and maintenance has been demonstrated by several laboratories (17, 132, 292, 364). Overexpression of the first 362 amino acids of titin in primary cultures of chicken cardiomyocytes resulted in complete disassembly of the sarcomeric cytoskeleton (364). Not only were Z-disks narrow, bent, and scattered, but A-bands were also disorganized or absent, suggesting that anchorage of full-length titin into the Z-disk is important for the formation and maintenance of the entire sarcomere. Similarly, overexpression of the entire integral Z-disk region of titin in the myogenic cell line, Hsk btsA58, led to sarcomere disassembly (292), and overexpression of just the first 200 amino acids of the Z-disk portion of titin, which contain repeats ZIg1 and ZIg2, in cultures of embryonic cardiac myocytes resulted in severe disruption of myofibrils and loss of contractile activity (132). Likewise, overexpression of individual Z-repeats in primary cultures of embryonic chick cardiomyocytes or quail skeletal myotubes also led to complete loss of existing myofibrils and inhibition of the formation of new ones (17).
All these studies suggest that overexpression of the Z-disk portion of titin affects not only the assembly of Z-disks and associated structures, such as the organization of actin filaments into I-bands, but also the organization of thick filaments into A-bands. These observations are consistent with the idea of “cross-talk” between the two ends of this gigantic molecule during sarcomere formation, and further imply that disruption of the COOH-terminal region of titin, located at the M-band, will also affect the organization of the Z-disk. Consistent with this, gene targeting of the extreme COOH terminus of titin in cultured skeletal myotubes demonstrated that the organization of both M-bands and Z-disks was severely affected (243). Although a number of studies have postulated that thin and thick filaments assemble independently, at least during early stages of myofibril formation (148, 178), the need for a structure that interacts with both filamentous systems and ultimately coordinates their integration into sarcomeres is obvious. Titin could fulfill such a role, as it directly interacts with both sets of filaments.
The studies summarized above indicate that the NH2-terminal domains of titin play a key role during myofibril formation by serving as “molecular blueprints” that coordinate the assembly and organization of proteins at the Z-disk as well as the M- and A-bands, and that continue to play a role in stabilizing these structures in mature myofibrils.
B. The I-Band Region of Titin
The central I-band region of titin is the most well studied portion of this gigantic molecule and has been the focus of many reviews, which discuss its molecular composition and structure, its elastic properties, its many binding partners, and the signaling pathways it may control through them. We discuss key features of this region of titin below, but refer the reader to earlier and recent reviews for additional details (83, 115, 117–119, 121–123, 185, 195, 205, 209, 211).
1. Molecular structure and elastic properties of the central I-band region
Titin's I-band region begins ~100 nm away from the center of the Z-disk, is encoded by exons 28–251, and undergoes extensive alternative splicing, to give rise to isoforms with distinct elastic properties and molecular masses, ranging from 3 to 4 MDa (Fig. 2) (209, 215–217). In the absence of external forces, the I-band region of titin is highly folded. During stretch, however, it gradually extends, developing passive tension, in contrast to the other regions of titin, which appear to be inextensible.
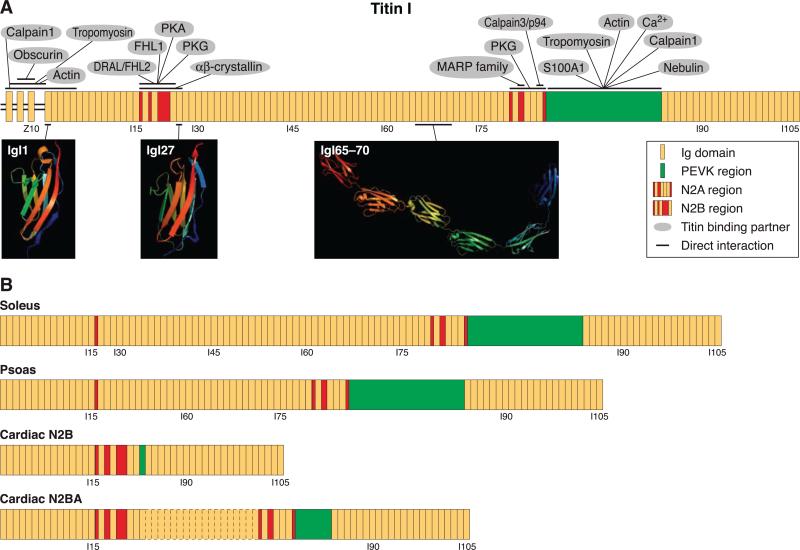
FIG. 2.
Schematic representation of titin spanning the I-band, illustrating its domain orientation and the structure of some of its domains, as well as identifying its binding partners. The I-band region of titin, encoded by exons 28–251 of the TTN gene, is the most highly alternatively spliced region of titin. Exons 45–48 are excluded from titin in striated muscle and are alternatively spliced to produce smaller isoforms, termed Novex-1, -2, and -3. A: the hypothetical protein comprising all the other exons, with binding partners and their sites of interaction indicated. Some of the domains along the I-band region of titin have been characterized structurally, either by NMR or X-ray crystallography, and are shown as ribbon diagrams. B: actual variants of titin that are expressed in different muscle tissues and have been characterized by RT-PCR (soleus, psoas, and two forms found in cardiac muscle, N2B and N2BA). The I-band region of titin is composed primarily of Ig domains. The Ig domains at the Z-I junction (Z8-Z10, encoded by exon 28) are flanked by sequences of unknown structure and are followed by the first 15 Ig domains found at the level of the I-band which are present in all striated muscles. Immediately downstream of this group of Ig domains is the N2B region, composed of nonrepetitive sequence and Ig domains, which is present only in some isoforms of titin. Following the N2B region is another stretch of Ig domains (I27-I79), the N2A region, which also contains nonrepetitive sequence and Ig domains, and the PEVK domain, which is largely responsible for titin's elastic properties. This middle stretch of Ig domains varies greatly among muscle-specific isoforms. Slow-twitch fibers of the soleus muscle, for example, contain all the Ig domains linked to this region and the longest PEVK region of any known titin isoform. The psoas, which is a fast-twitch muscle, contains 19 fewer Ig domains and has a much shorter PEVK domain. Cardiac N2B possesses only 2 Ig domains and a very short PEVK region. Many isoforms of cardiac N2BA, containing both the N2B and N2A regions, have been detected. The differences among them can be attributed to the number of Ig domains between the N2B and N2A regions, which can be as few as 13 or as many as 25 (as shown by the Ig domains outlined by a dotted line). COOH terminal to the PEVK domain is the last set of Ig domains in the I-band region of titin; all isoforms contain these same 22 Ig domains.
Four springlike elements have been characterized within the I-band region of titin. These function as bidirectional springs and contribute to the development of passive and restoring forces that maintain a constant resting sarcomere length, even after stretch or contraction (please refer to the reviews, cited above, for more details). They include tandemly arranged proximal and distal Ig-domain regions of variable lengths, multiple PEVK motifs, ranging from 183 residues in the human heart to 2,174 residues in the human soleus muscle, enriched in proline (P), glutamic acid (E), valine (V), and lysine (K) residues, an N2A element present in all skeletal and some cardiac isoforms, and an N2B element expressed exclusively in cardiac variants (119, 121–123). Thus mammalian skeletal muscle has titins with N2A domains that are 3–4 MDa in mass, whereas cardiac muscle has different ratios of N2B (~3 MDa) and N2BA (3.2–3.7 MDa) isoforms, the relative amounts of which vary with development. As the ratios of different cardiac isoforms change in the hearts of hibernating grizzly bears (267), the expression of these splice forms is also likely to be regulated metabolically.
Elegant studies with the atomic force microscope of single regions of titin, expressed in bacteria, have demonstrated that the four spring elements of titin have distinct bending rigidities and as a result do not extend uniformly with stretch. As stretch is initiated, the proximal and distal Ig-domain regions extend by straightening of linker sequences. Unfolding of individual Ig domains is highly unlikely, however, as it would entail repeated unfolding and refolding during each cycle of stretch and release, which would be energetically unfavorable (113, 114, 119, 121–123, 210, 212, 214, 216, 217). As stretch forces rise, random coil sequences within the PEVK segment extend, followed at higher forces by the extension of random coil sequences in the N2B element (112, 206, 216, 262, 358, 360, 380, 395). This stepwise extension of titin's I-band region generates a unique passive force-extension curve that is shallow close to slack sarcomere lengths, but steeper at higher degrees of extension (48, 114, 145, 212).
The contribution of titin's central I-band region to the generation of passive tension has been examined during myocardial stretch and ventricular filling (112, 115–117, 119, 121, 124, 144, 146, 204, 205). In normal myocardium, I-band titin behaves as a bidirectional spring by restoring sarcomeres to their slack or resting length after systole, and by limiting the lengths to which sarcomeres can be stretched during early diastole. Although titin's central I-band segment is the predominant source of passive tension at relatively short sarcomere lengths (~1.9 μm), extracellular matrix components, with collagens I and III being the main contributors, together with titin determine the passive myocardial stiffness near the upper limit of sarcomere lengths (~2.2 μm) achieved during diastolic ventricular filling (120, 417). Consistent with this, increased collagen type I and I/III ratios have been associated with increased diastolic chamber stiffness (411).
Recently, Radke et al. (312) developed the first genetically engineered mouse model for the I-band portion of titin. Specifically, they generated a partial knockout mouse in which exon 49 of titin, which encodes the N2B region, was deleted, but in which the rest of the titin gene was left intact. The mutant mice survived to adulthood, indicating that the N2B region is dispensable for the development or contractile activity of striated muscles. Although the hearts lacking the N2B region had smaller ventricles, they produced normal ejection volumes, most likely due to a compensatory increase in ejection fraction. Moreover, the slack sarcomere length was reduced and the passive stiffness was increased. The increase in passive stiffness was attributed to an additional extension of the remaining spring elements, with the PEVK region extending the most, followed by the proximal and distal Ig segments. Thus it appears that the N2B region is essential for the diastolic, but not the systolic, function of the heart through its contribution to passive tension.
2. Phosphorylation and Ca2+ regulators of titin-based passive tension
In addition to differential splicing of its elastic elements, phosphorylation and changes in Ca2+ concentration also regulate the mechanical properties of titin's I-band region. Phosphorylation of the N2B region by protein kinase A (PKA) following stimulation with β-adrenergic agonists reduces passive tension (84, 419). This has been attributed to a phosphorylation-induced increase of the functional length of the N2B element, resulting in lower passive force (184, 199, 419). Consistent with this, PKA increases ventricular compliance in a titin-dependent manner during β-adrenergic stimulation (84).
Contrary to the effect of PKA-mediated phosphorylation, Ca2+ binding to the PEVK region of titin lowers its bending rigidity and augments its passive stiffness during stretch (189, 418). This effect of Ca2+ requires the presence of E-rich motifs within the PEVK segment. Accordingly, N2B titin, which primarily contains PPAK repeats but not E-rich motifs within its PEVK segment, is largely insensitive to [Ca2+] fluctuations, whereas N2BA titin, which contains several E-rich motifs, shows increased passive tension following Ca2+ binding (81).
Ca2+ also affects passive stiffness indirectly by modulating the interaction of the PEVK region of titin with actin (81, 188, 213, 215, 261, 418). In vitro motility assays and passive cardiac myocyte mechanics showed that as the thin filament slides relative to titin, a dynamic interaction between actin and the PEVK segment slows filament sliding and contributes to the generation of passive force (418). This effect requires S100A1, a soluble Ca2+ binding protein of the EF-hand family that is abundantly expressed in the cytoplasm of cardiomyocytes. S100A1 inhibits the interaction of actin with the PEVK segment in a Ca2+-dependent manner, increasing the passive stiffness of cardiomyocytes during diastole as Ca2+ levels decay (346, 347, 418). Thus, during the heart's pumping cycle, oscillation of free Ca2+ in the presence of S100A1 may modulate the PEVK/actin interaction, which in turn may provide a means to increase passive tension under dynamic conditions.
3. Binding partners of the I-band region of titin
All the findings discussed above indicate that the central I-band region of titin behaves as a spring that generates passive tension. It also contains binding sites for many proteins involved in distinct cellular processes that help to integrate the mechanical and contractile activity of heart and skeletal muscle with regulatory mechanisms that control metabolism and gene expression. To date, the ligands of the I-band portion of titin include actin, tropomyosin, nebulin, αβ-crystallin, DRAL/FHL2, FHL-1, calpains 1 and 3, and members of the muscle ankyrin repeat proteins (MARPs) family (Fig. 2, Table 2).
A) ACTIN
Two sites in titin's I-band region interact with sarcomeric actin, one near the Z-disk involving Ig domains and the second in the PEVK segment. An ~100-nm-long stretch of F-actin binds in a Ca2+-dependent manner to titin fragments that, depending on the splice form, contain variable numbers of Ig domains located at the junction between the Z-disk and the I-band (169, 213, 359, 361). Although the physiological role of the binding of titin to actin at this site is not known, it may contribute to the assembly and regular alignment of the thin filaments in the I-band, and at the same time may provide a firm and secure anchor for the extensible I-band segment of titin.
A second binding site for sarcomeric actin within titin's PEVK segment has been extensively studied both in the cardiac and skeletal isoforms. Studies by Yamasaki et al. (418) and Linke et al. (215) indicated that the right ventricular form of titin, which contains the N2B domain but has a low number of E-rich motifs in its PEVK region, binds actin more avidly than the atrial N2BA form of titin, which has a high poly-E content (93). In contrast, Nagy et al. (261) demonstrated that different portions of the N2A-PEVK fragment of titin from skeletal muscle have distinct affinities for sarcomeric actin, with the middle portion, which has a higher preponderance of poly-E motifs, exhibiting the strongest binding. These results together suggest that the poly-E motifs alone are not responsible for actin binding, but the additional sites in this region of titin that can influence binding have not yet been identified. In a subsequent study, the same investigators used optical tweezers to verify the presence of multiple actin binding sites along the PEVK fragment with different binding avidities and capacities (29). Consequently, they suggested that this region may be promiscuous for actin binding, and thus may provide a viscoelastic scaffold that maintains sarcomeric structural integrity during stress and relaxation.
As the interaction between the PEVK region of titin and sarcomeric actin must accommodate the movement of the thin filaments during contraction and relaxation, it has been proposed that their binding is transitory and weak. Consistent with this, Astier et al. (13) calculated an apparent binding affinity (KD) in the low micromolar range for the binding of actin to titin's PEVK region, and Kulke et al. (188) and Yamasaki et al. (418) proposed that this binding might be modulated by dynamic extrinsic factors, such as local ionic strength and temperature. Using in vitro motility assays and cosedimentation studies, they demonstrated that increases in ionic strength or temperature augmented binding significantly, which suggested the involvement of hydrophobic interactions in the association of actin with the PEVK region of titin (188, 418).
The functional significance of the binding of the PEVK region of titin to actin has been investigated using both in vitro motility assays and studies of passive mechanics in cardiac myocytes (188, 418). These studies highlighted a crucial role for this interaction in the production of viscous force production and the passive stiffness of myocytes, which are both dynamically modulated by Ca2+ and S100A1 (see also sect. iiB2). Thus the two different types of interactions between titin and actin may facilitate distinct functions: binding of titin's Ig domains, located at the interface of the Z-disk and I-band, to actin filaments may anchor its adjacent elastic I-band region, whereas binding of its PEVK domain to actin filaments may regulate the development of passive force.
B) TROPOMYOSIN
Using a solid phase binding assay, Raynaud et al. (313) showed that tropomyosin binds to the I-band portion of titin at two independent sites with affinities in the low micromolar range. One site is located near the Z-disk at the N1 line and the other in the PEVK region near the N2 line. Binding is independent of Ca2+ and actin, although both sites can also interact with actin. The latter observation and the fact that tropomyosin lines the grooves of thin filaments suggested that these regions of titin may bind to the tropomyosin-actin complex. Cosedimentation assays, however, demonstrated that the binding of the PEVK region of titin to actin inhibited the binding of tropomyosin to actin when it was added before tropomyosin but had no effect when added after the formation of the tropomyosin-actin complex. Thus it appears that titin makes transitory contacts with actin and with the tropomyosinactin complex that may regulate the sliding of the thin filaments during contraction.
C) NEBULIN
The proline-rich sequences in the PEVK region of titin contain binding sites that have micromolar affinity for the COOH-terminal SH3 domain of nebulin, as shown by circular dichroism, fluorescence spectroscopy, and nuclear magnetic resonance techniques (227, 305). In fact, the multiple copies of poly-proline sequences arranged in tandem along the PEVK region suggest the presence of numerous SH3 binding motifs, a possibility that remains to be examined (226). The physiological significance of the interaction of titin's proline-rich sequences with nebulin's SH3 domain is unclear, especially as SH3 domains can bind to proline-rich sequences nonspecifically, and nebulin's SH3 domain lies within the Z-disk while titin's proline-rich sequences lie hundreds of nanometers away, in the I-band. Thus any links that form between these two domains should be transitional, occurring, for example, during myofibril assembly. Consistent with this, it has been speculated that their interaction may play a critical role in the recruitment, orientation, and integration of nebulin into the I-band (227).
D) α B-CRYSTALLIN
αB-crystallin is a small heat shock protein that oligomerizes and binds to partially unfolded proteins to prevent denaturation (40, 298). During ischemia, αB-crystallin translocates to the N1-region of the myofibrillar I-band, where it associates with titin, as demonstrated by immunoelectron microscopy and biochemical approaches (106, 107). The precise binding site of αB-crystallin on I-band titin was identified by Bullard et al. (44), who employed a combination of immunofluorescent and immunoelectron microscopy and in vitro binding assays. Their studies showed that, at physiological sarcomere lengths, αB-crystallin bound specifically to titin's N2B region and the two Ig domains located COOH-terminally to N2B (i.e., Ig26/Ig27), but not to the PEVK fragment. To study the stabilizing effects of αB-crystallin on titin, these investigators used atomic force microscopy to stretch a block of eight Ig domains (Ig91-Ig98) from the distal Ig domains portion of I-band titin. Higher stretching forces were needed to unfold these domains in the presence of αB-crystallin, which implied that the latter might protect I-band titin under conditions of extreme stress that otherwise might cause domain unfolding and protein denaturation. Related studies of the effects of binding of αB-crystallin to titin's N2B domain and nearby regions, including studies of the effects of pathogenic mutations in αB-crystallin, have recently appeared (427).
E) DRAL/FHL-2 AND FHL-1
In addition to binding αB-crystallin, the N2B region of titin also interacts with the cardiac-specific four and a half LIM domain protein, DRAL/FHL2 (50, 101, 197). DRAL/FHL2 localizes in broad bands at the ends of sarcomeres, at the Z/I interface, and in fainter striations in the middle of sarcomeres, at the M-bands. The interaction of DRAL/FHL-2 with the N2B domain of titin is likely to be functionally significant, as the expression of DRAL/FHL-2 is inhibited in the N2B-knockout mouse and may contribute to the reduced size of the N2B-deficient heart (312). By implication, it may also contribute to hypertrophic and atrophic responses in wild-type hearts. The association of DRAL/FHL-2 with several glycolytic enzymes involved in the synthesis of ATP and with titin at sites overlying the I-band suggests that its binding to titin may couple ATP production via glycolysis to the contractile cytoskeleton, and thus may help to maintain high local concentrations of ATP near sites of contractile activity.
Interestingly, a recent study further demonstrated that the N2B region of titin also binds to FHL-1, another member of the four and a half LIM domain proteins, that localizes at the I-band and plays important roles in the progression of pathological cardiac hypertrophy (332). As FHL1 interacts with titin's N2B region and proteins of the Gαq-MAPK pathway, it may sense biomechanical stress responses in the sarcomere, via its interaction with titin, and alter signaling cascades mediated by the Gαq-MAPK pathway, leading to pathological hypertrophy (332).
F) CALPAINS-1 AND -3
Calpain-1 is a ubiquitously expressed Ca2+-dependent protease that is tightly linked to the myofibrillar Z-disk and I-band through its direct interaction with titin (57, 314). Immunofluorescent and immunoelectron microscopic studies as well as biochemical studies by Raynaud et al. (314) demonstrated that calpain-1 associates with titin at two different locations: one that includes the NH2-terminal ZIg8-IIg5 domains, located at the level of the N1 line, which is close to the Z-disk, and another that contains the PEVK fragment and flanking sequences, located at the N2 line in the middle of the I-band (314). Both interactions are Ca2+ dependent, as their affinity drops from the nanomolar to the micromolar range when Ca2+ is removed. Notably, both titin subfragments are efficiently cleaved by calpain-1 in the presence of Ca2+ (314). Thus it appears that calpain-1 through its interaction with titin's I-band regions preferentially accumulates at the myofibrillar N1 and N2 structures. These sites along the sarcomere contain high amounts of Ca2+ deposits (354, 420) and act as the main postmortem proteolytic cleavage sites, where muscle protein breakdown and the initial steps of sarcomere disassembly take place (12, 153).
The N2A region of the I-band portion of titin, and specifically Ig domain 83, supports binding to the inserted domain 2 (IS2) of calpain-3, which is located between domains IIa and IIb of its papain-type proteolytic motif (140, 283, 341). Calpain-3/p94 is a nonlysosomal Ca2+-dependent cysteine protease that is specific to skeletal muscle. It is unique among calpains and other proteases, as it undergoes rapid and exhaustive autolysis and has a half-life of <1 h (61). Loss-of-function mutations in calpain-3 lead to limb-girdle muscular dystrophy type 2A (LGMD2A), a common form of muscular dystrophy characterized by progressive muscle weakness, atrophy of the shoulder and pelvic girdle musculature, and extensive degeneration and regeneration of muscle (64) (see below).
The functional significance of the binding of calpain-3 to titin and whether titin is itself a substrate for the protease remain unknown. Although coexpression experiments and in vitro studies have shown that titin can be cleaved by calpain-3, this observation has not yet been confirmed in vivo (140, 355). Conversely, titin has been suggested to inhibit the proteolytic autoactivation of calpain-3 (61, 140, 283, 355). The signal that leads to activation of calpain-3 remains elusive, and much debate has focused on the possible role of Ca2+ or exercise in this process (65). Overexpression of calpain-3 in mice suffering from muscular dystrophy with myositis (mdm), a model carrying a deletion within domain Ig83 of titin (the binding site for calpain-3 in the N2A region of titin) dramatically worsened their dystrophic phenotype (92, 152). Conversely, overexpression of calpain-3 in wild-type mice or muscle cells in culture did not result in any apparent phenotype, suggesting that muscle cells contain high “buffering capacity” for the activity of calpain-3 activity, which has been attributed to titin (24, 343). Taken together, these findings indicate that binding of calpain-3 to the N2A domain (and perhaps to the MIg9/Mis7 region; see below) of titin may regulate its activity and that titin may act as a reservoir of inactive calpain-3 (281).
G) MARP
Cardiac ankyrin repeat protein (CARP), ankrd-2/Arpp, and diabetes-related ankyrin repeat protein (DARP) are conserved members of the MARP family of proteins. CARP, ankrd-2/Arpp, and DARP localize at the myofibrillar I-band and in the nucleus, and their expression is upregulated in both skeletal and cardiac muscles after mechanical or metabolic stress (244). All three MARP proteins contain a conserved titin-binding motif within their second ankyrin repeat, which directly interacts with a unique tyrosine-rich sequence between Ig80 and Ig81 of the N2A region of titin (244). As the binding affinities of the MARP proteins for the N2A region of titin are similar, it has been speculated that the nature of the MARPs associated with titin is regulated in a developmental fashion or, alternatively, may depend on the differential expression of these proteins upon exposure to different stress stimuli.
Given their dual distribution at the I-band and the nucleus, their direct regulation by stress, and their diverse binding partners, MARPs may function as stretch sensors to link myofibrillar-stress responses through their interaction with N2A titin to muscle gene expression, through their association with transcription factors (172, 244, 408). Studies with the atomic force microscope to examine the effects of the MARPs on the extensibility of titin could test this idea experimentally.
C. The A-Band Region of Titin
1. Molecular composition of the A-band portion of titin
The COOH-terminal 2 MDa of titin are located in the sarcomeric A-band. This portion of titin is highly repetitive and relatively inextensible. It is composed of two types of super-repeats, both consisting of regular patterns of Ig and FN-III motifs (87, 191, 252, 363). Unlike Ig domains, which are found over the entire length of titin, FN-III repeats are found exclusively in its A-band segment and with the Ig domains make up ~70% of the A-band portion of titin (Fig. 3).
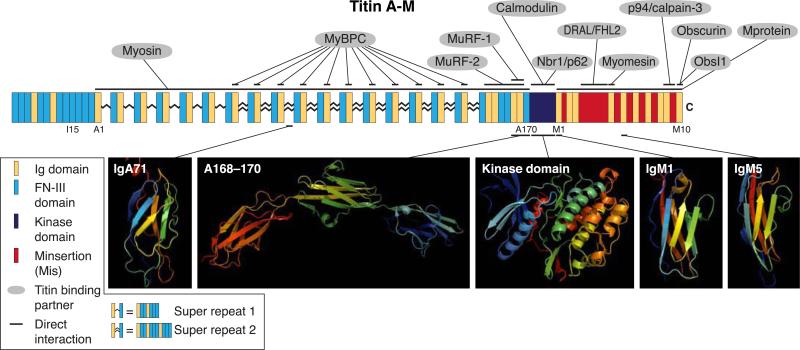
FIG. 3.
Schematic representation of titin spanning the A- and M-bands, showing its domain orientation and the structure of some of its key domains, as well as identifying its binding partners. This portion of titin is encoded by exons 252–363 of the TTN gene. The A-band region of titin, including domain A1 through the kinase domain, is composed of multiple Ig and FN-III domains. They are arranged in two types of super repeats in which stretches of FN-III domains are bisected by single Ig domains. The M-band region, from the end of the kinase domain to the COOH terminus of the molecule, lacks FN-III domains and is composed solely of Ig domains and M-insertions (Mis; please see key for a complete list of the domains, with color-coding). Binding partners and interaction sites that have been mapped to this region of titin are indicated. Myosin binding protein-C (MyBP-C) binds titin repeatedly along the length of the A-band, specifically to the first Ig domain of each of the second type of super repeat. The precise location of the binding site on titin for myosin is unknown, but myosin does bind several of titin's FN-III domains throughout the A band, with the affinity increasing with increasing numbers of the FN-III domains with which it interacts. The domains in this region of titin that have been characterized structurally, by NMR or X-ray crystallography, are represented as ribbon diagrams.
The first super-repeat is found in the D-zone of the A-band and comprises six copies of a 7-domain repeat arranged as Ig-(FN-III)2-Ig-(FN-III)3 (93, 191, 193). The second super-repeat, located COOH-terminally to the first, is found in the C-zone of the A-band and contains an 11-domain motif, arranged as Ig-(FN-III)2-Ig-(FN-III)3-Ig-(FN-III)3, that is repeated 11 times (93, 191, 193). A striking feature of the 11-domain super-repeat is that it shows a periodicity of ~43–44 nm, which correlates well with the 11 ~43 nm structural elements formed by myosin and accessory proteins within the thick filament. This suggests that the 11-domain super-repeat of titin associates laterally with the thick filament (28, 141) (see also below). Consistent with this, the individual domains at similar positions within the two super-repeats have higher sequence homology than the domains within the same super-repeat. This super-repeat pattern of titin at the A-band is broken only in two places: one near the beginning of the Ig/FN-III-rich region, where, following the first two groups of the first repeat, there is a stretch of six FN-III domains, and the other at the end of the Ig/FN-III region, specifically at the junction of the A-band and M-band portions of titin, where the pattern is more varied.
Unlike the elastic I-band portion of titin, the A-band portion of titin is inextensible and remains fixed with respect to the M-band as sarcomere length varies (88, 402). This suggests that the A-band region of titin is tightly associated with the thick filament, where it may play a critical role in the regulation of its length and structure.
2. Ligands of titin at the A-band
The repetitive patterns of the two super-repeats in the A-band region of titin provide regularly spaced binding sites for integral components of the thick filaments, such as sarcomeric myosin and MyBP-C, as well as proteins with diverse activities, associated with additional cytoplasmic compartments, such as muscle-specific RING-finger protein-1 and -2 (MuRF-1 and -2), which could link thick filaments to signaling pathways (Fig. 3, Table 2).
A) SARCOMERIC MYOSIN
Early studies by Isaacs et al. (158) used pulse-labeling, immunoprecipitation, and reversible cross-linking procedures to demonstrate that titin and sarcomeric myosin are chemically cross-linked into large, detergent-resistant complexes within minutes of their synthesis, suggesting that the two proteins associate in situ. Since these original observations, several laboratories have studied the direct interaction of titin and sarcomeric myosin with cosedimentation and solid-phase binding assays, spectroscopy, and electron microscopy. These studies confirmed the tight association of the two proteins and identified the regions on titin and on the heavy chains of myosin that mediate binding.
In 1995, Houmeida et al. (150) demonstrated that full-length myosin specifically interacts with the 0.8-μm fragment of titin located in its A-band, but not its I-band, region. Two distinct binding sites for titin were identified on myosin's heavy chain. Three groups found that the last ~20 nm section of the light meromyosin (LMM) portion of the molecule, that forms the backbone of the thick filament, binds to the A-band region of titin (150, 191, 342), whereas two other groups showed that binding to this region was mediated by the S1 fragment of the head domain of myosin (252, 392). Binding of the S1 fragment to titin is stronger than binding of the LMM segment and increases with the number of FN-III domains present. The differences between these studies mainly arise from the different portions of the A-band portion of titin that were assayed. The studies of LMM used either purified, full-length titin or pieces that included variable combinations of Ig domains and partial FN-III motifs, whereas the studies of the S1 fragment focused on the role of the FN-III domains, found exclusively in the A-band portion of titin. To reconcile this discrepancy, Muhle-Goll et al. (252) proposed that unique sites, distinct from the FN-III domains and possibly within the Ig motifs, mediate the interaction with LMM.
The strong binding of the S1 portion of the myosin head domain to titin may have important functional consequences for the topography of the actomyosin filaments, as it would position the head domain closer to the backbone of the thick filament and further away from its binding site to actin, altering Ca2+ sensitivity and reducing both the probability of cross-bridge formation and force generation.
B) MYOSIN BINDING PROTEIN-C
MyBP-C was originally discovered by Offer et al. (277) in 1973 as a contaminant of myosin preparations. Subsequent studies further characterized its interaction with myosin, identified the minimal binding sites that mediate their tight association, and postulated that binding of MyBP-C to myosin plays a critical role in maintaining the normal structure of thick filaments and regulating contraction by controlling the formation and cycling of cross-bridges (67, 271, 403).
The interaction of MyBP-C with myosin heavy chain is not uniform along the length of the A-band but is restricted to the C-zone, where MyBP-C is arranged in 11 transverse stripes at regular intervals of ~43 nm (344). This periodicity corresponds to that of the 11-domain super-repeat present in the C-zone of titin, to which MyBP-C binds (90, 191). Thus these studies suggested that binding to titin's super-repeats specifies the subsarcomeric distribution of MyBP-C. Subsequent studies by Freiburg and Gautel (80) used recombinant titin domains and either native or recombinant subfragments of cardiac MyBP-C to demonstrate that the binding of titin and MyBP-C in vitro is mediated by the 11-domain super-repeats. Further dissection of the 11-domain super-repeat [Ig-(FN-III)2-Ig-(FN-III)3-Ig-(FN-III)3] into its three distinct Ig domains localized the primary binding site for MyBP-C to the first Ig domain, although contribution of flanking motifs was not excluded.
The minimal domain of MyBP-C required for binding to titin's 11-domain super-repeat was assigned to its COOH-terminal C8-C10 repeats (80), which also harbor the binding site for sarcomeric myosin (6, 68, 282, 401). These domains are deleted in patients suffering from familial hypertrophic cardiomyopathy linked to chromo-some 11 (33, 271, 272). The binding of MyBP-C to titin is significantly weaker than to myosin (282), suggesting that formation of a ternary complex may promote a strong association with titin at specific sites along the thick filament (80). There is no evidence that a three-way complex among these proteins can form, however.
C) MUSCLE RING FINGER PROTEINS 1 AND 2
Muscle ring finger protein (MURF)-1 is an E3-ubiquitin ligase that is expressed throughout muscle development (131). Recent studies have shown that MURF-1 is upregulated during muscle atrophy and may prevent cardiac hypertrophy via a protein kinase C (PKC)-dependent pathway (32). MURF-1 localizes at the myofibrillar Z-disk, the periphery of the M-band, and in soluble form in the cytoplasm (49, 238). It binds to sarcomeric and cytoskeletal proteins, enzymes involved in ATP production, regulators of nuclear transcription, and enzymes involved in sumoylation, which in turn modulate nuclear translocation, gene expression, and subcellular targeting (131, 407).
Consistent with its site of localization in the middle of the sarcomere, MURF-1 binds to titin repeats A168–169, located adjacent to the kinase domain at the periphery of the M-band (49, 249). Overexpression in chick cardiac myocytes of full-length MURF-1 or its central 144 amino acids, which contain the titin-binding site, specifically perturbed the structure of titin in the M-band region (238). Overexpression of the A168–170 repeats of titin produced a similar phenotype (238). In both cases, thick filaments and M-bands were also disrupted, but thin filaments and Z-disks were not. MURF-1 and its binding to titin are therefore likely to play a role in the assembly or stabilization of thick filaments and M-bands. As the binding site of titin on MURF-1 does not involve the latter's RING domain, required for its ubiquitin ligase activity, it was suggested that titin is not ubiquitinated by MURF-1 (167), but this has not yet been demonstrated. In light of the role of MURF-1 in muscle atrophy (32, 200), however, its binding to titin may regulate the degradation of myofibrils and the turnover of the contractile apparatus, processes that are vital for the structure and function of healthy muscle as well as under pathological conditions, like atrophy and hypertrophy.
MURF-2 is expressed in at least four isoforms in striated muscle (198, 304). It also binds to titin in vitro, specifically to domains A164–169 (49, 304). At early stages of myofibrillogenesis, MURF-2 colocalizes with stable, glutaminated microtubules but not with the dynamic pool of tyrosinated microtubules (304). At later stages, MURF-2 transiently associates with sarcomeric myosin and the A-band portion of titin, and then disappears as mature myofibrils form. MURF-2 may therefore transiently link microtubules, myosin, and titin during myofibril assembly, perhaps allowing the microtubules to act as a scaffold for the formation of mature A-bands (241).
Although there is so far no experimental evidence suggesting that MURF-3 also binds titin, and at least one group reported negative results for the binding of MURF-2 to titin (49), the ability of the MURF proteins to heteromultimerize may link the sarcomere, via the association of titin with MURF-1, to proteolysis, nuclear transport, and the functions of the microtubular network.
D. The M-Band Region of Titin
1. Molecular composition of the M-band portion of titin
In contrast to the regular pattern of structural motifs in the A-band portion of titin, the COOH-terminal 200 kDa of titin, localized to the M-band, has a complex structure (Fig. 3). This portion of titin consists of a serine/threonine kinase domain that is encoded within M-band exon 1 (Mex-1) and 10 Ig-CII globular motifs (MIg1-MIg10), each composed of seven antiparallel β-sheets, interspersed by unique interdomain sequences (Is1-Is7) of varying lengths and properties that are encoded within M-line exons 2–6 (Mex2-Mex6) (49, 98, 123, 130, 191). Five of the six exons that encode the M-band portion of titin are constitutively expressed in all types of muscles throughout embryonic development and in adulthood, but exon 5 (Mex5), which contains a binding site for calpain-3, is alternatively spliced and its expression varies widely among muscles (173, 174). For instance, 90% of titin molecules in heart, soleus, and psoas (slow-twitch muscles), but only 10% in extensor digitorum longus and tibialis anterior (fast-twitch muscles) contain Mex5. Like the binding activities in other regions of titin, most of the binding of the M-band section of titin to other proteins is mediated by the Ig repeats, although recent evidence has postulated that the Is insertions are also involved.
2. Structure and activation of the Ser/Thr kinase domain of titin
The kinase domain of titin belongs to the myosin light-chain kinase (MLCK) family of kinases and has been implicated in mechanochemical signal transduction pathways. It is composed of a catalytic core that contains the ATP- and substrate-binding sites and a downstream regulatory domain that contains an autoinhibitory domain and a binding domain for Ca2+-calmodulin (94). Elucidation of the kinase's crystal structure provided evidence about its regulation (125, 236). In the inactive state, its catalytic aspartate is blocked by a nearby tyrosine (Y170), and its ATP-binding site is blocked by the kinase's regulatory tail. It has been suggested that this autoinhibition is relieved by a dual mechanism that involves phosphorylation of tyrosine-170 by an unknown kinase, followed by binding of Ca2+-calmodulin to the regulatory tail, which displaces it from the ATP binding site. To date, this type of activation is unique to the Ser/Thr kinase domain of titin, which is the only known non-arginine/aspartate kinase to be activated by a phosphorylation event. The uniqueness of the mechanism regulating its activation suggests that control of titin's kinase activity is physiologically important. This is supported by studies of its ligands and the effects of targeted mutations.
3. Ligands of titin at the M-band
The COOH-terminal M-band region of titin binds to sarcomeric components, like myomesin and M-protein, proteins like DRAL/FHL2 and Nbr1 that move among compartments in the myoplasm, and Ca2+-dependent proteases, specifically calpain-3/p94 (Fig. 3, Table 2). Through these interactions, the M-band portion of titin has been implicated in diverse activities, including thick filament assembly and sarcomeric stability, transcriptional regulation, and mechanisms for sensing and transducing stress.
A) MYOMESIN AND M-PROTEIN
Myomesin, a ubiquitous protein of the M-band, binds directly to titin and the heavy chain of myosin and may act as an elastic cross-linker connecting the end of titin at the M-band with myosin thick filaments (265, 273–275). The binding between myomesin and titin is mediated by the FN-III domains My4-My6 of myomesin and the Ig domain 4, MIg4, of titin (274). Phosphorylation by cAMP-dependent protein kinase of a serine residue (Ser-482) in the sequence linking myomesin domains, My4 and My5, results in complete inhibition of binding. This finding suggests that sarcomerogenesis and turnover may be controlled by phosphorylation. Consistent with this, myomesin and the COOH terminus of titin coassemble into primitive M-bands early in the process of myofibrillogenesis, providing binding sites for additional components of the M-band and functioning as scaffolding structures for the integration of sarcomeric myosin into regular A-bands (179, 180, 208, 375, 376).
Like myomesin, M-protein, which is also present at M-bands and shares a similar domain structure, binds to both myosin and titin (273, 276, 379). Unlike myomesin, however, M-protein is only expressed in postnatal cardiac myofibers and in fast-twitch muscle fibers at all stages of development (2, 3). The sites on the two proteins that mediate their binding have not yet been characterized.
B) DRAL/FHL-2
Yeast two-hybrid experiments combined with in vitro binding assays demonstrated that DRAL/FHL-2 directly binds to two different sites of titin, one located in the central 270 amino acids of the N2B region in the I-band portion of titin (see above) and the other located in the Is2 region of the M-band region, between MIg3 and MIg4 (197). DRAL/FHL-2 also interacts with creatine kinase, phosphofructokinase, and adenylate cyclase, suggesting that it may tether these metabolic enzymes at sites of high-energy consumption through its association with titin (197). Consistent with this, a missense mutation in DRAL/FHL-2 (Gly48Ser) was identified in a patient with familial dilated cardiomyopathy (DCM) (9). In vitro binding studies indicated that this FHL-2 mutation dramatically reduced binding to titin's N2B and Is2 domains, suggesting that the Gly48Ser mutation results in DCM by reducing the recruitment of metabolic enzymes to the cardiac sarcomere, which in turn leads to impaired energy production and heart failure.
C) P94/CALPAIN-3
Titin interacts directly with calpain-3 at two distinct sites: one located at the I-band region (see above) and the other located at the M-band end of this gigantic molecule (171, 340, 341). Full-length calpain-3 is required for binding to the M-band portion of titin, as different deletion mutants did not show any binding activity (171). Later studies indicated, however, that elimination of insertion sequences 1 or 2 (IS1 or IS2) of calpain-3 potentiated binding (147). The minimal binding site for calpain-3 in the M-band portion of titin is confined to Ig motif 9 (MIg9) and the unique adjacent sequence Mis7, both of which are encoded by the alternatively spliced exon Mex5 (170). These findings suggest that the mechanisms of calpain-3 binding to the I-band and M-band regions of titin are different, with binding at the M-band site varying with muscle fiber type and developmental stage (see above).
A possible physiological link between calpain-3 and its binding site to the M-band region of titin is suggested by the recent discovery of a novel titinopathy, affecting both skeletal and cardiac muscles, that is linked to two different homozygous, out-of-frame deletions in exons 358 (Mex1) and 360 (Mex3) (46) (see below). These deletions led to the absence of titin's COOH-terminal epitopes and the incorporation into sarcomeres of truncated forms of the protein that lack a binding site in their M-band region for calpain-3. The fact that these patients develop severe DCM, despite the fact that calpain-3 is not normally expressed in mature heart muscle, suggests that this portion of titin may have other activities as well.
D) NBR1
The identification of binding partners and in vivo substrates of the kinase domain of titin has also been the focus of the research performed by several groups in the last decade. Recently, the zinc-finger protein nbr1 was identified as a ligand of the serine/threonine kinase domain of titin in a systematic yeast two-hybrid screen (198). The NH2-terminal Phox/Bem1p (PB1) motif of nbr1 recognizes the kinase domain, but only when it is in an open or active conformation, which is mechanically induced by stretch (198). P62, an nbr1-related zinc finger protein, which acts as a multivalent scaffold and plays important roles in controlling ubiquitin-mediated turnover and kinase signaling cascades (52), also binds to titin's kinase domain, as well as to nbr1. Both nbr1 and p62 can be phosphorylated by the titin kinase domain in vitro, although p62 is a significantly poorer substrate than nbr1 (198).
One of the many ligands of p62 is MURF-2, which also binds to titin's Ig domains A164–169 (see above). MURF-2 shuttles between the cytosol and the nucleus under atrophic conditions induced by mechanical arrest, and it is essential in primary myofibrillogenesis (241). MURF-2 also binds to the transactivation domain of the serum response factor (SRF) via its RING/B-box motif, which plays a crucial role in normal heart development and growth as well as in the adaptation of muscle to hypertrophic stimuli, including mechanical stress (207, 290). Binding of MURF-2 to SRF inhibits the nuclear targeting of the latter and represses its transcriptional activity (198). Consequently, mechanically induced hypertrophic responses elicited by SRF are suppressed. Over-expression of the kinase domain of titin in neonatal rat cardiomyocytes significantly disrupted the sarcomeric and nuclear localization of MURF-2 and reduced its expression, relieving the inhibitory action of nuclear MURF-2 on SRF-mediated gene expression and counteracting the effects of mechanical arrest (198). Interestingly, however, a recent study by Witt et al. (406) demonstrated that mice null for MURF-2 are healthy and have normal muscles, which raises questions about its physiological importance in the regulation of the signaling pathways discussed above.
An autosomal dominant hereditary myopathy with early respiratory failure (HMERF) has been linked to substitution of a highly conserved arginine with tryptophan in the regulatory tail of the kinase domain of titin (see below) (268). Examination of the subcellular localization of Nbr1 and MURF-2 in skeletal muscles from HMERF patients demonstrated that both proteins were absent from their respective sarcomeric structures and that Nbr1 was diffusely localized in the cytoplasm, whereas MURF-2 preferentially concentrated in centralized nuclei.
4. Functional properties of the Ser/Thr kinase domain of titin
The role of the Ser/Thr kinase domain of titin and flanking sequences has been extensively studied using gene-targeting approaches. In an elegant set of studies by Gotthardt et al. (111), the effects of conditionally eliminating the M-line exons, Mex1 and Mex2, which encode Ig169 and FN-III170 of the A-band, the kinase domain and MIg1-MIg7, respectively, were assessed in heart and skeletal muscle at different stages of embryonic development (111). Elimination of Mex1 and Mex2 in early embryonic development but after sarcomeric assembly had commenced was lethal; their excision late in embryonic development allowed mice to survive in utero but led to the development of a progressive myopathy and death at 5 wk of age. Ultrastructural evaluation of skeletal muscles lacking these exons revealed different degrees of myofibrillar disarray with widened and pale M-lines, that (if they were present at all) were devoid of M-bridges. Thus the M-line portion of titin has a critical role in sarcomeric integrity and maintenance both early in muscle development and postnatally.
In a follow-up study, the same group created mice with an inducible, tissue-specific knockout of the kinase domain and nearby regions of titin, to study the phenotype specifically in the adult heart (297). Hearts from these mice showed reduced β-adrenergic responsiveness and impaired contractile properties. This phenotype was most likely due to abnormal intracellular Ca2+ cycling, as Ca2+ uptake by the SR was severely reduced. Consistent with this, the levels of both the SR Ca2+ pump (SERCA2) and its regulator, phospholamban, were significantly downregulated. In contrast, PKC-δ, which has been linked to postischemic contractile dysfunction, development of hypertrophy, and disruption of the cytoskeleton, was up-regulated in titin kinase-deficient hearts, suggesting that there is cross-talk between the titin kinase domain and signaling cascades involving PKC-δ. Ultimately, these animals developed cardiac hypertrophy and heart failure.
In a third set of studies, Gotthard and colleagues (400) created mice constitutively lacking exons Mex1-Mex-2 to learn whether the kinase domain and flanking regions of titin are essential in the initial assembly of sarcomeres in developing striated muscles. Heterozygous recombinant animals were fertile and did not display any apparent morphological defects, but homozygous mice lacking the M-band region of titin, including the kinase domain, died in midgestation. Ultrastructural studies showed that myofibrils at embryonic day 9.5 (E9.5) formed regularly aligned Z-disks and M-bands, which, however, failed to grow laterally. By E11, mutant sarcomeres contained few filaments and were in disarray. Remarkably, the absence of the titin kinase domain did not affect the initial organization of other M-band proteins. Myomesin, for example, was present in M-band striations at E9.5, although by E10 its expression was reduced and it was more diffusely distributed. Thus the titin kinase domain is dispensable for the initial stages of sarcomero-genesis, but it is critical for maintaining sarcomeres and for their subsequent lateral growth. Notably, sarcomere length was not affected, which indicates that the kinase domain of titin is not responsible for determining the positioning of the M-band and suggests that other structural domains of titin that are not affected by the deletion, or perhaps other sarcomeric proteins, serve this function. Although neither mislocalization of A-bands nor misalignment of M-bands was detected during the initial stages of myofibril formation in the absence of the M-band portion of titin, this form of titin is incompatible with the maintenance of sarcomeric integrity.
Contrary to the results summarized above, Musa et al. (255) proposed that the entire M-line portion of titin is indispensable for the initial stages of myofibrillogenesis in developing cardiomyocytes derived in culture from embryonic stem cells. Targeted deletion of the entire M-band portion (i.e., the kinase domain along with MIg1-MIg10) in both titin alleles resulted in the failure of ES cells to differentiate. Myofibrillogenesis was arrested at an early stage, sarcomeric Z-disks and M-bands were abnormal, and sarcomeric myosin did not incorporate into A-bands. Due to the severity of the phenotype they observed, the authors suggested that the entire M-band portion of titin is essential for the initial organization and assembly of muscle sarcomeres.
To reconcile the different outcomes of these two sets of studies, Musa et al. (255) pointed out that M8-M10 were retained and expressed in the study by Weinert et al. (400). This cannot explain the different phenotypes that the two groups observed, however, as the MIg8-MIg10 fragment, although present in the mutant titin of the study by Weinert et al. (400), did not incorporate into M-bands and so could not contribute directly to their assembly. The reasons that the two groups observe these differences remain unknown but are perhaps due to the inherent differences of in vivo and in vitro models.
All the molecular, biochemical, and genetic studies of the titin kinase domain are consistent with the idea that there is a complex of signaling molecules associated with it, that links its kinase activity with sarcomere assembly and maintenance, stress-sensing mechanisms, transcriptional regulation, protein turnover, and hypertrophic responses. Consistent with this, mutations in the kinase domain of titin alter the levels of expression and the subcellular localization of several other proteins, including their dissociation from sites along the sarcomere normally created by titin, that result in unstable sarcomeric structures, compromised transcriptional responses, mechanochemical uncoupling, and dystrophic or myopathic phenotypes. These broad effects of mutations in the titin kinase domain underscore its importance in muscle physiology and pathophysiology.
In summary, the gigantic size of a single titin molecule, its unique location within the sarcomere, the multiple binding sites that it provides for diverse proteins, that immobilize those proteins at particular sites along the sarcomere, as well as its elastic nature and its role in governing the resting length of the sarcomere indicate that titin plays essential roles in a wide range of processes in muscle cells. Assembly and maintenance of contractile filaments, regulation of passive muscle stiffness, transmission of force, compartmentalization of metabolic enzymes, binding of chaperones, positioning of internal membrane systems, regulation of gene expression and myofibrillar signaling are some of the multiple functions that have been attributed to titin to date.
III. NEBULIN
A. Structure of Nebulin
Nebulin is a large protein (500–800 kDa) that binds along the lengths of the thin filaments of skeletal muscle. It is anchored by its COOH-terminal region in the Z-disk, with its NH2-terminal region located at the end of the thin filaments that form the I-band. Nebulin associates at either end with actin filament capping proteins, specifically CapZ, a barbed end capping protein located at the edge of the Z-disk, and tropomodulin, a pointed end capping protein located in the I-band (Fig. 4, Table 1). Nebulin's COOH-terminal region binds to titin and myopalladin, which is believed to anchor the protein in the Z-disk, but along most of its length it associates laterally with F-actin.
FIG. 4.
Schematic representation of nebulin, illustrating its domain orientation, structure, and binding partners. Nebulin is composed mainly of ~35-amino acid-long repeating motifs, i.e., M1-M185. The middle repeats, where alternative splicing occurs, are further organized into 22 super repeats, each containing 7 modules each. The super repeat region spans the majority of the I-band and harbors binding sites for the major contractile proteins, including actin and myosin. The NH2- and COOH-terminal repeats (denoted in yellow), located at the pointed and barbed ends of actin, at the tips of the thin filaments of the I-band and the Z-disk, respectively, differ in sequence from the middle repeats and do not form super repeats. The NH2-terminal sequence is rich in glutamic acid residues, whereas the COOH terminus carries a serine-rich region and an SH3 domain. Binding partners that interact directly with nebulin are depicted with a solid line pointing to their binding sites on nebulin.
Nebulin is encoded by the NEB gene, which is present as a single copy in the mammalian genome. NEB has been localized to a 249-kb region at chromosome 2q22 in humans (59, 295). Mutations in nebulin have been linked to nemaline myopathies (see below). The gene contains 183 exons and represents the product of extensive, tandem gene duplications, which yield a protein with a highly repetitive domain structure (59). In particular, the central ~8.2-kb region of the gene, spanning exons 82–89, shows evidence of two rounds of duplication, resulting in the generation of two segments, containing exons 90–97 and 98–105, that are 99% identical. Transcription and splicing produce an mRNA of ~20.8 kb with an open reading frame of ~20 kb (193, 345). Initiation of translation of the mRNA occurs in exon 3; the stop codon and the 3′-untranslated region (UTR) are located in exon 183. mRNAs can be alternatively spliced in several locations, including exons 63–66 (encoding nebulin super-repeat 11; see below) and exons 166–177 (encoding simple repeats 176–182) (59). Alternative splicing, which is regulated both developmentally and in a muscle-specific manner, generates protein products of different sizes, ranging from ~5to8 × 102 kDa (58, 59, 186, 192, 220). The protein nebulin, or NEB, is synthesized in skeletal muscle shortly after the initiation of the fusion of myoblasts to form myotubes (26, 89, 175). As myotubes begin to assemble their contractile proteins, nebulin associates with I-Z-I “brushes” (175, 280), the precursors of the Z-disks and I-bands, presumably via its COOH-terminal region (288). This occurs after the premyofibrils appear (see sect. iv), as mature myofibrils assemble and before the thin filaments reach the lengths typical of mature I-bands (175, 247, 280, 333, 387), consistent with the idea that nebulin helps determine the size and organization of the F-actin molecules that comprise the thin filaments (54, 76, 388, 389, 409).
As suggested by the fact that large sequences within the NEB gene have been duplicated, the protein is comprised largely of sequences that share significant homology, termed nebulin modules, domains, or repeats (Fig. 4). Nebulin modules are ~35 amino acids in length. The large majority are contained within the repeats numbered 9–162 and share the sequence SDXXYK, which promotes binding to actin (186, 192, 302, 350). Groups of 7 tandem modules within this region are further organized into 22 “super-repeats,” most of which share the sequence WLKGIGW (186, 192, 350). The order of the super-repeats reflects the pattern of duplication of exons within the NEB gene (see above), which is likely to have occurred by duplication of units of seven repeats, resulting in the preservation not only of the sequence homologies among the repeats and super-repeats but also their exon-intron boundaries (30).
The pattern of repeats and super-repeats suggested to early investigators that individual 35-amino acid modules can interact with actin and that a group of 7 repeats organized into a super-repeat interact with a stretch of F-actin containing 7 G-actin subunits bound to a tropomyosin-troponin complex (163, 186, 192, 193, 302, 350). Specifically, if nebulin repeats are α-helical, each repeat should extend ~5.5 nm, equivalent to the diameter of a G-actin monomer within the thin filament. Similarly, a super-repeat should extend ~38.5 nm, the length of a single tropomyosin molecule associated with seven actin monomers in the thin filament (182). Modeling studies as well as experiments with small fragments of nebulin support this idea by demonstrating the repetitive organization of nebulin repeats along the thin filaments, and the direct binding of short sets of repeats with F-actin (162, 163, 221, 301, 302, 350, 389). Modeling of the association of nebulin repeats with F-actin, ultrastructural studies, as well as consideration of the steric requirements placed on its association by the need for tropomyosin and the troponins to regulate actin-myosin binding in response to Ca2+, indicate that nebulin is oriented parallel to the long axis of the F-actin filament and the tropomyosin-troponin complex (221, 302, 350). Modeling suggests that short nebulin repeats sit in each of the symmetrically oriented grooves of the thin filament (302), but reconstruction of electron micrographs of filaments decorated with a set of four tandem nebulin repeats indicates that each of the repeats associates with a distinct site on the outer domains of the actin monomers, where they may bind to tropomyosin and troponin, as well as to actin itself (221). The latter experiments were conducted with nebulin repeats M170–173, from the COOH-terminal region of the protein, which associates with a segment of the thin filament that does not interact with myosin (see below). Structural studies of larger fragments of nebulin, including super-repeats associated with segments of the filaments that interact with myosin, will likely be needed to elucidate the roles of nebulin in organizing and regulating the activities of the thin filaments.
Modules 1–8 of nebulin, near its NH2 terminus, share some homology with the vast majority of modules in the protein, but they differ in their binding activities. They constitute the NH2-terminal 8 kDa of nebulin and are located in situ near the pointed end of F-actin. These modules harbor a binding site for tropomodulin, which caps actin filaments at their pointed ends (73, 187). The sequence of the 8-kDa NH2 terminus is unique to nebulin and is not found in closely related proteins, such as nebulin-related anchoring protein (N-RAP) or LIM and SH3 domain containing protein (LASP), in which it is replaced with an NH2-terminal LIM domain (222, 246, 356).
Modules COOH-terminal to repeat 162 are also distinct and contain binding sites for the Z-disk region of titin and for myopalladin, another protein of the Z-disk (284). Specifically, repeats M171-M183, in the COOH-terminal region of nebulin, share the S and YK residues of the typical nebulin repeats, but they lack the residues that define the super-repeats. They have therefore been termed “simple repeats” or “single repeats” (193, 350, 389). The repeats that link them to the super-repeat region are distinct, as well, and have been termed “linker repeats” (193, 350). Like repeats M1-M8 at the NH2 terminus, repeats M184 and M185 differ more extensively from the typical nebulin repeat. Adjacent to M185 is an SH3 domain, which forms the COOH-terminal domain of nebulin that binds myopalladin (22, 227), as well as a region of titin associated with the Z-disk (404). The COOH-terminal 20-kDa portion is unique to nebulin but is shared with closely related proteins, including nebulette, N-RAP, and LASP, which have homologous SH3 domains.
The orientation of nebulin, with respect to the Z-disk, I-band, and the pointed and barbed ends of the thin filaments in the I-band, has been established by a combination of in vitro binding assays, to identify its ligands in the contractile apparatus (see below), and by immunoelectron microscopic studies. Early ultrastructural studies with monoclonal antibodies directed to different regions of nebulin clearly established that it was organized between the Z-disk and the end of the I-band (186, 353, 391, 404, 409). These studies also showed that its COOH-terminal region is present within or at the periphery of the Z-disk and that its NH2-terminal region extends into the sarcomere at or near the ends of the F-actin filaments of the I-band, at distances of ~0.9 μm or more from the center of the nearest Z-disk (186, 409). Confirmation of the location of nebulin's NH2- and COOH-terminal regions has been obtained by immunoelectron microscopic studies of their ligands, specifically myopalladin (22), which binds to the SH3 domain at nebulin's COOH terminus and links it to the EF-hand domain of α-actinin, and tropomodulin, which binds to nebulin's NH2-terminal 8-kDa domain (129), capping the pointed end of the thin filaments. Immunoelectron microscopic studies place the SH3 domain 30–40 nm from the middle of the Z-disk, well within this structure (339). Thus nebulin extends from the Z-disk, where it is anchored at its COOH terminus, to the ends of the thin filaments in the I-band.
Although the evidence summarized above suggests that the SH3 domain of nebulin helps to anchor nebulin to the Z-disk, an alternative model was recently proposed in which modules 160–164 of nebulin are involved in anchoring. These modules were shown by in vitro binding studies to associate with CapZ, the barbed end actin binding protein (289). If this model is correct, these repeats should lie within or at the edge of the Z-disk, contrary to the results of ultrastructural studies (245). The latter used immunoelectron microscopic methods to examine the location of modules 177–181 of nebulin and placed them at the edge of the Z-disk, ~60 nm from its midline. By implication, modules 160–164, 17 repeats NH2-terminal, should be located ~94 nm further from the middle of the Z-disk, well within the I-band, where they would be unlikely to associate with CapZ. It is not clear how the binding of modules 160–164 to CapZ and its putative role in anchoring nebulin to the Z-disk (289) can be reconciled with the immunoelectron microscopic localization of modules 177–181 (245).
The idea that nebulin aligns laterally with actin microfilaments in the I-band, suggested by the spacing of the nebulin repeats and super-repeats (please see above) and the ability of much of the molecule to bind to actin (162, 163, 221, 302, 350, 388), has been strongly supported by immunoelectron microscopic studies. Of the monoclonal antibodies generated to nebulin, some label at a series of sites along the I-bands of skeletal myofibers. Analysis of the patterns generated by these antibodies showed a minimum spacing between these sites of 38–40 nm (186), close to the calculated distances covered by the nebulin super-repeats and to the periodicity of actin in thin filaments in situ. Although their epitopes have not been mapped, these results suggest that the monoclonal antibodies showing this periodic labeling recognize the WLKGIGW sequence that is common to nebulin's super-repeats, but not to the individual repeats. These results suggest that the mass of nebulin is arrayed more or less uniformly along the thin filaments (409), consistent with its forming an α-helical structure that associates laterally with the F-actin filament.
As mentioned above, although nebulin is made by a single gene in mammals, its mRNA can be alternatively spliced to produce nebulin proteins of different sizes in different muscles. There is a strong correlation between the sizes of nebulin in these muscles and the lengths of the thin filaments of the I-band (186, 192), which supports the idea that nebulin aligns laterally with thin filaments to help determine their overall length. In this case, epitopes shared by small and large forms of nebulin, located in the middle or near the NH2 terminus of the molecule, should localize nearer or farther from the center of the Z-disks, respectively. This has been confirmed for at least one such epitope (409), consistent with the idea that nebulin is arrayed co-linearly with thin filaments and plays a role in determining their overall length.
Ultrastructural results also indicate that nebulin is inextensible. Epitopes do not change their position relative to the center of the Z-disk as sarcomeres are stretched, even to the point of eliminating the overlap of thick and thin filaments (186). This is in sharp contrast to epitopes in the I-band region of titin, which move farther from the Z-disk as sarcomere length is increased (see above). The constancy of nebulin's relationship with the thin filaments of the I-band and with the Z-disk are consistent with a structural model in which nebulin associates laterally with thin filaments of the I-band along most of its length and anchors to the Z-disk at its COOH terminus.
Given the close association of nebulin with the thin filaments, nebulin would not be expected to remain in place when thin filaments are absent. Indeed, treatment of myofibrils with phalloidin, to alter the stability of actin filaments, causes the normally extended structure of nebulin to collapse (8). Nevertheless, gelsolin treatment of isolated myofibrils to fragment the thin filaments of the I-band leaves nebulin extended at its normal length. Remarkably, nebulin appears to remain organized normally after gelsolin treatment removes both thin filaments and Z-disks (186). These results suggest that nebulin's position in the sarcomere is determined in large part by its interactions with proteins of the Z-disk that remain in place when α-actinin is removed, and with proteins of the I-band that remain associated with the A- or M-band when actin is removed, probably titin (404). Although nebulin binds to myopalladin and other proteins of the Z-disk (see below), their fate in gelsolin-treated myofibrils has not been determined. Similarly, nebulin has been reported to interact with titin in the I-band region (226), but this is mediated by its SH3 domain, located at the Z-disk, and a mechanism that would maintain nebulin's relatively rigid structure in I-band-free sarcomeres through an association with the most extensible region of titin is not immediately apparent.
The close relationship of nebulin to thin filaments of the I-band in skeletal muscle also predicts that thin filaments would vary greatly in length when nebulin is absent. This is not the case, however. Two different groups have reported the formation of narrower I-bands with shorter thin filaments in nebulin-null muscle (21, 404), but remarkably in one of these null strains the filaments appear to be of relatively uniform size (21). As expected, nebulin-null skeletal muscle generates less contractile force than controls, with the null strain with uniform thin filaments showing a smaller decrement (105) than the more severely affected strain (287). Although the thin filaments that form the I-bands in cardiac muscle are not as uniform in size as those in skeletal muscle, they too form largely in the absence of nebulin, which in postnatal muscle is only found in atrial and in a few ventricular cardiomyocytes. Thus I-bands and their constituent thin filaments assemble and achieve some uniformity of organization, even when nebulin is absent. This strongly suggests that nebulin is not a template for formation and organization of thin filaments, but instead provides a mechanism for stabilizing thin filaments at a length determined by the size of the nebulin molecule, when, as in skeletal muscle, a fixed uniform length is important for function. The mechanisms responsible for determining the length of the thin filaments in cardiac ventricular muscle remain unknown.
Perhaps equally remarkably, nebulin-null skeletal muscle shows a ~70-fold increase in the expression of sarcolipin and a ~2-fold decrease in the expression of SERCA, without concomitant changes in two other Ca2+ regulatory or binding proteins, phospholamban or calsequestrin (285). Consistent with these changes, the rate of Ca2+ reuptake into the sarcoplasmic reticulum is reduced. Nebulin-null muscles also show a decrease in maximal contractile strength under tetanic stimulation, but it is not clear if this is due to changes in the function of the SR or in the interactions between the shorter thin filaments present in these muscles and myosin. The link between nebulin and the mechanisms controlling the expression of SERCA and sarcolipin, suggested by these experiments, remains to be studied.
B. Ligands of Nebulin
1. Nebulin-like repeats and binding partners
Work from several laboratories has demonstrated that nebulin has binding sites for an array of diverse proteins, some of which are components of thin or thick filaments and interact with the super-repeat domains of nebulin, and others of which associate with nebulin's unique NH2- and COOH-terminal regions, located at the ends of the thin filaments and in the Z-disks, respectively (Fig. 4, Table 3).
TABLE 3.
Nebulin binding partners
| Ligand | Binding Region of Ligand | Binding Region of Nebulin | Reference Nos. |
|---|---|---|---|
| NH2-terminal region (M1-M8) | |||
| Tropomodulin | M1–M3 | 240 | |
| Middle region (M9–M162) | |||
| Actin | SDXXYK motif of the central repeats | 162, 163, 186, 192 | |
| Tropomyosin | Central repeats | 389 | |
| Troponin | Central repeats | 389 | |
| Myosin | Central repeats | 162 | |
| NH2-terminal repeats | |||
| Myosin binding protein-C | Central repeats | 162 | |
| Calmodulin | NH2-terminal repeats | 319, 334 | |
| COOH-terminal repeats | |||
| COOH-terminal region (M163–M185, SH3) | |||
| Desmin | Coiled coil domain | M163-M170 | 20 |
| α-Actinin | Z-disk repeats | 264 | |
| CapZ | M160-M164 | 404 | |
| SH3 domain | 289 | ||
| Archvillin | COOH-terminal region | M184-M185 | 201 |
| Myopalladin | Proline-rich region of the 3rd insertion domain | SH3 domain | 22 |
| Titin | Zis1 | SH3 domain | 196 |
| PEVK | 305, 306 | ||
2. The central M9-M162 repeats contain binding sites for thin and thick filament proteins
A) ACTIN
The interaction of nebulin with actin has been extensively studied since the 1990s. A number of early studies had convincingly demonstrated that the conserved 35-amino acid-long modules of nebulin contain binding sites for actin (162, 163, 186, 192). Biochemical, biophysical, and structural approaches showed that individual, complete nebulin repeats, but not truncated repeats, bind actin (51, 301, 319). The helical conformation is strongly stabilized by the presence of anionic detergents, which may mimic the effects of the clusters of negatively charged amino acids in the environment encountered by nebulin when it is bound to actin (302).
If each repeat of 35 residues serves as an actin binding site, then the number of actin binding domains on intact nebulin may be as many as ~150 or more, assuming that each one of nebulin's central motifs behaves similarly with regards to actin binding (192, 301, 389). Such a linear lattice of binding domains should exhibit a high avidity for actin, resulting from the contribution of the large number of independent binding sites of moderate affinity along the length of the molecule, as well as the possibility of their cooperative interactions (51, 110, 163). Consequently, the possible one-to-one matching between single nebulin modules and actin monomers suggests that nebulin may function as an actin “zipper.” Given the strong correlation between the number of nebulin modules and the length of skeletal muscle thin filaments in different species, it was postulated that two nebulin molecules span the entire length of the thin filament, occupying symmetrical positions along the central cleft of the two actin strands, and operate as rulers by determining the length of thin filaments or stabilizing them at lengths consistent with the number of nebulin repeats present. This prediction has been supported by careful comparisons of thin filament lengths in different muscles with the sizes of the nebulin molecules they express (186, 391, 409, 425). Consistent with this, in vitro studies using RNA interference of nebulin expression in primary cultures of cardiomyocytes yielded thin filaments of irregular lengths (242). Elimination of nebulin by homologous recombination also alters thin filament lengths (21, 404), but, remarkably, in one nebulin-null strain muscle fibers have I-bands that, though shorter than controls, are relatively constant in length (see above).
B) TROPOMYOSIN/TROPONIN
Using solid-phase binding as-says, Wang et al. (389) performed a systematic screening to identify potential interactions of the portion of nebulin that contains the super-repeats, with other major myofibrillar proteins, besides actin. Using recombinant nebulin fragments containing two to eight modules, they found that both troponin and tropomyosin showed moderate binding affinities for the different nebulin fragments, ranging from low to high micromolar. Notably, troponin exhibited a stronger binding to the various nebulin fragments than tropomyosin, although both proteins bound more weakly than actin.
The ability of the central nebulin-like repeats of nebulin to interact with actin, tropomyosin, and troponin led the same authors to propose a model whereby a single nebulin-like module within each 7-module super-repeat binds an actin protomer through its SDXXYK signature peptide, while each one of the 22 super-repeats binds 1 tropomyosin/troponin complex through a conserved WLKGIGW peptide found once in every super-repeat (389). Thus it was proposed that the nebulin super-repeat might serve as a molecular template for the binding, orientation, and spacing of the actin/tropomyosin/troponin complex. Surprisingly, this hypothesis has not yet been subjected to rigorous testing.
C) α-ACTININ
α-Actinin, the major actin binding protein of the Z-disk, has been also shown to bind nebulin in blot overlay experiments of myofibrillar proteins obtained from skeletal muscle homogenates (264). The exact binding sites and the physiological significance of this interaction have not been determined, however.
D) MYOSIN AND MYOSIN BINDING PROTEIN-C
In addition to binding actin and proteins of the thin filament and Z-disk, the central region of nebulin also binds proteins of the thick filament and specifically myosin and MyBP-C (163, 186, 291, 319). Although its binding to MyBP-C has not been well characterized, binding of nebulin's super-repeat region to myosin and its effects on actomyosin interactions was well addressed by Wang and colleagues (163, 319). They found that cloned nebulin fragments that contained seven or eight nebulin-like repeats, examined in solid-phase assays, were able to bind myosin (and myosin heads) with high affinity, suggesting that nebulin may help to tether actin and myosin in rigor. The possibility that its interaction with myosin is physiological is suggested by the fact that the COOH-terminal modules of nebulin, located near the Z-disk and therefore at a considerable distance from the A-band, showed weak, if any, binding to myosin, whereas fragments encompassing more NH2-terminal modules, situated in the region of nebulin medial to the A-I junction, exhibited high-affinity binding to myosin (319). Consistent with this, the latter fragments, but not the former, inhibited the actomyosin ATPase activity and the sliding velocity of actin over myosin in motility assays, implying a role for the NH2-terminal super-repeats of nebulin in regulating actomyosin activity.
E) CALMODULIN
Contrary to the binding of nebulin to myosin, which appears to be mediated by nebulin-like repeats located in the region of the molecule where the thin and thick filaments overlap, calmodulin interacts with multimodular fragments of either NH2- or COOH-terminal repeats (319, 334). Binding of nebulin to calmodulin is independent of Ca2+, suggesting that they form a relatively stable complex. In the presence of Ca2+, however, calmodulin greatly reduced the binding of nebulin fragments to either actin or myosin, with a stronger effect on the latter, reversing the inhibitory effect of nebulin on actomyosin ATPase activity and accelerating the sliding of actin over myosin (319). Thus nebulin's ability to modulate actomyosin activity is likely to be regulated by calmodulin in a Ca2+-dependent manner.
3. The COOH-terminal M163-M185 repeats bind desmin
The COOH-terminal M163-M185 nebulin-like repeats, located at the edge of the Z-disk, are not arranged into super-repeats, suggesting that they may bind to proteins other than those of the thin and thick filaments (193, 301). Consistent with this, Bang et al. (20) found that this portion of nebulin interacts with the intermediate filament protein desmin in a yeast two-hybrid screen. The direct interaction between nebulin and desmin was confirmed in vitro, and their minimal binding sites were confined to nebulin's modules M163-M170 and desmin's ~19 kDa, central, coiled coil domain. Although the functional significance of their interaction remains unknown, Bang et al. (20) proposed that it may mediate links between Z-disks and the desmin-based intermediate filaments that surround them, helping to align Z-disks in adjacent myofibrils and promoting efficient force transmission and mechanochemical signaling.
4. The NH2-terminal modules M1-M3 bind the pointed end capping protein tropomodulin
Tropomodulin, a protein that caps thin filaments at their pointed ends, was originally identified as a tropomyosin-binding partner in the membrane-associated cytoskeleton of the mammalian erythrocyte (74, 75). Two isoforms have been characterized in striated muscle cells that are the products of different genes and show restricted tissue distribution: E-tropomodulin is present in embryonic skeletal muscle, slow-twitch myofibers, and heart cells, whereas Sk-tropomodulin is expressed in fast-twitch skeletal myofibers late in differentiation and into maturity (5, 77, 133). Both tropomodulin isoforms localize at the pointed ends of actin filaments, where they inhibit actin polymerization in a tropomyosin-dependent manner (133, 397–399).
Studies by McElhinny et al. (240) showed that tropomodulin participates in the maintenance of thin filaments and in the specification of their lengths, through its direct interaction with the NH2-terminal modules of nebulin. Using in vitro binding assays, they showed that the NH2-terminal M1-M2-M3 repeats of nebulin, situated with tropomodulin at the pointed ends of actin filaments, contain the minimal site for binding tropomodulin (240). Sk-tropomodulin showed higher affinity binding to nebulin (in the low nanomolar range) than E-tropomodulin (in the low micromolar range), although the physiological significance of this result is unclear. Notably, the targeting of E-tropomodulin to the pointed ends of the thin filaments was significantly altered in nebulin-deficient skeletal myofibers, but its expression levels were unaffected (404).
5. The COOH-terminal SH3 domain of nebulin provides binding sites for several sarcomeric proteins
Yeast two-hybrid screens and in vitro binding assays have shown that the COOH-terminal SH3 domain of nebulin can bind to a number of myofibrillar proteins, including the barbed end actin capping protein CapZ, the Z-disk protein myopalladin, as well as the PEVK and Zis2 domains of titin. The ability of the SH3 domain of nebulin to interact with such diverse proteins directly, and with their respective ligands indirectly, suggests the existence of a multicomponent anchoring system involved in myofibril assembly, mechanochemical signaling, and regulation of gene expression.
A) CAPZ
In addition to regulating capping of the pointed end of thin filaments, by binding tropomodulin at its NH2-terminal region (see above), nebulin also regulates capping at the barbed end through its COOH-terminal SH3 domain, which binds directly to the barbed end-capping protein CapZ (404). CapZ localizes at the edges of Z-disks, functions as an α/β heterodimer, and inhibits actin polymerization and depolymerization (23, 47, 396).
In contrast to the results of Witt et al. (404), Papas et al. (289) recently reported that CapZ binds directly to repeats M160-M164 of nebulin, rather than to the SH3 domain. Binding occurred with an affinity in the low nanomolar range (289), similar to the interaction between nebulin and tropomodulin (240). Studies with peptide arrays indicated that repeats M160-M164 contain two binding sites for CapZ and that each contributes to the interaction. Moreover, COOH-terminal truncation mutants of CapZ were able to bind nebulin's M160-M164 repeats with an affinity comparable to that of wild type, suggesting that the COOH-termini of the α/β CapZ heterodimer, which contain the binding sites for the barbed end of actin filaments, do not contribute to binding to nebulin (289, 396). Consistent with this, the actin-capping activity of CapZ was not affected by its interaction with nebulin.
Using small inhibitory RNA technology, Papas et al. (289) reduced the expression of nebulin in primary cultures of skeletal myotubes. Downregulation of nebulin resulted in significant disruption of the organization of CapZ at the Z-disk. As neither its transcript nor its protein levels were altered, this result suggests that the interaction of CapZ with nebulin is essential for its proper targeting to the Z-disk. This result, too, contrasts with that of an earlier study by Witt et al. (404), who demonstrated that the sarcomeric incorporation and organization of CapZ was only mildly affected in skeletal myofibers of nebulin-null mice. In the total absence of nebulin, these authors found that CapZ still assembled at Z-disks, although its staining appeared weaker and broader compared with wild-type muscle. A possible explanation for these variable outcomes may be the inherent differences between nebulin's acute downregulation in an in vitro system and chronic elimination in an in vivo animal model, where compensation by other proteins may alleviate the phenotypic severity of a genetic manipulation.
The results of Papas et al. (289), indicating that CapZ binds to repeats M160–164 in the Z-disk region of nebulin, are in sharp contrast to an earlier report by Millevoi et al. (245), who predicted that repeats M160-M164 localize outside the Z-disk, in the I-band. Papas et al. (289) proposed an alternate model in which M160-M164 repeats are located within the Z-disk. This model was based on their additional observations that desmin, an intermediate filament protein present at the periphery of the Z-disk, also interacts directly with M160-M164, and that α-actinin binds to repeats M160-M170 in yeast two-hybrid assays (289). Detailed studies of the localization of both CapZ and nebulin's M160-M164 repeats at the ultrastructural level will be required to resolve these discrepancies and to refine the mechanisms by which nebulin and CapZ cap the barbed ends of thin filaments.
B) MYOPALLADIN
Myopalladin is a ~145-kDa sarcomeric protein that was originally identified in a yeast two-hybrid screen for binding partners of the extreme COOH terminus of nebulin (22). Analysis of its primary sequence demonstrated that it contains five Ig domains, referred to as I–V, separated by six interdomain insertions, designated as IS1–IS6. Myopalladin is abundantly expressed in both cardiac and skeletal muscle cells, where it concentrates primarily at the Z-disk and to a lesser extent at the I-band. In isolated cardiomyocytes, myopalladin is also reported to be present in the nucleus (22).
Deletion analyses in combination with in vitro binding assays were used to identify the minimal sites on nebulin and myopalladin that mediate their interaction. These studies localized the binding sites to the COOH-terminal SH3 domain of nebulin and to a 42-residue, proline-rich sequence in the IS3 domain of myopalladin (22). Like tropomodulin and CapZ, the expression of myopalladin was not affected in skeletal myofibers of nebulin-deficient animals, but its incorporation into sarcomeres was altered, as its labeling at Z-disks was rather diffuse (404).
To date, the functional significance of the binding of myopalladin to nebulin remains speculative, but the ability of each protein to interact with several other ligands involved in stabilizing the Z-disk and regulating gene expression suggests that their interaction may modulate these processes in muscle. Consistent with this, Ig domains III, IV, and V of myopalladin interact specifically and directly with the COOH-terminal EF-hand region of sarcomeric α-actinin-2, and its NH2 terminus directly binds to the transcriptional regulator CARP, which is found at the central I-band as well as in the nucleus (22, 25, 161, 430).
C) TITIN
The SH3 domain of nebulin has also been shown to bind directly to titin's Zis2 and PEVK regions, as discussed above (see sect. ii, A2 and B3).
In summary, nebulin is a giant actin-binding protein that, through its interactions with proteins associated with the Z-disks and the thin and thick filaments, contributes to the regulation of muscle contraction, and through its interactions with thin filament capping proteins provides a mechanism for stabilizing the length of the actin filaments in skeletal myofibers.
IV. OBSCURIN
Obscurin is the third and most recently discovered member of the family of giant proteins expressed in vertebrate striated muscle (421). It derives its name from the fact that it was at first difficult to characterize due to its large size (~720–900 kDa) and insolubility in extracts of adult skeletal and cardiac muscle. Later studies showed that, when appropriate methods are used, obscurin can be readily solubilized in buffer preparatory to SDS-PAGE and analyzed by immunoblotting (181).
Like titin, obscurin has a modular architecture of adhesion and signaling domains arranged in tandem (Fig. 5, Table 1) (85, 176, 320, 421). The NH2-terminal half of the molecule contains repetitive Ig domains, which are 88–92 amino acids in length and joined without any obvious linker sequences. In contrast to the arrangement of the Ig domains in titin (93, 191), no super-repeat pattern of the Ig domains is apparent in obscurin either by multiple sequence alignment or phylogenic tree studies (85, 320, 348, 421). Obscurin also contains 2 FN-III domains together with the 59 Ig repeats present in the NH2-terminal half of the molecule. The COOH-terminal portion of the molecule consists of four additional Ig domains, flanked by nonmodular sequences and several signaling domains, including an IQ motif, which binds to calmodulin or calmodulin-like proteins, a src homology 3 (SH3) domain, and tandem Rho-guanine nucleotide exchange factor/pleckstrin homology motifs (Rho-GEF/PH), suggesting the protein's possible involvement in Ca2+-mediated and Rho-GTPase-regulated signaling pathways. The most COOH-terminal region consists of 2 additional Ig repeats followed by a nonmodular region of ~420 amino acids that contains several consensus phosphorylation motifs for ERK kinases. This form of the protein, which is predominant in adult skeletal muscle (320), has been termed “obscurin-A,” to distinguish it from other products of the obscurin gene.
FIG. 5.
Schematic representation of the known isoforms of obscurin, illustrating their motifs, structures, and binding partners. Alternative splicing of the OBSCN gene results in at least four forms of obscurin: two giant isoforms, Obscurin A and Obscurin B/giant (g) MLCK, and two smaller kinase containing isoforms, Obscurin tandem (t) MLCK and Obscurin single (s) MLCK. All isoforms are composed of multiple structural motifs and signaling domains (see key for complete domain list and color coding). The giant isoforms diverge at the COOH terminus. Obscurin A is composed of a nonmodular COOH terminus, which houses the ankyrin-binding site (maroon box). The COOH terminus of Obscurin B/gMLCK possesses two kinase domains along with two additional Ig domains and a FN-III domain. Only 11 of obscurin's many domains have been characterized structurally, either by NMR or X-ray crystallography, which are shown as a cartoon model with flat arrows and coils representing β-sheets and α-helices, respectively. Binding partners and interaction sites that have been mapped to obscurin are also shown. Binding partners shown to interact directly with obscurin are depicted with a gray background and solid line pointing to their binding site on obscurin. The interaction between obscurin and myosin is not as well defined and may only be indirect and is thus shown by a dotted line. The phosphatase SCPL-1 and the LIM-domain protein LIM-9 were shown to interact directly with the kinases and preceding Ig and FN-III domains of Unc89, obscurin's invertebrate homolog, and are shown with a white background.
A. Structure of the Obscurin Gene: Implications for Isoform Generation
Obscurin-A is encoded by the OBSCN gene on human chromosome 1q42 (85). The full-length protein is encoded by 91 exons, which are subject to extensive alternative splicing (Fig. 5). The splice donor and acceptor sites for the exons encoding many of the tandem Ig domains are compatible and can therefore produce a large number of potential isoforms, some of which have been identified and termed “obscurin-A1,” etc. (85, 421).
Obscurin-A is encoded by the same gene cluster that produces obscurin-MLCK, a set of proteins containing two serine/threonine kinase domains (320). Obscurin-MLCK can be expressed independently, as smaller proteins containing one or both kinase domains, or as part of a second giant form of obscurin in which the two MLCK motifs are arranged in tandem at the COOH terminus of the protein, replacing the nonmodular COOH-terminal region of obscurin-A (37, 85). This obscurin isoform, which has been referred to as the obscurin-MLCK giant kinase isoform, or obscurin-B, is generated through a more complex splicing procedure that occurs at the 3′ end of the OBSCN gene, to link the mRNA sequences encoding the signaling domains to the sequences encoding the two kinase domains to a FN-III domain and two additional Ig repeats (85). The two smaller forms of obscurin-MLCK containing the kinase domains, referred to as single and double kinase isoforms, are also encoded by the OBSCN gene, and their expression depends on alternative translation initiation sites (37). Obscurins A and B are expressed mainly in skeletal and cardiac muscle, although low levels of a form of the giant obscurin-MLCK protein have also been reported in the brain (256–259). Given the large number of potential alternative splice products of the obscurin-MLCK gene, it seems likely that some will be expressed in other tissues as well.
B. Subcellular Distribution of Obscurin Isoforms in Striated Muscle Cells
Initial studies on obscurin's localization in adult myocardium, using antibodies targeted to internal sequences, including Ig29/30, Ig58/59, and Ig61/62 (numbering conforms with the revised nomenclature in Ref. 85), as well as the Rho-GEF domain, revealed that the protein primarily concentrates at M-bands (19, 421). Its distribution is more variable during embryogenesis, however, with obscurin epitopes accumulating transiently at Z-disks early in development and concentrating preferentially at M-bands, with eventual loss of Z-disk staining later (421). Notably, the Z-disk labeling, whenever observed, occurred primarily with antibodies against Ig58/59, the obscurin's titin-binding region (19, 421), suggesting that different regions of obscurin might occupy distinct sites within the sarcomere or that certain regions might be spliced out at different developmental stages and therefore remain undetected.
Subsequent studies of adult rat cardiac and skeletal muscle fibers with antibodies directed to the COOH-terminal nonmodular region of obscurin-A, localized the protein simultaneously at M-bands and Z-disks (177). In contrast, antibodies to its NH2 terminus and the Rho-GEF domain localized obscurin-A predominantly at M-bands, similarly to the results of Young et al. (421), raising additional questions about the subcellular distribution of the protein and its possible alternatively spliced variants at maturity (42, 176).
To study the subcellular localization of obscurin further, Bowman et al. (42) examined its distribution in adult rat skeletal myofibers at resting and stretched sarcomere lengths and provided potential explanations for the diverse staining patterns observed with the different antibodies. In particular, using antibodies against the NH2-terminal, Rho-GEF and COOH-terminal regions of obscurin-A, and to the second kinase-like domain (SKII) of obscurin-B, they showed that at resting sarcomere lengths obscurin-A primarily concentrates at the M-band, whereas obscurin-B localizes at the M-band and the A/I junction. Interestingly, antibodies that recognized the NH2 terminus and Rho-GEF domains of obscurin labeled the M-band region preferentially at resting muscle lengths, but labeled structures at the I-band near the A/I junction when the muscle was stretched, suggesting that stretching of the muscle may either unmask previously hidden epitopes, or that obscurin can redistribute to distinct sites along the sarcomere as muscle is stretched. These studies also suggested the presence of additional obscurin variants, distinct from obscurin A and B, at the Z-disk and the Z/I interface, raising the possibility of unique isoforms that may contain a novel NH2-terminal structure with the same COOH-terminal region as obscurin-A, but without the Rho-GEF domain (42). Independent evidence is consistent with the possibility that this form of obscurin appears at late stages of myofibril formation (178). Collectively, these observations strongly suggest a preferential integration of different obscurin isoforms to certain regions of the sarcomere, and further imply specialized functions of these isoforms during myofibrillogenesis and at maturity.
Recently, the subcellular localization of obscurin was also examined in developing and mature human muscles (45). Similar to the above studies, antibodies to its IQ motif and titin binding domains (Ig58/59) also showed preferential labeling at the M-band in both developing and adult skeletal and heart muscles. The same antibodies revealed the presence of obscurin or closely related proteins at the sarcolemma and the postsynaptic region of the neuromuscular junction, suggesting a possible role of obscurin-like proteins at costameres and the synapse. The molecular identity of the obscurin isoforms present in these structures remains unknown.
Detailed analysis of the distribution pattern of the protein in cross sections of adult skeletal muscle fibers using antibodies against its NH2 and COOH termini as well as the Rho-GEF domain revealed a clear reticular pattern, which suggested that obscurin, or at least large regions of the protein, are positioned at the surface of the myofibril rather than within it (42, 45, 176, 177). This unique localization of obscurin, distinct from other proteins of the sarcomere, suggested that it might envelop myofibrils at the M-band, and possibly at the Z-disk. At ~200 nm in length (421), however, a single obscurin molecule is clearly not big enough to surround myofibrils as a monomer but is instead likely to associate with other molecules of the Z-disk and M-band in such a way as to allow at least its NH2- and COOH-terminal regions, and possibly other domains, to be exposed at the surface of these structures. Alternatively, obscurin may homo-oligomerize to form a “ring” big enough to encircle developing myofibrils. Although this is still speculative, the ability of obscurin to surround sarcomeres raises the possibility that it may play a role in specifying the periphery, and thus the diameter, of myofibrils. The idea that obscurin would have to oligomerize to surround the myofibril is consistent with the fact that these diameters vary broadly.
C. Molecular Map of the Interactions Between Obscurin and Muscle Proteins
The presence of multiple adhesion and signaling motifs in the obscurin sequence, along with its unique localization around the myofibrillar Z-disk and M-band, suggested that obscurin might interact with several sarcoplasmic components. Consistent with this, obscurin associates with a diverse set of proteins, including myofibrillar components, like titin, and sarcomeric myosin, as well as peripheral and integral membrane proteins, like small ankyrin-1 and ankyrin-2 (Fig. 5, Table 4).
Table 4.
Obscurin binding partners
| Ligand | Binding Region of Ligand | Binding Region of Obscurin | Reference Nos. |
|---|---|---|---|
| Titin | MIg10 | Ig1 | 86 |
| ZIg9-ZIg10 | Ig58–Ig59 | 421 | |
| MyBP-C slow variant-1 | C10 domain +26 COOH-terminal residues | Ig2 | 1 |
| Myomesin | My4–My5 linker | Ig3 | 86 |
| Novex-3 | Ig58–Ig59 | 19 | |
| Calmodulin | IQ motif | 421 | |
| RanBP9 | Rho-GEF domain | 41 | |
| RhoA | Rho-GEF domain | 72, 310 | |
| TC10 | Rho-GEF domain | 53 | |
| Ankyrin 1.5 | Residues 61–130 | Non-modular COOH terminus | 18, 181 |
| Ankyrin 1.9 | Non-modular COOH terminus | 11 | |
| Ankyrin 2.2 | Non-modular COOH terminus | 55 | |
| SCPL-1 | Kinase domains and preceding Ig and FN-III repeats | 311 | |
| LIM-9 | NH2-terminal kinase domain + | 413 | |
| Interkinase sequence |
1. Titin
Obscurin was serendipitously discovered in mammalian muscle in experiments that used a yeast two-hybrid screen to identify novel binding partners of titin (421). The peripheral Z-disk domains, ZIg9-ZIg10, of titin were found to interact with Ig domains 58–59 of obscurin (modified nomenclature according to Ref. 85), located just NH2-terminal to obscurin's second FN-III domain and signaling motifs (421). In vitro binding studies indicated that the paired domains of both proteins were necessary to form a functional binding site, as the interaction failed to occur when either titin Ig domains Z9 and Z10 or obsurin Ig domains 58 and 59 were assayed individually.
A similar interaction was identified between obscurin and a smaller ~700 kDa isoform of titin, novex-3, which extends between the Z-disk and the I-band (19). Ig domains, Ig58-Ig59, that interact with the full-length titin molecule, also interact with Ig-repeat 21 of novex-3. Immunoelectron microscopy with antibodies to this region of obscurin localized it to structures 60 nm from the edge of the Z-disks of sarcomeres 1.9 μm in length, but 120 nm from the edge in sarcomeres 2.2 μm in length. Thus these experiments, like those of Bowman et al. (42), suggest that the structures containing obscurin may be dynamic and move further from the Z-disk with increasing sarcomere length. Although the physiological significance of the interaction of obscurin with the different titin isoforms is still elusive, obscurin molecules in the vicinity of the Z-disk may bind to both large and small titin isoforms to promote the regular assembly of thin filaments (19).
2. Small ankyrin-1
In an effort to understand how the membranes of the SR become aligned with the nearby myofibrillar cytoskeleton, two groups independently used the yeast two-hybrid screen to search for potential binding partners of small ankyrin 1 (sAnk1, also known as Ank1.5) (18, 181), an integral membrane protein of the network SR (307). Both studies identified obscurin as the major ligand of the cytoplasmic domain of sAnk1, and for the first time demonstrated direct binding between proteins of the sarcomere and the SR.
The two sets of studies identified distinct binding sites for sAnk1 at the COOH terminus of obscurin, however. Kontrogianni-Konstantopoulos et al. (181) determined the binding site on obscurin to be within a ~120 amino acid segment, equivalent to residues 6312–6432 of the human obscurin ortholog (181). Extensive studies by the authors, using an array of in vitro binding assays, further confirmed that the binding of sAnk1 to obscurin is specific and direct, with a binding constant, determined by surface plasmon resonance, of ~130 nM. In contrast, Bagnato et al. (18) localized the sequence responsible for binding of obscurin to sAnk1 (referred to as ank1.5) to a distinct but nearby site, a region of 25 amino acids corresponding to residues 6236–6260. A likely explanation for this variability is the existence of more than one site of interaction in obscurin for sAnk1, which may be differentially regulated and possess different affinities for sAnk1.
In a follow-up study, Armani et al. (11) also explored the ability of other small products of the ANK1 gene, namely, ank1.6, ank1.7 and ank1.9, to bind to the COOH terminus of obscurin in vitro. In contrast to ank1.5 (sAnk1), ank1.6 and ank1.7 failed to bind to the COOH terminus of the protein; ank1.9 did bind, however, although with significantly lower apparent affinity than sAnk1/ank1.5. The weak association of ank1.9 with the COOH terminus of obscurin, as well as its low abundance in muscle cells, suggest that it is unlikely to play as large a role as ank1.5 in binding obscurin.
The minimal binding site on sAnk1 for obscurin is confined to the cytoplasmic region of sAnk1 that contains two stretches of positively charged amino acids, one that is unique to sAnk1 and another that is conserved among the smaller and larger ankyrin isoforms (18, 181, 426). These two regions, which have been modeled as ankyrin-like repeats, are both necessary and sufficient to support binding to obscurin, since elimination of either one abolishes binding completely (39). Consistent with this, sAnk1/ank1.5 and ank1.9 are the only known ankyrin 1 isoforms to contain both stretches of positively charged binding sequences, thus accounting for the selective binding of obscurin to these specific ankyrin variants (11, 39). Recently, however, a new alternatively spliced form of ankyrin 2 (also known as ankyrin B) was identified that also contains two such homologous stretches near its COOH terminus, and shown to interact with obscurin (55). As both sAnk1 binding sites identified on obscurin by Kontrogianni-Konstantopoulos and co-workers (39, 181) and Bagnato et al. (18) contain stretches of negatively charged amino acids, it seems likely that electrostatic interactions play an important role in the binding of sAnk1 to obscurin.
The discovery of a direct interaction between obscurin, a protein associated with the contractile filament, and sAnk1, an integral protein of the SR, provides the first evidence of a link between the contractile apparatus of the muscle and its surrounding SR membrane (18, 181). This is consistent with obscurin's unusual distribution at the periphery of myofibrils. As previously discussed, the COOH- and NH2-terminal regions of obscurin-A are exposed on the surface of the myofibrils, primarily at the level of M-bands, where they can interact with surrounding structures. Both binding sites identified on obscurin for sAnk1 are present in the nonmodular COOH-terminal region of obscurin-A (18, 181). Consistent with their interaction in situ, sAnk1 exhibits a similar distribution in striated muscle as these epitopes of obscurin (181, 426), and indeed, the COOH-terminal epitope of obscurin is located within molecular distances of the surface of the network compartment of the SR (176), where sAnk1 concentrates (426). Thus the COOH-terminal regions of the two molecules are sited appropriately to bind to each other. As kinetic studies have demonstrated that obscurin and sAnk1 bind with high affinity, it has been postulated that obscurin, by virtue of its tight association with sAnk1, may serve to anchor and orient the network SR around nearby contractile elements (39, 181). In agreement with this, SR membranes fail to organize around the contractile apparatus when obscurin's expression is inhibited by small inhibitory RNA (siRNA) constructs (180). As other structural elements of sarcomeres were altered when obscurin levels were reduced, however, additional experiments will be required to substantiate the role of obscurin in organizing sAnk1 and the network compartment of the SR. The possibility that other proteins may be involved has received recent support from a report that sAnk1 first appears at sites along developing sarcomeres that lack its binding region on obscurin (104).
A) RAN BINDING PROTEIN-9
Although the function of the Rho-GEF domain of obscurin has been a subject of speculation since it was first identified and sequenced in 2001 (421), it was only recently that biochemical and cellular transfection experiments demonstrated its direct binding to Ran binding protein-9 (RanBP9), suggesting one, of potentially many, physiologically significant interactions (41).
RanBP9 is a protein first identified in centrosomes and shown to bind to Ran GTPases (263). It is a scaffolding protein that can associate with a variety of proteins in different subcellular locations (270), including signaling proteins, cell surface and nuclear receptors, as well as protein kinases and other enzymes (254). The role of RanBP9 in skeletal muscle is not yet well understood, but Bowman et al. (41) clearly demonstrated its ability to bind directly to the Rho-GEF domain of obscurin, with an affinity of ~2 μM. RanBP9 also partially colocalizes with the Rho-GEF domain of obscurin near the Z-disk in developing myotubes, although it is not clear if obscurin or RanBP9 is the primary scaffolding protein in this case. Using adenoviral-mediated delivery of the Rho-GEF domain of obscurin and its binding site on RanBP9, together with immunofluorescence methods, Bowman et al. (41) showed further that overexpression of either of these domains inhibited the ability of the NH2-terminal portion of titin to integrate properly into developing Z-disks, although it had no effect on the integration of the COOH-terminal region of titin into developing M-bands. Thin-section electron microscopic studies confirmed that the integrity of the Z-disk was compromised when the Rho-GEF domain was overexpressed, but that the M-band was unaffected. These investigators also demonstrated that both the Rho-GEF domain and its binding region on RanBP9 can bind independently to the two NH2-terminal Ig domains of titin (ZIg1/ZIg2) and speculated that this binding might regulate titin's integration and maintenance at the Z-disk (41).
B) SMALL GTPASES
The most widely characterized role of GEF domains is the activation of small GTPases, but the mammalian GTPases that are activated by obscurin's Rho-GEF domain are just now being identified. Initial progress on this question was achieved in studies of the ortholog of obscurin in Caenorhabditis elegans, UNC-89, the first form of obscurin identified (27, 337). Those studies used the yeast exchange assay (a variant of the yeast three-hybrid assay) to identify Rho1 (RhoA in C. elegans) as a specific ligand of the paired double homology (DH, equivalent to Rho-GEF) and PH domains, and confirmed its identity by demonstrating direct binding and activation-Speelman of Rho1 by these domains of UNC-89 in vitro (310). Recent results of Ford-Speelman et al. (72) indicated that the Rho-GEF domain of mammalian obscurin specifically binds to and activates the mammalian ortholog of Rho1, RhoA. Overexpression of the Rho-GEF domain also increases the levels of RhoA and induces its redistribution from the level of M-bands, where RhoA normally concentrates, to structures near Z-disks, where it activates the downstream effector Rho kinase (ROCKs). Similar changes in RhoA localization and activity occur in muscle injured by large-strain lengthening contractions, suggesting that these changes may be physiological, perhaps linked to mechanisms mediating repair or hypertrophy. RhoA is not the only small GTPase capable of interacting with obscurin's Rho-GEF domain, however. TC10, a small GTPase not previously thought to influence the biology of striated muscle, is expressed in skeletal myotubes and is activated by obscurin's Rho-GEF domain. This is likely to play a role in later stages of differentiation, as inhibition of TC10 activity blocks myofibrillogenesis (53). The ability of obscurin to activate small GTPases in muscle, and the factors that influence this activation, are likely to play a wide range of important roles in the plasticity of muscle tissue, in particular during development, as well as in its response to injury or during atrophy or hypertrophy.
C) MYOMESIN AND TITIN
The prominent localization of obscurin at the M-band prompted Fukuzawa et al. (86) to identify binding partners of obscurin that reside at or in the vicinity of the M-band. Using the yeast two-hybrid screen followed by in vitro binding assays, the authors found that the most NH2-terminal Ig domain of obscurin (Ig1) binds directly to the most COOH-terminal Ig domain of titin (M10), located in the M-band, whereas the third Ig domain of obscurin (Ig3) interacts directly with the linker region present between two of myomesin's FN-III domains, My4 and My5.
Overexpression studies by the same investigators suggested that both regions of obscurin are likely to play important roles in assembling and stabilizing the M-band regions of sarcomeres. Transfection of neonatal cardiomyocytes with plasmids encoding the Ig1 domain of obscurin, harboring the binding site for titin, or its Ig3 domain, harboring the binding site for myomesin, inhibited the assembly of endogenous obscurin at M-bands either partially (Ig1) or completely (Ig3) (86). Similarly, overexpression of myomesin's My4-linker-My5 domains inhibited the incorporation of endogenous obscurin into M-bands, with the residual protein appearing in broad striations, whereas overexpression of titin's M10 domain led to a milder phenotype. It is not clear why these different treatments, which should affect the same protein complex, altered the organization of endogenous obscurin in such distinct ways. One possibility is that the use of two FN-III domains of myomesin and the intervening linker sequence, rather than the minimal binding site, may affect other protein-protein interactions as well (274). No apparent changes were observed in the localization of either titin or myomesin following overexpression of obscurin's Ig1 or Ig3 domains. These findings suggested that obscurin's targeting to the M-band in cultured cardiocytes may depend on its interactions with myomesin and titin, but not vice versa. This agrees with an earlier report that obscurin assembles at M-bands shortly after myomesin and the COOH-terminal region of titin (178). It will be of interest to examine the effects of overexpression of the three most NH2-terminal Ig domains of obscurin, which contain binding sites for at least two major proteins of the M-band, to learn if they alter assembly or stability of that structure. If, as suggested by experiments with siRNA (180), obscurin plays a role in stabilizing M-bands, then this region should inhibit the assembly of other major M-band components, including myomesin, and perhaps the COOH-terminal region of titin.
D) MYOSIN BINDING PROTEIN-C SLOW VARIANT-1
In addition to binding myomesin and titin at the M-band, obscurin also interacts with a novel isoform of the thick filament associated protein MyBP-C slow, specifically variant-1. This alternatively spliced form of MyBP-C slow preferentially concentrates at the periphery of M-bands in both developing and adult skeletal myofibers, where it codistributes with obscurin (1). MyBP-C slow variant-1 shares a common primary sequence and domain architecture with the prototypical forms of MyBP-C slow, but it has a unique COOH terminus, consisting of 26 amino acids following the last Ig domain (C10) present in all isoforms of MyBP-C slow. The second Ig domain of obscurin (Ig2) and the last Ig domain (C10) of MyBP-C slow variant-1 are both necessary and sufficient to mediate binding, but binding is significantly enhanced by the presence of the novel 26 amino acids in the COOH terminus of variant-1 (1). Over-expression of obscurin's Ig2 domain in primary cultures of skeletal myotubes severely disrupted the formation of M- and A-bands, but not of Z-disks and I-bands, suggesting that the binding of obscurin to the novel variant of MyBP-C slow is involved in the organization of thick filaments (1). Thus the studies by Fukuzawa et al. (86), discussed above, and Ackermann et al. (1) strongly suggest that obscurin, through its interactions with other components of the myofibrillar M-band, plays a critical role in the assembly and maintenance of this structure.
D. Role of Obscurin in Myofibrillogenesis
The spatial and temporal dynamics of the distribution of obscurin during de novo myofibrillogenesis have been studied in C2C12 myotubes and in primary cultures of cardiomyocytes (34, 35, 180). In C2C12 cells, obscurin initially organized into primitive striations that coincided with developing M-bands, within 24–48 h postdifferentiation. At this stage of development, myomesin and the COOH-terminal epitopes of titin had already assembled at M-bands, shortly after α-actinin and the NH2-terminal epitopes of titin had integrated into Z-disks (178). At this time, sarcomeric myosin had not yet assembled into mature A-bands, but instead accumulated in long filamentous structures with occasional periodicity. The presence of myosin in well-defined A-bands in developing C2C12 myotubes was not apparent until 72–96 h after initiation of differentiation, ~2 days after the assembly of obscurin in M-bands. Likewise, the localization of obscurin into Z-disks occurred from 72–96 h postdifferentiation, when fully striated myofibrils had already formed. As the forms of obscurin at Z-disks are likely to be distinct from the ones at M-bands (42), this suggests that different obscurin isoforms with potentially diverse activities localize to different sites in the developing sarcomere and play distinct roles in myofibrillogenesis. This is consistent with results of studies of the RhoGEF domain (41) and the effects of siRNA suppression of obscurin synthesis (180). Neonatal cardiomyocytes showed a similar temporal sequence of assembly of obscurin into sarcomeres, in which obscurin associated with M-bands at early stages of myofibrillogenesis and with Z-disks only later, when sarcomeres were nearly mature (34, 35).
Remarkably, the progressive incorporation of obscurin into M-bands and Z-disks at later stages of myofibrillogenesis coincides with the lateral alignment and fusion of myofibrils into larger bundles (34, 35). Thus obscurin may promote the lateral alignment of myofibrils. This is consistent with data obtained in zebrafish that developed from embryos treated with morpholinos to reduce the expression of obscurin (38), but it does not agree with results obtained in skeletal myotubes, in which the lateral alignment of Z-disks was only slightly affected by siRNA targeted to obscurin (180). These differences may be due to subtle differences in the process of myofibrillogenesis in cardiac and somitic myocytes, compared with skeletal myotubes. Borisov et al. (34) further reported that the association of desmin with the Z-disks of developing myofibrils occurred only after obscurin had accumulated around Z-disks, consistent with a role for obscurin in the proper positioning of desmin filaments.
In contrast to studies of the formation and alignment of Z-disks, in which the role of obscurin is both subtle and dependent on the muscle examined, obscurin's role in the formation of the M-band and A-bands is well established. This was first demonstrated by adenoviral-mediated overexpression of the COOH-terminal nonmodular region of obscurin-A in cultures of primary skeletal myotubes, in which myosin's assembly into A-bands was specifically inhibited (179). In treated myotubes, myosin either remained diffusely distributed or accumulated in cytoplasmic aggregates, and at best showed only occasional periodicity. Remarkably, the organization of other sarcomeric markers, including actin, α-actinin, myomesin, and epitopes of titin, at the Z-disk and the A/I junction were unaffected. Thus these observations pointed to a vital role for obscurin's COOH-terminal region in regulating the assembly of myosin during sarcomere formation.
A subsequent study revealed that inhibiting the expression of obscurin by siRNA treatment of primary cultures of skeletal myotubes also disrupted the assembly of myosin into A-bands and resulted in reduced levels of myosin in the cytoplasm (180). siRNA-treated myotubes also failed to assemble M-bands, whereas Z-disks appeared unaffected. This raises the possibility that the assembly of M-bands is required for A-bands to form properly, consistent with the appearance of M-band markers in developing myofibrils several days before clearly defined A-bands appear (63, 179, 180, 375). Thus obscurin seems to play a key role in the assembly and stabilization of both A-bands and M-bands (179, 180). In agreement with this hypothesis, obscurin and myosin exist in a complex in the cytoplasm of skeletal myofibers (179). It is not yet clear if obscurin modulates the assembly of A-bands directly by binding to myosin, possibly through its COOH-terminal region, or indirectly through interactions with other essential M-line proteins, including titin and myomesin (86). In either case, obscurin and possibly other M-line components with which it interacts (86) are likely to have an important role as a scaffold for the assembly of myosin thick filaments into A-bands.
In summary, obscurin is a very large protein of striated muscles that interacts with diverse protein partners located in distinct subcellular compartments within the cell including the SR and the sarcomere. Given its ability to associate tightly, selectively, and periodically with the periphery of the myofibril and with thick filaments, obscurin is ideally suited to coordinate the assembly and organization of the SR around the myofibrillar elements in the middle of the sarcomere.
E. Obscurin Homologs and Related Proteins
1. UNC-89
Obscurin closely resembles UNC-89, one of the largest muscle proteins identified in the nematode C. elegans (27). The similarities are manyfold. UNC-89 contains multiple tandem Ig modules as well as signaling domains, including SH3 and Rho-GEF/PH domains. UNC-89 is localized at the M-band region of striated muscle and has also been implicated in the organization of thick filaments, as mutations in the UNC-89 gene lead to disorganized A-bands, devoid of M-lines. At least six isoforms of UNC-89 are generated by use of three different promoters expressed in different sets of muscles and by alternative splicing; four of these contain double MLCK motifs, arranged in tandem (66, 337). Recently, both the MLCK-1 and MLCK-2 motifs of UNC-89 were shown to bind to the small COOH-terminal domain-phosphatase like-1 protein (SCPL-1), a novel protein phosphatase (311), while MLCK-1 and part of the interkinase region were shown to bind to LIM-9, the worm homolog of DRAL/FHL-2 (413). Moreover, the PH/Rho-GEF domains of UNC-89 were shown to specifically activate Rho-1 GTPase in C. elegans muscle cells (310). The presence of tandem MLCK motifs, shared by both proteins, provides additional evidence that UNC-89 is the counterpart of obscurin in invertebrates and that vertebrate obscurins evolved from UNC-89 or a similar protein (348). If so, significant differences were introduced into the vertebrate obscurins, most notably in the location of the Rho-GEF and PH domains, which are NH2-terminal in the nematode protein.
2. SPEG
Striated preferentially expressed gene (SPEG) encodes a protein by the same name that exhibits striking sequence homology to obscurin and also contains the hallmark Ig FN-III and dual MLCK domains that are characteristic of obscurin and UNC-89 (151, 348). Like obscurin, SPEG is predominantly expressed in cardiac and skeletal muscles of vertebrates. There is, however, no equivalent for SPEG in invertebrates. Given the close resemblance of the kinase domains in SPEG and obscurin-MLCK, SPEG may have been derived from obscurin via a gene duplication event that occurred after the initiation of vertebrate evolution (348). The presence of two tandemly arranged MLCK motifs, a feature shared by all three members of the obscurin-MLCK family and conserved across species, suggests a prominent role of these domains in signaling during myofibrillogenesis or in adult muscle.
3. OBSL1
The protein, obscurin like-1 (OBSCL-1), ranges in size between 130 and 230 kDa and, like SPEG, is related to obscurin (100). The OBSCL-1 gene is located in human chromosome 2q35 within 100 kb of the SPEG gene. Like its homologs, the OBSCL-1 mRNA undergoes extensive alternative splicing, giving rising to transcripts and proteins of different sizes. Unlike obscurin, UNC-89, and SPEG however, OBSCL-1 does not contain MLCK motifs and instead is composed only of tandem Ig repeats. Geisler et al. (100) reported that OBSCL-1 is expressed in a broad range of tissues, but most prominently in the heart, where it concentrates primarily at perinuclear regions and intercalated disks and to a lesser extent at Z-disks and M-bands. This led to the suggestion that it functions as an adaptor between cytoskeletal elements and membrane complexes present at the nuclear envelope and intercalated disks. In contrast, Fukuzawa et al. (86) reported that OBCSL-1 is predominantly, if not exclusively, present at the M-band. Consistent with this, these authors reported that, like Ig1 and Ig3 domains of obscurin, the Ig1 and Ig3 repeats of OBSCL-1 bind to the M10 domain of titin and the linker region between My4 and My5 of myomesin, respectively, suggesting that this activity is shared by obscurin and its close homologs. The different subcellular localizations of OBSCL-1 and the distinct roles suggested for it in these two reports are likely to be due to the differences in the ability of the antibodies used in each to recognize the range of splice variants of OBSCL-1 that are expressed in striated muscle during development and in adulthood. It will be of considerable interest to determine the binding affinities of these regions of obscurin and OBSCL-1 for their ligands, which should provide valuable insights into their relative roles in sarcomerogenesis.
V. MUSCLE GIANTS IN DISEASE
Given the many roles we have described above for titin, nebulin, and obscurin in striated muscle, and the effects of mutations or deletions that affect their activities in vivo and in vitro, it is not surprising that many human diseases of heart and skeletal muscle have been linked to these proteins (Table 5). Below we summarize the literature on the cardiac and skeletal myopathies and muscular dystrophies caused by mutations in the genes encoding these three giant proteins. In our summaries, we first describe the disease and then establish the genetic linkage. Consistent with studies of mice (see above, especially for titin), the severity of these diseases can vary from moderate to severe, depending on the nature of the mutation and the protein affected.
Table 5.
Diseases linked to mutations of the titin, nebulin, and obscurin genes
| Disease | Mutation(s) | Effect | Reference Nos. |
|---|---|---|---|
| Mutations of the titin gene | |||
| Tibial muscular dystrophy | Leu97786Pro, Ile97777Asn | Potential loss of titin binding to ligands at its COOH terminus | 137, 138, 371 |
| Limb girdle muscular dystrophy 2J | Gln97757Val, Val97758Lys, Thr97759Gln, Trp97760Lys | Potential loss of titin binding to ligands at its COOH terminus | 136 – 138 |
| Hereditary myopathy with early respiratory failure | Arg25026Trp | Loss of titin binding to nbr-1 | 198 |
| Dilated cardiomyopathy | Gln4053STOP | Truncation mutant (before the binding site for FHL2/DRAL) | 160 |
| Ser4465Asp | Decrease binding to FHL2 | 160 | |
| 2-bp insertion in exon 326 leading to early termination | Proteolytic cleavage within the PEVK region | 103 | |
| Trp930Arg | Unknown | 102 | |
| Frameshift mutation at nucleotide 62890 leading to early termination | Truncated protein degraded | 102 | |
| Ala743Val | Reduced binding to α-actinin | 160 | |
| Val54Met | Reduced binding to T-cap/telethonin | 160 | |
| Arg25618Gln | Unknown | 235 | |
| Hypertrophic cardiomyopathy | Ser3799Tyr | Increased binding to FHL2 | 160 |
| Arg740Leu | Increased binding to α-actinin | 327 | |
| Early-onset myopathy with fatal cardiomyopathy | 1-bp deletion in Mex3 | Truncated protein (after the kinase domain) | 46 |
| 8-bp deletion in Mex1 | Truncated protein (after the kinase domain) | 46 | |
| Mutations of the nebulin gene | |||
| Nemaline myopathy | For complete list or all 64 mutations, please see Ref. 202 | 7, 202, 293, 294 | |
| Distal myopathy | Thr5681Pro, Ser4665Ile | Possible disruption of actin binding | 384 |
| Mutations of the obscurin gene | |||
| Hypertrophic cardiomyopathy | Arg4344Gln, Ala4484The | Loss of titin binding | 10 |
A. Titin
Mutations in the TTN gene and protein have been linked to disorders of both skeletal and cardiac muscles.
1. Titinopathies and skeletal muscle
A) TIBIAL MUSCULAR DYSTROPHY
Tibial muscular dystrophy (TMD) (366) is an autosomal dominant distal myopathy that occurs most frequently (6 in 100,000) in the Finnish population (135). TMD presents after the age of 35 years, primarily with weakness and atrophy, with onset in the distal anterior compartment, specifically the tibialis anterior (135, 365). Nonspecific dystrophic changes along with adipose replacement are found in the affected muscles, although muscles of identical innervation may remain unaffected throughout a patient's lifetime (135). A mild foot drop may occur 10–15 years after onset, usually related to involvement of long toe extensors, but overall disability is mild and patients remain ambulatory throughout their lives (368). TMD has been assigned to chromo-some 2q31 and linked to mutations in the giant TTN gene (138, 139, 366).
The first mutation in TTN shown to cause TMD is an 11-bp change found in the Finnish population that alters four amino acids in Mex6, the 363rd and last exon encoding titin (137). Testing of 78 TMD heterozygotes, 3 TMD homozygotes, and 76 healthy first-degree relatives demonstrated that the mutation cosegregated with TMD in 12 unrelated pedigrees. The mutation did not occur in 216 control individuals. Muscle biopsies of TMD patients who were homozygous for the mutation in this study lacked epitopes at the COOH-terminal region of titin, as determined by immunohistochemical analysis, although antibodies recognizing the portion of the protein encoded by the Mex1 exon were present, indicating the proper incorporation of titin into sarcomeres at least up to this region (137).
Analysis of a large Finnish population for the Mex6 mutation revealed significant clinical variability of patients with the mutation (369). Homozygosity for the mutation will be discussed briefly below as limb girdle muscular dystrophy 2J. Dramatic variability of phenotype among the heterozygotes was found, with 91% having classic TMD, but 9% having a wide-ranging presentation of the disease, primarily due to the specific muscles affected, including asymmetry, or a much earlier onset than seen with classic TMD (369). These results suggest that a certain myopathic or dystrophic phenotype should not be excluded from being a titinopathy based on differences in presentation from TMD.
Other mutations of titin, characterized in French and Belgian families, also lead to TMD. Analysis of the Mex6 region on DNA samples from a French family did not show the 11-bp mutation found commonly in Finnish TMD patients, but instead revealed a point mutation in Mex6 that converts a leucine to a proline residue, that was absent in 93 unaffected controls (137). A third mutation in titin identified in a Belgian family that also that causes TMD. Sequencing of the Mex6 exon in these patients identified a point mutation leading to the substitution of an asparagine residue for an isoleucine. The effects of this mutation were subclinical in some family members, suggesting that other factors can influence the severity of TMD when the COOH-terminal region of titin is mutated (371).
Although the Mex6 exon is expressed in the heart, TMD patients do not exhibit cardiomyopathy, possibly because calpain-3, which binds to the COOH-terminal region of titin (see above), is not present in mature cardiac muscle. This suggests that the mutation(s) ultimately affect titin interactions with proteins specific to skeletal muscle (137). Alternatively, mutations in Mex6 may alter binding of titin to obscurin. Fukuzawa et al. (86) demonstrated that the French and Finnish, but not the Belgian, mutations described above substantially inhibit the binding of titin to the first NH2-terminal Ig domain of obscurin (86). Thus it is likely that the clinical phenotypes and variability seen in TMD patients are related to variable inhibition of the ability of the M-band region of titin to bind its ligands, including calpain-3 and obscurin.
B) LIMB GIRDLE MUSCULAR DYSTROPHY 2J
A subset of patients in TMD families have a more severe muscular dystrophy of the limb girdle (LGMD) type (139, 367, 369). LGMD differs from TMD by having an earlier age of onset, between the first and third decade, and the development of weakness and dystrophy in proximal muscles, with only mild effects on the distal muscles (138). Patients with LGMD occur in members of the Finnish family that are homozygous for the Mex6 mutation in titin associated with the 11-bp mutation that in a single copy causes TMD. This disorder is termed LGMD type 2J (137, 369).
C) HEREDITARY MYOPATHY WITH EARLY RESPIRATORY FAILURE
Hereditary myopathy with early respiratory failure (HMERF, MIM 603689) is linked to mutations in the COOH-terminal kinase domain of titin (198). HMERF is an autosomal dominant disease resembling LGMD, as the proximal muscles of the upper and lower limbs are primarily affected. HMERF patients also have severe weakness of the respiratory muscles, which frequently appears as the first symptom of the disease. This weakness can lead to respiratory failure and is the primary cause of death (62). The disease-causing locus of HMERF identified as 2q24–31 suggests titin as a possible candidate (412). Subsequent sequencing of titin in HMERF patients revealed a point mutation in the kinase domain of titin that caused a single amino acid substitution (Arg279Trp that corresponds to Arg25026Trp, accession no. CAA62188; Ref. 198). The mutation inhibits the interaction of the titin kinase domain with nbr1, a zinc finger protein that targets p62 to sarcomeres, where it binds MuRF-2 (muscle-specific RING-finger), a ligand for serum response transcription factor (198). The inhibition of the binding of the titin kinase domain to nbr1 is thought to prevent a critical link between the signaling properties of titin and the regulation of transcription.
D) MUSCULAR DYSTROPHY WITH MYOSITIS
Although muscular dystrophies or myopathies in humans have not yet been linked to the extensible regions of titin, a muscular dystrophy of this type has been identified in mice. The “muscular dystrophy with myositis” mouse (mdm) arose spontaneously and is characterized by severe and progressive muscle degeneration in the proximal and distal muscles, with the latter more affected. The mutation is inherited in an autosomal recessive pattern, and heterozygotes are phenotypically normal, although some gait abnormalities have been described (152). Analysis of mdm mice revealed a 779-bp genomic deletion and a 2.4-kb insertion of a 5′-truncated LINE-1 retrotransposon that results in the deletion of 83 amino acids from the N2A region of titin (92). Like the TMD mutation, mutation of the N2A sites renders mdm titin unable to interact with calpain-3 (405). Although overexpression of calpain-3 in mdm mice exacerbates the phenotype, calpain-3 is not responsible for pathogenesis, as the mdm phenotype remains unchanged in mice lacking calpain-3 due to homologous recombination (152). Overexpression of calpain-3 did, however, rescue the gait abnormalities in heterozygous mdm mice, suggesting a functional role for the interaction of calpain-3 with the N2A region of titin (152).
Several proteins are thought to be upregulated in mdm mice, including muscle LIM protein (MLP), cardiac ankyrin repeat protein (CARP), ankrd2/Arpp (CARP-like protein), and MURF-1, all of which associate with titin (405). CARP, which like calpain-3 also associates with the N2A region, is usually expressed in response to injury or hypertrophy and accumulates at the I-band region of the sarcomere in homozygous mdm skeletal muscle, but not heterozygotes or mdm cardiac muscle, suggesting that its presence there may be linked to some aspects of the mdm pathology (244, 405).
E) TITIN MISCELLANEOUS
In addition to the diseases directly linked to mutations in titin, discussed above, titin has been indirectly associated with a variety of pathologies, including chronic obstructive pulmonary disease (COPD) and myasthenia gravis (MG). Although it is unlikely that these diseases are due to mutations in titin, the involvement of titin (or in the case of MG, anti-titin antibodies) in both has been well established.
Inspiratory muscle weakness, including the diaphragm, is a significant contributor to cause of death in COPD patients. Analysis of biopsies of diaphragm muscle from COPD patients, including passive tension studies of single fibers, protein analysis, and microarray studies, showed that they generate less passive tension upon stretch at all fiber lengths compared with controls (286). Exon microarray studies demonstrated the up-regulation of seven exons specific to the PEVK region of titin (286), and gel electrophoretic analysis revealed larger titin isoforms in COPD patients, compared with controls (248). These results suggest that alternative splicing of the titin gene may be a contributing factor to the pathology in COPD patients and that understanding the regulation and pathophysiology of titin could provide insight into other diseases of respiratory dysfunction.
MG is an autoimmune disease caused in most cases by the presence of autoantibodies to the acetylcholine receptors of the neuromuscular junction. Typically, MG patients also produce antibodies to a number of other muscle proteins, including titin (168, 316–318, 349, 378). The major immunogenic region of titin in MG patients, termed “myasthenia gravis titin 30 kDa (MGT-30),” is located in the region of titin found near the A/I-junction (97).
Although the presence of anti-titin antibodies does not underlie MG, it may be clinically significant, as the presence of anti-titin antibodies is typical of more severe muscle weakness (336). Anti-titin antibodies are almost never found in patients with early-onset (prior to age 60) MG without thymoma (338, 416). Indeed, the absence of titin antibodies has a 99% negative predictive value, compared with 65% for computed tomography scans for thymoma (318). In patients with late-onset MG, however, the prevalence of anti-titin antibodies does not differ significantly with or without thymoma (43). Thus the presence of titin antibodies may be useful in detecting the presence of thymoma in early-onset MG patients.
2. Titinopathies and cardiac muscle
Cardiovascular disease frequently results in remodeling of the heart to maintain its ability to function as a pump. This remodeling generally occurs either as hypertrophy, manifested as an increase in the thickness of the left ventricular wall and interventricular septum due to interstitial fibrosis and an increase in myocyte size rather than number, or as dilation, characterized by a modest increase in ventricular wall thickness and expansion of intraventricular volume (4). These changes, initially compensatory, eventually become maladaptive. Although titin has been implicated in cardiomyopathies for some time (143, 260, 335), only recently has its direct role been elucidated.
A) DILATED CARDIOMYOPATHY
DCM is frequently caused by coronary artery disease, viral infection, or other environmental factors, but up to 35% of cases are thought to be familial, with several cases linked to mutations in titin. Currently, nine different mutations have been identified in titin in vertebrates that result in DCM (46, 103, 160, 235, 414). These include mutations in zebrafish as well as human. The pickwickm171 mutant of zebrafish was originally characterized by the development of abnormal sarcomeres in the heart and poor contractility from the first beat. This mutant is due to a T to G transversion in the unique sequence (is3) of the N2B region of titin that results in a premature stop codon (414). Injection of a morpholino antisense oligonucleotide, that targets the splice donor site at the 3′-end of the N2B exon, results in an exact phenotype of the pikm171 allele (414). These results suggest that the titin mutation is responsible for the cardiac phenotype in the zebrafish.
Mutations have also been identified in the N2B region in humans. Transversion of C to T in codon 4053 results in the conversion of a glutamine residue to a premature stop codon. Another point mutation in codon 4465 replaces a serine residue with an asparagine. These mutations were identified in DCM patients (160), but not in 520 controls. The premature stop codon terminated the protein NH2-terminal to the binding site for four-and-a-half-LIM domain protein 2 (DRAL/FHL2, see above) as determined by yeast two-hybrid studies (235).
Gerull et al. (103) analyzed two kindreds with autosomal dominant DCM and identified two unique mutations in titin. A 2-bp insertion mutation in exon 326, which causes a premature stop codon after the addition of four novel amino acids, leads to proteolytic degradation of titin, probably near or within the PEVK domain, as determined by antibody labeling (103). Another kindred had a point mutation in exon 18 that results in the conversion of a tryptophan residue to arginine (103), but the functional consequence of this mutation is unknown. The same group later discovered a frameshift mutation (62890delG) that also resulted in a premature stop codon, with the addition of 10 novel amino acids (102). The resulting phenotype was variable, with some patients remaining asymptomatic and others with disease onset occurring anywhere from age 20–80. Although the mutant mRNA is detected in patient samples, the truncated titin is not, suggesting that it is degraded (102).
Analysis of a Japanese family who presented with cardiac arrhythmias progressing to DCM identified a point mutation resulting in an alanine to valine conversion in codon 743 (160). Another Z-disk point mutation in exon 3 (or codon 54), encoding a region of titin found in the Z-disk, led to conversion of a valine residue to methionine (160). As these mutations occurred in the binding domains of titin to α-actinin and T-cap/telethonin, respectively, experiments were conducted to determine if the mutations affected their binding to titin. The Ala743Val mutation reduced binding to α-actinin binding by ~40%, as determined by the yeast two-hybrid assay (160). The Val54Met mutation reduced titin binding to T-cap/telethonin by ~60%, determined similarly (160). Although these results indicate that mutations in titin can reduce binding to T-cap/telethonin, it remains to be proven that this is sufficient to cause DCM.
A mutation in the Is2 region of titin also has been linked to DCM. The arginine to glutamine conversion at amino acid 25618 was found in two related individuals, both having a late-onset DCM (235). Although this polymorphism was not found in 288 controls, its pathogenicity has not yet been determined.
B) HYPERTROPHIC CARDIOMYOPATHY
The genetic heterogeneity of familial hypertrophic cardiomyopathy (HCM) has long been recognized, but it was not until recently that mutations in titin were linked to the disorder (159, 327). Sequencing of the DNA encoding the Z-disk region of titin in patients with unknown mutations in the other eight disease-causing genes revealed a G to T transversion in codon 740 that results in the replacement of an arginine residue with leucine (327). The mutation was not found in 520 normal chromosomes, suggesting it is not a polymorphism. Yeast two-hybrid assays showed the mutation increased binding to α-actinin by ~40% (327). Another mutation in titin (Ser3799Tyr), in the N2B region, was discovered in two siblings with familial HCM (160) and found to increase binding to FHL2 by 26% in a yeast two-hybrid assay (235).
The relationship between mutations that increase or decrease the ability of titin to bind to other proteins and the development of HCM or DCM, respectively, is remarkable (160, 235, 327). Matsumoto et al. (235) postulate that decreased binding leads to “loose” sarcomeres and reduced stretch response that eventually lead to DCM. Increased binding may lead to stiff sarcomeres, which may be highly susceptible to the hypertrophic response of cardiomyocytes against stretch that leads to HCM. The power of this hypothesis is limited by the methods used to quantitate binding in several of these studies, many of which relied on the yeast two-hybrid screen rather than more reliable quantitative techniques capable of providing definitive values for the changes in binding affinities caused by titin mutations. Furthermore, most of the published results do not demonstrate a direct effect of alterations in binding to the development of disease. Further studies are needed before we can conclude that mutations resulting in increased binding to sarcomeric proteins and mutations resulting in decreased binding to sarcomeric proteins lead to HCM and DCM, respectively.
3. Early-onset myopathy with fatal cardiomyopathy
Unlike the titinopathies described above, which affect skeletal or cardiac muscle but not both, a newly identified disorder, early-onset myopathy with fatal cardiomyopathy, has been described in two families diagnosed with congenital myopathy or congenital muscular dystrophy, linked to a COOH-terminal deletion in the titin gene (46). One family showed delayed motor milestones, symmetric generalized muscle weakness, including facial muscles, and a DCM that developed within the first decade of life. Arrhythmias led to the death of two siblings at ages 8 and 17, whereas the third sibling initially survived a heart transplant but died 2 years later, subsequent to postoperative complications. The other family had similar symptoms, as well as neonatal hypotonia, with onset of DCM in childhood leading to sudden cardiac death within the second decade. Ultrastructural studies of skeletal muscle from these patients demonstrated disruption of sarcomeres, more so at M-bands than at Z-disks, with a loss of mitochondria. Genetic analysis showed the defects in the titin gene to be homozygous. Specifically, the mutations consisted of a 1-bp deletion in exon 360 (Mex3) and an 8-bp deletion in exon 358 (Mex1), both of which left the titin kinase domain intact but resulted in premature stop codons and loss of the COOH-terminal 447 and 808 amino acids, respectively. These mutations in the titin gene are the first to be identified that produce both skeletal and cardiac muscle defects.
4. Isoform shift
As previously discussed, cardiac muscle expresses two predominant isoforms of titin, the N2B and N2BA isoforms, with the former promoting higher passive myocardial stiffness (48). The stiffness of the sarcomere is inversely proportional to the N2BA:N2B ratio, suggesting that changes in the relative amounts of these isoforms may play a significant role in heart disease. Consistent with this, heart failure patients with nonischemic DCM show almost a twofold increase in their N2BA:N2B ratios [from 0.56 in controls to 0.97 in DCM (260); from 0.47 in controls to 0.72 in DCM (228)]. This shift correlated significantly with end-diastolic volume, end-systolic volume, and ejection fraction. As expected, titin-based passive tension and stiffness are lower in patients with a higher N2BA:N2B ratio who have improved diastolic function linked to increased compliance (228, 260).
Similar studies have been done in ischemic hearts by analyzing human hearts with coronary artery disease (CAD), obtained after transplant, with significant infarcts, left ventricular dysfunction, and heart failure as well as human hearts transplanted for reasons other than ischemia. We refer to these hearts as CAD transplants and nonischemic transplants, respectively. These samples were compared with human donor hearts with and without CAD, referred to as CAD donors and normal donors, respectively. The N2BA isoform represented 47.0% of the total titin in CAD samples compared with 32.1% in hearts requiring transplant for reasons other than ischemia, 29.5% in donors with CAD without cardiomyopathy, and 28.1% in normal donors. Comparison of hearts from normal donors and CAD transplants demonstrate that the latter, which have increased N2BA:N2B ratios, are associated with decreased myofibrillar passive tension despite the overall increased stiffness of the heart, likely due to collagen deposition and fibrosis (266). These results conflict with data from animal models which have shown an overall decrease in N2BA:N2B ratio in pacing-induced failure in canines or spontaneously hypertensive rats (394, 410). Possible explanations for this discrepancy include the possibility that the isoform switch in spontaneously hypertensive rats (a decrease in the N2BA:N2B ratio) occurs earlier in the spectrum of disease progression than in human CAD transplant hearts (an increase in the N2BA:N2B ratio), which were all severe enough to require transplantation, suggesting that isoform ratios vary with disease progression (203). Additionally, the pathophysiology of the hypertension-induced hypertrophy likely varies from the human CAD transplant hearts analyzed by the laboratory of Linke et al. (266) and thus may represent two distinct disease processes.
5. Conclusion
Although they have long been a topic of speculation, titinopathies have only recently been discovered and characterized, and their pathophysiology is just beginning to be elucidated. They have provided considerable insights into the role of this protein in regulating the passive tension in skeletal and cardiac muscle and its role as a scaffold to anchor smaller proteins along its length. Of all the disease processes described above, understanding the isoform shift of titin and its physiological consequences is likely to have the greatest public health impact. Heart failure signified by impaired cardiac contraction or relaxation has a lifetime risk of 1 in 5 for both men and women (219). The ability to improve cardiac contractility even by a small percentage by regulating titin isoform expression could have profound public health consequences and reduction of disease burden.
B. Nebulin and Nemaline Myopathy
Nemaline myopathy (NM), the most common of the congenital myopathies, is identified by skeletal muscle weakness and the presence of nemaline bodies (rodlike structures) in skeletal muscle fibers (383). Nemaline myopathy is characterized by genetic heterogeneity and clinical variability of skeletal muscle weakness, ranging in the most severe cases from neonatal mortality to only mild muscle weakness in adulthood.
Nemaline myopathy has been divided into seven sub-types by the European Neuromuscular Centre: severe congenital, typical, intermediate congenital, mild/childhood/juvenile-onset, adult, Amish, and other (164, 381). All subtypes are characterized by the presence of nema-line rods that are typically composed of α-actinin and other components of the Z-disk and are usually present near the nucleus or sarcolemma (326).
Genetic analyses have further characterized the mutations underlying these disorders and shown that all mutations identified to date are in proteins of the thin filament or related proteins. Although many mutations are sporadic, inheritance can be both autosomal dominant and autosomal recessive and has been linked to the genes encoding α-tropomyosin, nebulin, α-actin, troponin T, β-tropomyosin, and cofilin-2. Genetic analysis of 45 families with autosomal recessive nemaline myopathy showed 41 to have linkage markers near nebulin, suggesting that nebulin is the most commonly mutated thin filament protein in nemaline myopathy (385). A recent study confirmed that patients with nemaline myopathy linked to mutations in nebulin show muscle weakness, consistent with the expression of reduced levels of nebulin, similar to that observed for mice lacking the protein (287).
Pelin et al. (294) were the first group to discover mutations in nebulin linked to nemaline myopathy (294). Their study of five Finnish families identified six mutations in the COOH-terminal region of nebulin, ranging from exons 163–185 and encoding the region of nebulin located near the Z-I junction. Remarkably, in all but one family the COOH-terminal SH3 epitope was retained, despite the upstream mutations. This observation, which was confirmed in additional patients (331), led to the idea that one of the regions of nebulin that is subject to extensive alternative splicing (M176-M181) may facilitate exon skipping, allowing the COOH terminus to be transcribed and translated normally. The presence of nemaline myopathies in the families that retained the SH3 domain epitope suggested that aberrant nebulins may lack the usual diversity of physiological isoforms created by the normal alternative splicing events that occur in healthy muscles (294).
Further work by Pelin et al. (293) identified 12 additional mutations in nebulin that cause autosomal recessive nemaline myopathy. These mutations occurred in exons 157–184 and led to nemaline myopathies ranging from mild to severe. Most were frameshift or nonsense mutations that resulted in premature stop codons, but one point mutation in exon 160 caused a threonine-to-proline transversion that was linked to a nemaline myopathy of moderate severity. Two of the mutations identified were present in introns and were proposed to cause abnormal splicing of the nebulin mRNA. These results, along with others (383), indicate that although nebulin mutations most frequently result in a typical nemaline myopathy of moderate severity, they can also lead to mild or severe forms of the disease (excluding the Amish type).
An additional autosomal recessive mutation of nebulin was later identified in the Ashkenazi Jewish population that results in a 2,502-bp deletion in exon 55 and introns 54 and 55 (7). As the deletion of exon 55 does not result in a frameshift mutation, the resulting nebulin transcript encodes 35 fewer amino acids, the size of a typical nebulin repeat, with the resulting deletion occurring from Arg-2478 to Asp-2512. This deletion is thought to affect the binding of nebulin to tropomyosin and results in a myopathy of intermediate severity. The carrier frequency of the mutation, 1 in 108, and its ease of detection suggest that it is a good candidate for carrier screening in the Ashkenazi Jewish population.
A more extensive study of nebulin mutations in autosomal recessive nemaline myopathies has identified an additional 45 mutations (202), leading to a total of 64 different nebulin mutations that are pathogenic. Of these, 55% are frameshift or nonsense mutations, 25% are point mutations, and 3% are deletions thought to affect conserved splice signals (202). Several mutations throughout the I-band region of nebulin, like that described in the Ashkenazi Jewish population, are thought to affect binding to actin or tropomyosin and perhaps the stability of thin filaments.
Nemaline myopathy is not the only disease of skeletal muscle linked to nebulin, however, as a distal myopathy with mutations in nebulin was recently described (384). Patients with this myopathy are affected primarily in their ankle dorsiflexors, finger extensors, and neck flexors. Biopsies from these patients showed only infrequent nemaline bodies or rimmed vacuoles, but electron microscopy revealed altered Z-disks in all muscle samples and occasional nemaline bodies in four of the five samples examined. Genetic analysis of four Finnish families revealed homozygous missense mutations in exon 151 and exon 122 that result in a threonine-to-proline (Thr5681Pro) mutation and a serineto-isoleucine mutation (Ser4665Ile), respectively. These mutations, combined with the more dramatic nebulin mutations (frameshift, nonsense, or mutations affecting splice sites), result in compound heterozygotes with typical or intermediate nemaline myopathy (202, 293).
In conclusion, although the size of the nebulin gene and the lack of mutation “hotspots” make mutational analysis of nebulin challenging, recent studies have led to the discovery of 66 different pathogenic mutations. The majority of these mutations result in nemaline myopathy, but two are associated with a distal myopathy in the Finnish population. Investigation into the physiological sequelae of these mutations is needed to elucidate the role of nebulin in normal and diseased muscle, and how changes in its sequence can lead to the misassembly of key structural proteins.
C. Obscurin and Muscle Disease
The role of obscurin in diseases of striated muscle is still unclear. Upregulation of different obscurin gene products, including full-length obscurin and several of the smaller MLCK variants, was reported to occur in mice with myocardial hypertrophy induced by aortic constriction (36). Likewise, other studies documented an upregulation of obscurin during cardiac hypertrophic responses to pressure overload and myopathic responses to mutations in titin (36, 228, 410). Whether these changes occur in response to hypertrophy or are linked causally to this condition has not been resolved.
More direct evidence for the involvement of obscurin in the development of heart disease was demonstrated in a single patient with HCM (10). Linkage analysis revealed a sequence variation in the OBSCN gene in the region encoding the titin binding site (Ig58/59), specifically an Arg4344Gln variant in the Ig58 domain of obscurin. In vitro studies showed that this variant resulted in decreased binding of obscurin to titin and also impaired the localization of obscurin to the Z-disk. Although this is a sole example, it suggests that, like titin and nebulin, mutations in the OBSCN gene may lead to myopathy and, by implication, suggest a critical role for obscurin in normal muscle development and physiology.
In conclusion, although obscurin has only recently been linked to the pathogenesis of muscle disease, its gigantic size and structural complexity suggest that, similar to titin and nebulin, mutations in its adhesion and signaling motifs may also lead to hereditary muscle diseases in mankind. Detailed genetic linkage analysis will be needed to identify such mutations.
VI. THE GIANT PROTEINS OF MUSCLE AND THEIR ROLE IN MYOFIBRILLOGENESIS
A. Myofibrillogenesis
The myoplasm of adult muscle fibers contains two major and highly ordered components: myofibrils and the membrane systems that surround them (16, 322–324).
Myofibrils assemble as sufficient concentrations of the necessary proteins accumulate in the cytoplasm of muscle cells shortly after the onset of differentiation. Two models of sarcomerogenesis have been proposed that invoke interactions of developing thin and thick filaments with other elements of the cytoskeleton. Holtzer and coworkers (148, 280, 328) have suggested that, during early myofibrillogenesis, thin and thick filaments assemble independently on stress fiberlike structures (SFLS), which develop into nonstriated myofibrils (NSMF). These progress to nascent striated myofibrils (naSMF) that in turn develop into fully mature, striated myofibrils (SMF). During the transition of NSMF to SMF, adjacent strands of thin and thick filaments align, initially at cell borders and subsequently throughout the cytoplasm. A key feature of this model is that the earliest precursors of mature thin and thick filaments form independently, yet concurrently, in the myoplasm, but that later stages of development proceed along common structures. Sanger and colleagues (56, 315) have postulated an alternative model that evokes three distinct structures involved in myofibrillogenesis premyofibrils, nascent myofibrils and mature myofibrils. Premyofibrils contain transitory arrays of I-Z-I complexes, consisting of precursors of Z-disks, termed “Z-bodies,” enriched in α-actinin, that anchor sarcomeric actin occupying primitive I-bands, that in turn interact with miniature A-bands, composed of nonmuscle myosin II. A number of dramatic changes take place as premyofibrils transform to nascent myofibrils (321–324). Precursor I-Z-I bodies transit into maturing I-Z-I bands with the cooperative binding and integration of at least four integral Z-band components, including titin and nebulin along with the (already present) sarcomeric α-actinin and α-actin. Muscle myosin II occupies the developing A-bands as nonmuscle myosin II filaments are gradually eliminated. Concurrently, titin associates with the maturing I-Z-I complexes via its NH2-terminal region and with myosin filaments through its COOH-terminal region, guiding their assembly into definitive A-bands and thus facilitating the coordinated integration of thin and thick filaments into sarcomeres. As nascent myofibrils develop into mature myofibrils, they go through a final series of transformations: 1) they align parallel to each other and assume a diameter of 1–3 μm; 2) they organize as a series of sarcomeres with sharply delineated Z-disks linked to highly ordered ~1 μm long, thin filaments containing α-actin and associated proteins, which form the I-bands; and 3) they assemble ~1.6 μm long, thick filaments, composed of muscle myosin but devoid of nonmuscle myosin II, that form the A-bands, which in turn are bisected by M-lines containing unique accessory proteins. A key feature of this model is that the precursors of thin and thick filaments form along the same structures, which together develop into mature sarcomeres.
Although the two proposed models are distinct, they share the view that structural proteins are essential to the proper assembly and incorporation of actin and myosin into mature myofibrils (60, 208, 372–376). Thus during the initial assembly of myofibrils, Z-bodies composed of α-actinin, N-RAP, the NH2-terminal region of titin and the COOH-terminal region of nebulin contribute to the polarized organization of thin actin filaments to form “I-Z-I brushes” that become incorporated into forming sarcomeres (63, 99, 132, 280, 321, 324). Likewise, proteins of the M-band, including myomesin, M-protein, the COOH-terminal region of titin and obscurin play a key role in the integration of myosin thick filaments into periodic A-bands (178, 372–376, 393).
The internal membrane systems of striated myofibers assemble as myofibrillogenesis proceeds. Striated muscles have two systems of internal membranes highly organized around each sarcomere, the SR and t-tubules that, with the sarcolemma, modulate cytosolic Ca2+ release and uptake during successive cycles of contraction and relaxation (69, 70).
A series of developmental events leads to the assembly and association of these membrane systems with the developing myofibrils, which assemble first. Initially, the endoplasmic reticulum (ER) differentiates into SR by the gradual displacement of generic ER proteins by massive amounts of SR specific proteins, including the Ca2+-pump SERCA the Ca2+ release channel ryanodine receptor (RyR) and the low-affinity, high-capacity Ca2+-binding protein calsequestrin (70, 78, 79, 154). Docking of a patch of SR to a corresponding patch of surface membrane follows, concurrently with the transition of premyofibrils to nascent myofibrils (70, 71, 351, 352). This event triggers the SR proteins to segregate into two functionally distinct, yet continuous compartments within the SR: the free SR, which contains high concentrations of SERCA and the junctional SR (jSR), which contains ordered arrays of RyR, calsequestrin, and associated proteins (70, 71, 308, 352). In parallel, plasma membrane invaginations differentiate into t-tubules as voltage-gated Ca2+ channels or dihydropyridine receptors (DHPR) accumulate in high amounts. Although they are formed initially by invagination of the sarcolemma in transverse orientation, t-tubules also branch longitudinally and maintain a longitudinal orientation in the cytoplasm until late in development (69–71, 351, 424). As nascent myofibrils transit to mature myofibrils, junctions between the jSR membrane and t-tubules form and develop into functional coupling units, which further differentiate into triads, consisting of two jSR cisternae and one t-tubule, or dyads, consisting of one jSR cistern and one t-tubule. Finally, as myotubes transform to myofibers, t-tubules lose their longitudinal orientation and align transversely, after myofibrils, SR membranes, and triadic or dyadic junctions have become transversely aligned.
At maturity, the SR of mammalian striated muscle fibers contains at least three morphologically and functionally distinct, yet continuous, compartments: the terminal cisternae or jSR, which is responsible for Ca2+-release during successive cycles of contraction/relaxation, the network SR, which controls Ca2+ uptake, and the longitudinal SR that connects the terminal cisternae with the network SR (156, 157). The SR compartments have specific locations around each sarcomere. In skeletal myofibers, the network SR is positioned over Z-disks and M-bands, whereas the terminal cisternae are located around the A-I junction, where triads or dyads form; in cardiac myofibers the network SR is present around M-bands and Z-disks, whereas the terminal cisternae surround the myofibrils at the level of the Z-disk, where the dyad or triad junctions reside (71, 78, 79). Unlike SR membranes, t-tubules are not associated with specific regions of the sarcomere of myofibers until late in development, when they finally acquire their mature topography, running between myofibrils at the level of the A-I junction or the Z-disk in skeletal and cardiac myofibers, respectively (71, 78, 79).
B. Muscle Giants: Templates, Rulers, Blueprints, or Scaffolds?
A major goal of muscle physiology has been the elucidation of the sequence of events that lead to the regular assembly of thin and thick filaments into sarcomeres. The precise organization and arrangement of these structures has led several researchers to propose the existence of “molecular templates” that govern their ordered assembly in repeating units. A number of features shared by the three giant muscle proteins (titin, nebulin, and obscurin) has suggested that they may serve as rulers or blueprints during sarcomerogenesis: their enormous sizes and unique locations within the sarcomere, their early appearance and incorporation into primitive sarcomeric structures, their multiple and diverse binding partners, the presence of signaling motifs within their sequences, and their ability to respond to metabolic and stress stimuli.
Using a combination of in vitro and in vivo studies, several laboratories have attempted to unravel the role of titin in directing myofibril assembly and examine its involvement in the initial formation, integration, and stabilization of these structures. Conflicting results have emerged from these studies that have led to the generation of opposing theories regarding titin's role as a template during myofibrillogenesis. Ehler et al. (63) studied titin during early development of neonatal cardiocytes in cultures and demonstrated that the COOH-terminal epitopes of titin become organized into M-bands later than its NH2-terminal epitopes into Z-disks. These findings supported a previously proposed model in which titin may serve as a molecular template for key features of the sarcomere, beginning at the Z-disk through the I- and A-bands to the M-band (194, 373, 374, 388). Later studies questioned this idea and demonstrated that the NH2- and COOH-terminal ends of titin are each targeted to their appropriate structures in developing sarcomeres at nearly the same time of differentiation, whereas the middle of the molecule assumes a periodic organization ~24 h later (178). These observations implied that, at early stages of myofibrillogenesis, an individual titin molecule might be well organized at nascent Z-disks or M-bands, but not at both simultaneously, while its middle portion does not assume its extended conformation until both ends of the same molecule are anchored to their respective structures. This progression in the organization of titin within sarcomeres is incompatible with its role as a molecular blueprint during early sarcomerogenesis, when the two ends of the same molecule are not yet stably associated with Z-disks and M-bands. Notably, however, this idea is in agreement with the model proposed by Holtzer et al. (148), which also questioned the role of titin as a molecular ruler during myofibril assembly, and which postulated that thin and thick filaments assemble concurrently, yet independently, at spatially distinct sites.
A complementary approach that several laboratories undertook to study the contribution of titin to the de novo formation of myofibrils involved either manipulation of the expression levels or elimination of portions of titin in vitro and in vivo. These studies have yielded conflicting results as well. Overexpression of the entire Z-disk portion of titin in cultures of neonatal cardiocytes affected not only the assembly of Z-disks and associated structures but also the organization of thick filaments into A-bands (132). Likewise, gene targeting of the extreme COOH terminus of titin in cultured skeletal myotubes disrupted the organization of both M-bands and Z-disks, suggesting that the entire M-band portion of titin is indispensable for the initial stages of myofibrillogenesis in developing cardiomyocytes derived in culture from embryonic stem cells (243, 255). These findings clearly suggested a key role for titin in the initial stages of myofibril formation, as alterations in one portion of the molecule, thought to interact with only one sarcomeric structure, affect other structures too.
In contrast, a number of recent studies do not support such a role for titin and indicate the need for a new model for titin's role in sarcomerogenesis. Failure of the extreme Z-disk portion of titin to integrate into sarcomeres following overexpression of obscurin's Rho-GEF domain did not affect the regular organization of the rest of the titin molecule and had mild effects on the structure of Z-disks with no apparent effects on the structure of A-, I-, and M-bands (41). These findings suggested that the proper incorporation and stable association of titin with Z-disks is not a prerequisite for the formation of these structures or the stable association of titin with M-bands, and that M-bands can form during myofibrillogenesis even when titin is not stably incorporated into Z-disks. Consistent with this, Fukuzawa et al. (86) demonstrated that the proper incorporation of the COOH terminus of titin into M-bands depends on the earlier integration of myomesin into these structures. Moreover, although mice lacking the M-band region of titin died in midgestation, they showed normal assembly of myofibrils during early stages of development (400). Similarly, a mouse expressing titin lacking the N2B region developed normal sarcomeres (312). These results are inconsistent with early models of myofibrillogenesis that viewed titin as a molecular ruler or template for sarcomeric assembly. Instead, they suggest important roles for titin, or parts of it, in the maintenance of sarcomeres and their integration with other cellular compartments. Thus it seems that titin is necessary but not sufficient to guide some aspects of myofibrillogenesis and that other molecules play important roles in this process. We will not be able to obtain more definitive information about titin's role in myofibrillogenesis until we can manipulate the expression and structure of the entire molecule in vivo, rather than parts of it, without causing lethality.
The tight association of nebulin with actin filaments throughout its length has suggested that together with the capping proteins tropomodulin and cap-Z, it can act as a molecular ruler for specifying and maintaining the precise lengths of thin filaments. This idea is supported by early observations indicating that nebulin assembles early during myofibrillogenesis, before actin filaments attain their mature lengths and organization (280). Moreover, different sizes of nebulin isoforms, generated by alternative splicing of the nebulin gene, have been correlated with the different thin filament lengths present in different types of muscle fibers and during muscle development (186).
Although this evidence strongly supports a key role for nebulin in determining thin filament length, there are two major and perplexing issues. The first concerns the low abundance, or absence, of nebulin in cardiac cells. Although cardiac thin filaments are not as uniform in size as those in skeletal muscle, their lengths are also highly regulated. A recent study by Witt et al. (404) demonstrated that, in postnatal heart muscle, nebulin is present in atrial cardiocytes, albeit in low amounts, but is absent from the great majority of ventricular cardiocytes. Thus actin filaments in most cardiac cells have defined lengths, although they form in the absence of nebulin. This indicates that, if nebulin determines the length and stability of the thin filaments in skeletal muscle, the molecular mechanisms for determining the length of thin filaments in cardiac muscle must be different. The second issue concerns one of the two mouse models that lack nebulin (21). In the absence of nebulin, I-bands in this mouse appear narrower with shorter thin filaments, which, however, are of uniform lengths. These observations strongly imply that nebulin is not a template for formation and organization of thin filaments in skeletal and cardiac muscle, but may instead stabilize thin filaments at a length determined by the size of the nebulin molecule.
The last of the three muscle giants, obscurin, has so far been implicated in the alignment of the contractile apparatus with internal membranes, the regular assembly of thick filaments, and the incorporation of titin's NH2-terminal region into Z-disks through its Rho-GEF domain (18, 41, 179–181). Unlike nebulin and titin, which are integral components of sarcomeres and extend longitudinally from Z-disks to M-bands and along thin filaments, respectively, obscurin is positioned at the surface of myofibrils, where it intimately surrounds them at the level of the Z-disk and the M-band (42, 45, 176, 181). This peripheral localization of obscurin, distinct from other proteins of the sarcomere, suggests that it might define their diameter. At ~200 nm in length, a single obscurin molecule is apparently not big enough to surround myofibrils as a monomer (421). Thus it is likely that obscurin oligomerizes to form a “ring” big enough to encircle developing myofibrils and perhaps define their periphery. Studies from our laboratory are now in progress to test this idea further.
C. Concluding Remarks
Although the evidence, summarized above, suggests that neither titin, nor nebulin, nor obscurin is alone sufficient to act as a molecule template, blueprint, or ruler in assembling the sarcomere or particular contractile elements in the sarcomere, it strongly suggests that these proteins contribute to sarcomerogenesis through their roles as molecular scaffolds. Each of these giant proteins interacts with a number of other proteins to anchor them at particular sites in the sarcomere (see Tables 2–4). These include proteins that are structural, involved in signaling and the maintenance of homeostasis, or associated with intracellular membranes, particularly those comprising compartments of the SR. Altering the amounts, sequences, or activities of any of these giant proteins can modify their roles as molecular scaffolds, with potentially serious effects on the structure and function of striated muscle cells. Remarkably, these giant molecules not only bind to thick and thin filaments, but also to each other and to other large scaffolding molecules, such as α-actinin, filamin, myomesin, MyBP-C, and RanBP9, to regulate sarcomerogenesis. The assembly of sarcomeres and their surrounding membranes is therefore likely to be an iterative process in which titin, obscurin, and nebulin (when it is present) serially bind and recruit additional sets of scaffolding and signaling molecules. Elucidating the mechanisms involved in sarcomero-genesis will require learning the order of these interactions, how they are regulated, and how they in turn regulate gene expression and homeostasis in striated muscle.
ACKNOWLEDGMENTS
GRANTS
Our research has been supported by National Institutes of Health (NIH) Grant R01-AR-52768 and Muscular Dystrophy Association Grant 4214 (to A. Kontrogianni-Konstantopoulos); NIH Grants R01-HL-64304, R01-AR-056330, and R01-HL-75106 and Muscular Dystrophy Association Grant 3932 (to R. J. Bloch); and fellowships provided by NIH Training Grants T32-GM-08181 and F30-NS-48648-02 (to A. L. Bowman).
Footnotes
NOTE ADDED IN PROOF
A number of papers on titin, nebulin, or obscurin have appeared since we completed our literature review in January 2009. Of these, we selected 3 to summarize in this note.
Costello et al. (Biophys J 96: 1856–1865, 2009) find that the ends of thin filaments in several different rabbit muscles, marked by tropomodulin, are notably farther from the Z-disk than the ends of nebulin molecule. They suggest that nebulin determines neither the sarcomeric location of tropomodulin nor the lengths of thin filaments.
Lange et al. (J Cell Sci 122: 2640–2650, 2009) describe the effects on skeletal muscle of eliminating obscurin by homologous recombination. “Knock-out” of obscurin disrupts the longitudinal sarcoplasmic reticulum (SR) and affects the expression and distribution of sAnk1 and other proteins of the SR, but it otherwise has no notable effects on sarcomeric organization. Most measures of contractile function are normal in young mice, but a mild myopathy appears with age.
Most recently, Granzier et al. (Circ Res Aug 13, 2009 e-publication ahead of print) report that eliminating 282 amino acids from the PEVK region of N2B titin leads to upregulation of FHL proteins in the heart, increased ventricular stiffness and hypertrophy, and diastolic dysfunction.
REFERENCES
- 1.Ackermann AA, Hu LY, Bowman AL, Bloch RJ, Kontrogianni-Konstantopoulos A. Obscurin interacts with a novel isoform of myosin binding protein-C slow that selectively targets at the periphery of M-bands to regulate the assembly of thick filaments. Mol Biol Cell. doi: 10.1091/mbc.E08-12-1251. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarkova I, Ehler E, Lange S, Schoenauer R, Perriard JC. M-band: a safeguard for sarcomere stability? J Muscle Res Cell Motil. 2003;24:191–203. doi: 10.1023/a:1026094924677. [DOI] [PubMed] [Google Scholar]
- 3.Agarkova I, Perriard JC. The M-band: an elastic web that crosslinks thick filaments in the center of the sarcomere. Trends Cell Biol. 2005;15:477–485. doi: 10.1016/j.tcb.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad F, Seidman JG, Seidman CE. The genetic basis for cardiac remodeling. Annu Rev Genomics Hum Genet. 2005;6:185–216. doi: 10.1146/annurev.genom.6.080604.162132. [DOI] [PubMed] [Google Scholar]
- 5.Almenar-Queralt A, Lee A, Conley CA, Ribas de Pouplana L, Fowler VM. Identification of a novel tropomodulin isoform, skeletal tropomodulin, that caps actin filament pointed ends in fast skeletal muscle. J Biol Chem. 1999;274:28466–28475. doi: 10.1074/jbc.274.40.28466. [DOI] [PubMed] [Google Scholar]
- 6.Alyonycheva TN, Mikawa T, Reinach FC, Fischman DA. Iso-form-specific interaction of the myosin-binding proteins (MyBPs) with skeletal and cardiac myosin is a property of the C-terminal immunoglobulin domain. J Biol Chem. 1997;272:20866–20872. doi: 10.1074/jbc.272.33.20866. [DOI] [PubMed] [Google Scholar]
- 7.Anderson SL, Ekstein J, Donnelly MC, Keefe EM, Toto NR, LeVoci LA, Rubin BY. Nemaline myopathy in the Ashkenazi Jewish population is caused by a deletion in the nebulin gene. Hum Genet. 2004;115:185–190. doi: 10.1007/s00439-004-1140-8. [DOI] [PubMed] [Google Scholar]
- 8.Ao X, Lehrer SS. Phalloidin unzips nebulin from thin filaments in skeletal myofibrils. J Cell Sci. 1995;108:3397–3403. doi: 10.1242/jcs.108.11.3397. [DOI] [PubMed] [Google Scholar]
- 9.Arimura T, Hayashi T, Matsumoto Y, Shibata H, Hiroi S, Nakamura T, Inagaki N, Hinohara K, Takahashi M, Manatsu SI, Sasaoka T, Izumi T, Bonne G, Schwartz K, Kimura A. Structural analysis of four and half LIM protein-2 in dilated cardiomyopathy. Biochem Biophys Res Commun. 2007;357:162–167. doi: 10.1016/j.bbrc.2007.03.128. [DOI] [PubMed] [Google Scholar]
- 10.Arimura T, Matsumoto Y, Okazaki O, Hayashi T, Takahashi M, Inagaki N, Hinohara K, Ashizawa N, Yano K, Kimura A. Structural analysis of obscurin gene in hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 2007;362:281–287. doi: 10.1016/j.bbrc.2007.07.183. [DOI] [PubMed] [Google Scholar]
- 11.Armani A, Galli S, Giacomello E, Bagnato P, Barone V, Rossi D, Sorrentino V. Molecular interactions with obscurin are involved in the localization of muscle-specific small ankyrin1 isoforms to subcompartments of the sarcoplasmic reticulum. Exp Cell Res. 2006;312:3546–3558. doi: 10.1016/j.yexcr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 12.Astier C, Labbe JP, Roustan C, Benyamin Y. Sarcomeric disorganization in post-mortem fish muscles. Comp Biochem Physiol B Comp Biochem. 1991;100:459–465. doi: 10.1016/0305-0491(91)90204-q. [DOI] [PubMed] [Google Scholar]
- 13.Astier C, Raynaud F, Lebart MC, Roustan C, Benyamin Y. Binding of a native titin fragment to actin is regulated by PIP2. FEBS Lett. 1998;429:95–98. doi: 10.1016/s0014-5793(98)00572-9. [DOI] [PubMed] [Google Scholar]
- 14.Atkinson RA, Joseph C, Dal Piaz F, Birolo L, Stier G, Pucci P, Pastore A. Binding of alpha-actinin to titin: implications for Z-disk assembly. Biochemistry. 2000;39:5255–5264. doi: 10.1021/bi991891u. [DOI] [PubMed] [Google Scholar]
- 15.Atkinson RA, Joseph C, Kelly G, Muskett FW, Frenkiel TA, Nietlispach D, Pastore A. Ca2+-independent binding of an EF-hand domain to a novel motif in the alpha-actinin-titin complex. Nat Struct Biol. 2001;8:853–857. doi: 10.1038/nsb1001-853. [DOI] [PubMed] [Google Scholar]
- 16.Auerbach D, Rothen-Ruthishauser B, Bantle S, Leu M, Ehler E, Helfman D, Perriard JC. Molecular mechanisms of myofibril assembly in heart. Cell Struct Funct. 1997;22:139–146. doi: 10.1247/csf.22.139. [DOI] [PubMed] [Google Scholar]
- 17.Ayoob JC, Turnacioglu KK, Mittal B, Sanger JM, Sanger JW. Targeting of cardiac muscle titin fragments to the Z-bands and dense bodies of living muscle and non-muscle cells. Cell Motil Cytoskeleton. 2000;45:67–82. doi: 10.1002/(SICI)1097-0169(200001)45:1<67::AID-CM7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 18.Bagnato P, Barone V, Giacomello E, Rossi D, Sorrentino V. Binding of an ankyrin-1 isoform to obscurin suggests a molecular link between the sarcoplasmic reticulum and myofibrils in striated muscles. J Cell Biol. 2003;160:245–253. doi: 10.1083/jcb.200208109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, Witt CC, Labeit D, Gregorio CC, Granzier H, Labeit S. The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ Res. 2001;89:1065–1072. doi: 10.1161/hh2301.100981. [DOI] [PubMed] [Google Scholar]
- 20.Bang ML, Gregorio C, Labeit S. Molecular dissection of the interaction of desmin with the C-terminal region of nebulin. J Struct Biol. 2002;137:119–127. doi: 10.1006/jsbi.2002.4457. [DOI] [PubMed] [Google Scholar]
- 21.Bang ML, Li X, Littlefield R, Bremner S, Thor A, Knowlton KU, Lieber RL, Chen J. Nebulin-deficient mice exhibit shorter thin filament lengths and reduced contractile function in skeletal muscle. J Cell Biol. 2006;173:905–916. doi: 10.1083/jcb.200603119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bang ML, Mudry RE, McElhinny AS, Trombitas K, Geach AJ, Yamasaki R, Sorimachi H, Granzier H, Gregorio CC, Labeit S. Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies. J Cell Biol. 2001;153:413–427. doi: 10.1083/jcb.153.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barron-Casella EA, Torres MA, Scherer SW, Heng HH, Tsui LC, Casella JF. Sequence analysis and chromosomal localization of human Cap Z. Conserved residues within the actin-binding domain may link Cap Z to gelsolin/severin and profilin protein families. J Biol Chem. 1995;270:21472–21479. doi: 10.1074/jbc.270.37.21472. [DOI] [PubMed] [Google Scholar]
- 24.Bartoli M, Bourg N, Stockholm D, Raynaud F, Delevacque A, Han Y, Borel P, Seddik K, Armande N, Richard I. A mouse model for monitoring calpain activity under physiological and pathological conditions. J Biol Chem. 2006;281:39672–39680. doi: 10.1074/jbc.M608803200. [DOI] [PubMed] [Google Scholar]
- 25.Beggs AH, Byers TJ, Knoll JH, Boyce FM, Bruns GA, Kunkel LM. Cloning and characterization of two human skeletal muscle alpha-actinin genes located on chromosomes 1 and 11. J Biol Chem. 1992;267:9281–9288. [PubMed] [Google Scholar]
- 26.Behr T, Fischer P, Muller-Felber W, Schmidt-Achert M, Pongratz D. Myofibrillogenesis in primary tissue cultures of adult human skeletal muscle: expression of desmin, titin, and nebulin. Clin Invest. 1994;72:150–155. doi: 10.1007/BF00184594. [DOI] [PubMed] [Google Scholar]
- 27.Benian GM, Tinley TL, Tang X, Borodovsky M. The Caenorhabditis elegans gene unc-89, required for muscle M-line assembly, encodes a giant modular protein composed of Ig and signal transduction domains. J Cell Biol. 1996;132:835–848. doi: 10.1083/jcb.132.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett PM, Gautel M. Titin domain patterns correlate with the axial disposition of myosin at the end of the thick filament. J Mol Biol. 1996;259:896–903. doi: 10.1006/jmbi.1996.0367. [DOI] [PubMed] [Google Scholar]
- 29.Bianco P, Nagy A, Kengyel A, Szatmari D, Martonfalvi Z, Huber T, Kellermayer MS. Interaction forces between F-actin and titin PEVK domain measured with optical tweezers. Biophys J. 2007;93:2102–2109. doi: 10.1529/biophysj.107.106153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjorklund AK, Ekman D, Elofsson A. Expansion of protein domain repeats. PLoS Comput Biol. 2006;2:e114. doi: 10.1371/journal.pcbi.0020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blomberg N, Baraldi E, Sattler M, Saraste M, Nilges M. Structure of a PH domain from the C. elegans muscle protein UNC-89 suggests a novel function. Structure. 2000;8:1079–1087. doi: 10.1016/s0969-2126(00)00509-8. [DOI] [PubMed] [Google Scholar]
- 32.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 33.Bonne G, Carrier L, Bercovici J, Cruaud C, Richard P, Hainque B, Gautel M, Labeit S, James M, Beckmann J, Weissenbach J, Vosberg HP, Fiszman M, Komajda M, Schwartz K. Cardiac myosin binding protein-C gene splice acceptor site mutation is associated with familial hypertrophic cardiomyopathy. Nat Genet. 1995;11:438–440. doi: 10.1038/ng1295-438. [DOI] [PubMed] [Google Scholar]
- 34.Borisov AB, Kontrogianni-Konstantopoulos A, Bloch RJ, Westfall MV, Russell MW. Dynamics of obscurin localization during differentiation and remodeling of cardiac myocytes: obscurin as an integrator of myofibrillar structure. J Histochem Cytochem. 2004;52:1117–1127. doi: 10.1369/jhc.3A6183.2004. [DOI] [PubMed] [Google Scholar]
- 35.Borisov AB, Martynova MG, Russell MW. Early incorporation of obscurin into nascent sarcomeres: implication for myofibril assembly during cardiac myogenesis. Histochem Cell Biol. 2008;129:463–478. doi: 10.1007/s00418-008-0378-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borisov AB, Raeker MO, Kontrogianni-Konstantopoulos A, Yang K, Kurnit DM, Bloch RJ, Russell MW. Rapid response of cardiac obscurin gene cluster to aortic stenosis: differential activation of Rho-GEF and MLCK and involvement in hypertrophic growth. Biochem Biophys Res Commun. 2003;310:910–918. doi: 10.1016/j.bbrc.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 37.Borisov AB, Raeker MO, Russell MW. Developmental expression and differential cellular localization of obscurin and obscurin-associated kinase in cardiac muscle cells. J Cell Biochem. 2008;103:1621–1635. doi: 10.1002/jcb.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borisov AB, Sutter SB, Kontrogianni-Konstantopoulos A, Bloch RJ, Westfall MV, Russell MW. Essential role of obscurin in cardiac myofibrillogenesis and hypertrophic response: evidence from small interfering RNA-mediated gene silencing. Histochem Cell Biol. 2006;125:227–238. doi: 10.1007/s00418-005-0069-x. [DOI] [PubMed] [Google Scholar]
- 39.Borzok MA, Catino DH, Nicholson JD, Kontrogianni-Konstantopoulos A, Bloch RJ. Mapping the binding site on small ankyrin 1 for obscurin. J Biol Chem. 2007;282:32384–32396. doi: 10.1074/jbc.M704089200. [DOI] [PubMed] [Google Scholar]
- 40.Bova MP, Yaron O, Huang Q, Ding L, Haley DA, Stewart PL, Horwitz J. Mutation R120G in alphaB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function. Proc Natl Acad Sci USA. 1999;96:6137–6142. doi: 10.1073/pnas.96.11.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowman AL, Catino DH, Strong JC, Randall WR, Kontrogianni-Konstantopoulos A, Bloch RJ. The rho-guanine nucleotide exchange factor domain of obscurin regulates assembly of titin at the Z-disk through interactions with Ran binding protein 9. Mol Biol Cell. 2008;19:3782–3792. doi: 10.1091/mbc.E08-03-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowman AL, Kontrogianni-Konstantopoulos A, Hirsch SS, Geisler SB, Gonzalez-Serratos H, Russell MW, Bloch RJ. Different obscurin isoforms localize to distinct sites at sarcomeres. FEBS Lett. 2007;581:1549–1554. doi: 10.1016/j.febslet.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckley C, Newsom-Davis J, Willcox N, Vincent A. Do titin and cytokine antibodies in MG patients predict thymoma or thymoma recurrence? Neurology. 2001;57:1579–1582. doi: 10.1212/wnl.57.9.1579. [DOI] [PubMed] [Google Scholar]
- 44.Bullard B, Ferguson C, Minajeva A, Leake MC, Gautel M, Labeit D, Ding L, Labeit S, Horwitz J, Leonard KR, Linke WA. Association of the chaperone alphaB-crystallin with titin in heart muscle. J Biol Chem. 2004;279:7917–7924. doi: 10.1074/jbc.M307473200. [DOI] [PubMed] [Google Scholar]
- 45.Carlsson L, Yu JG, Thornell LE. New aspects of obscurin in human striated muscles. Histochem Cell Biol. 2008;130:91–103. doi: 10.1007/s00418-008-0413-z. [DOI] [PubMed] [Google Scholar]
- 46.Carmignac V, Salih MA, Quijano-Roy S, Marchand S, Al Rayess MM, Mukhtar MM, Urtizberea JA, Labeit S, Guicheney P, Leturcq F, Gautel M, Fardeau M, Campbell KP, Richard I, Estournet B, Ferreiro A. C-terminal titin deletions cause a novel early-onset myopathy with fatal cardiomyopathy. Ann Neurol. 2007;61:340–351. doi: 10.1002/ana.21089. [DOI] [PubMed] [Google Scholar]
- 47.Casella JF, Torres MA. Interaction of Cap Z with actin. The NH2-terminal domains of the alpha 1 and beta subunits are not required for actin capping, and alpha 1 beta and alpha 2 beta heterodimers bind differentially to actin. J Biol Chem. 1994;269:6992–6998. [PubMed] [Google Scholar]
- 48.Cazorla O, Freiburg A, Helmes M, Centner T, McNabb M, Wu Y, Trombitas K, Labeit S, Granzier H. Differential expression of cardiac titin isoforms and modulation of cellular stiffness. Circ Res. 2000;86:59–67. doi: 10.1161/01.res.86.1.59. [DOI] [PubMed] [Google Scholar]
- 49.Centner T, Yano J, Kimura E, McElhinny AS, Pelin K, Witt CC, Bang ML, Trombitas K, Granzier H, Gregorio CC, Sorimachi H, Labeit S. Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. J Mol Biol. 2001;306:717–726. doi: 10.1006/jmbi.2001.4448. [DOI] [PubMed] [Google Scholar]
- 50.Chan KK, Tsui SK, Lee SM, Luk SC, Liew CC, Fung KP, Waye MM, Lee CY. Molecular cloning and characterization of FHL2, a novel LIM domain protein preferentially expressed in human heart. Gene. 1998;210:345–350. doi: 10.1016/s0378-1119(97)00644-6. [DOI] [PubMed] [Google Scholar]
- 51.Chen MJ, Shih CL, Wang K. Nebulin as an actin zipper. A two-module nebulin fragment promotes actin nucleation and stabilizes actin filaments. J Biol Chem. 1993;268:20327–20334. [PubMed] [Google Scholar]
- 52.Ciani B, Layfield R, Cavey JR, Sheppard PW, Searle MS. Structure of the ubiquitin-associated domain of p62 (SQSTM1) and implications for mutations that cause Paget's disease of bone. J Biol Chem. 2003;278:37409–37412. doi: 10.1074/jbc.M307416200. [DOI] [PubMed] [Google Scholar]
- 53.Coisy-Quivy M, Touzet O, Bourret A, Hipskind RA, Mercier J, Fort P, Philips A. TC10 controls human myofibril organization and is activated by the sarcomeric RhoGEF obscurin. J Cell Sci. 2009;122:947–956. doi: 10.1242/jcs.040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cuneo P, Trombetta G, Adami R, Grazi E. Is nebulin truly a component of the thin filament? FEBS Lett. 1996;397:136–138. doi: 10.1016/s0014-5793(96)01157-x. [DOI] [PubMed] [Google Scholar]
- 55.Cunha SR, Mohler PJ. Obscurin targets ankyrin-B and protein phosphatase 2A to the cardiac M-line. J Biol Chem. 2008;283:31968–31980. doi: 10.1074/jbc.M806050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dabiri GA, Turnacioglu KK, Sanger JM, Sanger JW. Myofibrillogenesis visualized in living embryonic cardiomyocytes. Proc Natl Acad Sci USA. 1997;94:9493–9498. doi: 10.1073/pnas.94.17.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delgado EF, Geesink GH, Marchello JA, Goll DE, Koohmaraie M. Properties of myofibril-bound calpain activity in longissimus muscle of callipyge and normal sheep. J Anim Sci. 2001;79:2097–2107. doi: 10.2527/2001.7982097x. [DOI] [PubMed] [Google Scholar]
- 58.Donner K, Nowak KJ, Aro M, Pelin K, Wallgren-Pettersson C. Developmental and muscle-type-specific expression of mouse nebulin exons 127 and 128. Genomics. 2006;88:489–495. doi: 10.1016/j.ygeno.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 59.Donner K, Sandbacka M, Lehtokari VL, Wallgren-Pettersson C, Pelin K. Complete genomic structure of the human nebulin gene and identification of alternatively spliced transcripts. Eur J Hum Genet. 2004;12:744–751. doi: 10.1038/sj.ejhg.5201242. [DOI] [PubMed] [Google Scholar]
- 60.Du A, Sanger JM, Linask KK, Sanger JW. Myofibrillogenesis in the first cardiomyocytes formed from isolated quail precardiac mesoderm. Dev Biol. 2003;257:382–394. doi: 10.1016/s0012-1606(03)00104-0. [DOI] [PubMed] [Google Scholar]
- 61.Duguez S, Bartoli M, Richard I. Calpain 3: a key regulator of the sarcomere? FEBS J. 2006;273:3427–3436. doi: 10.1111/j.1742-4658.2006.05351.x. [DOI] [PubMed] [Google Scholar]
- 62.Edstrom L, Thornell LE, Albo J, Landin S, Samuelsson M. Myopathy with respiratory failure and typical myofibrillar lesions. J Neurol Sci. 1990;96:211–228. doi: 10.1016/0022-510x(90)90134-9. [DOI] [PubMed] [Google Scholar]
- 63.Ehler E, Rothen BM, Hammerle SP, Komiyama M, Perriard JC. Myofibrillogenesis in the developing chicken heart: assembly of Z-disk, M-line and the thick filaments. J Cell Sci. 1999;112:1529–1539. doi: 10.1242/jcs.112.10.1529. [DOI] [PubMed] [Google Scholar]
- 64.Fardeau M, Eymard B, Mignard C, Tome FM, Richard I, Beckmann JS. Chromosome 15-linked limb-girdle muscular dystrophy: clinical phenotypes in Reunion Island and French metropolitan communities. Neuromuscul Disord. 1996;6:447–453. doi: 10.1016/s0960-8966(96)00387-2. [DOI] [PubMed] [Google Scholar]
- 65.Feasson L, Stockholm D, Freyssenet D, Richard I, Duguez S, Beckmann JS, Denis C. Molecular adaptations of neuromuscular disease-associated proteins in response to eccentric exercise in human skeletal muscle. J Physiol. 2002;543:297–306. doi: 10.1113/jphysiol.2002.018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferrara TM, Flaherty DB, Benian GM. Titin/connectin-related proteins in C. elegans: a review and new findings. J Muscle Res Cell Motil. 2005;26:435–447. doi: 10.1007/s10974-005-9027-4. [DOI] [PubMed] [Google Scholar]
- 67.Flashman E, Redwood C, Moolman-Smook J, Watkins H. Cardiac myosin binding protein C: its role in physiology and disease. Circ Res. 2004;94:1279–1289. doi: 10.1161/01.RES.0000127175.21818.C2. [DOI] [PubMed] [Google Scholar]
- 68.Flashman E, Watkins H, Redwood C. Localization of the binding site of the C-terminal domain of cardiac myosin-binding protein-C on the myosin rod. Biochem J. 2007;401:97–102. doi: 10.1042/BJ20060500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Flucher BE. Structural analysis of muscle development: transverse tubules, sarcoplasmic reticulum, and the triad. Dev Biol. 1992;154:245–260. doi: 10.1016/0012-1606(92)90065-o. [DOI] [PubMed] [Google Scholar]
- 70.Flucher BE, Phillips JL, Powell JA, Andrews SB, Daniels MP. Coordinated development of myofibrils, sarcoplasmic reticulum and transverse tubules in normal and dysgenic mouse skeletal muscle, in vivo and in vitro. Dev Biol. 1992;150:266–280. doi: 10.1016/0012-1606(92)90241-8. [DOI] [PubMed] [Google Scholar]
- 71.Flucher BE, Takekura H, Franzini-Armstrong C. Development of the excitation-contraction coupling apparatus in skeletal muscle: association of sarcoplasmic reticulum and transverse tubules with myofibrils. Dev Biol. 1993;160:135–147. doi: 10.1006/dbio.1993.1292. [DOI] [PubMed] [Google Scholar]
- 72.Ford-Speelman DL, Roche JA, Bowman AL, Bloch RJ. The rhoGEF domain of obscurin activates rhoA signaling in skeletal muscle. Mol Biol Cell. doi: 10.1091/mbc.E08-10-1029. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fowler VM. Capping actin filament growth: tropomodulin in muscle and nonmuscle cells. Soc Gen Physiol Ser. 1997;52:79–89. [PubMed] [Google Scholar]
- 74.Fowler VM. Identification and purification of a novel Mr 43,000 tropomyosin-binding protein from human erythrocyte membranes. J Biol Chem. 1987;262:12792–12800. [PubMed] [Google Scholar]
- 75.Fowler VM. Tropomodulin: a cytoskeletal protein that binds to the end of erythrocyte tropomyosin and inhibits tropomyosin binding to actin. J Cell Biol. 1990;111:471–481. doi: 10.1083/jcb.111.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fowler VM, McKeown CR, Fischer RS. Nebulin: does it measure up as a ruler? Curr Biol. 2006;16:R18–20. doi: 10.1016/j.cub.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 77.Fowler VM, Sussmann MA, Miller PG, Flucher BE, Daniels MP. Tropomodulin is associated with the free (pointed) ends of the thin filaments in rat skeletal muscle. J Cell Biol. 1993;120:411–420. doi: 10.1083/jcb.120.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Franzini-Armstrong C, Jorgensen AO. Structure and development of E-C coupling units in skeletal muscle. Annu Rev Physiol. 1994;56:509–534. doi: 10.1146/annurev.ph.56.030194.002453. [DOI] [PubMed] [Google Scholar]
- 79.Franzini-Armstrong C, Protasi F, Tijskens P. The assembly of calcium release units in cardiac muscle. Ann NY Acad Sci. 2005;1047:76–85. doi: 10.1196/annals.1341.007. [DOI] [PubMed] [Google Scholar]
- 80.Freiburg A, Gautel M. A molecular map of the interactions between titin and myosin-binding protein C. Implications for sarcomeric assembly in familial hypertrophic cardiomyopathy. Eur J Biochem. 1996;235:317–323. doi: 10.1111/j.1432-1033.1996.00317.x. [DOI] [PubMed] [Google Scholar]
- 81.Fujita H, Labeit D, Gerull B, Labeit S, Granzier HL. Titin isoform-dependent effect of calcium on passive myocardial tension. Am J Physiol Heart Circ Physiol. 2004;287:H2528–H2534. doi: 10.1152/ajpheart.00553.2004. [DOI] [PubMed] [Google Scholar]
- 82.Fukami K, Furuhashi K, Inagaki M, Endo T, Hatano S, Takenawa T. Requirement of phosphatidylinositol 4,5-bisphosphate for alpha-actinin function. Nature. 1992;359:150–152. doi: 10.1038/359150a0. [DOI] [PubMed] [Google Scholar]
- 83.Fukuda N, Granzier HL, Ishiwata S, Kurihara S. Physiological functions of the giant elastic protein titin in mammalian striated muscle. J Physiol Sci. 2008;58:151–159. doi: 10.2170/physiolsci.RV005408. [DOI] [PubMed] [Google Scholar]
- 84.Fukuda N, Wu Y, Nair P, Granzier HL. Phosphorylation of titin modulates passive stiffness of cardiac muscle in a titin isoform-dependent manner. J Gen Physiol. 2005;125:257–271. doi: 10.1085/jgp.200409177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fukuzawa A, Idowu S, Gautel M. Complete human gene structure of obscurin: implications for isoform generation by differential splicing. J Muscle Res Cell Motil. 2005;26:427–434. doi: 10.1007/s10974-005-9025-6. [DOI] [PubMed] [Google Scholar]
- 86.Fukuzawa A, Lange S, Holt M, Vihola A, Carmignac V, Ferreiro A, Udd B, Gautel M. Interactions with titin and myomesin target obscurin and obscurin-like 1 to the M-band: implications for hereditary myopathies. J Cell Sci. 2008;121:1841–1851. doi: 10.1242/jcs.028019. [DOI] [PubMed] [Google Scholar]
- 87.Furst DO, Gautel M. The anatomy of a molecular giant: how the sarcomere cytoskeleton is assembled from immunoglobulin super-family molecules. J Mol Cell Cardiol. 1995;27:951–959. doi: 10.1016/0022-2828(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 88.Furst DO, Osborn M, Nave R, Weber K. The organization of titin filaments in the half-sarcomere revealed by monoclonal antibodies in immunoelectron microscopy: a map of ten nonrepetitive epitopes starting at the Z line extends close to the M line. J Cell Biol. 1988;106:1563–1572. doi: 10.1083/jcb.106.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Furst DO, Osborn M, Weber K. Myogenesis in the mouse embryo: differential onset of expression of myogenic proteins and the involvement of titin in myofibril assembly. J Cell Biol. 1989;109:517–527. doi: 10.1083/jcb.109.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Furst DO, Vinkemeier U, Weber K. Mammalian skeletal muscle C-protein: purification from bovine muscle, binding to titin and the characterization of a full-length human cDNA. J Cell Sci. 1992;102:769–778. doi: 10.1242/jcs.102.4.769. [DOI] [PubMed] [Google Scholar]
- 91.Furukawa T, Ono Y, Tsuchiya H, Katayama Y, Bang ML, Labeit D, Labeit S, Inagaki N, Gregorio CC. Specific interaction of the potassium channel beta-subunit minK with the sarcomeric protein T-cap suggests a T-tubule-myofibril linking system. J Mol Biol. 2001;313:775–784. doi: 10.1006/jmbi.2001.5053. [DOI] [PubMed] [Google Scholar]
- 92.Garvey SM, Rajan C, Lerner AP, Frankel WN, Cox GA. The muscular dystrophy with myositis (mdm) mouse mutation disrupts a skeletal muscle-specific domain of titin. Genomics. 2002;79:146–149. doi: 10.1006/geno.2002.6685. [DOI] [PubMed] [Google Scholar]
- 93.Gautel M. The super-repeats of titin/connectin and their interactions: glimpses at sarcomeric assembly. Adv Biophys. 1996;33:27–37. doi: 10.1016/s0065-227x(96)90020-9. [DOI] [PubMed] [Google Scholar]
- 94.Gautel M, Castiglione Morelli MA, Pfuhl M, Motta A, Pastore A. A calmodulin-binding sequence in the C-terminus of human cardiac titin kinase. Eur J Biochem. 1995;230:752–759. [PubMed] [Google Scholar]
- 95.Gautel M, Goulding D. A molecular map of titin/connectin elasticity reveals two different mechanisms acting in series. FEBS Lett. 1996;385:11–14. doi: 10.1016/0014-5793(96)00338-9. [DOI] [PubMed] [Google Scholar]
- 96.Gautel M, Goulding D, Bullard B, Weber K, Furst DO. The central Z-disk region of titin is assembled from a novel repeat in variable copy numbers. J Cell Sci. 1996;109:2747–2754. doi: 10.1242/jcs.109.11.2747. [DOI] [PubMed] [Google Scholar]
- 97.Gautel M, Lakey A, Barlow DP, Holmes Z, Scales S, Leonard K, Labeit S, Mygland A, Gilhus NE, Aarli JA. Titin antibodies in myasthenia gravis: identification of a major immunogenic region of titin. Neurology. 1993;43:1581–1585. doi: 10.1212/wnl.43.8.1581. [DOI] [PubMed] [Google Scholar]
- 98.Gautel M, Leonard K, Labeit S. Phosphorylation of KSP motifs in the C-terminal region of titin in differentiating myoblasts. EMBO J. 1993;12:3827–3834. doi: 10.1002/j.1460-2075.1993.tb06061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gautel M, Mues A, Young P. Control of sarcomeric assembly: the flow of information on titin. Rev Physiol Biochem Pharmacol. 1999;138:97–137. doi: 10.1007/BFb0119625. [DOI] [PubMed] [Google Scholar]
- 100.Geisler SB, Robinson D, Hauringa M, Raeker MO, Borisov AB, Westfall MV, Russell MW. Obscurin-like 1, OBSL1, is a novel cytoskeletal protein related to obscurin. Genomics. 2007;89:521–531. doi: 10.1016/j.ygeno.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Genini M, Schwalbe P, Scholl FA, Remppis A, Mattei MG, Schafer BW. Subtractive cloning and characterization of DRAL, a novel LIM-domain protein down-regulated in rhabdomyosarcoma. DNA Cell Biol. 1997;16:433–442. doi: 10.1089/dna.1997.16.433. [DOI] [PubMed] [Google Scholar]
- 102.Gerull B, Atherton J, Geupel A, Sasse-Klaassen S, Heuser A, Frenneaux M, McNabb M, Granzier H, Labeit S, Thierfelder L. Identification of a novel frameshift mutation in the giant muscle filament titin in a large Australian family with dilated cardiomyopathy. J Mol Med. 2006;84:478–483. doi: 10.1007/s00109-006-0060-6. [DOI] [PubMed] [Google Scholar]
- 103.Gerull B, Gramlich M, Atherton J, McNabb M, Trombitas K, Sasse-Klaassen S, Seidman JG, Seidman C, Granzier H, Labeit S, Frenneaux M, Thierfelder L. Mutations of TTN, encoding the giant muscle filament titin, cause familial dilated cardiomyopathy. Nat Genet. 2002;30:201–204. doi: 10.1038/ng815. [DOI] [PubMed] [Google Scholar]
- 104.Giacomello E, Sorrentino V. Localization of ank1.5 in the sarcoplasmic reticulum precedes that of SERCA and RyR: relationship with the organization of obscurin in developing sarcomeres. Histochem Cell Biol. 2009;131:371–382. doi: 10.1007/s00418-008-0534-4. [DOI] [PubMed] [Google Scholar]
- 105.Gokhin DS, Bang ML, Zhang J, Chen J, Lieber RL. Reduced thin filament length in nebulin-knockout skeletal muscle alters isometric contractile properties. Am J Physiol Cell Physiol. 2009;296:C1123–C1132. doi: 10.1152/ajpcell.00503.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Golenhofen N, Arbeiter A, Koob R, Drenckhahn D. Ischemia-induced association of the stress protein alpha B-crystallin with I-band portion of cardiac titin. J Mol Cell Cardiol. 2002;34:309–319. doi: 10.1006/jmcc.2001.1513. [DOI] [PubMed] [Google Scholar]
- 107.Golenhofen N, Htun P, Ness W, Koob R, Schaper W, Drenckhahn D. Binding of the stress protein alpha B-crystallin to cardiac myofibrils correlates with the degree of myocardial damage during ischemia/reperfusion in vivo. J Mol Cell Cardiol. 1999;31:569–580. doi: 10.1006/jmcc.1998.0892. [DOI] [PubMed] [Google Scholar]
- 108.Golenhofen N, Ness W, Wawrousek EF, Drenckhahn D. Expression and induction of the stress protein alpha-B-crystallin in vascular endothelial cells. Histochem Cell Biol. 2002;117:203–209. doi: 10.1007/s00418-001-0378-7. [DOI] [PubMed] [Google Scholar]
- 109.Goll CM, Pastore A, Nilges M. The three-dimensional structure of a type I module from titin: a prototype of intracellular fibronectin type III domains. Structure. 1998;6:1291–1302. doi: 10.1016/s0969-2126(98)00129-4. [DOI] [PubMed] [Google Scholar]
- 110.Gonsior SM, Gautel M, Hinssen H. A six-module human nebulin fragment bundles actin filaments and induces actin polymerization. J Muscle Res Cell Motil. 1998;19:225–235. doi: 10.1023/a:1005372915268. [DOI] [PubMed] [Google Scholar]
- 111.Gotthardt M, Hammer RE, Hubner N, Monti J, Witt CC, Mc-Nabb M, Richardson JA, Granzier H, Labeit S, Herz J. Conditional expression of mutant M-line titins results in cardiomyopathy with altered sarcomere structure. J Biol Chem. 2003;278:6059–6065. doi: 10.1074/jbc.M211723200. [DOI] [PubMed] [Google Scholar]
- 112.Granzier H, Helmes M, Cazorla O, McNabb M, Labeit D, Wu Y, Yamasaki R, Redkar A, Kellermayer M, Labeit S, Trombitas K. Mechanical properties of titin isoforms. Adv Exp Med Biol. 2000;481:283–300. doi: 10.1007/978-1-4615-4267-4_17. [DOI] [PubMed] [Google Scholar]
- 113.Granzier H, Helmes M, Trombitas K. Nonuniform elasticity of titin in cardiac myocytes: a study using immunoelectron microscopy and cellular mechanics. Biophys J. 1996;70:430–442. doi: 10.1016/S0006-3495(96)79586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Granzier H, Kellermayer M, Helmes M, Trombitas K. Titin elasticity and mechanism of passive force development in rat cardiac myocytes probed by thin-filament extraction. Biophys J. 1997;73:2043–2053. doi: 10.1016/S0006-3495(97)78234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Granzier H, Labeit D, Wu Y, Labeit S. Titin as a modular spring: emerging mechanisms for elasticity control by titin in cardiac physiology and pathophysiology. J Muscle Res Cell Motil. 2002;23:457–471. doi: 10.1023/a:1023458406346. [DOI] [PubMed] [Google Scholar]
- 116.Granzier H, Labeit D, Wu Y, Witt C, Watanabe K, Lahmers S, Gotthardt M, Labeit S. Adaptations in titin's spring elements in normal and cardiomyopathic hearts. Adv Exp Med Biol. 2003;538:517–530. doi: 10.1007/978-1-4419-9029-7_46. [DOI] [PubMed] [Google Scholar]
- 117.Granzier H, Labeit S. Cardiac titin: an adjustable multi-functional spring. J Physiol. 2002;541:335–342. doi: 10.1113/jphysiol.2001.014381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Granzier H, Labeit S. Structure-function relations of the giant elastic protein titin in striated and smooth muscle cells. Muscle Nerve. 2007;36:740–755. doi: 10.1002/mus.20886. [DOI] [PubMed] [Google Scholar]
- 119.Granzier H, Wu Y, Siegfried L, LeWinter M. Titin: physiological function and role in cardiomyopathy and failure. Heart Fail Rev. 2005;10:211–223. doi: 10.1007/s10741-005-5251-7. [DOI] [PubMed] [Google Scholar]
- 120.Granzier HL, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J. 1995;68:1027–1044. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Granzier HL, Labeit S. The giant muscle protein titin is an adjustable molecular spring. Exerc Sport Sci Rev. 2006;34:50–53. doi: 10.1249/00003677-200604000-00002. [DOI] [PubMed] [Google Scholar]
- 122.Granzier HL, Labeit S. The giant protein titin: a major player in myocardial mechanics, signaling, and disease. Circ Res. 2004;94:284–295. doi: 10.1161/01.RES.0000117769.88862.F8. [DOI] [PubMed] [Google Scholar]
- 123.Granzier HL, Labeit S. Titin and its associated proteins: the third myofilament system of the sarcomere. Adv Protein Chem. 2005;71:89–119. doi: 10.1016/S0065-3233(04)71003-7. [DOI] [PubMed] [Google Scholar]
- 124.Granzier HL, Wu Y, Trombitas K, Witt C, Labeit S, Bell S, LeWinter M. Variable titin-based stiffness adjustment in heart disease. Circulation. 2003;108:e23. doi: 10.1161/01.CIR.0000081439.94575.E3. [DOI] [PubMed] [Google Scholar]
- 125.Grater F, Shen J, Jiang H, Gautel M, Grubmuller H. Mechanically induced titin kinase activation studied by force-probe molecular dynamics simulations. Biophys J. 2005;88:790–804. doi: 10.1529/biophysj.104.052423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Greaser M. Identification of new repeating motifs in titin. Proteins. 2001;43:145–149. doi: 10.1002/1097-0134(20010501)43:2<145::aid-prot1026>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 127.Greaser ML, Berri M, Warren CM, Mozdziak PE. Species variations in cDNA sequence and exon splicing patterns in the extensible I-band region of cardiac titin: relation to passive tension. J Muscle Res Cell Motil. 2002;23:473–482. doi: 10.1023/a:1023410523184. [DOI] [PubMed] [Google Scholar]
- 128.Greaser ML, Wang SM, Berri M, Mozdziak P, Kumazawa Y. Sequence and mechanical implications of titin's PEVK region. Adv Exp Med Biol. 2000;481:53–63. doi: 10.1007/978-1-4615-4267-4_4. [DOI] [PubMed] [Google Scholar]
- 129.Gregorio CC, Fowler VM. Mechanisms of thin filament assembly in embryonic chick cardiac myocytes: tropomodulin requires tropomyosin for assembly. J Cell Biol. 1995;129:683–695. doi: 10.1083/jcb.129.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gregorio CC, Granzier H, Sorimachi H, Labeit S. Muscle assembly: a titanic achievement? Curr Opin Cell Biol. 1999;11:18–25. doi: 10.1016/s0955-0674(99)80003-9. [DOI] [PubMed] [Google Scholar]
- 131.Gregorio CC, Perry CN, McElhinny AS. Functional properties of the titin/connectin-associated proteins, the muscle-specific RING finger proteins (MURFs), in striated muscle. J Muscle Res Cell Motil. 2005;26:389–400. doi: 10.1007/s10974-005-9021-x. [DOI] [PubMed] [Google Scholar]
- 132.Gregorio CC, Trombitas K, Centner T, Kolmerer B, Stier G, Kunke K, Suzuki K, Obermayr F, Herrmann B, Granzier H, Sorimachi H, Labeit S. The NH2 terminus of titin spans the Z-disc: its interaction with a novel 19-kD ligand (T-cap) is required for sarcomeric integrity. J Cell Biol. 1998;143:1013–1027. doi: 10.1083/jcb.143.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gregorio CC, Weber A, Bondad M, Pennise CR, Fowler VM. Requirement of pointed-end capping by tropomodulin to maintain actin filament length in embryonic chick cardiac myocytes. Nature. 1995;377:83–86. doi: 10.1038/377083a0. [DOI] [PubMed] [Google Scholar]
- 134.Gutierrez-Cruz G, Van Heerden AH, Wang K. Modular motif, structural folds and affinity profiles of the PEVK segment of human fetal skeletal muscle titin. J Biol Chem. 2001;276:7442–7449. doi: 10.1074/jbc.M008851200. [DOI] [PubMed] [Google Scholar]
- 135.Hackman JP, Vihola AK, Udd AB. The role of titin in muscular disorders. Ann Med. 2003;35:434–441. doi: 10.1080/07853890310012797. [DOI] [PubMed] [Google Scholar]
- 136.Hackman P, Marchand S, Sarparanta J, Vihola A, Penisson-Besnier I, Eymard B, Pardal-Fernandez JM, Hammouda el H, Richard I, Illa I, Udd B. Truncating mutations in C-terminal titin may cause more severe tibial muscular dystrophy (TMD). Neuromuscul Disord. 2008;18:922–928. doi: 10.1016/j.nmd.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 137.Hackman P, Vihola A, Haravuori H, Marchand S, Sarparanta J, De Seze J, Labeit S, Witt C, Peltonen L, Richard I, Udd B. Tibial muscular dystrophy is a titinopathy caused by mutations in TTN, the gene encoding the giant skeletal-muscle protein titin. Am J Hum Genet. 2002;71:492–500. doi: 10.1086/342380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Haravuori H, Makela-Bengs P, Udd B, Partanen J, Pulkkinen L, Somer H, Peltonen L. Assignment of the tibial muscular dystrophy locus to chromosome 2q31. Am J Hum Genet. 1998;62:620–626. doi: 10.1086/301752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Haravuori H, Vihola A, Straub V, Auranen M, Richard I, Marchand S, Voit T, Labeit S, Somer H, Peltonen L, Beckmann JS, Udd B. Secondary calpain3 deficiency in 2q-linked muscular dystrophy: titin is the candidate gene. Neurology. 2001;56:869–877. doi: 10.1212/wnl.56.7.869. [DOI] [PubMed] [Google Scholar]
- 140.Hayashi C, Ono Y, Doi N, Kitamura F, Tagami M, Mineki R, Arai T, Taguchi H, Yanagida M, Hirner S, Labeit D, Labeit S, Sorimachi H. Multiple molecular interactions implicate the connectin/titin N2A region as a modulating scaffold for p94/calpain 3 activity in skeletal muscle. J Biol Chem. 2008;283:14801–14814. doi: 10.1074/jbc.M708262200. [DOI] [PubMed] [Google Scholar]
- 141.Head JG, Houmeida A, Knight PJ, Clarke AR, Trinick J, Brady RL. Stability and folding rates of domains spanning the large A-band super-repeat of titin. Biophys J. 2001;81:1570–1579. doi: 10.1016/s0006-3495(01)75811-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hein S, Schaper J. Weakness of a giant: mutations of the sarcomeric protein titin. Trends Mol Med. 2002;8:311–313. doi: 10.1016/s1471-4914(02)02372-9. [DOI] [PubMed] [Google Scholar]
- 143.Hein S, Scholz D, Fujitani N, Rennollet H, Brand T, Friedl A, Schaper J. Altered expression of titin and contractile proteins in failing human myocardium. J Mol Cell Cardiol. 1994;26:1291–1306. doi: 10.1006/jmcc.1994.1148. [DOI] [PubMed] [Google Scholar]
- 144.Helmes M, Lim CC, Liao R, Bharti A, Cui L, Sawyer DB. Titin determines the Frank-Starling relation in early diastole. J Gen Physiol. 2003;121:97–110. doi: 10.1085/jgp.20028652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Helmes M, Trombitas K, Centner T, Kellermayer M, Labeit S, Linke WA, Granzier H. Mechanically driven contour-length adjustment in rat cardiac titin's unique N2B sequence: titin is an adjustable spring. Circ Res. 1999;84:1339–1352. doi: 10.1161/01.res.84.11.1339. [DOI] [PubMed] [Google Scholar]
- 146.Helmes M, Trombitas K, Granzier H. Titin develops restoring force in rat cardiac myocytes. Circ Res. 1996;79:619–626. doi: 10.1161/01.res.79.3.619. [DOI] [PubMed] [Google Scholar]
- 147.Herasse M, Ono Y, Fougerousse F, Kimura E, Stockholm D, Beley C, Montarras D, Pinset C, Sorimachi H, Suzuki K, Beckmann JS, Richard I. Expression and functional characteristics of calpain 3 isoforms generated through tissue-specific transcriptional and posttranscriptional events. Mol Cell Biol. 1999;19:4047–4055. doi: 10.1128/mcb.19.6.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Holtzer H, Hijikata T, Lin ZX, Zhang ZQ, Holtzer S, Protasi F, Franzini-Armstrong C, Sweeney HL. Independent assembly of 1.6 microns long bipolar MHC filaments and I-Z-I bodies. Cell Struct Funct. 1997;22:83–93. doi: 10.1247/csf.22.83. [DOI] [PubMed] [Google Scholar]
- 149.Horowits R. Nebulin regulation of actin filament lengths: new angles. Trends Cell Biol. 2006;16:121–124. doi: 10.1016/j.tcb.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 150.Houmeida A, Holt J, Tskhovrebova L, Trinick J. Studies of the interaction between titin and myosin. J Cell Biol. 1995;131:1471–1481. doi: 10.1083/jcb.131.6.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hsieh CM, Fukumoto S, Layne MD, Maemura K, Charles H, Patel A, Perrella MA, Lee ME. Striated muscle preferentially expressed genes alpha and beta are two serine/threonine protein kinases derived from the same gene as the aortic preferentially expressed gene-1. J Biol Chem. 2000;275:36966–36973. doi: 10.1074/jbc.M006028200. [DOI] [PubMed] [Google Scholar]
- 152.Huebsch KA, Kudryashova E, Wooley CM, Sher RB, Seburn KL, Spencer MJ, Cox GA. Mdm muscular dystrophy: interactions with calpain 3 and a novel functional role for titin's N2A domain. Hum Mol Genet. 2005;14:2801–2811. doi: 10.1093/hmg/ddi313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Huff-Lonergan E, Mitsuhashi T, Beekman DD, Parrish FC, Jr, Olson DG, Robson RM. Proteolysis of specific muscle structural proteins by mu-calpain at low pH and temperature is similar to degradation in postmortem bovine muscle. J Anim Sci. 1996;74:993–1008. doi: 10.2527/1996.745993x. [DOI] [PubMed] [Google Scholar]
- 154.Imanaka-Yoshida K, Amitani A, Ioshii SO, Koyabu S, Yamakado T, Yoshida T. Alterations of expression and distribution of the Ca2+-storing proteins in endo/sarcoplasmic reticulum during differentiation of rat cardiomyocytes. J Mol Cell Cardiol. 1996;28:553–562. doi: 10.1006/jmcc.1996.0051. [DOI] [PubMed] [Google Scholar]
- 155.Improta S, Politou AS, Pastore A. Immunoglobulin-like modules from titin I-band: extensible components of muscle elasticity. Structure. 1996;4:323–337. doi: 10.1016/s0969-2126(96)00036-6. [DOI] [PubMed] [Google Scholar]
- 156.Inui M, Wang S, Saito A, Fleischer S. Characterization of junctional and longitudinal sarcoplasmic reticulum from heart muscle. J Biol Chem. 1988;263:10843–10850. [PubMed] [Google Scholar]
- 157.Inui M, Wang S, Saito A, Fleischer S. Junctional and longitudinal sarcoplasmic reticulum of heart muscle. Methods Enzymol. 1988;157:100–106. doi: 10.1016/0076-6879(88)57072-6. [DOI] [PubMed] [Google Scholar]
- 158.Isaacs WB, Kim IS, Struve A, Fulton AB. Association of titin and myosin heavy chain in developing skeletal muscle. Proc Natl Acad Sci USA. 1992;89:7496–7500. doi: 10.1073/pnas.89.16.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Itoh Y, Suzuki T, Kimura S, Ohashi K, Higuchi H, Sawada H, Shimizu T, Shibata M, Maruyama K. Extensible and less-extensible domains of connectin filaments in stretched vertebrate skeletal muscle sarcomeres as detected by immunofluorescence and immunoelectron microscopy using monoclonal antibodies. J Biochem. 1988;104:504–508. doi: 10.1093/oxfordjournals.jbchem.a122499. [DOI] [PubMed] [Google Scholar]
- 160.Itoh-Satoh M, Hayashi T, Nishi H, Koga Y, Arimura T, Koyanagi T, Takahashi M, Hohda S, Ueda K, Nouchi T, Hiroe M, Marumo F, Imaizumi T, Yasunami M, Kimura A. Titin mutations as the molecular basis for dilated cardiomyopathy. Biochem Biophys Res Commun. 2002;291:385–393. doi: 10.1006/bbrc.2002.6448. [DOI] [PubMed] [Google Scholar]
- 161.Jeyaseelan R, Poizat C, Baker RK, Abdishoo S, Isterabadi LB, Lyons GE, Kedes L. A novel cardiac-restricted target for doxorubicin. CARP, a nuclear modulator of gene expression in cardiac progenitor cells and cardiomyocytes. J Biol Chem. 1997;272:22800–22808. doi: 10.1074/jbc.272.36.22800. [DOI] [PubMed] [Google Scholar]
- 162.Jin JP, Wang K. Cloning, expression, and protein interaction of human nebulin fragments composed of varying numbers of sequence modules. J Biol Chem. 1991;266:21215–21223. [PubMed] [Google Scholar]
- 163.Jin JP, Wang K. Nebulin as a giant actin-binding template protein in skeletal muscle sarcomere. Interaction of actin and cloned human nebulin fragments. FEBS Lett. 1991;281:93–96. doi: 10.1016/0014-5793(91)80366-b. [DOI] [PubMed] [Google Scholar]
- 164.Johnston JJ, Kelley RI, Crawford TO, Morton DH, Agarwala R, Koch T, Schaffer AA, Francomano CA, Biesecker LG. A novel nemaline myopathy in the Amish caused by a mutation in troponin T1. Am J Hum Genet. 2000;67:814–821. doi: 10.1086/303089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Joseph C, Stier G, O'Brien R, Politou AS, Atkinson RA, Bianco A, Ladbury JE, Martin SR, Pastore A. A structural characterization of the interactions between titin Z-repeats and the alpha-actinin C-terminal domain. Biochemistry. 2001;40:4957–4965. doi: 10.1021/bi002739r. [DOI] [PubMed] [Google Scholar]
- 166.Kazmierski ST, Antin PB, Witt CC, Huebner N, McElhinny AS, Labeit S, Gregorio CC. The complete mouse nebulin gene sequence and the identification of cardiac nebulin. J Mol Biol. 2003;328:835–846. doi: 10.1016/s0022-2836(03)00348-6. [DOI] [PubMed] [Google Scholar]
- 167.Kedar V, McDonough H, Arya R, Li HH, Rockman HA, Patterson C. Muscle-specific RING finger 1 is a bona fide ubiquitin ligase that degrades cardiac troponin I. Proc Natl Acad Sci USA. 2004;101:18135–18140. doi: 10.1073/pnas.0404341102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Keesey JC. Clinical evaluation and management of myasthenia gravis. Muscle Nerve. 2004;29:484–505. doi: 10.1002/mus.20030. [DOI] [PubMed] [Google Scholar]
- 169.Kellermayer MS, Granzier HL. Elastic properties of single titin molecules made visible through fluorescent F-actin binding. Biochem Biophys Res Commun. 1996;221:491–497. doi: 10.1006/bbrc.1996.0624. [DOI] [PubMed] [Google Scholar]
- 170.Kinbara K, Sorimachi H, Ishiura S, Suzuki K. Muscle-specific calpain, p94, interacts with the extreme C-terminal region of connectin, a unique region flanked by two immunoglobulin C2 motifs. Arch Biochem Biophys. 1997;342:99–107. doi: 10.1006/abbi.1997.0108. [DOI] [PubMed] [Google Scholar]
- 171.Kinbara K, Sorimachi H, Ishiura S, Suzuki K. Skeletal muscle-specific calpain, p49: structure and physiological function. Biochem Pharmacol. 1998;56:415–420. doi: 10.1016/s0006-2952(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 172.Kojic S, Medeot E, Guccione E, Krmac H, Zara I, Martinelli V, Valle G, Faulkner G. The Ankrd2 protein, a link between the sarcomere and the nucleus in skeletal muscle. J Mol Biol. 2004;339:313–325. doi: 10.1016/j.jmb.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 173.Kolmerer B, Olivieri N, Witt CC, Herrmann BG, Labeit S. Genomic organization of M line titin and its tissue-specific expression in two distinct isoforms. J Mol Biol. 1996;256:556–563. doi: 10.1006/jmbi.1996.0108. [DOI] [PubMed] [Google Scholar]
- 174.Kolmerer B, Witt CC, Freiburg A, Millevoi S, Stier G, Sorimachi H, Pelin K, Carrier L, Schwartz K, Labeit D, Gregorio CC, Linke WA, Labeit S. The titin cDNA sequence and partial genomic sequences: insights into the molecular genetics, cell biology and physiology of the titin filament system. Rev Physiol Biochem Pharmacol. 1999;138:19–55. doi: 10.1007/BFb0119623. [DOI] [PubMed] [Google Scholar]
- 175.Komiyama M, Zhou ZH, Maruyama K, Shimada Y. Spatial relationship of nebulin relative to other myofibrillar proteins during myogenesis in embryonic chick skeletal muscle cells in vitro. J Muscle Res Cell Motil. 1992;13:48–54. doi: 10.1007/BF01738427. [DOI] [PubMed] [Google Scholar]
- 176.Kontrogianni-Konstantopoulos A, Bloch RJ. Obscurin: a multitasking muscle giant. J Muscle Res Cell Motil. 2005;26:419–426. doi: 10.1007/s10974-005-9024-7. [DOI] [PubMed] [Google Scholar]
- 177.Kontrogianni-Konstantopoulos A, Bloch RJ. The hydrophilic domain of small ankyrin-1 interacts with the two N-terminal immunoglobulin domains of titin. J Biol Chem. 2003;278:3985–3991. doi: 10.1074/jbc.M209012200. [DOI] [PubMed] [Google Scholar]
- 178.Kontrogianni-Konstantopoulos A, Catino DH, Strong JC, Bloch RJ. De novo myofibrillogenesis in C2C12 cells: evidence for the independent assembly of M bands and Z disks. Am J Physiol Cell Physiol. 2006;290:C626–C637. doi: 10.1152/ajpcell.00442.2005. [DOI] [PubMed] [Google Scholar]
- 179.Kontrogianni-Konstantopoulos A, Catino DH, Strong JC, Randall WR, Bloch RJ. Obscurin regulates the organization of myosin into A bands. Am J Physiol Cell Physiol. 2004;287:C209–C217. doi: 10.1152/ajpcell.00497.2003. [DOI] [PubMed] [Google Scholar]
- 180.Kontrogianni-Konstantopoulos A, Catino DH, Strong JC, Sutter S, Borisov AB, Pumplin DW, Russell MW, Bloch RJ. Obscurin modulates the assembly and organization of sarcomeres and the sarcoplasmic reticulum. FASEB J. 2006;20:2102–2111. doi: 10.1096/fj.06-5761com. [DOI] [PubMed] [Google Scholar]
- 181.Kontrogianni-Konstantopoulos A, Jones EM, Van Rossum DB, Bloch RJ. Obscurin is a ligand for small ankyrin 1 in skeletal muscle. Mol Biol Cell. 2003;14:1138–1148. doi: 10.1091/mbc.E02-07-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Korn ED. Actin polymerization and its regulation by proteins from nonmuscle cells. Physiol Rev. 1982;62:672–737. doi: 10.1152/physrev.1982.62.2.672. [DOI] [PubMed] [Google Scholar]
- 183.Kruger M, Kotter S, Grutzner A, Lang P, Andresen C, Redfield MM, Butt E, Dos Remedios CG, Linke WA. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res. 2009;104:87–94. doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- 184.Kruger M, Linke WA. Protein kinase-A phosphorylates titin in human heart muscle and reduces myofibrillar passive tension. J Muscle Res Cell Motil. 2006;27:435–444. doi: 10.1007/s10974-006-9090-5. [DOI] [PubMed] [Google Scholar]
- 185.Kruger M, Linke WA. Titin-based mechanical signalling in normal and failing myocardium. J Mol Cell Cardiol. 2009;46:490–498. doi: 10.1016/j.yjmcc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 186.Kruger M, Wright J, Wang K. Nebulin as a length regulator of thin filaments of vertebrate skeletal muscles: correlation of thin filament length, nebulin size, and epitope profile. J Cell Biol. 1991;115:97–107. doi: 10.1083/jcb.115.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kuhlman PA, Fowler VM. Purification and characterization of an alpha 1 beta 2 isoform of CapZ from human erythrocytes: cytosolic location and inability to bind to Mg2+ ghosts suggest that erythrocyte actin filaments are capped by adducin. Biochemistry. 1997;36:13461–13472. doi: 10.1021/bi970601b. [DOI] [PubMed] [Google Scholar]
- 188.Kulke M, Fujita-Becker S, Rostkova E, Neagoe C, Labeit D, Manstein DJ, Gautel M, Linke WA. Interaction between PEVK-titin and actin filaments: origin of a viscous force component in cardiac myofibrils. Circ Res. 2001;89:874–881. doi: 10.1161/hh2201.099453. [DOI] [PubMed] [Google Scholar]
- 189.Labeit D, Watanabe K, Witt C, Fujita H, Wu Y, Lahmers S, Funck T, Labeit S, Granzier H. Calcium-dependent molecular spring elements in the giant protein titin. Proc Natl Acad Sci USA. 2003;100:13716–13721. doi: 10.1073/pnas.2235652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Labeit S, Barlow DP, Gautel M, Gibson T, Holt J, Hsieh CL, Francke U, Leonard K, Wardale J, Whiting A. A regular pattern of two types of 100-residue motif in the sequence of titin. Nature. 1990;345:273–276. doi: 10.1038/345273a0. [DOI] [PubMed] [Google Scholar]
- 191.Labeit S, Gautel M, Lakey A, Trinick J. Towards a molecular understanding of titin. EMBO J. 1992;11:1711–1716. doi: 10.1002/j.1460-2075.1992.tb05222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Labeit S, Gibson T, Lakey A, Leonard K, Zeviani M, Knight P, Wardale J, Trinick J. Evidence that nebulin is a protein-ruler in muscle thin filaments. FEBS Lett. 1991;282:313–316. doi: 10.1016/0014-5793(91)80503-u. [DOI] [PubMed] [Google Scholar]
- 193.Labeit S, Kolmerer B. The complete primary structure of human nebulin and its correlation to muscle structure. J Mol Biol. 1995;248:308–315. doi: 10.1016/s0022-2836(95)80052-2. [DOI] [PubMed] [Google Scholar]
- 194.Labeit S, Kolmerer B. Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science. 1995;270:293–296. doi: 10.1126/science.270.5234.293. [DOI] [PubMed] [Google Scholar]
- 195.Labeit S, Kolmerer B, Linke WA. The giant protein titin. Emerging roles in physiology and pathophysiology. Circ Res. 1997;80:290–294. doi: 10.1161/01.res.80.2.290. [DOI] [PubMed] [Google Scholar]
- 196.Labeit S, Lahmers S, Burkart C, Fong C, McNabb M, Witt S, Witt C, Labeit D, Granzier H. Expression of distinct classes of titin isoforms in striated and smooth muscles by alternative splicing, and their conserved interaction with filamins. J Mol Biol. 2006;362:664–681. doi: 10.1016/j.jmb.2006.07.077. [DOI] [PubMed] [Google Scholar]
- 197.Lange S, Auerbach D, McLoughlin P, Perriard E, Schafer BW, Perriard JC, Ehler E. Subcellular targeting of metabolic enzymes to titin in heart muscle may be mediated by DRAL/FHL-2. J Cell Sci. 2002;115:4925–4936. doi: 10.1242/jcs.00181. [DOI] [PubMed] [Google Scholar]
- 198.Lange S, Xiang F, Yakovenko A, Vihola A, Hackman P, Rostkova E, Kristensen J, Brandmeier B, Franzen G, Hedberg B, Gunnarsson LG, Hughes SM, Marchand S, Sejersen T, Richard I, Edstrom L, Ehler E, Udd B, Gautel M. The kinase domain of titin controls muscle gene expression and protein turnover. Science. 2005;308:1599–1603. doi: 10.1126/science.1110463. [DOI] [PubMed] [Google Scholar]
- 199.Leake MC, Grutzner A, Kruger M, Linke WA. Mechanical properties of cardiac titin's N2B-region by single-molecule atomic force spectroscopy. J Struct Biol. 2006;155:263–272. doi: 10.1016/j.jsb.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 200.Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- 201.Lee MA, Joo YM, Lee YM, Kim HS, Kim JH, Choi JK, Ahn SJ, Min BI, Kim CR. Archvillin anchors in the Z-line of skeletal muscle via the nebulin C-terminus. Biochem Biophys Res Commun. 2008;374:320–324. doi: 10.1016/j.bbrc.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 202.Lehtokari VL, Pelin K, Sandbacka M, Ranta S, Donner K, Muntoni F, Sewry C, Angelini C, Bushby K, Van den Bergh P, Iannaccone S, Laing NG, Wallgren-Pettersson C. Identification of 45 novel mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Hum Mutat. 2006;27:946–956. doi: 10.1002/humu.20370. [DOI] [PubMed] [Google Scholar]
- 203.LeWinter MM. Titin isoforms in heart failure: are there benefits to supersizing? Circulation. 2004;110:109–111. doi: 10.1161/01.CIR.0000137284.17083.93. [DOI] [PubMed] [Google Scholar]
- 204.LeWinter MM. Titin: the “missing link” of diastole. J Mol Cell Cardiol. 2000;32:2111–2114. doi: 10.1006/jmcc.2000.1286. [DOI] [PubMed] [Google Scholar]
- 205.LeWinter MM, Wu Y, Labeit S, Granzier H. Cardiac titin: structure, functions and role in disease. Clin Chim Acta. 2007;375:1–9. doi: 10.1016/j.cca.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 206.Li H, Linke WA, Oberhauser AF, Carrion-Vazquez M, Kerkvliet JG, Lu H, Marszalek PE, Fernandez JM. Reverse engineering of the giant muscle protein titin. Nature. 2002;418:998–1002. doi: 10.1038/nature00938. [DOI] [PubMed] [Google Scholar]
- 207.Li S, Czubryt MP, McAnally J, Bassel-Duby R, Richardson JA, Wiebel FF, Nordheim A, Olson EN. Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specific gene deletion in mice. Proc Natl Acad Sci USA. 2005;102:1082–1087. doi: 10.1073/pnas.0409103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lin Z, Lu MH, Schultheiss T, Choi J, Holtzer S, DiLullo C, Fischman DA, Holtzer H. Sequential appearance of muscle-specific proteins in myoblasts as a function of time after cell division: evidence for a conserved myoblast differentiation program in skeletal muscle. Cell Motil Cytoskeleton. 1994;29:1–19. doi: 10.1002/cm.970290102. [DOI] [PubMed] [Google Scholar]
- 209.Linke WA. Sense and stretchability: the role of titin and titin-associated proteins in myocardial stress-sensing and mechanical dysfunction. Cardiovasc Res. 2008;77:637–648. doi: 10.1016/j.cardiores.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 210.Linke WA, Bartoo ML, Ivemeyer M, Pollack GH. Limits of titin extension in single cardiac myofibrils. J Muscle Res Cell Motil. 1996;17:425–438. doi: 10.1007/BF00123359. [DOI] [PubMed] [Google Scholar]
- 211.Linke WA, Fernandez JM. Cardiac titin: molecular basis of elasticity and cellular contribution to elastic and viscous stiffness components in myocardium. J Muscle Res Cell Motil. 2002;23:483–497. doi: 10.1023/a:1023462507254. [DOI] [PubMed] [Google Scholar]
- 212.Linke WA, Granzier H. A spring tale: new facts on titin elasticity. Biophys J. 1998;75:2613–2614. doi: 10.1016/S0006-3495(98)77706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Linke WA, Ivemeyer M, Labeit S, Hinssen H, Ruegg JC, Gautel M. Actin-titin interaction in cardiac myofibrils: probing a physiological role. Biophys J. 1997;73:905–919. doi: 10.1016/S0006-3495(97)78123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Linke WA, Ivemeyer M, Mundel P, Stockmeier MR, Kolmerer B. Nature of PEVK-titin elasticity in skeletal muscle. Proc Natl Acad Sci USA. 1998;95:8052–8057. doi: 10.1073/pnas.95.14.8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Linke WA, Kulke M, Li H, Fujita-Becker S, Neagoe C, Manstein DJ, Gautel M, Fernandez JM. PEVK domain of titin: an entropic spring with actin-binding properties. J Struct Biol. 2002;137:194–205. doi: 10.1006/jsbi.2002.4468. [DOI] [PubMed] [Google Scholar]
- 216.Linke WA, Rudy DE, Centner T, Gautel M, Witt C, Labeit S, Gregorio CC. I-band titin in cardiac muscle is a three-element molecular spring and is critical for maintaining thin filament structure. J Cell Biol. 1999;146:631–644. doi: 10.1083/jcb.146.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Linke WA, Stockmeier MR, Ivemeyer M, Hosser H, Mundel P. Characterizing titin's I-band Ig domain region as an entropic spring. J Cell Sci. 1998;111:1567–1574. doi: 10.1242/jcs.111.11.1567. [DOI] [PubMed] [Google Scholar]
- 218.Littlefield RS, Fowler VM. Thin filament length regulation in striated muscle sarcomeres: pointed-end dynamics go beyond a nebulin ruler. Semin Cell Dev Biol. 2008;19:511–519. doi: 10.1016/j.semcdb.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 220.Locker RH, Wild DJ. A comparative study of high molecular weight proteins in various types of muscle across the animal kingdom. J Biochem. 1986;99:1473–1484. doi: 10.1093/oxfordjournals.jbchem.a135617. [DOI] [PubMed] [Google Scholar]
- 221.Lukoyanova N, VanLoock MS, Orlova A, Galkin VE, Wang K, Egelman EH. Each actin subunit has three nebulin binding sites: implications for steric blocking. Curr Biol. 2002;12:383–388. doi: 10.1016/s0960-9822(02)00678-4. [DOI] [PubMed] [Google Scholar]
- 222.Luo G, Zhang JQ, Nguyen TP, Herrera AH, Paterson B, Horowits R. Complete cDNA sequence and tissue localization of N-RAP, a novel nebulin-related protein of striated muscle. Cell Motil Cytoskeleton. 1997;38:75–90. doi: 10.1002/(SICI)1097-0169(1997)38:1<75::AID-CM7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 223.Luther PK. Three-dimensional structure of a vertebrate muscle Z-band: implications for titin and alpha-actinin binding. J Struct Biol. 2000;129:1–16. doi: 10.1006/jsbi.1999.4207. [DOI] [PubMed] [Google Scholar]
- 224.Luther PK, Padron R, Ritter S, Craig R, Squire JM. Heterogeneity of Z-band structure within a single muscle sarcomere: implications for sarcomere assembly. J Mol Biol. 2003;332:161–169. doi: 10.1016/s0022-2836(03)00883-0. [DOI] [PubMed] [Google Scholar]
- 225.Luther PK, Squire JM. Muscle Z-band ultrastructure: titin Z-repeats and Z-band periodicities do not match. J Mol Biol. 2002;319:1157–1164. doi: 10.1016/S0022-2836(02)00372-8. [DOI] [PubMed] [Google Scholar]
- 226.Ma K, Forbes JG, Gutierrez-Cruz G, Wang K. Titin as a giant scaffold for integrating stress and Src homology domain 3-mediated signaling pathways: the clustering of novel overlap ligand motifs in the elastic PEVK segment. J Biol Chem. 2006;281:27539–27556. doi: 10.1074/jbc.M604525200. [DOI] [PubMed] [Google Scholar]
- 227.Ma K, Wang K. Interaction of nebulin SH3 domain with titin PEVK and myopalladin: implications for the signaling and assembly role of titin and nebulin. FEBS Lett. 2002;532:273–278. doi: 10.1016/s0014-5793(02)03655-4. [DOI] [PubMed] [Google Scholar]
- 228.Makarenko I, Opitz CA, Leake MC, Neagoe C, Kulke M, Gwathmey JK, del Monte F, Hajjar RJ, Linke WA. Passive stiffness changes caused by upregulation of compliant titin iso-forms in human dilated cardiomyopathy hearts. Circ Res. 2004;95:708–716. doi: 10.1161/01.RES.0000143901.37063.2f. [DOI] [PubMed] [Google Scholar]
- 229.Marino M, Svergun DI, Kreplak L, Konarev PV, Maco B, Labeit D, Mayans O. Poly-Ig tandems from I-band titin share extended domain arrangements irrespective of the distinct features of their modular constituents. J Muscle Res Cell Motil. 2005;26:355–365. doi: 10.1007/s10974-005-9017-6. [DOI] [PubMed] [Google Scholar]
- 230.Maruyama K. Connectin, an elastic filamentous protein of striated muscle. Int Rev Cytol. 1986;104:81–114. doi: 10.1016/s0074-7696(08)61924-5. [DOI] [PubMed] [Google Scholar]
- 231.Maruyama K. Connectin, an elastic protein from myofibrils. J Biochem. 1976;80:405–407. doi: 10.1093/oxfordjournals.jbchem.a131291. [DOI] [PubMed] [Google Scholar]
- 232.Maruyama K. [Connectin, muscle elastic protein]. Tanpakushitsu Kakusan Koso. 1988;33:2579–2584. [PubMed] [Google Scholar]
- 233.Maruyama K. Connectin/titin, giant elastic protein of muscle. FASEB J. 1997;11:341–345. doi: 10.1096/fasebj.11.5.9141500. [DOI] [PubMed] [Google Scholar]
- 234.Maruyama K, Endo T, Kume H, Kawamura Y, Kanzawa N, Kimura S, Kawashima S. A partial connectin cDNA encoding a novel type of RSP motifs isolated from chicken embryonic skeletal muscle. J Biochem. 1994;115:147–149. doi: 10.1093/oxfordjournals.jbchem.a124290. [DOI] [PubMed] [Google Scholar]
- 235.Matsumoto Y, Hayashi T, Inagaki N, Takahashi M, Hiroi S, Nakamura T, Arimura T, Nakamura K, Ashizawa N, Yasunami M, Ohe T, Yano K, Kimura A. Functional analysis of titin/connectin N2-B mutations found in cardiomyopathy. J Muscle Res Cell Motil. 2005;26:367–374. doi: 10.1007/s10974-005-9018-5. [DOI] [PubMed] [Google Scholar]
- 236.Mayans O, van der Ven PF, Wilm M, Mues A, Young P, Furst DO, Wilmanns M, Gautel M. Structural basis for activation of the titin kinase domain during myofibrillogenesis. Nature. 1998;395:863–869. doi: 10.1038/27603. [DOI] [PubMed] [Google Scholar]
- 237.Mayans O, Wuerges J, Canela S, Gautel M, Wilmanns M. Structural evidence for a possible role of reversible disulphide bridge formation in the elasticity of the muscle protein titin. Structure. 2001;9:331–340. doi: 10.1016/s0969-2126(01)00591-3. [DOI] [PubMed] [Google Scholar]
- 238.McElhinny AS, Kakinuma K, Sorimachi H, Labeit S, Gregorio CC. Muscle-specific RING finger-1 interacts with titin to regulate sarcomeric M-line and thick filament structure and may have nuclear functions via its interaction with glucocorticoid modulatory element binding protein-1. J Cell Biol. 2002;157:125–136. doi: 10.1083/jcb.200108089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.McElhinny AS, Kazmierski ST, Labeit S, Gregorio CC. Nebulin: the nebulous, multifunctional giant of striated muscle. Trends Cardiovasc Med. 2003;13:195–201. doi: 10.1016/s1050-1738(03)00076-8. [DOI] [PubMed] [Google Scholar]
- 240.McElhinny AS, Kolmerer B, Fowler VM, Labeit S, Gregorio CC. The N-terminal end of nebulin interacts with tropomodulin at the pointed ends of the thin filaments. J Biol Chem. 2001;276:583–592. doi: 10.1074/jbc.M005693200. [DOI] [PubMed] [Google Scholar]
- 241.McElhinny AS, Perry CN, Witt CC, Labeit S, Gregorio CC. Muscle-specific RING finger-2 (MURF-2) is important for microtubule, intermediate filament and sarcomeric M-line maintenance in striated muscle development. J Cell Sci. 2004;117:3175–3188. doi: 10.1242/jcs.01158. [DOI] [PubMed] [Google Scholar]
- 242.McElhinny AS, Schwach C, Valichnac M, Mount-Patrick S, Gregorio CC. Nebulin regulates the assembly and lengths of the thin filaments in striated muscle. J Cell Biol. 2005;170:947–957. doi: 10.1083/jcb.200502158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Miller G, Musa H, Gautel M, Peckham M. A targeted deletion of the C-terminal end of titin, including the titin kinase domain, impairs myofibrillogenesis. J Cell Sci. 2003;116:4811–4819. doi: 10.1242/jcs.00768. [DOI] [PubMed] [Google Scholar]
- 244.Miller MK, Bang ML, Witt CC, Labeit D, Trombitas C, Watanabe K, Granzier H, McElhinny AS, Gregorio CC, Labeit S. The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and DARP as a family of titin filament-based stress response molecules. J Mol Biol. 2003;333:951–964. doi: 10.1016/j.jmb.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 245.Millevoi S, Trombitas K, Kolmerer B, Kostin S, Schaper J, Pelin K, Granzier H, Labeit S. Characterization of nebulette and nebulin and emerging concepts of their roles for vertebrate Z-discs. J Mol Biol. 1998;282:111–123. doi: 10.1006/jmbi.1998.1999. [DOI] [PubMed] [Google Scholar]
- 246.Mohiddin SA, Lu S, Cardoso JP, Carroll S, Jha S, Horowits R, Fananapazir L. Genomic organization, alternative splicing, and expression of human and mouse N-RAP, a nebulin-related LIM protein of striated muscle. Cell Motil Cytoskeleton. 2003;55:200–212. doi: 10.1002/cm.10123. [DOI] [PubMed] [Google Scholar]
- 247.Moncman CL, Wang K. Assembly of nebulin into the sarcomeres of avian skeletal muscle. Cell Motil Cytoskeleton. 1996;34:167–184. doi: 10.1002/(SICI)1097-0169(1996)34:3<167::AID-CM1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 248.Moore AJ, Stubbings A, Swallow EB, Dusmet M, Goldstraw P, Porcher R, Moxham J, Polkey MI, Ferenczi MA. Passive properties of the diaphragm in COPD. J Appl Physiol. 2006;101:1400–1405. doi: 10.1152/japplphysiol.01614.2005. [DOI] [PubMed] [Google Scholar]
- 249.Mrosek M, Labeit D, Witt S, Heerklotz H, von Castelmur E, Labeit S, Mayans O. Molecular determinants for the recruitment of the ubiquitin-ligase MuRF-1 onto M-line titin. FASEB J. 2007;21:1383–1392. doi: 10.1096/fj.06-7644com. [DOI] [PubMed] [Google Scholar]
- 250.Mueller-Dieckmann J. The open-access high-throughput crystallization facility at EMBL Hamburg. Acta Crystallogr D Biol Crystallogr. 2006;62:1446–1452. doi: 10.1107/S0907444906038121. [DOI] [PubMed] [Google Scholar]
- 251.Mues A, van der Ven PF, Young P, Furst DO, Gautel M. Two immunoglobulin-like domains of the Z-disc portion of titin interact in a conformation-dependent way with telethonin. FEBS Lett. 1998;428:111–114. doi: 10.1016/s0014-5793(98)00501-8. [DOI] [PubMed] [Google Scholar]
- 252.Muhle-Goll C, Habeck M, Cazorla O, Nilges M, Labeit S, Granzier H. Structural and functional studies of titin's fn3 modules reveal conserved surface patterns and binding to myosin S1—a possible role in the Frank-Starling mechanism of the heart. J Mol Biol. 2001;313:431–447. doi: 10.1006/jmbi.2001.5017. [DOI] [PubMed] [Google Scholar]
- 253.Muller S, Lange S, Gautel M, Wilmanns M. Rigid conformation of an immunoglobulin domain tandem repeat in the A-band of the elastic muscle protein titin. J Mol Biol. 2007;371:469–480. doi: 10.1016/j.jmb.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 254.Murrin LC, Talbot JN. RanBPM, a scaffolding protein in the immune and nervous systems. J Neuroimmune Pharmacol. 2007;2:290–295. doi: 10.1007/s11481-007-9079-x. [DOI] [PubMed] [Google Scholar]
- 255.Musa H, Meek S, Gautel M, Peddie D, Smith AJ, Peckham M. Targeted homozygous deletion of M-band titin in cardiomyocytes prevents sarcomere formation. J Cell Sci. 2006;119:4322–4331. doi: 10.1242/jcs.03198. [DOI] [PubMed] [Google Scholar]
- 256.Nagase T, Kikuno R, Hattori A, Kondo Y, Okumura K, Ohara O. Prediction of the coding sequences of unidentified human genes. XIX. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 2000;7:347–355. doi: 10.1093/dnares/7.6.347. [DOI] [PubMed] [Google Scholar]
- 257.Nagase T, Kikuno R, Ishikawa K, Hirosawa M, Ohara O. Prediction of the coding sequences of unidentified human genes. XVII. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 2000;7:143–150. doi: 10.1093/dnares/7.2.143. [DOI] [PubMed] [Google Scholar]
- 258.Nagase T, Kikuno R, Ishikawa KI, Hirosawa M, Ohara O. Prediction of the coding sequences of unidentified human genes. XVI. The complete sequences of 150 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 2000;7:65–73. doi: 10.1093/dnares/7.1.65. [DOI] [PubMed] [Google Scholar]
- 259.Nagase T, Kikuno R, Nakayama M, Hirosawa M, Ohara O. Prediction of the coding sequences of unidentified human genes. XVIII. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 2000;7:273–281. doi: 10.1093/dnares/7.4.271. [DOI] [PubMed] [Google Scholar]
- 260.Nagueh SF, Shah G, Wu Y, Torre-Amione G, King NM, Lahmers S, Witt CC, Becker K, Labeit S, Granzier HL. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation. 2004;110:155–162. doi: 10.1161/01.CIR.0000135591.37759.AF. [DOI] [PubMed] [Google Scholar]
- 261.Nagy A, Cacciafesta P, Grama L, Kengyel A, Malnasi-Csizmadia A, Kellermayer MS. Differential actin binding along the PEVK domain of skeletal muscle titin. J Cell Sci. 2004;117:5781–5789. doi: 10.1242/jcs.01501. [DOI] [PubMed] [Google Scholar]
- 262.Nagy A, Grama L, Huber T, Bianco P, Trombitas K, Granzier HL, Kellermayer MS. Hierarchical extensibility in the PEVK domain of skeletal-muscle titin. Biophys J. 2005;89:329–336. doi: 10.1529/biophysj.104.057737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Nakamura M, Masuda H, Horii J, Kuma K, Yokoyama N, Ohba T, Nishitani H, Miyata T, Tanaka M, Nishimoto T. When over-expressed, a novel centrosomal protein, RanBPM, causes ectopic microtubule nucleation similar to gamma-tubulin. J Cell Biol. 1998;143:1041–1052. doi: 10.1083/jcb.143.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Nave R, Furst DO, Weber K. Interaction of alpha-actinin and nebulin in vitro. Support for the existence of a fourth filament system in skeletal muscle. FEBS Lett. 1990;269:163–166. doi: 10.1016/0014-5793(90)81144-d. [DOI] [PubMed] [Google Scholar]
- 265.Nave R, Furst DO, Weber K. Visualization of the polarity of isolated titin molecules: a single globular head on a long thin rod as the M band anchoring domain? J Cell Biol. 1989;109:2177–2187. doi: 10.1083/jcb.109.5.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Neagoe C, Kulke M, del Monte F, Gwathmey JK, de Tombe PP, Hajjar RJ, Linke WA. Titin isoform switch in ischemic human heart disease. Circulation. 2002;106:1333–1341. doi: 10.1161/01.cir.0000029803.93022.93. [DOI] [PubMed] [Google Scholar]
- 267.Nelson OL, Robbins CT, Wu Y, Granzier H. Titin isoform switching is a major cardiac adaptive response in hibernating grizzly bears. Am J Physiol Heart Circ Physiol. 2008;295:H366–H371. doi: 10.1152/ajpheart.00234.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Nicolao P, Xiang F, Gunnarsson LG, Giometto B, Edstrom L, Anvret M, Zhang Z. Autosomal dominant myopathy with proximal weakness and early respiratory muscle involvement maps to chromosome 2q. Am J Hum Genet. 1999;64:788–792. doi: 10.1086/302281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Niggli V, Gimona M. Evidence for a ternary interaction between alpha-actinin, (meta)vinculin and acidic-phospholipid bilayers. Eur J Biochem. 1993;213:1009–1015. doi: 10.1111/j.1432-1033.1993.tb17848.x. [DOI] [PubMed] [Google Scholar]
- 270.Nishitani H, Hirose E, Uchimura Y, Nakamura M, Umeda M, Nishii K, Mori N, Nishimoto T. Full-sized RanBPM cDNA encodes a protein possessing a long stretch of proline and glutamine within the N-terminal region, comprising a large protein complex. Gene. 2001;272:25–33. doi: 10.1016/s0378-1119(01)00553-4. [DOI] [PubMed] [Google Scholar]
- 271.Oakley CE, Chamoun J, Brown LJ, Hambly BD. Myosin binding protein-C: enigmatic regulator of cardiac contraction. Int J Biochem Cell Biol. 2007;39:2161–2166. doi: 10.1016/j.biocel.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 272.Oakley CE, Hambly BD, Curmi PM, Brown LJ. Myosin binding protein C: structural abnormalities in familial hypertrophic cardiomyopathy. Cell Res. 2004;14:95–110. doi: 10.1038/sj.cr.7290208. [DOI] [PubMed] [Google Scholar]
- 273.Obermann WM, Gautel M, Steiner F, van der Ven PF, Weber K, Furst DO. The structure of the sarcomeric M band: localization of defined domains of myomesin, M-protein, and the 250-kD carboxy-terminal region of titin by immunoelectron microscopy. J Cell Biol. 1996;134:1441–1453. doi: 10.1083/jcb.134.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Obermann WM, Gautel M, Weber K, Furst DO. Molecular structure of the sarcomeric M band: mapping of titin and myosin binding domains in myomesin and the identification of a potential regulatory phosphorylation site in myomesin. EMBO J. 1997;16:211–220. doi: 10.1093/emboj/16.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Obermann WM, Plessmann U, Weber K, Furst DO. Purification and biochemical characterization of myomesin, a myosin-binding and titin-binding protein, from bovine skeletal muscle. Eur J Biochem. 1995;233:110–115. doi: 10.1111/j.1432-1033.1995.110_1.x. [DOI] [PubMed] [Google Scholar]
- 276.Obermann WM, van der Ven PF, Steiner F, Weber K, Furst DO. Mapping of a myosin-binding domain and a regulatory phosphorylation site in M-protein, a structural protein of the sarcomeric M band. Mol Biol Cell. 1998;9:829–840. doi: 10.1091/mbc.9.4.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Offer G, Moos C, Starr R. A new protein of the thick filaments of vertebrate skeletal myofibrils. Extractions, purification and characterization. J Mol Biol. 1973;74:653–676. doi: 10.1016/0022-2836(73)90055-7. [DOI] [PubMed] [Google Scholar]
- 278.Ohtsuka H, Yajima H, Maruyama K, Kimura S. Binding of the N-terminal 63 kDa portion of connectin/titin to alpha-actinin as revealed by the yeast two-hybrid system. FEBS Lett. 1997;401:65–67. doi: 10.1016/s0014-5793(96)01432-9. [DOI] [PubMed] [Google Scholar]
- 279.Ohtsuka H, Yajima H, Maruyama K, Kimura S. The N-terminal Z repeat 5 of connectin/titin binds to the C-terminal region of alpha-actinin. Biochem Biophys Res Commun. 1997;235:1–3. doi: 10.1006/bbrc.1997.6534. [DOI] [PubMed] [Google Scholar]
- 280.Ojima K, Lin ZX, Zhang ZQ, Hijikata T, Holtzer S, Labeit S, Sweeney HL, Holtzer H. Initiation and maturation of I-Z-I bodies in the growth tips of transfected myotubes. J Cell Sci. 1999;112:4101–4112. doi: 10.1242/jcs.112.22.4101. [DOI] [PubMed] [Google Scholar]
- 281.Ojima K, Ono Y, Hata S, Koyama S, Doi N, Sorimachi H. Possible functions of p94 in connectin-mediated signaling pathways in skeletal muscle cells. J Muscle Res Cell Motil. 2005;26:409–417. doi: 10.1007/s10974-005-9023-8. [DOI] [PubMed] [Google Scholar]
- 282.Okagaki T, Weber FE, Fischman DA, Vaughan KT, Mikawa T, Reinach FC. The major myosin-binding domain of skeletal muscle MyBP-C (C protein) resides in the COOH-terminal, immunoglobulin C2 motif. J Cell Biol. 1993;123:619–626. doi: 10.1083/jcb.123.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Ono Y, Torii F, Ojima K, Doi N, Yoshioka K, Kawabata Y, Labeit D, Labeit S, Suzuki K, Abe K, Maeda T, Sorimachi H. Suppressed disassembly of autolyzing p94/CAPN3 by N2A connectin/titin in a genetic reporter system. J Biol Chem. 2006;281:18519–18531. doi: 10.1074/jbc.M601029200. [DOI] [PubMed] [Google Scholar]
- 284.Otey CA, Rachlin A, Moza M, Arneman D, Carpen O. The palladin/myotilin/myopalladin family of actin-associated scaffolds. Int Rev Cytol. 2005;246:31–58. doi: 10.1016/S0074-7696(05)46002-7. [DOI] [PubMed] [Google Scholar]
- 285.Ottenheijm CA, Fong C, Vangheluwe P, Wuytack F, Babu GJ, Periasamy M, Witt CC, Labeit S, Granzier H. Sarcoplasmic reticulum calcium uptake and speed of relaxation are depressed in nebulin-free skeletal muscle. FASEB J. 2008;22:2912–2919. doi: 10.1096/fj.07-104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Ottenheijm CA, Heunks LM, Hafmans T, van der Ven PF, Benoist C, Zhou H, Labeit S, Granzier HL, Dekhuijzen PN. Titin and diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:527–534. doi: 10.1164/rccm.200507-1056OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Ottenheijm CA, Witt CC, Stienen GJ, Labeit S, Beggs AH, Granzier H. Thin filament length dysregulation contributes to muscle weakness in nemaline myopathy patients with nebulin deficiency. Hum Mol Genet. 2009;18:2359–2369. doi: 10.1093/hmg/ddp168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Panaviene Z, Deng XA, Esham M, Moncman CL. Targeting of nebulin fragments to the cardiac sarcomere. Exp Cell Res. 2007;313:896–909. doi: 10.1016/j.yexcr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 289.Pappas CT, Bhattacharya N, Cooper JA, Gregorio CC. Nebulin interacts with CapZ and regulates thin filament architecture within the Z-disc. Mol Biol Cell. 2008;19:1837–1847. doi: 10.1091/mbc.E07-07-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Parlakian A, Tuil D, Hamard G, Tavernier G, Hentzen D, Concordet JP, Paulin D, Li Z, Daegelen D. Targeted inactivation of serum response factor in the developing heart results in myocardial defects and embryonic lethality. Mol Cell Biol. 2004;24:5281–5289. doi: 10.1128/MCB.24.12.5281-5289.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Patel K, Strong PN, Dubowitz V, Dunn MJ. Calmodulin-binding profiles for nebulin and dystrophin in human skeletal muscle. FEBS Lett. 1988;234:267–271. doi: 10.1016/0014-5793(88)80095-4. [DOI] [PubMed] [Google Scholar]
- 292.Peckham M, Young P, Gautel M. Constitutive and variable regions of Z-disk titin/connectin in myofibril formation: a dominant-negative screen. Cell Struct Funct. 1997;22:95–101. doi: 10.1247/csf.22.95. [DOI] [PubMed] [Google Scholar]
- 293.Pelin K, Donner K, Holmberg M, Jungbluth H, Muntoni F, Wallgren-Pettersson C. Nebulin mutations in autosomal recessive nemaline myopathy: an update. Neuromuscul Disord. 2002;12:680–686. doi: 10.1016/s0960-8966(02)00066-4. [DOI] [PubMed] [Google Scholar]
- 294.Pelin K, Hilpela P, Donner K, Sewry C, Akkari PA, Wilton SD, Wattanasirichaigoon D, Bang ML, Centner T, Hanefeld F, Odent S, Fardeau M, Urtizberea JA, Muntoni F, Dubowitz V, Beggs AH, Laing NG, Labeit S, de la Chapelle A, Wallgren-Pettersson C. Mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Proc Natl Acad Sci USA. 1999;96:2305–2310. doi: 10.1073/pnas.96.5.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Pelin K, Ridanpaa M, Donner K, Wilton S, Krishnarajah J, Laing N, Kolmerer B, Millevoi S, Labeit S, de la Chapelle A, Wallgren-Petterson C. Refined localisation of the genes for nebulin and titin on chromosome 2q allows the assignment of nebulin as a candidate gene for autosomal recessive nemaline myopathy. Eur J Hum Genet. 1997;5:229–234. [PubMed] [Google Scholar]
- 296.Pelin K, Wallgren-Pettersson C. Nebulin–a giant chameleon. Adv Exp Med Biol. 2008;642:28–39. [PubMed] [Google Scholar]
- 297.Peng J, Raddatz K, Molkentin JD, Wu Y, Labeit S, Granzier H, Gotthardt M. Cardiac hypertrophy and reduced contractility in hearts deficient in the titin kinase region. Circulation. 2007;115:743–751. doi: 10.1161/CIRCULATIONAHA.106.645499. [DOI] [PubMed] [Google Scholar]
- 298.Perng MD, Muchowski PJ, van Den IP, Wu GJ, Hutcheson AM, Clark JI, Quinlan RA. The cardiomyopathy and lens cataract mutation in alphaB-crystallin alters its protein structure, chaperone activity, and interaction with intermediate filaments in vitro. J Biol Chem. 1999;274:33235–33243. doi: 10.1074/jbc.274.47.33235. [DOI] [PubMed] [Google Scholar]
- 299.Pfuhl M, Gautel M, Politou AS, Joseph C, Pastore A. Secondary structure determination by NMR spectroscopy of an immunoglobulin-like domain from the giant muscle protein titin. J Biomol NMR. 1995;6:48–58. doi: 10.1007/BF00417491. [DOI] [PubMed] [Google Scholar]
- 300.Pfuhl M, Pastore A. Tertiary structure of an immunoglobulin-like domain from the giant muscle protein titin: a new member of the I set. Structure. 1995;3:391–401. doi: 10.1016/s0969-2126(01)00170-8. [DOI] [PubMed] [Google Scholar]
- 301.Pfuhl M, Winder SJ, Castiglione Morelli MA, Labeit S, Pastore A. Correlation between conformational and binding properties of nebulin repeats. J Mol Biol. 1996;257:367–384. doi: 10.1006/jmbi.1996.0169. [DOI] [PubMed] [Google Scholar]
- 302.Pfuhl M, Winder SJ, Pastore A. Nebulin, a helical actin binding protein. EMBO J. 1994;13:1782–1789. doi: 10.1002/j.1460-2075.1994.tb06446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Pinotsis N, Petoukhov M, Lange S, Svergun D, Zou P, Gautel M, Wilmanns M. Evidence for a dimeric assembly of two titin/ telethonin complexes induced by the telethonin C-terminus. J Struct Biol. 2006;155:239–250. doi: 10.1016/j.jsb.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 304.Pizon V, Iakovenko A, Van Der Ven PF, Kelly R, Fatu C, Furst DO, Karsenti E, Gautel M. Transient association of titin and myosin with microtubules in nascent myofibrils directed by the MURF2 RING-finger protein. J Cell Sci. 2002;115:4469–4482. doi: 10.1242/jcs.00131. [DOI] [PubMed] [Google Scholar]
- 305.Politou AS, Millevoi S, Gautel M, Kolmerer B, Pastore A. SH3 in muscles: solution structure of the SH3 domain from nebulin. J Mol Biol. 1998;276:189–202. doi: 10.1006/jmbi.1997.1521. [DOI] [PubMed] [Google Scholar]
- 306.Politou AS, Spadaccini R, Joseph C, Brannetti B, Guerrini R, Helmer-Citterich M, Salvadori S, Temussi PA, Pastore A. The SH3 domain of nebulin binds selectively to type II peptides: theoretical prediction and experimental validation. J Mol Biol. 2002;316:305–315. doi: 10.1006/jmbi.2001.5312. [DOI] [PubMed] [Google Scholar]
- 307.Porter NC, Resneck WG, O'Neill A, Van Rossum DB, Stone MR, Bloch RJ. Association of small ankyrin 1 with the sarcoplasmic reticulum. Mol Membr Biol. 2005;22:421–432. doi: 10.1080/09687860500244262. [DOI] [PubMed] [Google Scholar]
- 308.Protasi F, Sun XH, Franzini-Armstrong C. Formation and maturation of the calcium release apparatus in developing and adult avian myocardium. Dev Biol. 1996;173:265–278. doi: 10.1006/dbio.1996.0022. [DOI] [PubMed] [Google Scholar]
- 309.Puchner EM, Alexandrovich A, Kho AL, Hensen U, Schafer LV, Brandmeier B, Grater F, Grubmuller H, Gaub HE, Gautel M. Mechanoenzymatics of titin kinase. Proc Natl Acad Sci USA. 2008;105:13385–13390. doi: 10.1073/pnas.0805034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Qadota H, Blangy A, Xiong G, Benian GM. The DH-PH region of the giant protein UNC-89 activates RHO-1 GTPase in Caenorhabditis elegans body wall muscle. J Mol Biol. 2008;383:747–752. doi: 10.1016/j.jmb.2008.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Qadota H, McGaha LA, Mercer KB, Stark TJ, Ferrara TM, Benian GM. A novel protein phosphatase is a binding partner for the protein kinase domains of UNC-89 (Obscurin) in Caenorhabditis elegans. Mol Biol Cell. 2008;19:2424–2432. doi: 10.1091/mbc.E08-01-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Radke MH, Peng J, Wu Y, McNabb M, Nelson OL, Granzier H, Gotthardt M. Targeted deletion of titin N2B region leads to diastolic dysfunction and cardiac atrophy. Proc Natl Acad Sci USA. 2007;104:3444–3449. doi: 10.1073/pnas.0608543104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Raynaud F, Astier C, Benyamin Y. Evidence for a direct but sequential binding of titin to tropomyosin and actin filaments. Biochim Biophys Acta. 2004;1700:171–178. doi: 10.1016/j.bbapap.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 314.Raynaud F, Fernandez E, Coulis G, Aubry L, Vignon X, Bleimling N, Gautel M, Benyamin Y, Ouali A. Calpain 1-titin interactions concentrate calpain 1 in the Z-band edges and in the N2-line region within the skeletal myofibril. FEBS J. 2005;272:2578–2590. doi: 10.1111/j.1742-4658.2005.04683.x. [DOI] [PubMed] [Google Scholar]
- 315.Rhee D, Sanger JM, Sanger JW. The premyofibril: evidence for its role in myofibrillogenesis. Cell Motil Cytoskeleton. 1994;28:1–24. doi: 10.1002/cm.970280102. [DOI] [PubMed] [Google Scholar]
- 316.Romi F, Aarli JA, Gilhus NE. Myasthenia gravis patients with ryanodine receptor antibodies have distinctive clinical features. Eur J Neurol. 2007;14:617–620. doi: 10.1111/j.1468-1331.2007.01785.x. [DOI] [PubMed] [Google Scholar]
- 317.Romi F, Skeie GO, Aarli JA, Gilhus NE. The severity of myasthenia gravis correlates with the serum concentration of titin and ryanodine receptor antibodies. Arch Neurol. 2000;57:1596–1600. doi: 10.1001/archneur.57.11.1596. [DOI] [PubMed] [Google Scholar]
- 318.Romi F, Skeie GO, Gilhus NE, Aarli JA. Striational antibodies in myasthenia gravis: reactivity and possible clinical significance. Arch Neurol. 2005;62:442–446. doi: 10.1001/archneur.62.3.442. [DOI] [PubMed] [Google Scholar]
- 319.Root DD, Wang K. Calmodulin-sensitive interaction of human nebulin fragments with actin and myosin. Biochemistry. 1994;33:12581–12591. doi: 10.1021/bi00208a008. [DOI] [PubMed] [Google Scholar]
- 320.Russell MW, Raeker MO, Korytkowski KA, Sonneman KJ. Identification, tissue expression and chromosomal localization of human Obscurin-MLCK, a member of the titin and Dbl families of myosin light chain kinases. Gene. 2002;282:237–246. doi: 10.1016/s0378-1119(01)00795-8. [DOI] [PubMed] [Google Scholar]
- 321.Sanger JM, Sanger JW. The dynamic Z bands of striated muscle cells. Sci Signal. 2008;1:pe37. doi: 10.1126/scisignal.132pe37. [DOI] [PubMed] [Google Scholar]
- 322.Sanger JW, Ayoob JC, Chowrashi P, Zurawski D, Sanger JM. Assembly of myofibrils in cardiac muscle cells. Adv Exp Med Biol. 2000;481:89–105. doi: 10.1007/978-1-4615-4267-4_6. [DOI] [PubMed] [Google Scholar]
- 323.Sanger JW, Chowrashi P, Shaner NC, Spalthoff S, Wang J, Freeman NL, Sanger JM. Myofibrillogenesis in skeletal muscle cells. Clin Orthop Relat Res. 2002:S153–162. doi: 10.1097/00003086-200210001-00018. [DOI] [PubMed] [Google Scholar]
- 324.Sanger JW, Kang S, Siebrands CC, Freeman N, Du A, Wang J, Stout AL, Sanger JM. How to build a myofibril. J Muscle Res Cell Motil. 2005;26:343–354. doi: 10.1007/s10974-005-9016-7. [DOI] [PubMed] [Google Scholar]
- 325.Sanger JW, Sanger JM. Fishing out proteins that bind to titin. J Cell Biol. 2001;154:21–24. doi: 10.1083/jcb.200106072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sanoudou D, Beggs AH. Clinical and genetic heterogeneity in nemaline myopathy—a disease of skeletal muscle thin filaments. Trends Mol Med. 2001;7:362–368. doi: 10.1016/s1471-4914(01)02089-5. [DOI] [PubMed] [Google Scholar]
- 327.Satoh M, Takahashi M, Sakamoto T, Hiroe M, Marumo F, Kimura A. Structural analysis of the titin gene in hypertrophic cardiomyopathy: identification of a novel disease gene. Biochem Biophys Res Commun. 1999;262:411–417. doi: 10.1006/bbrc.1999.1221. [DOI] [PubMed] [Google Scholar]
- 328.Schultheiss T, Lin ZX, Lu MH, Murray J, Fischman DA, Weber K, Masaki T, Imamura M, Holtzer H. Differential distribution of subsets of myofibrillar proteins in cardiac nonstriated and striated myofibrils. J Cell Biol. 1990;110:1159–1172. doi: 10.1083/jcb.110.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Sebestyen MG, Fritz JD, Wolff JA, Greaser ML. Primary structure of the kinase domain region of rabbit skeletal and cardiac muscle titin. J Muscle Res Cell Motil. 1996;17:343–348. doi: 10.1007/BF00240931. [DOI] [PubMed] [Google Scholar]
- 330.Sebestyen MG, Wolff JA, Greaser ML. Characterization of a 5.4 kb cDNA fragment from the Z-line region of rabbit cardiac titin reveals phosphorylation sites for proline-directed kinases. J Cell Sci. 1995;108:3029–3037. doi: 10.1242/jcs.108.9.3029. [DOI] [PubMed] [Google Scholar]
- 331.Sewry CA, Brown SC, Pelin K, Jungbluth H, Wallgren-Pettersson C, Labeit S, Manzur A, Muntoni F. Abnormalities in the expression of nebulin in chromosome-2 linked nemaline myopathy. Neuromuscul Disord. 2001;11:146–153. doi: 10.1016/s0960-8966(00)00172-3. [DOI] [PubMed] [Google Scholar]
- 332.Sheikh F, Raskin A, Chu PH, Lange S, Domenighetti AA, Zheng M, Liang X, Zhang T, Yajima T, Gu Y, Dalton ND, Mahata SK, Dorn GW, 2nd, Heller-Brown J, Peterson KL, Omens JH, McCulloch AD, Chen J. An FHL1-containing complex within the cardiomyocyte sarcomere mediates hypertrophic biomechanical stress responses in mice. J Clin Invest. 2008;118:3870–3880. doi: 10.1172/JCI34472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Shimada Y, Komiyama M, Begum S, Maruyama K. Development of connectin/titin and nebulin in striated muscles of chicken. Adv Biophys. 1996;33:223–233. [PubMed] [Google Scholar]
- 334.Sinz A, Wang K. Mapping protein interfaces with a fluorogenic cross-linker and mass spectrometry: application to nebulin-calmodulin complexes. Biochemistry. 2001;40:7903–7913. doi: 10.1021/bi010259+. [DOI] [PubMed] [Google Scholar]
- 335.Siu BL, Niimura H, Osborne JA, Fatkin D, MacRae C, Solomon S, Benson DW, Seidman JG, Seidman CE. Familial dilated cardiomyopathy locus maps to chromosome 2q31. Circulation. 1999;99:1022–1026. doi: 10.1161/01.cir.99.8.1022. [DOI] [PubMed] [Google Scholar]
- 336.Skeie GO, Romi F, Aarli JA, Bentsen PT, Gilhus NE. Pathogenesis of myositis and myasthenia associated with titin and ryan-odine receptor antibodies. Ann NY Acad Sci. 2003;998:343–350. doi: 10.1196/annals.1254.039. [DOI] [PubMed] [Google Scholar]
- 337.Small TM, Gernert KM, Flaherty DB, Mercer KB, Borodovsky M, Benian GM. Three new isoforms of Caenorhabditis elegans UNC-89 containing MLCK-like protein kinase domains. J Mol Biol. 2004;342:91–108. doi: 10.1016/j.jmb.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 338.Somnier FE, Engel PJ. The occurrence of anti-titin antibodies and thymomas: a population survey of MG 1970–1999. Neurology. 2002;59:92–98. doi: 10.1212/wnl.59.1.92. [DOI] [PubMed] [Google Scholar]
- 339.Sorimachi H, Freiburg A, Kolmerer B, Ishiura S, Stier G, Gregorio CC, Labeit D, Linke WA, Suzuki K, Labeit S. Tissue-specific expression and alpha-actinin binding properties of the Z-disc titin: implications for the nature of vertebrate Z-discs. J Mol Biol. 1997;270:688–695. doi: 10.1006/jmbi.1997.1145. [DOI] [PubMed] [Google Scholar]
- 340.Sorimachi H, Kimura S, Kinbara K, Kazama J, Takahashi M, Yajima H, Ishiura S, Sasagawa N, Nonaka I, Sugita H, Maruyama K, Suzuki K. Structure and physiological functions of ubiquitous and tissue-specific calpain species. Muscle-specific calpain, p94, interacts with connectin/titin. Adv Biophys. 1996;33:101–122. doi: 10.1016/s0065-227x(96)90026-x. [DOI] [PubMed] [Google Scholar]
- 341.Sorimachi H, Kinbara K, Kimura S, Takahashi M, Ishiura S, Sasagawa N, Sorimachi N, Shimada H, Tagawa K, Maruyama K. Muscle-specific calpain, p94, responsible for limb girdle muscular dystrophy type 2A, associates with connectin through IS2, a p94-specific sequence. J Biol Chem. 1995;270:31158–31162. doi: 10.1074/jbc.270.52.31158. [DOI] [PubMed] [Google Scholar]
- 342.Soteriou A, Gamage M, Trinick J. A survey of interactions made by the giant protein titin. J Cell Sci. 1993;104:119–123. doi: 10.1242/jcs.104.1.119. [DOI] [PubMed] [Google Scholar]
- 343.Spencer MJ, Guyon JR, Sorimachi H, Potts A, Richard I, Herasse M, Chamberlain J, Dalkilic I, Kunkel LM, Beckmann JS. Stable expression of calpain 3 from a muscle transgene in vivo: immature muscle in transgenic mice suggests a role for calpain 3 in muscle maturation. Proc Natl Acad Sci USA. 2002;99:8874–8879. doi: 10.1073/pnas.132269299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Squire JM, Luther PK, Knupp C. Structural evidence for the interaction of C-protein (MyBP-C) with actin and sequence identification of a possible actin-binding domain. J Mol Biol. 2003;331:713–724. doi: 10.1016/s0022-2836(03)00781-2. [DOI] [PubMed] [Google Scholar]
- 345.Stedman H, Browning K, Oliver N, Oronzi-Scott M, Fischbeck K, Sarkar S, Sylvester J, Schmickel R, Wang K. Nebulin cDNAs detect a 25-kilobase transcript in skeletal muscle and localize to human chromosome 2. Genomics. 1988;2:1–7. doi: 10.1016/0888-7543(88)90102-4. [DOI] [PubMed] [Google Scholar]
- 346.Stuyvers BD, Miura M, Jin JP, ter Keurs HE. Ca(2+)-dependence of diastolic properties of cardiac sarcomeres: involvement of titin. Prog Biophys Mol Biol. 1998;69:425–443. doi: 10.1016/s0079-6107(98)00018-2. [DOI] [PubMed] [Google Scholar]
- 347.Stuyvers BD, Miura M, ter Keurs HE. Diastolic viscoelastic properties of rat cardiac muscle: involvement of Ca2+. Adv Exp Med Biol. 1997;430:13–28. doi: 10.1007/978-1-4615-5959-7_2. [DOI] [PubMed] [Google Scholar]
- 348.Sutter SB, Raeker MO, Borisov AB, Russell MW. Orthologous relationship of obscurin and Unc-89: phylogeny of a novel family of tandem myosin light chain kinases. Dev Genes Evol. 2004;214:352–359. doi: 10.1007/s00427-004-0413-5. [DOI] [PubMed] [Google Scholar]
- 349.Suzuki S, Utsugisawa K, Nagane Y, Satoh T, Terayama Y, Suzuki N, Kuwana M. Classification of myasthenia gravis based on autoantibody status. Arch Neurol. 2007;64:1121–1124. doi: 10.1001/archneur.64.8.1121. [DOI] [PubMed] [Google Scholar]
- 350.Suzuki T, Yajima H, Maruyama K, Kimura SA. 7.5-kb 3′-Terminal cDNA sequence of chicken skeletal muscle nebulin reveals its actin binding regions. Zool Sci. 2000;17:1095–1099. doi: 10.2108/zsj.17.1095. [DOI] [PubMed] [Google Scholar]
- 351.Takekura H, Flucher BE, Franzini-Armstrong C. Sequential docking, molecular differentiation, and positioning of T-tubule/SR junctions in developing mouse skeletal muscle. Dev Biol. 2001;239:204–214. doi: 10.1006/dbio.2001.0437. [DOI] [PubMed] [Google Scholar]
- 352.Takekura H, Sun X, Franzini-Armstrong C. Development of the excitation-contraction coupling apparatus in skeletal muscle: peripheral and internal calcium release units are formed sequentially. J Muscle Res Cell Motil. 1994;15:102–118. doi: 10.1007/BF00130422. [DOI] [PubMed] [Google Scholar]
- 353.Tatsumi R, Hattori A, Takahashi K. Substructure of nebulin filaments: localization and characterization of subfragments produced by 0.1 mM CaCl2. J Biochem. 1993;113:797–804. doi: 10.1093/oxfordjournals.jbchem.a124121. [DOI] [PubMed] [Google Scholar]
- 354.Tatsumi R, Maeda K, Hattori A, Takahashi K. Calcium binding to an elastic portion of connectin/titin filaments. J Muscle Res Cell Motil. 2001;22:149–162. doi: 10.1023/a:1010349416723. [DOI] [PubMed] [Google Scholar]
- 355.Taveau M, Bourg N, Sillon G, Roudaut C, Bartoli M, Richard I. Calpain 3 is activated through autolysis within the active site and lyses sarcomeric and sarcolemmal components. Mol Cell Biol. 2003;23:9127–9135. doi: 10.1128/MCB.23.24.9127-9135.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Terasaki AG, Suzuki H, Nishioka T, Matsuzawa E, Katsuki M, Nakagawa H, Miyamoto S, Ohashi K. A novel LIM and SH3 protein (lasp-2) highly expressing in chicken brain. Biochem Biophys Res Commun. 2004;313:48–54. doi: 10.1016/j.bbrc.2003.11.085. [DOI] [PubMed] [Google Scholar]
- 357.Thompson TG, Chan YM, Hack AA, Brosius M, Rajala M, Lidov HG, McNally EM, Watkins S, Kunkel LM. Filamin 2 (FLN2): a muscle-specific sarcoglycan interacting protein. J Cell Biol. 2000;148:115–126. doi: 10.1083/jcb.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Trombitas K, Freiburg A, Centner T, Labeit S, Granzier H. Molecular dissection of N2B cardiac titin's extensibility. Biophys J. 1999;77:3189–3196. doi: 10.1016/S0006-3495(99)77149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Trombitas K, Granzier H. Actin removal from cardiac myocytes shows that near Z line titin attaches to actin while under tension. Am J Physiol Cell Physiol. 1997;273:C662–C670. doi: 10.1152/ajpcell.1997.273.2.C662. [DOI] [PubMed] [Google Scholar]
- 360.Trombitas K, Greaser M, French G, Granzier H. PEVK extension of human soleus muscle titin revealed by immunolabeling with the anti-titin antibody 9D10. J Struct Biol. 1998;122:188–196. doi: 10.1006/jsbi.1998.3984. [DOI] [PubMed] [Google Scholar]
- 361.Trombitas K, Greaser ML, Pollack GH. Interaction between titin and thin filaments in intact cardiac muscle. J Muscle Res Cell Motil. 1997;18:345–351. doi: 10.1023/a:1018626210300. [DOI] [PubMed] [Google Scholar]
- 362.Tskhovrebova L, Trinick J. Giant proteins: sensing tension with titin kinase. Curr Biol. 2008;18:R1141–1142. doi: 10.1016/j.cub.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 363.Tskhovrebova L, Trinick J. Properties of titin immunoglobulin and fibronectin-3 domains. J Biol Chem. 2004;279:46351–46354. doi: 10.1074/jbc.R400023200. [DOI] [PubMed] [Google Scholar]
- 364.Turnacioglu KK, Mittal B, Dabiri GA, Sanger JM, Sanger JW. Zeugmatin is part of the Z-band targeting region of titin. Cell Struct Funct. 1997;22:73–82. doi: 10.1247/csf.22.73. [DOI] [PubMed] [Google Scholar]
- 365.Udd B. Molecular biology of distal muscular dystrophies—sarcomeric proteins on top. Biochim Biophys Acta. 2007;1772:145–158. doi: 10.1016/j.bbadis.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 366.Udd B, Haravuori H, Kalimo H, Partanen J, Pulkkinen L, Paetau A, Peltonen L, Somer H. Tibial muscular dystrophy—from clinical description to linkage on chromosome 2q31. Neuromuscul Disord. 1998;8:327–332. doi: 10.1016/s0960-8966(98)00024-8. [DOI] [PubMed] [Google Scholar]
- 367.Udd B, Kaarianen H, Somer H. Muscular dystrophy with separate clinical phenotypes in a large family. Muscle Nerve. 1991;14:1050–1058. doi: 10.1002/mus.880141103. [DOI] [PubMed] [Google Scholar]
- 368.Udd B, Lamminen A, Somer H. Imaging methods reveal unexpected patchy lesions in late onset distal myopathy. Neuromuscul Disord. 1991;1:279–285. doi: 10.1016/0960-8966(91)90102-x. [DOI] [PubMed] [Google Scholar]
- 369.Udd B, Vihola A, Sarparanta J, Richard I, Hackman P. Titinopathies and extension of the M-line mutation phenotype beyond distal myopathy and LGMD2J. Neurology. 2005;64:636–642. doi: 10.1212/01.WNL.0000151853.50144.82. [DOI] [PubMed] [Google Scholar]
- 370.Valle G, Faulkner G, De Antoni A, Pacchioni B, Pallavicini A, Pandolfo D, Tiso N, Toppo S, Trevisan S, Lanfranchi G. Telethonin, a novel sarcomeric protein of heart and skeletal muscle. FEBS Lett. 1997;415:163–168. doi: 10.1016/s0014-5793(97)01108-3. [DOI] [PubMed] [Google Scholar]
- 371.Van den Bergh PY, Bouquiaux O, Verellen C, Marchand S, Richard I, Hackman P, Udd B. Tibial muscular dystrophy in a Belgian family. Ann Neurol. 2003;54:248–251. doi: 10.1002/ana.10647. [DOI] [PubMed] [Google Scholar]
- 372.Van der Loop FT, Schaart G, Langmann W, Ramaekers FC, Viebahn C. Expression and organization of muscle specific proteins during the early developmental stages of the rabbit heart. Anat Embryol. 1992;185:439–450. doi: 10.1007/BF00174082. [DOI] [PubMed] [Google Scholar]
- 373.Van der Loop FT, van der Ven PF, Furst DO, Gautel M, van Eys GJ, Ramaekers FC. Integration of titin into the sarcomeres of cultured differentiating human skeletal muscle cells. Eur J Cell Biol. 1996;69:301–307. [PubMed] [Google Scholar]
- 374.Van der Loop FT, Van Eys GJ, Schaart G, Ramaekers FC. Titin expression as an early indication of heart and skeletal muscle differentiation in vitro. Developmental re-organisation in relation to cytoskeletal constituents. J Muscle Res Cell Motil. 1996;17:23–36. doi: 10.1007/BF00140321. [DOI] [PubMed] [Google Scholar]
- 375.Van der Ven PF, Ehler E, Perriard JC, Furst DO. Thick filament assembly occurs after the formation of a cytoskeletal scaffold. J Muscle Res Cell Motil. 1999;20:569–579. doi: 10.1023/a:1005569225773. [DOI] [PubMed] [Google Scholar]
- 376.Van der Ven PF, Furst DO. Assembly of titin, myomesin and M-protein into the sarcomeric M band in differentiating human skeletal muscle cells in vitro. Cell Struct Funct. 1997;22:163–171. doi: 10.1247/csf.22.163. [DOI] [PubMed] [Google Scholar]
- 377.Van der Ven PF, Obermann WM, Lemke B, Gautel M, Weber K, Furst DO. Characterization of muscle filamin isoforms suggests a possible role of gamma-filamin/ABP-L in sarcomeric Z-disc formation. Cell Motil Cytoskeleton. 2000;45:149–162. doi: 10.1002/(SICI)1097-0169(200002)45:2<149::AID-CM6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 378.Vincent A, Leite MI, Farrugia ME, Jacob S, Viegas S, Shiraishi H, Benveniste O, Morgan BP, Hilton-Jones D, Newsom-Davis J, Beeson D, Willcox N. Myasthenia gravis seronegative for acetylcholine receptor antibodies. Ann NY Acad Sci. 2008;1132:84–92. doi: 10.1196/annals.1405.020. [DOI] [PubMed] [Google Scholar]
- 379.Vinkemeier U, Obermann W, Weber K, Furst DO. The globular head domain of titin extends into the center of the sarcomeric M band. cDNA cloning, epitope mapping and immunoelectron microscopy of two titin-associated proteins. J Cell Sci. 1993;106:319–330. doi: 10.1242/jcs.106.1.319. [DOI] [PubMed] [Google Scholar]
- 380.Von Castelmur E, Marino M, Svergun DI, Kreplak L, Ucurum-Fotiadis Z, Konarev PV, Urzhumtsev A, Labeit D, Labeit S, Mayans O. A regular pattern of Ig super-motifs defines segmental flexibility as the elastic mechanism of the titin chain. Proc Natl Acad Sci USA. 2008;105:1186–1191. doi: 10.1073/pnas.0707163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Wallgren-Pettersson C. Neuromuscul Disord; 72nd ENMC International Workshop: myotubular myopathy; Hilversum, The Netherlands. 1–3 October 1999; 2000. pp. 525–529. [DOI] [PubMed] [Google Scholar]
- 382.Wallgren-Pettersson C. Nemaline and myotubular myopathies. Semin Pediatr Neurol. 2002;9:132–144. doi: 10.1053/spen.2002.33804. [DOI] [PubMed] [Google Scholar]
- 383.Wallgren-Pettersson C, Donner K, Sewry C, Bijlsma E, Lammens M, Bushby K, Giovannucci Uzielli ML, Lapi E, Odent S, Akcoren Z, Topaloglu H, Pelin K. Mutations in the nebulin gene can cause severe congenital nemaline myopathy. Neuromuscul Disord. 2002;12:674–679. doi: 10.1016/s0960-8966(02)00065-2. [DOI] [PubMed] [Google Scholar]
- 384.Wallgren-Pettersson C, Lehtokari VL, Kalimo H, Paetau A, Nuutinen E, Hackman P, Sewry C, Pelin K, Udd B. Distal myopathy caused by homozygous missense mutations in the nebulin gene. Brain. 2007;130:1465–1476. doi: 10.1093/brain/awm094. [DOI] [PubMed] [Google Scholar]
- 385.Wallgren-Pettersson C, Pelin K, Hilpela P, Donner K, Porfirio B, Graziano C, Swoboda KJ, Fardeau M, Urtizberea JA, Muntoni F, Sewry C, Dubowitz V, Iannaccone S, Minetti C, Pedemonte M, Seri M, Cusano R, Lammens M, Castagna-Sloane A, Beggs AH, Laing NG, de la Chapelle A. Clinical and genetic heterogeneity in autosomal recessive nemaline myopathy. Neuromuscul Disord. 1999;9:564–572. doi: 10.1016/s0960-8966(99)00061-9. [DOI] [PubMed] [Google Scholar]
- 386.Wallgren-Pettersson C, Pelin K, Nowak KJ, Muntoni F, Romero NB, Goebel HH, North KN, Beggs AH, Laing NG. Geno-type-phenotype correlations in nemaline myopathy caused by mutations in the genes for nebulin and skeletal muscle alpha-actin. Neuromuscul Disord. 2004;14:461–470. doi: 10.1016/j.nmd.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 387.Wang J, Sanger JM, Kang S, Thurston H, Abbott LZ, Dube DK, Sanger JW. Ectopic expression and dynamics of TPM1alpha and TPM1kappa in myofibrils of avian myotubes. Cell Motil Cytoskeleton. 2007;64:767–776. doi: 10.1002/cm.20221. [DOI] [PubMed] [Google Scholar]
- 388.Wang K. Titin/connectin and nebulin: giant protein rulers of muscle structure and function. Adv Biophys. 1996;33:123–134. [PubMed] [Google Scholar]
- 389.Wang K, Knipfer M, Huang QQ, van Heerden A, Hsu LC, Gutierrez G, Quian XL, Stedman H. Human skeletal muscle nebulin sequence encodes a blueprint for thin filament architecture. Sequence motifs and affinity profiles of tandem repeats and terminal SH3. J Biol Chem. 1996;271:4304–4314. doi: 10.1074/jbc.271.8.4304. [DOI] [PubMed] [Google Scholar]
- 390.Wang K, McClure J, Tu A. Titin: major myofibrillar components of striated muscle. Proc Natl Acad Sci USA. 1979;76:3698–3702. doi: 10.1073/pnas.76.8.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Wang K, Wright J. Architecture of the sarcomere matrix of skeletal muscle: immunoelectron microscopic evidence that suggests a set of parallel inextensible nebulin filaments anchored at the Z line. J Cell Biol. 1988;107:2199–2212. doi: 10.1083/jcb.107.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Wang SM, Jeng CJ, Sun MC. Studies on the interaction between titin and myosin. Histol Histopathol. 1992;7:333–337. [PubMed] [Google Scholar]
- 393.Wang SM, Lo MC, Shang C, Kao SC, Tseng YZ. Role of M-line proteins in sarcomeric titin assembly during cardiac myofibrillo-genesis. J Cell Biochem. 1998;71:82–95. doi: 10.1002/(sici)1097-4644(19981001)71:1<82::aid-jcb9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 394.Warren CM, Jordan MC, Roos KP, Krzesinski PR, Greaser ML. Titin isoform expression in normal and hypertensive myocardium. Cardiovasc Res. 2003;59:86–94. doi: 10.1016/s0008-6363(03)00328-6. [DOI] [PubMed] [Google Scholar]
- 395.Watanabe K, Nair P, Labeit D, Kellermayer MS, Greaser M, Labeit S, Granzier H. Molecular mechanics of cardiac titin's PEVK and N2B spring elements. J Biol Chem. 2002;277:11549–11558. doi: 10.1074/jbc.M200356200. [DOI] [PubMed] [Google Scholar]
- 396.Wear MA, Yamashita A, Kim K, Maeda Y, Cooper JA. How capping protein binds the barbed end of the actin filament. Curr Biol. 2003;13:1531–1537. doi: 10.1016/s0960-9822(03)00559-1. [DOI] [PubMed] [Google Scholar]
- 397.Weber A. Actin binding proteins that change extent and rate of actin monomer-polymer distribution by different mechanisms. Mol Cell Biochem. 1999;190:67–74. [PubMed] [Google Scholar]
- 398.Weber A, Pennise CR, Babcock GG, Fowler VM. Tropomodulin caps the pointed ends of actin filaments. J Cell Biol. 1994;127:1627–1635. doi: 10.1083/jcb.127.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Weber A, Pennise CR, Fowler VM. Tropomodulin increases the critical concentration of barbed end-capped actin filaments by converting ADP. P(i)-actin to ADP-actin at all pointed filament ends. J Biol Chem. 1999;274:34637–34645. doi: 10.1074/jbc.274.49.34637. [DOI] [PubMed] [Google Scholar]
- 400.Weinert S, Bergmann N, Luo X, Erdmann B, Gotthardt M. M line-deficient titin causes cardiac lethality through impaired maturation of the sarcomere. J Cell Biol. 2006;173:559–570. doi: 10.1083/jcb.200601014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Welikson RE, Fischman DA. The C-terminal IgI domains of myosin-binding proteins C and H (MyBP-C and MyBP-H) are both necessary and sufficient for the intracellular crosslinking of sarcomeric myosin in transfected non-muscle cells. J Cell Sci. 2002;115:3517–3526. doi: 10.1242/jcs.115.17.3517. [DOI] [PubMed] [Google Scholar]
- 402.Whiting A, Wardale J, Trinick J. Does titin regulate the length of muscle thick filaments? J Mol Biol. 1989;205:263–268. doi: 10.1016/0022-2836(89)90381-1. [DOI] [PubMed] [Google Scholar]
- 403.Winegrad S. Cardiac myosin binding protein C. Circ Res. 1999;84:1117–1126. doi: 10.1161/01.res.84.10.1117. [DOI] [PubMed] [Google Scholar]
- 404.Witt CC, Burkart C, Labeit D, McNabb M, Wu Y, Granzier H, Labeit S. Nebulin regulates thin filament length, contractility, and Z-disk structure in vivo. EMBO J. 2006;25:3843–3855. doi: 10.1038/sj.emboj.7601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Witt CC, Ono Y, Puschmann E, McNabb M, Wu Y, Gotthardt M, Witt SH, Haak M, Labeit D, Gregorio CC, Sorimachi H, Granzier H, Labeit S. Induction and myofibrillar targeting of CARP, and suppression of the Nkx2.5 pathway in the MDM mouse with impaired titin-based signaling. J Mol Biol. 2004;336:145–154. doi: 10.1016/j.jmb.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 406.Witt CC, Witt SH, Lerche S, Labeit D, Back W, Labeit S. Cooperative control of striated muscle mass and metabolism by MuRF1 and MuRF2. EMBO J. 2008;27:350–360. doi: 10.1038/sj.emboj.7601952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Witt SH, Granzier H, Witt CC, Labeit S. MURF-1 and MURF-2 target a specific subset of myofibrillar proteins redundantly: towards understanding MURF-dependent muscle ubiquitination. J Mol Biol. 2005;350:713–722. doi: 10.1016/j.jmb.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 408.Witt SH, Labeit D, Granzier H, Labeit S, Witt CC. Dimerization of the cardiac ankyrin protein CARP: implications for MARP titin-based signaling. J Muscle Res Cell Motil. 2005;26:401–408. doi: 10.1007/s10974-005-9022-9. [DOI] [PubMed] [Google Scholar]
- 409.Wright J, Huang QQ, Wang K. Nebulin is a full-length template of actin filaments in the skeletal muscle sarcomere: an immunoelectron microscopic study of its orientation and span with site-specific monoclonal antibodies. J Muscle Res Cell Motil. 1993;14:476–483. doi: 10.1007/BF00297210. [DOI] [PubMed] [Google Scholar]
- 410.Wu Y, Bell SP, Trombitas K, Witt CC, Labeit S, LeWinter MM, Granzier H. Changes in titin isoform expression in pacing-induced cardiac failure give rise to increased passive muscle stiffness. Circulation. 2002;106:1384–1389. doi: 10.1161/01.cir.0000029804.61510.02. [DOI] [PubMed] [Google Scholar]
- 411.Wu Y, Cazorla O, Labeit D, Labeit S, Granzier H. Changes in titin and collagen underlie diastolic stiffness diversity of cardiac muscle. J Mol Cell Cardiol. 2000;32:2151–2162. doi: 10.1006/jmcc.2000.1281. [DOI] [PubMed] [Google Scholar]
- 412.Xiang F, Nicolao P, Chapon F, Edstrom L, Anvret M, Zhang Z. A second locus for autosomal dominant myopathy with proximal muscle weakness and early respiratory muscle involvement: a likely chromosomal locus on 2q21. Neuromuscul Disord. 1999;9:308–312. doi: 10.1016/s0960-8966(99)00030-9. [DOI] [PubMed] [Google Scholar]
- 413.Xiong G, Qadota H, Mercer KB, McGaha LA, Oberhauser AF, Benian GM. A LIM-9 (FHL)/SCPL-1 (SCP) complex interacts with the C-terminal protein kinase regions of UNC-89 (obscurin) in Caenorhabditis elegans muscle. J Mol Biol. 2009;386:976–988. doi: 10.1016/j.jmb.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Xu X, Meiler SE, Zhong TP, Mohideen M, Crossley DA, Burggren WW, Fishman MC. Cardiomyopathy in zebrafish due to mutation in an alternatively spliced exon of titin. Nat Genet. 2002;30:205–209. doi: 10.1038/ng816. [DOI] [PubMed] [Google Scholar]
- 415.Yajima H, Ohtsuka H, Kume H, Endo T, Maruyama K, Kimura S. Molecular cloning of a partial cDNA clone encoding the C terminal region of chicken breast muscle connectin. Zool Sci. 1996;13:119–123. doi: 10.2108/zsj.13.119. [DOI] [PubMed] [Google Scholar]
- 416.Yamamoto AM, Gajdos P, Eymard B, Tranchant C, Warter JM, Gomez L, Bourquin C, Bach JF, Garchon HJ. Anti-titin antibodies in myasthenia gravis: tight association with thymoma and heterogeneity of nonthymoma patients. Arch Neurol. 2001;58:885–890. doi: 10.1001/archneur.58.6.885. [DOI] [PubMed] [Google Scholar]
- 417.Yamamoto K, Masuyama T, Sakata Y, Nishikawa N, Mano T, Yoshida J, Miwa T, Sugawara M, Yamaguchi Y, Ookawara T, Suzuki K, Hori M. Myocardial stiffness is determined by ventricular fibrosis, but not by compensatory or excessive hypertrophy in hypertensive heart. Cardiovasc Res. 2002;55:76–82. doi: 10.1016/s0008-6363(02)00341-3. [DOI] [PubMed] [Google Scholar]
- 418.Yamasaki R, Berri M, Wu Y, Trombitas K, McNabb M, Keller-mayer MS, Witt C, Labeit D, Labeit S, Greaser M, Granzier H. Titin-actin interaction in mouse myocardium: passive tension modulation and its regulation by calcium/S100A1. Biophys J. 2001;81:2297–2313. doi: 10.1016/S0006-3495(01)75876-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H. Protein kinase A phosphorylates titin's cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res. 2002;90:1181–1188. doi: 10.1161/01.res.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]
- 420.Yarom R, Meiri U. N lines in striated muscle: a site of intracellular Ca2+. Nat New Biol. 1971;234:254–256. doi: 10.1038/newbio234254a0. [DOI] [PubMed] [Google Scholar]
- 421.Young P, Ehler E, Gautel M. Obscurin, a giant sarcomeric Rho guanine nucleotide exchange factor protein involved in sarcomere assembly. J Cell Biol. 2001;154:123–136. doi: 10.1083/jcb.200102110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Young P, Ferguson C, Banuelos S, Gautel M. Molecular structure of the sarcomeric Z-disk: two types of titin interactions lead to an asymmetrical sorting of alpha-actinin. EMBO J. 1998;17:1614–1624. doi: 10.1093/emboj/17.6.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Young P, Gautel M. The interaction of titin and alpha-actinin is controlled by a phospholipid-regulated intramolecular pseudoligand mechanism. EMBO J. 2000;19:6331–6340. doi: 10.1093/emboj/19.23.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Yuan SH, Arnold W, Jorgensen AO. Biogenesis of transverse tubules and triads: immunolocalization of the 1,4-dihydropyridine receptor, TS28, and the ryanodine receptor in rabbit skeletal muscle developing in situ. J Cell Biol. 1991;112:289–301. doi: 10.1083/jcb.112.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Zhang JQ, Luo G, Herrera AH, Paterson B, Horowits R. cDNA cloning of mouse nebulin. Evidence that the nebulin-coding sequence is highly conserved among vertebrates. Eur J Biochem. 1996;239:835–841. doi: 10.1111/j.1432-1033.1996.0835u.x. [DOI] [PubMed] [Google Scholar]
- 426.Zhou D, Birkenmeier CS, Williams MW, Sharp JJ, Barker JE, Bloch RJ. Small, membrane-bound, alternatively spliced forms of ankyrin 1 associated with the sarcoplasmic reticulum of mammalian skeletal muscle. J Cell Biol. 1997;136:621–631. doi: 10.1083/jcb.136.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Zhu Y, Bogomolovas J, Labeit S, Granzier H. Single molecule force spectroscopy of cardiac titin's N2B element—effects of the molecular chaperone alpha B-crystallin with disease causing mutations. J Biol Chem. 2009;284:13914–13923. doi: 10.1074/jbc.M809743200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Zou P, Gautel M, Geerlof A, Wilmanns M, Koch MH, Svergun DI. Solution scattering suggests cross-linking function of telethonin in the complex with titin. J Biol Chem. 2003;278:2636–2644. doi: 10.1074/jbc.M210217200. [DOI] [PubMed] [Google Scholar]
- 429.Zou P, Pinotsis N, Lange S, Song YH, Popov A, Mavridis I, Mayans OM, Gautel M, Wilmanns M. Palindromic assembly of the giant muscle protein titin in the sarcomeric Z-disk. Nature. 2006;439:229–233. doi: 10.1038/nature04343. [DOI] [PubMed] [Google Scholar]
- 430.Zou Y, Evans S, Chen J, Kuo HC, Harvey RP, Chien KR. CARP, a cardiac ankyrin repeat protein, is downstream in the Nkx2–5 homeobox gene pathway. Development. 1997;124:793–804. doi: 10.1242/dev.124.4.793. [DOI] [PubMed] [Google Scholar]