Abstract
The survival of individual Pseudomonas syringae cells was determined on bean leaf surfaces maintained under humid conditions or periodically exposed to desiccation stress. Cells of P. syringae strain B728a harboring a GFP marker gene were visualized by epifluorescence microscopy, either directly in situ or after recovery from leaves, and dead cells were identified as those that were stained with propidium iodide in such populations. Under moist, conducive conditions on plants, the proportion of total live cells was always high, irrespective of their aggregated state. In contrast, the proportion of the total cells that remained alive on leaves that were periodically exposed to desiccation stress decreased through time and was only ≈15% after 5 days. However, the fraction of cells in large aggregates that were alive on such plants in both condition was much higher than more solitary cells. Immediately after inoculation, cells were randomly distributed over the leaf surface and no aggregates were observed. However, a very aggregated pattern of colonization was apparent within 7 days, and >90% of the living cells were located in aggregates of 100 cells or more. Our results strongly suggest that, although conducive conditions favor aggregate formation, such cells are much more capable of tolerating environmental stresses, and the preferential survival of cells in aggregates promotes a highly clustered spatial distribution of bacteria on leaf surfaces.
Recent studies have provided considerable evidence that cell aggregates are quantitatively important features of bacterial populations on leaves. Aggregates of bacterial cells have been observed on the surfaces of several plant species (1–4), and recent studies have found that the majority of the bacteria present on a leaf are in various states of aggregation (4, 5). Morris et al. (6) reported that as many as 70% of the bacteria on naturally colonized leaves are in aggregates. Direct in situ observation of epiphytic bacterial populations revealed that, when plants are maintained under high relative humidity (RH), up to 50% of the epiphytic bacterial cells are in aggregates containing 103 cells or more (5). The presence of the majority of epiphytic bacteria in large cell aggregates has many ecological implications and raises questions about the possible differential behavior of aggregated and more solitary cells on leaves.
Like microbial biofilms observed in aquatic environments, bacterial aggregates present in the phyllosphere are characterized by a localized high spatial density of cells embedded in a mucoid matrix (3). It is now well documented that the micro-environment of cells in biofilms is markedly different from planktonic conditions. The extensive exopolysaccharidic matrix associated with biofilms can trap nutrients, concentrate enzymes leading to an increased metabolic activity, and act as a physical barrier to protect bacteria from diverse chemical, biological, or environmental stresses (7, 8). Cells within biofilms are phenotypically different from that of planktonic cells of the same species (7, 9, 10). Bacteria in close spatial proximity probably also have ample opportunity to communicate with each other via small diffusible signal molecules to coordinate physiological processes or responses in a density-dependent manner. Both the biological differences of planktonic cells and cells in biofilms and the protective environment within the biofilm may enhance their survival and growth under hostile conditions.
The spatial and temporal variations in water availability on leaves are particularly important challenges to successful epiphytic colonization by bacteria. Although epiphytic bacterial aggregates share structural characteristics with aquatic biofilms, their environment is probably drastically different. The leaf surface is a nonsaturated habitat characterized by constantly changing physical conditions (11, 12). Bacteria have to cope with spatial and temporal changes in water and hence in nutrient availability, temperature, RH, and irradiation levels during the course of the day and throughout leaf development (12). Yet, despite this, a large diversity of bacterial species are apparently well adapted to their habitat and are able to grow and to maintain large population sizes on leaves. Although the traits that influence the ability of bacteria to survive and grow in this dynamic ecosystem remain obscure, coping with limited water availability seems to be an important attribute of epiphytic bacteria. Several studies have reported that rains or high RH trigger the rapid multiplication of epiphytic bacteria (12), and that exposure of plants to low RH caused a significant decrease in epiphytic population sizes (12–15). However, even when water is unavailable, at least a part of the epiphytic bacterial population is able to survive.
Several strategies have been proposed to explain how epiphytic bacteria could avoid or tolerate stresses on leaves (for review, see refs. 11 and 16). Our recent findings that epiphytic bacterial cells are predominantly in aggregates with cluster sizes ranging from 1 to >104 cells (5) suggests that aggregated cells could have a different stress tolerance than more solitary cells. Bacterial aggregates are preferentially formed at the base of glandular trichomes and in the crevices between cells along the veins (5), sites that apparently provide adequate nutrients for microbial growth but also may retain water on the plant surface (17). The formation of large aggregates surrounded by an exopolymeric matrix may provide a protected microenvironment for bacteria on leaf surfaces (3). However, bacterial aggregates on leaves exhibit a strong right-hand-skewed frequency distribution of sizes with a large number of small aggregates and a few very large clusters (5). Because the viability of the more solitary cells was not assessed, it cannot be assumed that they are alive and may in fact represent unsuccessful colonists after stressful conditions are imposed.
The objective of this study was to evaluate the differential survival of epiphytic bacteria in various states of aggregation when periodically exposed to dry conditions on plants. To ensure that we examined all, but only, cells of the common epiphyte Pseudomonas syringae, our experiments were conducted with a strain harboring a gfp marker gene conferring constitutive green fluorescence. Both the aggregation state of cells and their viability were determined directly on leaves by using epifluorescence microscopy. We show that cells in large aggregates and more solitary cells differ greatly in survival on leaves and that periodic changes in water availability on leaves may drive the establishment of the highly aggregated epiphytic bacterial populations.
Experimental Procedures
Bacterial Strains and Culture Media. P. syringae pv. syringae B728a is a spontaneous rifampicin-resistant derivative of a strain isolated from a bean leaflet. Its characteristics have been reported (12, 18). Cultures were stored at -80°C in 15% glycerol (vol/vol) in 10 mM potassium phosphate buffer, pH 7.0 (PB), and routinely grown on King's medium B (KB) (19). Plasmid DNA was isolated from Escherichia coli DH5 (pKT-trp) (20) by using a DNA isolation kit (Qiagen, Valencia, CA) and was transferred into P. syringae B728a by electroporation according to standard procedures (21). The plasmid pKT-trp consists of a gfp marker gene driven by the trp promoter from Salmonella typhimurium (20) that yields constitutive expression in P. syringae in the stable plasmid vector pPROBE-KT (22). Wild-type strain B728a and its derivative B728a (pKT-trp) exhibit no significant differences in their epiphytic fitness after inoculation on leaves (23).
Plant Inoculation and Growth Conditions. All experiments were conducted with 2-week-old, greenhouse-grown bean plants (Phaseolus vulgaris cv. Bush Blue Lake 274) and incubated under controlled conditions in the laboratory. The bacteria were applied by immersing the plants in a suspension of P. syringae B728a (pKT-trp) [105 colony-forming units (cfu)/ml in PB] for ≈3 s. The plants were then kept in a moist chamber maintained close to 100% RH at 22°C. After allowing the bacteria to grow for 2 days, half of the plants were repeatedly exposed to low RH. Each desiccation stress cycle consisted of maintaining the plants under high RH for 16 h and then exposing then to low RH (<50% RH on a laboratory bench) for 8 h. After 3 cycles, the plants were placed back into the moist chamber for 2 days. The remaining half of the plants were maintained under high RH conditions for the entire duration of the experiment.
Formation of Bacterial Aggregates in Vitro. Aggregates of different sizes were artificially obtained in vitro by spreading bacterial cells on microscope slides. Cells grown on KB were resuspended in 1 ml of PB, placed in Eppendorf microfuge tubes, centrifuged for 10 min at 5,000 × g, rinsed twice with filter-sterilized PB, and centrifuged again. Supernatants were discarded and different amounts of the pelleted cells were harvested with a sterile toothpick and unevenly spread on sterile microscope slides to obtain a wide range of cluster sizes. Slides were then placed inside 90-mm sterile Petri plates and exposed to low RH conditions on a laboratory bench for 8 h. The fraction of dead and living cells was determined for the suspension of cells before inoculation on slides and on slides as a function of aggregate size after exposure to low RH. The suspension used to inoculate the slides was adjusted to a concentration of 107 cfu/ml and stained with propidium iodide (10 μg/ml) for 10 min in the dark at room temperature. Five microliters of the suspension were mixed with 5 μl of Polymount (Polysciences), deposited on a slide, covered with a coverslip, and immediately observed under the microscope. At least 20 random fields of view were observed, and the total number of dead (red) and living (green) cells was counted. For each treatment, three slides each covered by three different coverslips were prepared for microscopic observation. Ten microliters of a solution of propidium iodide (10 μg/ml) in Polymount was placed on the center of each coverslip, which was then gently pressed down onto the slide. The mountings were kept in the dark at room temperature for 10 min and then observed by epifluorescence microscopy. A stratified sampling was performed where the total number of dead cells was related to the total number of cells per aggregate for 10–20 aggregates within size classes of 2–10, 10–50, 50–100, 100–200, 200–500, and 500 or more cells per aggregate. In addition, the fraction of dead and living solitary cells was also counted for 20 randomly selected fields of view on each slide observed.
Microscopy of Bacteria Inoculated onto Bean Leaves. The fraction of dead and living cells in aggregates of different sizes was determined directly in situ and after recovery of cells from leaves. In situ measurements were obtained from the upper leaf surfaces of plants maintained under high RH and after exposure of plants to low RH, at different times after inoculation with P. syringae. Segments of ≈1 × 1 cm were randomly cut from leaves and placed on top of 100 μl of melted water agar (1%) on a microscope slide to stabilize leaf segments and ensure a flat surface for microscopic observations. After solidification of the agar (in ≈20 s), 10 μl of a solution of propidium iodide (10 μg/ml) in Polymount was placed on the center of a coverslip, which was then gently pressed down onto the leaf segment. Propidium iodide allowed a rapid in situ visualization of nonviable cells directly on leaf surfaces (23). The mountings were kept in the dark at room temperature for 10 min and then observed by epifluorescence microscopy. A total of five segments randomly cut from each of three leaves were observed at each sampling time. For each segment, the size of each bacterial aggregate and the number of dead (red) and living (green) cells in each aggregate present in 15 randomly selected fields of view were determined. All of the solitary and aggregated cells present on each field of view were accounted for.
Bacterial cells were also recovered from bean leaves maintained under high RH for 7 days after inoculation with P. syringae, using a method adapted from Morris et al. (4). Individual bean leaves were gently washed in 100 ml of PB for 10 min by placing the flasks on a shaker at 120 rpm. Three aliquots of 10, 20, and 60 ml were separately filtered through TMTP Isopore Polycarbonate filters (5-μm-diameter pore size; Millipore). Solitary cells in the filtrates were recovered by refiltration through black polycarbonate filters (0.2-μm-diameter pore size; Millipore). All filters were then placed on Whatman paper to remove excess PB, transferred to microscope slides placed inside Petri plates, and kept under low RH conditions for 8 h. Ten microliters of a solution of propidium iodide (10 μg/ml) in Polymount was placed on the center of each coverslip, which was then gently pressed down onto the filters. Both the total number of dead cells and the total number of cells per aggregate were noted for 20 aggregates of each size class as noted above.
Epifluorescence Microscopy. Samples were observed by epifluorescence microscopy using an Axiophot microscope equipped with a ×100, 1.30-numerical-aperture Plan-Neofluar oil immersion objective (Zeiss). A filter set for fluorescein [excitation filter, 450–490 nm; dichroic mirror, 510 nm; emission filter, long pass (LP) 520 nm] was used to visualize green and red fluorescent cells in the same field of view. In some cases, when dead and living cells were not clearly distinguishable, the same field of view was observed by using separate filter sets for GFP (excitation filter, 450–490 nm; dichroic mirror, LP 495 nm; emission filter, 500–550 nm) and rhodamine (excitation filter, 526–566 nm; dichroic mirror, 580 nm; emission filter, LP 590 nm) to count the total number of GFP-marked P. syringae cells and the number of dead cells, respectively. In addition, for direct observation of bacterial cells on the leaf surface, a 650-nm short pass filter (Melles Griot, Irvine, CA) was used to improve the quality of images by partially blocking the strong red autofluorescence of the leaf. Some images were captured with an Optronics DEI-750 video camera (Optronics International, Chelmsford, MA), transferred to a PC platform, and processed with photopaint software (Corel, Ottawa). Processed images were obtained by combining three images of the same field of view captured by using the filter sets for GFP and rhodamine and a bright-field image of the leaf surface converted to gray scale.
Statistics. Data management and computation were performed by using Microsoft EXCEL. Descriptive statistics, analysis of variance of the arcsine square-root-transformed measurements of the proportion of living cells, and regression analysis were all performed with STATISTICA (StatSoft, Tulsa, OK).
Results
Effect of RH on Survival of Epiphytic Bacteria and Aggregate Formation. Although a high fraction of epiphytic cells were alive on moist plants, irrespective of how long they had colonized a leaf, a majority of the cells died rapidly on imposition of desiccation stress on leaves. The proportion of viable cells of P. syringae (pKT-trp) that had colonized bean leaves during a 2-day moist period rapidly decreased through time when leaves were subsequently exposed to low RH conditions periodically over the next several days. Immediately after inoculation of P. syringae onto bean leaves, 98.5 ± 0.0% (mean ± SE) of the total number of P. syringae cells observed were alive. The percentage of these cells recovered from leaves maintained under high RH that remained alive did not decrease significantly with time (85.7 ± 0.1%, 85.4 ± 0.6%, 88.0 ± 0.9%, and 86.8 ± 0.1%, 3, 4, 5, and 7 days after inoculation, respectively). In contrast, the percentage of cells recovered from leaves exposed to low RH that were alive decreased quickly after leaves were dried (42.8 ± 1.4%, 28.1 ± 0.2%, and 15.3 ± 1.3%, 3, 4, and 5 days after inoculation, respectively). After three exposures to low RH (at which point only a small fraction of cells were still alive) plants were maintained under high RH for an additional 2 days; the percentage of live cells subsequent to transfer to humid conditions did not differ significantly (17.0 ± 4.5%) from those on leaves before transfer (15.3 ± 1.3%).
Direct in situ observation of epiphytic bacteria revealed that RH affected aggregate formation. Although the average size of bacterial aggregates formed under both humid or stressful dry conditions increased through time, aggregate sizes were consistently larger at a given incubation time on plants maintained under high RH. The largest aggregates on plants kept under high RH conditions for 5 days contained ≈5,000 cells but aggregates contained only ≈600 cells on plants periodically exposed to low RH. Cells of P. syringae were not randomly distributed on the leaf surface and occurred in a wide range of cluster sizes (data not shown). However, by 5 days after inoculation, the large majority of aggregates consisted of only a few cells; 99.0% and 99.7% of aggregates observed on plants maintained under conducive conditions or periodically exposed to low RH, respectively, consisted of 100 cells or less.
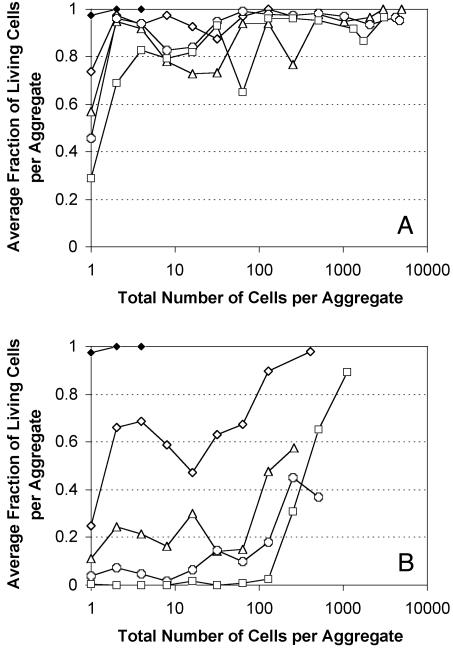
Differential Survival of Solitary and Aggregated Cells on Leaf Surfaces. The fraction of live and dead cells was determined in cell aggregates of different sizes on plants exposed to conducive and stressful conditions for different periods of time. Propidium iodide allowed us to distinguish membrane-compromised cells, stained in red, from viable cells, which retained only green fluorescence (Fig. 1). The proportion of dead cells did not differ among aggregates of different sizes when plants were maintained under high RH. However, the fraction of live solitary cells decreased with time and was significantly smaller than the fraction of live cells in aggregates of any size (ranging from 2 to >5,000 cells per aggregate) at the same sampling time (Fig. 2A). Cells in aggregates of less than ≈32 cells appeared to have a different survival than did those in larger aggregates so we arbitrarily defined “small” aggregates (2–32 cells) and “large” aggregates (>33 cells) by using this threshold. Regression analysis between the fraction of living cells per aggregate and aggregate size for small aggregates at a given sampling time resulted in positive slope values, which increased with time after inoculation when plants were maintained under high RH. For larger aggregates, this relationship was not significantly different from 0 at any sampling time (Table 1). In contrast, when plants were periodically exposed to low RH, the average fraction of live cells in an aggregate of a given size decreased through time (Fig. 2B). However, the proportion of live cells was usually higher in larger aggregates and increased with increasing aggregate size (Fig. 2B). Regression analysis revealed a positive correlation between the fraction of live cells and aggregate size for both small and large aggregates (Table 1). At each sampling time, the proportion of live cells in aggregates containing 100 cells or more was significantly greater than that in smaller aggregates or of solitary cells.
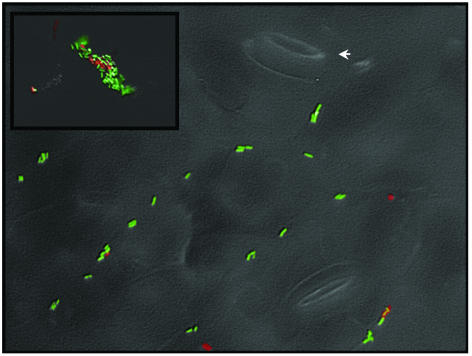
Fig. 1.
Visualization of viable and nonviable cells of P. syringae strain B728a directly on bean leaf surfaces. P. syringae cells harbored a gfp marker gene and propidium iodide was used as a cell viability indicator. P. syringae cells appear green, and nonviable cells appear red. A bright-field image of the leaf surface provides spatial context for cell location. The arrow points at one of the two stomates present in the field of view. (Inset) Aggregated cells (detail of a larger field of view). (Magnification, ×500.)
Fig. 2.
Average fraction of living cells in relation to the total number of cells per aggregate on plants maintained under humid conditions (A) or periodically exposed to low RH (B), 0 (black diamonds), 3 (open diamonds), 4 (triangles), 5 (circles), and 7 (squares) days after inoculation. The standard errors of the average fractions of living cells per aggregate were removed for clarity but averaged ≈0.06.
Table 1. Regression analysis of the fraction of living cells as a function of aggregate size.
| Day after inoculation (slope values†)
|
|||||
|---|---|---|---|---|---|
| Treatment | Aggregate size | 3 | 4 | 5 | 7 |
| High RH | Small‡ | 0.298** | 0.196** | 0.596** | 0.669** |
| Large§ | 0.071 | 0.061 | –0.062 | –0.030 | |
| Low RH | Small | 0.202** | 0.155* | 0.106* | 0.049* |
| Large | 0.534 | 0.840** | 0.445* | 0.762** | |
, 0.001 < P < 0.05
, P ≤ 0.001
Values reported represent the slope and the corresponding level of significance when compared to the null hypothesis of independence of aggregate size and fraction of living cells (corresponding to a slope value of 0)
Cell aggregates of 32 cells or less
Cells aggregates of 33 cells or more
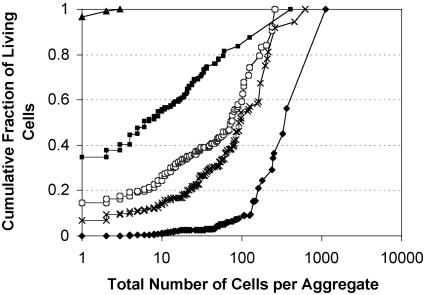
Distribution of Live Cells After Repeated Exposure to Stressful Conditions. Exposure of epiphytic bacteria to low RH on plants greatly affected the size distribution of aggregates in which living cells could be found. After three cycles of periodic low RH stress, the large majority of live bacteria were found only in aggregates. Immediately after inoculation and before imposition of low RH stress, solitary cells constituted >96% of the total population of living cells and no aggregates of more than three cells were found. However, as a result of growth and differential survival of bacteria as a function of aggregate size, solitary cells constituted only 35% of the total living cells observed after one cycle of exposure to low RH and <7% after 5 days and 3 cycles of low RH (Fig. 3). After 7 days of incubation on leaf surfaces, solitary cells constituted only 0.18% of the total live cells observed. The cumulative fraction of live cells found in larger aggregates increased with time after repeated exposure to low RH; 0.0%, 16.6%, 54.1%, 63.1%, and 93.9% of live cells were located in aggregates containing 64 cells or more, 0, 3, 4, 5, and 7 days after inoculation, respectively.
Fig. 3.
Cumulative fraction of living P. syringae cells as a function of aggregate size. Leaves of plants periodically exposed to desiccation stress were visualized at 0 (triangles), 3 (squares), 4 (circles), 5 (crosses), and 7 (diamonds) days after inoculation for plants periodically exposed to stress. Plants were exposed three times to low RH for 8 h prior sampling at 3, 4, and 5 days and otherwise maintained under humid conditions.
Differential Survival of Solitary and Aggregated Cells on Slides. To determine whether aggregation per se and not leaf location was responsible for higher rates of cell survival in larger aggregates, we deposited mechanically generated cell aggregates of different sizes from cultured cells on slides and determine their survival as a function of aggregate size when slides were exposed to low RH. Over 98% of the cells inoculated onto the slides were initially alive. After exposure of the slides to low RH for 8 h, only 47.4% of the cells observed remained alive. In contrast to what was observed on leaves, no correlation between the fraction of live cells and size of aggregates in which they were found was observed (Fig. 4). The slope value obtained by regression analysis was 0.040 and was not significantly different from 0 (P < 0.469). Similar results were obtained when slides were exposed to low RH for different periods of time (data not shown).
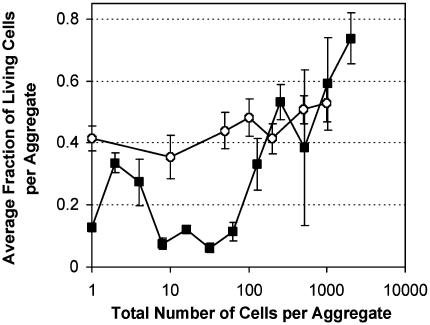
Fig. 4.
Fraction of living cells in relation to the total number of cells in the aggregate in which they occurred for cell aggregates artificially generated from cultured cells on slides (circles) or for aggregates recovered from plants and deposited onto membranes filters (squares). Slides and filters were exposed to low RH for 8 h after cell deposition. Vertical bars represent the standard error of the mean fraction of living cells in aggregates of different sizes.
Differential Survival of Solitary and Aggregated Cells After Recovery of Bacteria from Leaves. To determine whether aggregated cells survived better on leaves as a consequence of their localization at sites conducive for aggregate formation, we recovered solitary cells and cell aggregates from leaves in a nondestructive way and exposed them to low RH in a uniform environment on membrane filters. Over 98% of the cells initially inoculated onto leaves were alive. After incubation of leaves for 7 days under humid conditions, 92.1 ± 1.1% of the cells recovered from such leaves and deposited onto membrane filters were still alive. However, after exposure of recovered bacteria on membranes to low RH for 8 h, only 37.7 ± 0.7% of the cells remained alive. Similar to the results obtained for bacteria observed directly on leaf surfaces, the proportion of cells in large aggregates that survived low RH stress was much higher than that of more solitary cells. The fraction of cells that survived low RH treatment increased with an aggregate size greater than ≈32 cells (Fig. 4). Regression analysis revealed a positive correlation between the fraction of live cells and aggregate size for clusters containing >32 cells, with a slope of 0.801, which was significantly different from 0 (P < 0.001).
Discussion
Survival on dry leaf surfaces is a challenge for epiphytic bacteria. Enumeration of bacteria on leaf surfaces by culturable cell counts revealed that after exposure to low RH, surface populations often decrease, whereas internal populations remain constant (15, 16, 24). It has been hypothesized that bacterial populations residing in internal microsites, such as substomatal chambers or intercellular spaces, may provide a physical protection from environmental stresses (16). However, our results bring insight and demonstrate the significance of aggregates in the survival of epiphytic populations on the surface of leaves. This study provides a description of the survival of epiphytic bacteria at the single-cell level, directly on leaf surfaces, and demonstrates a role for aggregates in the survival of epiphytic populations periodically exposed to desiccation stress.
Although aggregates contribute to the survival of epiphytic bacteria, our study also reveals the importance of RH in aggregate formation. Aggregates of P. syringae cells formed on leaves of plants maintained under high RH were more numerous and larger than aggregates formed on plants periodically exposed to low RH. As reported (5), cells occurred in a wide range of cluster sizes, apparently reflecting the localized spatial heterogeneity of nutrients on leaf surfaces (25).
Although no difference was observed in the proportion of nonviable cells as a function of aggregate size when plants were maintained under humid, conducive conditions, such a relation was seen clearly when plants were periodically exposed to low RH (Fig. 2). Because cell viability increased with an increasing number of cells constituting an aggregate, they were either more tolerant of the stress because of physiological differences from cells in smaller aggregates or experienced less stress because of their physical location in the aggregate. Dead cells were sometimes located on the surface of aggregates, but no recurrent pattern in the spatial distribution of dead cells within an aggregate could be identified. In some cases, dead cells formed microcolony-like structures within an aggregate and were localized either on the edges or in the center of an aggregate. We hypothesize that these different patterns resulted from the successive alternation of conducive conditions and stressful conditions. Although it is probable that cells located on the surface of an aggregate are more exposed and therefore more sensitive to stress than cells in the center of an aggregate, the growth of remaining viable cells between exposures to desiccation stress might conceal this pattern. We expect that successive episodes of stress on a leaf would cause a preferential loss of the most solitary cells and an increasingly aggregated pattern of occurrence of viable cells on a leaf. As a consequence of cell growth and differential survival of solitary and aggregated cells, the spatial distribution of epiphytic bacteria would be expected to shift from solitary cells randomly distributed on the leaf surface to a highly clustered distribution were the vast majority of live cells were found in a few large aggregates. After 7 days of incubation and three successive exposures to low RH, >90% of the viable cells were located in aggregates constituted of 100 cells or more (Fig. 2).
The combined effects of several factors might explain the differential survival of solitary and aggregated cells on leaf surfaces. We discount the idea that aggregated cells might survive better merely because aggregates are preferentially formed in sites on the leaf surface that provide protection from a harsh external environment. When we exposed aggregates originally formed on leaves to desiccation stress in a uniform environment on membranes we still observed a much higher proportion of live cells in large aggregates compared with more solitary cells (Fig. 4). In fact, the relationship between cell viability and aggregate size observed in situ and on membranes was strikingly similar (Figs. 2 and 4). Thus, the localization of aggregates on the leaf surface could not explain the higher fraction of live cells observed in larger aggregates. We did note that the fraction of live cells in aggregates desiccated on membranes was about half that of cells in aggregates observed in situ. Perhaps this difference reflects the higher humidity that is thought to exist in the laminar layer on leaves compared with that of the bulk air on a membrane surface. Alternatively, by detaching aggregates from the leaf, we may have exposed those cells that were at the base of the aggregate (in contact with the leaf surface), and that may have been protected from the external environment, to desiccation stress. It is important to note that the method used to recover aggregates from leaves did not appear to disrupt large aggregates and that the frequency distribution of aggregate sizes determined in situ and after recovery of cells were very similar (data not shown). However, we found a higher proportion of solitary cells after washing from the leaf, probably resulting from the disruption of aggregates in the recovery process. The relatively higher fraction of live cells observed in aggregates of two or four cells (Fig. 4) recovered from leaves may indicate that those cells derive from larger aggregates whose component cells were more tolerant of stress.
A second factor that could explain the higher fraction of live cells in larger aggregates is aggregation per se. Aggregates observed on leaves are large clusters of densely organized cells. One might think that bacteria located on the surface could act as a physical barrier against environmental stresses and protect bacteria located underneath. However, when aggregates of different sizes artificially obtained by spreading washed cells on slides were exposed to desiccation stress we saw no relation between the survival of cells and the number of cells per aggregate (Fig. 4). The fraction of dead cells was independent of aggregate size and no pattern in the spatial distribution of nonviable cells could be determined. Such artificially generated bacterial aggregates, although presumably composed of cells of a uniform physiological state because they were harvested from culture plates of a uniform chemical and physical environment, probably differ from cell aggregates found on leaves in several ways. To generate the artificial aggregates, cells were washed extensively after harvest and cell pastes were spread onto slides to yield spatially aggregated cell masses. Such aggregates would not likely retain significant amounts of exopolysaccharides (EPS) that probably characterize the original cultured cells or cell aggregates on leaves (3). EPS has been suggested as an important protectant of cells against desiccation stress (26, 27). Local EPS abundance would be expected to accumulate with increasing aggregate size in situ. Thus, the “naked” cells of uniform physiological state may not have retained sufficient EPS to confer protection against low RH. Alternatively, the increased tolerance of low RH by cell aggregates in situ may be explained by a difference in metabolic status among the cells on leaves. Cells at localized sites of sugar abundance on leaves, probably where the largest cell aggregates are formed, have a higher ribosomal RNA content than those cells at sites lacking abundant sugar (25). Perhaps such cells are capable of more effective expression of phenotypes that are protective of desiccation tolerance than are cells without such nutrient resources. Thus, because aggregate size on leaves is probably directly related to metabolic rate of epiphytes, those most active cells might also be most tolerant of environmental stresses.
The physiology of the aggregated cell population on leaves might be influenced by the localized high cell density. Cells within biofilms in aquatic environment are phenotypically different from planktonic cells of the same species, and some traits including EPS biosynthesis are expressed selectively within biofilms (7, 9, 10). Although the methods used in this study did not allow us to visualize a matrix embedding the cells, there is evidence that bacterial aggregates on leaves are surrounded by a copious exopolymeric matrix (2, 3). We hypothesize that the presence of an exopolymeric matrix could provide a microenvironment that protects aggregated cells against desiccation, by fostering water retention. In addition to the high cell density, the physiology of the aggregated population might also be influenced by their alleged copiotrophic environment. We also found that aggregated cells of P. syringae were significantly larger than solitary cells (23), consistent with the hypothesis that such cells experience more nutrient resources than more solitary cells. This is further evidence that the physiological state of the bacteria on a leaf may vary with their location on a leaf. We had hypothesized that although epiphytic bacteria that occur in large aggregates may escape stress, other more exposed cells may adapt to the harsh conditions of epiphytic life by modulating their size and concomitantly express traits that allow them to be more resistant to stress. This study, however, seems to suggest that solitary cells of P. syringae are not able to cope with desiccation stress and consequently that the fate of immigrant cells may depend largely on the nature of their landing site on plants.
The development of microscopy techniques adapted to the observation of epiphytic bacteria in their natural environment has proven to be an extremely valuable tool that has brought new insight to phyllosphere microbiology. Although this approach has shown that bacterial aggregates are apparently a ubiquitous feature on leaf surfaces, this study suggests that the solitary cells seen on leaves usually represent unsuccessful colonization events that ended in their death under stressful conditions. The fate of solitary and aggregated cells on leaves is clearly different. This study strongly suggests that large aggregates constitute a distinct ecological niche for epiphytic bacteria, allowing them to escape harsh environmental conditions encountered on the leaf surface. Such a niche is probably, at least partially, a result of the modification of the leaf surface habitat by the epiphytic colonists. Whereas this study revealed aggregate-mediated differences in desiccation stress, it should be fruitful to determine whether aggregates also confer protection from other stresses, such as bactericidal treatments. In addition, the highly clustered spatial distribution of viable bacteria on leaves resulting from periodic exposure to environmental stress may limit microbial interactions to a few sites and could explain in part the incomplete efficiency of biological control of disease by antagonists applied to plants (28).
Acknowledgments
We thank Drs. Steve Ruzin and Denise Schichnes of the Biological Imaging Facility at the University of California, Berkeley, for their assistance with microscopy. This study was supported by U.S. Department of Agriculture National Research Initiative Grant 99-35303-8633, Department of Energy Grant DR-F603-86ER13518, and Torrey Mesa Research Institute, Syngenta Research and Technology, San Diego.
Abbreviations: PB, 10 mM potassium phosphate buffer, pH 7.0; RH, relative humidity.
References
- 1.Fett, W. F. (2000) J. Food Protect. 63, 625-632. [DOI] [PubMed] [Google Scholar]
- 2.Gras, M. H., Druet-Michaud, C. & Cerf, O. (1994) Sci. Aliments 14, 173-188. [Google Scholar]
- 3.Morris, C. E., Monier, J.-M. & Jacques, M.-A. (1997) Appl. Environ. Microbiol. 63, 1570-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris, C. E., Monier, J.-M. & Jacques, M.-A. (1998) Appl. Environ. Microbiol. 64, 4789-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monier, J.-M. & Lindow, S. E. (2003) Appl. Environ. Microbiol., in press.
- 6.Morris, C. E., Barnes, M. B. & McLean, R. J. C. (2001) in Phyllosphere Microbiology, eds. Lindow, S. E., Hecht-Poinar, E. I. & Elliot, V. J. (Am. Phytopathol. Soc., St. Paul), pp. 138-154.
- 7.Costerton, J. W., Lewandowski, Z., Caldwell, D. E., Korber, R. & Lappinscott, H. M. (1995) Annu. Rev. Microbiol. 49, 711-745. [DOI] [PubMed] [Google Scholar]
- 8.Costerton, J. W., Stewart, P. S. & Greenberg, E. P. (1999) Science 284, 1318-1322. [DOI] [PubMed] [Google Scholar]
- 9.De Kievit, T. R., Gillis, R., Marx, S., Brown, C. & Iglewski, B. H. (2001) Appl. Environ. Microbiol. 67, 1865-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potera, C. (1999) Science 283, 1838-1839. [DOI] [PubMed] [Google Scholar]
- 11.Beattie, G. A. & Lindow, S. E. (1995) Annu. Rev. Phytopathol. 33, 145-172. [DOI] [PubMed] [Google Scholar]
- 12.Hirano, S. S. & Upper, C. D. (2000) Microbiol. Mol. Biol. Rev. 64, 624-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirano, S. S. & Upper, C. D. (1983) Annu. Rev. Phytopathol. 21, 243-269. [Google Scholar]
- 14.Hirano, S. S. & Upper, C. D. (1990) Annu. Rev. Phytopathol. 28, 155-177. [Google Scholar]
- 15.Timmer, L. W., Marois, J. J. & Achor, D. (1987) Phytopathology 77, 1341-1345. [Google Scholar]
- 16.Beattie, G. A. & Lindow, S. E. (1999) Phytopathology 89, 353-359. [DOI] [PubMed] [Google Scholar]
- 17.Brewer, C. A., Smith, W. K. & Vogelmann, T. C. (1991) Plant Cell Environ. 14, 955-962. [Google Scholar]
- 18.Loper, J. E. & Lindow, S. E. (1987) Phytopathology 77, 1449-1454. [Google Scholar]
- 19.King, E. O., Ward, M. K. & Rainey, D. E. (1954) J. Lab. Clin. Med. 44, 301-307. [PubMed] [Google Scholar]
- 20.Hallman, J., Quadt-Hallmann, A., Miller, W. G., Sikora, R. A. & Lindow, S. E. (2001) Phytopathology 91, 415-422. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook J., Fritsch, E. F. & Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 22.Miller, W. G., Leveau, J. H. J. & Lindow, S. E. (2000) Mol. Plant–Microbe Interact. 13, 1243-1250. [DOI] [PubMed] [Google Scholar]
- 23.Monier, J.-M. & Lindow, S. E. (2003) Phytopathology 93, 1209-1216. [DOI] [PubMed] [Google Scholar]
- 24.Beattie, G. A. & Lindow, S. E. (1994) Appl. Environ. Microbiol. 60, 3790-3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leveau, J. H. J. & Lindow, S. E. (2001) Proc. Natl. Acad. Sci. USA 98, 3446-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ophir, T. & Gutnick, D. L. (1994) Appl. Environ. Microbiol. 60, 740-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnider-Keel, U., Lejbolle, K. B., Baehler, E., Haas, D. & Keel, C. (2001) Appl. Environ. Microbiol. 67, 5683-5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson, K. B. (1994) Phytopathology 84, 780-784. [Google Scholar]