Abstract
There is substantial evidence implicating environmental factors in the progression of prostate cancer. The metabolic consequences of a western lifestyle, such as obesity, insulin resistance and abnormal hormone production have been linked to prostate carcinogenesis through multiple overlapping pathways. Insulin resistance results in raised levels of the mitogens insulin and insulin-like growth factor-1, both of which may affect prostate cancer directly, or through their effect on other metabolic regulators. Obesity is associated with abnormal levels of adipocyte-derived peptides (adipokines), sex hormones and inflammatory cytokines. Adipokines have been shown to influence prostate cancer in both cell culture studies and observational, population level studies. Testosterone appears to have a complex relationship with prostate carcinogenesis, and it has been suggested that the lower levels associated with obesity may select for more aggressive androgen independent prostate cancer cells. Prostatic inflammation, caused by infection, urinary reflux or dietary toxins, frequently occurs prior to cancer development and may influence progression to advanced disease. High levels of ω-6 fatty acids in the diet may lead to the production of further inflammatory molecules that may influence prostate cancer. Increased fatty acid metabolism occurs within tumour cells, providing a potential target for prostate cancer therapies. Aberrations in amino acid metabolism have also been identified in prostate cancer tissue, particularly in metastatic cancer. This evidence indicates lifestyle interventions may be effective in reducing the incidence of clinical disease. However, much more research is needed before recommendations are made.
Keywords: Prostate cancer, obesity, adipokines, insulin-like growth factors, diabetes, inflammation, metabolism
Introduction
The prostate is a male accessory sex gland situated at the base of the bladder surrounding the urethra. Its function is to produce several components of semen that aid sperm survival, function and motility [1,2]. Pathology of the prostate is a frequent cause of morbidity and mortality; prostatitis affects around 15% of men at some point in their lives [3] and clinical benign prostate hyperplasia around 20-40% – although histological prevalence is much higher [4]. Prostate cancer is the most common cancer in men, with 217,730 new cases estimated in the US in 2010 (http://www.cancer.gov/cancertopics/types/prostate). A prostate cancer diagnosis is, however, not a death sentence. While some cancers do progress and become invasive, many do not impact on the natural lifespan of the patient – men are more likely to die with prostate cancer than from it [5]. The estimated case-fatality rate of screen detected prostate cancer is 16% [6] but when considered in absolute terms, given the high incidence rate, prostate cancer is still a significant cause of death (it is estimated that 32,050 men will die from prostate cancer in the US in 2010 [http://www.cancer.gov/cancertopics/types/prostate]).
Age is the most significant prostate cancer risk factor; diagnosis in men under 40 years is extremely rare but rates rise steeply after middle age. Ethnicity also affects risk considerably; in the US and the UK black men are around 2-3 times more likely to develop prostate cancer than white men [7]. Family history is also an indicator of risk; inherited susceptibility traits may cause around 5-10% of all cases (http://info.cancerresearchuk.org/cancer-statsindex.htm). In addition to these, the effect of the western lifestyle and its metabolic consequences on prostate cancer progression is gaining recognition [8,9,10]. The current evidence and possible mechanisms supporting a role for obesity and other important metabolic sequelae of westernisation, such as insulin resistance and abnormal hormone production, in advanced and aggressive prostate cancer are explored in this review.
Environmental causes of prostate cancer
Rapid industrialisation facilitated by agricultural and technological advances has dramatically changed our dietary composition and physical activity patterns – qualitatively and quantitatively [11]. This is most notable in developed (‘western’) countries, where the consumption of large volumes of energy dense food combined with sedentary lifestyles has lead to a substantial burden of obesity and metabolic and hormonal imbalance [9]. These conditions put individuals at increased risk of a variety of chronic diseases including diabetes, coronary heart disease and some cancers [12,8,13]. The geographic distribution of industrialisation and urban development appears to mirror variations in prostate cancer incidence and mortality [9], which has led many to believe there may be a causal connection.
Autopsy studies of prostate cancer prevalence have universally found an unexpectedly high prevalence of latent prostate carcinomas in men over 50 years of age when compared to incidence rates [14,15]. Although there is some variation by geographical location, even populations with low clinical prostate cancer incidence, such as Japan, have high clinically-undetected prevalence rates [16]. This suggests that there may be some environmental factor (or factors) influencing development of clinically evident disease in those populations where incidence is high.
Studies of immigrants from populations with a low risk of prostate cancer to those with a high risk appear to support this environmental theory [17]. Risk profiles of migrants often converge towards those of locals [17,18,19,20], indicating that factors other than genetics are important in development of clinical prostate cancer. The rising levels of clinical disease in Asian countries have been attributed to westernisation [21,8,22]. However, the results of migration studies should be considered with caution as several other factors that vary between country of origin and country of residence may substantially affect incident rates; for example more regular health checks, higher prostate-specific antigen (PSA) testing rates and more complete cancer registries.
Obesity and prostate cancer
For many years failure to stratify by disease stage or grade (Box 1), or by incidence versus mortality masked the complex relationship now emerging between adiposity and prostate cancer [23]. A meta-analysis of 43 observational studies that investigated the relationship between body mass index (BMI) and prostate cancer incidence revealed an overall rate ratio (RR) of 1.05 per 5 kg/m2 (95% CI 1.01-1.23) [24]. However, when stratified by stage, a stronger relationship with advanced disease (RR 1.12, 95% CI 1.01-1.23) and no relationship with localised disease (RR 0.96, 95% CI 0.89-1.03) was observed. The majority of studies published since this meta-analysis have also found positive associations between body mass and advanced, aggressive and/or fatal prostate cancer [25,26,27,28], though some smaller studies did not [29,30].
Box 1 Grade and Stage
Gleason grade: Tumour grade is a measure of how ‘normal’, or well differentiated, the cancer cells appear under microscopic examination. The more abnormal, the higher the grade, and the more likely the tumour is to grow, metastasise and lead to the death of the patient. Gleason grade is a specific scale used for grading prostatic adenocarcinoma that is calculated from the sum of the two most common grades seen within the sample.
Stage: Tumour staging is a measure of the extent and spread of tumour throughout the body. In prostate cancer the TNM scale is used, in which the extent of the tumour (T 1-4), the spread to lymph nodes (N 0-3) and presence of metastases (M 0-1) are included. For example, Tl and 2 are organ confined (localised within the prostate). Tumour stage indicates what treatment options would be of benefit to the patient and the likelihood of progression.
Often this pattern of association has been attributed to detection bias – more obese men have lower PSA levels and larger prostates, impairing detection of prostate cancer during the early stages [10]. Therefore cancers may present at a more advanced stage in obese men, when prognosis is poorer; hence the positive association of BMI with prostate cancer mortality. However, several plausible biological mechanisms linking obesity and clinically evident prostate cancer have also been proposed.
Overview of potential mechanisms
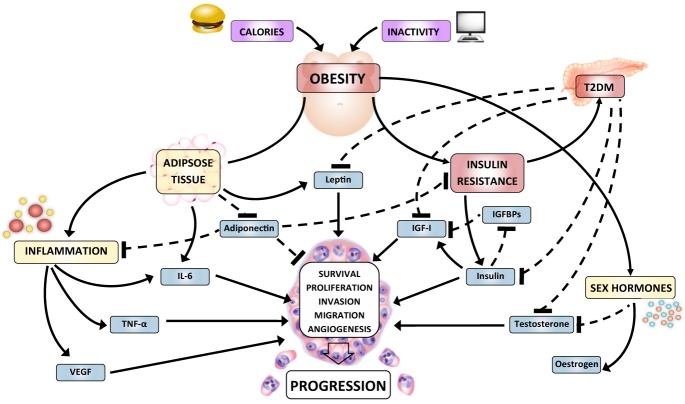
Multiple overlapping metabolic and hormonal pathways are disrupted in obesity (Figure 1). Insulin resistance is an increasingly common consequence, which is accompanied by raised insulin and bio-available insulin-like growth factor (IGF)-I – both of which may positively influence prostate cancer growth and progression as well as the expression of other growth factors. Excess adiposity results in increases in adipo-cyte-derived peptides such as leptin and interleukin (IL)-6, which have been associated with prostate cancer progression. In contrast, the proposed ‘anti-cancer’ adipokine, adiponectin, is decreased in obesity. Aberrations in adiponectin levels may affect prostate carcinogenesis directly and through linkage with multiple other metabolic pathways. Obesity is associated with lower testosterone and higher oestrogen levels due to peripheral aromatisation of androgens to oestrogens in the adipose tissue. It has been hypothesised that low testosterone levels may select for androgen independent cells and promote progression. Additionally, the metabolic consequences of a western lifestyle are coupled with a chronic low grade inflammatory state which may substantially contribute to a hormonal milieu, along with other adipokines and growth factors, which favours cancer cell survival and progression. Disturbances in lipid and amino acid metabolism also appear to be linked to prostate cancer.
Figure 1.
Overview of potential pathways linking metabolic disruption to prostate cancer progression. Arrows: stimulates/up regulates. Dashed lines: inhibits/down regulates. Abbreviations: T2DM type 2 diabetes mellitus, IGF insulin-like growth factor, IGFBP IGF binding protein, IL-6 interleukin-6, TNF-α tumour necrosis factor alpha, VEGF vascular endothelial growth factor.
The remainder of this review outlines in vitro, in vivo and epidemiological evidence for a link between specific obesity-related hormonal systems and prostate cancer progression. The review is based on a search of the Medline electronic bibliographical database, which included the following terms: “adipokines”, “adiponectin”, “leptin”, “insulin”, “insulin-like growth factor”, “interleukin-6”, “diabetes”, “metabolism”, “inflammation” AND “prostatic neoplasms”. This was supplemented by the authors' citation of relevant papers.
Adipokines - adipose tissue as an endocrine organ
Adipocytes are the major constituent of white adipose tissue, the main site of energy storage in mammals (in contrast, brown adipose tissue mainly generates heat). However, adipocytes also synthesise and secrete a range of bioactive molecules termed adipokines [9]. The adipokines, including leptin, adiponectin and inter-leukin (IL)-6, can act both locally through autocrine and paracrine signal transduction and systemically through endocrine pathways [9,31]. Several properties of adipokines could influence prostate carcinogenesis, in particular progression. Leptin, the first adipokine to be discovered, has been shown by several studies to induce proliferation, cell migration and invasion and/or prevent apoptosis when administered to the androgen independent prostate cancer cell lines PC3 or DU145 [32,33,34,35]. Intriguingly, leptin has not been found to enhance proliferation of androgen dependent LNCaP cells by most studies [32,31,36], (although not all [35]) indicating possible differential effects on non-aggressive and aggressive prostate cancer phenotypes. Leptin also induces expression of several angiogenic growth factors including vascular endothelial growth factor (VEGF), transforming growth factor-betal (TGF-(β1), and basic fibroblast growth factor (bFGF) [34] and has itself been found to have angiogenic activity [37].
Similarly, IL-6 has been implicated in the progression of hormone refractory prostate cancer, but may promote differentiation in androgen dependent cells [38,39]. Chronic exposure of LNCaP cells to IL-6 produces LNCaP cells that express IL-6, which develop into tumour xenographs that grow more rapidly and to larger volumes than their IL-6 negative equivalents [40,41].
In contrast to IL- 6 and leptin, adiponectin is inversely associated with BMI [42]. It has been shown to inhibit proliferation of prostate cancer cells in vitro regardless of their androgen sensitivity and to antagonise the proliferative effects of leptin and IGF-I on androgen independent (DU145) prostate cancer cells [42]. Adiponectin has also been reported to restore expression of the tumour suppressor gene p53 in LNCaP cells, which had been inhibited by administration of leptin [36]. Adiponectin is a potent inhibitor of angiogenesis, in both in vitro and in vivo models and has also been shown to inhibit cell migration [43]. Adiponectin is modulated by and modulates various components of other metabolic and hormonal pathways; it is athero-protective [43] and involved in sensitisation of insulin [44], which is related to prostate cancer (see section on Insulin-IGF system). It also possesses anti-inflammatory properties, including inhibition of IL-6 and tumour necrosis factor-a (TNF-α) [45,46]. Through its downstream effector 5′-AMP-activated protein kinase (AMPK), adiponectin signalling may also inhibit the lipo-genic enzyme fatty acid synthase (FASN) and protein synthesis through inhibition of mammalian target of rapamycin (mTOR) [45,46]. Testosterone negatively regulates adiponectin levels [44], while calorie restriction and weight loss increase levels [45]. Furthermore, there is some evidence to suggest physical activity and calorie restriction may protect against prostate cancer progression [12,47]. Taken together, the in vitro evidence indicates adiponectin may have potential as a therapeutic agent across the spectrum of prostate cancer phenotypes [42].
Several observational studies have examined circulating levels of adipokines in men with prostate cancer, with mixed findings (Tables 1 to 4). Few studies of leptin and prostate cancer incidence found clear evidence of associations. Three found positive associations [48,49,50], although one of these was not a dose response relationship and risk attenuated at very high leptin levels [50]. Three studies found no evidence of an association [51,52,53], and a further three found no clear evidence but risk estimates were suggestive of an inverse relationship [54,55,56]. No studies of leptin and advanced, aggressive or fatal disease reported inverse associations, but many were underpowered to provide robust evidence. Only two small studies reported positive associations [57,49] and three others were indicative of such a relationship but the evidence was insufficient to be conclusive [51,55,58]. Two small studies of prostate cancer stage [57,49] and one larger study of prostate cancer volume [59] found positive associations with leptin levels, and two studies of lethal [51] or aggressive [48] disease indicated a possible weak positive relationship. However, several leptin polymorphisms have been associated with prostate cancer risk [60].
Table 1.
Leptin and prostate cancer incidence observational studies
| Prostate Cancer Incidence | ||||||
|---|---|---|---|---|---|---|
| Study, Country | Authors | Year | Design | Size | Results - RR/OR (95% CI)* | Comments |
| Greece [56] | Lagiou | 1998 | CC | 43 PCa: 48 HC | OR = 0.70 (0.32-1.55) | Adjusted for age, height, education, BMI, E, T, DHT, DHEAS, SHBG and IGF-1 |
| China [53] | Hsing | 2001 | CC | 128 PCa: 304 HC | ORTS vs T1 = 1.10 (0.59-2.07) | Adjusted for age, education, BMI, WHR and I |
| WHO-MONICA and VIP, Sweden [50] | Statin | 2001 | NCC | 149 PCa: 298 HC | RRQ2-3 vs Q1 = 2.4 (1.3-4.5) | Adjusted for BMI and I |
| WHO-MONICA and VIP, Sweden [50] | Statin | 2001 | NCC | 149 PCa: 298 HC | RRQ4-5 vs Q1 = 1.5 (0.7-3.2) | Adjusted for BMI and I. Risk attenuated at higher leptin levels |
| Turkey [49] | Saglam | 2003 | CC | 21 PCa: 50 HC | PCa Mean 27.33 ± SE 12.50, HC Mean 17.55 ± 7.20 p<0.001 (ng/ml) | |
| Janus Project, Norway [52] | Statin | 2003 | NCC | 200 PCa:397 HC | ORQ4 vs Q1 = 0.9 (0.6-1.6) | Adjusted for T, E and SHBG |
| SABOR, US [55] | Baillargeon | 2006 | NCC | 125 PCa: 125 HC | ORTS vs T1 = 0.77 (0.43-1.37) | Adjusted for age |
| SABOR, US [54] | Parekh | 2007 | NCC | 123 PCa: 127 HC | OR 0.88 (p=0.16) | |
| VIP, Sweden [48] | Stocks | 2007 | NCC | 366 PCa:376 HC | ORQ4 vs Q1 = 0.55 (0.36-0.84) | |
| PHS, US [51] | Li | 2010 | NCC | 635 PCa:635 HC | RRQ5 vs Q1 = 1.05 (0.73-1.51) | Adjusted for age and smoking |
Unless otherwise stated. Abbreviations: CC, case control; NCC, nested case control; PCa, prostate cancer case; HC, healthy control; OR, odds ratio; RR, relative risk; 95% CI, 95% confidence interval; SE, standard error of the mean; Q, quantile (quartile or quintile); T, tertile; vs versus; BMI, body mass index; WHR, waist to hip ratio; I, serum insulin; T, serum testosterone; E, serum estrodiol; SHBG, serum sex hormone binding globulin; DHT, dihydrotestosterone; DHEAS, dihydroepiandrosterone sulphate; IGF-1, insulin-like growth factor 1.
Table 4.
Adiponectin and prostate cancer progression observational studies
| Study, Country | Authors | Year | Design | Outcome | Size | Results - RR/OR (95% CI)* | Comments |
|---|---|---|---|---|---|---|---|
| US [65] | Freedland | 2005 | CC | Grade (all men) | 65 HG PCa: 171 LG PCa | ORQ4 vs Q1 = 0.67 (0.29-1.53) | Adjusted for age and BMI |
| US [65] | Freedland | 2005 | CC | Grade (BMI<25) | unclear | OR = 1.05 (0.94-1.18) | Adjusted for age and BMI |
| US [65] | Freedland | 2005 | CC | Grade (BMI>25) | unclear | OR = 0.94 (0.87-1.01) | Adjusted for age and BMI |
| US [65] | Freedland | 2005 | CC | Stage (all men) | 78 Adv PCa: 158 Loc PCa | ORQ4 vs Q1 = 1.01 (0.47-2.16) | Adjusted for age and BMI |
| US [65] | Freedland | 2005 | CC | Stage (BMI<25) | 21 Adv PCa : 52 Loc PCa | OR = 1.14 (1.02-1.29) | Adjusted for age and BMI |
| US [65] | Freedland | 2005 | CC | Stage (BMI>25) | 57 Adv PCa: 106 Loc PCa | OR = 0.97 (0.91-1.04) | Adjusted for age and BMI |
| US [65] | Freedland | 2005 | CC | Grade and Stage (all men) | 39 HG and Adv | ORQ4 vs Q1 = 0.94 (CI 0.33-2.70) | Adjusted for age and BMI |
| US [65] | Freedland | 2005 | CC | Grade and Stage (BMI<25) | unclear | OR = 1.14 (0.98-1.33) | Adiponectin as continuous variable of the median of the quartiles |
| US [65] | Freedland | 2005 | CC | Grade and Stage (BMI>25) | unclear | OR = 0.95 (0.87-1.04) | Adiponectin as continuous variable of the median of the quartiles |
| Turkey [61] | Goktas | 2005 | CC | Stage | 16 Adv PCa: 14 Loc PCa | Loc PCa Mean 6.0 SD ± 1.7 : Adv PCa Mean 4.7 SD ± 1.2 p < 0.012 (μg/ml) | |
| Czech Republic [63] | Housa | 2008 | CC | Stage | 26 Adv PCa: 17 Loc PCa | Loc PCa Mean 14.51, SD ± 4.92: Adv PCa Mean 21.41, SD ± 8.12 p = 0.003 (ng/ml) | |
| US [64] | Sher | 2008 | CC | Biopsy Grade | 253 HG PCa: 286 LG PCa | OR = 0.98 (0.70-1.37) | Adiponectin dichotomized at the median |
| US [64] | Sher | 2008 | CC | Pathological Grade | 101 HG PCa: 98 LG PCa | OR = 2.04 (1.1-3.58) | Adiponectin dichotomized at the median |
| Turkey [57] | Arisan | 2009 | CC | Grade | 8 HG: 32 MG: 10 LG: 50 HC | HG Mean 4.1 , MG Mean 6.2, LG Mean 9.2, HC Mean 18.4 (μg/ml) | Results unclear |
| Turkey [57] | Arisan | 2009 | CC | Stage | 18 Adv PCa: 32 Loc PCa: 50 HC | Adv Mean 5.5, Loc Mean 8.5, HC Mean 18.4 (μg/ml) | Results unclear |
| SABOR, US [55] | Baillargeon | 2009 | NCC | Grade | 40 HG PCa: 85 LG PCa | ORT3 vs T1 = 1.93 (CI 0.74-5.10) | Adjusted for age |
| PHS, US [51] | Li | 2010 | NCC | Grade | 115 HG: 115 Controls | RRQ5 vs Q1 = 0.49 (0.20-1.22) | Adjusted for age and smoking |
| PHS, US [51] | Li | 2010 | NCC | Lethal PCa | 117 Lethal: 117 Controls | RRQ5 vs Q1 = 0.25 (0.07-0.87) | Adjusted for age and smoking |
Unless otherwise stated. Abbreviations: CC, case control; NCC, nested case control; BMI, body mass index; PCa, prostate cancer case; HC, healthy control; HG, high grade; MG, medium grade; LG, low grade; Adv, advanced; Loc, localised; Lethal, metastatic OR fatal; OR, odds ratio; RR, relative risk; 95% CI, 95% confidence interval; SD, standard deviation; Q, quantile (quartile OR quintile); T, tertile; vs versus
Table 2.
Leptin and prostate cancer progression observational studies
| High Gleason grade, advanced stage and/or fatal prostate cancer | |||||||
|---|---|---|---|---|---|---|---|
| Study, Country | Authors | Year | Design | Outcome | Size | Results - RR/OR (95% CI)* | Comments |
| US [59] | Chang | 2001 | CC | Volume | 160 HV PCa: 48 LV PCa | OR>median vs <median = 2.06 (0.93-4.58) | Adjusted for BMI |
| Turkey [49] | Saglam | 2003 | CC | Stage | 10 Adv: 11 Loc | Adv Mean 36.47 ± SE 12.73, Loc Mean 19.01 ± S.E. 2.72, p<0.001 (ng/ml) | |
| Turkey [49] | Saglam | 2003 | CC | Grade | 7 HG PCa: 5 LG PCa | HG PCa Mean 33.15 ± SE 6.36, LG PCa Mean 19.52 ± 2.02 p=0.003 (ng/ml) | |
| SABOR, US [55] | Baillargeon | 2007 | NCC | Grade | 40 HG PCa: 85 LG PCa | ORT3 vs T1 = 1.20 (0.48-3.01) | Adjusted for age |
| VIP, Sweden [48] | Stocks | 2007 | NCC | Non-Aggressive PCa | 278 Non-Aggressive PCa: 376 HC | ORT3 vs T1 = 0.51 (0.32-0.79) | |
| VIP, Sweden [48] | Stocks | 2007 | NCC | Aggressive PCa | 144 Aggressive PCa: 376 HC | ORT3 vs T1 = 1.15 (0.60-2.24) | |
| Turkey [57] | Arisan | 2009 | CC | Grade | 8 HG: 32 MG: 10 LG: 50 HC | HG Mean 15.98 , MG Mean 14.67, LG Mean 13.90, HC Mean 12.98 (ng/ml) | Results unclear |
| Turkey [57] | Arisan | 2009 | CC | Stage | 18 Adv: 32 Loc: 50 HC | Adv Mean 15.24, Loc Mean 14.78, HC Mean 12.98 (ng/ml) | Results unclear |
| PHS, US [51] | Li | 2010 | NCC | Grade | 124 HG:124 Controls | RRQ5 vs Q1 = 1.74 (0.76-4.00) | Adjusted for age and smoking |
| PHS, US [51] | Li | 2010 | NCC | Lethal PCa | 121 Lethal :121 Controls | RRQ5 vs Q1 = 1.69 (0.67-4.23) | Adjusted for age and smoking |
| PCPT, US [58] | Neuhouser | 2010 | NCC | High Grade | 486 HG PCa: 1 778 HC | ORQ4 vs Q1 = 1.18 (0.88-1.57) | Adjusted for age, race, FHx, treatment arm, smoking, insulin use |
| PCPT, US [58] | Neuhouser | 2010 | NCC | Low Grade | 1224 LG PCa: 1 778 HC | ORQ4 vs Q1 = 0.72 (0.58-0.90) | Adjusted for age, race, FHx, treatment arm, smoking, insulin use |
Unless otherwise stated. Abbreviations: CC, case control; NCC, nested case control; PCa, prostate cancer case; HC, healthy control; Adv, advanced; Loc, localised; HG, high grade; LG, low grade; Lethal, metastatic or fatal; HV, high volume; LV, low volume; Aggressive, high grade, advanced PSA>50ng/ml or fatal; OR, odds ratio; RR, relative risk; 95% CI, 95% confidence interval; SD, standard deviation; SE, standard error of the mean; Q, quantile (quartile or quintile); T, tertile; vs versus; BMI, body mass index; FHx, family history of prostate cancer
Table 3.
Adiponectin and prostate cancer incidence observational studies
| Study, Country | Authors | Year | Design | Size | Results - RR/OR (95% CI)* | Comments |
|---|---|---|---|---|---|---|
| Turkey [61] | Goktas | 2005 | CC | 30 PCa: 3 HC | HC Mean 16.2 SD ± 4.1 : PCa Mean 5.3 SD ± 1.6 μg/ml p <0.001 | |
| Greece [62] | Michalakis | 2007 | CC | 75 cases: 150 HCs | ORQ4 vs Q1 = 0.29 (0.12-0.73) | |
| SABOR, US [54] | Parekh | 2007 | NCC | 124 PCa: 127 HC | OR 0.76 (P=0.12) | |
| SABOR, US [55] | Baillargeon | 2008 | NCC | 125 PCa: 125 HC | ORT3 vs T1 = 0.87 (CI 0.46-1.65) | Adjusted for age |
| Czech Republic [63] | Housa | 2008 | CC | 43 PCa: 25 BPH | BPH Mean 20.47, SD ± 10.13: PCa Mean 18.68, SD ± 7.75 p = 0.64 (ng/ml ) | |
| PHS, US [51] | Li | 2010 | NCC | 599 PCa: 599 HC | RRQ5 vs Q1 = 0.69 (0.47-1.03) | Adjusted for age and smoking |
Unless otherwise stated. Abbreviations: CC, case control; NCC, nested case control; PCa, prostate cancer case; HC, healthy control; BPH, benign prostate hyperplasia; OR, odds ratio; RR, relative risk; 95% CI, 95% confidence interval; SD, standard deviation; Q, quantile (quartile or quintile); T, tertile; vs versus
Six studies of prostate cancer incidence and adiponectin levels were found; two reported inverse relationships [61,62], three others were indicative of an inverse relationship but confidence intervals were wide and included the possibility of no association [51,55,54], and one study found no evidence of an association [63]. Studies of Gleason grade and adiponectin levels have been mixed. Biopsy Gleason grade was not associated with adiponectin levels in one study [64], but within the same patients levels were found to positively predict pathological Gleason grade upgrading following radical prostatectomy. Another study reported no association with grade in all men and normal weight men when stratified by BMI, but a possible inverse association in overweight men [65]. No clear associations were found in two other studies [55,51], but risk estimates were indicative of both positive [55] and inverse [51] relationships and a further, small, study reported an inverse relationship [57]. Stage has been found to be positively [63], inversely [57,61] and not [65] related to adiponectin levels, although a positive association was found in the later study for normal weight men only. Li et al. [51] reported an inverse relationship with lethal (metastatic or fatal) prostate cancer.
It has also been suggested that the leptin:adiponectin relationship may be worthy of investigation as the co-administration of adiponectin reduced the ability of leptin to induce PC3 prostate cancer cell proliferation in vitro [9]. Only one small observational study was found that assessed this ratio in relation to prostate cancer [57] and the findings were not reported in full. However, there appeared to be a positive association with both grade and stage of disease (leptin:adiponectin ratios 0.71, 1.79, 2.77, 1.51, 2.37, 3.90 in controls, localised, advanced, low grade, medium grade and high grade cases respectively).
Findings have clearly been mixed and larger studies and meta-analyses are needed help to establish the true relationships amongst these varied findings as summarized in Box 2.
Box 2 Summary
Leptin displays a range of pro-cancer properties in cell culture studies, but observational studies have found mixed results.
Adiponectin possesses many anti-cancer properties and interacts with several other pathways implicated in prostate cancer, but, again, evidence from epidemiological studies has been mixed.
Many of the studies have been small and under-powered to detect differences; larger studies and meta-analyses stratified by stage, grade or mortality are needed to clarify the nature of any relationships.
Leptin to adiponectin ratio may provide a more sensitive marker of risk and should be investigated in future studies of adipokines and prostate cancer.
Inflammatory cytokines
Adipose tissue also contains connective tissue, vascular cells and nerve cells and is infiltrated by immune cells which secrete additional adipokines, including TNF-α and VEGF [9]. In addition, adipocytes have been shown to affect the growth patterns and cytokine expression of prostate cancer cells; PC3 cells cultured with adipocytes formed larger clusters, showed pleomorphism and 20-fold VEGF and platelet-derived growth factor expression [66]. A chronic, low grade inflammatory state reflected by increased expression, production and release of pro-inflammatory cytokines such as interleukins and TNF-α is associated with obesity, insulin resistance and diabetes [8,67,68,69,70]. These peptides may also contribute to a hormonal milieu that favours the proliferation and survival of prostate cancer cells through growth signalling and protection from cell death [32,71,72,39,73,74,75]. Many of these markers have demonstrated roles in tumorigenesis and progression in vitro [73]. Epidemiological studies have found associations of circulating levels of IL-6, TNF-α and VEGF with prostate cancer incidence [76,77] and makers of progression such as grade [78], volume [79] and presence of metastases [76,78,77]. IL-6 and VEGF have also been found to predict biochemical progression in localised disease [78,76,80]. In addition, TNF-α gene polymorphisms have been associated with prostate cancer [81,82], and other inflammatory gene polymorphisms may also affect risk [83,84]. See Box 3 for summary.
Box 3 Summary
Obesity, and insulin resistance are associated with a chronic, low grade inflammatory state.
Inflammatory cytokines, together with adipokines, may produce a hormonal milieu that supports and encourages prostate cancer growth, survival and progression.
Sex steroids
Adipose tissue produces the enzyme aromatase, which catalyses the conversion of androgens to oestrogens. As a consequence, obesity is associated with lower total testosterone and higher oestrogen levels [8,9,10], which is a challenging concept in the association of prostate cancer and obesity.
Androgens are essential in the normal development, differentiation and proliferation of prostatic tissue [85]. Multiple studies also found them to be important in prostate cancer development [86] and androgen blockade is a common and successful treatment in advanced and metastatic prostate cancer [85]. However, a pooled analysis of 18 prospective observational studies has not shown circulating androgen levels to be associated with risk of prostate cancer [87]. Lowering of androgen levels through inhibition of 5-α-reductase was found to protect against development of prostate cancer in two trials [88,86] but also to increase the risk of higher grade disease in one trial [86]. However, interpretation of these findings has been controversial and further studies have found lower testosterone levels to be associated with higher grade disease [10]. It has been hypothesised that lower androgen concentrations may provide a microenvironment which favours and selects for more aggressive and/or androgen-independent tumour cells - indicating one route via which obesity may influence disease progression [86,8,9]. Alternatively, lower serum testosterone levels may be a bystander effect of the metabolic imbalance which is on the true causal pathway to prostate carcinogenesis [8] or associations may be being masked by complex and inverse relationships with metabolic hormones such as leptin, insulin and IGF-I [89]. However, intraprostatic conversion of testosterone to 5α-dihydrotestosterone, which is not strongly related to circulating androgen levels, may be more influential in prostate cancer development than androgens in the circulation [90]. See Box 4 for summary.
Box 4 Summary
Androgens are important in normal prostatic development, function and carcinogenesis.
Pooled epidemiological data did not provide evidence of an association between prostate cancer risk and circulating androgen levels, but levels within the prostate may be more relevant to risk or interactions with other pathways may be complicating the relationship.
Further work is needed to unravel and explore this relationship.
Insulin-IGF system
Abnormalities in carbohydrate metabolism such as insulin resistance and type II diabetes mellitus (T2DM) are commonly associated with the western lifestyle and obesity and may also be linked to prostate cancer. In insulin resistance and the early stages of T2DM circulating levels of insulin and IGF-I are high [91,92,27]. Insulin may promote prostate cancer growth and progression both directly, through its action as a growth factor for prostate cancer [93], and indirectly, through its effect on the expression of other growth factors and their regulators [94,93] or through drug-interactions.
Serum insulin has been associated with more aggressive or more advanced disease [95] and plasma c-peptide, a surrogate marker of insulin secretion, has been positively associated with high grade prostate cancer [58] and prostate cancer mortality [96]. A meta-analysis of 19 studies of diabetes and risk of prostate cancer revealed a reduced risk among established diabetics [93] and 3 large studies published since this review have also found a history of diabetes to be inversely related to prostate cancer risk [27,91,97]. Although initially hyperinsulinaemic, patients with long-term T2DM progress to hypoinsulinaemia, which may be protective against prostate cancer development [27,91,93]. Indeed, time since diagnosis of diabetes has been repeatedly associated with decreasing risk of prostate cancer [93,98]. Studies of T2DM susceptibility genes and prostate cancer risk have had mixed results, which may reflect the complex aetiology and diagnostic criteria of diabetes [99]. For example, the glucokinase single nucleotide polymorphism (SNP) rs 1799884 is positively associated with both T2DM and prostate cancer [99]. Although this may seem to be contradictory to the majority of evidence, this SNP is not associated with insulin secretion but with high fasting plasma glucose, a central diagnostic criterion in diabetes. In a further example, a SNP within the TCF2 gene that is known to protect against T2DM, rs7501939, was found to increase risk of prostate cancer [100].
In cell culture, insulin was been found to be mitogenic for both normal rat prostate epithelial cells [101] and rat prostate adenocarcinoma cells [102] as well as primary cultures of human prostate epithelial cells [103]. Furthermore, the insulin receptor is highly homologous to the IGF-I receptor and stimulates similar intracellular signalling pathways, as described below (also see Figure 2).
Figure 2.
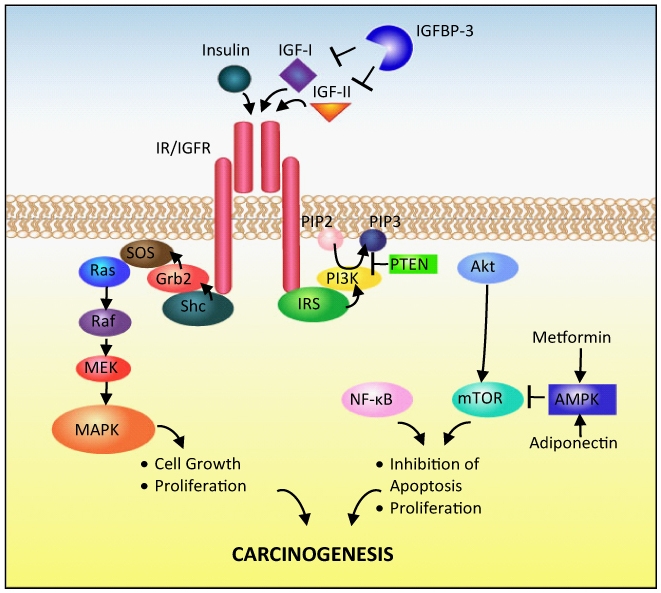
Overview of insulin/IGF signalling in prostate carcinogenesis. Abbreviations: IGF insulin-like growth factor, IGFBP IGF binding protein, IR insulin receptor, IGFR IGF receptor, IRS insulin receptor substrate , PI3K phosphatidylinositol 3′kinase , PIP2 phosphatidylinositol 4,5-diphosphate, PIP3 phosphatidylinositol 3,4,5-triphosphate, PTEN phosphatase and tensin homolog, Akt protein kinase B, NF-κB nuclear factor-κB, mTOR mammalian target of rapamycin, AMPK 5′-AMP-activated protein kinase, Grb2 growth factor receptor-bound protein 2, SOS son of sevenless, MAPK mitogen-activated protein kinase, MEK MAPK kinase.
Insulin suppresses production of some of the IGF binding proteins (IGFBPs) and stimulates hepatic production of IGF-I [94] therefore increasing bioavailable IGF-I. Advanced T2DM and its accompanying hypoinsulinaemia are associated with low levels of IGF-I and high levels of IGFBP-3 [91]. IGF-I is a potent prostate cancer mitogen [104]. The insulin and IGF-I receptors are widely expressed in both normal tissue [105] and neoplastic prostatic tissue [106,107], and cancer cell lines have been shown to be mitogenically responsive to physiological doses of insulin and IGF-I [105]. Both a recent meta-analysis of observational studies [108] and a pooled analysis of prospective studies [109] found IGF-I to be positively associated with prostate cancer risk. However, the meta-analysis found stronger associations between IGF-I and advanced disease than localised and IGFBP-3 to be inversely related over-all risk. The pooled analysis reported stronger associations between IGF-I and localised disease, and a positive association between IGFBP-3 and over-all risk which was attenuated after controlling for IGF-I level. Positive associations are also seen for height and prostate cancer [110], which are believed to be mediated by increased circulating IGF-I. IGF-I is nutritionally modifiable; high milk and calcium diets are associated with both increased IGF-I levels and prostate cancer, especially advanced or aggressive disease [111,12].
Binding of IGF-I or -II or insulin to the insulin/IGF family of receptors induces activation of an intrinsic tyrosine kinase [112] (Figure 2). The kinase phosphorylates the receptor and its substrates – insulin receptor substrate (IRS) and She. Phosphorylated She activates the Ras/Raf/Mitogen-activated protein kinase (MAPK) pathway which stimulates cell growth and proliferation [112,113]. Phosphorylated IRS activates the phosphatidylinositol 3′kinase (PI3K)/Akt pathway, leading to inhibition of apoptosis and stimulation of cell proliferation through downstream mediators including nuclear factor-κB (NF–κB) and mTOR [105,112,113]. PI3K is inhibited by the tumour suppressor gene phosphatase and tensin homolog (PTEN), which is often lost in advanced prostate cancer [114,115] and predicts progression [116]. Although IGFBP-2 is inversely associated with obesity and insulin resistance [117], it may play a role in prostate cancer progression [118] because IGFBP-2 free from IGF-II has been found to suppress PTEN in breast cancer cells [119]. Substantially increased IGFBP-2 levels occur in men with prostate cancer [120,121,122,123], particularly advanced [121] compared with early disease [124,125]. In a small study, serial IGFBP-2 rose in parallel with rises in PSA in advanced prostate cancer [121], and fell after radical prostatectomy [122], indicating a potential for IGFBP-2 in monitoring tumour load. In another, IGFBP-2 predicted biochemical recurrence after radical prostatectomy independent of stage, Gleason score or PSA and predicted survival in an androgen-dependent manner [126]. IGFBP-2 may stimulate prostate cancer cell growth [127] and its production is reported to be regulated by PTEN [128]. Serum IGFBP-2 measures may therefore provide an accessible marker of PTEN status and disease progression and indeed in a screen for markers of PTEN status in prostate cancer IGFBP-2 was identified as the strongest candidate [129].
AMPK inhibits mTOR signalling, a pathway which may be particularly important in prostate cancer [114,45]. As mentioned above, adiponectin, the putative ‘anti-cancer’ adipokine, stimulates AMPK signalling. Metformin, the glucose and insulin lowering diabetes drug, also activates AMPK [105] and its use has been found to be associated with reduced risk of prostate cancer in Caucasian men with diabetes [130].
Hypoinsulinaemia is associated with decreased leptin levels [131] and T2DM is also associated with reduced bioavailable testosterone, both of which may affect prostate cancer risk [91]. There is further evidence to suggest that IGF-I has synergistic effects on leptin stimulation of androgen independent cell lines [32]. See summary in Box 5.
Box 5 Summary
Hyperinsulinaemia associated with obesity and insulin resistance may increase prostate cancer risk directly, and through effects on other growth factors – especially IGF-I.
IGF-I is a potent mitogen and has been associated with prostate cancer progression.
The Insulin-IGF system can potentially be targeted through dietary and lifestyle interventions.
Drugs that inhibit IGF signalling may be useful in the prevention or treatment of prostate cancer – several trials are ongoing and are high lighted in Pollak [105].
Lipid metabolism
Lipid metabolism may be involved in prostate cancer aetiology or progression through several mechanisms.
The fatty acid composition of the western diet has been implicated in prostate carcinogenesis [132,9]. (ω-6 polyunsaturated fatty acids (PUFAs) such as arachidonic acid and linoleic acid are the primary PUFAs of the western diet. In a reaction partially catalysed by cyclooxygenase (COX) they are converted to proinflam-matory eicosanoids, some of which have been associated with prostate cancer [132].ω-3 PUFAs can also be converted via these pathways to eicosinoids, but the end products are believed to be less potent [133], therefore ω3 PUFAs provide competitive inhibition to the production of potent proinflammatory molecules [132]. Increasing the ω -3 to ω-6 PUFA ratio of the diet was found to decrease production of ω-6 PUFA derived eicosinoids [133]. Therefore ω-3 PUFAs are potential anti-inflammatory and anti-prostate cancer agents.
Fatty acid synthesis often occurs at high rates in tumour cells, and over-expression of FASN is a sign of poor prognosis in many cancers [134]. In vitro, over-expression of FASN in combination with androgen receptor (AR) expression in prostate cancer cells has been reported to increase proliferation and inhibit apoptosis [134]. De novo androgen synthesis by tumours allows AR activation following androgen ablation therapy and both cholesterol and fatty acid regulatory genes (including HMG-CoA reductase and FASN) appear to be involved in this process [135]. FASN has been suggested as a target for anti-cancer therapies, but initial studies in animal models have found FASN inhibition to cause hypophagia and weight loss [136]. HMG CoA reductase inhibitors, statins, have been investigated as potential anti-prostate cancer agents but meta-analyses failed to find evidence of any associations between risk of prostate cancer and statin use, particularly in randomised controlled trials [137,138]. There may be a differential effect on advanced prostate cancer compared to total prostate cancer, especially metastatic or fatal disease, such as that reported by in the Health Professionals Follow-up Study [139]. Meta-analyses stratified by stage, grade or mortality may clarify this relationship. See Box 6 for summary.
Box 6 Summary
High ω-6 to ω-3 fatty acids in the western diet may increase proinflammatory eicosanoid production and consequently prostate cancer risk.
Modification of fatty acid intake, or disruption of eicosanoid production through inhibition of COX may provide some protection against prostate cancer development
Fatty acid synthesis has also been implicated in prostate cancer progression but early studies have found FASN inhibition to produce adverse side effects.
AminoAcid Metabolism
Recent evidence has suggested that amino acid metabolism may be another metabolic pathway involved in prostate cancer progression. Sreekumar [140] compared the metabolomes of tissues from benign prostate, prostate cancer and metastatic prostate cancer and found substantial differences in the metabolomic profiles of the tissues. When these were mapped to their respective biochemical pathways, amino acid and nitrogen breakdown pathways were seen to be increased in metastatic prostate cancer samples. One particular metabolite highlighted by this study, sarcosine, was elevated in 42% of prostate cancer samples and 79% of metastatic samples, indicating a possible role in prostate cancer progression. They also measured sarcosine in benign prostate and prostate cancer cell lines and found levels were increased in prostate cancer cells and correlated with cell invasiveness. Addition of sarcosine to benign prostate epithelial cells induced invasive characteristics in these cells and GNMT-knockdown substantially reduced both intracellular sarcosine levels and cell invasion. Sarcosine is produced from glycine through transfer of a methyl group from S-adenosylmethionone by the enzyme glycine-N-methyltransferase (GNMT). Genetic variants in the GNMT gene had previously been reported to be associated with prostate cancer [141]. GNMT regulates the cellular levels of S-adenosylmethionone, which is a methyl donor for several key cell regulatory reactions, in particular those involved in epigenetic regulation of gene expression [142]. Transcriptional regulators central to prostate cancer progression (androgen receptor and Ets gene fusions) appeared to directly control transcription of sarcosine regulatory enzymes and therefore intracellular sarcosine levels. However, much more research in clinical and epidemiological studies will be required to verify the role of sarcosine in prostate cancer progression, particularly because of its potential clinical role in prognostication. See Box 7 for summary.
Box 7 Summary
Increased amino acid and nitrogen breakdown is a feature of prostate cancer.
Sarcosine has recently been identified as a potential prostate cancer marker, but further research is needed to identify its role and verify its use as a prognostic tool.
Metabolism versus immune system
Evidence is emerging directly linking chronic inflammation and prostate carcinogenesis. Prostatitis and history of sexually transmitted infections, regardless of the aetiological organism, have been positively associated with risk of prostate cancer [149,150]. This indicates that inflammation, rather than a specific infectious agent, is likely to be driving tumorigenesis [151]. Inflammation is characterised by local infiltration of white blood cells (WBCs) and production of signalling molecules including cyto-kines and chemokines. Through activation of downstream effectors, such as NF-κB and inducible nitric oxide synthase (iNOS), cytokines can influence tumour initiation and progression [74,152,153]. Furthermore, in response to cyto-kine signalling, activated WBCs generate reactive oxygen and nitrogen species that can induce mutations in cellular DNA, from which the cell may gain survival advantages.
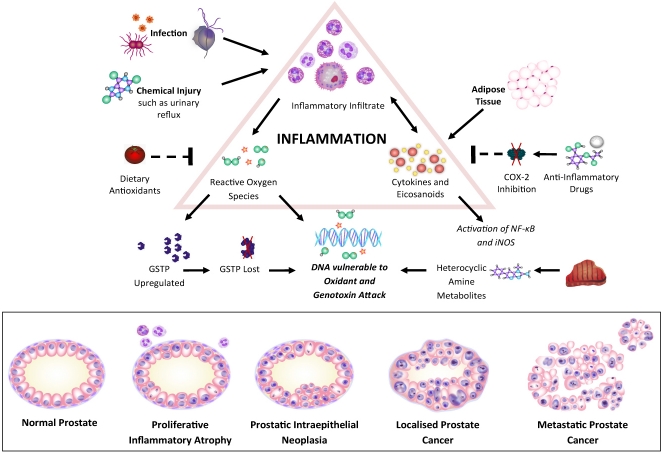
Infection and chemically-induced prostatic injury can lead to lesions of proliferative inflammatory atrophy (PIA), in which focal atrophy is accompanied by epithelial proliferation and inflammatory infiltration [154] (See Figure 3). Transitions between PIA, high-grade prostatic intraepithial neoplasia (PIN) and adenocarcinoma have been observed [154]. Cells within PIA lesions are subjected to substantial oxidative stress, in response to which the detoxifying enzyme π-class glutathione-S-transferase (GSTP) is upregulated [151]. Expression of GSTP1 is frequently lost in PIN and prostate cancer following CpG island hypermethylation of the GSTP1 gene, leaving the cellular DNA vulnerable to oxidant attack and consequently somatic mutations can accumulate [155]. Chronic exposure to oxidants and other genotoxins such as metabolites of hetero-cyclic amines (produced from charred meat) can therefore both initiate and perpetuate prostate cancer development [156,157,158]. Hence, the considerable influence of dietary and lifestyle factors on prostate carcinogenesis may be a consequence of initial GSTP1 loss [143].
Figure 3.
Inflammation in prostate carcinogenesis. Arrows: stimulates. Dashed lines: inhibits. Abbreviations: COX-2 cyclooxygenase-2, GSTP glutathione-S-transferase, NF-κB nuclear factor-κB, iNOS inducible nitric oxide synthase.
Germline genetic mutations in genes involved in viral and bacterial recognition and defence may leave some individuals more susceptible to infection, prostatic inflammation, PIA and eventual prostate cancer. Indeed, mutations in RNASEL, which encodes an endonuclease capable of degrading viral DNA, and MSR1, which encodes the macrophage scavenger receptor 1 that recognises bacterial antigens, have been indentified in linkage studies of hereditary prostate cancers [151,159]. Candidate gene analysis of Toll-like Receptor (TLR) genes have identified several SNPs in these regions associated with prostate cancer [160]. As TLRs are important mediators of innate immunity this provides further evidence, through increased susceptibility to infection, that inflammation is linked to prostate cancer.
The protective effect of antioxidant intake (in the form of lycopene and selenium) on prostate cancer risk provides indirect evidence of the role of oxidative stress in prostate carcinogenesis [12]. There is some observational evidence that non-steroidal anti-inflammatory drugs (NSAIDS) such as aspirin reduce risk of prostate cancer [161]. Inhibition of the growth factor and cytokine inducible enzyme COX-2 (and therefore suppression of eicosinoid production) has been proposed as the biological mechanism behind these observations and there is in vitro evidence to support this theory [162]. Furthermore, COX-2 has been implicated in prostate cancer progression to androgen independence during androgen deprivation therapy [163]. However, a large cross-sectional case-control analysis, based on the Prostate testing for cancer and Treatment (ProtecT) study, found no evidence that NSAIDs reduce the risk of PSA-detected prostate cancer [164]. See Box 8 for summary.
Box 8 Summary
Chronic inflammation may initiate prostate cell transformation.
Loss of detoxifying enzyme GSPT1 may make cells particularly vulnerable to endogenous and dietary oxidant attack.
Increased anti-oxidant intake and anti-inflammatory drug use may be protective, but more evidence is needed.
Future prospects for interventions
Lifestyle changes
Much of this evidence indicates that weight loss and dietary interventions aimed at restoring metabolic and hormonal balance and reducing inflammation may be important tools in prostate cancer prevention and control of localised disease, and potential targets for population level interventions. Gastric bypass surgery, which results in substantial and sustained weight loss, has been associated with reduced cancer incidence and mortality [165], indicating weight loss can be an affective protective tool.
High levels of physical activity have been associated with reduced risk of advanced or aggressive disease [12]. Low fat diets and exercise can reduce serum insulin, IGF-I and leptin levels and increase adiponectin levels [94,45,166]. As there is evidence that the IGF system can be manipulated from very early in life, there is potential for population-based interventions starting from childhood to prevent cancer [111,167]. Indeed, high dietary milk and calcium intake (including in childhood) are associated with both high IGF levels and prostate cancer [111,12]. However, the manipulation of nutritionally dependent pathways, particularly in early life, may have adverse perturbations. For example, childhood IGF-I levels have been found to be positively associated with Intelligence Quotient (IQ) [168], therefore dietary intervention aimed at reducing levels may have detrimental effects on cognitive function.
Calorie restriction and increased consumption of long chain ω-3 polyunsaturated fatty acids can reduce circulating levels of inflammatory markers [169]. LNCaP cell cultured in serum from subjects on a low-fat starch diet and taking daily exercise showed increased apoptosis and decreased proliferation [94]. A further study by this group found a low fat, high fibre and soy protein supplemented diet versus a western diet decreased serum ω-6 and increased serum ω-3 fatty acids [170]. When LNCaP cells were incubated in the subjects' serum these fatty acid changes correlated with decreased growth of the cells. Further to this, there is some observational evidence to suggest that consumption of pulses, including soya products, may protect against prostate cancer – possibly through their effect on sex hormones [12]. There is a growing body of evidence that heterocyclic amines from charred meat are prostate carcinogens [156]. Dietary antioxidants, lycopene and selenium, may protect against prostate cancer [12] but a large randomised controlled trial which included selenium supplementation for primary prostate cancer prevention found no association [171]. See Box 9 for summary.
Box 9 Summary of WCRF Judgements on dietary factors that modify the risk of prostate cancer
-
Increase risk:
- Probable: Diets high in calcium.
- Limited evidence: Milk, dairy products and processed meat
-
Decrease risk
- Probable: Food containing lycopene and selenium and selenium supplements
- Limited evidence: Pulses, foods containing vitamin E and alpha-tocopherol supplements.
Chemoprevention and therapy
Given its anti-cancer effects in vitro, adiponectin may have potential as an adjuvant prostate cancer therapy [42,43] although Metformin, which also acts via activation of AMPK, is already widely used for the treatment of diabetes, has an established excellent safety record and there is evidence that its use in men with diabetes reduces their risk of prostate cancer (128). Weight loss may be preferable to chemotherapy as it is also associated with reversal of other adverse adipokine concentrations [9]. Use of NSAIDS and selective COX-2 inhibitors in prostate cancer treatment is at present limited by adverse gastrointestinal or cardiovascular effects, but trials are ongoing [162]. Targeting of IGF signalling in cancer prevention or treatment is currently being investigated in a number of studies and trials [136,105,113]. Interventions under study include reducing ligand availability or activity, inhibition of receptor stimulation and activators of AMPK, including metformin (which reduces circulating insulin and suppresses insulin- and IGF-stimulated proliferation) [136]. mTOR inhibitors Temsirolimus and Everolimus have already been licensed for the treatment of renal cell carcinoma, and various other PI3k/Akt/mTOR inhibitory compounds are undergoing Phase I and II trials [136].
Future research directions
The main conclusion that can be drawn from this review is that further studies are needed to identify the specific dietary and lifestyle factors that are influencing the high levels of clinical prostate cancer in developed countries.
Further observational studies of adipokine levels, which are large, prospective, include repeated exposure measures and focus on factors influencing progression would help elucidate associations. Attention should focus on biomarker collections, including blood, urine and tissue. Randomised trials would assess the potential benefits of interventions such as long-term anti-inflammatory drug use and dietary modifications, including increased omega 3 fatty acid intake, on prostate cancer risk and prognosis. However, before large-scale trials are conducted, proper assessment of the feasibility of such trials would be a logical next step. We are currently conducting a feasibility study of a trial of dietary modification (PRODiet – ISRCTN 95931417). Pooling of the available data in meta-analyses may also be valuable.
An alternative, or complement, to these types of epidemiological studies is Mendelian Randomization [172,173,174]. Confounding due to measurement error, lifestyle factors (such as smoking, socioeconomic status, diet) and reverse causation are concerns when conducting any observational epidemiological study involving biological measurements. As alleles are allocated at random during meiosis, the use of genetic variants that are associated with phenotypic changes has been identified as a useful tool by which the impact of confounding and reverse causality can be minimised. The identification and analysis of genetic variants as ‘instrumental variables’ can allow more robust causal inferences to be made – a concept known as Mendalian Randomization. For example, a SNP in the Leptin gene (LEP), is associated with both hyperleptinaemia [175] and prostate cancer incidence and advanced disease [176] providing unconfounded evidence for a role for leptin in aetiology and progression of PCa. Genetic variants may also encode products with altered functional characteristics; functional polymorphisms in genes encoding enzymes, binding proteins, receptors and transcriptional activators can affect bioavailability, cellular uptake and cellular response to peptides and hormones, providing insight into important biological pathways. Further genetic studies may help clarify associations and identify key components of these pathways in prostate cancer progression.
Experimental, epidemiological and clinical evidence all suggest that environmental factors play a key role in prostate cancer progression. The evidence presented indicates that metabolic and hormonal imbalances are plausible mechanisms, but whether there are one or two specific elements or a cumulative effect of multiple aspects of these pathways remains to be investigated.
Acknowledgments
AB is recipient of an MRC 4 year PhD studentship at the MRC Centre for Causal Analysis in Translational Epidemiology. Funding for additional research has been received from the World Cancer Research Fund, Cancer Research UK, the UK NIHR Health Technology Assessment Programme, the University of Bristol Cancer Research Fund and the National Cancer Research Institute (formed by the Department of Health, the Medical Research Council and Cancer Research UK).
References
- 1.Owen DH, Katz DF. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J Androl. 2005;26(4):459–469. doi: 10.2164/jandrol.04104. [DOI] [PubMed] [Google Scholar]
- 2.Balk SP, Ko YJ, Bubley GJ. Biology of prostate-specific antigen. J Clin Oncol. 2003;21(2):383–391. doi: 10.1200/JCO.2003.02.083. [DOI] [PubMed] [Google Scholar]
- 3.Krieger JN, Riley DE, Cheah PY, Liong ML, Yuen KH. Epidemiology of prostatitis: new evidence for a world-wide problem. World Journal of Urology. 2003;21(2):70–74. doi: 10.1007/s00345-003-0329-0. [DOI] [PubMed] [Google Scholar]
- 4.Lepor H. Pathophysiology, epidemiology, and natural history of benign prostatic hyperplasia. Rev Urol. 2004;6(Suppl 9):S3–S10. [PMC free article] [PubMed] [Google Scholar]
- 5.Frankel S, Smith GD, Donovan J, Neal D. Screening for prostate cancer. The Lancet. 2003;361(9363):1122–1128. doi: 10.1016/S0140-6736(03)12890-5. [DOI] [PubMed] [Google Scholar]
- 6.McGregor M, Hanley JA, Boivin JF, McLean RG. Screening for prostate cancer: estimating the magnitude of overdetection. CMAJ. 1998;159(11):1368–1372. [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Shlomo Y, Evans S, Ibrahim F, Patel B, Anson K, Chinegwundoh F, Corbishley C, Dorling D, Thomas B, Gillatt D, Kirby R, Muir G, Nargund V, Popert R, Metcalfe C, Persad R. The risk of prostate cancer amongst black men in the United Kingdom: the PROCESS cohort study. Eur Urol. 2008;53(1):99–105. doi: 10.1016/j.eururo.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 8.Hsing AW, Sakoda LC, Chua SC., Jr Obesity, metabolic syndrome, and prostate cancer. Am J Clin Nutr. 2007;86(3):843S–8857. doi: 10.1093/ajcn/86.3.843S. [DOI] [PubMed] [Google Scholar]
- 9.Mistry T, Digby JE, Desai KM, Randeva HS. Obesity and prostate cancer: a role for adipokines. Eur Urol. 2007;52(1):46–53. doi: 10.1016/j.eururo.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 10.Freedland SJ, Platz EA. Obesity and prostate cancer: making sense out of apparently conflicting data. Epidemiol Rev. 2007;29:88–97. doi: 10.1093/epirev/mxm006. [DOI] [PubMed] [Google Scholar]
- 11.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O'Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81(2):341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 12.World Cancer Research Fund/American Institute for Cancer Research, editor. 2007. Second Expert Report, Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective.
- 13.Simmons RK, Alberti KG, Gale EA, Colagiuri S, Tuomilehto J, Qiao Q, Ramachandran A, Tajima N, Brajkovich MI, Ben-Nakhi A, Reaven G, Hama SB, Mendis S, Roglic G. The metabolic syndrome: useful concept or clinical tool? Report of a WHO Expert Consultation. Diabetologia. 2010;53(4):600–605. doi: 10.1007/s00125-009-1620-4. [DOI] [PubMed] [Google Scholar]
- 14.Haas GP, Delongchamps N, Brawley OW, Wang CY, de la Roza G. The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can J Urol. 2008;15(1):3866–3871. [PMC free article] [PubMed] [Google Scholar]
- 15.Breslow N, Chan CW, Dhom G, Drury RA, Franks LM, Gellei B, Lee YS, Lundberg S, Sparke B, Sternby NH, Tulinius H. Latent carcinoma of prostate at autopsy in seven areas. The International Agency for Research on Cancer, Lyons, France. Int J Cancer. 1977;20(5):680–688. doi: 10.1002/ijc.2910200506. [DOI] [PubMed] [Google Scholar]
- 16.Yatani R, Shiraishi T, Nakakuki K, Kusano I, Takanari H, Hayashi T, Stemmermann GN. Trends in Frequency of Latent Prostate Carcinoma in Japan from 1965-1979 to 1982-1986. J Natl Cancer Inst. 1988;80(9):683–687. doi: 10.1093/jnci/80.9.683. [DOI] [PubMed] [Google Scholar]
- 17.Peto J. Cancer epidemiology in the last century and the next decade. Nature. 2001;411(6835):390–395. doi: 10.1038/35077256. [DOI] [PubMed] [Google Scholar]
- 18.Iwasaki M, Mameri CP, Hamada GS, Tsugane S. Cancer mortality among Japanese immigrants and their descendants in the state of Sao Paulo, Brazil, 1999-2001. Jpn J Clin Oncol. 2004;34(11):673–680. doi: 10.1093/jjco/hyh123. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Demissie K, Lu SE, Rhoads GG. Cancer incidence among Korean-American immigrants in the United States and native Koreans in South Korea. Cancer Control. 2007;14(1):78–85. doi: 10.1177/107327480701400111. [DOI] [PubMed] [Google Scholar]
- 20.Rastogi T, Devesa S, Mangtani P, Mathew A, Cooper N, Kao R, Sinha R. Cancer incidence rates among South Asians in four geographic regions: India, Singapore, UK and US. Int J Epidemiol. 2008;37(1):147–160. doi: 10.1093/ije/dym219. [DOI] [PubMed] [Google Scholar]
- 21.Pu YS, Chiang HS, Lin CC, Huang CY, Huang KH, Chen J. Changing trends of prostate cancer in Asia. Aging Male. 2004;7(2):120–132. doi: 10.1080/13685530412331284687. [DOI] [PubMed] [Google Scholar]
- 22.Tominaga S, Kuroishi T. An ecological study on diet/nutrition and cancer in Japan. Int J Cancer. 1997;(Suppl 10):2–6. doi: 10.1002/(sici)1097-0215(1997)10+<2::aid-ijc2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 23.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 24.MacInnis RJ, English DR. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. 2006;17(8):989–1003. doi: 10.1007/s10552-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 25.Wright ME, Chang SC, Schatzkin A, Albanes D, Kipnis V, Mouw T, Hurwitz P, Hollenbeck A, Leitzmann MF. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007;109(4):675–684. doi: 10.1002/cncr.22443. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez C, Freedland SJ, Deka A, Jacobs EJ, McCullough ML, Patel AV, Thun MJ, Calle EE. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2007;16(1):63–69. doi: 10.1158/1055-9965.EPI-06-0754. [DOI] [PubMed] [Google Scholar]
- 27.Gong Z, Neuhouser ML, Goodman PJ, Albanes D, Chi C, Hsing AW, Lippman SM, Platz EA, Pollak MN, Thompson IM, Kristal AR. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiology, Biomarkers & Prevention. 2006;15(10):1977–1983. doi: 10.1158/1055-9965.EPI-06-0477. [DOI] [PubMed] [Google Scholar]
- 28.Magheli A, Rais-Bahrami S, Trock BJ, Humphreys EB, Partin AW, Han M, Gonzalgo ML. Impact of body mass index on biochemical recurrence rates after radical prostatectomy: an analysis utilizing propensity score matching. Urology. 2008;72(6):1246–1251. doi: 10.1016/j.urology.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Littman AJ, White E, Kristal AR. Anthropometrics and prostate cancer risk. Am J Epidemiol. 2007;165(11):1271–1279. doi: 10.1093/aje/kwm013. [DOI] [PubMed] [Google Scholar]
- 30.Wallstrom P, Bjartell A, Gullberg B, Olsson H, Wirfalt E. A prospective Swedish study on body size, body composition, diabetes, and prostate cancer risk. Br J Cancer. 2009;100(11):1799–1805. doi: 10.1038/sj.bjc.6605077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Housa D, Housova J, Vernerova Z, Haluzik M. Adipocytokines and cancer. Physiol Res. 2006;55(3):233–244. doi: 10.33549/physiolres.930848. [DOI] [PubMed] [Google Scholar]
- 32.Onuma M, Bub JD, Rummel TL, Iwamoto Y. Prostate Cancer Cell-Adipocyte Interaction: Leptin mediates androgen-independent prostate cancer cell proliferation through c-Jun NH2-terminal kinase. J Biol Chem. 2003;278(43):42660–42667. doi: 10.1074/jbc.M304984200. [DOI] [PubMed] [Google Scholar]
- 33.Hoda MR, Popken G. Mitogenic and antiapoptotic actions of adipocyte-derived hormone leptin in prostate cancer cells. BJU Int. 2008;102(3):383–388. doi: 10.1111/j.1464-410X.2008.07534.x. [DOI] [PubMed] [Google Scholar]
- 34.Frankenberry KA, Somasundar P, McFadden DW, Vona-Davis LC. Leptin induces cell migration and the expression of growth factors in human prostate cancer cells. Am J Surg. 2004;188(5):560–565. doi: 10.1016/j.amjsurg.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 35.Deo DD, Rao AP, Bose SS, Ouhtit A, Baliga SB, Rao SA, Trock BJ, Thouta R, Raj MH, Rao PN. Differential effects of leptin on the invasive potential of androgen-dependent and - independent prostate carcinoma cells. J Biomed Biotechnol. 2008;2008 doi: 10.1155/2008/163902. 163902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mistry T, Digby JE, Desai KM, Randeva HS. Leptin and adiponectin interact in the regulation of prostate cancer cell growth via modulation of p53 and bcl-2 expression. BJU Int. 2008;101(10):1317–1322. doi: 10.1111/j.1464-410X.2008.07512.x. [DOI] [PubMed] [Google Scholar]
- 37.Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, Flores-Riveros JR. Biological Action of Leptin as an Angiogenic Factor. Science. 1998;281(5383):1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 38.Culig Z, Steiner H, Bartsch G, Hobisch A. Interleukin-6 regulation of prostate cancer cell growth. J Cell Biochem. 2005;95(3):497–505. doi: 10.1002/jcb.20477. [DOI] [PubMed] [Google Scholar]
- 39.Smith PC, Hobisch A, Lin DL, Culig Z, Keller ET. Interleukin-6 and prostate cancer progression. Cytokine Growth Factor Rev. 2001;12(1):33–40. doi: 10.1016/s1359-6101(00)00021-6. [DOI] [PubMed] [Google Scholar]
- 40.Wegiel B, Bjartell A, Culig Z, Persson JL. Interleukin-6 activates PI3K/Akt pathway and regulates cyclin A1 to promote prostate cancer cell survival. Int J Cancer. 2008;122(7):1521–1529. doi: 10.1002/ijc.23261. [DOI] [PubMed] [Google Scholar]
- 41.Steiner H, Godoy-Tundidor S, Rogatsch H, Berger AP, Fuchs D, Comuzzi B, Bartsch G, Hobisch A, Culig Z. Accelerated in Vivo Growth of Prostate Tumors that Up-Regulate Interleukin-6 Is Associated with Reduced Retinoblastoma Protein Expression and Activation of the Mitogen-Activated Protein Kinase Pathway. Am J Pathol. 2003;162(2):655–663. doi: 10.1016/S0002-9440(10)63859-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bub JD, Miyazaki T, Iwamoto Y. Adiponectin as a growth inhibitor in prostate cancer cells. Biochemical and Biophysical Research Communications. 2006;340(4):1158–1166. doi: 10.1016/j.bbrc.2005.12.103. [DOI] [PubMed] [Google Scholar]
- 43.Brakenhielm E, Veitonmaki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, Funahashi T, Cao Y. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2004;101(8):2476–2481. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baillargeon J, Rose DP. Obesity, adipokines, and prostate cancer (review) Int J Oncol. 2006;28(3):737–745. [PubMed] [Google Scholar]
- 45.Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer. 2006;94(9):1221–1225. doi: 10.1038/sj.bjc.6603051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86(3):s858–s866. doi: 10.1093/ajcn/86.3.858S. [DOI] [PubMed] [Google Scholar]
- 47.Frankel S, Gunnell DJ, Peters TJ, Maynard M, Smith GD. Childhood energy intake and adult mortality from cancer: the boyd orr cohort study. BMJ. 1998;316(7130):499–504. doi: 10.1136/bmj.316.7130.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stocks T, Lukanova A, Rinaldi S, Biessy C, Dossus L, Lindahl B, Hallmans G, Kaaks R, Stattin P. Insulin resistance is inversely related to prostate cancer: a prospective study in Northern Sweden. Int J Cancer. 2007;120(12):2678–2686. doi: 10.1002/ijc.22587. [DOI] [PubMed] [Google Scholar]
- 49.Saglam K, Aydur E, Yilmaz M, Goktas S. Leptin influences cellular differentiation and progression in prostate cancer. J Urol. 2003;169(4):1308–1311. doi: 10.1097/01.ju.0000055903.18400.25. [DOI] [PubMed] [Google Scholar]
- 50.Stattin P, Soderberg S, Hallmans G, Bylund A, Kaaks R, Stenman UH, Bergh A, Olsson T. Leptin Is Associated with Increased Prostate Cancer Risk: A Nested Case-Referent Study. J Clin Endocrinol Metab. 2001;86(3):1341–1345. doi: 10.1210/jcem.86.3.7328. [DOI] [PubMed] [Google Scholar]
- 51.Li H, Stampfer MJ, Mucci L, Rifai N, Qiu W, Kurth T, Ma J. A 25-year prospective study of plasma adiponectin and leptin concentrations and prostate cancer risk and survival. Clin Chem. 2010;56(1):34–43. doi: 10.1373/clinchem.2009.133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stattin P, Kaaks R, Johansson R, Gislefoss R, Soderberg S, Alfthan H, Stenman UH, Jellum E, Olsson T. Plasma Leptin Is Not Associated with Prostate Cancer Risk. Cancer Epidemiol Biomarkers Prev. 2003;12(5):474–475. [PubMed] [Google Scholar]
- 53.Hsing AW, Chua S, Jr, Gao YT, Gentzschein E, Chang L, Deng J, Stanczyk FZ. Prostate cancer risk and serum levels of insulin and lepta population-based study. J Natl Cancer Inst. 2001;93(10):783–789. doi: 10.1093/jnci/93.10.783. [DOI] [PubMed] [Google Scholar]
- 54.Parekh DJ, Ankerst DP, Baillargeon J, Higgins B, Platz EA, Troyer D, Hernandez J, Leach RJ, Lokshin A, Thompson IM. Assessment of 54 biomarkers for biopsy-detectable prostate cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(10):1966–1972. doi: 10.1158/1055-9965.EPI-07-0302. [DOI] [PubMed] [Google Scholar]
- 55.Baillargeon J, Platz EA, Rose DP, Pollock BH, Ankerst DP, Haffner S, Higgins B, Lokshin A, Troyer D, Hernandez J, Lynch S, Leach RJ, Thompson IM. Obesity, adipokines, and prostate cancer in a prospective population-based study. Cancer Epidemiol Biomarkers Prev. 2006;15(7):1331–1335. doi: 10.1158/1055-9965.EPI-06-0082. [DOI] [PubMed] [Google Scholar]
- 56.Lagiou P, Signorello LB, Trichopoulos D, Tzonou A, Trichopoulou A, Mantzoros CS. Leptin in relation to prostate cancer and benign prostatic hyperplasia. Int J Cancer. 1998;76(1):25–28. doi: 10.1002/(sici)1097-0215(19980330)76:1<25::aid-ijc5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 57.Arisan ED, Arisan S, Atis G, Palavan-Unsal N, Ergenekon E. Serum adipocytokine levels in prostate cancer patients. Urol Int. 2009;82(2):203–208. doi: 10.1159/000200801. [DOI] [PubMed] [Google Scholar]
- 58.Neuhouser ML, Till C, Kristal A, Goodman P, Hoque A, Platz EA, Hsing AW, Albanes D, Parnes HL, Pollak M. Finasteride modifies the relation between serum C-peptide and prostate cancer risk: results from the Prostate Cancer Prevention Trial. Cancer Prev Res (Phila Pa) 2010;3(3):279–289. doi: 10.1158/1940-6207.CAPR-09-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang S, Hursting SD, Contois JH, Strom SS, Yamamura Y, Babaian RJ, Troncoso P, Scardino PS, Wheeler TM, Amos CI, Spitz MR. Leptin and prostate cancer. Prostate. 2001;46(1):62–67. doi: 10.1002/1097-0045(200101)46:1<62::aid-pros1009>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 60.Moore SC, Leitzmann MF, Albanes D, Weinstein SJ, Snyder K, Virtamo J, Ahn J, Mayne ST, Yu H, Peters U, Gunter MJ. Adipokine genes and prostate cancer risk. Int J Cancer. 2009;124(4):869–876. doi: 10.1002/ijc.24043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goktas S, Yilmaz MI, Caglar K, Sonmez A, Kilic S, Bedir S. Prostate cancer and adiponectin. Urology. 2005;65(6):1168–1172. doi: 10.1016/j.urology.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 62.Michalakis K, Williams CJ, Mitsiades N, Blakeman J, Balafouta-Tselenis S, Giannopoulos A, Mantzoros CS. Serum adiponectin concentrations and tissue expression of adiponectin receptors are reduced in patients with prostate cancer: a case control study. Cancer Epidemiol Biomarkers Prev. 2007;16(2):308–313. doi: 10.1158/1055-9965.EPI-06-0621. [DOI] [PubMed] [Google Scholar]
- 63.Housa D, Vernerova Z, Heracek J, Prochazka B, Cechak P, Kuncova J, Haluzik M. Adiponectin as a potential marker of prostate cancer progression: studies in organ-confined and locally advanced prostate cancer. Physiol Res. 2008;57(3):451–458. doi: 10.33549/physiolres.931156. [DOI] [PubMed] [Google Scholar]
- 64.Sher DJ, Oh WK, Jacobus S, Regan MM, Lee GS, Mantzoros C. Relationship between serum adiponectin and prostate cancer grade. Prostate. 2008;68(14):1592–1598. doi: 10.1002/pros.20823. [DOI] [PubMed] [Google Scholar]
- 65.Freedland SJ, Sokoll LJ, Platz EA, Mangold LA, Bruzek DJ, Mohr P, Yiu SK, Partin AW. Association between serum adiponectin, and pathological stage and grade in men undergoing radical prostatectomy. J Urol. 2005;174(4 Pt 1):1266–1270. doi: 10.1097/01.ju.0000173093.89897.97. [DOI] [PubMed] [Google Scholar]
- 66.Tokuda Y, Satoh Y, Fujiyama C, Toda S, Sugihara H, Masaki Z. Prostate cancer cell growth is modulated by adipocyte-cancer cell interaction. BJU Int. 2003;91(7):716–720. doi: 10.1046/j.1464-410x.2003.04218.x. [DOI] [PubMed] [Google Scholar]
- 67.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 68.Lehrke M, Lazar MA. Inflamed about obesity. Nat Med. 2004;10(2):126–127. doi: 10.1038/nm0204-126. [DOI] [PubMed] [Google Scholar]
- 69.Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004;15(11):2792–2800. doi: 10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]
- 70.Mena S, Ortega A, Estrela JM. Oxidative stress in environmental-induced carcinogenesis. Mutat Res. 2009;674(1-2):36–44. doi: 10.1016/j.mrgentox.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 71.Nunez C, Cansino JR, Bethencourt F, Perez-Utrilla M, Fraile B, Martinez-Onsurbe P, Olmedilla G, Paniagua R, Royuela M. TNF/IL-1/ NIK/NF-kappa B transduction pathway: a comparative study in normal and pathological human prostate (benign hyperplasia and carcinoma) Histopathology. 2008;53(2):166–176. doi: 10.1111/j.1365-2559.2008.03092.x. [DOI] [PubMed] [Google Scholar]
- 72.Ricote M, Garcia-Tunon I, Bethencourt FR, Fraile B, Paniagua R, Royuela M. Interleukin- 1 (IL-1alpha and IL-1beta) and its receptors (IL-1RI, IL-1RII, and IL-1Ra) in prostate carcinoma. Cancer. 2004;100(7):1388–1396. doi: 10.1002/cncr.20142. [DOI] [PubMed] [Google Scholar]
- 73.Bouraoui Y, Ricote M, Garcia-Tunon I, Rodriguez-Berriguete G, Touffehi M, Rais NB, Fraile B, Paniagua R, Oueslati R, Royuela M. Pro-inflammatory cytokines and prostate-specific antigen in hyperplasia and human prostate cancer. Cancer Detect Prev. 2008;32(1):23–32. doi: 10.1016/j.cdp.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 74.Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat Res. 2008;659(1 -2):15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 75.Maitland NJ, Collins AT. Inflammation as the primary aetiological agent of human prostate cancer: a stem cell connection? J Cell Biochem. 2008;105(4):931–939. doi: 10.1002/jcb.21843. [DOI] [PubMed] [Google Scholar]
- 76.Shariat SF, Anwuri VA, Lamb DJ, Shah NV, Wheeler TM, Slawin KM. Association of Preoperative Plasma Levels of Vascular Endothelial Growth Factor and Soluble Vascular Cell Adhesion Molecule-1 With Lymph Node Status and Biochemical Progression After Radical Prostatectomy. J Clin Oncol. 2004;22(9):1655–1663. doi: 10.1200/JCO.2004.09.142. [DOI] [PubMed] [Google Scholar]
- 77.Michalaki V, Syrigos K, Charles P, Waxman J. Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer. 2004;90(12):2312–2316. doi: 10.1038/sj.bjc.6601814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shariat SF, Andrews B, Kattan MW, Kim J, Wheeler TM, Slawin KM. Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology. 2001;58(6):1008–1015. doi: 10.1016/s0090-4295(01)01405-4. [DOI] [PubMed] [Google Scholar]
- 79.Svatek RS, Jeldres C, Karakiewicz PI, Suardi N, Walz J, Roehrborn CG, Montorsi F, Slawin KM, Shariat SF. Pre-treatment biomarker levels improve the accuracy of postprostatectomy nomogram for prediction of biochemical recurrence. Prostate. 009;69(8):886–894. doi: 10.1002/pros.20938. [DOI] [PubMed] [Google Scholar]
- 80.Vergis R, Corbishley CM, Norman AR, Bartlett J, Jhavar S, Borre M, Heeboll S, Horwich A, Huddart R, Khoo V, Eeles R, Cooper C, Sydes M, Dearnaley D, Parker C. Intrinsic markers of tumour hypoxia and angiogenesis in localised prostate cancer and outcome of radical treatment: a retrospective analysis of two randomised radiotherapy trials and one surgical cohort study. Lancet Oncol. 2008;9(4):342–351. doi: 10.1016/S1470-2045(08)70076-7. [DOI] [PubMed] [Google Scholar]
- 81.Oh BR, Sasaki M, Perinchery G, Ryu SB, Park YI, Carroll P, Dahiya R. Frequent genotype changes at -308, and 488 regions of the tumor necrosis factor-alpha (TNF-alpha) gene in patients with prostate cancer. J Urol. 2000;163(5):1584–1587. [PubMed] [Google Scholar]
- 82.Saenz-Lopez P, Carretero R, Cozar JM, Romero JM, Canton J, Vilchez JR, Tallada M, Garrido F, Ruiz-Cabello F. Genetic polymorphisms of RANTES, IL1-A, MCP-1 and TNF-A genes in patients with prostate cancer. BMC Cancer. 2008;8:382. doi: 10.1186/1471-2407-8-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun J, Turner A, Xu J, Gronberg H, Isaacs W. Genetic variability in inflammation pathways and prostate cancer risk. Urol Oncol. 2007;25(3):250–259. doi: 10.1016/j.urolonc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 84.Pierce BL, Biggs ML, DeCambre M, Reiner AP, Li C, Fitzpatrick A, Carlson CS, Stanford JL, Austin MA. C-reactive protein, interleukin- 6, and prostate cancer risk in men aged 65 years and older. Cancer Causes Control. 2009;20(7):1193–1203. doi: 10.1007/s10552-009-9320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Platz EA, Giovannucci E. The epidemiology of sex steroid hormones and their signaling and metabolic pathways in the etiology of prostate cancer. The Journal of Steroid Biochemistry and Molecular Biology. 2004;92(4):237–253. doi: 10.1016/j.jsbmb.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 86.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA., Jr The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349(3):215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 87.Roddam AW, Allen NE, Appleby P, Key TJ, Prostate Cancer Collaborative Grou Endogenous Sex Hormones and Prostate Cancer: A Collaborative Analysis of 18 Prospective Studies. J Natl Cancer Inst. 2008;100(3):170–183. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, Pettaway CA, Tammela TL, Teloken C, Tindall DJ, Somerville MC, Wilson TH, Fowler IL, Rittmaster RS. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362(13):1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 89.Kaaks R, Lukanova A, Rinaldi S, Biessy C, Soderberg S, Olsson T, Stenman UH, Riboli E, Hallmans G, Stattin P. Interrelationships between plasma testosterone, SHBG, IGF-I, insulin and leptin in prostate cancer cases and controls. Eur J Cancer Prev. 2003;12(4):309–315. doi: 10.1097/00008469-200308000-00011. [DOI] [PubMed] [Google Scholar]
- 90.Kaaks R, Stattin P. Obesity, endogenous hormone metabolism, and prostate cancer risk: a conundrum of “highs” and “lows”. Cancer Prev Res (Phila Pa) 2010;3(3):259–262. doi: 10.1158/1940-6207.CAPR-10-0014. [DOI] [PubMed] [Google Scholar]
- 91.Calton BA, Chang SC, Wright ME, Kipnis V, Lawson K, Thompson FE, Subar AF, Mouw T, Campbell DS, Hurwitz P, Hollenbeck A, Schatzkin A, Leitzmann MF. History of diabetes mellitus and subsequent prostate cancer risk in the NIH-AARP Diet and Health Study. Cancer Causes Control. 2007;18(5):493–503. doi: 10.1007/s10552-007-0126-y. [DOI] [PubMed] [Google Scholar]
- 92.Rodriguez C, Patel AV, Mondul AM, Jacobs EJ, Thun MJ, Calle EE. Diabetes and risk of prostate cancer in a prospective cohort of US men. Am J Epidemiol. 2005;161(2):147–152. doi: 10.1093/aje/kwh334. [DOI] [PubMed] [Google Scholar]
- 93.Kasper JS, Giovannucci E. A Meta-analysis of Diabetes Mellitus and the Risk of Prostate Cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2056–2062. doi: 10.1158/1055-9965.EPI-06-0410. [DOI] [PubMed] [Google Scholar]
- 94.Barnard RJ, Aronson WJ, Tymchuk CN, Ngo TH. Prostate cancer: another aspect of the insulin-resistance syndrome? Obes Rev. 2002;3(4):303–308. doi: 10.1046/j.1467-789x.2002.00081.x. [DOI] [PubMed] [Google Scholar]
- 95.Lehrer S, Diamond EJ, Stagger S, Stone NN, Stock RG. Increased serum insulin associated with increased risk of prostate cancer recurrence. Prostate. 2002;50(1):1–3. doi: 10.1002/pros.10026. [DOI] [PubMed] [Google Scholar]
- 96.Ma J, Li H, Giovannucci E, Mucci L, Qiu W, Nguyen PL, Gaziano JM, Pollak M, Stampfer MJ. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 2008;9(11):1039–1047. doi: 10.1016/S1470-2045(08)70235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Turner E, Lane A, Donovan J, Davis M, Metcalfe C, Neal D, Hamdy F, Martin RM. Association of diabetes mellitus with prostate cancer: Nested case-control study (ProtecT: Prostate testing for cancer and treatment) Int J Cancer. 2010 doi: 10.1002/ijc.25360. In press. [DOI] [PubMed] [Google Scholar]
- 98.Baradaran N, Ahmadi H, Salem S, Lotfi M, Jahani Y, Baradaran N, Mehrsai AR, Pourmand G. The protective effect of diabetes mellitus against prostate cancer: Role of sex hormones. Prostate. 2009;69(16):1744–1750. doi: 10.1002/pros.21023. [DOI] [PubMed] [Google Scholar]
- 99.Murad A, Davey Smith G, Lewis S, Cox A, Donovan J, Neal D, Hamdy F, Martin RM. A polymorphism in the glucokinase gene that raises plasma fasting glucose, rs1799884, is associated with diabetes mellitus and prostate cancer: findings from a population-based, case control study (the ProtecT study) International Journal of Molecular Epidemiology and Genetics. 2010;1(3):175–183. [PMC free article] [PubMed] [Google Scholar]
- 100.Gudmundsson J, Sulem P, Steinthorsdottir V, Bergthorsson JT, Thorleifsson G, Manolescu A, Rafnar T, Gudbjartsson D, Agnarsson BA, Baker A, Sigurdsson A, Benediktsdottir KR, Jakobsdottir M, Blondal T, Stacey SN, Helgason A, Gunnarsdottir S, Olafsdottir A, Kristinsson KT, Birgisdottir B, Ghosh S, Thorlacius S, Magnusdottir D, Stefansdottir G, Kristjansson K, Bagger Y, Wilensky RL, Reilly MP, Morris AD, Kimber CH, Adeyemo A, Chen Y, Zhou J, So WY, Tong PC, Ng MC, Hansen T, Andersen G, Borch-Johnsen K, Jorgensen T, Tres A, Fuertes F, Ruiz-Echarri M, Asin L, Saez B, van BE, Klaver S, Swinkels DW, Aben KK, Graif T, Cashy J, Suarez BK, van Vierssen TO, Frigge ML, Ober C, Hofker MH, Wijmenga C, Christiansen C, Rader DJ, Palmer CN, Rotimi C, Chan JC, Pedersen O, Sigurdsson G, Benediktsson R, Jonsson E, Einarsson GV, Mayordomo JI, Catalona WJ, Kiemeney LA, Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K. Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet. 2007;39(8):977–983. doi: 10.1038/ng2062. [DOI] [PubMed] [Google Scholar]
- 101.McKeehan WL, Adams PS, Rosser MP. Direct Mitogenic Effects of Insulin, Epidermal Growth Factor, Glucocorticoid, Cholera Toxin, Unknown Pituitary Factors and Possibly Prolactin, but not Androgen, on Normal Rat Prostate Epithelial Cells in Serum-free, Primary Cell Culture. Cancer Res. 1984;44(5):1998–2010. [PubMed] [Google Scholar]
- 102.Polychronakos C, Janthly U, Lehoux JG, Koutsilieris M. Mitogenic effects of insulin and insulin-like growth factors on PA-III rat prostate adenocarcinoma cells: characterization of the receptors involved. Prostate. 1991;19(4):313–321. doi: 10.1002/pros.2990190405. [DOI] [PubMed] [Google Scholar]
- 103.Cohen P, Peehl DM, Lamson G, Rosenfeld RG. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins in primary cultures of prostate epithelial cells. J Clin Endocrinol Metab. 1991;73(2):401–407. doi: 10.1210/jcem-73-2-401. [DOI] [PubMed] [Google Scholar]
- 104.Gennigens C, Menetrier-Caux C, Droz JP. Insulin-Like Growth Factor (IGF) family and prostate cancer. Crit Rev Oncol Hematol. 2006;58(2):124–145. doi: 10.1016/j.critrevonc.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 105.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8(12):915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 106.Hellawell GO, Turner GD, Davies DR, Poulsom R, Brewster SF, Macaulay VM. Expression of the type 1 insulin-like growth factor receptor is up-regulated in primary prostate cancer and commonly persists in metastatic disease. Cancer Res. 2002;62(10):2942–2950. [PubMed] [Google Scholar]
- 107.Cox ME, Gleave ME, Zakikhani M, Bell RH, Piura E, Vickers E, Cunningham M, Larsson O, Fazli L, Pollak M. Insulin receptor expression by human prostate cancers. Prostate. 2009;69(1):33–40. doi: 10.1002/pros.20852. [DOI] [PubMed] [Google Scholar]
- 108.Rowlands MA, Gunnell D, Harris R, Vatten LJ, Holly JM, Martin RM. Circulating insulin-like growth factor peptides and prostate cancer risk: a systematic review and metaanalysis. Int J Cancer. 2009;124(10):2416–2429. doi: 10.1002/ijc.24202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roddam AW, Allen NE, Appleby P, Key TJ, Ferrucci L, Carter HB, Metter EJ, Chen C, Weiss NS, Fitzpatrick A, Hsing AW, Lacey JV, Jr, Helzlsouer K, Rinaldi S, Riboli E, Kaaks R, Janssen JA, Wildhagen MF, Schroder FH, Platz EA, Pollak M, Giovannucci E, Schaefer C, Quesenberry CP, Jr, Vogelman JH, Severi G, English DR, Giles GG, Stattin P, Hallmans G, Johansson M, Chan JM, Gann P, Oliver SE, Holly JM, Donovan J, Meyer F, Bairati I, Galan P. Insulin-like growth factors, their binding proteins, and prostate cancer risk: analysis of individual patient data from 12 prospective studies. Ann Intern Med. 2008;149(7):461–468. doi: 10.7326/0003-4819-149-7-200810070-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zuccolo L, Harris R, Gunnell D, Oliver S, Lane JA, Davis M, Donovan J, Neal D, Hamdy F, Beynon R, Savovic J, Martin RM. Height and prostate cancer risk: a large nested casecontrol study (ProtecT) and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17(9):2325–2336. doi: 10.1158/1055-9965.EPI-08-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gunnell D, Oliver SE, Peters TJ, Donovan JL, Persad R, Maynard M, Gillatt D, Pearce A, Hamdy FC, Neal DE, Holly JM. Are dietprostate cancer associations mediated by the IGF axis? A cross-sectional analysis of diet, IGF -I and IGFBP-3 in healthy middle-aged men. Br J Cancer. 2003;88(11):1682–1686. doi: 10.1038/sj.bjc.6600946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kojima S, Inahara M, Suzuki H, Ichikawa T, Furuya Y. Implications of insulin-like growth factor-I for prostate cancer therapies. Int J Urol. 2009;16(2):161–167. doi: 10.1111/j.1442-2042.2008.02224.x. [DOI] [PubMed] [Google Scholar]
- 113.Meinbach DS, Lokeshwar BL. Insulin-like growth factors and their binding proteins in prostate cancer: Cause or consequence? Urologic Oncology: Seminars and Original Investigations. 2007;24(4):294–306. doi: 10.1016/j.urolonc.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 114.van der Poel HG. Molecular markers in the diagnosis of prostate cancer. Crit Rev Oncol Hematol. 2007;61(2):104–139. doi: 10.1016/j.critrevonc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 115.McMenamin ME, Soung P, Perera S, Kaplan I, Loda M, Sellers WR. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res. 1999;59(17):4291–4296. [PubMed] [Google Scholar]
- 116.Koksal IT, Dirice E, Yasar D, Sanlioglu AD, Ciftcioglu A, Gulkesen KH, Ozes NO, Baykara M, Luleci G, Sanlioglu S. The assessment of PTEN tumor suppressor gene in combination with Gleason scoring and serum PSA to evaluate progression of prostate carcinoma. Urol Oncol. 2004;22(4):307–312. doi: 10.1016/j.urolonc.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 117.Martin RM, Holly JM, Davey SG, Gunnell D. Associations of adiposity from childhood into adulthood with insulin resistance and the insulin-like growth factor system: 65-year follow-up of the Boyd Orr Cohort. J Clin Endocrinol Metab. 2006;91(9):3287–3295. doi: 10.1210/jc.2006-0745. [DOI] [PubMed] [Google Scholar]
- 118.Degraff DJ, Aguiar AA, Sikes RA. Disease evidence for IGFBP-2 as a key player in prostate cancer progression and development of osteosclerotic lesions. Am J Transl Res. 2009;1(2):115–130. [PMC free article] [PubMed] [Google Scholar]
- 119.Perks CM, Vernon EG, Rosendahl AH, Tonge D, Holly JM. IGF-II and IGFBP-2 differentially regulate PTEN in human breast cancer cells. Oncogene. 2007;26(40):5966–5972. doi: 10.1038/sj.onc.1210397. [DOI] [PubMed] [Google Scholar]
- 120.Cohen P, Peehl DM, Stamey TA, Wilson KF, Clemmons DR, Rosenfeld RG. Elevated levels of insulin-like growth factor-binding protein-2 in the serum of prostate cancer patients. J Clin Endocrinol Metab. 1993;76(4):1031–1035. doi: 10.1210/jcem.76.4.7682560. [DOI] [PubMed] [Google Scholar]
- 121.Ho PJ, Baxter RC. Insulin-like growth factor-binding protein-2 in patients with prostate carcinoma and benign prostatic hyperplasia. Clin Endocrinol (Oxf) 1997;46(3):333–342. [PubMed] [Google Scholar]
- 122.Shariat SF, Lamb DJ, Kattan MW, Nguyen C, Kim J, Beck J, Wheeler TM, Slawin KM. Association of preoperative plasma levels of insulin-like growth factor I and insulin-like growth factor binding proteins-2 and -3 with prostate cancer invasion, progression, and metastasis. J Clin Oncol. 2002;20(3):833–841. doi: 10.1200/JCO.2002.20.3.833. [DOI] [PubMed] [Google Scholar]
- 123.Kanety H, Madjar Y, Dagan Y, Levi J, Papa MZ, Pariente C, Goldwasser B, Karasik A. Serum insulin-like growth factor-binding protein- 2 (IGFBP-2) is increased and IGFBP-3 is decreased in patients with prostate cancer: correlation with serum prostate-specific antigen. J Clin Endocrinol Metab. 1993;77(1):229–233. doi: 10.1210/jcem.77.1.7686915. [DOI] [PubMed] [Google Scholar]
- 124.Stattin P, Bylund A, Rinaldi S, Biessy C, Dechaud H, Stenman UH, Egevad L, Riboli E, Hallmans G, Kaaks R. Plasma insulin-like growth factor-I, insulin-like growth factorbinding proteins, and prostate cancer risk: a prospective study. J Natl Cancer Inst. 2000;92(23):1910–1917. doi: 10.1093/jnci/92.23.1910. [DOI] [PubMed] [Google Scholar]
- 125.Oliver SE, Gunnell D, Donovan J, Peters TJ, Persad R, Gillatt D, Pearce A, Neal DE, Hamdy FC, Holly J. Screen-detected prostate cancer and the insulin-like growth factor axis: results of a population-based case-control study. Int J Cancer. 2004;108(6):887–892. doi: 10.1002/ijc.11631. [DOI] [PubMed] [Google Scholar]
- 126.Inman BA, Harel F, Audet JF, Meyer F, Douville P, Fradet Y, Lacombe L. Insulin-like growth factor binding protein 2: an androgendependent predictor of prostate cancer survival. Eur Urol. 2005;47(5):695–702. doi: 10.1016/j.eururo.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 127.Moore MG, Wetterau LA, Francis MJ, Peehl DM, Cohen P. Novel stimulatory role for insulin-like growth factor binding protein-2 in prostate cancer cells. Int J Cancer. 2003;105(1):14–19. doi: 10.1002/ijc.11015. [DOI] [PubMed] [Google Scholar]
- 128.Levitt RJ, Georgescu MM, Pollak M. PTENinduction in U251 glioma cells decreases the expression of insulin-like growth factor binding protein-2. Biochem Biophys Res Commun. 2005;336(4):1056–1061. doi: 10.1016/j.bbrc.2005.08.229. [DOI] [PubMed] [Google Scholar]
- 129.Mehrian-Shai R, Chen CD, Shi T, Horvath S, Nelson SF, Reichardt JK, Sawyers CL. Insulin growth factor-binding protein 2 is a candidate biomarker for PTEN status and PI3K/Akt pathway activation in glioblastoma and prostate cancer. Proc Natl Acad Sci U S A. 2007;104(13):5563–5568. doi: 10.1073/pnas.0609139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wright J, Stanford J. Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer Causes and Control. 2009;20(9):1617–1622. doi: 10.1007/s10552-009-9407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sandoval DA, Davis SN. Leptmetabolic control and regulation. J Diabetes Complications. 2003;17(2):108–113. doi: 10.1016/s1056-8727(02)00167-8. [DOI] [PubMed] [Google Scholar]
- 132.Fradet V, Cheng I, Casey G, Witte JS. Dietary omega-3 fatty acids, cyclooxygenase-2 genetic variation, and aggressive prostate cancer risk. Clin Cancer Res. 2009;15(7):2559–2566. doi: 10.1158/1078-0432.CCR-08-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 Suppl):1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 134.Migita T, Ruiz S, Fornari A, Fiorentino M, Priolo C, Zadra G, Inazuka F, Grisanzio C, Palescandolo E, Shin E, Fiore C, Xie W, Kung AL, Febbo PG, Subramanian A, Mucci L, Ma J, Signoretti S, Stampfer M, Hahn WC, Finn S, Loda M. Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer. J Natl Cancer Inst. 2009;101(7):519–532. doi: 10.1093/jnci/djp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Locke JA, Guns ES, Lehman ML, Ettinger S, Zoubeidi A, Lubik A, Margiotti K, Fazli L, Adomat H, Wasan KM, Gleave ME, Nelson CC. Arachidonic acid activation of intratumoral steroid synthesis during prostate cancer progression to castration resistance. Prostate. 2009;70(3):239–251. doi: 10.1002/pros.21057. [DOI] [PubMed] [Google Scholar]
- 136.Tennant DA, Duran RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer. 2010;10(4):267–277. doi: 10.1038/nrc2817. [DOI] [PubMed] [Google Scholar]
- 137.Browning DR, Martin RM. Statins and risk of cancer: a systematic review and metaanalysis. Int J Cancer. 2007;120(4):833–843. doi: 10.1002/ijc.22366. [DOI] [PubMed] [Google Scholar]
- 138.Kuoppala J, Lamminpaa A, Pukkala E. Statins and cancer: A systematic review and meta-analysis. Eur J Cancer. 2008;44(15):2122–2132. doi: 10.1016/j.ejca.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 139.Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, Giovannucci E. Statin Drugs and Risk of Advanced Prostate Cancer. J Natl Cancer Inst. 2006;98(24):1819–1825. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 140.Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457(7231):910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 141.Huang YC, Lee CM, Chen M, Chung MY, Chang YH, Huang WJ, Ho DM, Pan CC, Wu TT, Yang S, Lin MW, Hsieh JT, Chen YM. Haplotypes, loss of heterozygosity, and expression levels of glycine N-methyltransferase in prostate cancer. Clin Cancer Res. 2007;13(5):1412–1420. doi: 10.1158/1078-0432.CCR-06-1551. [DOI] [PubMed] [Google Scholar]
- 142.Abate-Shen C, Shen MM. Diagnostics: The prostate-cancer metabolome. Nature. 2009;457(7231):799–800. doi: 10.1038/457799a. [DOI] [PubMed] [Google Scholar]
- 143.Nelson WG, De Marzo AM, Yegnasubramanian S. Epigenetic alterations in human prostate cancers. Endocrinology. 2009;150(9):3991–4002. doi: 10.1210/en.2009-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kang GH, Lee S, Lee HJ, Hwang KS. Aberrant CpG island hypermethylation of multiple genes in prostate cancer and prostatic intraepithelial neoplasia. J Pathol. 2004;202(2):233–240. doi: 10.1002/path.1503. [DOI] [PubMed] [Google Scholar]
- 145.Phe V, Cussenot O, Roupret M. Methylated genes as potential biomarkers in prostate cancer. BJU Int. 2010 doi: 10.1111/j.1464-410X.2009.09167.x. [DOI] [PubMed] [Google Scholar]
- 146.Li LC, Carroll PR, Dahiya R. Epigenetic changes in prostate cancer: implication for diagnosis and treatment. J Natl Cancer Inst. 2005;97(2):103–115. doi: 10.1093/jnci/dji010. [DOI] [PubMed] [Google Scholar]
- 147.Shi XB, Tepper CG, White RW. MicroRNAs and prostate cancer. J Cell Mol Med. 2008;12(5A):1456–1465. doi: 10.1111/j.1582-4934.2008.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saini S, Majid S, Dahiya R. Diet, microRNAs and prostate cancer. Pharm Res. 2010;27(6):1014–1026. doi: 10.1007/s11095-010-0086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dennis LK, Lynch CF, Torner JC. Epidemiologic association between prostatitis and prostate cancer. Urology. 2002;60(1):78–83. doi: 10.1016/s0090-4295(02)01637-0. [DOI] [PubMed] [Google Scholar]
- 150.Daniels NA, Ewing SK, Zmuda JM, Wilt TJ, Bauer DC. Correlates and prevalence of prostatitis in a large community-based cohort of older men. Urology. 2005;66(5):964–970. doi: 10.1016/j.urology.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 151.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349(4):366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 152.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431(7007):461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 153.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15(2):425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 154.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7(4):256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nelson WG, DeWeese TL, DeMarzo AM. The diet, prostate inflammation, and the development of prostate cancer. Cancer Metastasis Rev. 2002;21(1):3–16. doi: 10.1023/a:1020110718701. [DOI] [PubMed] [Google Scholar]
- 156.De Marzo AM, DeWeese TL, Platz EA, Meeker AK, Nakayama M, Epstein JI, Isaacs WB, Nelson WG. Pathological and molecular mechanisms of prostate carcinogenesis: implications for diagnosis, detection, prevention, and treatment. J Cell Biochem. 2004;91(3):459–477. doi: 10.1002/jcb.10747. [DOI] [PubMed] [Google Scholar]
- 157.Wagenlehner FM, Elkahwaji JE, Algaba F, Bjerklund-Johansen T, Naber KG, Hartung R, Weidner W. The role of inflammation and infection in the pathogenesis of prostate carcinoma. BJU Int. 2007;100(4):733–737. doi: 10.1111/j.1464-410X.2007.07091.x. [DOI] [PubMed] [Google Scholar]
- 158.De Marzo AM, Nakai Y, Nelson WG. Inflammation, atrophy, and prostate carcinogenesis. Urol Oncol. 2007;25(5):398–400. doi: 10.1016/j.urolonc.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 159.De Marzo AM, Meeker AK, Zha S, Luo J, Nakayama M, Platz EA, Isaacs WB, Nelson WG. Human prostate cancer precursors and pathobiology. Urology. 2003;62(5 Suppl 1):55–62. doi: 10.1016/j.urology.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 160.El-Omar EM, Ng MT, Hold GL. Polymorphisms in Toll-like receptor genes and risk of cancer. Oncogene. 2008;27(2):244–252. doi: 10.1038/sj.onc.1210912. [DOI] [PubMed] [Google Scholar]
- 161.Jacobs EJ, Thun MJ, Bain EB, Rodriguez C, Henley SJ, Calle EE. A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence. J Natl Cancer Inst. 2007;99(8):608–615. doi: 10.1093/jnci/djk132. [DOI] [PubMed] [Google Scholar]
- 162.James N. Cyclooxygenase-2 inhibitors and prostate cancer. Lancet Oncol. 2007;8(10):859–860. doi: 10.1016/S1470-2045(07)70291-7. [DOI] [PubMed] [Google Scholar]
- 163.Cai Y, Lee YF, Li G, Liu S, Bao BY, Huang J, Hsu CL, Chang C. A new prostate cancer therapeutic approach: combination of androgen ablation with COX-2 inhibitor. Int J Cancer. 2008;123(1):195–201. doi: 10.1002/ijc.23481. [DOI] [PubMed] [Google Scholar]
- 164.Murad A, Down L, Davey Smith G, Donovan J, Lane A, Hamdy F, Neal D, Martin RM. Associations of asprins, non-steroidal antiinflammatory drug and paracetomol use with PSA-detected prostate cancer: findings from a large, population-based, case-control study (the ProtecT study) International Journal of Cancer. 2010 doi: 10.1002/ijc.25465. In press. [DOI] [PubMed] [Google Scholar]
- 165.Adams TD, Stroup AM, Gress RE, Adams KF, Calle EE, Smith SC, Halverson RC, Simper SC, Hopkins PN, Hunt SC. Cancer incidence and mortality after gastric bypass surgery. Obesity (Silver Spring) 2009;17(4):796–802. doi: 10.1038/oby.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: the role of the insulin-IGF axis. Trends Endocrinol Metab. 2006;17(8):328–336. doi: 10.1016/j.tem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 167.Gunnell D, Oliver SE, Donovan JL, Peters TJ, Gillatt D, Persad R, Hamdy FC, Neal DE, Holly JM. Do height-related variations in insulin-like growth factors underlie the associations of stature with adult chronic disease? J Clin Endocrinol Metab. 2004;89(1):213–218. doi: 10.1210/jc.2003-030507. [DOI] [PubMed] [Google Scholar]
- 168.Gunnell D, Miller LL, Rogers I, Holly JM. Association of insulin-like growth factor I and insulin-like growth factor-binding protein-3 with intelligence quotient among 8- to 9-yearold children in the Avon Longitudinal Study of Parents and Children. Pediatrics. 2005;116(5):e681–e686. doi: 10.1542/peds.2004-2390. [DOI] [PubMed] [Google Scholar]
- 169.Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91(2):439–446. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 170.Aronson WJ, Barnard RJ, Freedland SJ, Henning S, Elashoff D, Jardack PM, Cohen P, Heber D, Kobayashi N. Growth Inhibitory Effect of Low Fat Diet on Prostate Cancer Cells: Results of a Prospective, Randomized Dietary Intervention Trial in Men With Prostate Cancer. The Journal of Urology. 2010;183(1):345–350. doi: 10.1016/j.juro.2009.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, III, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Jr, Baker LH, Coltman CA., Jr Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Thomas DC, Conti DV. Commentary: the concept of ‘Mendelian Randomization’. Int J Epidemiol. 2004;33(1):21–25. doi: 10.1093/ije/dyh048. [DOI] [PubMed] [Google Scholar]
- 173.Smith GD, Ebrahim S. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol. 2004;33(1):30–42. doi: 10.1093/ije/dyh132. [DOI] [PubMed] [Google Scholar]
- 174.Ebrahim S, Davey SG. Mendelian randomization: can genetic epidemiology help redress the failures of observational epidemiology? Hum Genet. 2008;123(1):15–33. doi: 10.1007/s00439-007-0448-6. [DOI] [PubMed] [Google Scholar]
- 175.Hoffstedt J, Eriksson P, Mottagui-Tabar S, Arner P. A polymorphism in the leptin promoter region (-2548 G/A) influences gene expression and adipose tissue secretion of leptin. Horm Metab Res. 2002;34(7):355–359. doi: 10.1055/s-2002-33466. [DOI] [PubMed] [Google Scholar]
- 176.Ribeiro R, Vasconcelos A, Costa S, Pinto D, Morais A, Oliveira J, Lobo F, Lopes C, Medeiros R. Overexpressing leptin genetic polymorphism (-2548 G/A) is associated with susceptibility to prostate cancer and risk of advanced disease. Prostate. 2004;59(3):268–274. doi: 10.1002/pros.20004. [DOI] [PubMed] [Google Scholar]