Abstract
Candida albicans is the leading fungal pathogen causing invasive disease in immunocompromised patients including the neonate. A reliable animal model for disseminated candidiasis in the neonate is needed to study the unique aspects of this host-pathogen interaction. To establish such a model, two day old BALB/c mouse pups were given intraperitoneal injections with varied inocula of C. albicans or saline control. Pups were examined every 3-8 hours for death. Surviving pups were sacrificed at 72 hours. Kidney, lung, spleen, liver and brain were homogenized and plated for colony counts and/or fixed for histological staining. Intraperitoneal injection of C. albicans led to mortality in a dose-dependent fashion. Disseminated infection was confirmed by colony counts of homogenized kidney, lung, and brain, as well as by histological examination. Infection with a C. albicans mutant lacking the cell surface adhesin, Als3p, led to significant reduction in mortality relative to wild-type (P = 0.03). This model will be useful to study the unique aspects of antifungal defense in a neonatal host and will provide a means to test novel therapeutic strategies.
Introduction
Candida albicans is the leading fungal pathogen in immunocompromised patients (1), and the third most common pathogen overall causing late-onset sepsis in premature infants (2). Well described risk factors for disseminated disease in this population include gastrointestinal (GI) colonization, prolonged hospitalization, broad spectrum antibiotic use, central venous catheters and parenteral nutrition (3-5). Colonization of preterm infants has been documented to occur through both vertical (from mother to infant) and horizontal routes (6). Even with antifungal therapy, candidiasis is often fatal among premature infants and is associated with neurodevelopmental impairment among survivors. Follow-up examinations at 18-22 months corrected age show significant increases in rates of cerebral palsy, blindness, deafness, and mental retardation (7). The severity of these infections has led to development of prophylactic strategies to reduce colonization and limit invasive disease (8-11). Although effective, these strategies require prolonged exposure to antifungal agents with their associated risks (12-13). Novel therapeutic strategies are needed to improve these outcomes. However, the mechanisms leading to immune compromise in the neonate are likely different from other patient populations at risk (14). An animal model of disseminated candidiasis in a neonatal host is therefore needed to recapitulate unique aspects of this host-pathogen interaction.
Murine models have been a feasible and reliable method to study the pathogenesis of candidiasis, but have their limitations. Unlike humans, the mouse is not naturally colonized in the GI tract with C. albicans. In order to achieve persistent colonization, the animals must be treated with antibiotics and/or immunosuppressive agents, and dissemination is uncommon (15-16). Two strategies have been employed to circumvent these issues. The most widely used model involves intravenous injection of adult animals via the lateral tail vein. This model has been used extensively to study virulence properties of the organism and immunological adaptations of the animal in response to hematogenous infection (15, 17). A second strategy is gastric inoculation of neonatal mice, which leads to persistent, albeit decreasing colonization over time with some dissemination and mortality. However, mortality was strain dependent and amounted to approximately 50% or less in these studies (18-19).
In the present study, we sought to develop a model that would result in more reliable disease burden while still maintaining some clinical relevance. The goal was to avoid pharmacological immunosuppression so as to allow inquiry into inherent immune status in infected neonates, as well as to obviate the variability and technical challenges inherent to GI colonization. GI pathology including abdominal surgery is an independent risk factor for disseminated disease (3, 5). When the integrity of the bowel mucosa is compromised, translocation of the organism is likely facilitated with spread via the enteric or lymphatic circulation. Direct inoculation of the peritoneum can also occur in the setting of bowel perforation with similar routes to dissemination. This study was structured to test the hypothesis that disseminated disease and subsequent mortality could be induced in a reliable and reproducible fashion by the intraperitoneal (i.p.) route in neonatal mice without additional immunosuppression. This model provides the framework to study the unique host-pathogen interface in neonatal candidiasis as well as the development of novel therapeutic strategies.
Methods
Strains and Media
C. albicans strains used in this study include wild type strain SC5314 (20) and strain 1843 containing a homozygous deletion in ALS3 (iro1-ura3Δ ∷ λimm434/iro1-ura3Δ∷λimm434als3laΔ/als3saΔ-URA3) (21) generously provided by Lois Hoyer. Starter cultures for injection were grown 16 h at 37°C with vigorous agitation in YEPD medium comprised of 1% yeast extract, 2% peptone and 2% dextrose (Difco Laboratories; Becton, Dickinson and Company; Franklin Lakes, NJ). Cultures were predominantly (>99%) yeast forms following this incubation. Prior to inoculation, overnight cultures of C. albicans were washed, enumerated on a hemacytometer, and resuspended in pyrogen-free saline (Hospira, Inc.; Lake Forest, IL). The concentration was adjusted such that the desired unit dose per gram could be delivered in a volume of 10 μl.
Injection of Neonatal Mice
Timed pregnant BALB/c mice were obtained from Charles River Laboratories (Wilmington, MA). Pregnant dams were maintained in individual cages with unlimited access to food and water. Mice were monitored to determine the date of parturition. Pups were delivered in litters ranging from 3-9 pups and were randomized prior to inoculation to either sterile saline (control) or 10x colony forming units (CFU)/g of C. albicans yeast. Randomization was performed within cages rather than by litter to account for maternal and litter variations. Although cross-contamination of pups assigned to different experimental groups was theoretically possible, this risk was minimized by the short duration of the experiment. Further, in experiments using an endpoint such as mortality that is influenced by many variables, the risk of cross-contamination was outweighed by the risk of confounding effects of maternal and litter variability inherent to a litter-based randomization scheme. Pups were injected on post-partum day 2. Just prior to injection, each pup was weighed to the nearest tenth of a gram. Weight of pups was closely clustered around 2 g, so each pup received a standard dose of 20 μl yeast or sterile saline injected i.p. in the lower half of the abdomen. After injection, pups were examined every 3-8 hours for death or signs of illness. The pups were dissected at the time of natural death or sacrificed for dissection when found moribund. All surviving animals were sacrificed at 72 hours after injection. A single kidney and lung were harvested and immediately homogenized for colony counts. If the time of natural death could not be accurately determined within 2 hours, the organ colony counts were excluded from analysis. The remaining kidney, lung, spleen, liver and brain were fixed in 10% buffered formalin (Fisher Scientific, Kalamazoo, MI) for subsequent histology. In selected studies, brain tissue was also collected for homogenization and colony counts.
Selected tissues underwent histological preparation and silver staining at the institutional core facility. Kidney, lung, and brain were homogenized with a FastPrep-24 Instrument (MP Biomedicals, Inc., Solon, OH) using Lysing Matrix D (Qbiogene, MP Biomedicals, Inc., Solon, OH) in 1 ml sterile saline and appropriate dilutions were plated on YEPD. Colony counts were performed following an overnight incubation at 37°C. All animal studies were reviewed and approved by the Lifespan Institutional Animal Care and Use Committee, which oversees the animal care facility where animals were housed for this study.
Results
C. albicans infection in neonatal mice
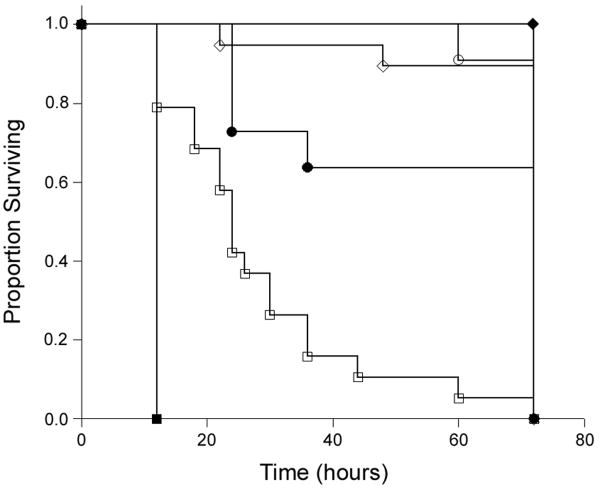
Mouse pups were injected i.p. on post-partum day 2 with concentrations of C. albicans strain SC5314 ranging from 104 to 108 CFU/g or with saline. Mice were followed closely for signs of illness and sacrificed at 72 hours following injection. Figure 1 shows the Kaplan-Meier survival curve summarizing these experiments. Doses of 106 CFU/g and below were not associated with mortality, and caused no apparent clinical symptoms. Doses above 106 CFU/g caused increased mortality in a dose dependent fashion. Because 107 CFU/g led to near complete mortality by study end point with a range of time of death throughout the observation period, this dose was selected for subsequent experiments.
Figure 1. Kaplan-Meier survival curve by dose injected.
Two day old mouse pups were injected i.p. with the following doses of wild-type C. albicans and survival curves were plotted: ■: 1 × 108 CFU/g (n=3); □:1 × 107 CFU/g (n=19); ●: 5 × 106 CFU/g (n=11); ○:1 × 106 CFU/g (n=11); ◆: 1 × 104 CFU/g (n=3); ◇: saline (n=19). Mortality occurred in a dose-dependent fashion at doses higher than 106 CFU/g.
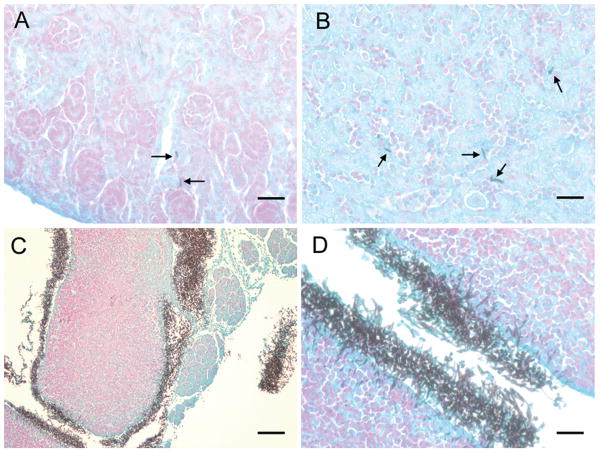
Tissue sections of kidney, spleen, liver, and brain were silver stained to detect fungal elements (figure 2). Yeast and hyphal elements were scattered in the kidney and liver parenchyma with no specific anatomic relationship (figure 2, panel A and B). There was no fungus detected in brain by histology (not shown). In the low power view of the spleen, abundant fungal elements were diffusely present in the capsular region (figure 2, panel C). A high power view of the same region demonstrated prominent hyphae with penetration into the spleen parenchyma (figure 2, panel D).
Figure 2. Tissue histology.
Silver stain of representative sections from kidney (A), liver (B), and spleen ((C) 10× magnification, (D) 40× magnification) are depicted from an animal injected with 108 CFU/g wild-type C. albicans. Arrows indicate hyphal elements visible within the organ parenchyma. Heavy involvement of the capsular and subcapsular regions of the spleen was seen. Panel A, B, D: bar = 25 microns; Panel C: bar = 100 microns.
Colony counts were obtained from homogenized kidney and lung and were highly variable (table 1). In general, colony counts were higher in kidney compared to lung at a given dose, and extent of fungal burden was proportional to dose injected. Statistical analysis using a negative binomial model supported this dose response relationship with P = 0.0005 for kidney and P = 0.003 for lung. Although no fungal elements were detected in histological studies of the brain, an additional experiment was conducted to examine brain involvement by colony counts, a more sensitive measure. Among 12 pups injected, all died by study end point and 9 pups (75%) had colony counts ranging from 100-240 colonies per brain. Although a lower fungal burden was seen relative to kidney and lung, these data support some involvement of the central nervous system. No organisms were recovered from any organs taken from animals in the saline control groups, providing reassurance in regard to the possibility of cross-contamination among animals in the same litter.
Table 1. Tissue burden by dose of wild-type C. albicans injected.
| Kidney (CFU/organ) | Lung (CFU/organ) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose (CFU/g) |
Mean | Median | Minimum | Maximum | Mean | Median | Minimum | Maximum | n |
| 107 | 10,442 | 600 | 0 | 71,300 | 1446 | 500 | 0 | 6100 | 13 |
| 5×106 | 8734 | 70 | 0 | 66,000 | 1055 | 30 | 0 | 7800 | 8 |
| 106 | 806 | 20 | 0 | 8000 | 20 | 25 | 0 | 40 | 11 |
| 104 | 13 | 20 | 0 | 20 | 0 | 0 | 0 | 0 | 3 |
Assessment of ALS3 mutant in neonatal model
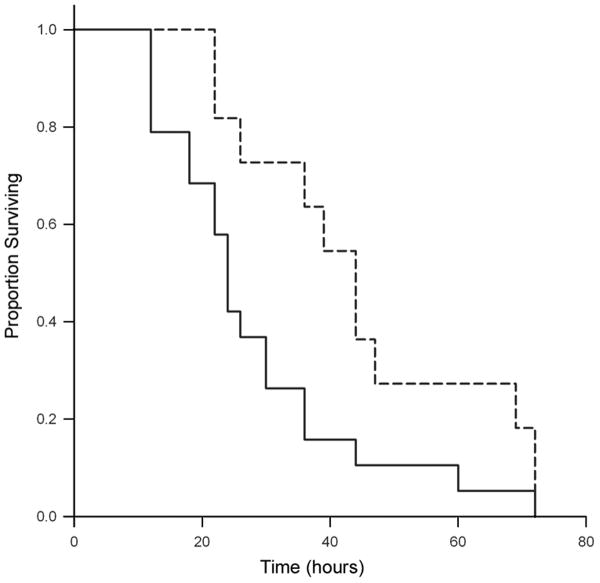
To determine the utility of this model in assessing virulence determinants of C. albicans, a mutant (1843) carrying a homozygous deletion of the adhesin gene, ALS3, was evaluated in the neonatal mouse model. Prior work demonstrated that this strain had reduced adhesion to epithelial and endothelial cells in vitro (22). The als3 mutant strain yielded a statistically significant reduction in mortality relative to wild-type (figure 3, P = 0.03). The median survival for the wild-type was 24 hours compared to 44 hours for the mutant. Tissue burden in kidney and lung was also compared in these animals (table 2). Because colony count data were again highly disperse and not normally distributed, a negative binomial model was used for analysis. Again, tissue burden was higher in kidney than in lung for both strains. Although trends toward higher colony counts in mice injected with wild-type vs. mutant could be identified, there was no significant difference in tissue fungal burden in these animals.
Figure 3. Kaplan-Meier survival curve comparing wild-type C. albicans and als3 deletion mutant.
Two day old mouse pups were injected i.p. with 107 CFU/g wild-type C. albicans (solid line, n=19) or als3 deletion mutant (dashed line, n=11) and survival curves were plotted. Median survival was 24 hours and 44 hours, respectively (P = 0.03 by log-rank test).
Table 2. Tissue burden in mice infected with wild-type vs. mutant (als3-/-) C. albicans.
| WT (CFU/organ) n=13 |
als3-/- (CFU/organ) n=10 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | Minimum | Maximum | Mean | Median | Minimum | Maximum | |
| Kidney* | 10,442 | 600 | 0 | 71,300 | 3950 | 1100 | 0 | 13,500 |
| Lung** | 1446 | 500 | 0 | 6100 | 518 | 350 | 0 | 2200 |
P = 0.29 for wild-type vs. mutant.
P = 0.14 for wild-type vs. mutant.
Discussion
Invasive candidiasis portends a poor prognosis despite available antifungal agents. A sophisticated understanding of host-pathogen interactions will be required to make additional progress in treatment and prevention of these infections. The model described here will be useful in studies to explore the unique aspects of the neonatal host in this disease. Neonatal mouse models have been successfully used to study sepsis with other microorganisms including group B Streptococcus (GBS) (23), E. coli (24), Pseudomonas aeruginosa (25), and Listeria monocytogenes (26-27). In some cases these studies have uncovered significant differences that can be defined between the neonatal and adult host and demonstrate the importance of neonatal models to study invasive disease.
Previous studies of C. albicans in the neonatal mouse utilized gastric inoculation as the route of infection. Using neonatal mice, Pope et al. provided the first report of lethal candidiasis in an animal model following GI colonization without any additional measures to compromise immunity in the animals (18). In this study, five to six day old mouse pups were inoculated via intragastric injection (5 × 108 CFU) and systemic spread of infection was seen in selected organs. Fungal invasion was seen in liver, kidney, and spleen within six hours of inoculation, suggesting timely passage across the digestive tract wall or entry into the systemic circulation, possibly through the lymphatics. However, mortality was approximately 50% or less in this model and tissue burden decreased over time. A subsequent study using a lower dose (2 × 107 CFU) in 6 day old pups by the same route also led to recovery of C. albicans from kidney, liver, lung, and spleen, but in relatively smaller numbers and no mortality (19). A dose of 1 × 107 CFU led to still fewer or no recovery of fungi from these organs. However, long term GI colonization was demonstrated in these animals and early colonization led to protective immune responses after later IV challenge with C. albicans as adults. In another study, six day old mouse pups were orally inoculated with C. albicans following cortisone induced immunosuppression. Histology of the entire gastrointestinal tract showed highest frequency of invasion of mucosa by Candida in the jejunum. Only GI tract organs were studied in this model (28).
GI colonization models using adult mice show important differences from the neonatal models. Broad spectrum antibiotic administration for 3 days prior to oral inoculation with C. albicans was necessary for GI colonization to develop (29). Additionally, once colonized, extraintestinal dissemination in these animals was very infrequent. When these animals were treated with dexamethasone, however, dissemination to kidney and mesenteric lymph nodes did occur, but mortality remained low (16). Features of disease associated with infection by the i.p. route in adult mice are also available. Vonk et al. studied the role of tumor necrosis factor-α (TNF) and lymphotoxin-α (LT) by injecting C. albicans i.p. in adult TNF-LT double knockout mice and their wild-type littermates (30). Unlike the present study in neonates, disseminated disease only occurred if immunosuppression was induced with cyclosporine prior to infection. Otherwise, the adult mice formed local abscesses that were cleared without disseminated disease. Taken together, these studies demonstrate that features of candidiasis in mouse models differ dramatically in the neonate compared to the adult, likely due to the relative immaturity of host defenses in the neonatal period. Such differences support the notion that features of disease unique to the neonatal host can be manifest by such an approach. However, there are additional factors placing preterm infants at risk for disseminated candidiasis that will be difficult to emulate in a murine model. Interventions such as indwelling catheters, parenteral nutrition, lack of enteral nutrition/breast milk, and many others that increase risk in the NICU are difficult to model and somewhat limit the applicability of host defense studies at this stage of mouse development to that of the preterm human.
In this study, the heavy involvement of the spleen with scattered foci in other organs supports a hematogenous route of dissemination, perhaps initiating in the spleen. Although involvement of the spleen was detected in the neonatal gastric inoculation model, colonization was similar or less than other organs (18). Presumably, the i.p. route of infection is responsible for the heavy spleen involvement in our model, either by direct contact with the organ or through lymphatic channels. Brieland et al. inoculated C. albicans (5 × 106 CFU/mouse) via lateral tail vein injections into adult, immunocompetent mice and reported growth of C. albicans in various organs (31). The kidney was noted to have logarithmic growth in fungal burden. However, the liver and heart fungal burden declined quickly over time. The brain, lung, and spleen were all noted to have steady fungal loads with no significant change over the duration of the infection. Consistent with these data, the kidney counts in this study were higher than in lung tissue. The Brieland study collected data over a 21-day post-infection time course. In our model, mortality occurred within 72 hours, and any surviving pups had generally cleared the infection by the 72 hour time point. The kinetics of infection were therefore quite different from the Brieland model.
The Brieland study described multiple foci of hyphal invasion in kidneys, hearts, brains and spleens of infected mice, with the largest fungal burden in the kidney. We found the largest foci of hyphae around the splenic capsule. We also did not visualize hyphal elements in brain of neonatal mice by histology, while the adult model showed brain involvement within 48 hours post-infection. Brain colony counts yielded consistent but reduced fungal burdens when compared to lung and kidney, suggesting that the fungal burden of the brain is not high enough to be detected by histology. Because tissue homogenates were the only way to assess fungal burden, involvement of vascular structures in the brain rather than the parenchyma itself is also possible. The differences in tissue distribution between these models likely relate to the route of infection and/or the dose of inoculation, but may also be influenced by developmental stage of the animal.
We have previously described a single-chain variable fragment, scFv3, which is specific to Als3p (22). Als3p is a cell wall protein expressed on C. albicans hyphae, which belongs to the Als family of adhesins (32). Als3p enables adherence to both epithelial and endothelial host cells through interaction with E-cadherin and N-cadherin respectively (33). Strain 1843, carrying a homozygous deletion of ALS3 demonstrates reduced adhesion to human epithelial and endothelial cells. Treatment of wild-type C. albicans with scFv3 resulted in reduced adherence, similar to the als3 mutant (22). In our model, the als3 deletion mutant showed somewhat attenuated mortality. Antibodies against Als3p such as scFv3 may therefore be useful to confer protection from disseminated candidiasis. This model provides fertile grounds to test this and other therapeutic strategies. Experiments are underway to evaluate scFv3 and other Als3p specific antibodies for their capacity to provide protection. Novel chemotherapeutic agents against fungi could also be tested in this model for efficacy and to assess any unique toxicity that may arise in a neonatal setting. Studies of pathogenesis with C. albicans frequently find differences among strains. This model can be used to extend these observations and make comparisons among isolates that are presumed to be different in pathogenic potential. Additionally, as non-albicans species increase in prevalence in the neonatal intensive care unit, this model will have utility to compare the pathogenic features of the different Candida species and potentially tailor appropriate therapies.
Acknowledgments
We thank Lois Hoyer for providing strain 1843 and helpful discussions and Monique DePaepe for pathological interpretation of histology.
Financial Support: This work was supported by an NIH COBRE grant, number P20 RR018728.
Abbreviations
- CFU
colony forming units
- GI
gastrointestinal
- scFv3
single-chain variable fragment 3
Contributor Information
Nancy Y. Tsai, Department of Pediatrics, Women & Infants Hospital of Rhode Island, Warren Alpert Medical School of Brown University, Providence, RI 02905
Sonia S. Laforce-Nesbitt, Department of Pediatrics, Women & Infants Hospital of Rhode Island, Warren Alpert Medical School of Brown University, Providence, RI 02905
Richard Tucker, Department of Pediatrics, Women & Infants Hospital of Rhode Island, Warren Alpert Medical School of Brown University, Providence, RI 02905.
Joseph M. Bliss, Department of Pediatrics, Women & Infants Hospital of Rhode Island, Warren Alpert Medical School of Brown University, Providence, RI 02905; Graduate Program in Pathobiology, Brown University, Providence, RI 02912
References
- 1.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, Lemons JA, Donovan EF, Stark AR, Tyson JE, Oh W, Bauer CR, Korones SB, Shankaran S, Laptook AR, Stevenson DK, Papile LA, Poole WK. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 3.Saiman L, Ludington E, Dawson JD, Patterson JE, Rangel-Frausto S, Wiblin RT, Blumberg HM, Pfaller M, Rinaldi M, Edwards JE, Wenzel RP, Jarvis W. Risk factors for Candida species colonization of neonatal intensive care unit patients. Pediatr Infect Dis J. 2001;20:1119–1124. doi: 10.1097/00006454-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Reef SE, Lasker BA, Butcher DS, McNeil MM, Pruitt R, Keyserling H, Jarvis WR. Nonperinatal nosocomial transmission of Candida albicans in a neonatal intensive care unit: prospective study. J Clin Microbiol. 1998;36:1255–1259. doi: 10.1128/jcm.36.5.1255-1259.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feja KN, Wu F, Roberts K, Loughrey M, Nesin M, Larson E, Della-Latta P, Haas J, Cimiotti J, Saiman L. Risk factors for candidemia in critically ill infants: a matched case-control study. J Pediatr. 2005;147:156–161. doi: 10.1016/j.jpeds.2005.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bliss JM, Basavegowda KP, Watson WJ, Sheikh AU, Ryan RM. Vertical and horizontal transmission of Candida albicans in very low birth weight infants using DNA fingerprinting techniques. Pediatr Infect Dis J. 2008;27:231–235. doi: 10.1097/INF.0b013e31815bb69d. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin DK, Jr, Stoll BJ, Fanaroff AA, McDonald SA, Oh W, Higgins RD, Duara S, Poole K, Laptook A, Goldberg R. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117:84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Donowitz LG. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med. 2001;345:1660–1666. doi: 10.1056/NEJMoa010494. [DOI] [PubMed] [Google Scholar]
- 9.Manzoni P, Stolfi I, Pugni L, Decembrino L, Magnani C, Vetrano G, Tridapalli E, Corona G, Giovannozzi C, Farina D, Arisio R, Merletti F, Maule M, Mosca F, Pedicino R, Stronati M, Mostert M, Gomirato G. A multicenter, randomized trial of prophylactic fluconazole in preterm neonates. N Engl J Med. 2007;356:2483–2495. doi: 10.1056/NEJMoa065733. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Grossman LB. Twice weekly fluconazole prophylaxis for prevention of invasive Candida infection in high-risk infants of <1000 grams birth weight. J Pediatr. 2005;147:172–179. doi: 10.1016/j.jpeds.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 11.Clerihew L, Austin N, McGuire W. Prophylactic systemic antifungal agents to prevent mortality and morbidity in very low birth weight infants. Cochrane Database Syst Rev. 2007:CD003850. doi: 10.1002/14651858.CD003850.pub3. [DOI] [PubMed] [Google Scholar]
- 12.Long SS, Stevenson DK. Reducing Candida infections during neonatal intensive care: management choices, infection control, and fluconazole prophylaxis. J Pediatr. 2005;147:135–141. doi: 10.1016/j.jpeds.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 13.Manzoni P, Mostert M, Jacqz-Aigrain E, Farina D. The use of fluconazole in neonatal intensive care units. Arch Dis Child. 2009;94:983–987. doi: 10.1136/adc.2008.154385. [DOI] [PubMed] [Google Scholar]
- 14.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 15.Clancy CJ, Cheng S, Nguyen MH. Animal models of candidiasis. Methods Mol Biol. 2009;499:65–76. doi: 10.1007/978-1-60327-151-6_8. [DOI] [PubMed] [Google Scholar]
- 16.Bendel CM, Wiesner SM, Garni RM, Cebelinski E, Wells CL. Cecal colonization and systemic spread of Candida albicans in mice treated with antibiotics and dexamethasone. Pediatr Res. 2002;51:290–295. doi: 10.1203/00006450-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Romani L. Immunology of invasive candidiasis. In: Calderone RA, editor. Candida and Candidiasis. ASM Press; Washington, D.C.: 2002. pp. 223–241. [Google Scholar]
- 18.Pope LM, Cole GT, Guentzel MN, Berry LJ. Systemic and gastrointestinal candidiasis of infant mice after intragastric challenge. Infect Immun. 1979;25:702–707. doi: 10.1128/iai.25.2.702-707.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domer JE. Intragastric colonization of infant mice with Candida albicans induces systemic immunity demonstrable upon challenge as adults. J Infect Dis. 1988;157:950–958. doi: 10.1093/infdis/157.5.950. [DOI] [PubMed] [Google Scholar]
- 20.Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 21.Zhao X, Oh SH, Cheng G, Green CB, Nuessen JA, Yeater K, Leng RP, Brown AJ, Hoyer LL. ALS3 and ALS8 represent a single locus that encodes a Candida albicans adhesin; functional comparisons between Als3p and Als1p. Microbiology. 2004;150:2415–2428. doi: 10.1099/mic.0.26943-0. [DOI] [PubMed] [Google Scholar]
- 22.Laforce-Nesbitt SS, Sullivan MA, Hoyer LL, Bliss JM. Inhibition of Candida albicans adhesion by recombinant human antibody single-chain variable fragment specific for Als3p. FEMS Immunol Med Microbiol. 2008;54:195–202. doi: 10.1111/j.1574-695X.2008.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodewald AK, Onderdonk AB, Warren HB, Kasper DL. Neonatal mouse model of group B streptococcal infection. J Infect Dis. 1992;166:635–639. doi: 10.1093/infdis/166.3.635. [DOI] [PubMed] [Google Scholar]
- 24.Cox F, Taylor L. Prevention of Escherichia coli K1 bacteremia in newborn mice by using topical vaginal carbohydrates. J Infect Dis. 1990;162:978–981. doi: 10.1093/infdis/162.4.978. [DOI] [PubMed] [Google Scholar]
- 25.Tang HB, DiMango E, Bryan R, Gambello M, Iglewski BH, Goldberg JB, Prince A. Contribution of specific Pseudomonas aeruginosa virulence factors to pathogenesis of pneumonia in a neonatal mouse model of infection. Infect Immun. 1996;64:37–43. doi: 10.1128/iai.64.1.37-43.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Nakane A, Minagawa T. Recombinant murine gamma interferon induces enhanced resistance to Listeria monocytogenes infection in neonatal mice. Infect Immun. 1989;57:2345–2349. doi: 10.1128/iai.57.8.2345-2349.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genovese F, Mancuso G, Cuzzola M, Biondo C, Beninati C, Delfino D, Teti G. Role of IL-10 in a neonatal mouse listeriosis model. J Immunol. 1999;163:2777–2782. [PubMed] [Google Scholar]
- 28.Cole GT, Seshan KR, Pope LM, Yancey RJ. Morphological aspects of gastrointestinal tract invasion by Candida albicans in the infant mouse. J Med Vet Mycol. 1988;26:173–185. [PubMed] [Google Scholar]
- 29.Kinneberg KM, Bendel CM, Jechorek RP, Cebelinski EA, Gale CA, Berman JG, Erlandsen SL, Hostetter MK, Wells CL. Effect of INT1 gene on Candida albicans murine intestinal colonization. J Surg Res. 1999;87:245–251. doi: 10.1006/jsre.1999.5755. [DOI] [PubMed] [Google Scholar]
- 30.Vonk AG, Netea MG, van Krieken JH, van der Meer JW, Kullberg BJ. Delayed clearance of intraabdominal abscesses caused by Candida albicans in tumor necrosis factor-alpha- and lymphotoxin-alpha-deficient mice. J Infect Dis. 2002;186:1815–1822. doi: 10.1086/345818. [DOI] [PubMed] [Google Scholar]
- 31.Brieland J, Essig D, Jackson C, Frank D, Loebenberg D, Menzel F, Arnold B, DiDomenico B, Hare R. Comparison of pathogenesis and host immune responses to Candida glabrata and Candida albicans in systemically infected immunocompetent mice. Infect Immun. 2001;69:5046–5055. doi: 10.1128/IAI.69.8.5046-5055.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoyer LL. The ALS gene family of Candida albicans. Trends Microbiol. 2001;9:176–180. doi: 10.1016/s0966-842x(01)01984-9. [DOI] [PubMed] [Google Scholar]
- 33.Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, Ibrahim AS, Edwards JE, Jr, Filler SG. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007;5:e64. doi: 10.1371/journal.pbio.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]