Abstract
Background and Purpose
Enrollment in the ALIAS Trial was suspended in late 2007 due to a safety concern. Here we present the safety data of that Trial (“Part 1”) and the rationale for the design of Part 2.
Methods
ALIAS Part 1 was designed to assess whether 25% albumin (ALB) begun within 5h of stroke onset would confer neuroprotection in subjects with acute ischemic stroke and baseline NIH Stroke Scale of 6 or above. Exclusion criteria included recent or current congestive heart failure, myocardial infarction, or cardiac surgery. The study comprised 2 cohorts -- subjects who received thrombolysis and those who did not -- each with 1:1 randomization to ALB or placebo. The primary outcome was the NIHSS and modified Rankin scales at 90 days. The intended sample size was 1,800.
Results
434 subjects were enrolled, and 424 were used in the safety analysis (ALB 207, saline 217). There were 36 deaths within the first 30 days in the ALB group, and 21 in the saline group. In contrast, death rates after 30 days were similar by treatment. Large strokes were the predominant cause of early death in both groups. In subjects older than 83 years, 90-day death rates were 2.3-fold higher with ALB than with saline (95% CI, 1.04-5.12). Similarly, 90-day deaths in subjects receiving excessive fluids were 2.10-fold greater with ALB than with saline (CI, 1.10-3.98).
Conclusions
The ALIAS Part 2 Trial, which commenced in early 2009, was modified as follows to enhance safety: upper age limit of 83 years; requirement for normal baseline serum troponin level; restriction of total IV fluids in the first 48 hours to 4200 ml or less; mandatory diuretic at 12-24h; and detailed site re-training. Because of insufficient non-thrombolysed subjects (22%) in Part 1, the two-cohort design was eliminated. The DSMB has reviewed the safety data of Part 2 three times and has approved continuation of the trial.
Keywords: albumin, neuroprotectant, ischemic stroke, randomized controlled trial
Introduction
In the clinical management of acute ischemic stroke, there is a compelling, unmet need for safe and effective neuroprotective strategies to limit brain injury, facilitate brain repair, and improve functional outcome 1. Extensive animal studies have shown human albumin (ALB) in moderate-to-high doses to be a promising neuroprotectant in focal and global cerebral ischemia and traumatic brain injury 2-7. In focal ischemia, ALB diminished total infarct volume by two-thirds and reduced brain edema by three-quarters or more, with a therapeutic window of efficacy extending to four hours 6; ameliorated brain swelling 2,3,6; improved blood flow to critically perfused brain regions 8; enhanced microvascular perfusion 9,10; reduced postischemic microvascular blood-element adhesion 11; and helped to transport important free fatty acids to the postischemic brain 12. An NINDS-funded phase 1 pilot clinical trial was subsequently conducted in 82 acute ischemic-stroke subjects who received 25% human albumin in doses that were escalated into the experimentally neuroprotective range 13,14. ALB therapy was safely tolerated: mild-to-moderate pulmonary edema occurred in 13% of subjects but responded readily to medical management. Exploratory efficacy analysis suggested that a beneficial treatment effect might exist 14.
Following the pilot trial, the ALIAS (Albumin In Acute Stroke) Trial (ClinicalTrials.gov Identifier NCT00235495) was begun as a randomized, double-blind, placebo-controlled trial whose primary aim was to ascertain whether high-dose ALB therapy (2 g/kg) administered within 5 hours of stroke onset would increase the proportion of subjects with favorable outcome at 3 months compared to saline-placebo 15. The ALIAS trial was sponsored by NINDS and operated under an FDA Investigational New Drug license. The first ALIAS subject was recruited in July 2006. In December 2007, at the first interim analysis of 3-month follow-up data in 225 subjects (but after 434 subjects had been enrolled) at 62 North American clinical sites, the trial’s independent Data and Safety Monitoring Board (DSMB) recommended to NINDS that enrollment be suspended due to safety concerns, and that the study team consider revising the protocol so as to enable the trial to resume with increased safety. The ALIAS principals were granted permission to review the safety data in an unblinded manner. After extensive internal conferences and discussions with external advisors, the ALIAS Executive Committee (EC) developed a revised protocol and analysis plan together with a comprehensive site-training program. These changes were approved by the DSMB in July 2008 and by the FDA in September 2008. The ALIAS Trial then began as a separate, new study referred to as “Part 2,” and enrolled its first subject in February 2009.
Revisions to the protocol were based on our unblinded analysis of the Part 1 safety data and were implemented in an effort to improve the safety profile of trial participants. This paper presents the results of our evaluation of the safety data from Part 1 and sets forth the rationale for the design changes instituted in the currently ongoing Part 2.
Methods
The ALIAS Part 1 Trial was originally designed as two separate but concurrently-implemented double-blind, phase III multicenter trials with 1:1 randomization to ALB or saline-placebo. The objective was to assess whether 25% ALB therapy (2g/kg intravenously administered over 120 minutes) compared to an equal volume of 0.9% normal saline conferred neuroprotection in acute ischemic stroke, over and above the standard of care, in two cohorts of patients with acute ischemic stroke. One cohort consisted of subjects who received standard-of-care thrombolytic therapy (IV tPA, intra-arterial tPA, endovascular mechanical thrombolysis with approved devices and catheters, or a combination of IV and endovascular treatment). The other cohort consisted of subjects who were not thrombolysed. The administration of thrombolytic therapy was based on local clinical judgment informed by then-current guidelines 16. The rationale for this design stemmed in part from preclinical evidence that ALB did not require induced reperfusion in order to confer neuroprotection 7; and from an observed trend in the ALIAS Pilot Trial of better 3-month outcome with higher doses of ALB in both the tPA and the non-tPA cohorts 14. The study design of the ALIAS Part 1 Trial was identical for the two cohorts (thrombolysis and non-thrombolysis). The eligibility criteria are presented in Table 1.
Table 1.
Inclusion and Exclusion Criteria, ALIAS Part 1 Trial
Inclusions
|
Exclusions
|
A centralized step-forward, web-based 1:1 randomization process was employed. All study personnel and patients were blinded. A biased-coin minimization algorithm adjusted for clinical site within each cohort 17. Study-drug kits consisted of a 500 ml and a 250 ml bottle of either 25% ALB or saline encased in blinding boxes and delivered via tinted IV tubing 18. Albumin was manufactured for the trial by Baxter Healthcare Corp., Westlake Village, CA. A bedside nurse or other personnel not involved with the trial administered the study-drug [8 ml/kg] by constant IV infusion over 2 hours (± 15 min). Subjects weighing 94 kg or more received a maximum volume of 750 ml.
Vital signs were monitored frequently during and after study-drug administration. Serum chemistries were collected at 24 and 48 hours. Intravenous fluid intake was recorded at 24 and 48 hours. A follow-up brain CT or MRI scan was obtained at 24 hours. An ECG was repeated at 24-48 hours. Neurological and cardiac status, including NIHSS score, was assessed at 24 and 48 hours and at 7 days or discharge, whichever came first. Diuretic treatment was not mandated, but administration of a loop diuretic such as furosemide in an initial dose of 10-20 mg IV was recommended if clinically indicated. Antiplatelet therapy was recommended in all subjects within 48 hours of their stroke. Blood pressure was managed according to the local standard of care.
Subjects were followed for 1 year. At 3 months (± 14 days) post-randomization, subjects were required to come to the clinic, where the NIHSS, modified Rankin score (mRS), Barthel Index, Stroke-Specific Quality of Life (SS-QOL) instrument 19 and Trailmaking A and B 20 were assessed by a site investigator who was certified in outcome-scale completion and blinded to the subject’s admission treatment assignment and hospital course. Subjects were also followed by telephone contact at 1 month (± 7 days), 6 months (± 14 days), 9 months (± 14 days), and 1 year (± 14 days) post-randomization to assess the mRS, record serious adverse events (SAEs) and complete the EuroQol 21 at 3 months and 1 year and the Questionnaire to Validate a Stroke-Free Status (QVSFS) 22 at 3, 6, 9, and 12 months. We assessed blinding by asking the rater to indicate what treatment assignment they thought the patient had received. The raters’ responses were correct 52.4% of the time (177 correct out of 338 responses), indicating that the outcome assessment had been truly blinded.
A favorable outcome was defined as an NIHSS score of 0-1 and/or a mRS score of 0-1 at 90 days post-randomization. With two-sided type I error probability of 0.05, power of 80% to detect a 10% absolute effect-size difference in the primary outcome, and an assumption of the control group’s favorable-outcome proportion to be 40%, the required sample size was 900 in each cohort, or a total of 1,800 subjects. Since the primary analysis was based on the intent-to-treat principle, the sample size included inflation to account for crossovers and missing data as well as for three interim analyses for overwhelming efficacy or futility.
Due to premature suspension of the trial after 434 subjects had been enrolled, neither the thrombolytic (N=327) nor the non-thrombolytic cohort (N=97) had sufficient power to test the primary hypothesis. Thus, we evaluated the safety data by combining the cohorts.
Results
There were 434 randomized subjects, 215 allocated to ALB and 219 to saline treatment. Of these, 424 received at least 20% of the study drug (ALB 207, saline 217) and were used in the safety analysis. The baseline characteristics of the safety cohort, shown in Table 2, were very similar to those of the entire population. [A CONSORT diagram 23 of the Part 1 Trial is available online as a Supplementary Figure.]
Table 2.
Baseline characteristics of safety cohort
| Albumin (N=207) | Saline (N=217) | |
|---|---|---|
| Age | 69.3 ± 13.6 (SD) years (max, 97) |
69.8 ± 146 (SD) years (max, 97) |
| Gender | 56.0% male | 50.2% male |
| Race | 86.5% Caucasian, 7.7% African-American |
81.6% Caucasian, 12.0% African-American |
| Time from stroke onset to study-drug treatment | 202 ± 50 min | 206 ± 50 min |
| Time from stroke onset to IV tPA | 137 ± 33 min | 138 ± 36 min |
| Baseline NIHSS score | Median, 11. NIHSS 6-10, 43.5%; 11-15, 23.7%; 16- 20, 17.9%; 21-25, 9.7%; >25, 5.3% |
Median, 11. NIHSS 6-10, 42.4%; 11-15, 24.0%; 16- 20, 18.9%; 21-25, 11.1%; >25, 3.7% |
| Medical history: | ||
| Hypertension | 73.4% | 75.1% |
| Ischemic heart disease | 15.9% | 18.4% |
| Atrial fibrillation | 21.7% | 24.9% |
| Previous stroke; previous TIA | 16.9%; 10.6% | 18.4%; 15.2% |
| Diabetes mellitus | 20.3% | 22.6% |
| Oxfordshire stroke classification: | ||
| Partial anterior circulation | 50.2% | 54.0% |
| Total anterior circulation | 30.0% | 27.4% |
| Lacunar | 13.5% | 11.6% |
| Posterior circulation | 5.8% | 6.5% |
| Baseline systolic blood pressure | 160 ± 29 mmHg | 157 ± 29 mmHg |
| Baseline plasma glucose | 7.4 ± 3 mmol/L | 7.5 ± 2.9 mmol/L |
| CT-based ASPECTS score 33 of 0-7 (central reader) |
33.2% | 27.7% |
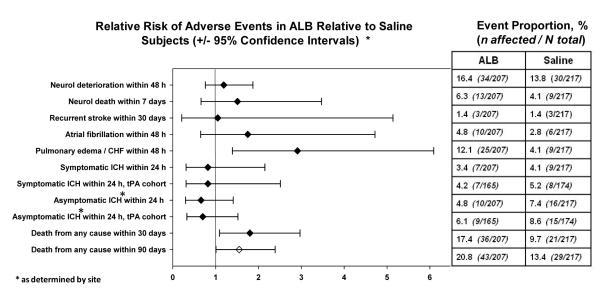
Figure 1 presents the major safety events by treatment assignment. As anticipated, pulmonary edema occurred approximately 3-fold more often in ALB- than in saline-treated subjects; the event rate of 12.1% in ALB subjects is similar to the 13% incidence observed in the ALIAS Pilot Trial 13. In the thrombolytic cohort, the proportion of subjects with symptomatic intracranial hemorrhage (ICH) was similar in ALB (4.2%) and saline subjects (5.2%) and similar to that of the NINDS tPA trial 16. Symptomatic hemorrhage was defined as the occurrence of intracranial hemorrhage within 24 ± 6 hours of randomization, proven by neuroimaging (MRI or CT) and associated with deterioration in neurological status. In the investigator’s opinion, the hemorrhage must have been thought to be the primary cause of the subject’s deterioration.
Figure 1. Adverse events in ALB- and saline-treated subjects.
Diamond points denote the Relative Risk of each adverse event, and the whiskers denote the lower and upper 95% Confidence Intervals of this estimate.
Although the DSMB did not disclose the details of its confidential deliberations, we believe that its recommendation to suspend subject recruitment in ALIAS-Part 1 was based primarily on an observed imbalance in overall deaths in the two groups (Figure 1, Table 3). Deaths in the ALB and saline groups were similar on days 1-4 after randomization, while between days 5 and 30 there were more deaths in the ALB group compared to the saline group (Table 3). By contrast, death rates beyond 30 days were virtually identical in the two treatment groups. Safety data of all subjects who died were reviewed in a treatment-blinded fashion (by MDG and MDH) in order to assign a primary cause of death. Large strokes (with or without medical complications) were the predominant cause of death throughout days 1-30, and these were more frequent in ALB- than in saline-treated subjects (Table 3). No single cause of death, however, completely explained the difference in deaths by treatment assignment.
Table 3.
Deaths by treatment assignment, day, and adjudicated cause of death
| Days 1-4 | Days 5-10 | Days 11-20 | Days 21-30 | Days 31-365 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ALB | Saline | ALB | Saline | ALB | Saline | ALB | Saline | ALB | Saline | |
| Large stroke | 6 | 4 | 8 | 4 | 3 | 1 | 1 | 1 | ||
| Large stroke + complications | 1 | 2 | 3 | 1 | 2 | 1 | 2 | 2 | 1 | 4 |
| ICH as primary cause | 1 | 2 | 1 | 1 | 1 | |||||
| Primary cardiac cause | 1 | 2 | 1 | 1 | 3 | |||||
| Medical complication | 1 | 2 | 7 | 13 | ||||||
| Other known cause | 2 | |||||||||
| Unknown cause | 1 | 1 | 1 | 2 | ||||||
| COLUMN TOTALS | 9 | 9 | 15 | 6 | 8 | 4 | 4 | 2 | 16 | 18 |
To assess potential treatment-related factors, we conducted univariate analyses of 90-day deaths for various baseline variables. The relative risks (RR) and 95% confidence intervals (CI) are: age (RR 1.04, CI 1.02-1.06), baseline NIHSS (1.13, 1.09-1.17), plasma glucose (1.03, 0.97-1.10), baseline ASPECTS score of 8-10 vs. 0-7 (0.34, 0.21-0.55), onset to study-drug treatment (1.00, 0.99-1.01), and cohort (1.25, 0.67-2.33). A multivariable model incorporating treatment, baseline NIHSS (bNIHSS), and age showed a non-significant effect of treatment (1.49, 0.93-2.38), but significant effects of bNIHSS (1.10, 1.06-1.14) and age (1.03, 1.01-1.05).
Influence of advanced age and fluid excess: Because the multivariable analysis showed a significant treatment effect for age, we explored the differential death rate in the safety cohort by various dichotomized age groups beginning at age 80. Ninety-day death rates did not differ significantly in ALB and saline subjects aged 83 or younger, while 90-day deaths in subjects older than 83 years were 2.3-fold higher in the ALB than in the saline group (Table 4a). We also hypothesized that differences in IV fluids may have contributed to ALB-associated deaths. We considered fluid excess to be present if a subject received > 4200 ml of total IV fluids during the first 48 hours (based upon an assumed 75 ml/h IV fluid rate plus the volumes of study drug and tPA). Among subjects without excessive fluids, 90-day death rates were not significantly associated with treatment assignment, while among subjects with fluid excess, death rates were two-fold greater in ALB than in saline subjects (RR 2.10, CI 1.10-3.98) (Table 4b). However, since fluid administration occurred during and after study-drug administration, it is possible that excess fluid volume is a merely a marker of worse outcome rather than its cause.
Table 4a.
Dichotomization analyses of deaths at 90 days in the safety population. Dichotomization by age
| Age <=83 | Age > 83 | |||
|---|---|---|---|---|
| ALB | Saline | ALB | Saline | |
| Total N | 178 | 178 | 29 | 39 |
| N dead at 90 days | 31 | 22 | 12 | 7 |
| % dead at 90 days | 17.4% | 12.4% | 41.4% | 17.9% |
| RR (95% CI) | 1.41 (0.85-2.34) | 2.31 (1.04-5.12) | ||
Table 4b.
Dichotomization analyses of deaths at 90 days in the safety population. Dichotomization by intravenous fluids
| 48-h IV Fluids <= 4200 ml | 48-h IV Fluids > 4200 ml | |||
|---|---|---|---|---|
| ALB | Saline | ALB | Saline | |
| Total N | 131 | 126 | 76 | 91 |
| N dead at 90 days | 22 | 17 | 21 | 12 |
| % dead at 90 days | 16.8% | 13.5% | 27.6% | 13.2% |
| RR (95% CI) | 1.24 (0.69-2.23) | 2.10 (1.10-3.98) | ||
These results suggested the potential for minimizing excess deaths by attention to these two factors. This was modeled by comparing the 90-day death rates in the entire safety cohort vs. the subgroup with out-of-hospital stroke, age <84 and with 48-h IV fluids <= 4200 ml (Table 4c). (Subjects with in-hospital strokes were excluded based on our impression that these patients tended to be more ill at baseline and to suffer more adverse events.) The result suggested that implementation of the age and fluid restrictions and the exclusion of in-hospital strokes would eliminate significant treatment-related differences in 90-day deaths.
Table 4c.
Dichotomization analyses of deaths at 90 days in the safety population. Entire safety cohort, vs. safety cohort with age <84 AND IV fluids <= 4200 ml AND only out-of hospital strokes
| Entire Safety Cohort | Safety Cohort with age < 84 and 48-h IV Fluids ≤ 4200 ml and only out-of-hospital strokes |
|||
|---|---|---|---|---|
| ALB | Saline | ALB | Saline | |
| Total N | 207 | 217 | 114 | 100 |
| N dead at 90 days | 43 | 29 | 17 | 13 |
| % dead at 90 days | 20.8% | 13.4% | 14.8% | 12.9% |
| RR (95% CI) | 1.55 (1.01-2.39) | 1.15 (0.59-2.24) | ||
Serious Adverse Event (SAEs) occurred in 53.6% of ALB subjects and 46.5% of placebo subjects. Cardiovascular SAEs (CV-SAEs) were coded more commonly in ALB subjects (21.7%) than in the placebo group (8.3%) (RR, 2.60; 95% CI, 1.57-4.37), primarily due to higher SAE rates of pulmonary edema (6.8% vs. 2.8%) and acute coronary syndrome (8.2% vs. 0.5%) in ALB-treated subjects than in saline-treated subjects. Among subjects with CV-SAEs, myocardial infarction (MI) was diagnosed in 15 ALB-treated subjects (33%) but in only 2 saline-treated cases (11%). Of the 15 ALB-treated subjects with MI, 7 were diagnosed acutely, on days 1 or 2; 6 of those subjects died, although in only 2 of these cases could the death be directly attributed to an acute cardiac cause (i.e., progressive hypoxemia; hemodynamic instability leading to shock). Among those CV-SAE subjects without a clinical diagnosis of myocardial infarction on days 1-2, elevated serum troponin levels at 24 and/or 48 hours were noted in 11 of 39 ALB-subjects (28%) but in only 1 of 16 saline-treated subjects (6%). While we concluded that cardiopulmonary pathophysiology must have played a role in the excess mortality, we were unable to show convincingly that it was the sole or specific cause.
Redesign of ALIAS Trial – Part 2
Part 2 of the ALIAS Trial – a stand-alone study -- retains many structural design features of the Part 1 Trial: i.e., it is a concurrently controlled, parallel two-arm trial of ALB versus saline with a 1:1 randomization ratio. The primary efficacy measure remains unchanged, and we maintain 40% as the control group’s presumed proportion of good outcome. The major modifications from the Part 1 protocol are listed below, and the rationale for their implementation discussed.
Upper age limit of 83 years at the time of randomization: See Table 4a.
Requirement that baseline serum troponin level be 0.1 mcg/L or less. Elevated baseline troponin levels may occur in a minority of ischemic stroke patients, denotes some degree of cardiac injury, and may therefore be associated with cardiac adverse events. The ALIAS DSMB recommended that troponin levels be reviewed before randomizing a patient in the ALIAS Part 2 Trial.
Exclusion of patients with in-hospital strokes. These patients have significant co-morbid illness and are more likely to suffer serious adverse events and less likely to respond to treatment.
Imposition of strict IV fluid management guidelines: total IV fluids in first 48 hours not to exceed 4200 ml (unless cogent medical indications exist); strict monitoring of IV fluid intake; and the mandatory administration of a loop diuretic (typically furosemide, 20 mg IV) between 12 and 24 hours after study-drug treatment: Age is associated with loss of left ventricular compliance with associated diastolic dysfunction and, therefore, potentially reduced ability to compensate in the face of a fluid challenge, particularly a prolonged one such as is seen with high-dose ALB administration.
Implementation of a detailed re-training module for clinical site staff, including a mandatory certification test and site-PI attestation form: Mandatory re-training (for all clinical sites that participated in Part 1) and mandatory training (for clinical sites that did not participate in Part 1) was required for participation in Part 2. A web-based training and testing module was developed. Each clinical site PI provided a signed attestation that he/she would provide re-training to all involved clinical personnel.
Inclusion of baseline NIHSS score as a covariate in the primary efficacy and safety analyses: The clinical trials literature has repeatedly cited the benefits of covariate-adjusted analysis for improving statistical power, particularly for covariates that are highly correlated with the outcome measure 24,25.
-
Combining subjects with and without thrombolytic treatment into a single cohort; inclusion of thrombolysis treatment status in the primary efficacy analysis model: The original premise of the ALIAS Trial, that ALB is effective in patients who received thrombolytic treatment as well as those who did not, has not changed. However, we assumed that we would see a greater effect in the former group (possibly due to a synergistic effect of tPA and ALB 14), and hence, we had designed Part 1 of the trial to conduct separate studies in these two cohorts of patients. Because of the insufficient enrollment into Part 1 of subjects who did not receive thrombolytic treatment, we are combining the two groups in Part 2. It is possible that we may observe a modest effect (e.g., 5-10% treatment effect) in the non-thrombolysis stratum and a very large effect (e.g., 20-30%) in the thrombolysis stratum. In such a scenario, we might find a statistically significant interaction effect; however, we would wish to conclude that there is a significant study treatment effect overall. Hence, we shall consider a statistically significant interaction effect only if it is also qualitative, i.e., if the treatment effect is in the opposite direction in the two strata. In such a case, the primary efficacy analysis will be based on the thrombolysis stratum only.
We conducted multiple simulations to determine the sample size needed to address adequately the power for the interaction effect and the main study treatment effect for the entire study, as well as for the thrombolysis stratum only. We concluded that a total sample size of 1,100 will provide sufficient power (80%) and minimize Type I error probability for the overall Part 2 Trial. The clinically significant interaction effect is defined as a 20% differential treatment effect between the thrombolysis and non-thrombolysis strata. We believe that this value of 20% is justified because in the ALIAS Pilot Trial 14, a 26.1% absolute difference in good outcome occurred in the high-dose ALB tiers of the thrombolysis and non-thrombolysis cohorts. The sample size of 1,100 was determined via simulation to ensure that the 20% interaction effect could be detected with 80% power at a two-sided alpha=0.10.
Inclusion of statistical safety monitoring guideline: In addition to stopping guidelines based on efficacy and futility, we established a statistical stopping guideline for safety based on the 30-day mortality rate. The rationale for basing the stopping guidelines on the number of events (referred to as reverse sampling method) rather than on the number of subjects is because we are unsure of the precise estimate of the event rates. Safety assessments based on the number of subjects may yield a decision-making process based on unstable estimates, since a relatively small (approximately 10%) event rate is anticipated in the control group. With 100 subjects enrolled in the study, for instance, a treatment group differential of only one death (which can happen by chance) would exaggerate the relative risk unnecessarily.
Planned meta-analysis of Parts 1 and 2: After completion of Part 2 and analysis of its data, we plan to conduct a meta-analysis of Parts 1 and 2, using summary statistics from the two cohorts of Part 1 and the Part 2 study cohort weighted using the inverse normal method. We also plan to conduct pooled analysis of individual data from both parts, adjusting for the study and cohort/strata effect.
Current Status of ALIAS Part 2 Trial
The ALIAS Part 2 Trial randomized its first subject in February 2009, and has enrolled approximately 225 subjects in the ensuing year. The ratio of thrombolysed to non-thrombolysed subjects currently exceeds 5:1. The DSMB has reviewed the safety data of the ALIAS Part 2 Trial three times since its initiation and has approved the continuation of the trial on each occasion. In addition, safety analyses based on deaths within 30 days were conducted in December 2009 (based on first 15 deaths) and in April 2010 (based on the first 30 deaths), and there were no safety concerns based on the predefined guidelines specified in the Safety Monitoring Plan and Statistical analysis Plan of the ALIAS Part 2 Trial. Thus, we are confident that the changes introduced in the ALIAS Part 2 Trial have resulted in an improved participant safety profile.
Discussion
In Part 1 of the ALIAS Trial, the 90-day death rate was greater in ALB- than in saline-treated subjects (Table 3, Figure 1), and this was chiefly accounted for by 15 excess deaths occurring on days 5-30 post-randomization. This timing suggests that the excess deaths were not the direct consequence of ALB-associated volume expansion but rather were due to indirectly-acting mechanisms. Although these deaths occurred predominantly in older subjects with large ischemic strokes who had received larger volumes of intravenous fluids in the first 48 hours (Table 4b), careful adjudication failed to identify specific additional factors contributing to deaths of individual ALB-treated subjects. Cardiovascular SAEs occurred more commonly following ALB administration than with saline – in particular, acute coronary syndrome and pulmonary edema, although the event-rate of the latter was no greater than expected from the ALIAS Pilot Trial 13. In subjects with CV-SAEs, the clinical diagnosis of myocardial infarction was more common in those receiving ALB (15 cases: 7 acute, 6 late) than in saline-treated subjects (2 acute or subacute cases); asymptomatic troponin elevations also tended to be more common in the former group. Nonetheless, deaths even in ALB-treated subjects with acute MI were much more commonly attributed to complications of a large stroke than to a direct cardiac mechanism (Table 3). These results, taken together, suggest that ALB treatment tended to predispose susceptible subjects to a degree of myocardial stress, which acting indirectly and in combination with other predisposing factors, increased mortality in the 5-30 day period after treatment. This was supported by a comparison of overall mortality in the ALIAS-Part 1 safety subjects who had not experienced a CV-SAE: with ALB, 33 deaths in 154 subjects (21.4%); with saline, 35 deaths in 178 subjects (19.7%). That is, the entire difference in treatment-related death rates could be attributed to those ALB-treated subjects who experienced CV-SAEs.
The protocol modifications instituted in Part 2 were largely intended to diminish ALB-associated mortality in the very elderly and in subjects with fluid excess, and to reduce the likelihood of including subjects at higher risk of cardiovascular events. (In order to maximize the applicability of ALB treatment to ischemic stroke, we chose not to impose a ceiling on the permissible baseline NIHSS score.) As this proposal involved major protocol modifications, the DSMB, NINDS and FDA concurred in the decision that the study go forward from this point on as a separate trial – “the ALIAS Part 2 Trial” – and that the ALIAS-Part 1 data eventually be used in a pooled analysis after completion of Part 2.
It is appropriate that we devote brief attention to the bioethical considerations that guided our decision-making as to publication. As laid forth in the Declaration of Helsinki 26, clinical investigators have an ethical duty to make publicly available the results of human-subjects research, whether positive, negative, or inconclusive. The National Institutes of Health’s Belmont Report 27 reaffirmed the concept of beneficence as a fundamental guiding principle of biomedical research 28. Randomized clinical trials tend to place doctors in the ethically challenging position of acting both as physicians and as scientists 29. In so doing, investigators must necessarily adopt a utilitarian approach that maximizes societal benefit while minimizing the risk to individual subjects. Thus, the challenge to the physician-investigator is to maintain clinical equipoise – a state of uncertainty about the relative merits of treatments A vs. B - during his/her participation in a randomized controlled trial so as to avoid forming fixed beliefs about a novel treatment whose benefit has yet to be established 29,30.
In publishing the safety results of the ALIAS Trial - Part 1 at this time, we fulfill an ethical duty to publicize the results of our trial, but we are cognizant that these results may pose risks to clinical equipoise in the stroke-neurology community, despite the fact that the DSMB, the clinical trials leadership at NINDS, and the FDA have all given their approval to continue the ALIAS Trial as Part 2. Baron 31 has applied decision analysis to illustrate the implications of various decision-making scenarios. In our case, publication of the ALIAS Trial - Part 1 data at this time poses the potential risk that certain centers might decide not to participate in Part 2 of the ALIAS Trial or to participate with reduced enthusiasm; and that publication might reinforce the beliefs of neuroprotection-nihilists. By contrast, deferring publication might engender suspicion among participants despite our well-intentioned rationale. We have decided, on balance, that clinical equipoise is best maintained by publishing this report at this time.
The ALIAS Trial is a clinical trial that was adapted in mid-course. As these changes were not preplanned, the trial does not meet the definition of “adaptive design” 32. Nonetheless, we believe that describing the process which led to Part 2 may benefit other acute stroke clinical trials experiencing a similar predicament and thus needing to consider the potential incorporation of changes, particularly with respect to safety factors, into their study design in order to make it more efficient and truly adaptive.
Supplementary Material
Acknowledgment
Supported by NIH grants U01 NS040406 (to MDG) and U01 NS054630 (to YYP).
APPENDIX
ALIAS Part 1 Executive Committee
Myron D. Ginsberg, University of Miami, Miami, FL (Study Chair and PI, Clinical Coordinating Center);
Yuko Y. Palesch, Medical University of South Carolina, Charleston, SC (PI, Statistics and Data Coordinating Center);
Michael D. Hill, University of Calgary, Calgary, Canada (Director, Canadian Coordinating Center);
Bonnie D. Waldman, Medical University of South Carolina, Charleston, SC (Project Manager);
Lynn Patterson (ex officio), Medical University of South Carolina, Charleston, SC (Project Manager Assistant);
Richard Leinster, Medical University of South Carolina, Charleston, SC (Data Manager);
Isabel Mendez, University of Miami, Miami, FL (Financial Manager);
Diego Tamariz, University of Miami, Miami, FL (Clinical Project Coordinator);
Karla J. Ryckborst, University of Calgary, Calgary, Canada (Study Coordinator);
Claudia S. Moy, NINDS, NIH, Rockville, MD (NINDS Liaison)
Unblinded Study Statisticians
Renee H. Martin, Medical University of South Carolina, Charleston, SC;
Sharon D. Yeatts, Medical University of South Carolina, Charleston, SC
External Safety Monitors
Stephan A. Mayer, Columbia University, New York, NY;
Andrew M. Naidech, Northwestern University, Chicago, IL;
Alejandro A. Rabinstein, Mayo Clinic, Rochester, MN
Data and Safety Monitoring Committee (DSMB)
Patrick D. Lyden, Cedars-Sinai Medical Center, Los Angeles, CA (Chair);
Christopher S. Coffey, University of Iowa, Iowa City, IA;
Marco DiTullio, Columbia University, New York, NY;
Christine Wijman, Stanford University, Palo Alto, CA;
Janice Cordell (ex officio, NINDS, NIH, Rockville, MD)
Enrolling clinical centers, ALIAS Part 1 Trial (with numbers of subjects contributed)
M. Hill, University of Calgary, Calgary, Canada (56);
M. Clark, Oregon Health Sciences University, Portland, OR (36);
A. Shuaib, University of Alberta, Edmonton, Canada (36);
D. Selchen, Trillium Health Centre, Mississauga, Canada (35);
M. Concha, Intercoastal Neurology, Sarasota, FL (15);
J.-M. Boulanger, Hopital Charles LeMoyne, Greenfield Park, Canada (14);
S. Silliman, University of Florida/Shands, Jacksonville, FL (13);
A. Forteza, University of Miami, Miami, FL (12);
L. Pettigrew, University of Kentucky, Lexington, KY (12);
K. Remmel, University of Louisville, Louisville, KY (10);
M. Rymer, St. Luke’s Hospital, Kansas City, MO (10);
V. Hachinski, London Health Sciences Centre, London, Canada (9);
S. Hanson, Park Nicollet Institute, Minneapolis, MN (9);
N. Bayer, St. Michael’s Hospital, Toronto, Canada (8);
D. Chiu, Methodist Hospital, Houston, TX (8);
P. Teal, Vancouver Coastal Health Authority, Vancouver, Canada (8);
R. Englander, Sacred Heart Hospital, Eugene, OR (7);
L. Wechsler, University of Pittsburgh Medical Center, Pittsburgh, PA (7);
R. Dafer, Loyola University, Maywood, IL (6);
J. Gebel, Jewish Hospital, Louisville, KY (6);
G. Gubitz, Dalhousie University, Halifax, Canada (6);
D. Laskowitz, Duke University, Durham, NC (6);
S. Cruz-Flores, St. Louis University, St. Louis, MO (5);
G. Howell, Villages Research Group, Ocala, FL (5);
M. Jacoby, Ruan Neurology Clinic, Des Moines, IA (5);
J. Schindler, Yale University, New Haven, CT (5);
R. Zweifler, University of South Alabama, Mobile, AL (5);
M. Beaudry, CSSS de Chicoutimi, Saguenay, Canada (4);
C. Ionita, Millard Fillmore Gates Hospital, Buffalo, NY (4);
G. Lopez, Baylor College of Medicine, Houston, TX (4);
S. Messe, University of Pennsylvania, Philadelphia, PA (4);
S. Sen, University of North Carolina, Chapel Hill, NC (4);
R. Stephens, John Muir Medical Center, Walnut Creek & Concord, CA (4);
J. Teitelbaum, Montreal Neurological Institute, Montreal, Canada (4);
C. Voll, University of Saskatchewan, Saskatoon, Canada (4);
D. Camp, Seton Family of Hospitals, Austin, TX (3);
T. Collier, Royal Island Hospital, Kamloops, Canada (3);
B. Coull, University of Arizona, Tucson, AZ (3);
M. Flaster, St. Joseph’s Hospital, Phoenix, AZ (3);
R. Kelley, LSU Health Sciences Center, Shreveport, LA (3);
S. Mallenbaum, Neurological Consultants of Virginia Beach, Virginia Beach, VA (3);
N. Solenski, University of Virginia, Charlottesville, VA (3);
S. Starkman, UCLA, Los Angeles, CA (3);
G. Stotts, Ottawa Hospital, Ottawa, Canada (3);
S. Bansil, Overlook Hospital, Summit, NJ (2);
R. Fessler, St. Joseph Mercy Oakland, Southfield, MI (2);
J. Hanna, MetroHealth Medical Center, Cleveland, OH (2);
J. Harris, Neurologic Consultants, Ft. Lauderdale, FL (2);
H. Kirshner, Vanderbilt University, Nashville, TN (2);
E. Leira, University of Iowa, Iowa City, IA (2);
C. Lewandowski, Henry Ford Health System, Detroit, MI (2);
R. Welch, Wayne State University, Detroit, MI (2);
M. Aguilar, Mayo Clinic Hospital, Phoenix, AZ (1);
D. Brenner, University of Alabama, Birmingham, AL (1);
E. Feen, University Hospitals, Cleveland, OH (1);
K. O’Phelan, Queens Medical Center, Honolulu, HI (1);
D. Weisman, Abington Memorial Hospital, Abington, PA (1)
Footnotes
Disclosures/Conflicts of interest: none.
Clinical Trial Registration—URL: http://www.clinicaltrials.gov. Unique identifier: NCT00235495
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belayev L, Busto R, Zhao W, Clemens JA, Ginsberg MD. Effect of delayed albumin hemodilution on infarction volume and brain edema after transient middle cerebral artery occlusion in rats. J Neurosurg. 1997;87:595–601. doi: 10.3171/jns.1997.87.4.0595. [DOI] [PubMed] [Google Scholar]
- 3.Belayev L, Zhao W, Pattany PM, Weaver RG, Huh PW, Lin B, Busto R, Ginsberg MD. Diffusion-weighted magnetic resonance imaging confirms marked neuroprotective efficacy of albumin therapy in focal cerebral ischemia. Stroke. 1998;29:2587–2599. doi: 10.1161/01.str.29.12.2587. [DOI] [PubMed] [Google Scholar]
- 4.Belayev L, Alonso OF, Huh PW, Zhao W, Busto R, Ginsberg MD. Posttreatment with high-dose albumin reduces histopathological damage and improves neurological deficit following fluid percussion brain injury in rats. J Neurotrauma. 1999;16:445–453. doi: 10.1089/neu.1999.16.445. [DOI] [PubMed] [Google Scholar]
- 5.Belayev L, Saul I, Huh PW, Finotti N, Zhao W, Busto R, Ginsberg MD. Neuroprotective effect of high-dose albumin therapy against global ischemic brain injury in rats. Brain Res. 1999;845:107–111. doi: 10.1016/s0006-8993(99)01952-6. [DOI] [PubMed] [Google Scholar]
- 6.Belayev L, Liu Y, Zhao W, Busto R, Ginsberg MD. Human albumin therapy of acute ischemic stroke : marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke. 2001;32:553–560. doi: 10.1161/01.str.32.2.553. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Belayev L, Zhao W, Busto R, Belayev A, Ginsberg MD. Neuroprotective effect of treatment with human albumin in permanent focal cerebral ischemia: histopathology and cortical perfusion studies. Eur J Pharmacol. 2001;428:193–201. doi: 10.1016/s0014-2999(01)01255-9. [DOI] [PubMed] [Google Scholar]
- 8.Huh PW, Belayev L, Zhao W, Busto R, Saul I, Ginsberg MD. The effect of high-dose albumin therapy on local cerebral perfusion after transient focal cerebral ischemia in rats. Brain Res. 1998;804:105–113. doi: 10.1016/s0006-8993(98)00674-x. [DOI] [PubMed] [Google Scholar]
- 9.Nimmagadda A, Park H-P, Prado R, Ginsberg MD. Albumin therapy improves local vascular dynamics in a rat model of primary microvascular thrombosis: a two-photon laser-scanning microscopy study. Stroke. 2008;39:198–204. doi: 10.1161/STROKEAHA.107.495598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park HP, Nimmagadda A, DeFazio RA, Busto R, Prado R, Ginsberg MD. Albumin therapy augments the effect of thrombolysis on local vascular dynamics in a rat model of arteriolar thrombosis: a two-photon laser-scanning microscopy study. Stroke. 2008;39:1556–1562. doi: 10.1161/STROKEAHA.107.502195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belayev L, Pinard E, Nallet H, Seylaz J, Liu Y, Riyamongkol P, Zhao W, Busto R, Ginsberg MD. Albumin therapy of transient focal cerebral ischemia: in vivo analysis of dynamic microvascular responses. Stroke. 2002;33:1077–1084. doi: 10.1161/hs0402.105555. [DOI] [PubMed] [Google Scholar]
- 12.de Turco EB Rodriguez, Belayev L, Liu Y, Busto R, Parkins N, Bazan NG, Ginsberg MD. Systemic fatty acid responses to transient focal cerebral ischemia: influence of neuroprotectant therapy with human albumin. J Neurochem. 2002;83:515–524. doi: 10.1046/j.1471-4159.2002.01121.x. [DOI] [PubMed] [Google Scholar]
- 13.Ginsberg MD, Hill MD, Palesch YY, Ryckborst KJ, Tamariz D. The ALIAS Pilot Trial: a dose-escalation and safety study of albumin therapy for acute ischemic stroke. I. Physiological responses and safety results. Stroke. 2006;37:2100–2106. doi: 10.1161/01.STR.0000231388.72646.05. [DOI] [PubMed] [Google Scholar]
- 14.Palesch YY, Hill MD, Ryckborst KJ, Tamariz D, Ginsberg MD. The ALIAS Pilot Trial: a dose-escalation and safety study of albumin therapy for acute ischemic stroke. II. Neurological outcome and efficacy-analysis. Stroke. 2006;37:2107–2114. doi: 10.1161/01.STR.0000231389.34701.b5. [DOI] [PubMed] [Google Scholar]
- 15.Ginsberg MD, Palesch YY, Hill MD. The ALIAS (ALbumin In Acute Stroke) Phase III randomized multicentre clinical trial: design and progress report. Biochem Soc Trans. 2006;34:1323–1326. doi: 10.1042/BST0341323. [DOI] [PubMed] [Google Scholar]
- 16.NINDS rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 17.Zhao W, Ciolino J, Palesch Y. Step-forward randomization in multi-site emergency treatment clinical trials. Academic Emergency Medicine. 2010 doi: 10.1111/j.1553-2712.2010.00746.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 19.Williams LS, Weinberger M, Harris LE, Clark DO, Biller J. Development of a stroke-specific quality of life scale. Stroke. 1999;30:1362–1369. doi: 10.1161/01.str.30.7.1362. [DOI] [PubMed] [Google Scholar]
- 20.Guadino E, Geisler M, Squires N. Construct validity in the Trailmaking Test: what makes Part B harder? J Clin Exp Neuropsychol. 1995;17:529–535. doi: 10.1080/01688639508405143. [DOI] [PubMed] [Google Scholar]
- 21.EuroQol--a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 22.Jones WJ, Williams LS, Meschia JF. Validating the Questionnaire for Verifying Stroke-Free Status (QVSFS) by neurological history and examination. Stroke. 2001;32:2232–2236. doi: 10.1161/hs1001.096191. [DOI] [PubMed] [Google Scholar]
- 23.Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, Gotzsche PC, Lang T. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134:663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 24.Permutt T. Testing for imbalance of covariates in controlled experiments. Stat Med. 1990;9:1455–1462. doi: 10.1002/sim.4780091209. [DOI] [PubMed] [Google Scholar]
- 25.Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med. 2002;21:2917–2930. doi: 10.1002/sim.1296. [DOI] [PubMed] [Google Scholar]
- 26.World Medical Association Declaration of Helsinki. Internet. 2009 [Google Scholar]
- 27.National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, National Institutes of Health The Belmont Report -- Ethical principles and guidelines for the protection of human subjects of research. Internet. 1979 [PubMed] [Google Scholar]
- 28.Emanuel EJ, Crouch RA, Arras JD, Moreno JD, Grady C. Ethical and Regulatory Aspects of Clinical Research. Johns Hopkins University Press; Baltimore: 2003. [Google Scholar]
- 29.Hellman S, Hellman DS. Of mice but not men. Problems of the randomized clinical trial. N Engl J Med. 1991;324:1585–1589. doi: 10.1056/NEJM199105303242208. [DOI] [PubMed] [Google Scholar]
- 30.Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987;317:141–145. doi: 10.1056/NEJM198707163170304. [DOI] [PubMed] [Google Scholar]
- 31.Baron J. Against Bioethics. MIT Press; Cambridge: 2006. [Google Scholar]
- 32.Gallo P, Chuang-Stein C, Dragalin V, Gaydos B, Krams M, Pinheiro J. Adaptive designs in clinical drug development--an Executive Summary of the PhRMA Working Group. J Biopharm Stat. 2006;16:275–283. doi: 10.1080/10543400600614742. [DOI] [PubMed] [Google Scholar]
- 33.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.