Abstract
Background
Many breast cancer survivors experience long term sequelae, including fatigue, decreased physical functioning, pain, and psychological distress. Physical activity can ameliorate these problems, but there is little research on how activity should be performed to be most beneficial. This study explores how dimensions of physical activity (total energy expenditure, frequency, and duration) are associated with symptoms among breast cancer survivors.
Methods
We conducted secondary analysis of data on physical activity behavior and symptoms in a cross-sectional study (n = 148) of breast cancer survivors who were off treatment and had been diagnosed within the past 5 years.
Results
Multivariate analyses showed that total energy expenditure was associated with better general health (p=0.006) and fewer depressive symptoms (p=0.014), while frequency of activity was linearly related to physical functioning (p=0.047), pain (0.057), general health (p<0.001), and depressive symptoms (p<0.001). Duration was related to physical functioning, pain, and general health, but the worst outcomes were reported by the participants with the shortest and longest duration of activity (quadratic trend p values = 0.002, 0.003, 0.008, respectively).
Discussion/Conclusions
Greater total energy expenditure, higher physical activity frequency, and moderate duration were associated with better outcomes for most variables, although there was no relationship between any of the dimensions of physical activity and fatigue.
Implications for Cancer Survivors
The association of better outcomes with higher energy expenditure, higher frequency of activity, and moderate duration indicates that increasing activity through multiple short bouts may be the most beneficial for breast cancer survivors. However, randomized studies are needed to confirm this finding.
Keywords: symptoms, depression, physical activity, breast cancer survivors
Introduction
Breast cancer survivors may experience a number of long-term difficulties that can be attributed to the disease or its treatment. For example, many survivors have decreased physical functioning (1-5) with problems being more pronounced in those who received systemic adjuvant therapy (6). Breast cancer survivors have poorer physical health and slightly more functional problems than similarly aged women without cancer (7). They also are more likely to have a medical condition that limits their activities (8), and to report higher rates of pain and physical symptoms than healthy women (5). Physical functioning problems can persist and worsen (2), and may endure as late as 8 years post-diagnosis (4). Cancer-related fatigue, a common problem, causes distress, impairs quality of life, and delays return to regular functioning (9-11)(12). It can remain a serious problem several years post-diagnosis (2, 3). For example, the Healthy Eating, Activity, and Lifestyle (HEAL) study, a cohort study of breast cancer survivors showed that 41% of the sample experienced significant levels of fatigue 2-5 years after diagnosis. The fatigued survivors had poorer quality of life than both non-fatigued survivors and normative samples (12).
Survivors may have ongoing emotional problems due to fears of recurrence or death and changes in social relationships (13). While some studies show that breast cancer survivors score within normal ranges on measures of emotional well-being (2, 5, 8, 14), others indicate that mood disturbances persist even after most physical problems have resolved (3, 15, 16). Though as a whole, breast cancer survivors have good emotional well-being, as many as 25% may experience ongoing distress (5, 6); percentages may be higher in those who have had both mastectomy and chemotherapy (1).
The results of a growing number of studies (17-21) demonstrate that exercise and physical activity can improve many of the symptoms and health problems experienced by breast cancer survivors. Exercise interventions for survivors of breast cancer have been evaluated in randomized trials and have been found to have significant positive effects on functional capacity and physical functioning (17-20, 22, 23), fatigue (19, 24-27), pain (20), body image (28), psychologic adjustment (19, 21, 29, 30), sleep problems (19), and overall health and quality of life (17, 20, 21).
While considerable progress has been made in investigating the benefits of physical activity for cancer survivors, gaps in our knowledge remain. Most existing studies evaluated aerobic exercise of consistent intensity, frequency, and duration, supervised by research staff. The studies were not designed to determine how variations in amount, frequency, and duration affect breast cancer survivors' symptoms and treatment sequelae. For example, we have no answers to questions such as how much exercise is needed to improve psychological distress, or whether greater improvements in fatigue would be attained with a few long walks or several short walks each week Studies of how the frequency, intensity, and duration of physical activity affect cardiovascular disease and mortality risk in healthy populations (31, 32) have led to changes in the physical activity recommendations for the general public. Early recommendations emphasized vigorous exercise for at least 20 minutes, but current recommendations also endorse moderate activity done for at least 30 minutes on most days of the week, allowing people to accumulate this activity in short bouts throughout the day. The latter regimen decreases the risk of cardiovascular disease and mortality (33) but what has not been studied is how altering dimensions of activity such as frequency, duration, and total energy expenditure might affect the specific benefits of exercise for cancer survivors. For certain outcomes total energy expenditure might have a stronger relationship to outcomes and be mediated by fitness differences, but for others, especially those associated with emotional health, other factors might come into play. For example, the distraction hypothesis (34) postulates that physical activity improves emotional well-being due to the distraction it provides, rather than the physical effects of the activity itself. If distraction accounts for improvements in outcomes, it is likely that frequency of exercise will be a critical predictor since mood disruption can potentially occur daily, making frequent distraction necessary to have a lasting impact on emotional well-being.
We conducted a secondary analysis of a cross-sectional study to explore whether dose, frequency, and duration of physical activity are associated with breast cancer survivors' symptoms and health status. We hypothesized that there would be a linear relationship between the physical activity variables and symptoms and health status; that greater total energy expenditure, higher frequency of activity, and longer activity duration would be associated with better outcomes. Because research has shown that breast cancer survivors experience difficulties with fatigue, physical functioning, pain, depression, and general health, we have focused on these outcomes for this paper.
Method
Design and Participants
Participants in the cross-sectional study were 148 female breast cancer survivors who were off treatment and who had been diagnosed within 5 years of the start of the study. They also had to be aware of the date, their location and their name (which provides a general indication of cognitive function); over 18; and speak and read English. The protocol was reviewed and approved by the Institutional Review Boards at the University of Texas M. D. Anderson Cancer Center and the Harris County Hospital District. Participants were recruited from The University of Texas M. D. Anderson Cancer Center, the Houston chapter of the Sisters' Network (a support and advocacy group for African-American breast cancer survivors), The Rose (a non-profit agency that provides breast cancer screening and diagnosis and support groups to women with breast cancer), and Lyndon B. Johnson (L.B.J.) Harris County Hospital District General Hospital in Houston, Texas.
Procedures
At M. D. Anderson Cancer Center, breast cancer survivors who were scheduled for follow-up appointments between January and October, 2002 with either of 2 medical oncologists in the Nellie B. Connally Breast Center or with physicians at the Cancer Prevention Center were mailed a letter introducing the study and provided with a number to call if they were not interested in being contacted further about the study. Patients who had not declined to participate were later called by the research coordinator to schedule an interview appointment, either on the same day as the next clinic appointment or at a more convenient time.
At the interview appointment, a research coordinator met with the patient, obtained written informed consent, and administered a 7-day physical activity recall interview and a battery of self-report questionnaires assessing physical and psychological symptoms and quality of life.
Measures
Demographic and health information
Questionnaires were used to determine participants' demographic (age, ethnicity, education, and marital status) and health information (non-cancer medical history, cancer disease stage at diagnosis, recurrence [yes or no], cancer treatment [surgery, with or without radiation therapy, chemotherapy, or both]), and other medical information.
Participant reported outcomes
Participants completed a number of questionnaires related to the primary study objective. However, for the purpose of this secondary analysis, we selected depression, fatigue, pain, physical functioning, and overall health as the priorities to analyze, given that the literature on breast cancer survivors indicates that these are common problems. Participants completed the Medical Outcomes Study Short Form-36 (SF-36 (35, 36), a 36-item self-report measure of health-related quality of life that includes 8 subscales measuring diverse health concepts (physical functioning, social functioning, pain, mental health, vitality, general health, role limitations because of physical problems, and role limitations because of emotional problems) and has high internal consistency reliability (Cronbach's alpha= 0.78-0.93) (37). The subscales measuring physical functioning, vitality, bodily pain, and general health were used for this analysis. On all subscales a higher score indicates a better outcome (i.e., better physical functioning, less pain). Participants also completed the Center for Epidemiological Studies Depression Scale, a well-validated, 20-item self-report depression measure, with scores from 0 to 60. A score of 16 or higher indicates a need for clinical evaluation. It has high internal consistency (Cronbach's alpha = 0.84-0.90), moderate test-retest reliability (kappa = 0.51-0.70), and good construct validity with other measures of depression (38).
Physical activity
The 7-Day Physical Activity Recall Questionnaire (7-DPARQ) is a valid and reliable (39, 40) interviewer-administered measure used to assess physical activity participation in the past week, including household and occupational activities, leisure activity, and intentional exercise. The amount of time spent in sleep and moderate-, hard-, and very hard-intensity activities is gathered through the interview process, and the amount of time spent in light-intensity activities is imputed from the other activities. Total energy expenditure calculated from 7-day recall interviews have been shown to correlate with VO2max (40, 41), body fat (40), past year activity (41), and objective measures of physical activity (42). Test-retest reliability is adequate, although it is lower for moderate intensity activity than hard and very hard intensity activity (39).
Research coordinators attended a 2-hour training session in which the recall interview was demonstrated; they then conducted 3-5 practice interviews with each other, interviewed the trainer, and received feedback on their performances. Patient assessments were audiotaped so the trainer could provide the research coordinators with corrective feedback if necessary.
We included the following data in our analysis: total energy expenditure for the week (kcal/kg/week), frequency (number of days with at least 30 minutes of physical activity), and duration (number of minutes of physical activity on days it was performed)
Analysis
The association between total energy expenditure, frequency of physical activity, and duration of physical activity was calculated using one-way analysis of variance, with tests for linear and quadradic trends. An unadjusted analysis was conducted first, followed by an analysis of covariance controlling for potential confounding variables and (for the analysis of frequency and duration) total energy expenditure. The potential confounding variables were selected by analyzing the associations between the independent variable (total energy expenditure, frequency of activity, and duration of activity) and potential confounding variables (disease stage, diagnosis of recurrence or second primary, received chemotherapy, mastectomy, education level, age, time since diagnosis, African-American race, Latina ethnicity, and comorbidity score). Any potential confounding variables that had an association with a particular independent variable with a p value of <0.20 were included in an analysis of covariance model testing that independent variable
Results
Recruitment and Demographic data
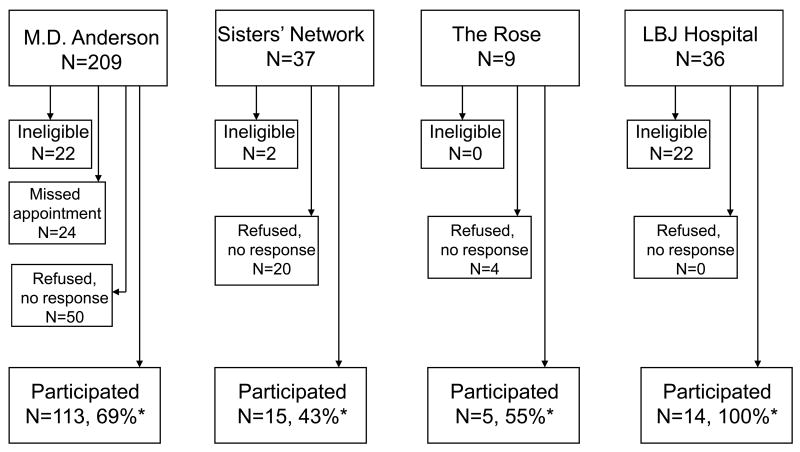
A flow chart of participant recruitment is provided in Figure 1. Participation rates (participating survivors/ survivors identified as eligible) ranged from 43 – 100% depending on the source of recruitment. Of those survivors who refused, the reasons included not being interested in participating in research and living outside of the area. Of the 148 women who participated, 11% had ductal carcinoma in situ at diagnosis. The others had stage I (39%), stage II (35%), stage III (11%), or stage IV (3%) breast cancer. Eighty-one participants had undergone radiation therapy, 104 had undergone chemotherapy, and 69 had received both. Participants had been diagnosed a mean of 4.5 years before the study began and had a mean BMI of 26.1 (SD = 5.2). The sample was fairly diverse in terms of race and ethnicity. Sixty percent of participants were white, 21% African American, 15% Hispanic, 1% Asian, and 3% other. The mean age of the participants was 54.2 years (SD = 10.3) and approximately half had at least a bachelor's degree. Most were either married (61%) or living with a significant other (4%).
Figure 1.
Recruitment flow chart for survey of breast cancer survivors' physical activity of breast cancer survivors conducted in four settings. (*Participating percentages calculated with number of eligible women as the denominator. For the M. D. Anderson sample, the patients who missed their appointments during the recruiting period were not included in the denominator)
The average total energy expenditure in the sample for the entire 7 days was 244.9 kcal/kg. Forty-one percent of the participants reported engaging five or more days of moderate-intensity or greater activity (for at least 30 minutes) during the week, while 36% reported 2-4 days and 23% were active on one day or not at all. The mean duration of activity among participants reporting activity on at least one day was 1.72 hours (SD = 1.16) on days activity was reported.
Relationship of total energy expenditure to outcomes
Table 1 shows the associations between total energy expenditure and physical activity frequency and physical functioning, vitality, pain, general health, and depression. Several significant linear trends were found for energy expenditure; increasing energy expenditure was associated with better physical functioning and general health, and less pain and depression. Most of the improvements in outcomes occurred between the 1st (<229 kcal/kg/week) and 2nd (229-237 kcal/kg/week) quartiles. Post hoc analyses showed that for physical functioning and pain, the first quartile was significantly different than the fourth quartile (>253 kcal/kg/week) but no other quartile groups differed significantly (although the comparison between the 1st and 2nd quartile neared significance (p=0.052 for physical functioning, p=0.079 for pain). For general health and depression, the first quartile group was significantly different from all other quartiles, but quartiles 2-4 did not differ from each other. Because comorbidity score, having experienced a recurrence, and education level were associated with total energy expenditure (p<0.20), we repeated the analysis controlling for these variables. After adjusting for potential confounding variables a similar pattern emerged. Energy expenditure quartile was significantly related to general health (p=0.006) and depression (p=0.014), while the linear associations between energy expenditure quartile and physical functioning and pain approached significance (p=0.102 and p=0.094, respectively).
Table 1.
Differences in outcomes among participants with varying levels of energy expenditure.
| Quartiles of energy expenditure | p value2 | |||||
|---|---|---|---|---|---|---|
| 1st Mean (CI) |
2nd Mean (CI) |
3rd Mean (CI) |
4th Mean (CI) |
p value1 | ||
| Physical functioning | 64.4 (54.6, 74.2) | 79.3 (71.6, 86.9) | 76.5 (69.2, 83.8) | 82.4 (75.4,89.4) | 0.004 | 0.102 |
| Vitality | 53.9 (46.6, 61.3) | 65.7 (57.8, 73.6) | 63.7 (57.4, 70.4) | 62.3 (54.0, 70.5) | 0.165 | 0.262 |
| Pain | 62.0 (52.7, 71.3) | 76.4 (68.0, 84.7) | 76.4 (68.9, 83.9) | 78.1 (70.3, 85.9) | 0.009 | 0.094 |
| General health | 66.4 (59.3, 73.4) | 77.7 (72.5, 82.9) | 79.0 (73.4, 84.7) | 81.2 (75.8, 86.7) | 0.001 | 0.006 |
| Depression | 14.2 (10.2, 18.4) | 6.6 (5.1, 8.2) | 8.6 (6.7, 10.6) | 7.9 (5.4, 10.4) | 0.005 | 0.014 |
p value for the test of the linear trend, unadjusted
p value for the test of the linear trend, adjusted for co morbidities, ever had a recurrence, education.
Effects of frequency of activity on outcomes
The relationship between frequency of physical activity and outcome variables was analyzed by comparing participants who were sedentary (30 minutes or more of moderate or more intense activity 1 day or less in the past week), low active (30 minutes or more of moderate or more intense activity on 2 to 4 days in the past week), and meeting recommendations (30 minutes or more of moderate or more intense activity on 5 or more in the past week). With regard to activity frequency, the means increased as the frequency of activity increased for most outcome variables; as shown in Table 2 the linear trend was statistically significant for physical functioning (p = 0.010), pain (p = 0.023), and general health (p = 0.001). Depression scores decreased as the frequency of physical activity increased (p = 0.001). The largest differences occurred between the sedentary group and the low active group. For physical functioning, general health, and depression, pairwise comparisons using the Bonferroni correction showed that the sedentary group had significantly poorer outcomes than the low active survivors or those who were active and meeting recommendations (p<0.05). For pain, the difference between the sedentary and active meeting recommendations group was statistically significant (p<0.05) and the difference between the sedentary and low active groups approached significance (p=0.066). We also conducted analyses controlling for total energy expenditure and potential confounding variables that had an association with activity frequency (having had a mastectomy, education level, and African-American race). In the adjusted analysis, the linear trend for physical functioning, general health, and depression remained significant, and the trend for pain approached significance (p=0.057).
Table 2.
Outcome variables by frequency of physical activity in past week (# of days with >30 minutes activity)
| Outcome variable | Days with physical activity | Adjusted p value2 | |||
|---|---|---|---|---|---|
| 0 -1 day (n = 33) | 2-4 days (n = 54) | 5 or more (n = 60) | |||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | p value1 | ||
| Physical functioning | 64.7 (54.5, 74.9) | 78.1 (71.6, 84.6) | 79.5 (74.0, 85.0) | 0.010 | 0.047 |
| Vitality | 54.7 (46.0, 63.4) | 62.9 (56.3, 96.4) | 63.7 (58.6, 68.7) | 0.088 | 0.154 |
| Pain | 63.0 (53.8, 72.3) | 75.7 (68.9, 82.5) | 76.5 (70.1, 82.8) | 0.023 | 0.057 |
| General health | 66.5 (59.8, 73.2) | 77.2 (72.5, 81.9) | 80.3 (75.8, 84.7) | 0.001 | 0.000 |
| CES-D | 14.4 (10.0, 18.8) | 8.1 (6.3, 10.0) | 7.7 (6.1, 9.3) | 0.001 | 0.000 |
p value for linear trend
p value for linear trend, adjusted for total energy expenditure, mastectomy, educational level, and African-American race
Effects of physical activity duration on outcomes
To test the relationship between duration of activity, we categorized patients into quartiles based on the number of minutes moderate or greater intensity activity on the days physical activity was performed. Means and confidence intervals for the outcome variables by duration quartile are provided in Table 3. The linear trend was not significant for any of the variables; however, a significant quadradic trend was observed for physical functioning, pain, and general health. For these variables, outcomes were poorer for participants in the 1st and 4th quartile than in the 2nd and 3rd quartile. Analysis of the pairwise comparisons did not show any significant differences except for between the 3rd and 4th quartiles for physical functioning. In the analysis that controlled for total energy expenditure and potential confounding variables (time since diagnosis, past diagnosis of a recurrence), the quadratic trends for physical functioning, pain, and general health remained significant. There was also a statistically significant linear trend for physical functioning.
Table 3.
Outcome variables by duration of physical activity (hours of moderate or more intense physical activity averaged over days on which it was reported).
| Duration of activity | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1st quartile | 2nd quartile | 3rd quartile | 4th quartile | |||||
| Median=36 min | Median=64 min | Median=91 min | Median=162 min | p value | p value | Adjusted p value1 | Adjusted p value1 | |
| Outcome variable | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Linear trend | Quadradic trend | Linear trend | Quadratic trend |
| Physical functioning | 72.8 (64.2, 81.5) | 79.1 (71.7, 86.4) | 83.4 (76.8, 89.9) | 67.3 (57.8, 76.8) | 0.494 | 0.007 | 0.035 | 0.002 |
| Vitality | 63.1 (54.7,71.6) | 62.9 (57.2, 68.6) | 64.8 (57.2, 72.4) | 54.3 (46.1, 62.5) | 0.144 | 0.172 | 0.076 | 0.099 |
| Pain | 66.1 (56.8, 75.5) | 79.7 (73.1, 86.4) | 78.4 (71.2, 85.7) | 67.2 (57.1, 77.3) | 0.927 | 0.003 | 0.725 | 0.003 |
| General health | 73.0 (66.5, 79.5) | 78.5 (72.6, 84.3) | 79.8 (74.8, 84.8) | 72.8 (65.8, 79.7) | 0.941 | 0.040 | 0.086 | 0.008 |
| CES-D | 9.2 (6.5, 12.0) | 8.6 (6.1, 11.2) | 10.0 (6.4, 13.6) | 9.6 (7.1, 12.2) | 0.685 | 0.909 | 0.179 | 0.634 |
p value, trend analysis adjusted for total energy expenditure, recurrence, time since diagnosis
Discussion
Most breast cancer survivors in this sample participated in some type of physical activity, which included household and occupational activities, leisure time physical activity, and intentional exercise. Approximately 78% participated in at least two days of moderate or greater intensity activity, but only 41% reported physical activity levels at the currently recommended frequency and duration (43). This percentage is slightly lower than U.S. women in the general population; national data shows that approximately 50% of women between the ages of 35 and 44, and 46% of those between 45 and 64 engage in leisure time physical activity of moderate to vigorous intensity for 30 minutes on at least 5 days per week (44).
Results of this study showed that total energy expenditure from all types and intensity levels of activity was associated with better physical functioning, overall health, and less pain and depression. Results were very similar for frequency of activity. These results are more consistent than those reported from the HEAL study, which found associations primarily with physical functioning and selected fatigue variables. Our results are consistent with our hypotheses and other research showing an association between exercise and symptoms and health outcomes in cancer survivors (17-21). The finding that both energy expenditure and frequency were related to outcomes suggests that both mechanisms related to the total volume of physical activity and distraction from distress may be operational.
Our results highlight two unique points. First, we found relationships between outcomes and total physical activity, which includes activity such as occupational and household activity as well as exercise. Most previous studies have looked only at exercise (planned physical activity performed with an intent to increase fitness). Second, the analysis of both total energy expenditure and frequency of activity showed that the largest differences in outcomes occurred between survivors with the lowest levels of energy expenditure and frequency of activity, and those with slightly more. Our results are similar to those reported by Brown et al. (45). They found that respondents to the BRFSS surveys who reported any physical activity were less likely to report poor physical or mental health in the past 30 days than respondents who were completely sedentary. This suggests that increasing activity in the sedentary cancer survivor population should be a higher intervention priority than helping those who are already active enhance their physical activity. Currently, few studies target only sedentary cancer survivors, which, in the long run, may be the most cost-effective and best use of resources, although this requires further study.
The results for duration of activity (average time per day spent in moderate or more intense activity) were surprising. A linear relationship was expected, with greater duration associated with better outcomes, but this study found that, in general, outcomes improved with increasing duration for the first three quartiles, but were poor for participants with the highest duration of physical activity. These survivors may have extensive household or occupational activity, causing them to be active to the point of exhaustion, resulting in decrements in their quality of life.
Studies of moderate-intensity activity for breast cancer survivors have been shown to improve their physical function and quality of life (17-21) with some suggesting a possible dose response effect (46). The findings of this study are also consistent with studies showing fitness benefits from multiple short bouts of activity (47, 48). Such a regimen may produce improvements in symptoms without compromising fitness benefits, and may be easier for some survivors to adopt and maintain (47). To date, most studies on exercise for cancer survivors have used exercise programs of a fixed frequency, duration, and intensity, which does not allow for testing the effects of these variables in a randomized fashion. Most of these studies have prescribed moderate to vigorous exercise 3 to 5 days per week, with sessions lasting 20 to 30 minutes (49). More research is needed, including randomized studies, to better determine is the frequency and duration of activity necessary to achieve optimal outcomes among cancer survivors.
A surprising finding is that there was no consistent relationship between the physical activity variable and fatigue as indicated by low scores on the SF-36 vitality subscale. This is particularly surprising given the existing evidence of the positive effects of exercise interventions on fatigue, as summarized in a recent Cochrane review (50). We used only a single unidimensional measure of fatigue, the SF-36 vitality subscale, so it is possible that it is not an adequate fatigue measures. However, other studies of fatigue in cancer survivors have found it correlates highly with other commonly used fatigue measures (12). Our study may have failed to show such a relationship because it measured total physical activity, including household and occupational activity, as well as exercise. A large study of breast cancer survivors and physical activity found that while sports and recreational activity was related to sensory aspects of fatigue, household activity was not (46). Intentional exercise or leisure time physical activity may provide distraction that household activity does not, thus leading to different effects on fatigue. It is also possible that household activity is not done at a consistently moderate or vigorous intensity, and thus does not affect fitness which may relate to fatigue.
Although total energy expenditure and frequency of activity was found to be associated with symptoms and health status, the cross-sectional design of this study makes it difficult to determine causal effects. It is unclear whether experiencing symptoms and poor health leads survivors to be less active or whether physical activity improves these outcomes. Indeed, both directions of influence are likely present to some extent. While controlling for medical variables partially addresses this, the direction of causation is still questionable in a cross-section study. A longitudinal study examining the effect of physical activity on symptoms and health status would provide stronger support for the influential effects of specific dimensions of physical activity, allowing an examination of changes in physical activity and outcomes.
In summary, the higher total energy expenditure and more frequent physical activity was more consistently associated with outcomes than was greater duration of the activity. When encouraging cancer survivors to participate in exercise, emphasizing increases in frequency of moderate intensity activity follows safe practices, and may have positive implications for symptoms and health status. However, our results are based on a fairly small sample, and should be interpreted accordingly. Because our measure of physical activity included all forms of physical activity, not only planned exercise, a more controlled study with a different measurement approach may reveal different effects. This issue could be further examined using randomized design comparing activity regimens that vary in frequency and duration. More systematic investigation is needed on the effects varying dimensions of activity explored in these studies. Future studies should investigate the optimal frequency and duration of various types of physical activity for improving symptoms frequently experienced by breast cancer survivors.
References
- 1.Ganz PA, Kwan L, Stanton AL, et al. Quality of life at the end of primary treatment of breast cancer: First results from the Moving Beyond Cancer randomized trial. Journal of the National Cancer Institute. 2004;96(5):376–387. doi: 10.1093/jnci/djh060. [DOI] [PubMed] [Google Scholar]
- 2.Ganz PA, Coscarelli A, Fred C, Kahn B, Polinsky ML, Petersen L. Breast cancer survivors: Psychosocial concerns and quality of life. Breast Cancer Research and Treatment. 1996;38:183–199. doi: 10.1007/BF01806673. [DOI] [PubMed] [Google Scholar]
- 3.Dow KH, Ferell BR, Leigh S, Ly J, Gulasekaram P. An evaluation of the quality of life among long-term survivors of breast cancer. Breast Cancer Research and Treatment. 1996;39:261–273. doi: 10.1007/BF01806154. [DOI] [PubMed] [Google Scholar]
- 4.Polinsky ML. Functional status of long-term breast cancer survivors: Demonstrating chronicity. Health and Social Work. 1994;19(3):165–173. doi: 10.1093/hsw/19.3.165. [DOI] [PubMed] [Google Scholar]
- 5.Ganz PA, Rowland JH, Desmond K, Meyerowitz BE, Wyatt GE. Life after breast cancer: Understanding women's health-related quality of life and sexual functioning. Journal of Clinical Oncology. 1998;16(2):501–514. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- 6.Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. Quality of life in long-term, disease-free survivors of breast cancer: A follow-up study. Journal of the National Cancer Institute. 2002;94(1):39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 7.Dorval M, Maunsell E, Deschenes L, Brisson J, Masse B. Long-term quality of life after breast cancer: Comparison of 8-year survivors with population controls. Journal of Clinical Oncology. 1998;16(2):487–494. doi: 10.1200/JCO.1998.16.2.487. [DOI] [PubMed] [Google Scholar]
- 8.Vinokur AD, Threatt BA, Caplan RD, Zimmerman BL. Physical and psychosocial functioning and adjustment to breast cancer. Long-term follow-up of a screening population. Cancer. 1989;63:394–405. doi: 10.1002/1097-0142(19890115)63:2<394::aid-cncr2820630233>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Hann DM, Garovoy N, Finkelstein B, Jacobsen PB, Azzarello LM, Fields KK. Fatigue and quality of life in breast cancer patients undergoing autologous stem cell transplantation: A longitudinal comparative study. Journal of Pain and Symptom Management. 1999;17(5):311–319. doi: 10.1016/s0885-3924(99)00007-x. [DOI] [PubMed] [Google Scholar]
- 10.Bower JE, Ganz PA, Desmond Ka, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. Journal of Clinical Oncology. 2000;18(4):743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 11.Servaes P, Verhagen S, Bleijenberg G. Determinants of chronic fatigue in disease-free breast cancer patients: A cross-sectional study. Annals of Oncology. 2002;13:589–598. doi: 10.1093/annonc/mdf082. [DOI] [PubMed] [Google Scholar]
- 12.Meeske K, Smith AW, Alfano CM, et al. Fatigue in breast cancer survivors two to five years post diagnosis: a HEAL Study report. Qual Life Res. 2007;16(6):947–60. doi: 10.1007/s11136-007-9215-3. [DOI] [PubMed] [Google Scholar]
- 13.Aaronson NK, Meyerowitz BE, Bard M, Bloom JR, Fawzy FI, et al. Quality of life research in oncology. Past achievements and future priorities. Cancer. 1991;67(Suppl 3):839–843. doi: 10.1002/1097-0142(19910201)67:3+<839::aid-cncr2820671415>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Saleeba AK, Weitzner MA, Meyers CA. Subclinical psychological distress in long-term survivors of breast cancer: A preliminary communication. Journal of Psychosocial Oncology. 1996;14(1):83–93. [Google Scholar]
- 15.Wyatt G, Friedman LL. Long-term female cancer survivors: Quality of life issues and clinical implications. Cancer Nursing. 1996;19(1):1–7. doi: 10.1097/00002820-199602000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Weitzner MA, Meyers CA, Stuebing KK, Saleeba AK. Relationship between quality of life and mood in long-term survivors of breast cancer treated with mastectomy. Support Care Center. 1997;5:241–248. doi: 10.1007/s005200050067. [DOI] [PubMed] [Google Scholar]
- 17.Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones LW, Fairey AS. A randomized trial of exercise and quality of life in colorectal cancer survivors. Eur J Cancer Care (Engl) 2003;12(4):347–57. doi: 10.1046/j.1365-2354.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 18.Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stage I and II breast cancer: Results of a randomized controlled trial. Journal of Clinical Oncology. 2001;19(No. 3):657–665. doi: 10.1200/JCO.2001.19.3.657. [DOI] [PubMed] [Google Scholar]
- 19.Mock V, Dow KH, Meares CJ, et al. Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncology Nursing Forum. 1997;24(6):991–1000. [PubMed] [Google Scholar]
- 20.Basen-Engquist K, Taylor CLC, Rosenblum C, et al. Randomized pilot test of a lifestyle physical activity intervention for breast cancer survivors. Patient Education and Counseling. 2006;64(1-3):225–234. doi: 10.1016/j.pec.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Daley AJ, Crank H, Saxton JM, Mutrie N, Coleman R, Roalfe A. Randomized trial of exercise therapy in women treated for breast cancer. J Clin Oncol. 2007;25(13):1713–21. doi: 10.1200/JCO.2006.09.5083. [DOI] [PubMed] [Google Scholar]
- 22.Dimeo F, Fetscher S, Lange W, Mertelsmann R, Keul J. Effects of aerobic exercise on the physical performance and incidence of treatment-related complications after high-dose chemotherapy. Blood. 1997;90(9):3390–3394. [PubMed] [Google Scholar]
- 23.Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH. Home-based physical activity intervention for breast cancer patients. J Clin Oncol. 2005;23(15):3577–87. doi: 10.1200/JCO.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 24.Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. Journal of Clinical Oncology. 2003;21(9):1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 25.Dimeo FC, Stieglitz R, Novelli-Fischer U, Fetscher S, Keul J. Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer. 1999;85(10):2273–2277. [PubMed] [Google Scholar]
- 26.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: Cardiopulmonary and quality of life outcomes. Journal of Clinical Oncology. 2003;21(9):1660–1668. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 27.Pinto BM, Trunzo J, Rabin C, Bucknam L, Cram R, Marcus B. Moving Forward: A randomized trial of a home-based physical activity program for breast cancer patients. Annals of Behavioral Medicine. 2003;25(Suppl):53. [Google Scholar]
- 28.Pinto BM, Clark MM, Maruyama NC, Feder SI. Psychological and fitness changes associated with exercise participation among women with breast cancer. Psycho-Oncology. 2003;12:118–126. doi: 10.1002/pon.618. [DOI] [PubMed] [Google Scholar]
- 29.Courneya KS. Exercise interventions during cancer treatment: biopsychosocial outcomes. Exerc Sport Sci Rev. 2001;29(2):60–4. doi: 10.1097/00003677-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Dimeo F, Schmittel A, Fietz T, et al. Physical performance, depression, immune status and fatigue in patients with hematological malignancies after treatment. Annals of Oncology. 2004;15(8):1237–42. doi: 10.1093/annonc/mdh314. [DOI] [PubMed] [Google Scholar]
- 31.Paffenbarger RS, Hyde RT, Wing AL, Hsieh C. Physical activity, all-cause mortality, and longevity of college alumni. New England Journal of Medicine. 1986;314:605–613. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 32.Blair SN, Kohl HW, Gordon NF, Paffenbarger RS. How much physical activity is good for health? Annu Rev Physiol. 1992;13:99–126. doi: 10.1146/annurev.pu.13.050192.000531. [DOI] [PubMed] [Google Scholar]
- 33.USDHHS. (Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion) Physical activity and health: A report of the Surgeon General. 1996 [Google Scholar]
- 34.Morgan WP. Affective beneficence of vigorous physical activity. Med Sci Sports Exerc. 1985;17(1):94–100. [PubMed] [Google Scholar]
- 35.Ware JE, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: Summary of results from the Medical Outcomes Study. Medical Care. 1995;33(4):AS264–AS279. [PubMed] [Google Scholar]
- 36.Ware JE, Jr, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User's Manual. Boston, Massachusetts: The Health Institute, New England Medical Center; 1994. [Google Scholar]
- 37.McHorney CA, Ware JE, Raczek AE. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 39.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City project. American Journal of Epidemiology. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 40.Blair SN, Haskell WL, Ho P, et al. Assessment of habitual physical activity by seven-day recall in a community survey and controlled experiments. American Journal of Epidemiology. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 41.Dishman RK, Steinhardt M. Reliability and concurrent validity for a 7-d re-call of physical activity in college students. Med Sci Sports Exerc. 1988;20(1):14–25. doi: 10.1249/00005768-198802000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Rauh MJ, Hovell MF, Hofstetter CR, Sallis JF, Gleghorn A. Reliability and validity of self-reported physical activity in Latinos. Int J Epidemiol. 1992;21(5):966–71. doi: 10.1093/ije/21.5.966. [DOI] [PubMed] [Google Scholar]
- 43.United States Department of Health and Human Services. (US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion) Physical Activity and Health: A Report of the Surgeon General. 1996 [Google Scholar]
- 44.Kruger J, Kohl HW, III, Miles IJ. Prevalence of regular physical activity among adults -- United States, 2001 and 2005. Morbidity and Mortality Weekly Report. 2007;56(46):1209–1212. [PubMed] [Google Scholar]
- 45.Brown DW, Brown DR, Heath GW, et al. Associations between physical activity dose and health-related quality of life. Med Sci Sports Exerc. 2004;36(5):890–6. doi: 10.1249/01.mss.0000126778.77049.76. [DOI] [PubMed] [Google Scholar]
- 46.Alfano CM, Smith AW, Irwin ML, et al. Physical activity, long-term symptoms, and physical health-related quality of life among breast cancer survivors: A prospective analysis. Journal of Cancer Survivorship. 2007;1:116–128. doi: 10.1007/s11764-007-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jakicic JM, Wing RR, Butler BA, Robertson RJ. Prescribing exercise in multiple short bouts versus one continuous bout: Effects on adherence, cardiorespiratory fitness, and weight loss in overweight women. International Journal of Obesity. 1995;19:893–901. [PubMed] [Google Scholar]
- 48.DeBusk RF, Stenestrand U, Sheehan M, Haskell WL. Training effects of long versus short bouts of exercise in healthy subjects. Am J Cardiol. 1990;65(15):1010–3. doi: 10.1016/0002-9149(90)91005-q. [DOI] [PubMed] [Google Scholar]
- 49.Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiology, Biomarkers & Prevention. 2005;14(7):1588–95. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 50.Cramp F, Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2008;(2):CD006145. doi: 10.1002/14651858.CD006145.pub2. [DOI] [PubMed] [Google Scholar]