Abstract
Although Zn2+ homeostasis in neurons is tightly regulated and its destabilization has been linked to a number of pathologies including Alzheimer's disease and ischemic neuronal death, the primary mechanisms affecting intracellular Zn2+ concentration ([Zn2+]i) in neurons exposed to excitotoxic stimuli remain poorly understood. The present work addressed these mechanisms in cultured hippocampal neurons exposed to glutamate and glycine (Glu/Gly). [Zn2+]i and [Ca2+]i were monitored simultaneously using FluoZin-3 and Fura2-FF and intracellular pH (pHi) was studied in parallel experiments using 2',7'-bis-(2-carboxyethyl)-5(6)-carboxyfluorescein. Glu/Gly applications under Na+-free conditions (Na+ substituted with N-methyl-D-glucamine+) caused Ca2+ influx, pHi drop, and Zn2+ release from intracellular stores. Experimental maneuvers resulting in a pHi increase during Glu/Gly applications, such as stimulation of Na+-dependent pathways of H+ efflux, forcing H+ efflux via gramicidin-formed channels, or increasing extracellular pH counteracted [Zn2+]i elevations. In the absence of Na+, the rate of [Zn2+]i decrease could be correlated with the rate of pHi increase. In the presence of Na+, the rate of [Zn2+]i decrease was about twice as fast as expected from the rate of pHi elevation. The data suggest that Glu/Gly-induced cytosolic acidification promotes [Zn2+]i elevations and that Na+ counteracts the latter by promoting pHi-dependent and pHi-independent mechanisms of cytosolic Zn2+ clearance.
Keywords: Fura-2FF, FluoZin-3, BCECF, TPEN, intracellular pH, intracellular Zn2+ stores
Introduction
Increasing evidence indicates that destabilization of Zn2+ homeostasis can play a major role in neuronal death in vitro and in vivo (Yokoyama et al. 1986; Choi et al. 1988; Tonder et al. 1990; Koh et al. 1996; Aizenman et al. 2000; Lee et al. 2000; Sheline et al. 2000; Medvedeva et al. 2009; Sensi et al. 2009). Chelation of extracellular Zn2+ was neuroprotective in animal models of stroke (Koh et al. 1996; Lee et al. 2002) and global brain ischemia (Calderone et al. 2004). Recently, Zn2+ influx was found to promote spreading depression in brain slices (Dietz et al. 2008) and elevated extracellular Zn2+ levels have also been implicated in the formation of Alzheimer beta-amyloid plaques (Bush 2003; Sensi et al. 2009). However, the mechanisms maintaining Zn2+ homeostasis in neurons remain poorly understood. Two families of Zn2+ transporters are recognized, ZnT with the systematic name of SLC30 (Palmiter and Huang 2004) and Zip with the systematic name of SLC39 (Eide 2006). Among the ZnT transporters expressed in the brain, the best characterized is localized in the plasma membrane ZnT-1 (Palmiter and Findley 1995). The remaining ZnTs are found in intracellular compartments (Beyersmann and Haase 2001; Eide 2006), usually associated with endosomes, Golgi apparatus, or endoplasmic reticulum (Cousins et al. 2006), or in the case of ZnT-3, synaptic vesicles (Palmiter 2004). The molecular mechanism of ZnT-mediated transport involves an exchange of Zn2+ for H+ or K+ (Guffanti et al. 2002; Chao and Fu 2004; Ohana et al. 2009).
Transcripts of several Zip family members, Zip1, Zip9, Zip10, and Zip14, are robustly expressed in the mouse brain (Gyulkhandanyan et al. 2006) and Zip1 and Zip3 were recently found in neurons (Qian et al. 2011). While the cellular localization of the Zip proteins is yet unclear, in non-neuronal cells, Zip2 and Zip4 have been found in plasma membranes (Gaither and Eide 2000; Dufner-Beattie et al. 2003). The mechanism of Zn2+ transport by Zip2 involves a Zn2+-HCO3− co-transport (Gaither and Eide 2000) and the Zip proteins generally mediate Zn2+ influx (Eide 2006; Sensi et al. 2009). For example, Zip4 mediates zinc influx leading to lysosomal Zn2+ sequestration (Emmetsberger et al. 2010).
Early studies suggested that plasmalemmal Na+/Ca2+ exchangers may transport Zn2+ (Sensi et al. 1997; Cheng and Reynolds 1998) and the inhibition of the Na+/Ca2+ exchange by Zn2+ was also reported (Colvin 1998). However, Ohana et al. (2004) described what appears to be a genuine Na+/Zn2+ exchanger in HEK 293 cells and in cultured cortical neurons. Also in cultured cortical neurons, Qin et al. (2008) described distinct mechanisms of Na+-dependent Zn2+ efflux that were inhibited by 10 μM La3+ and Ca2+-free medium. None of the Na+/Zn2+ exchangers has been yet cloned.
Activation of glutamate receptors in cultured neurons leads to intracellular Zn2+ concentration ([Zn2+]i) elevations representing a Ca2+-dependent Zn2+ release from intracellular stores (Sensi et al. 2003; Dineley et al. 2008). The present work tested the impact of Na+ and pHi on Glu/Gly-induced [Zn2+]i elevations. It was found that the [Zn2+]i elevations critically depend on the Glu/Gly-induced pHi drop and that the Na+-induced pHi increase plays an important role in cytosolic Zn2+ clearance.
Materials and Methods
Measurement of Zn2+ concentration in Locke's buffer
Locke's buffer contained (in mM) NaCl (157.6), KCl (2.0), KHCO3 (3.6), MgCl2 (1.0), CaCl2 (1.3), HEPES (10), glucose (5), and pH 7.4 adjusted with Tris. To measure [Zn2+], the buffer was supplemented with 10 nM FluoZin-3 tetrapotassium salt, and F488 fluorescence (488 nm excitation, 510 nm emission) was monitored at 25°C using a PTI QuantaMaster spectrofluorometer (Photon Technology International, Inc., Birmingham, NJ). Minimal F488 (Fmin), and maximal F488 (Fmax) values were measured after adding 100 μM N,N,N',N'-tetrakis(2-pyridalmethyl)ethylenediamine (TPEN) or 100 μM ZnSO4, respectively, to the buffer. The formula [Zn2+] = Kd (F488 – Fmin) / (Fmax – F488) with the Kd value that was assumed to be 15 nM (Gee et al. 2002) was used to calculate Zn2+ concentration in the buffer.
The Fura-2FF isosbestic point and the effects of pH on Fura-2FF fluorescence
A solution of Fura-2FF K+ salt (1 μM) in a buffer containing (in mM) KCl (159.6), KHCO3 (3.6), HEPES (10), and pH 7.4 adjusted with Tris was supplemented with 1 mM EGTA, or 1 mM CaCl2, and/or 0.1 mM ZnCl2 to obtain a Ca2+/Zn2+-free, a Ca2+-containing, a Zn2+-containing, or a Ca2+ and Zn2+-containing solution of Fura-2FF, respectively. Using the PTI QuantaMaster spectrofluorometer (Birmingham, NJ), excitation spectra (300 – 400 nm excitation, 515 nm emission) were obtained at 25°C. The excitation wavelength at which the spectra of Ca2+-containing and Zn2+-containing solutions intersected the spectrum of Ca2+/Zn2+-free solution was taken as the respective isosbestic point.
To determine the impact of the pH drop from 7.4 to 5.5 on Fura-2FF fluorescence, analogous (300 – 400 nm excitation, 515 nm emission) spectra of 1 μM Fura-2FF (K+ salt) dissolved in the above described but nominally Ca2+-free (not supplemented with EGTA), or supplemented with 30 μM CaCl2 buffer were obtained at pH 7.4 and 5.5. First the spectrum at pH 7.4 was measured, than 1 μl of 5M HCl per 1 ml of the solution was added to the cuvette, which dropped the pH to 5.5 as determined using a pH meter and the spectrum was measured again.
Primary cultures of mouse hippocampal neurons
The cultures were plated using a SPOT™ culture kit from the University of Illinois at Chicago Research Resources Center (http://www.rrc.uic.edu/portal/SPOT_Culture_Kit). The kit contains cryopreserved hippocampal neurons from embryonic day 16 c57/bl/6 mice. The neurons were cultured in circular spots of about 4 mm diameter in the center 35 mm plastic dishes (BD Falcon™ 353001), according to the manufacturer's instructions. For the ion imaging experiments, the SPOT™ cultures were plated in a center of 25 mm glass coverslips (Fisher Scientific 12-545-86) placed in 35 mm dishes. After plating, the cells were cultured at 37°C in 2 ml of Neurobasal Medium (Gibco Invitrogen, Carlsbad, CA) supplemented with 2% B-27 (Gibco Invitrogen, Carlsbad, CA) and 2 mM glutamine in a humidified incubator (MCO-5M, Sanyo, Wood Dale, IL) maintaining 5% CO2 and 5% O2 balanced with N2. Two days after plating, glial proliferation was curtailed by adding 10 μg/ml of 5-fluoro-2'-deoxyuridine (FURD) to the cultures. The next day and once a week thereafter, half the culture medium was replaced using an analogous medium but containing 2 mM glutamax (Gibco Invitrogen, Carlsbad, CA) instead of glutamine and not supplemented with FURD. Cells were used for experiments after 13 days in vitro.
Monitoring Fura2-FF and FluoZin-3 fluorescence in neurons
Coverslips with SPOT™ cultures were placed in custom-made 50 μl imaging chambers and the cells were loaded at 37°C with 0.1 μM FluoZin-3 AM and 0.1 μM Fura-2FF AM for 5 – 6 minutes. The concentrations of the indicators and the time of loading were adjusted such that at the end of the experiments during in situ calibration, fluorescent signals did not exceed the dynamic range of the camera.
Imaging chambers with loaded cells were placed on a Zeiss Axiovert 100 microscope stage and prior to monitoring fluorescence, a Hoffman modulation contrast image of the cells was taken using a Zeiss LD A-Plan 20×/0.3 HMC objective. Fluorescence was monitored using a Zeiss Fluar 20×, NA 0.75 objective and an Attofluor digital imaging system (Atto Instruments, Rockville, MD). Superfusion media were delivered directly onto the cells via an 8-channel manifold (MPRE-8, Cell MicroControls, Norfolk, VA) with a computer-controlled flow using an 8-channel valve switch (cFlow8, Cell MicroControls). Temperature was maintained at 37°C using a bipolar temperature controller (TC2BIP, Cell MicroControls).
For time-lapse imaging, cells were exposed every 5 seconds to a sequence of 340, 380, 488 and 356 nm excitation and the images of fluorescence emitted at >520 nm (F340, F380, F488, and F356) were saved on a computer hard-drive for off-line analysis. Cells were superfused at a rate of 0.5 ml/min, initially with Locke's buffer and then with the experimental media. Glutamate receptors were activated using Mg-free Locke's buffer supplemented with 100 μM glutamate and 10 μM glycine (Gly/Glu). Na+-free buffers were made by replacing NaCl with chloride salts of N-methyl-D-glucamine (NMDG) or lithium or cesium, as indicated in the text. In Ca2+- and Zn2+-free buffers, CaCl2 was omitted and 100 μM EGTA was added. To remove Zn2+ but not Ca2+ from the buffers, 1 mM CaEDTA was used.
All fluorescence data were background-corrected by subtracting the fluorescence measured in cell-free areas from the fluorescence measured in the regions of interest positioned on neuronal somata. At the end of each experiment, the maximal F340/F380 ratio (Rmax) of Fura-2FF and the maximal F488 (Fmax) of FluoZin-3 was measured during “Ca saturation” and “Zn saturation,” respectively. During Ca saturation, Locke's buffer was supplemented with 10 μM ionomycin, 10 μM TPEN, 2 μM carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP) and 10 mM CaCl2. During Zn saturation, Locke's buffer was supplemented with 20 μM 1-hydroxypyridine-2-thione zinc salt and 100 μM ZnCl2.
In some experiments, an attempt was made to calibrate FluoZin-3 fluorescence in terms of [Zn2+]i. In these experiments, before performing Zn saturation to measure Fmax, minimal F488 (Fmin) was measured. To this end, the cells were exposed to 10 μM TPEN. [Zn2+]i was calculated as described above (measurement of Zn2+ concentration in Locke's buffer). In a majority of the experiments, the FluoZin-3 and Fura-2FF data were routinely expressed as a percentage of Fmax and Rmax, respectively, for the reasons explained in the text.
To compare the rates of cytoslolic Zn2+ clearance following 5 min exposure to Glu/Gly under Na+-free conditions, F488 data were normalized according to the formula: normalized F488 = (F488 – F488basal)*100/(F4885min – F488basal), where F488basal is the fluorescence measured before Glu/Gly application and F4885min is the fluorescence measured at the end of the 5th minute of exposure to Glu/Gly, just before the Zn2+ clearance-inducing media described in the text were applied. The rates of F488 drop were calculated with the linear regression method using the linear portion of the data.
Monitoring intracellular pH (pHi) in neurons
Coverslips with SPOT™ cultures were placed in custom-made 50 μl imaging chambers and the cells were loaded for 5 minutes at 37 °C with 1 μM BCECF-AM. After the loading, the cells were superfused and the fluorescence was measured using the above described instruments. For time-lapse imaging, cells were exposed every 5 seconds to 488 and 440 nm excitation and the images of fluorescence emitted at >520 nm (F488 and F440) were saved on a computer hard-drive for off-line analysis. In most experiments, the background-corrected F488/F440 ratio was converted to pHi values. To this end, a four point in situ calibration in the pH range of 5.8 – 8.0 was performed at the end of the experiments. The pH calibrating solutions contained 5μM monensin, 5μM nigericin, 2μM FCCP, 134.2 mM K-gluconate, 25.4 mM KCl, 1.3 mM CaCl2, 1 mM MgCl2, 3.6 mM KHCO3, and 10 mM 2-[N-morpholino]ethanesulfonic acid (MES), or 10 mM PIPES or 10 mM HEPES. MES was used to adjust the pH of the calibrating solutions to a range of 5.5 to 6.5; PIPES to a range of 6.6 –7.0 and HEPES to a range of 7.2 to 8.0. Using SigmaPlot 10.0 software (Systat Software Inc., Richmond, CA), the data were fitted to the four-parameter equation of a sigmoidal curve: F488/F440 = y + a /(1+ exp(−(pH−x)/b)). The rates of pHi increase were calculated with the linear regression method using the linear portion of the data.
Statistical analysis
A SigmaStat 3.5 package (Systat Software Inc., Richmond, CA) was used for statistical analysis. Normally distributed data were analyzed using one-way analysis of variance (ANOVA) followed by the Student-Newman-Keuls test. Data that were not normally distributed were analyzed using a Kruskal-Wallis one-way ANOVA on ranks followed by Dunn's test.
Reagents
Fura-2FF AM and Fura-2FF (K+ salt) were obtained from Teflabs (Austin, TX, USA). Neurobasal medium, B-27 supplement, BCECF-AM, FluoZin-3 AM and FluoZin-3 tetrapotassium salt were from Invitrogen (Carlsbad, CA, USA). All other reagents were from Sigma-Aldrich (St Louis, MO, USA) unless otherwise stated.
Results
Simultaneous monitoring of FluoZin-3 and Fura-2FF fluorescence
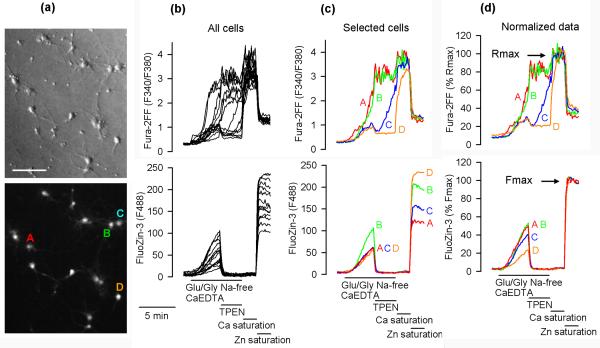
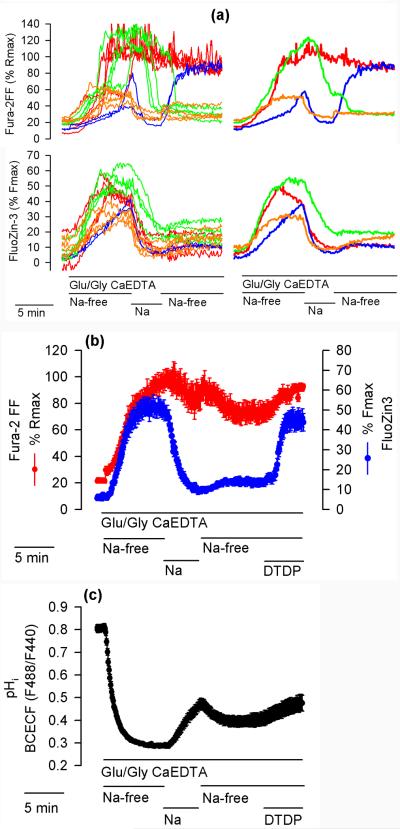
Fig. 1 illustrates the data generation and processing method used in this report. At the beginning of each experiment, the neuronal morphology of the cells was verified using the Hoffman modulation contrast (Fig. 1a, upper panel). At the end of each experiment, the maximal Fura-2FF F340/F380 ratio (Rmax) was measured in each cell after saturating Fura-2FF with Ca2+ (Ca saturation), which was followed by measuring the maximal FluoZin-3 F488 signal (Fmax) after saturating FluoZin-3 with Zn2+ (Zn saturation). The lower panel of Fig. 1a shows a F488 image of the cells captured during Zn saturation. The cells were exposed for 4 minutes to 100 μM glutamate and 10 μM glycine (Glu/Gly) under Na+-and Zn2+-free conditions (Na+ substituted with NMDG+ and extracellular zinc chelated by CaEDTA). Upon Glu/Gly application, both F340/F380 ratio and F488 began to increase. When 10 μM TPEN (a plasma membrane-permeable chelator) was added to chelate the intracellular zinc, F488 promptly dropped in all cells but the response in F340/F380 ratio varied greatly among the cells. In 50 neurons tested (3 experiments), the pattern of F340/F380 ratio response could be divided into three categories: category 1 (17 neurons, 34%) – the F340/F380 ratio failed to drop or kept increasing; category 2 (14 neurons, 28%) – the F340/F380 ratio initially dropped but then started to increase; category 3 (19 neurons, 38%) – the F340/F380 ratio dropped and remained low. Exemplar cells representing the three categories are indicated in the lower panel of Fig. 1a, and data from these cells are presented in Fig. 1c. Cells A (red) and B (green) represent category 1, cell C (blue) represents category 2, and cell D (orange) category 3.
Figure 1.
Simultaneous monitoring of FluoZin-3 and Fura-2FF fluorescence in single hippocampal neurons. (a) Upper panel, Hoffman modulation contrast image of 14 hippocampal neurons cultured for 14 days. Bar = 100 μm. Lower panel, F488 fluorescence during Zn saturation in the same neurons. Indicated A, B, C, D are four exemplar neurons that are discussed in the text. (b) Fura-2FF F340/F380 ratio and Fluo-Zin-3 fluorescence (F488) data from all 14 neurons shown in (a). The neurons were exposed to 100 μM glutamate and 10 μM glycine (Glu/Gly) under Na+-free conditions with Na+ substituted with N-methyl-D-glucamine (NMDG+) and in the presence of 1 mM CaEDTA to chelate extracellular Zn2+. Where indicated, 10 μM TPEN was added to chelate intracellular Zn2+. At the end of the experiment, Fura-2FF was saturated with Ca2+ to measure the maximal F340/F380 ratio (Rmax) followed by saturation of FluoZin-3 with Zn2+ to measure the maximal F488 fluorescence (Fmax). (c) Fluorescence data from the four exemplar cells indicated in (a). (d) Normalized Fura-2 FF and FluoZin-3 data expressed as a percentage of Rmax and Fmax, respectively.
Since the Glu/Gly-induced FluoZin-3 signal increase took place in the absence of extracellular Zn2+ (in the presence of 1 mM CaEDTA), the [Zn2+]i elevations represent Zn2+ release from intracellular stores (Fig. 1c lower panel). Dineley et al. (2008) recently reported that such an intracellular Zn2+ release depends on the Glu/Gly-induced Ca2+ influx. Therefore, one would expect that cells showing a faster rate and/or onset of [Ca2+]i (F340/F380 ratio) elevation would show a faster rate of [Zn2+]i (F488) increase. However, looking at the raw FluoZin-3 F488 data, this was not the case. Note that in cell A (red) and cell B (green), there is an F340/F380 ratio data overlap, which suggests that [Ca2+]i increases in both cells at the same rate. However, the rate of F488 increase is much faster in cell B than in cell A, suggesting that more Zn2+ is released in cell B (Fig. 1c). On the other hand, the F488 traces in cells A, C, and D overlap despite the very different patterns of F340/F380 ratio increases in these cells (Fig. 1c), suggesting that the rates of [Zn2+]i and [Ca2+]i elevations in these cells are independent from each other. Interestingly, Fmax values measured during Zn saturation greatly differed among the cells. For example, Fmax was much higher in cell B than in cell A (Fig. 1c bottom panel). Since FluoZin-3 fluorescence intensity during Fmax measurement is a function of FluoZin-3 concentration, the data indicate that cell B loaded more FluoZin-3 than cell A. Such uneven FluoZin-3 loading among the cells creates an artifact. The cells that loaded more of the indicator elevated F488 at a faster rate, which could be misinterpreted to indicate that [Zn2+]i elevates faster in these cells. This artifact could be normalized for by expressing F488 data as a percentage of Fmax. After such normalization, the rates of FluoZin-3 fluorescence increase could be related to the onset and/or rate of [Ca2+]i increase, the fastest in cells A and B and the slowest in cell D (Fig. 1d).
In neurons in which Glu/Gly-induced elevations of Fura-2FF signal were small (did not exceed 30% of Rmax), TPEN applications promptly decreased the signal, indicating that Zn2+ rather than Ca2+ contributed to the signal (for example cells C and D in Fig. 1c). Since in this case the Fura-2FF signal represented primarily [Zn2+]i elevations, no attempt was made to calibrate the Fura-2FF data in terms of [Ca2+]i. Instead, to relate the Fura-2FF signal to the maximal signal, the F340/F380 data were expressed as a percentage of Rmax (Fig. 1d upper panel). When Zn saturation was performed after Ca saturation, the Fura-2FF F340/F380 signal dropped to about 30% of Rmax (Fig. 1b,c,d upper panel). This drop was expected because Devinney II et al. (2005) already demonstrated that Zn2+ and Ca2+ affect the Fura2-FF excitation spectrum differently, which is confirmed by the data shown in Supplementary Fig. 1a. The F340/F380 ratio of Fura-2FF saturated with Zn2+ in vitro (Supplementary Fig. 1b), similarly as the one measured in vivo (Fig. 1d), can reach only about 30% of the ratio measured when the indicator is saturated with Ca2+. These data suggest that when the Fura-2FF signal exceeds 30% of Rmax, the signal results primarily from Ca2+ binding to the indicator. This can explain why Zn2+ chelation with TPEN fails to affect the signal when the latter exceeds 30% of Rmax (Fig. 1c, cells A and B).
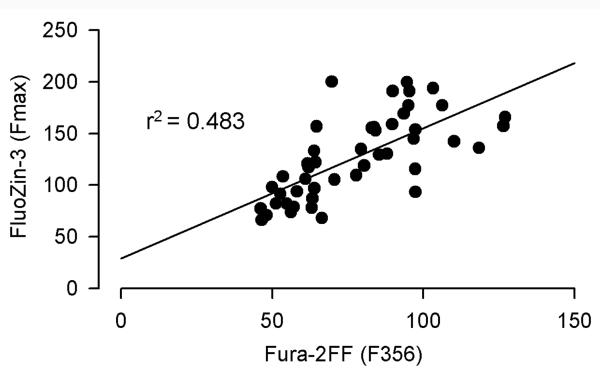
The expression of FluoZin-3 data as a percentage of Fmax does not take into account additional artifacts associated with the use of a nonratiometric [ion] indicator. Namely, fluorescence intensity is also affected by cell swelling/shrinking or dye bleaching or leakage from the cells during experimentation. Such artifacts can be accounted for by measuring fluorescence at the isosbestic point excitation of a ratiometric indicator, which provides a measure of indicator concentration independent of intracellular [ion]. Using the approach described in the Methods, the Ca2+ isosbestic point of Fura-2FF excitation was determined to be 356 nm (Supplementary Fig. 1a). To account for the artifacts associated with cell swelling, dye leakage, or bleaching, we considered expressing FluoZin-3 fluorescence data as a FluoZin-3 F488/Fura-2FF F356 ratio. This method would generate valid results only if both indicators are loaded, metabolized, and/or leaked at the same rates. If this were the case, there would be a very good correlation between FluoZin-3 Fmax and Fura-2FF F356 measured during Zn saturation. Fig. 2 shows that although on average the cells that loaded more FluoZin-3 also loaded more Fura-2FF, the correlation between Fura-2FF F356 and FluoZin-3 Fmax was not perfect, r2=0.483. Therefore, the idea of using FluoZin-3 F488/Fura-2FF F356 ratio as an index of [Zn2+]i was abandoned.
Figure 2.
Correlation between FluoZin-3 Fmax and Fura-2FF fluorescence excited at the Ca2+ isosbestic point (F356) and measured during Zn saturation in 47 hippocampal neurons (3 coverslips).
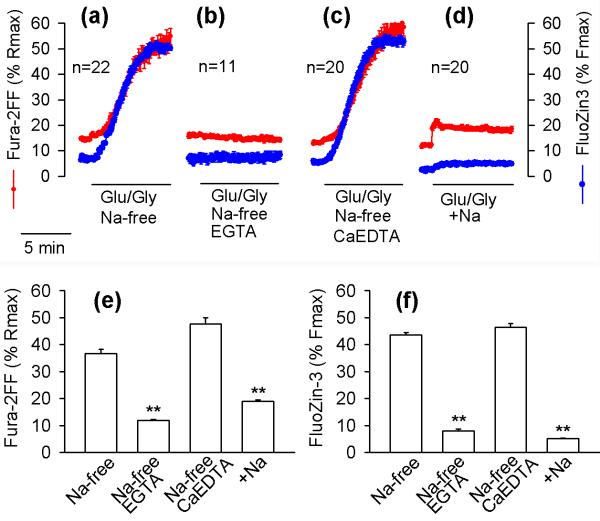
Glu/Gly-induced [Zn2+]i elevations depend on Ca2+ and Na+
Free Zn2+ available to the high affinity Zn2+ indicator FluoZin-3 in Locke's buffer was estimated to be 38 nM (for details see Methods). However, the extracellular Zn2+ did not affect the Glu/Gly-induced [Zn2+]i elevations because chelation with 1 mM CaEDTA had no effect on Glu/Gly-induced elevations of the FluoZin-3 signal (Fig. 3a,c,f). Glu/Gly-induced [Zn2+] and [Ca2+]i elevations were eliminated by preventing Ca2+ influx (Fig. 3b,e), which confirms the recent data of Dineley et al. (2008), indicating that exposure of neurons to Glu/Gly causes Zn2+ release from intracellular stores and that the Zn2+ release is triggered by Ca2+ influx. The Glu/Gly-induced [Ca2+]i elevations were greatly enhanced when extracellular Na+ was substituted with NMDG+ (Fig. 3a,e). This outcome was expected, as under such conditions the Glu/Gly-induced destabilization of Ca2+ homeostasis is exacerbated by a number of factors. These factors include a lack of Ca2+ extrusion by plasmalemmal Na+/Ca2+ exchangers, a lack of competition between Na+ and Ca2+ for NMDA and other Na+- and Ca2+-permeable channels, and an increased electrochemical driving force for Ca2+ influx due to a lack of Na+-dependent depolarization of the plasma membrane (Mattson et al. 1989; Kiedrowski 1999). Interestingly, when Na+ was present in the medium, the Glu/Gly-induced elevation of FluoZin-3 F488 signal was also greatly decreased (Fig. 3d,e,f), and further experiments were designed to address the mechanisms responsible for this outcome.
Figure 3.
Glu/Gly-induced [Zn2+]i elevations are Ca2+- and Na+-dependent. The effects of Glu/Gly on Fura-2FF (red) and FluoZin-3 (blue) fluorescence in hippocampal neurons were monitored as explained in Fig. 1, under Na+-free conditions with Na+ substituted with NMDG+ (a–c) or in the presence of 158 mM Na+ (d). In (a) and (d), 1.3 mM CaCl2 was present in the medium. In (b), Ca2+ was omitted from the medium and 0.1 mM EGTA was added. In (c), the medium contained 1.3 mM CaCl2 and 1 mM CaEDTA. The y-axes and the 5-min time bar apply to all panels. The data are means ± standard error from the indicated number of neurons monitored in a single experiment. All experiments were repeated at least 3 times with similar results. Panels (e) and (f) show Fura-2FF and FluoZin-3 signals, respectively, measured at the end of the 5th minute of Glu/Gly application. The data are means ± standard errors from 47 – 99 individual neurons (3 to 5 separate experiments). ** p < 0.01 versus Na+-free conditions; Kruskal-Wallis one-way ANOVA on Ranks followed by Dunn's test.
Na+-dependent [Zn2+]i clearance
An addition of Na+ to cells that have been exposed to Glu/Gly under Na+-free conditions (NMDG+ was removed when Na+ was added) caused a prompt decrease of the FluoZin-3 F488 signal (Fig. 4a blue trace), whereas the response in Fura-2FF F340/F480 ratio greatly varied among the cells (Fig. 4a red trace). The Na+-induced F488 decrease represented a [Zn2+]i decrease rather than a signal decrease due to cell swelling or dye leakage because a simultaneously monitored Fura-2FF F356 signal, the Ca2+ isosbestic point, was not affected (Fig. 4a green trace). Whereas the Fura2-FF F356 signal was not affected by Na+, there was a marked, 30 – 40%, drop in this signal during the first 4 minutes of Glu/Gly application. Since [Zn2+]i was increasing during that time (blue trace), one might be concerned that the drop in F356 was caused by Zn2+ binding to Fura-2FF. Such a decrease in F356 signal due to Zn2+ binding to Fura-2FF is unlikely, however, because the Zn2+ isosbestic point of Fura-2FF was found to be 364 nm (Supplementary Fig. 1a). Consequently, Zn2+ binding to Fura-2FF increases the fluorescence excited at the wavelengths lower than 364 nm, namely the F356 – the Ca2+ isosbestic point. Since the latter was decreasing, the decrease could not be caused by Zn2+ binding to the indicator. To further verify this line of reasoning, it was tested whether Zn2+ chelation with TPEN affects the F356 signal in neurons exposed to Glu/Gly. As shown in Fig. 4b, TPEN failed to affect Fura-2FF F356 signal (green trace) but as expected, caused a prompt drop in the FluoZin-3 F488 signal (blue trace). The data confirmed that the drop in the F356 signal during Glu/Gly exposure could not be caused by Zn2+ binding to Fura-2FF.
Figure 4.
Effects of Na+ and TPEN on FluoZin-3 F488 (blue), Fura-2FF F340/F380 ratio (red), and the Fura-2FF Ca2+ isosbestic point, F356 (green). (a) Hippocampal neurons co-loaded with FluoZin-3 and Fura-2FF were exposed to Glu/Gly under Na+-free conditions (Na+ substituted with NMDG+) followed by an application of 158 mM Na+ and removal of NMDG+. The data are means ± standard error from 17 neurons monitored in a single experiment. The experiment was repeated 5 times with similar results. (b) The cells were treated as in (a) except that 10 μM TPEN was applied instead of Na+. The data are means ± standard error from 22 neurons monitored in a single experiment. The experiment was repeated 5 times with similar results.
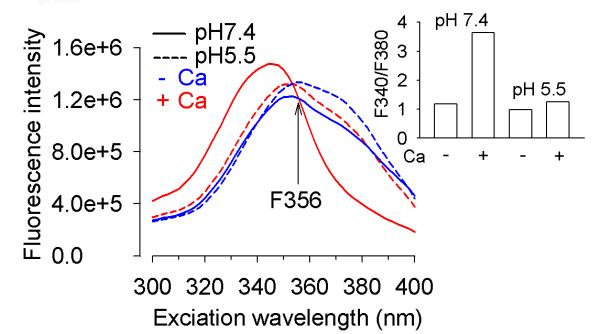
It was then considered that the F356 decrease could be caused by the major pHi drop taking place under such conditions (Kiedrowski 1999) and the impact of pH on the Fura-2FF F356 signal was examined. To this end, excitation spectra of Fura-2FF at pH 7.4 and 5.5 in vitro were obtained. The data show that the Fura-2FF fluorescence intensity excited at 356 nm was slightly higher at pH 5.5 than at pH 7.4 (Fig. 5), although the pH drop greatly compromised the ability of Fura-2FF to report [Ca2+] increase (Fig. 5, inset). Since the F356 signal was not decreased by the pH drop, the data argue against the idea that the decrease of the F356 signal during the first 4 minutes of Glu/Gly application (Fig. 4a,b) could be caused by a pHi decrease.
Figure 5.
Excitation spectra of Fura-2FF solution under nominally Ca2+-free conditions (−Ca, blue), in the presence of 30 μM CaCl2 (+Ca, red), at pH 7.4 (solid lines), and pH 5.5 (dashed lines). Note that acidification does not decrease the isosbestic point fluorescence (F356). Inset, acidification compromises the ability of Fura-2FF to report [Ca2+] increase. To calculate the F340/F380 ratio the data shown in the main figure were used. Note that the F340/F380 ratio is greatly increased by 30 μM Ca2+ (Ca+) at pH 7.4 but not at pH 5.5. The assay was repeated 3 times with similar results.
Most likely, the F356 signal decrease represents a Fura-2FF leakage. Similar leakage was observed by others (Vander Jagt et al. 2008; Medvedeva et al. 2009). It appears that this leakage occurs through pannexin-1 hemichannels which open following NMDA receptor activation and permeate a number of large molecules, including calcein (Thompson et al. 2008). One may envision that FluoZin-3 also leaks through these channels. Although such FluoZin-3 leakage is not apparent from the data (Fig. 4a,b), one has to consider that the basal FluoZin-3 F488 was so low in these experiments that any additional decrease of the signal could not be detected. Moreover, when [Zn2+]i starts to elevate, the detection of any F488 signal drop due to FluoZin-3 leakage is complicated by the simultaneous F488 signal increase due to Zn2+ binding to the indicator.
Additional experiments were performed to better understand the relationships between [Ca2+]i and [Zn2+]i changes during Glu/Gly applications. In these experiments, the neurons were exposed to Glu/Gly for 20 minutes. During the first 8 minutes, the medium was Na+-free, during the next 4 minutes, Na+ (158 mM) was present in the medium, and during the last 8 minutes, the medium was Na+-free again. Fig. 6a shows data from a representative experiment. As in the experiment shown in Fig. 1, there was a great variability in Fura-2FF signal changes (Fig. 6a upper panel). The pattern of signal changes could be divided into four categories. Of 131 cells tested in 7 experiments, in 53 cells (40%) the Fura-2FF signal increased all the way to Rmax during the first Na+-free period and remained at this level for the rest of the experiment (Fig. 6a upper, red). In 20 cells (15%), the Fura-2FF signal increased (often to Rmax) during the first Na+-free period, but then decreased when Na+ was added and remained low during the second Na+-free period (Fig. 6a upper, green). In 25 cells (19%), the Fura-2FF signal increased to variable levels during the first Na+-free period, then decreased when Na+ was added and increased again when Na+ was removed (Fig. 6a upper, blue). In 33 cells (25%), the Fura-2FF signal showed a moderate (about 50% of Rmax) increase during the first Na+-free period, decreased when Na+ was added and remained low when Na+ was removed (Fig. 6a upper, orange). To simplify the picture, average Fura-2FF and FluoZin-3 data from the representative experiment are shown in the right panel of Fig. 6a. In over 53% of neurons, the Fura-2FF signal remained low (below 30% of Rmax) during the second Na+-free period (green and orange traces). The persistence of low [Ca2+]i in these cells could be explained in terms of a decreased Ca2+ influx, increased Ca2+ efflux, or both. Since an increased Ca2+ efflux under Na+-free conditions (when plasmalemmal Na+/Ca2+exchangers do not remove Ca2+ from the cells) is unlikely, the persistence of low [Ca2+]i can be best explained in terms of a decreased Ca2+ influx as the NMDA currents are known to run down (Rosenmund and Westbrook 1993; Li et al. 2002).
Figure 6.
Effects of Na+ and DTDP on FluoZin-3 and Fura-2FF fluorescence, and on pHi. (a) The neurons were exposed to Glu/Gly and 1 mM CaEDTA under Na+-free conditions (Na+ substituted with NMDG+). Where indicated, 158 mM Na+ (Na) was transiently applied while NMDG+ was removed. The patterns of Fura-2FF fluorescence change could be divided into four color-coded categories that are discussed in the text. The left panel shows Fura-2FF and FluoZin-3 fluorescence changes in individual neurons and the right panel shows average fluorescence changes in neurons representing each category. The experiment was repeated 7 times with similar results. (b) Experiment analogous to the one shown in (a) except that 100 μM DTDP was added during the second Na+-free period, where indicated. The data are means ± standard error from 16 neurons. The experiment was repeated 4 times with similar results. (c) Experiment analogous to the one shown in (b) except that pHi (BCECF F488/F440 ratio) was monitored. The data are means ± standard error from 23 neurons. The experiment was repeated 3 times with similar results.
The bottom panel of Fig. 6a shows that the FluoZin-3 signal increased during the first Na+-free period and decreased following Na+ application, as expected. Interestingly, during the second Na+-free period, the FluoZin-3 signal remained low in all neurons despite the above discussed variability in the Fura-2FF signal among the neurons. Since virtually all cells failed to increase [Zn2+]i during the second Na+-free period (Fig. 6a bottom), it appears that either the intracellular store from which Zn2+ was released during the first Na+-free period had been depleted or that the mechanism involved in Zn2+ release is inactive during the second Na+-free period. To clarify this, it was tested whether an oxidizing agent, 2,2'dithiopyridine (DTDP) known to induce Zn2+ release from internal stores (Aizenman et al. 2000), is able to elevate [Zn2+]i during the second Na+-free period. As shown in Fig. 6b, DTDP application promptly increased the FluoZin-3 signal, indicating that prior to DTDP addition the intracellular stores still contained Zn2+.
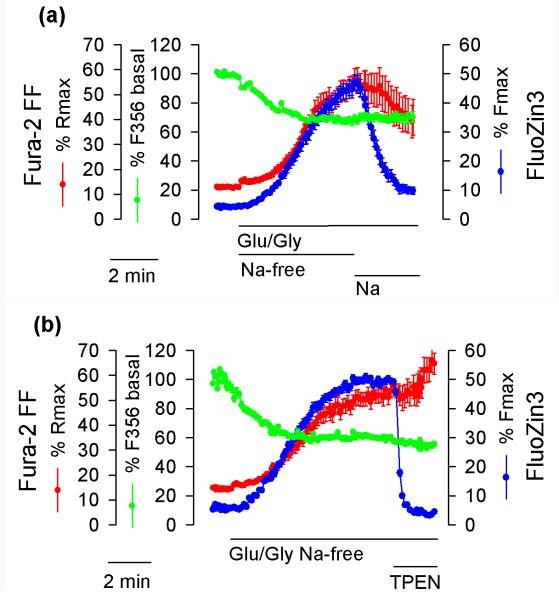
pHi increase plays a role in Na+-dependent [Zn2+]i clearance
A pHi drop is expected to promote Zn2+ release from intracellular thiols (Li et al. 1954) and other ligand types as well as promote Zn2+ efflux from organelles, for example from the Golgi apparatus, by activating H+/Zn2+ exchange (Ohana et al. 2009). Therefore, it was considered that the difference in [Zn2+]i elevation between the first and the second Na+-free period (Fig. 6b) could be explained in terms of a different pHi. To this end, using BCECF fluorescence, pHi was monitored under analogous experimental conditions. Glu/Gly application profoundly decreased BCECF F488/F440 ratio, indicating a pHi decrease, and Na+ application caused the pHi to increase. However, during the second Na+-free period, the Glu/Gly-induced pHi drop was less profound than during the first Na+-free period (Fig. 6c). This result may be interpreted to indicate that the pHi decrease during the second Na+-free period was insufficient to elevate [Zn2+]i. No pHi drop was associated with DTDP application (Fig. 6c), confirming that the mechanism of [Zn2+]i elevation induced by this agent represents oxidation (Aizenman et al. 2000), not a pHi decrease.
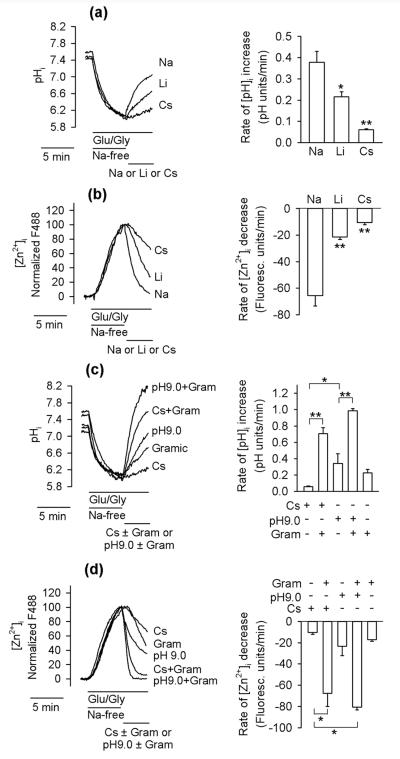
To clarify the role of pHi in [Zn2+]i changes, the relationships between pHi and [Zn2+]i were studied in a greater detail. To this end, the effects of Na+ versus Li+ and Cs+ on the rates of [Zn2+]i drop and pHi increase were compared. The efficacy of these cations in elevating pHi (Fig. 7a) and clearing [Zn2+]i (Fig. 7b) was similar, namely, Na+>Li+>Cs+. This result confirms that pHi fluctuations may play a role in the mechanism of [Zn2+]i changes. To further explore this possibility, gramicidin was used as an experimental tool. Gramicidin is an antibiotic that forms channels that permeate small monovalent but not divalent cations (Hladky and Haydon 1972) and therefore when pHi is low, gramicidin is expected to increase it by promoting H+ efflux. Indeed, as shown in Fig. 7c, the rate of pHi elevation increased when gramicidin was added. The rate of pHi elevation increased even more when gramicidin was co-applied with Cs+ (NMDG+ was removed when Cs+ was added). Gramicidin also greatly increased the rate of pHi elevation when extacellular pH was increased from 7.4 to 9.0 (Fig. 7c). As shown in Fig. 7d, the experimental maneuvers leading to the pHi increase also resulted in [Zn2+]i clearance and the efficacy of these maneuvers in provoking [Zn2+]i decrease and pHi increase was similar, namely, gramicidin + pH 9.0 > gramicidin + Cs+ > pH 9.0 alone > gramicidin alone.
Figure 7.
The relationships between pHi and [Zn2+]i in neurons exposed to Glu/Gly. (a) Effects of Na+, Li+, and Cs+ on pHi in neurons treated with Glu/Gly. During the Na+-free period, Na+ was substituted with NMDG+. The left panel shows averages from 17 to 24 neurons monitored in a single experiment. The right panel shows average rates of pHi increase calculated from three such experiments. **p < 0.01, *p < 0.05; one-way ANOVA followed by Student–Newman–Keuls test. (b) Analogous experiments as those shown in (a) except that [Zn2+]i was monitored. **p < 0.01, one-way ANOVA followed by Student– Newman–Keuls test. (c) Analogous experiments as those shown in (a) except that the effects of cesium ± gramicidin (Gram; 5 µM) or pH 9.0 ± gramicidin on the rate of pHi increase were studied. *p < 0.05; Kruskal–Wallis one-way ANOVA on Ranks followed by Dunn's test. (d) Analogous experiments as those shown in (c) except that [Zn2+]i was monitored. **p < 0.01, one-way ANOVA followed by Student–Newman– Keuls test.
The faster rate of gramicidin-induced pHi increase in the presence of Cs+ than in the presence of NMDG+ was expected. The gramicidin-formed channels do not permeate NMDG+. Therefore, K+ plus H+ efflux through the channels generates electrical potential that hampers exit of these ions. This does not occur in the presence of Cs+ (or another cation that permeates gramicidin channels) because the electrical potential generated by the K+ plus H+ efflux is dissipated by the Cs+ influx via the gramicidin channels.
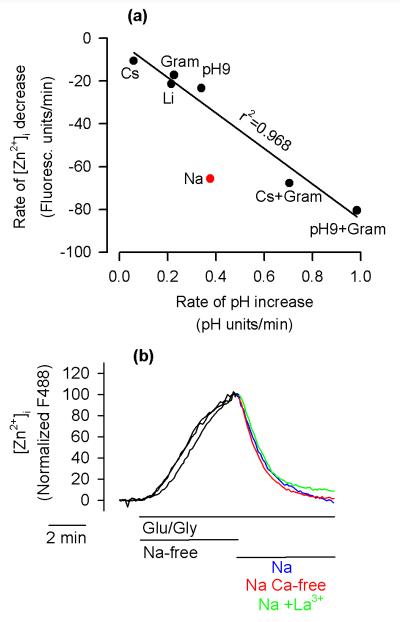
The data shown in Fig. 7 indicate that pHi fluctuations play a role in [Zn2+]i changes. However, should the pHi increase be the sole mechanism responsible for the clearance of cytosolic Zn2+, there should be a perfect correlation between the rate of pHi increase and the rate of [Zn2+]i drop. As shown in Fig. 8a, this was indeed the case for all experimental conditions except for the Na+ application. The rate of Na+-induced [Zn2+]i decrease was about twice as fast as expected from the rate of the Na+-induced pHi increase. This suggests that while the pHi increase plays a role in Na+-dependent [Zn2+]i decrease, Na+ also promotes additional pHi-independent mechanisms of cytosolic Zn2+clearance.
Figure 8.
Na+-dependent [Zn2+]i clearance involves pHi-dependent and pHi-independent mechanisms. (a) Correlation between the rate of pHi increase and the rate of [Zn2+]i decrease using the data from Fig. 7(a–d) (right panels). The linear regression with r2 = 0.968 is calculated for all the data except Na+ (the red point). Note that the rate of Na+-induced [Zn2+]i drop is about twice as fast as could be predicted based on the rate of Na+-induced pHi increase. (b) The Na+-dependent [Zn2+]i clearance (Na, blue trace) is not inhibited by 10 µM La3+ (Na + La3+, green trace) and is fully active under Ca-free conditions (Na Ca-free, red trace). The experimental details are the same as in Fig. 4(a). The data are averages from 18 to 23 neurons. The experiments were repeated four times with similar results.
In cortical neurons, Qin et al. (2008) recently described a Na+-dependent mechanism of Zn2+ efflux, which requires the presence of extracellular Ca2+ and is inhibited by 10 μM La3+. To test whether this mechanism plays a role in Na+-induced cytosolic Zn2+ clearance, the impact of 10 μM La3+ and the Ca2+-free medium on the rate of Na+-dependent [Zn2+]i decrease was tested. However as shown in Fig. 8b, the rate of the Na+-dependent [Zn2+]i decrease was not affected by either treatment.
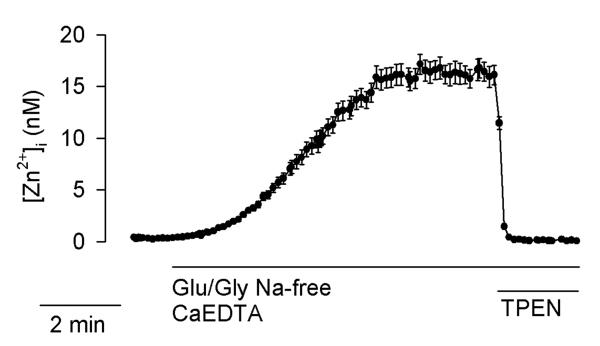
Glu/Gly-induced [Zn2+]i elevations do not exceed 20 nM
In an attempt to calibrate FluoZin-3 signals in terms of [Zn2+]i, possible complications from overloading neurons with indicator (Dineley et al. 2002) were minimized by loading the neurons with very low concentrations of FluoZin-3 AM (0.1 μM) for only 5–6 minutes. Using the calibration approach described in Methods, [Zn2+]i elevations appeared to reach the low nanomolar range (Fig. 9). However, as mentioned earlier, FluoZin-3 most likely leaks from the Glu/Gly-treated neurons. Therefore, Fmax values measured at the end of the experiments and used to calibrate the data are likely underestimated. Nevertheless, such calibration defines the upper limit of [Zn2+]i increase in these experiments. While the exact value of [Zn2+]i elevation is not known, one can be confident that it does not exceed 20 nM (Fig. 9).
Figure 9.
FluoZin-3 fluorescence data calibrated for [Zn2+]i. Experimental conditions were analogous to those described in Fig. 1. The data are means ± standard error from 20 cells monitored in a single experiment. The experiment was repeated 5 times with similar results.
Discussion
Earlier work showed that reversal of the Na+ concentration gradient across the plasma membrane greatly enhances [Zn2+]i elevations resulting from the applications of extracellular Zn2+ (Sensi et al. 1997; Cheng and Reynolds 1998), suggesting that Na+/Zn2+ exchanger may mediate Zn2+ influx under such conditions. More recently, Ohana et al. (2004) reported that under physiological conditions when extracellular Na+ is high and intracellular low, such Na+/Zn2+ exchanger extrudes Zn2+ from the cells. Additional mechanisms of Na+-dependent Zn2+ efflux that are inhibited by La3+ and require extracellular Ca2+ for operation were also characterized (Qin et al. 2008), but those do not seem to play a role in the mechanism of Na+-dependent Zn2+ clearance reported here (Fig. 8b).
The major finding of the present report is that Na+-dependent [Zn2+]i clearance may result not only from activation of plasmalemmal Na+/Zn2+ exchangers but also from Na+-induced pHi increase. The pathways of Na+-dependent pHi regulation in neurons are well recognized and include plasmalemmal Na+/H+ exchangers (Tolkovsky and Richards 1987; Raley-Susman et al. 1991) and Na+-dependent Cl−-HCO3− exchangers (Schwiening and Boron 1994). Application of Na+, Li+ or Cs+ to neurons that had been exposed to Glu/Gly under Na+-free conditions (Na+ substituted with NMDG+) may also increase pHi due to Na+, Li+ or Cs+ influx and K+ plus H+ efflux via NMDA and other channels that permeate these cations - a mechanism analogous to the above discussed acceleration of pHi increase by gramicidin co-applied with Cs+.
The Glu/Gly-induced pHi drop depends on Ca2+ influx because activation of NMDA receptors under Ca2+-free conditions fails to induce any major drop in pHi (Hartley and Dubinsky 1993; Irwin et al. 1994; Kiedrowski 1999; Wu et al. 1999). Since the present data show the correlation between [Zn2+]i and pHi (Fig. 8a), one may conclude that the pHi drop plays a critical role in the mechanism of Ca2+-dependent [Zn2+]i elevation in this experimental model.
Several mechanisms may participate in the acidification-induced increase of [Zn2+]i. For example, Zn2+ may be displaced from a number of intracellular binding sites as intracellular Zn2+ is buffered by interactions of the ion with a variety of ligand types, including sulfur from cysteine (glutathione, metallothionein), nitrogen from histidine, and oxygen from, for example, glutamate or aspartate (Berg and Shi 1996), and also phosphate (Binder et al. 2001). Moreover, cytosolic acidification favors Zn2+ release from organelles that use the H+/Zn2+ exchange to store Zn2+ (Ohana et al. 2009). These organelles are expected to release Zn2+ when pHi drops sufficiently. The resulting [Zn2+]i increase is expected to persist at low pHi because low pHi compromises ligand-dependent Zn2+ buffering (Li et al. 1954; Sensi et al. 2003). On the other hand, a pHi increase favors Zn2+ re-loading to the organelles, which is supported by the data showing that re-loading of the organelles with Zn2+ takes place at pH 7.4 but not 6.0 (Colvin 2002).
Whereas the present author observed only minor [Zn2+]i increases in neurons exposed to Glu/Gly and Na+ (Fig. 3d), Dineley et al. (2008) found major statistically significant elevations of FluoZin-3 signal in cortical neurons exposed to Glu/Gly despite the presence of Na+ in the medium. This discrepancy could be due in part to the fact that in the cortical neurons used by Dineley et al. (2008), [Ca2+]i increased to Fura-2FF-saturating levels within 3 minutes of Glu/Gly application, but much smaller increases of the Fura-2FF signal were observed in the majority of hippocampal neurons used in the present study (Fig. 3d). As the release of Zn2+ from intracellular stores is triggered by Ca2+ influx (Dineley et al. 2008), one would expect that neurons that increase [Ca2+]i sooner and faster also show a faster rate of [Zn2+]i elevations. Another reason that the FluoZin-3 signal increase in Fig. 3d is small is that the data are expressed as the percentage of Fmax. The practical advantage of such data presentation is that it normalizes for differences in FluoZin-3 loading among the cells. These differences, if overlooked, may lead to data misinterpretation because as shown in Fig. 1c, [Zn2+]i seemingly elevates at a faster rate in cells that loaded higher levels of FluoZin-3. Another reason for not using the common way of expressing FluoZin-3 data as the F488/F488basal ratio was that this author intended to measure the FluoZin-3 Fmax at the end of the experiments. Therefore, the cells needed to be loaded with very low concentrations of FluoZin-3 (see Methods) so that the F488 light intensity during the Fmax measurements was within the dynamic range of the camera. After such limited FluoZin-3 loading, F488basal was very low, close to or at background levels, and subtracting the background F488 from such low F488basal values often yielded zero. Loading more FluoZin-3 to the cells (to increase F488basal) resulted in Fmax values being higher than the maximal fluorescence intensity that could be measured using our Attofluor instrument. Presenting F488 data as a percentage of Fmax (to normalize for differences in FluoZin-3 loading) as we did is not an ideal solution either. As indicated in the Results, the Fmax measured at the end of the experiments is most likely decreased due to dye leakage similar to that observed for Fura-2FF (Fig. 4a,b, green traces). Moreover, phenomena such as bleaching and/or cell swelling also decrease Fmax. These problems could be avoided by using a ratiometric Zn2+ indicator with a high affinity for zinc. Unfortunately, the ratiometric Zn2+ indicator we had access to, zinbo-5 (Taki et al. 2004), could not be used to monitor [Zn2+]i in this project. Zinbo-5 binds Zn2+ and permeates the plasma membrane. We found that it acts as an ionophore transporting Zn2+ across the plasma membrane according to the ion concentration gradient (data not shown). A similar problem was reported for another fluorescent Zn2+ indicator, zinquin (Snitsarev et al. 2001).
The current work is a follow up to our earlier report on the impact of Na+ on Glu/Gly-induced [Ca2+]i elevations (Kiedrowski 1999). At the time of that publication, Ca2+-dependent and Glu/Gly-induced Zn2+ release from intracellular stores was not known. Therefore, the extent to which Fura-2 signals in that study were affected by [Zn2+]i versus [Ca2+]i elevations was not addressed. Since Glu/Gly-induced [Ca2+]i elevations in neurons saturate Fura-2, the present study employed a low affinity Ca2+ indicator, Fura-2FF, which also binds Zn2+ with high affinity (Hyrc et al. 2000). The data indicate that Glu/Gly-induced [Zn2+]i elevations did not exceed 20 nM (Fig. 9). However, even much higher Zn2+ concentrations elevated the F340/F380 ratio to no more than 30% of Rmax (Fig. 1d; Supplementary Fig. 1b). The data indicate that the increase of Fura-2FF F340/F380 ratio above 30% of Rmax represents [Ca2+]i elevations.
In conclusion, the present study found that the Glu/Gly-induced pHi decrease plays a critical role in Ca2+-dependent [Zn2+]i elevations and suggests that the major extra- and intracellular acidification taking place in the ischemic brain (Smith et al. 1986) may destabilize Zn2+ homeostasis. The Na+-dependent Zn2+ clearance may be important for the restoration of Zn2+ homeostasis during brain recovery from ischemia.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health Grant R21 NS065305 and American Heart Association Grant-in-Aid 0855825G and was presented on poster 56.11, 2010 at the Society for Neuroscience Meeting. The author is grateful to Dr. C. William Shuttleworth for his critical reading of the manuscript, Dr. Peter G.W. Gettins for the access to PTI QuantaMaster spectrofluorometer, and to Dr. Thomas V. O'Halloran for providing zinbo-5. The author declares his involvement in the SPOT™ culture kit production at the University of Illinois at Chicago Research Resources Center.
Abbreviations used
- BCECF
2',7'-bis-(2-carboxyethyl)-5(6)-carboxyfluorescein
- [Ca2+]i and [Zn2+]i
intracellular Ca2+ and Zn2+ concentration
- CaEDTA
calcium EDTA
- DTDP
2,2'dithiopyridine
- FCCP
carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone
- F340, F356, F380, F440, F488
fluorescence emitted after 340, 356, 380, 440, and 488 nm excitation
- Fmin and Fmax
minimal and maximal F488
- FURD
5-fluoro-2'-deoxyuridine
- Glu/Gly
cell exposure to 100 μM glutamate plus 10 μM glycine
- MK-801
dizocilpine
- NMDG
N-methyl-D-glucamine
- pHi
intracellular pH
- Rmax
maximal F340/F380 ratio
- TPEN
N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine
References
- Aizenman E, Stout AK, Hartnett KA, Dineley KE, McLaughlin B, Reynolds IJ. Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release. J. Neurochem. 2000;75:1878–1888. doi: 10.1046/j.1471-4159.2000.0751878.x. [DOI] [PubMed] [Google Scholar]
- Berg JM, Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science. 1996;271:1081–1085. doi: 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]
- Beyersmann D, Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals. 2001;14:331–341. doi: 10.1023/a:1012905406548. [DOI] [PubMed] [Google Scholar]
- Binder H, Arnold K, Ulrich AS, Zschornig O. Interaction of Zn2+ with phospholipid membranes. Biophys. Chem. 2001;90:57–74. doi: 10.1016/s0301-4622(01)00130-2. [DOI] [PubMed] [Google Scholar]
- Bush AI. The metallobiology of Alzheimer's disease. Trends Neurosci. 2003;26:207–214. doi: 10.1016/S0166-2236(03)00067-5. [DOI] [PubMed] [Google Scholar]
- Calderone A, Jover T, Mashiko T, Noh KM, Tanaka H, Bennett MV, Zukin RS. Late calcium EDTA rescues hippocampal CA1 neurons from global ischemia-induced death. J. Neurosci. 2004;24:9903–9913. doi: 10.1523/JNEUROSCI.1713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y, Fu D. Kinetic study of the antiport mechanism of an Escherichia coli zinc transporter, ZitB. J. Biol. Chem. 2004;279:12043–12050. doi: 10.1074/jbc.M313510200. [DOI] [PubMed] [Google Scholar]
- Cheng C, Reynolds IJ. Calcium-sensitive fluorescent dyes can report increases in intracellular free zinc concentration in cultured forebrain neurons. J. Neurochem. 1998;71:2401–2410. doi: 10.1046/j.1471-4159.1998.71062401.x. [DOI] [PubMed] [Google Scholar]
- Choi DW, Yokoyama M, Koh J. Zinc neurotoxicity in cortical cell culture. Neuroscience. 1988;24:67–79. doi: 10.1016/0306-4522(88)90312-0. [DOI] [PubMed] [Google Scholar]
- Colvin RA. Zinc inhibits Ca2+ transport by rat brain Na+/Ca2+ exchanger. Neuroreport. 1998;9:3091–3096. doi: 10.1097/00001756-199809140-00032. [DOI] [PubMed] [Google Scholar]
- Colvin RA. pH dependence and compartmentalization of zinc transported across plasma membrane of rat cortical neurons. Am. J. Physiol. Cell Physiol. 2002;282:C317–329. doi: 10.1152/ajpcell.00143.2001. [DOI] [PubMed] [Google Scholar]
- Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J. Biol. Chem. 2006;281:24085–24089. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- Devinney MJ, 2nd, Reynolds IJ, Dineley KE. Simultaneous detection of intracellular free calcium and zinc using fura-2FF and FluoZin-3. Cell Calcium. 2005;37:225–232. doi: 10.1016/j.ceca.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Dietz RM, Weiss JH, Shuttleworth CW. Zn2+ influx is critical for some forms of spreading depression in brain slices. J. Neurosci. 2008;28:8014–8024. doi: 10.1523/JNEUROSCI.0765-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineley KE, Malaiyandi LM, Reynolds IJ. A reevaluation of neuronal zinc measurements: artifacts associated with high intracellular dye concentration. Mol. Pharmacol. 2002;62:618–627. doi: 10.1124/mol.62.3.618. [DOI] [PubMed] [Google Scholar]
- Dineley KE, Devinney MJ, 2nd, Zeak JA, Rintoul GL, Reynolds IJ. Glutamate mobilizes [Zn2+] through Ca2+-dependent reactive oxygen species accumulation. J. Neurochem. 2008;106:2184–2193. doi: 10.1111/j.1471-4159.2008.05536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufner-Beattie J, Wang F, Kuo YM, Gitschier J, Eide D, Andrews GK. The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J. Biol. Chem. 2003;278:33474–33481. doi: 10.1074/jbc.M305000200. [DOI] [PubMed] [Google Scholar]
- Eide DJ. Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta. 2006;1763:711–722. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Emmetsberger J, Mirrione MM, Zhou C, Fernandez-Monreal M, Siddiq MM, Ji K, Tsirka SE. Tissue plasminogen activator alters intracellular sequestration of zinc through interaction with the transporter ZIP4. J. Neurosci. 2010;30:6538–6547. doi: 10.1523/JNEUROSCI.6250-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaither LA, Eide DJ. Functional expression of the human hZIP2 zinc transporter. J. Biol. Chem. 2000;275:5560–5564. doi: 10.1074/jbc.275.8.5560. [DOI] [PubMed] [Google Scholar]
- Gee KR, Zhou ZL, Ton-That D, Sensi SL, Weiss JH. Measuring zinc in living cells. A new generation of sensitive and selective fluorescent probes. Cell Calcium. 2002;31:245–251. doi: 10.1016/S0143-4160(02)00053-2. [DOI] [PubMed] [Google Scholar]
- Guffanti AA, Wei Y, Rood SV, Krulwich TA. An antiport mechanism for a member of the cation diffusion facilitator family: divalent cations efflux in exchange for K+ and H+ Mol. MicroBiol. 2002;45:145–153. doi: 10.1046/j.1365-2958.2002.02998.x. [DOI] [PubMed] [Google Scholar]
- Gyulkhandanyan AV, Lee SC, Bikopoulos G, Dai F, Wheeler MB. The Zn2+-transporting pathways in pancreatic beta-cells: a role for the L-type voltage-gated Ca2+ channel. J. Biol. Chem. 2006;281:9361–9372. doi: 10.1074/jbc.M508542200. [DOI] [PubMed] [Google Scholar]
- Hartley Z, Dubinsky JM. Changes in intracellular pH associated with glutamate excitotoxicity. J. Neurosci. 1993;13:4690–4699. doi: 10.1523/JNEUROSCI.13-11-04690.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladky SB, Haydon DA. Ion transfer across lipid membranes in the presence of gramicidin A. I. Studies of the unit conductance channel. Biochim Biophys. Acta. 1972;274:294–312. doi: 10.1016/0005-2736(72)90178-2. [DOI] [PubMed] [Google Scholar]
- Hyrc KL, Bownik JM, Goldberg MP. Ionic selectivity of low-affinity ratiometric calcium indicators: mag- Fura-2, Fura-2FF and BTC. Cell Calcium. 2000;27:75–86. doi: 10.1054/ceca.1999.0092. [DOI] [PubMed] [Google Scholar]
- Irwin RP, Lin S-Z, Long RT, Paul SM. N-methyl-D-aspartate induces a rapid, reversible, and calcium dependent intracellular acidosis in cultured fetal rat hippocampal neurons. J. Neurosci. 1994;14:1352–1357. doi: 10.1523/JNEUROSCI.14-03-01352.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiedrowski L. N-methyl-D-aspartate excitotoxicity: relationships among plasma membrane potential, Na+/Ca2+ exchange, mitochondrial Ca2+ overload, and cytoplasmic concentrations of Ca2+, H+, and K+ Mol. Pharmacol. 1999;56:619–632. doi: 10.1124/mol.56.3.619. [DOI] [PubMed] [Google Scholar]
- Koh J-Y, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- Lee JM, Zipfel GJ, Park KH, He YY, Hsu CY, Choi DW. Zinc translocation accelerates infarction after mild transient focal ischemia. Neuroscience. 2002;115:871–878. doi: 10.1016/s0306-4522(02)00513-4. [DOI] [PubMed] [Google Scholar]
- Lee JY, Cole TB, Palmiter RD, Koh JY. Accumulation of zinc in degenerating hippocampal neurons of ZnT3-null mice after seizures: evidence against synaptic vesicle origin. J. Neurosci. 2000;20:RC79. doi: 10.1523/JNEUROSCI.20-11-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Chen N, Luo T, Otsu Y, Murphy TH, Raymond LA. Differential regulation of synaptic and extra-synaptic NMDA receptors. Nat. Neurosci. 2002;5:833–834. doi: 10.1038/nn912. [DOI] [PubMed] [Google Scholar]
- Li NC, Gawron O, Bascuas G. Stability of zinc complexes with glutathione and oxidized glutathione. J. Am. Chem. Soc. 1954;76:225–229. [Google Scholar]
- Mattson MP, Guthrie PB, Kater SB. A role for Na+-dependent Ca2+ extrusion in protection against neuronal excitotoxicity. FASEB J. 1989;3:2519–2526. doi: 10.1096/fasebj.3.13.2572500. [DOI] [PubMed] [Google Scholar]
- Medvedeva YV, Lin B, Shuttleworth CW, Weiss JH. Intracellular Zn2+ accumulation contributes to synaptic failure, mitochondrial depolarization, and cell death in an acute slice oxygen-glucose deprivation model of ischemia. J. Neurosci. 2009;29:1105–1114. doi: 10.1523/JNEUROSCI.4604-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohana E, Segal D, Palty R, Ton-That D, Moran A, Sensi SL, Weiss JH, Hershfinkel M, Sekler I. A sodium zinc exchange mechanism is mediating extrusion of zinc in mammalian cells. J Biol Chem. 2004;279:4278–4284. doi: 10.1074/jbc.M309229200. [DOI] [PubMed] [Google Scholar]
- Ohana E, Hoch E, Keasar C, Kambe T, Yifrach O, Hershfinkel M, Sekler I. Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J. Biol. Chem. 2009;284:17677–17686. doi: 10.1074/jbc.M109.007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD. Protection against zinc toxicity by metallothionein and zinc transporter 1. Proc. Natl. Acad. Sci. U S A. 2004;101:4918–4923. doi: 10.1073/pnas.0401022101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Findley SD. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. Embo J. 1995;14:639–649. doi: 10.1002/j.1460-2075.1995.tb07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Huang L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Arch. 2004;447:744–751. doi: 10.1007/s00424-003-1070-7. [DOI] [PubMed] [Google Scholar]
- Qian J, Xu K, Yoo J, Chen T, Andrews GK, Noebels J. Knockout of Zn transporters Zip-1 and Zip-3 attenuates seizure-induced CA1 neurodegeneration. J. Neurosci. 2011;31:97–104. doi: 10.1523/JNEUROSCI.5162-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Thomas D, Fontaine CP, Colvin RA. Mechanisms of Zn2+ efflux in cultured cortical neurons. J. Neurochem. 2008;107:1304–1313. doi: 10.1111/j.1471-4159.2008.05700.x. [DOI] [PubMed] [Google Scholar]
- Raley-Susman KM, Cragoe EJ, Jr., Sapolsky RM, Kopito RR. Regulation of intracellular pH in cultured hippocampal neurons by an amilioride-insensitive Na+/H+ exchanger. J. Biol. Chem. 1991;266:2739–2745. [PubMed] [Google Scholar]
- Rosenmund C, Westbrook GL. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993;10:805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- Schwiening CJ, Boron WF. Regulation of intracellular pH in pyramidal neurones from the rat hippocampus by Na+-dependent Cl−-HCO3-exchange. J. Physiol. 1994;475:59–67. doi: 10.1113/jphysiol.1994.sp020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensi SL, Canzoniero LM, Yu SP, Ying HS, Koh JY, Kerchner GA, Choi DW. Measurement of intracellular free zinc in living cortical neurons: routes of entry. J. Neurosci. 1997;17:9554–9564. doi: 10.1523/JNEUROSCI.17-24-09554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensi SL, Paoletti P, Bush AI, Sekler I. Zinc in the physiology and pathology of the CNS. Nat Rev Neurosci. 2009;10:780–791. doi: 10.1038/nrn2734. [DOI] [PubMed] [Google Scholar]
- Sensi SL, Ton-That D, Sullivan PG, Jonas EA, Gee KR, Kaczmarek LK, Weiss JH. Modulation of mitochondrial function by endogenous Zn2+ pools. Proc. Natl. Acad. Sci. U S A. 2003;100:6157–6162. doi: 10.1073/pnas.1031598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline CT, Behrens MM, Choi DW. Zinc-induced cortical neuronal death: contribution of energy failure attributable to loss of NAD+ and inhibition of glycolysis. J. Neurosci. 2000;20:3139–3146. doi: 10.1523/JNEUROSCI.20-09-03139.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, von Hanwehr R, Siesjo BK. Changes in extra- and intracellular pH in the brain during and following ischemia in hyperglycemic and in moderately hypoglycemic rats. J. Cereb. Blood Flow Metab. 1986;6:574–583. doi: 10.1038/jcbfm.1986.104. [DOI] [PubMed] [Google Scholar]
- Snitsarev V, Budde T, Stricker TP, Cox JM, Krupa DJ, Geng L, Kay AR. Fluorescent detection of Zn2+-rich vesicles with Zinquin: mechanism of action in lipid environments. Biophys. J. 2001;80:1538–1546. doi: 10.1016/S0006-3495(01)76126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki M, Wolford JL, O'Halloran TV. Emission ratiometric imaging of intracellular zinc: design of a benzoxazole fluorescent sensor and its application in two-photon microscopy. J. Am. Chem. Soc. 2004;126:712–713. doi: 10.1021/ja039073j. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, MacDonald JF, MacVicar BA. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science. 2008;322:1555–1559. doi: 10.1126/science.1165209. [DOI] [PubMed] [Google Scholar]
- Tolkovsky AM, Richards CD. Na+/H+ exchange is the major mechanism of pH regulation in cultured sympathetic neurons: measurements in single cell bodies and neurites using a fluorescent pH indicator. Neuroscience. 1987;22:1093–1102. doi: 10.1016/0306-4522(87)92984-8. [DOI] [PubMed] [Google Scholar]
- Tonder N, Johansen FF, Frederickson CJ, Zimmer J, Diemer NH. Possible role of zinc in the selective degeneration of dentate hilar neurons after cerebral ischemia in the adult rat. Neurosci. Lett. 1990;109:247–252. doi: 10.1016/0304-3940(90)90002-q. [DOI] [PubMed] [Google Scholar]
- Vander Jagt TA, Connor JA, Shuttleworth CW. Localized loss of Ca2+ homeostasis in neuronal dendrites is a downstream consequence of metabolic compromise during extended NMDA exposures. J. Neurosci. 2008;28:5029–5039. doi: 10.1523/JNEUROSCI.5069-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ML, Chen JH, Chen WH, Chen YJ, Chu KC. Novel role of the Ca2+-ATPase in NMDA-induced intracellular acidification. Am. J. Physiol. 1999;277:C717–727. doi: 10.1152/ajpcell.1999.277.4.C717. [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Koh J, Choi DW. Brief exposure to zinc is toxic to cortical neurons. Neurosci. Lett. 1986;71:351–355. doi: 10.1016/0304-3940(86)90646-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.