Abstract
This review considers the 250+ papers concerning the association of the angiotensin converting enzyme (ACE) gene insertion/deletion polymorphism (rs1799752) and various disease conditions published in 2009. The deletion allele occurs in approximately 55% of the population and is associated with increased activity of the ACE enzyme. It might be predicted that the D allele, therefore, might be associated with pathologies involving increased activity of the renin-angiotensin system. The D allele was seen to be associated with an increased risk of hypertension, pre-eclampsia, heart failure, cerebral infarct, diabetic nephropathy, encephalopathy, asthma, severe hypoglycaemia in diabetes, gastric cancer (in Caucasians) and poor prognosis following kidney transplant. On the positive side, the D allele appears to offer protection against schizophrenia and chronic periodontitis and confers greater up-per-body strength in old age. The I allele, meanwhile, offers improved endurance/athletic performance and aerobic capacity as determined by lung function tests, although it does increase the risk of oral squamous cell carcinoma and obstructive sleep apnoea in hypertensives.
Keywords: Angiotensin converting enzyme, ACE gene polymorphism, renin-angiotensin system, hypertension, heart failure, dementia, depression
Introduction
The unravelling of the physiology of the renin-angiotensin system (RAS) started over 100 years ago when in 1898 the Finnish physiologists Robert Tigerstedt and Per Bergman published evidence that intravenous injection of rabbit kidney extracts raised blood pressure; they postulated that the substance responsible for this effect was a protein which they named renin [1]. It wasn't until 1934 that Goldblatt showed in animal models that clamping of the renal arteries raised blood pressure [2] and in 1938 Pickering described the partial purification of renin and its ability to increase blood pressure [3]. In1940 Braun-Menendez and colleagues [4] and, independently Page and colleagues [5], proposed that renin itself was not directly responsible for its pressor effect but was in fact an enzyme. The names “hypertensin” and “angiotonin” were given independently to the pressor substance produced by the enzymatic action of renin. Subsequently, it was agreed that the term “angiotensin” would be used to describe this substance.
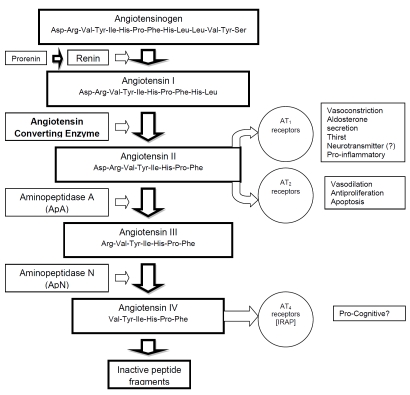
In 1956, Elliott and Peart [6] and Skeggs and colleagues [7] discovered that the product of renin action was a decapeptide which actually required further enzymatic breakdown to form the active pressor substance, an octapeptide. The terms angiotensin I and angiotensin II were introduced and the enzyme responsible was named angiotensin converting enzyme (ACE). Figure 1 illustrates the renin-angiotensin system and its physiological roles.
Figure 1.
A schematic representation of the renin-angiotensin system, its associated receptors and physiological effects. IRAP represents Insulin Regulated Amino Peptidase, the putative site of action of angiotensin IV; angiotensin IV inhibits its enzymatic activity.
It eventually became established that the RAS was involved in the homeostasis of fluid balance and blood pressure via effects on aldosterone secretion by the adrenal cortex and direct vasoconstriction. The RAS was thus implicated in the aetiology of cardiovascular disorders (eg renal artery stenosis), and became the target of research for antihypertensive medications.
Since then, however, the role of the RAS in other aspects of physiology has been investigated. There is convincing evidence, for example, that angiotensin II and its metabolites play an important role in the central nervous system and it has been implicated in aspects such as cognition, dementia, depression, anxiety and epilepsy [8, 9]. The brain RAS has recently, therefore, become the focus of studies targeted at the development of novel drugs for the treatment of the above mentioned conditions. The RAS has also been implicated in the inflammatory response, and with cancers [10].
To date, all drugs targeted at the RAS have similar aims: to decrease RAS function. It had been noted that venom from the Brazilian viper Bothrops jararaca caused severe hypotension as one of the effects of envenomation and in 1965 it was found that the venom potentiated the effects of bradykinin [11]. This lead to the presumption that substances contained in the snake venom were inhibitors of kininase, an enzyme that had been described a few years earlier which inactivated bradykinin [11]; it was also found that peptides from the same venom were able to inhibit ACE [12]. Eventually medicinal chemists developed SQ14225 which later became known as captopril. Captopril and other ACE inhibitors are now first line drug therapies for the treatment of hypertension and heart failure. More recently an inhibitor of renin has been marketed, aliskiren, which similarly ultimately prevents the synthesis of angiotensin II and is also used as an antihypertensive medication.
The other approach to pharmacological manipulation of the RAS is to use antagonists of the angiotensin receptors. Early drugs such as saralasin were peptides which were nonselective for AT1 and AT2 receptors, but modern drugs are metabolically-stable non-peptides with selectivity for the receptor subtypes, for example losartan.
The focus of this review is the insertion / deletion polymorphism of the gene encoding for ACE. The remainder will therefore focus on ACE and ACE inhibitors.
ACE, ACE inhibitors and cardiovascular disease
ACE inhibitors are first line therapies for the treatment of hypertension and heart failure. The current UK national guidelines for the treatment of hypertension (National Institute for Clinical Excellence, NICE) state that ‘In hypertensive patients younger than 55, the first choice for initial therapy should be an ACE inhibitor (or an angiotensin-II receptor antagonist if an ACE inhibitor is not tolerated). In hypertensive patients aged 55 or over, or black patients of any age (including both Black African and Black Caribbean patients, not Asian, Chinese, mixed-race, or other ethnic groups), the first choice for initial therapy should be either a calcium-channel blocker or a thiazide-type diuretic.’
In addition, because administration of drugs such as ACE inhibitors which cause peripheral vasodilation results in a reduced cardiac work-load, such drugs are routinely used for the relief of heart failure. The NICE guidelines for the treatment of heart failure give ACE inhibitors as the first-line treatment, but in this instance there are no additional considerations for age nor ethnic origin. The mechanism of action of the ACE inhibitors is heart failure is predominantly peripheral vasodilatory, but there is also evidence that the effect of reducing angiotensin synthesis also affects ventricular remodeling as a consequence of changes in AT2 receptor activity.
The question of ethnic differences in responses to antihypertensive medication warrants further investigation. There is evidence that Black patients gain lower antihypertensive benefit from ACE inhibitors and beta-adrenoceptor antagonists (“Beta Blockers”) than other ethnic groups [13], with diuretics producing a greater decrease in systolic blood pressure in Black patients than ACE inhibitors [14]. In one study, one year therapy with the ACE inhibitor enalapril was associated with significant reductions in blood pressure amongst the White patients, but not amongst the Black patients [15]. Using cardiovascular consequences such as fatal and nonfatal myocardial infarction and heart failure as end-points, NICE does not recommend the use of ACE inhibitors to treat hypertension in Black patients because ‘results of a subgroup analysis comprising Black patients from one randomised controlled trial (ALLHAT) indicated that therapy with the ACE inhibitor lisinopril was associated with a higher incidence of stroke (RR 1.40; 1.17 - 1.68 95% CI) and cardiovascular events (RR 1.19; 1.09 - 1.3095% CI) compared to the thiazide-type diuretic chlorthalidone.’
The case for ethnic differences in heart failure and its treatment are less clear. Black individuals are at much greater risk of heart failure than Whites: a 20-year prospective study of heart failure in over 5000 individuals recently reported that heart failure developed in 27 individuals, all but one of whom were Black, mean age of onset 39 years [16]. By the age of 50, the incidence of heart failure was more than 10 times greater in the Black men and women, than in the White. Amongst the Black patients, 75% of the heart failure patients had developed hypertension before the age of 40. Earlier studies of ethnic differences in responses to treatments for heart failure had produced conflicting responses. Exner et al [15] reported results from a study of 800 Black patients and almost 1200 White patients over a period of nearly 3 years showing that although there were no differences in demographic variables, the Black patients had higher rates of death from any cause (12.2 vs. 9.7 per 100 person-years) and of hospitalization for heart failure (13.2 vs. 7.7 per 100 person-years). Therapy with the ACE inhibitor enalapril was associated with a significant decrease in the risk of hospitalization for heart failure amongst the White patients but not in the Black patients. A meta-analysis of the available data, however, produced different results with ACE inhibitors being seen to reduce all-cause heart failure mortality in both Black and White patients [17]. ACE inhibitors and beta blockers (which act partially by decreasing renin secretion) are thus now considered as the essential drugs to improve the prognosis of heart failure both in Blacks and Whites [18]
Several hypotheses have been formulated to explain the increased risk of heart failure in the Black population and the failure of response to antihypertensive therapy with ACE inhibitors. Because the phenomenon was first observed in African Americans, one early hypothesis was that there was a naturally-selected ‘robust’ RAS as a consequence of mortality induced by dehydration during the transport of African slaves to the US. More recent hypotheses, however have turned to gene polymorphisms, and the RAS in particular, and it is now become clear that the ‘ethnic’ differences in cardiovascular risk and response to therapy may not be limited to ‘Black Africans’. This review concentrates on the insertion / deletion polymorphism of the gene encoding for ACE, and a literature review in late December 2009 indicated that there were over 250 publications on the subject of this polymorphism in 2009 (January to December). The review was conducted using ISI Web Of Knowledge and the search terms ACE and Polymorphism. As illustrated in Table 1, 250 publications on the subject of the ACE gene polymorphism in 2009 represents a decrease in the annual publication rate from a peak of approximately 600 per year between 1999 and 2003. This review will consider the recent (2009) publications only, for earlier reviews see Catellon and Hendi [19] and Jeunmaitre [20].
Table 1.
The number of publications concerning the ACE gene I/D polymorphism since 1989
| Search Terms | 1989-1993 | 1994-1998 | 1999-2003 | 2004-2008 |
|---|---|---|---|---|
| ACE polymorphism + Hypertension | 11 | 351 | 682 | 596 |
| ACE polymorphism + Coronary | 2 | 35 | 94 | 97 |
| ACE polymorphism + Infarct* | 12 | 322 | 432 | 294 |
| ACE polymorphism + Kidney Disease | - | 85 | 244 | 172 |
| ACE polymorphism + Dementia | - | 4 | 28 | 33 |
| ACE polymorphism + Depression | - | - | 12 | 12 |
| ACE polymorphism + Parkinson* | - | - | 6 | 10 |
| ACE polymorphism + Inflam* | 1 | 15 | 42 | 78 |
| ACE polymorphism + Asthma | - | 5 | 13 | 12 |
| ACE polymorphism + Diabetes | - | 152 | 289 | 244 |
| ACE polymorphism + Cancer | - | 4 | 11 | 58 |
| ACE polymorphism + Performance | - | 1 | 38 | 50 |
| Total | 26 | 974 | 1885 | 1664 |
The insertion/deletion polymorphism of the ACE gene is defined as either the presence (insertion, I) or absence (deletion, D), of a 287 base pair insert in intron 16 of the gene on chromosome 17q23 [21]. It is commonly described by the rs number rs1799752, although it is also sometimes represented as rs4340, rs13447447 and rs4646994. The insertion appears to reduce ACE expression, thus DD homozygotes have 65% more, and ID heterozygotes 31% more ACE than II homozygotes [22]. A meta-analysis of prevalence studies indicates that the overall frequency of the D allele is 54%, being unrelated to gender but there are ethnic differences[23], [24]. In Arab populations (Egyptians, Jordanians and Syrians) for example, frequencies of the D allele are approximately 65% [25]. Although there is evidence that an increase in ACE activity associated with the D allele does not result in increased synthesis of angiotensin II [26], it might be expected that increased ACE activity would be associated with increased incidence of cardiovascular disease and resistance to ACE inhibitor therapy.
Hypertension
Recent studies of the association of hypertension with ACE genotype have shown a positive correlation of the D allele with hypertension in a south Indian population [27], and in a south China population [28], but not in Punjabi [29], juvenile American [30], Turkish [31] or Sardinian [32] populations. These findings suggest that in some populations, presumably in the face of various environmental variables, the D allele of the ACE gene, which elevates the activity of ACE, may be associated with hypertension. The correlation, however is not robust and in a sample of Brazilian women, the I allele has been seen to be associated with a systolic blood pressure elevated by approximately 11mmHg [33]. In the special case of gestational hypertension and pre-eclampsia, in a study undertaken in approximately 700 pregnant Italian women, of which 204 suffered from pre-eclampsia and 56 from gestational diabetes, the D allele was significantly more prevalent in women suffering from mild pre-eclampsia than controls. There were no significant differences between gestational hypertension cases and controls [34].
When considering the responses of hypertensive patients to antihypertensive medications, that is, why Black patients show decreased response to ACE inhibitors, the antihypertensive effects of a calcium channel antagonist, a betaadrenoceptor antagonist, a diuretic and an angiotensin receptor antagonist were all seen to be unaffected by ACE I/D genotype in White hypertensive men [35] and the same results were found for the same drug classes, including ACE inhibitors in Dutch patients [36]. No influence of the ACE genotype was found on the magnitude of the treatment-induced blood pressure reduction in older Brazilian women [33] and 12-week therapy with the angiotensin receptor antagonist eprosartan has been seen to be associated with a good antihypertensive effect, regardless of ACE gene I/D polymorphism in Uzbek men [37]. The evidence is thus consistent in that there is no association of ACE I/D genotype with response to antihypertensive therapy. Even when considering a common adverse effect of ACE inhibitors, cough, no correlation with ACE genotype has been found [38]. This polymorphism is therefore probably not responsible for the observed ethnic differences in responses to ACE inhibitors.
It is well recognised that in healthy individuals, perturbations of blood pressure are normally rectified as part of the homeostatic processes, and indeed the RAS is perhaps the major homeostatic process. It is perhaps, not surprising therefore that a single gene polymorphism associated with increased activity of ACE, but probably no change in circulating angiotensin II [26], is not inevitably associated with increased incidence of hypertension or decreased response to antihypertensive drugs. The effects of angiotensin on the heart, however, are more complicated. Angiotensin, acting via AT1 receptors induces vasoconstriction and salt retention, resulting in increased blood pressure and therefore increased load on the heart. Angiotensin, acting via AT2 receptors on the heart, however, has different effects: notably anti-apoptotic and anti-inflammatory. It might be therefore postulated that increased ACE activity associated with a gene polymorphism might alter cardiac function. A recent study in Han Chinese patients with diastolic heart failure demonstrated that the concomitant presence of ACE DD and AT1R 1166 CC genotypes synergistically increased the predisposition to the heart failure [39]. This finding is of interest as the AT1 gene polymorphism has no effect on receptor function or density, although it may alter sensitivity to angiotensin.
Other cardiovascular disease
The association of the ACE gene polymorphism with other aspects of cardiovascular pathology have been explored with conceivable justification. There have recently been 3 studies of association with coronary artery disease / stroke, all from Turkey, but with different authors. Two found no association between genotype and disease [31, 40], whilst the third found a higher frequency of the ACE D/D genotype in all patients than in the healthy population [41]. A meta-analysis of 58 studies concluded that there was no association between stroke and ACE genotype [42]; the Women's Health Study in 25,000 White US women similarly found no association of ACE gene I/D status and the incidence of cardiovasular disease or stroke at the 11 year follow-up [43].
Similarly for myocardial infarct (MI), no association was found between ACE genotype and the disease in the US Women's Health Study [43] nor in young Han Chinese patients [44]. Although DD genotype was associated with higher risk of recurrent MI, life-threatening MI complications, and severe heart failure in a Russian population [45]. A study of sudden cardiac death in Russia revealed a decreased prevalence of II genotype amongst victims compared with the normal population [46], again suggesting an adverse ‘effect’ of the D allele.
Moving away from the coronary vasculature, to more peripheral vessels, the D-allele has been associated with a greater arterial stiffness as demonstrated by lower distensibility and higher pulse pressure [47]. The retinal arteriolar diameter was also significantly narrower in subjects with the DD genotype [48]. Paradoxical the frequencies of the D allele are also significantly higher in patients with aortic dissection, a condition involving separation of the arterial wall [49], although it has been reported that the ACE I/D gene polymorphism is not a susceptibility factor to aortoiliac occlusive disease. The polymorphism may, however, be an important factor in the development of abdominal aortic aneurysm when coexisting with hypertension [50].
Still with blood vessels. It is suggested that that the DD genotype may be a risk factor for cerebral infarction in Han Chinese population [51]. Migraine is also a condition associated with changes in cerebrovascular haemodynamics: the US Women's Health Study found no association of ACEI/D status with migraine [43], although in a smaller study of 150 migraine patients in Northern India, Joshi et al reported an increased prevalence of DD genotype amongst sufferers [52].
Kidney disease
As described above, the physiology of the RAS was initially studied with respect to renal homeostasis, it is not surprising, therefore, that it has been implicated in chronic kidney disease. In a small study in approximately 100 patients and controls in Southern India, Anbazhagan and colleagues reported that there was no association of ACE I/D genotype with the disease [27]. In terms of treatment of the condition, however, there may be differences. In a small study in 93 Japanese patients treated for approximately 3 years with a combination of ACE inhibitors and angiotensin receptor antagonists, proteinurea decreased only in II, and ID individuals, not in DD, although there was no difference in the decrease in blood pressure between the two groups [53]. In a larger study in 1000 Italian patients, over 10 years, almost opposite findings were reported, with ACE inhibitors realising greater health benefit in DD individuals than in II or ID [54]. Ironically the DD genotype has been associated with significantly poorer renal function in kidney transplant patients 18-30 months following transplantation in German Caucasians [55].
In the specific case of diabetic nephropathy the frequency of the D allele and the DD genotype was significantly increased in Asian Indian patients compared with diabetic without nephropathy and was associated with increased risk of nephropathy [56]; a similar higher frequency of the D allele was seen in Tunisian diabetic nephropathy patients [57]. Considering albuminurea as a correlate of nephropathy severity, in Iranian patients it has been reported that the frequency of the II genotype decreased and that of the DD increased with increasing severity of albuminurea and the D allele independently increased the odds of macroalbuminuria versus microalbumin [58]. In 435 Mexican patients there were no differences in ACE I/D genotype distribution between patients with and without albuminuria, although when the subgroup of female patients were studied separately, the DD genotype was more prevalent amongst albumin-urea patients than those without albuminuria [59]. A meta-analysis concluded that II subjects had a 22% lower risk of diabetic nephropathy than D allele carriers, but that Asians (Chinese, Japanese, Koreans) derived greater protection (36%), than Caucasians (11%) [60].
The central nervous system
In addition to roles in cardiovascular and renal homeostasis, the RAS has also been implicated in psychiatric and neurological conditions, with the use of ACE inhibitors to treat hypertension being seen to be associated with decreased depression and improved cognition. The ACE gene has therefore been an obvious candidate gene in studies of the aetiology of depression, dementia etc.
Pandey et al reported a study of I/D allele distribution between vascular dementia patients, degenerative dementia and age-matched controls. No differences were seen between the groups [61]. A meta-analysis came to similar conclusions [62]. Results reported by Halbecque et al [63], and Miners et al [64] for Alz-heimer's Disease, a specific form of dementia, indicated a similar lack of association of the disease with ACE I/D genotype. Consideration of the association of the ACE gene I/D polymorphism and Alzheimer's disease is still ongoing (see http://www.Alzgene)
The association between ACE genotype and depression and suicide has also been explored recently. We have previously reported a lack of association between ACE genotype and depression in a Lebanese population [65]. A similar lack of association was more recently reported in a Finnish sample of treatment-resistant depressed patients who were referred to electroconvulsive therapy. The distribution of genotypes and alleles did not differ between patients and healthy (non-depressed) controls nor did they influence response to treatment [66]. More recent studies have concentrated on suicide which is often used as a proxy measure of depression. In a study in Caucasians, Fudalej et al reported an association of the I allele with depression and a greater frequency of that allele amongst successful male and female suicide victims in comparison to age-matched controls [67]. Sparks et al, however, in a European sample, reported opposite results: in a study of ‘suicide completers’, ‘suicide attempers’ and matched controls, the risk of suicide was significantly associated with the DD genotype [68].
Harvey [69] recently suggested that depression may be a form of encephalopathy. Kehoe et al [70] have subsequently reported that the D al-lele was significantly more frequent in Chernobyl clean-up workers who subsequently developed encephalopathy than those that did not.
It has been known for some years that brain angiotensin affects dopamine, increasing concentrations in the nigrostriatal region, but not, or much less, the mesolimbic system [71]. An association of ACE gene I/D polymorphisms with Parkinson's disease (nigrostriatal) would therefore be more likely than an association with schizophrenia (mesolimbic). No differences in ACE I/D genotype frequencies were found between between Italian Parkinson's patients and controls [72], but the D allele was identified as a protective against schizophrenia in a Spanish population. The allele significantly reduced the risk of developing schizophrenia by 50%, the protection increased with the number of D alleles [73]. The association between the ACE gene I/D polymorphism and schizophrenia is under on-going consideration at SZgene (http://www.szgene.org).
Inflammation
Angiotensin has also been associated with a pro-inflammatory effect [10], thus a genetic predisposition to decreased angiotensin synthesis, eg possession of the ACE I allele, might be predicted to be associated with protection against inflammatory disorders. In the case of two dermatological disorders, psoriasis and vitiligo, no association has been found between ACE I/D genotype and either condition in either Spanish or Turkish sufferers respectively [74], [75]. Similarly, in Turkish patients, distribution of ID alleles was seen not to be associated with generalised, aggressive periodontitis [76], although in the same population the frequency of the D allele was significantly lower in a chronic periodontitis group than in healthy controls [77].
In asthma, another inflammatory condition, the DD genotype has been seen to be more prevalent in both a Turkish and a Chinese sample [78], [79]. Whilst in the case of another inflammatory condition, pancreatitis, no significant differences were found in the prevalence of the ACE I/D genotype frequencies between patients with alcoholic, non-alcoholic, and acute pancreatitis and controls [80] [81]. Diabetes, however, presents a different story. In type I diabetes, which is characterised by loss of pancreatic function, the D-allele was seen to be associated with severe hypoglycaemia requiring emergency treatment [82] and in type II diabetes, an association was seen with the D allele in a Tunisian population in both Arabs and Berbers [83], although the same relationship was not seen in a Lebanese population [84], nor was there any association of genotype with insulin resistance in a very small study conducted in a Turkish population [85]. Metabolic syndrome in elderly Slovaks and obesity in Saudi subjects were similarly unassociated with ACE I/D genotype status, [86] and [87] respectively.
Cancer
There is a large body of work concerned with the possible association of the gene polymorphism with the incidence and progression of various cancers. The I/D genotype was less frequently associated with breast cancer in a study of 101 Brazilian patients than were the DD or II genotypes [88]. Meanwhile in colorectal cancer patients in a Romanian population the distribution of neither genotype nor alleles differed between cancer patients and controls [89]; the II genotype was more frequent in a sample of Greek and German oral squamous cell carcinoma patients than in matched controls [90].
A meta-analysis of gene polymorphisms associated with gastric cancer determined that the effects of the different ACE I/D genotypes differed between Asians and Caucasians [91], although it actually only included two publications concerning the ACE I/D polymorphism: the first which found no difference in the distribution of genotypes between Japanese patients and controls [92], and the second which found an greater frequency of DD genotypes amongst Caucasian gastric cancer patients [93].
Athletic performance
A study of Japanese track athletes found that in males, but not females, the I allele was overrepresented amongst endurance athletes [94]. Similarly in Polish rowers the frequency of II was 31% compared with normal controls where it was 19% [95]. In Spain, endurance runners were again found to have a significantly greater frequency of the I allele than professional footballers [96], proving that the relationship is with endurance sport, not physical fitness or strength alone. In Portuguese short distance elite swimmers, the D allele was more common than in controls and elite middle-distance swimmers [97], again demonstrating the link of the I allele with endurance. A possible mechanistic reason for the relationship was elucidated with the finding, in Asian rugby players, that the I allele confers advantages in aerobic capacity as determined by lung function tests [98].
This relationship, however, does not carry-over into the non-elite population. A Brazilian study of policemen undertaking a 17-week exercise programme found no influence of ACE gene I/D status on left ventricular hypertrophy following exercise [99] and in a study of tourists scaling Mount Kilimanjaro, the likelihood of success was not associated with ACE genotype [100]. In normal Korean women undertaking a 12-week exercise regime, however, those with DD genotype showed greater increase in intimal thickness than the other two genotypes [101] and in elderly Japanese individuals, greater upper-body strength, as defined by grip strength etc, was associated with the DD genotype [102].
A few studies have also been reported where there is no immediate reason for predicting an association with ACE genotype. A study of polycystic ovary disease found no association [103], as did a study of spontaneous miscarriages [104]. No differences in gene frequencies were seen in normotensive individuals suffering obstructive sleep apnoea compared to normal controls, although the I allele was associated with mild to moderate obstructive sleep apnoea in hypertensive individuals [105]. Finally no association between the polymorphisms and different ‘constitutions’ defined within an oriental medicine system [106].
Conclusions
This review has concentrated on publications in 2009 only, primarily to illustrate the extent to which associations are being sought between common gene polymorphisms and illnesses. It should be remembered that approximately 55% of individuals carry the D allele, it is therefore unlikely that it will account for a large proportion of the variance associated with disorders affecting perhaps less than 5% of the population. That being said, based on the publications of 2009 is might be surmised that: The D allele carries with it an increased risk of hypertension, pre-eclampsia, heart failure, cerebral infarct, diabetic nephropathy, encephalopathy, asthma, severe hypoglycaemia in diabetes, gastric cancer (in Caucasians) and poor prognosis following kidney transplant, On the positive side the D allele appears to offer protection against schizophrenia and chronic periodontitis and confers greater upper-body strength in old age.
The I allele, meanwhile, offers improved endurance / athletic performance and aerobic capacity as determined by lung function tests, although it does increase the risk of oral squamous cell carcinoma and obstructive sleep apnoea in hypertensives.
Perhaps the final statement should come from Yang and colleagues, who, in a study in Han Chinese reported that there was no association between ACE gene I/D polymorphism and longevity [107].
References
- 1.Tigerstedt R, Bergman P. Niere und kreislauf. Scand Arch Physiol. 1898;8:223–70. [Google Scholar]
- 2.Goldblatt H, Lynch J, Hanzal R, Summerville W. Studies on experimental hypertension: 1. The production of persistent elevation of systolic blood pressure by means of renal ischaemia. J Exp Med. 1934;59:347–80. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pickering G, et al. Some observations on renin, a pressor substance contained in normal kidney, together with a method for its biological assay. Clin Sci. 1938;3:211–27. [Google Scholar]
- 4.Braun-Menendez E, Fasciolo J, Leloir L, Munoz J. The substance causing renal hypertension. J Physiol (Lond) 1940;98:293–8. doi: 10.1113/jphysiol.1940.sp003850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Page I, Helmer O. A crystalline pressor substance (angiotonin) resulting from the action between renin and renin-activator. J Exp Med. 1940;71:29–42. doi: 10.1084/jem.71.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott D, Peart W. Amino acid sequence in a hypertensin. Nature. 1956;177:527–8. doi: 10.1038/177527a0. [DOI] [PubMed] [Google Scholar]
- 7.Skeggs L, Kahn J, Shumway N. Preparation and function of the hypertensin-converting enzyme. J Exp Med. 1956;103:295–9. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gard PR. The role of angiotensin II in cognition and behaviour. Eur J Pharmacol. 2002;438:1–14. doi: 10.1016/s0014-2999(02)01283-9. [DOI] [PubMed] [Google Scholar]
- 9.Stragier B, Clinckers R, Meurs A, De Bundel D, Sarre S, Ebinger G, Michotte Y, Smolders I. Involvement of the somatostatin-2 receptor in the anti-convulsant effect of angiotensin IV against pilocarpine-induced limbic seizures in rats. J Neurochem. 2006;98:1100–13. doi: 10.1111/j.1471-4159.2006.03942.x. [DOI] [PubMed] [Google Scholar]
- 10.Benicky J, Sanchez-Lemus E, Pavel J, Saavedra JM. Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cell Mol Neurobiol. 2009;29:781–92. doi: 10.1007/s10571-009-9368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira S. A bradykinin-potentiating factor (BPF) present in the venom of Bothrops jararaca. Br J Pharmacol. 1965;24:163–9. doi: 10.1111/j.1476-5381.1965.tb02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erdos E, Sloane E. An enzyme in human blood plasma that inactivates bradykinin and kallidins. Biochem Pharmacol. 1962;11:39–43. doi: 10.1016/0006-2952(62)90119-3. [DOI] [PubMed] [Google Scholar]
- 13.Gibbs CR, Beevers DG, Lip GYH. The management of hypertensive disease in black patients. Q J Med. 1999;92:187–92. doi: 10.1093/qjmed/92.4.187. [DOI] [PubMed] [Google Scholar]
- 14.Wright JT, Dunn JK, Cutler JA, Davis BR, Cushman WC, Ford CE, et al. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA. 2005;293:1595–607. doi: 10.1001/jama.293.13.1595. [DOI] [PubMed] [Google Scholar]
- 15.Exner DV, Dries DL, Domanski MJ, Cohn JN. Lesser response to angiotensin-converting-enzyme inhibitor therapy in black as compared with white patients with left ventricular dysfunction. New Engl J Med. 2001;344:1351–57. doi: 10.1056/NEJM200105033441802. [DOI] [PubMed] [Google Scholar]
- 16.Bibbins-Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD, Hulley SB. Racial differences in incident heart failure among young adults. New Engl J Med. 2009;360:1179–90. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shekelle PG, Rich MW, Morton SC, Atkinson SW, Tu WL, Maglione M, Rhodes S, Barrett M, Fonarow GC, Greenberg B, Heidenreich PA, Knabel T, Konstam MA, Steimle A, Stevenson LW. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status - a meta-analysis of major clinical trials. J Am Coll Cardiol. 2003;41:1529–38. doi: 10.1016/s0735-1097(03)00262-6. [DOI] [PubMed] [Google Scholar]
- 18.Latado AL, Lopes MB, Passos LCS, Lopes AA. Is there evidence to treat heart failure based on race or ethnicity? Revista Da Associacao Medica Brasileira. 2009;55:110–16. doi: 10.1590/s0104-42302009000200010. [DOI] [PubMed] [Google Scholar]
- 19.Castellon R, Hamdi HK. Demystifying the ace polymorphism: From genetics to biology. Curr Pharm Design. 2007;13:1191–98. doi: 10.2174/138161207780618902. [DOI] [PubMed] [Google Scholar]
- 20.Jeunemaitre X. Genetics of the human renin angiotensin system. J Mol Med. 2008;86:637–41. doi: 10.1007/s00109-008-0344-0. [DOI] [PubMed] [Google Scholar]
- 21.Rieder MJ, Taylor SL, Clark AG, Nickerson DA. Sequence variation in the human angiotensin converting enzyme. Nature Genet. 1999;22:59–62. doi: 10.1038/8760. [DOI] [PubMed] [Google Scholar]
- 22.Rigat B, Hubert C, Alhencgelas F, Cambien F, Corvol P, Soubrier F. An insertion deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–46. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staessen JA, Ginocchio G, Wang JG, Saavedra AP, Soubrier F, Vlietinck R, Fagard R. Genetic variability in the renin-angiotensin system: Prevalence of alleles and genotypes. J Cardiovasc Risk. 1997;4:401–22. [PubMed] [Google Scholar]
- 24.Saab YB, Gard PR, Overall ADJ. The geographic distribution of the ACE II genotype: A novel finding. Genet Res. 2007;89:259–67. doi: 10.1017/S0016672307009019. [DOI] [PubMed] [Google Scholar]
- 25.Salem AH, Batzer MA. High frequency of the D allele of the angiotensin-converting enzyme gene in arabic populations. BMC Res Notes. 2009;2:99. doi: 10.1186/1756-0500-2-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danser AHJ, Deinum J, Osterop A, Admiraal PJJ, Schalekamp M. Angiotensin I to angiotensin II conversion in the human forearm and leg. Effect of the angiotensin converting enzyme gene insertion/deletion polymorphism. J Hypertens. 1999;17:1867–72. doi: 10.1097/00004872-199917121-00014. [DOI] [PubMed] [Google Scholar]
- 27.Anbazhagan K, Sampathkumar K, Ramakrishnan M, Gomathi P, Gomathi S, Selvam GS. Analysis of polymorphism in renin angiotensin system and other related genes in south indian chronic kidney disease patients. Clinica Chimica Acta. 2009;406:108–12. doi: 10.1016/j.cca.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Jiang X, Sheng H, Li J, Xun P, Cheng Y, Huang J, Xiao H, Zhan Y. Association between renin-angiotensin system gene polymorphism and essential hypertension: A community-based study. J Hum Hypertens. 2009;23:176–81. doi: 10.1038/jhh.2008.123. [DOI] [PubMed] [Google Scholar]
- 29.Alvi FM, Hasnain S. Ace I/D and G2350A polymorphisms in Pakistani hypertensive population of Punjab. Clin Exper Hypertens. 2009;31:471–80. doi: 10.1080/10641960902825479. [DOI] [PubMed] [Google Scholar]
- 30.Eisenmann JC, Sarzynski MA, Glenn K, Rothschild M, Heelan KA. ACE I/D genotype, adiposity, and blood pressure in children. Cardiovasc Diabetol. 2009;8:14. doi: 10.1186/1475-2840-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tascilar N, Dursun A, Ankarali H, Mungan G, Ekem S, Baris S. Angiotensin-converting enzyme insertion/deletion polymorphism has no effect on the risk of atherosclerotic stroke or hypertension. J Neurol Sci. 2009;285:137–41. doi: 10.1016/j.jns.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Filigheddu F, Argiolas G, Bulla E, Troffa C, Bulla P, Fadda S, Zaninello R, Degortes S, Frau F, Pitzoi S, Glorioso N. Clinical variables, not RAAS polymorphisms, predict blood pressure response to ACE inhibitors in Sardinians. Pharmacogenomics. 2008;9:1419–27. doi: 10.2217/14622416.9.10.1419. [DOI] [PubMed] [Google Scholar]
- 33.Moraes CF, Souza ER, Souza VC, Medeiros EFF, Goncalves TF, Toledo JO, Karnikowski M, Gomes L, Larnikowski MGO, Cordova C, Nobrega OT. A common polymorphism in the renin angiotensin system is associated with differential outcome of antihypertensive pharmacotherapy prescribed to Brazilian older women. Clinica Chimica Acta. 2008;396:70–75. doi: 10.1016/j.cca.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Mando C, Antonazzo P, Tabano S, Zanutto S, Pileri P, Somigliana E Colleoni F, Martinelli A, Zolin A, Benedetto C, Marozio L, Neri I, Facchinetti F, Miozzo M, Cetin I. Angiotensin-converting enzyme and adducin-1 polymorphisms in women with preeclampsia and gestational hypertension. Reprod Sci. 2009;16:819–26. doi: 10.1177/1933719109336612. [DOI] [PubMed] [Google Scholar]
- 35.Suonsyrja T, Hannila-Handelberg T, Fodstad H, Donner K, Kontula K, Hiltunen TP. Renin-angiotensin system and alpha-adducin gene polymorphisms and their relation to responses to antihypertensive drugs: Results from the genres study. Am J Hypertens. 2009;22:169–75. doi: 10.1038/ajh.2008.343. [DOI] [PubMed] [Google Scholar]
- 36.Schelleman H, Klungel OH, van Duijn CM, Witteman JCM, Hofman A, de Boer A, Stricker BHC. Drug-gene interaction between the inser-tion/deletion polymorphism of the angiotensin-converting enzyme gene and anti hypertensive therapy. Ann Pharmacother. 2006;40:212–18. doi: 10.1345/aph.1G316. [DOI] [PubMed] [Google Scholar]
- 37.Kurbanova DR, Srozhidinova NZ, Tursunova NB, Eliseeva MR. Pharmacogenetic aspects of eprosartan therapy and polymorphic markers of renin-angiotensin-aldosterone system genes in uzbek patients with essential arterial hypertension. Cardiovasc Therapy Prevent. 2009;8:41–46. [Google Scholar]
- 38.Woo SW, Bang S, Chung MW, Jin SK, Kim YS, Lee SH. Lack of association between ACE and bradykinin B2 receptor gene polymorphisms and ACE inhibitor-induced coughing in hypertensive Koreans. J. Clin. Pharm. Therapeutics. 2009;34:561–67. doi: 10.1111/j.1365-2710.2009.01028.x. [DOI] [PubMed] [Google Scholar]
- 39.Wu CK, Luo JL, Wu XM, Tsai CT, Lin JW, Hwang JJ, Lin JL, Tseng CD, Chiang FT. A propensity score-based case-control study of renin-angiotensin system gene polymorphisms and diastolic heart failure. Atherosclerosis. 2009;205:497–502. doi: 10.1016/j.atherosclerosis.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 40.Sipahi T, Guldiken B, Guldiken S, Ustundag S, Turgut N, Budak M, Cakina S, Ozkan H, Sener S. The association of gene polymorphisms of the angiotensin-converting enzyme and angiotensin II receptor type 1 with ischemic stroke in Turkish subjects of Trakya region. Trakya Universitesi Tip Fakultesi Dergisi. 2009;26:1–8. [Google Scholar]
- 41.Celiker G, Can U, Verdi H, Yazici AC, Ozbek N, Atca FB. Prevalence of thrombophilic mutations and ACE I/D polymorphism in Turkish ischemic stroke patients. Clin Appl Thromb Hemost. 2009;15:415–20. doi: 10.1177/1076029608315163. [DOI] [PubMed] [Google Scholar]
- 42.Kitsios G, Zintzaras E. ACE (I/D) polymorphism and response to treatment in coronary artery disease: A comprehensive database and meta-analysis involving study quality evaluation. BMC Med Genet. 2009;10:50. doi: 10.1186/1471-2350-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schurks M, Zee RYL, Buring JE, Kurth T. ACE D/I polymorphism, migraine, and cardiovascular disease in women. Neurology. 2009;72:650–56. doi: 10.1212/01.wnl.0000342517.97178.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rallidis LS, Gialeraki A, Varounis C, Dagres N, Kotakos C, Travlou A, Lekakis J, Kremastinos DT. Lack of association of angiotensin-converting enzyme insertion/deletion polymorphism and myocardial infarction at very young ages. Bio-markers. 2009;14:401–05. doi: 10.1080/13547500903039966. [DOI] [PubMed] [Google Scholar]
- 45.Malygina NA, Kostomarova IV, Melentyev IA, Melentyev AS, Vershinin AA, Serova LD. Molecular and genetic markers for coronary heart disease prognosis in elderly patients. Russian J Cardiol. 2009;4:68–72. [Google Scholar]
- 46.Voevoda MI, Kulikov IV, Maximov VN, Malyutina SK, Shakhtshneider EV, Kulishova LM, Novoselov VP, Romashchenko AG. Sudden cardiac death and polymorphism of genes-candidates of cardiovascular diseases. Kardiologiya. 2009;49:52–57. [PubMed] [Google Scholar]
- 47.Sie MPS, Yazdanpanah M, Mattace-Raso FUS, Uitterlinden AG, Hofman A, Hoeks APG, Reneman RS, Asmar R, van Duijn CM, Witteman JCM. Genetic variation in the renin-angiotensin system and arterial stiffness. The Rotterdam study. Clin Exper Hypertens. 2009;31:389–99. doi: 10.1080/10641960802668706. [DOI] [PubMed] [Google Scholar]
- 48.Tanabe Y, Kawasaki R, Wang JJ, Wong TY, Mitchell P, Daimon M, Oizumi T, Kato T, Kawata S, Kayama T, Yamashita H. Angiotensin-converting enzyme gene and retinal arteriolar narrowing: The Funagata study. J Hum Hypertens. 2009;23:788–93. doi: 10.1038/jhh.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalay N, Caglayan O, Akkaya H, Ozdogru I, Dogan A, Inanc MT, Kaya MG, Ergin A, Topsakal R, Cicek D, Eryol NK, Tasdemir K, Oguzhan A, Dundar M. The deletion polymorphism of the angiotensin -converting enzyme gene is associated with acute aortic dissection. Tohoku J Exp Med. 2009;219:33–37. doi: 10.1620/tjem.219.33. [DOI] [PubMed] [Google Scholar]
- 50.Korcz A, Mikolajczyk-Stecyna J, Gabriel M, Zowczak-Drabarczyk M, Pawlaczyk K, Kalafirov M, Oszkinis G, Slomski R. Angiotensin-converting enzyme (ACE, I/D) gene polymorphism and susceptibility to abdominal aortic aneurysm or aortoiliac occlusive disease. J Surg Res. 2009;153:76–82. doi: 10.1016/j.jss.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 51.Tao HM, Shao B, Chen GZ. Meta-analysis of the ACE gene polymorphism in cerebral infarction. Can J Neurol Sci. 2009;36:20–25. doi: 10.1017/s0317167100006259. [DOI] [PubMed] [Google Scholar]
- 52.Joshi G, Pradhan S, Mittal B. Role of the ACE ID and MTHFR C677T polymorphisms in genetic susceptibility of migraine in a North Indian population. J Neurol Sci. 2009;277:133–37. doi: 10.1016/j.jns.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 53.Nakayama Y, Nonoguchi H, Kohda Y, Inoue H, Memetimin H, Izumi Y, Tomita K. Different mechanisms for the progression of CKD with ACE gene polymorphisms. Nephron Clin Pract. 2009;111:C240–C46. doi: 10.1159/000209150. [DOI] [PubMed] [Google Scholar]
- 54.Vegter S, Perna A, Hiddema W, Ruggenenti P, Remuzzi G, Navis G, Postma MJ. Cost-effectiveness of ACE inhibitor therapy to prevent dialysis in nondiabetic nephropathy: Influence of the ACE insertion/deletion polymorphism. Pharmacogenet Genomics. 2009;19:695–703. doi: 10.1097/FPC.0b013e3283307ca0. [DOI] [PubMed] [Google Scholar]
- 55.Siekierka-Harreis M, Kuhr N, Willers R, Ivens K, Grabensee B, Mondry A, Loh MCS, Rump LC, Blume C. Impact of genetic polymorphisms of the renin-angiotensin system and of non-genetic factors on kidney transplant function - a single-center experience. Clin Transplant. 2009;23:606–15. doi: 10.1111/j.1399-0012.2009.01033.x. [DOI] [PubMed] [Google Scholar]
- 56.Ahluwalia TS, Ahuja M, Rai TS, Kohli HS, Bhansali A, Sud K, Khullar M. ACE variants interact with the RAS pathway to confer risk and protection against type 2 diabetic nephropathy. DNA Cell Biol. 2009;28:141–50. doi: 10.1089/dna.2008.0810. [DOI] [PubMed] [Google Scholar]
- 57.Ezzidi I, Mtiraoui N, Kacem M, Chaieb M, Mahjoub T, Almawi WY. Identification of specific angiotensin-converting enzyme variants and haplotypes that confer risk and protection against type 2 diabetic nephropathy. Diabetes-Metab Res Rev. 2009;25:717–24. doi: 10.1002/dmrr.1006. [DOI] [PubMed] [Google Scholar]
- 58.Nikzamir A, Esteghamati A, Feghhi M, Nakhjavani M, Rashidi A, Reza JZ. The insertion/deletion polymorphism of the angiotensin-converting enzyme gene is associated with progression, but not development, of albuminuria in Iranian patients with type 2 diabetes. JRAAS. 2009;10:109–14. doi: 10.1177/1470320309104872. [DOI] [PubMed] [Google Scholar]
- 59.Palomo-Pinon S, Gutierrez-Rodriguez ME, Diaz-Flores M, Sanchez-Barrera R, Valladares-Salgado A, Utrera-Barillas D, Utrera-Barillas D, Duran-Reyes G, Galvan-Duarte RE, Trinidad-Ramos P, Cruz M. DD genotype of angiotensin-converting enzyme in type 2 diabetes mellitus with renal disease in Mexican mestizos. Nephrol. 2009;14:235–39. doi: 10.1111/j.1440-1797.2008.01034.x. [DOI] [PubMed] [Google Scholar]
- 60.Ng D, Ramli SNB, Chia KS, Koh D, Tai BC. Genetic variation at the angiotensin-I converting enzyme locus and the risk for diabetic nephropathy: A study based on 53 studies and 17 791 subjects. Salud I Ciencia. 2009;16:751–54. [Google Scholar]
- 61.Pandey P, Pradhan S, Modi DR, Mittal B. MTHFR and ACE gene polymorphisms and risk of vascular and degenerative dementias in the elderly. Brain Cog. 2009;71:295–99. doi: 10.1016/j.bandc.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 62.Liu H, Liu M, Li W, Wu B, Zhang S-H, Fang Y, Wang Y. Association of ACE I/D gene polymorphism with vascular dementia: A meta-analysis. J Geriatr Psychiatry Neurol. 2009;22:10–22. doi: 10.1177/0891988708328221. [DOI] [PubMed] [Google Scholar]
- 63.Helbecque N, Codron V, Cottel D, Amouyel P. An age effect on the association of common variants of ACE with Alzheimer's disease. Neurosci Lett. 2009;461:181–84. doi: 10.1016/j.neulet.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 64.Miners S, Ashby E, Baig S, Harrison R, Tayler H, Speedy E, Prince JA, Love S, Kehoe PG. An-giotensin-converting enzyme levels and activity in Alzheimer's disease: Differences in brain and CWSF ACE and association with ACE1 genotypes. Am J Transl Res. 2009;1:163–77. [PMC free article] [PubMed] [Google Scholar]
- 65.Saab YB, Gard PR, Yeoman MS, Mfarrej B, El-Moalem H, Ingram MJ. Renin-angiotensin-system gene polymorphisms and depression. Prog. Neuro-Psychopharmacol. Biol Psychiatry. 2007;31:1113–18. doi: 10.1016/j.pnpbp.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 66.Stewart JA, Kampman O, Huuhka M, Anttila S, Huuhka K, Lehtimaki T, Leinonen E. ACE polymorphism and response to electroconvulsive therapy in major depression. Neurosci Lett. 2009;458:122–25. doi: 10.1016/j.neulet.2009.04.057. [DOI] [PubMed] [Google Scholar]
- 67.Fudalej S, Fudalej M, Kostrzewa G, Kuzniar P, Franaszczyk M, Wojnar M, Krajewski P, Ploski R. Angiotensin-converting enzyme polymorphism and completed suicide: An association in Caucasians and evidence for a link with a method of self-injury. Neuropsychobiology. 2009;59:151–58. doi: 10.1159/000218077. [DOI] [PubMed] [Google Scholar]
- 68.Sparks DL, Hunsaker JC, Amouyel P, Malafosse A, Bellivier F, Leboyer M. Courtet P and Helbecque N. Angiotensin I-converting enzyme I/D polymorphism and suicidal behaviors. Am J Med Genet Part B-Neuropsychiatric Genetics. 2009;150B:290–94. doi: 10.1002/ajmg.b.30793. [DOI] [PubMed] [Google Scholar]
- 69.Harvey BH. Is major depressive disorder a metabolic encephalopathy? Hum Psychopharmacol Clin Exp. 2008;23:371–84. doi: 10.1002/hup.946. [DOI] [PubMed] [Google Scholar]
- 70.Kehoe AD, Nikiforov AM, Alexanin SS, Neronov EG, Tikhomirova OV, Shun'kov VB, Makarova NV, Rabinovich E, Usmanova NM, Kazakov VI, Slozina NM, Montgomery HE. Angiotensinconverting enzyme genotype and encephalopathy in chernobyl cleanup workers. Eur J Neurol. 2009;16:95–100. doi: 10.1111/j.1468-1331.2008.02355.x. [DOI] [PubMed] [Google Scholar]
- 71.Braszko JJ, Holy ZZ, Kupryszewski G, Witczuk B. Effect of angiotensin-II, its 2-8, 3-8, 4-8 fragments and saralasin on the level and turnover of dopamine in the striatum and olfactory tubercle of the rat. Asia Pac J Pharmacol. 1991;6:243–47. [Google Scholar]
- 72.Pascale E, Purcaro C, Passarelli E, Guglielmi R, Vestri AR, Passarelli F, Meco G. Genetic polymorphism of angiotensin-converting enzyme is not associated with the development of Parkinson's disease and of L-dopa-induced adverse effects. J Neurol Sci. 2009;276:18–21. doi: 10.1016/j.jns.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 73.Crescenti A, Gasso P, Mas S, Abellana R, Deulofeu R, Parellada E, Bernardo M, Lafuente A. Insertion/deletion polymorphism of the angiotensin-converting enzyme: Gene is associated with schizophrenia in a Spanish population. Psychiatry Res. 2009;165:175–80. doi: 10.1016/j.psychres.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 74.Coto-Segura P, Alvarez V, Soto-Sanchez J, Morales B, Coto E, Santos-Juanes J. Lack of association between angiotensin I-converting enzyme insertion/deletion polymorphism and psoriasis or psoriatic arthritis in Spain. Int J Dermatol. 2009;48:1320–23. doi: 10.1111/j.1365-4632.2009.04245.x. [DOI] [PubMed] [Google Scholar]
- 75.Pehlivan S, Ozkinay F, Alper S, Onay H, Yuksel E, Pehlivan M, Ozkinay C. Association between IL4 (−590), ACE (I)/(D), CCR5 (Delta 32), CTLA4 (+49) and IL1-RN (VNTR intron 2) gene polymorphisms and vitiligo. Eur J Dermatol. 2009;19:126–28. doi: 10.1684/ejd.2008.0578. [DOI] [PubMed] [Google Scholar]
- 76.Gurkan A, Emingil G, Saygan BH, Atilla G, Kose T, Baylas H, Berdeli A. Angiotensin-converting enzyme (ACE), angiotensinogen (AGT), and angiotensin II type 1 receptor (AT1R) gene polymorphisms in generalized aggressive periodontitis. Arch Oral Biol. 2009;54:337–44. doi: 10.1016/j.archoralbio.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 77.Gurkan A, Emingil G, Saygan BH, Atilla G, Kose T, Baylas H, Berdeli A. Renin-angiotensin gene polymorphisms in relation to severe chronic periodontitis. J Clin Periodontol. 2009;36:204–11. doi: 10.1111/j.1600-051X.2008.01379.x. [DOI] [PubMed] [Google Scholar]
- 78.Eryuksel E, Ceyhan BB, Bircan R, Avsar M, Cirakoglu B. Angiotensin converting enzyme gene polymorphism in Turkish asthmatic patients. J Asthma. 2009;46:335–38. doi: 10.1080/02770900802660972. [DOI] [PubMed] [Google Scholar]
- 79.Guo S, Zhang JH, Yan YD, Ding YF, Sheng YY. Association between renin-angiotensin system gene polymorphism and recurrent wheezing in Chinese children: A 4-year follow-up study. J Int Med Res. 2009;37:351–58. doi: 10.1177/147323000903700209. [DOI] [PubMed] [Google Scholar]
- 80.Hucl T, Kylanpaa M-L, Kunzli B, Witt H, Lempinen M, Schneider A, Kemppainen E, Lohr M, Haas SL, Friess H, Ockenga J, Rosendahl J, Schulz HU, Gress T, Singer MV, Pfutzer RH. Angiotensin-converting enzyme insertion/deletion polymorphism in patients with acute and chronic pancreatitis. Eur J Gastroenterol Hepatol. 2009;21:1032–5. doi: 10.1097/MEG.0b013e328326f586. [DOI] [PubMed] [Google Scholar]
- 81.Oruc N, Papachristou GI, Avula H, Slivka A, Lamb J, Whitcomb DC. Angiotensin-converting enzyme gene DD genotype neither increases susceptibility to acute pancreatitis nor influences disease severity. HPB (Oxford) 2009;11:45–9. doi: 10.1111/j.1477-2574.2008.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pedersen-Bjergaard U, Nielsen SL, Akram K, Perrild H, Nordestgaard BG, Montgomery HE, Pramming S, Thorsteinsson B. Angiotensin-converting enzyme and angiotensin II receptor subtype 2 genotypes in type 1 diabetes and severe hypoglycaemia requiring emergency treatment: A case cohort study. Pharmacogenet Genomics. 2009;19:864–68. doi: 10.1097/FPC.0b013e328331e67b. [DOI] [PubMed] [Google Scholar]
- 83.Baroudi T, Bouhaha R, Moran-Moguel C, Sanchez-Corona J, Maiz HB, Abid HK, Benammar-Elgaaied A. Association of the insertion/deletion polymorphism of the angiotensin-converting enzyme gene with type 2 diabetes in two ethnic groups of Jerba island in Tunisia. JRAAS. 2009;10:35–40. doi: 10.1177/1470320309102314. [DOI] [PubMed] [Google Scholar]
- 84.Chmaisse HN, Jammal M, Fakhoury H, Fakhoury R. A study on the association between angiotensin-I converting enzyme I/D dimorphism and type-2 diabetes mellitus. Saudi J Kidney Dis Transpl. 2009;20:1038–46. [PubMed] [Google Scholar]
- 85.Akin F, Turgut S, Dursunoglu D, Turgut G, Karasu U, Gur S. ACE gene polymorphism and cardiac structure in patients with insulin resistance. Mol Biol Rep. 2009;36:623–29. doi: 10.1007/s11033-008-9222-7. [DOI] [PubMed] [Google Scholar]
- 86.Sivakova D, Lajdova A, Basistova Z, Cvicelova M, Blazicek P. ACE insertion/deletion polymorphism and its relationships to the components of metabolic syndrome in elderly Slovaks. Anthropologischer Anzeiger. 2009;67:1–11. [PubMed] [Google Scholar]
- 87.Settin AA, Algasham A, Dowaidar M, Ismail H. Methylene tetrahydrofolate reductase and angiotensin converting enzyme gene polymorphisms related to overweight/obesity among Saudi subjects from Qassim region. Dis Markers. 2009;27:97–102. doi: 10.3233/DMA-2009-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alves Correa SA, Ribeiro de Noronha SM, Nogueira-de-Souza NC, Valleta de Carvalho C, Massad Costa AM, Juvenal Linhares J, Vieira Gomes MT, Guerreiro da Silva IDC. Association between the angiotensin-converting enzyme (insertion/deletion) and angiotensin II type 1 receptor (A1166C) polymorphisms and breast cancer among Brazilian women. JRAAS. 2009;10:51–8. doi: 10.1177/1470320309102317. [DOI] [PubMed] [Google Scholar]
- 89.Toma M, Cimponeriu D, Apostol P, Stavarachi M, Cojocaru M, Belusica L, Craciun AM, Radu I, Gavrila L. Lack of association between ACE ID polymorphism and colorectal cancer in Romanian patients. Chirurgia. 2009;104:553–56. [PubMed] [Google Scholar]
- 90.Vairaktaris E, Serefoglou Z, Avgoustidis D, Yapijakis C, Critselis E, Vylliotis A, Spyridonidou S, Derka S, Vdssfou S, Nkenke E, Patsouris E. Gene polymorphisms related to angiogenesis, inflammation and thrombosis that influence risk for oral cancer. Oral Oncol. 2009;45:247–53. doi: 10.1016/j.oraloncology.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 91.Loh M, Koh KX, Yeo BH, Song CM, Chia KS, Zhu F, Yeoh KG, Hill J, Iacopetta B, Soong R. Meta-analysis of genetic polymorphisms and gastric cancer risk: Variability in associations according to race. Eur J Cancer. 2009;45:2562–68. doi: 10.1016/j.ejca.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 92.Sugimoto M, Furuta T, Shirai N, Ikuma M, Sugimura H, Hishida A. Influences of chymase and angiotensin I-converting enzyme gene polymorphisms on gastric cancer risks in Japan. Cancer Epidemiol Biomarkers Prev. 2006;15:1929–34. doi: 10.1158/1055-9965.EPI-06-0339. [DOI] [PubMed] [Google Scholar]
- 93.Ebert MPA, Lendeckel U, Westphal S, Dierkes J, Glas J, Folwaczny C, Roessner A, Stolte M, Malfertheiner P, Rocken C. The angiotensin I-converting enzyme gene insertion/deletion polymorphism is linked to early gastric cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2987–89. doi: 10.1158/1055-9965.EPI-05-0411. [DOI] [PubMed] [Google Scholar]
- 94.Min S-K, Takahashi K, Ishigami H, Hiranuma K, Mizuno M, Ishii T, Kim C-S, Nakazato K. Is there a gender difference between ACE gene and race distance? Appl Physiol Nutr Metab. 2009;34:926–32. doi: 10.1139/H09-097. [DOI] [PubMed] [Google Scholar]
- 95.Cieszczyk P, Krupecki K, Maciejewska A, Sawczuk M. The angiotensin converting enzyme gene I/D polymorphism in Polish rowers. Int J Sports Med. 2009;30:624–27. doi: 10.1055/s-0029-1202825. [DOI] [PubMed] [Google Scholar]
- 96.Juffer P, Furrer R, Gonzalez-Freire M, Santiago C, Verde Z, Serratosa L, Morate FJ, Rubio JC, Martin MA, Ruiz JR, Arenas J, Gomez-Gallego F, Lucia A. Genotype distributions in top-level soccer players: A role for ACE? Int J Sports Med. 2009;30:387–92. doi: 10.1055/s-0028-1105931. [DOI] [PubMed] [Google Scholar]
- 97.Costa AM, Silva AJ, Garrido ND, Louro H, de Oliveira RJ, Breitenfeld L. Association between ACE D allele and elite short distance swimming. Eur J App Physiol. 2009;106:785–90. doi: 10.1007/s00421-009-1080-z. [DOI] [PubMed] [Google Scholar]
- 98.Goh KP, Chew K, Koh A, Guan M, Wong YS, Sum CF. The relationship between ACE gene ID polymorphism and aerobic capacity in Asian rugby players. Singapore Med J. 2009;50:997–1003. [PubMed] [Google Scholar]
- 99.Alves GB, Oliveira EM, Alves CR, Rached HRS, Mota GFA, Pereira AC, Rondon MU, Hashimoto NY, Azevedo LF, Krieger JE, Negrao CE. Influence of angiotensinogen and angiotensin-converting enzyme polymorphisms on cardiac hypertrophy and improvement on maximal aerobic capacity caused by exercise training. Eur J Cardiovasc Prev Rehabil. 2009;16:487–92. doi: 10.1097/HJR.0b013e32832c5a8a. [DOI] [PubMed] [Google Scholar]
- 100.Kalson NS, Thompson J, Davies AJ, Stokes S, Earl MD, Whitehead A, Tyrrell-Marsh I, Frost H, Montgomery H. The effect of angiotensin-converting enzyme genotype on acute mountain sickness and summit success in trekkers attempting the summit of Mt. Kilimanjaro (5,895 m) Eur J App Physiol. 2009;105:373–79. doi: 10.1007/s00421-008-0913-5. [DOI] [PubMed] [Google Scholar]
- 101.Kim K. Association of angiotensin-converting enzyme insertion/deletion polymorphism with obesity, cardiovascular risk factors and exercise-mediated changes in Korean women. Eur J App Physiol. 2009;105:879–87. doi: 10.1007/s00421-008-0973-6. [DOI] [PubMed] [Google Scholar]
- 102.Yoshihara A, Tobina T, Yamaga T, Ayabe M, Yoshitake Y, Kimura Y, Shimada M, Nishimuta M, Nakagawa N, Ohashi M, Hanada N, Tanaka H, Kiyonaga A, Miyazaki H. Physical function is weakly associated with angiotensin-converting enzyme gene I/D polymorphism in elderly Japanese subjects. Gerontology. 2009;55:387–92. doi: 10.1159/000222429. [DOI] [PubMed] [Google Scholar]
- 103.Sun J, Fan HJ, Che YN, Cao YX, Wu XK, Sun HX, Liang FJ, Yi L, Wang Y. Association between ACE gene I/D polymorphisms and hyperandrogenism in women with polycystic ovary syndrome (PCOS) and controls. BMC Med Genet. 2009;10:64. doi: 10.1186/1471-2350-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goodman C, Hur J, Goodman CS, Jeyendran RS, Coulam C. Are polymorphisms in the ACE and PAI-1 genes associated with recurrent spontaneous miscarriages? Am J Reprod Immunol. 2009;62:365–70. doi: 10.1111/j.1600-0897.2009.00744.x. [DOI] [PubMed] [Google Scholar]
- 105.Koyama RG, Drager LF, Lorenzi-Filho G, Cintra FD, Pereira AC, Poyares D, Krieger JE, Castro RMRPS, Tufik S, de Mello MT, Pedrazzoli M. Reciprocal interactions of obstructive sleep apnea and hypertension associated with ACE I/D polymorphism in males. Sleep Med. 2009;10:1107–11. doi: 10.1016/j.sleep.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 106.Lee SY, Kang BY. DNA variations of renin-angiotensin system genes in sasang constitution. Gazzetta Medica Italiana Archivio per le Scienze Mediche. 2009;168:81–87. [Google Scholar]
- 107.Yang JK, Gong YY, Xie L, Lian SG, Yang J, Xu LY, Gao SJ, Zhang Y P. Lack of genetic association between the angiotensin-converting enzyme gene insertion/deletion polymorphism and longevity in a Han Chinese population. JRAAS. 2009;10:115–18. doi: 10.1177/1470320309104873. [DOI] [PubMed] [Google Scholar]