Abstract
The sirtuins are a family of proteins remarkably conserved from yeast to humans. In organisms such as yeast, worms and flies it is quite well established that the activity of sirtuins prolongs lifespan. As a result of promising findings in simple organisms, sirtuins are now investigated in higher organisms in relation to the ageing process. In mammals there are seven different sirtuin proteins each encoded by individual genes (SIRT1-7). Although sirtuins share a highly conserved catalytic domain, they differ in their biological function. Some mammalian sirtuins have been implicated in different ageing pathways and their modulation has been deemed to be beneficial in different models of age-associated diseases. Overall, sirtuins could contribute to mechanisms of human longevity and avoid or delay the onset of age-associated disorders. Here we review and discuss the potential impact of genetic variation in the sirtuin genes in relation to human longevity and age-related diseases.
Keywords: Ageing, longevity, sirtuin, SIRT1, SIRT3, SIRT4, genetic variation
Introduction
The sirtuins belong to a family of conserved genes found in organisms ranging from bacteria to mammals [1]. The mammal genome contains seven sirtuin genes (SIRT1-7) which share a highly conserved NAD+-binding catalytic domain [2]. Despite their homology, sirtuins seem to have specific and individual biological functions due to their different substrates, subcellular localization and patterns of expression, as summarized in TABLE 1 [3–20]. The first member of this gene family to be identified was SIR2, from yeast Saccharomyces cerevisiae (Sc). In 1999, not long after its identification, SIR2 was shown to extend the lifespan of Sc by repressing genome instability [21, 22]. A functional role of SIR2 in ageing was later demonstrated in other model organisms including Caenorhabditis ele-gans and Drosophila melanogaster [23]. Recent findings have further highlighted the involvement of mammalian sirtuins in age-associated disorders such as metabolic diseases, cancer, cardiovascular disease, neurodegeneration and inflammation, thus suggesting a promising role for sirtuins as therapeutic targets against ageing-linked pathologies (reviewed in [24]). In the light of these findings, a growing interest to understand the involvement of sirtuins in ageing and age-associated diseases has arisen. This review summarizes the impact of variation in the sirtuin genes that support a role for human sirtuins in modulating lifespan and disease susceptibility at older ages. In particular, we focus on SIRT1, the most researched sirtuin to date, while the relevance of the other sirtuins is less well established and a matter of debate.
Table 1.
Sirtuin localization, activity, cellular targets, biological effect and possible pathologic area
| Sirtuin | Localization | Activity | Targets | Biological effect | Disease area |
|---|---|---|---|---|---|
| SIRT1 | Nucleus | Deacetylase | PGC-1α, FOXO1, LXR, PPARγ, UCP2, PTP1B, MyoD | Gluconeogenesis (+), Fatty acid oxidation (+), Cholesterol scavenging (+), Adipogenesis (-), Fat storage (-), Mitochondrial activity (+), Thermogenesis (+), Insulin secretion (+), Myogenesis (-) [3] | Metabolic |
| NF-Kb, Ku70, FOXO3, FOXO4, p53 | Stress response (+) , cell survival (+) [4] | Neurological | |||
| P53, c-Myc | Cell proliferation (+,−) [5, 6] | Cancer | |||
| SIRT2 | Cytosol | Deacetylase | Tubulin, Histone H4, FOXO3a | Microtubule stability (+) [7], Cell cycle (+) [8], Stress response (+) [9] | Neurological |
| ART | FOXO1 | Adipogenesis (−) [10] | Metabolic | ||
| SIRT3 | Mitochondria | Deacetylase | ACS2, GDH | Metabolism [11, 12] | Metabolic |
| PGC-1α | Thermogenesis (+) [13] | ||||
| SIRT4 | Mitochondria | ART | GDH, IDE | Insulin secretion (−) [14, 15] | Metabolic |
| SIRT5 | Mitochondria | Deacetylase | CPS1 | Urea cycle (+) [16] | Neurological |
| SIRT6 | Nucleus | Deacetylase | Histone H3k9 | DNA repair (+) [17] | Cancer |
| ART | Histone H3k9 | Glucose homeostasis (+) [18] | Metabolic | ||
| SIRT7 | Nucleolus | Deacetylase | p53 | Stress resistance (+) [19] | Cardiovascular |
| Pol I | rDNA transcription (+) [20] |
ART, ADP-ribosyl-transferase; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1 α, FOXO, forkhead box O; LXR, liver X receptor; PPARγ, peroxisome proliferator-activated receptor γ; UCP2, uncoupling protein 2; PTP1B, protein tyrosine phosphatase 1B; NF-kb, nuclear factor kappa B; ACS2, acetyl-CoA synthetase; GDH, glutamate dehydrogenase; IDE, insulin degrading enzyme; CPS1, carbamoyl phosphate synthetase 1; PolI, DNA polymerase I.
+, − positive or negative regulation
In order to undertake a review on this topic as comprehensive as possible, we conducted a literature review using PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) with the keywords “sirtuin”, “SIRT1”, “SIRT2”, “SIRT3”, “SIRT4”, “SIRT5”, “SIRT6”, “SIRT7”, “polymorphism”, “longevity”, “ageing” and “age-associated disease”.
Sirtuin gene variability and human longevity
Mammalian sirtuins were first investigated for their involvement in calorie restriction (CR), a nutritional regimen that involves the reduction of daily food intake by approximately 30%, suggested to prolong life expectation and delay age -associated disorders [25]. Several studies support a link between sirtuins and the CR pheno-type. It has been shown that SIRT1 null mice have a shorter lifespan than their wild-type (WT) littermates and do not benefit from CR to increase their lifespan [26]. Other in vitro evidence has also indicated the involvement of the other mammalian sirtuins (e.gSIRT3, SIRT4 and SIRT5) in CR phenotype [27, 28].
The life-prolonging effects that genes homologous to SIR2 exert on various model organisms and the emerging evidence of links between sirtuins and CR has prompted a number of investigations into whether common allelic variations in the SIRT genes are associated with exceptional longevity in humans.
SIRT1 and longevity
Flachsbart and coworkers compared 1026 unrelated German long-lived individuals (mean age: 98.3 years) with 547 German control ‘younger’ subjects (mean age: 67.2 years) [29]. They analyzed five known single nucleotide polymorphisms (SNPs) (four of which were tagging SNPs) distributed across the entire SIRT1 gene, designed to capture the majority of the variation in SIRT1 which has been shown to have high levels of linkage disequilibrium (LD) across the gene [29]. No evidence of a genetic association was detected between any of the SNPs tested individually and longevity nor with any of the five haplotypes identified (with a frequency > 1%). A similar result was observed in a Japanese population [30] and by two other European based studies (the Leiden 85-Plus study [31] and the Rotterdam study [32]), where SIRT1 variation was not associated to a reduced risk of mortality.
SIRT3 and longevity
SIRT3 may have a role in modulating longevity not due to its homology to SIR2 but also because it maps to chromosome 11p15.5 that has previously been implicated with life extension by association studies [33]. Rose and co-workers analyzed the genotypic distribution of the SIRT3 silent variation G477T (corresponding to Ser159Ser; AF083108) in relation to longevity [34]. They found a sex-specific relationship between this polymorphism and survival in the elderly in an Italian cohort from Calabria. The association was strongest in elderly males where the homozygous genotype for the minor allele (TT) increased survival (p= 0.0272) while the heterozygous GT genotype appeared to be associated with decreased survival (p= 0.0391). Because of the silent nature of the polymorphism, no functional effect could be suggested for the G477T variation, however, the authors also found another polymorphism, consisting of a variable number of tandem repeats (VNTR) with a 72-bp repeat core which resided in intron 5 of SIRT3 which was highly associated with G477T and appeared to have a putative enhancer function. The authors suggested that this might be the ‘true’ variant responsible for the association [35]. The VNTR polymorphism has six possible alleles depending on the number of core repeats; four alleles were relatively common (alleles 1 to 4 which corresponded to 1-4 repeats), while two alleles of 5 or 6 repeats were more rare. Moreover, the authors also described a functional single nucleotide substitution (T/C) located in the second repeat, 63 base pairs from the starting point of the VNTR. The VNTR region showed an allele-specific enhancer activity depending on the presence of the T/C variant and the number of repeats. Interestingly, by comparing the genotypic and allelic frequencies between two groups of subjects aged 20 to 80 years and 90 to 106-years, the allele lacking the enhancer activity was found to be absent in males older than 90 years, while it was still present in the younger group. This finding suggested that this allele may have a deleterious effect on male longevity.
In a different multicentre study that recruited elderly participants from Central Italy, South Italy and Germany (for a total of 1321 centenarians and 1140 younger subjects) to investigate the 11p15.5 chromosomal region in conjunction with longevity, different SNPs on SIRT3 showed significant association with longevity in the Italian females and in the German male subgroups [36]. However, this result was not supported by an attempted replication study that tested eight polymorphisms in the SIRT3 gene (rs2293168, rs511744, rs3782115, rs7934919, rs1045288, rs939915, rs559422, rs3817630) in a French sample of 546 centenarians and 315 younger subjects [36]. An additional meta-analysis of SIRT3 genetic variation showed a positive association for one polymorphisms only (rs939915) [36].
Other sirtuins and longevity
None of the other sirtuins (SIRT2, SIRT4 to 7) have yet been investigated in relation to human longevity. Thus, the main genetic evidence implicating sirtuins to longevity arises from SIRT3 variability in modulating lifespan in males. This gender-specific contribution to longevity has already been reported in genetic studies addressing this topic [37–39]. Although the evidence is somewhat limited, not least because there are currently only a limited number of ageing cohorts that can be investigated, the current findings still allow the formulation of a hypothesis that these observed associations may be related to the enhancer activity of the VNTR polymorphism but more than likely only contributes to a small extent to what is an highly complex trait like longevity. For this reason, the limited availability of study cohorts and the considerable number of confounders, successful replications may be unlikely and may also explain current inconsistencies in the data.
What is perhaps more surprising is the current lack of evidence of association between longevity and SIRT1 variation. The failure to find any association with longevity despite the high coverage of SIRT1 variability and the large number of subjects used in the German study [29], serves to undermine the likelihood of SIRT1 modulating human longevity. Yet, if SIRT1 were to exert only a small effect (which may still be possible), the issue surrounding the likely number of confounders requiring consideration would suggest this study would actually be considerably underpowered. Indeed, other evidence supporting possible links between SIRT1 and modulatory effects on longevity comes from the evaluation of the genetic variability of FOXO3a, a molecular target of SIRT1 and transcription factor that regulates the scavenging of reactive oxygen species (ROS) and resistance to oxida-tive stress [40]. FOXO3A variation has also been strongly associated with longevity in two independent studies performed in Japanese [30] and German populations [41]. These findings may represent indirect support for SIRT1 involvement in longevity although it is perhaps more likely that any perceived association between SIRT1 action and longevity could be as a result of its interaction with FOXO3a which may be the true effector of longevity modulation.
Sirtuin variability and age-related diseases
Sirtuins post-translationally modulate the function of many important cellular proteins, such as p53, forkhead box class O (FOXO) transcription factors, peroxisome proliferator activated receptor PPARy co-activator 1α (PGC-1α), nuclear factor NF-kB and others, which are closely linked to some age-related disorders (reviewed in [42, 43]). Moreover, sirtuin genetic or pharmacological modulation has shown efficacy in preclinical models of metabolic, inflammatory and cardiovascular diseases, cancer and neu-rodegeneration (reviewed in [44]).
One study which focused on factors affecting the prevalence of age-related diseases (the Leiden 85-Plus study) evaluated SIRT1 genetic variation in two separate elderly cohorts, both including subjects older than 85 years, for a total of 1245 participants [31]. The study outcomes were mortality and different parameters concerning the metabolic profile, cardiovascular pathologies and diabetes. All participants were analyzed for 5 SNPs (rs3758391, rs3740051, rs2236319, rs2273773 and rs3818291) covering the SIRT1 gene region in equally spaced intervals. Two of the selected SNPs were the same as those genotyped in the German study previously described [29]. The analysis of mortality risk and SIRT1 haplotypes revealed no effect on total mortality rates, but highlighted a trend towards lower cardiovascular mortality in subjects carrying the TAATG haplotype compared to the reference haplotype (i.e. the most frequent one) [31]. The same association was also found for heterozygote of rs3758391 following univariate analysis, however, this association did not correspond to differences in the prevalence of cardiovascular pathologies. No association was also found between SIRT1 haplotypes and various metabolic profile parameters tested, except for LDL cholesterol and the TAATA haplotype where carriers of this haplotype had lower LDL levels than carriers of the more common reference haplotype [31]. Moreover, an interesting association was found between rs3758391 and cognitive performance, where people homozygous for the T allele of rs3758391 performed better on all tests measuring cognitive function for immediate and delayed memory.
Sirtuins and metabolic diseases
As previously stated, sirtuins are involved in the regulation of metabolism. Abnormal metabolic homeostasis can have severe consequences and it is an important risk factor for the development of severe diseases like obesity and diabetes. Moreover, signs of metabolic imbalance, such as insulin resistance, are increasing during ageing [45].
SIRT1 and metabolic diseases
SIRT1 has been widely investigated in the field of metabolism showing a pleiotropic activity in different key organs. SIRT1 promotes gluconeo-genesis through the deacetylation of PGC-1α and FOXO1 in liver [46, 47]. SIRT1 also enhances fatty acid oxidation in liver and skeletal muscle acting again through PGC-1α [48, 49]; attenuates adipogenesis and fat storage in white adipose tissue [50] and improves mito-chondrial activity in brown adipose tissue and skeletal muscle [51]. Furthermore, SIRT1 enhances insulin secretion by repressing UCP2 in the pancreas [52] and increases insulin sensitivity in the skeletal muscle [53].
On the basis of these collective findings, SIRT1 variation has been explored in the context of metabolism. A study in 2006 found that the minor allele of three SIRT1 SNPs (rs3740051, rs2236319 and rs2272773) was significantly associated with higher basal energy expenditure in a Finnish cohort of healthy, normal weight non-diabetic offspring (mean age: 34 years) of patients with type II diabetes [51]. Association was also observed, but in the opposite direction, in another study based on 917 non-diabetic individuals from southern Germany (TUEF cohort) [54]. In this case, carriers of the minor allele of two different SNPs (rs12413112 and rs7069102) presented with lower basal energy expenditure (p= 0.04 and p= 0.05 respectively) compared to people who were homozygous for the major allele. In the same study, no significant association of SIRT1 variants with predia-betic traits was found. However, the authors evaluated also 196 individuals belonging to a population which was at risk of diabetes (aged 45.8 ± 11.2 years – TULIP cohort) involved in a lifestyle intervention program for 9 months [54]. The longitudinal evaluation highlighted that rs 12413112 minor allele carriers were resistant against lifestyle-induced improvement of fasting plasma glucose (p= 0.04), had a weaker increase in insulin sensitivity (p= 0.05) and remarkably attenuated decline in liver fat content (p= 0.01). Thus, the authors concluded that SIRT1 does not affect prediabetic traits but influences the response to a lifestyle intervention. The authors also suggested that the different findings between their data and that derived from the Finnish cohort may be due to the very low linkage disequilibrium between the different SNPs in both studies. This would allow the associated SNPs in the two studies to act in the opposite direction as for the measured outcome (energy expenditure). Of course another explanation is that the associations observed were type I (false positive) or type II (false negative) statistical errors.
In a more recent investigation based on a longitudinal study in a cohort of 4573 participants (the Rotterdam study, mean age: 67.6 ± 7.7 years), SIRT1 variation (assessed by three tagging SNPs: rs7895833, rs1467568, and rs497849) did not influence the all-cause mortality risk [32]. Instead, in a subgroup with type II diabetes at baseline (n= 413), SIRT1 variability was related to survival rate. Diabetic subjects homozygous for the most common haplotype had a higher mortality risk (HR 1.5, 95% CI 1.1-2.1) compared to non homozygous. In particular, cardiovascular mortality in diabetic subjects was higher than in homozygous carriers. This risk further increased for diabetic participants when evaluated in association with smoking and dietary niacin consumption [32]. The same SIRT1 SNPs were also analyzed in an intended replication cohort of 2347 participants (Erasmus Rucphen Family study) in relation to BMI and risk of obesity (defined as BMI≥ 30 Kg/m2) [55], two well-known risk factors for different age-related pathologies (diabetes, cadiovascular diseases, etc) and early death. Minor alleles of rs7895833 and rs1467568 were associated with lower BMI (p= 0.002 for both SNPs in the two cohort combined) and with a 0.2-0.4 Kg/m2 decrease in BMI per allele copy. Moreover, carriers of these alleles had a 13-18% decreased risk of obesity (for rs7895833 p= 0.02 and for rs1467568 p= 0.0009 in the two studies combined). In the Rotterdam Study, the two variants were also found to be associated with a lower BMI increase during a 6.4 year follow-up (p= 0.01 and 0.08 respectively) [55].
Further evidence implicating SIRT1 in metabolic diseases came from another Belgian cohort in which an association between the SIRT1 gene and obesity was found. In 1068 obese subjects and 313 individuals older than 20 years, carriers of the C-allele of rs7069102 had a lower risk of being obese than non-carriers (p= 0.025). Moreover, in a multifactorial study that analyzed 3575 participants (20-59 years) for a relationship between obesity and 327 SNPs across 239 genes that belong to pathways that regulate fatty acid and glucose metabolism, the rs2273773 SNP of SIRT1 gene was associated with BMI but in women only, supporting a potential role of SIRT1 in obesity [56].
Overall, the literature supports the involvement of SIRT1 genetic variants in influencing metabolic parameters such as energy expenditure, BMI, LDL cholesterol and risk of obesity. Moreover, although SIRT1 is not directly involved in glucose metabolism, variability in the gene that encodes for it may modulate the outcome of a lifestyle intervention in diabetic patients and could also be relevant for the risk of cardiovascular mortality or the responsiveness of people to different forms of cardiovascular treatment. In this respect, it is not clear whether the perceived risk is concomitant with metabolic diseases, since in the Rotterdam study an association was found in diabetic patients only, whereas in the Leiden 85-Plus Study there was no correspondence with metabolic parameters.
Mitochondrial sirtuins and metabolic diseases
Among the seven sirtuins, SIRT3, SIRT4 and SIRT5 were reported to prevalently localise in the mitochondria. Due to their localisation, mitochondrial-associated sirtuins are potentially involved in metabolism and metabolic disfunction. The investigation of 58 tagging SNPs in 13 genes involved in mitochondrial function for an association with type II diabetes mellitus (T2DM) revealed that only rs2522138 in SIRT4 was significantly associated p= 0.01 [57]. The study was performed with a two-stage design. The first stage examined 519 controls and 480 cases (mean age: 65±8 and 67±8 years, respectively) for all 58 SNPs, while the second stage included 7620 cases and 2544 controls (mean age: 63±12 and 68±12 years, respectively) but only focused on the SNPs showing evidence for association with T2DM from the first stage. Meta-analysis of the data derived from the two cohorts resulted in a significant association between the G allele of rs2522138 in SIRT4 and T2DM. However, an effort to further replication these findings in an independent sample population (514 controls and 706 cases, 57±10 and 59±10 years, respectively) was not found to be significant although evidence toward an effect could be suggested (p= 0.06). The authors suggested that in the context of testing multiple SNPs, their positive result should be interpreted with caution and could be deemed consistent with statistical noise [57]. Besides, meta-analysis of three genome wide associations studies (GWAS) of T2DM, intended to offer greater sensitivity to detect modest effect size variations (the DIAGRAM GWAS meta-analysis), no evidence to support association between SIRT4 and diabetes (p= 0.72 for the G allele of rs2522138 of SIRT4) was observed [58]. Similarly, no positive association was found between SIRT3 and T2DM in a previous study [57].
In summary, these studies collectively suggest that an association between mitochondrial-related sirtuins and diabetes is, if present, likely to be only of small effect. Yet, the inconsistencies of the findings for SIRT4 and the in vitro evidence linking SIRT3 and SIRT4 to metabolism suggest that this route of investigation may warrant further study until there is less ambiguity in the data.
Sirtuins and cancer
SIRT1 involvement in cancer is still contentious. Recent findings show that SIRT1 is a tumor suppressor gene [59], whereas SIRT1 has been previously suggested to be a tumor promoter [60, 61]. It may be the case that SIRT1 may have dual functions in this manner in different tissue contexts as has also been suggested [62].
SIRT1 sequence variation has been investigated in gastric cancer (GC) and in colorectal cancer (CRC) characterized by gene microsatellite instability (MSI). The attention was focused on a region in exon III of SIRT1 characterized by two sets of 7A repeats (nucleotides 703-709 and 760-766). Analyzing 45 patients with GC and 48 patients with CRC a new deletion mutation was found in two cancer patients in the A7 repeat sequences. This deletion resulted in a frameshift and the introduction of a premature stop codon [63]. However, this study was small and the frequency of the new mutation rare and thus is unlikely to play a significant role in cancer pathogenesis involving MSI. To better assess the role of SIRT1 in cancer development, mutational analysis of the entire coding region of SIRT1 in groups of people with and without human cancer might be a strategy worthy of future pursuit.
Sirtuins and neurodegenerative disorders
Sirtuins have been found to work as disease-modifiers in various models of neurodegenera-tion such as in Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD) and Amyotrophic lateral sclerosis (ALS) (review in [64]). Some genetic studies have also been published.
The candidacy SIRT1 as a possible risk factor in relation to AD risk was due to its mapping to chromosome 10 (10q21.3), a region previously reported to be important to AD according to linkage studies [65, 66] and where other novel AD-associated genes have been reported, some of which have yet to be replicated and others not replicated [67–73]. SIRT1 association to late-onset AD was evaluated in a genetic screen of novel candidate genes on chromosome 10. The screening was performed on 1160 AD patients (mean age at onset 75.6 ± 6.84 years) and 1389 control subjects from the British population. No evidence supporting association between SIRT1 variation and AD risk was found [74]. These findings were supported by an independent study in the Finnish population on 326 AD cases (mean age at onset 72.1 ± 6.8 years) and 463 controls (mean age 69.6 ± 5.1 years). In this study five SIRT1 SNPs were genotyped (rs12778366; rs3740051; rs2236319; rs2273773 and rs10997870) [75]. A weak positive association with AD was reported in the subgroup of women older than 65 years for three different genotypes in the SIRT1 gene: rs3740051 (A/G), rs2236319 (A/G), rs2273773 (C/T). Moreover the TGGCG haplo-type from the 5 SNPs studied was over-represented in AD women older than 65 years compared to controls (p= 0.026). However, these associations may be a false positive because of the stratification with gender and age and indeed, following Bonferroni's correction of the data there no longer was evidence to support any association [75].
Collectively these data suggest that SIRT1 variability is not important with respect to AD risk, however given the increasing amount of in vivo and cellular data about sirtuins and AD, sirtuin associated pathways may still be implicated and an initial starting point might be the investigation of all seven sirtuin genes as risk factors for AD onset. In keeping with this, SIRT3 promoter methylation state has been studied in AD patients but no evidence supporting the existing of any peculiar methylation state in comparison to controls was found [76].
While the majority of genetic studies of neurodegenerative disease to-date have focused on AD, the same arguments can be applied to other forms of neurodegenerative disease, such as Parkinson's disease where evidence from in vitro studies suggests the involvement of SIRT2 [77]. Thus study of the sirtuin genes in these other diseases could be worthwhile.
Conclusions
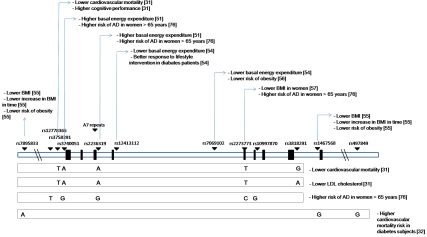
Despite the increasing amount of cellular and in vivo evidence linking sirtuins to age-associated diseases, only weak, often unreplicated evidence has been found to support the sirtuin involvement as being related to genetic variability. However, in some ways the failure to find association may be due to the complexity of ageing and other conditions they are involved with that are likely to be influenced by many other non-genetic factors, such as social behavior, diet, physical exercise and environment. The genetic contribution in such instances is likely to only be a small component. Indeed susceptibility genes of large effect in any complex disease are very rare as has been suggested following on from recent large GWAS studies of AD where other genes of similar effect size to the well recognized apolipoprotein-E epsilon 4 allele (APOE ε4) have not materialised [78]. Even if genetic variability in sirtuins is not found to be important in various clinical contexts, where much more work can be done given the paucity of information on sirtuins other than SIRT1 (Figure 1), the non-genetic evidence implicating sirtuins in various ageing and disease models suggests that sirtuin related pathways are important and this might direct future research focus towards other molecular targets upstream or downstream of sirtuin action.
Figure 1.
SIRT1 genetic variability related to ageing and age-associated diseases. Schematic structure of the SIRT1 gene where introns and exons are represented by white and black boxes respectively. SIRT1 SNPs reported to be associated with ageing and age-related diseases are represented across SIRT1 gene by black triangles and the relative rs number. SIRT1 haplotypes associated with age-related diseases are reported below the gene scheme.
Acknowledgments
This work was supported by Monzino Foundation (Milan, Italy).
References
- 1.North BJ, Verdin E. Sirtuins: Sir2-related NAD-dependent protein deacetylases. Genome Biol. 2004;5:224. doi: 10.1186/gb-2004-5-5-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys ResCommun. 2000;273:793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, Auwerx J. The role of sirtuins in the control of metabolic homeostasis. Ann N Y Acad Sci. 2009;1173(Suppl 1):E10–19. doi: 10.1111/j.1749-6632.2009.04952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Outeiro TF, Marques O, Kazantsev A. Therapeutic role of sirtuins in neurodegenerative disease. Biochim Biophys Acta. 2008;1782:363–369. doi: 10.1016/j.bbadis.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Stunkel W, Peh BK, Tan YC, Nayagam VM, Wang X, Salto-Tellez M, Ni B, Entzeroth M, Wood J. Function of the SIRT1 protein deacetylase in cancer. Biotechnol J. 2007;2:1360–1368. doi: 10.1002/biot.200700087. [DOI] [PubMed] [Google Scholar]
- 6.Yuan J, Minter-Dykhouse K, Lou Z. A c-Myc-SIRT1 feedback loop regulates cell growth and transformation. J Cell Biol. 2009;185:203–211. doi: 10.1083/jcb.200809167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schemies J, Sippl W, Jung M. Histone deacetylase inhibitors that target tubulin. Cancer Lett. 2009;280:222–232. doi: 10.1016/j.canlet.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 8.Vaquero A, Sternglanz R, Reinberg D. NAD+-dependent deacetylation of H4 lysine 16 by class III HDACs. Oncogene. 2007;26:5505–5520. doi: 10.1038/sj.onc.1210617. [DOI] [PubMed] [Google Scholar]
- 9.Wang F, Nguyen M, Qin FX, Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell. 2007;6:505–514. doi: 10.1111/j.1474-9726.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- 10.Jing E, Gesta S, Kahn CR. SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation. Cell Metab. 2007;6:105–114. doi: 10.1016/j.cmet.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallows WC, Lee S, Denu JM. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc Natl Acad Sci U S A. 2006;103:10230–10235. doi: 10.1073/pnas.0604392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CF, Steegborn C. Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J Mol Biol. 2008;382:790–801. doi: 10.1016/j.jmb.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 13.Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. 2005;280:13560–13567. doi: 10.1074/jbc.M414670200. [DOI] [PubMed] [Google Scholar]
- 14.Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, Wolberger C, Prolla TA, Weindruch R, Alt FW, Guarente L. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 15.Ahuja N, Schwer B, Carobbio S, Waltregny D, North BJ, Castronovo V, Maechler P, Verdin E. Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. J Biol Chem. 2007;282:33583–33592. doi: 10.1074/jbc.M705488200. [DOI] [PubMed] [Google Scholar]
- 16.Nakagawa T, Guarente L. Urea cycle regulation by mitochondrial sirtuin, SIRT5. Aging (Albany NY) 2009;1:578–581. doi: 10.18632/aging.100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCord RA, Michishita E, Hong T, Berber E, Boxer LD, Kusumoto R, Guan S, Shi X, Gozani O, Burlingame AL, Bohr VA, Chua KF. SIRT6 stabilizes DNA-dependent protein kinase at chromatin for DNA double-strand break repair. Aging (Albany NY) 2009;1:109–121. doi: 10.18632/aging.100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong L, D'Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, Guimaraes A, Marinelli B, Wikstrom JD, Nir T, Clish CB, Vaitheesvaran B, Iliopoulos O, Kurland I, Dor Y, Weissleder R, Shirihai OS, Ellisen LW, Espinosa JM, Mostoslavsky R. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell. 140:280–293. doi: 10.1016/j.cell.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, Braun T, Bober E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102:703–710. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- 20.Grob A, Roussel P, Wright JE, McStay B, Hernandez-Verdun D, Sirri V. Involvement of SIRT7 in resumption of rDNA transcription at the exit from mitosis. J Cell Sci. 2009;122:489–498. doi: 10.1242/jcs.042382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinclair DA, Guarente L. Extrachromosomal rDNA circles–a cause of aging in yeast. Cell. 1997;91:1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 22.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- 24.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boily G, Seifert EL, Bevilacqua L, He XH, Sabourin G, Estey C, Moffat C, Crawford S, Saliba S, Jardine K, Xuan J, Evans M, Harper ME, McBurney MW. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS One. 2008;3:e1759. doi: 10.1371/journal.pone.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crujeiras AB, Parra D, Goyenechea E, Martinez JA. Sirtuin gene expression in human mono-nuclear cells is modulated by caloric restriction. Eur J Clin Invest. 2008;38:672–678. doi: 10.1111/j.1365-2362.2008.01998.x. [DOI] [PubMed] [Google Scholar]
- 28.Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flachsbart F, Croucher PJ, Nikolaus S, Hampe J, Cordes C, Schreiber S, Nebel A. Sirtuin 1 (SIRT1) sequence variation is not associated with exceptional human longevity. Exp Gerontol. 2006;41:98–102. doi: 10.1016/j.exger.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci U S A. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuningas M, Putters M, Westendorp RG, Slagboom PE, van Heemst D. SIRT1 gene, age-related diseases, and mortality: the Leiden 85-plus study. J Gerontol A Biol Sci Med Sci. 2007;62:960–965. doi: 10.1093/gerona/62.9.960. [DOI] [PubMed] [Google Scholar]
- 32.Zillikens MC, van Meurs JB, Sijbrands EJ, Rivadeneira F, Dehghan A, van Leeuwen JP, Hofman A, van Duijn CM, Witteman JC, Uitterlinden AG, Pols HA. SIRT1 genetic variation and mortality in type 2 diabetes: interaction with smoking and dietary niacin. Free Radic Biol Med. 2009;46:836–841. doi: 10.1016/j.freeradbiomed.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 33.De Luca M, Rose G, Bonafe M, Garasto S, Greco V, Weir BS, Franceschi C, De Benedictis G. Sex-specific longevity associations defined by Tyrosine Hydroxylase-Insulin-Insulin Growth Factor 2 haplotypes on the 11p15.5 chromosomal region. Exp Gerontol. 2001;36:1663–1671. doi: 10.1016/s0531-5565(01)00146-2. [DOI] [PubMed] [Google Scholar]
- 34.Rose G, Dato S, Altomare K, Bellizzi D, Garasto S, Greco V, Passarino G, Feraco E, Mari V, Barbi C, BonaFe M, Franceschi C, Tan Q, Boiko S, Yashin AI, De Benedictis G. Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp Gerontol. 2003;38:1065–1070. doi: 10.1016/s0531-5565(03)00209-2. [DOI] [PubMed] [Google Scholar]
- 35.Bellizzi D, Rose G, Cavalcante P, Covello G, Dato S, De Rango F, Greco V, Maggiolini M, Feraco E, Mari V, Franceschi C, Passarino G, De Benedictis G. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics. 2005;85:258–263. doi: 10.1016/j.ygeno.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Lescai F, Blanche H, Nebel A, Beekman M, Sahbatou M, Flachsbart F, Slagboom E, Schreiber S, Sorbi S, Passarino G, Franceschi C. Human longevity and 11p15.5: a study in 1321 centenarians. Eur J Hum Genet. 2009;17:1515–1519. doi: 10.1038/ejhg.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Passarino G, Montesanto A, Dato S, Giordano S, Domma F, Mari V, Feraco E, De Benedictis G. Sex and age specificity of susceptibility genes modulating survival at old age. Hum Hered. 2006;62:213–220. doi: 10.1159/000097305. [DOI] [PubMed] [Google Scholar]
- 38.Cederholm T, Persson M, Andersson P, Stenvinkel P, Nordfors L, Madden J, Vedin I, Wretlind B, Grimble RF, Palmblad J. Polymorphisms in cytokine genes influence long-term survival differently in elderly male and female patients. J Intern Med. 2007;262:215–223. doi: 10.1111/j.1365-2796.2007.01803.x. [DOI] [PubMed] [Google Scholar]
- 39.Albani D, Batelli S, Polito L, Vittori A, Pesaresi M, Gajo GB, De Angeli S, Zanardo A, Gallucci M, Forloni G. A polymorphic variant of the insulin-like growth factor 1 (IGF-1) receptor correlates with male longevity in the Italian population: a genetic study and evaluation of circulating IGF-1 from the “Treviso Longeva (TRELONG)” study. BMC Geriatr. 2009;9:19. doi: 10.1186/1471-2318-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nemoto S, Finkel T. Redox regulation of fork-head proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 41.Flachsbart F, Caliebe A, Kleindorp R, Blanche H, von Eller-Eberstein H, Nikolaus S, Schreiber S, Nebel A. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci U S A. 2009;106:2700–2705. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126:257–268. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins–novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7:841–853. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- 45.Ryan AS. Insulin resistance with aging: effects of diet and exercise. Sports Med. 2000;30:327–346. doi: 10.2165/00007256-200030050-00002. [DOI] [PubMed] [Google Scholar]
- 46.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 47.Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280:20589–20595. doi: 10.1074/jbc.M412357200. [DOI] [PubMed] [Google Scholar]
- 48.Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci U S A. 2007;104:12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. Embo J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 52.Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A, Easlon EJ, Lin SJ, Guarente L. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 54.Weyrich P, Machicao F, Reinhardt J, Machann J, Schick F, Tschritter O, Stefan N, Fritsche A, Haring HU. SIRT1 genetic variants associate with the metabolic response of Caucasians to a controlled lifestyle intervention–the TULIP Study. BMC Med Genet. 2008;9:100. doi: 10.1186/1471-2350-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zillikens MC, van Meurs JB, Rivadeneira F, Amin N, Hofman A, Oostra BA, Sijbrands EJ, Witteman JC, Pols HA, van Duijn CM, Uitterlinden AG. SIRT1 genetic variation is related to BMI and risk of obesity. Diabetes. 2009;58:2828–2834. doi: 10.2337/db09-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van den Berg SW, Dolle ME, Imholz S, van der AD, van't Slot R, Wijmenga C, Verschuren WM, Strien C, Siezen CL, Hoebee B, Feskens EJ, Boer JM. Genetic variations in regulatory pathways of fatty acid and glucose metabolism are associated with obesity phenotypes: a population-based cohort study. Int J Obes (Lond) 2009;33:1143–1152. doi: 10.1038/ijo.2009.152. [DOI] [PubMed] [Google Scholar]
- 57.Reiling E, van Vliet-Ostaptchouk JV, van't Riet E, van Haeften TW, Arp PA, Hansen T, Kremer D, Groenewoud MJ, van Hove EC, Romijn JA, Smit JW, Nijpels G, Heine RJ, Uitterlinden AG, Pedersen O, Slagboom PE, Maassen JA, Hofker MH, t Hart LM, Dekker JM. Genetic association analysis of 13 nuclear-encoded mitochondrial candidate genes with type II diabetes mellitus: the DAMAGE study. Eur J Hum Genet. 2009;17:1056–1062. doi: 10.1038/ejhg.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeggini E, Scott LJ, Saxena R, Voight BF, Marchini JL, Hu T, de Bakker PI, Abecasis GR, Almgren P, Andersen G, Ardlie K, Bostrom KB, Bergman RN, Bonnycastle LL, Borch-Johnsen K, Burtt NP, Chen H, Chines PS, Daly MJ, Deodhar P, Ding CJ, Doney AS, Duren WL, Elliott KS, Erdos MR, Frayling TM, Freathy RM, Gianniny L, Grallert H, Grarup N, Groves CJ, Guiducci C, Hansen T, Herder C, Hitman GA, Hughes TE, Isomaa B, Jackson AU, Jorgensen T, Kong A, Kubalanza K, Kuruvilla FG, Kuusisto J, Langenberg C, Lango H, Lauritzen T, Li Y, Lindgren CM, Lyssenko V, Marvelle AF, Meisinger C, Midthjell K, Mohlke KL, Morken MA, Morris AD, Narisu N, Nilsson P, Owen KR, Palmer CN, Payne F, Perry JR, Pettersen E, Platou C, Prokopenko I, Qi L, Qin L, Rayner NW, Rees M, Roix JJ, Sandbaek A, Shields B, Sjogren M, Steinthorsdottir V, Stringham HM, Swift AJ, Thorleifsson G, Thorsteinsdottir U, Timpson NJ, Tuomi T, Tuomilehto J, Walker M, Watanabe RM, Weedon MN, Willer CJ, Illig T, Hveem K, Hu FB, Laakso M, Stefansson K, Pedersen O, Wareham NJ, Barroso I, Hattersley AT, Collins FS, Groop L, McCarthy MI, Boehnke M, Altshuler D. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. 2008;40:638–645. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C, Kim S, Xu X, Zheng Y, Chilton B, Jia R, Zheng ZM, Appella E, Wang XW, Ried T, Deng CX. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008;14:312–323. doi: 10.1016/j.ccr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deng CX. SIRT1, is it a tumor promoter or tumor suppressor? Int J Biol Sci. 2009;5:147–152. doi: 10.7150/ijbs.5.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 62.Liu T, Liu PY, Marshall GM. The critical role of the class III histone deacetylase SIRT1 in cancer. Cancer Res. 2009;69:1702–1705. doi: 10.1158/0008-5472.CAN-08-3365. [DOI] [PubMed] [Google Scholar]
- 63.Kim YR, Kim SS, Yoo NJ, Lee SH. Frameshift mutation of SIRT1 gene in gastric and colorectal carcinomas with microsatellite instability. Apmis. 118:81–82. doi: 10.1111/j.1600-0463.2009.02560.x. [DOI] [PubMed] [Google Scholar]
- 64.Han SH. Potential role of sirtuin as a therapeutic target for neurodegenerative diseases. J Clin Neurol. 2009;5:120–125. doi: 10.3988/jcn.2009.5.3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kehoe P, Wavrant-De Vrieze F, Crook R, Wu WS, Holmans P, Fenton I, Spurlock G, Norton N, Williams H, Williams N, Lovestone S, Perez-Tur J, Hutton M, Chartier-Harlin MC, Shears S, Roehl K, Booth J, Van Voorst W, Ramic D, Williams J, Goate A, Hardy J, Owen MJ. A full genome scan for late onset Alzheimer's disease. Hum Mol Genet. 1999;8:237–245. doi: 10.1093/hmg/8.2.237. [DOI] [PubMed] [Google Scholar]
- 66.Bertram L, Blacker D, Mullin K, Keeney D, Jones J, Basu S, Yhu S, McInnis MG, Go RC, Vekrellis K, Selkoe DJ, Saunders AJ, Tanzi RE. Evidence for genetic linkage of Alzheimer's disease to chromosome 10q. Science. 2000;290:2302–2303. doi: 10.1126/science.290.5500.2302. [DOI] [PubMed] [Google Scholar]
- 67.Myers A, Wavrant De-Vrieze F, Holmans P, Hamshere M, Crook R, Compton D, Marshall H, Meyer D, Shears S, Booth J, Ramic D, Knowles H, Morris JC, Williams N, Norton N, Abraham R, Kehoe P, Williams H, Rudrasingham V, Rice F, Giles P, Tunstall N, Jones L, Lovestone S, Williams J, Owen MJ, Hardy J, Goate A. Full genome screen for Alzheimer disease: stage II analysis. Am J Med Genet. 2002;114:235–244. doi: 10.1002/ajmg.10183. [DOI] [PubMed] [Google Scholar]
- 68.Liang X, Slifer M, Martin ER, Schnetz-Boutaud N, Bartlett J, Anderson B, Zuchner S, Gwirtsman H, Gilbert JR, Pericak-Vance MA, Haines JL. Genomic convergence to identify candidate genes for Alzheimer disease on chromosome 10. Hum Mutat. 2009;30:463–471. doi: 10.1002/humu.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Finckh U, van Hadeln K, Muller-Thomsen T, Alberici A, Binetti G, Hock C, Nitsch RM, Stoppe G, Reiss J, Gal A. Association of late-onset Alzheimer disease with a genotype of PLAU, the gene encoding urokinase-type plasminogen activator on chromosome 10q22.2. Neurogenetics. 2003;4:213–217. doi: 10.1007/s10048-003-0157-9. [DOI] [PubMed] [Google Scholar]
- 70.Myers AJ, Marshall H, Holmans P, Compton D, Crook RJ, Mander AP, Nowotny P, Smemo S, Dunstan M, Jehu L, Wang JC, Hamshere M, Morris JC, Norton J, Chakraventy S, Tunstall N, Lovestone S, Petersen R, O'Donovan M, Jones L, Williams J, Owen MJ, Hardy J, Goate A. Variation in the urokinase-plasminogen activator gene does not explain the chromosome 10 linkage signal for late onset AD. Am J Med Genet B Neuropsychiatr Genet. 2004;124B:29–37. doi: 10.1002/ajmg.b.20036. [DOI] [PubMed] [Google Scholar]
- 71.Papassotiropoulos A, Lambert JC, Wavrant-De Vrieze F, Wollmer MA, von der Kammer H, Streffer JR, Maddalena A, Huynh KD, Wolleb S, Lutjohann D, Schneider B, Thal DR, Grimaldi LM, Tsolaki M, Kapaki E, Ravid R, Konietzko U, Hegi T, Pasch T, Jung H, Braak H, Amouyel P, Rogaev EI, Hardy J, Hock C, Nitsch RM. Cholesterol 25-hydroxylase on chromosome 10q is a susceptibility gene for sporadic Alzheimer's disease. Neurodegener Dis. 2005;2:233–241. doi: 10.1159/000090362. [DOI] [PubMed] [Google Scholar]
- 72.Kuwano R, Miyashita A, Arai H, Asada T, Imagawa M, Shoji M, Higuchi S, Urakami K, Kakita A, Takahashi H, Tsukie T, Toyabe S, Akazawa K, Kanazawa I, Ihara Y. Dynamin-binding protein gene on chromosome 10q is associated with late-onset Alzheimer's disease. Hum Mol Genet. 2006;15:2170–2182. doi: 10.1093/hmg/ddl142. [DOI] [PubMed] [Google Scholar]
- 73.Dreses-Werringloer U, Lambert JC, Vingtdeux V, Zhao H, Vais H, Siebert A, Jain A, Koppel J, Rovelet-Lecrux A, Hannequin D, Pasquier F, Galimberti D, Scarpini E, Mann D, Lendon C, Campion D, Amouyel P, Davies P, Foskett JK, Campagne F, Marambaud P. A polymorphism in CALHM1 influences Ca2+ homeostasis, Abeta levels, and Alzheimer's disease risk. Cell. 2008;133:1149–1161. doi: 10.1016/j.cell.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morgan AR, Turic D, Jehu L, Hamilton G, Hollingworth P, Moskvina V, Jones L, Lovestone S, Brayne C, Rubinsztein DC, Lawlor B, Gill M, O'Donovan MC, Owen MJ, Williams J. Association studies of 23 positional/functional candidate genes on chromosome 10 in late-onset Alzheimer's disease. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:762–770. doi: 10.1002/ajmg.b.30509. [DOI] [PubMed] [Google Scholar]
- 75.Helisalmi S, Vepsalainen S, Hiltunen M, Koivisto AM, Salminen A, Laakso M, Soininen H. Genetic study between SIRT1, PPARD, PGC-1alpha genes and Alzheimer's disease. J Neurol. 2008;255:668–673. doi: 10.1007/s00415-008-0774-1. [DOI] [PubMed] [Google Scholar]
- 76.Bellizzi D, Dato S, Cavalcante P, Covello G, Di Cianni F, Passarino G, Rose G, De Benedictis G. Characterization of a bidirectional promoter shared between two human genes related to aging: SIRT3 and PSMD13. Genomics. 2007;89:143–150. doi: 10.1016/j.ygeno.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 77.Alcain FJ, Villalba JM. Sirtuin inhibitors. Expert Opin Ther Pat. 2009;19:283–294. doi: 10.1517/13543770902755111. [DOI] [PubMed] [Google Scholar]
- 78.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schurmann B, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frolich L, Hampel H, Hull M, Rujescu D, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhovennan C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Muhleisen TW, Nothen MM, Moebus S, Jockel KH, Klopp N, Wichmann HE, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O'Donovan M, Owen MJ, Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]