Abstract
Recent reports have supported the existence of neural stem cells in the adult mammalian CNS. Important features of such cells are self-renewal and multipotency, i.e., they can give rise to neurons, astrocytes, and oligodendrocytes and thus in principle replace lost cells in the CNS. Observations in several animal models of CNS diseases have shown that by unknown mechanisms endogenous as well as exogenous precursor cells preferentially migrate to damaged areas. Microglia are immunoreactive cells of nonneural lineage resident in the CNS. After injury to the CNS, microglia are rapidly activated and found concentrated at the sites of injury. In the present article we show, in two different assays, that soluble factors released from mouse microglial cells direct the migration of neural CNS precursor cells. We also provide evidence that microglia have the capacity to influence the differentiation of both adult and embryonic neural precursor cells toward a neuronal phenotype. Given that an invariant feature of pathological processes in CNS is the activation of microglia, these results indicate an important and unique role for microglia in directing the replacement of damaged or lost cells in the CNS.
The discovery of neural stem cells and their capacity to regenerate neural cells in the adult CNS have altered old dogmas in neurobiology and raised hopes for novel treatment in medicine. Nonetheless, it is clear that damage to the CNS in, e.g., neurodegenerative diseases is not matched by the ability of endogenous precursor cells to replace lost cells. To reach this therapeutic ideal, much additional knowledge has to be gained regarding the processes regulating replacement of damaged cells.
In the adult CNS, there are two principle locations of neural stem cells: in the subventricular zone and in the subgranular zone of the dentate gyrus in the hippocampus (reviewed in ref. 1). In addition, precursor cells with varying capacity for self-renewal and differentiation have also been isolated from other regions of the CNS, including from human white matter (2–4). In avian and rodent CNS, the new cells formed in the subventricular zone migrate in the rostral migratory stream from the ventricular walls to the olfactory bulb where they replace granule cells and periglomerular cells, whereas precursor cells in the subgranular zone mainly differentiate to granular cells (5, 6). In response to a number of experimental lesions in rodent's brain, including stroke, ischemia, and seizure, precursor cells in the neurogenic centers start to proliferate and migrate (7–9). Interestingly, by unknown mechanisms, new cells preferentially end up at the sites of pathology, indicating that agents present in the damaged tissue guide the migration of precursor cells (7, 10). Besides the proliferation and migration of endogenous precursor cells, exogenously propagated cells are similarly capable of migrating and repopulating damaged areas of the adult CNS (11–14).
The signals that direct the migration of precursor cells are largely unknown. In the rostral migratory stream, the secreted protein Slit has been suggested to function as a repellant for young migrating GABAergic neurons (15). Other molecules shown to guide neuronal precursor cells include SDF-1, which guides embryonic cerebellar neurons (16, 17), and recently unidentified signals from the prenatal cortex have been shown to guide interneurons to the cerebral cortex (18). The effect of these and other signals have mainly been studied on committed, immature neurons, whereas the signals that direct the migration of multipotent neural precursor cells remain elusive.
Another important issue is the correct differentiation of precursor cells to the cell type that should be replaced to regain function. Recent studies show a remarkable flexibility of stem cells regarding the cell type they will differentiate to. For example, exogenously propagated neural precursor cells administrated to an experimental model of multiple sclerosis were observed as oligodendrocytes in the damaged white matter (19, 20), whereas other studies have shown that precursor cells injected in neurogenic areas of the adult brain develops into neurons, gaining attributes appropriate to their location (21, 22). It is reasonable to believe that interactions between precursor cells and the microenvironment faced by the new cells influence their differentiation. In accordance with this, astrocytes were recently shown to induce a neuronal phenotype on adult stem cells from mice, both as a result of cell–cell contact and by secreted factors (23).
However, little is known about how a pathological environment affects the differentiation of precursor cells. An invariant feature of damage to the CNS is the migration of microglia cells to the site of injury and their subsequent activation; also astrocytes may be activated. The activation of microglia can result in either neuroprotective or neurotoxic effects, or both. Among such protective features, microglia can express and secrete neurotrophins such as brain-derived neurotrophic factor, as well as several cytokines and chemokines (24, 25). Because of the relocation of microglia to the site of injury and their rapid activation as response to CNS abnormalities, we reasoned that these cells may be involved in the attraction of precursor cells as well as in effecting their differentiation; we here present data that support this hypothesis.
Materials and Methods
Cell Culture and Conditioned Media. All cell culture reagents were from Invitrogen unless otherwise stated in the text. Cell cultures were kept at 37°C in 10% CO2 and 90% relative humidity. Primary microglia (PM) was prepared from embryonic day (E) 16 to E17 C57/BL mice, with some modifications, as described (26). In summary, isolated tissue was mechanically dissociated between the frosted ends of two glass slides, filtered trough a 70-μm nylon mesh (Falcon), and washed in culture medium (DMEM 10% FCS/0.45% glucose) before seeded in poly(L)lysine-coated flasks. When confluence was reached, microglial cells were harvested by gently shaking the flasks and transferring the resulting cell suspension, after washing, to poly(L)-lysine coated petridishes, changing one-half the medium to serum-free Neurobasal medium supplemented with B27 (NB27). After seeding (1 day), the enriched microglial {90–95% microglia [Iba-1+ cells]/<5% astrocytes [glia fibrillary acidic protein (GFAP)-positive cells]} cultures were washed with fresh NB27 and then cultivated in NB27 with or without lipopolysaccharide (LPS, Sigma) for 24 h. Conditioned media was harvested, cleared from free-floating cells by centrifugation for 5 min at 1,000 × g, and sterile filtered. Conditioned media was kept at 4°C until use.
The mouse microglia cell lines BV-2 and N13 and the astrosarcoma cell line C6 were cultured in NB27. When reaching confluence, fresh media were added with or without LPS (10 ng/ml), harvested after 24 h, centrifuged as described above, and passed through a 0.2-μm filter.
Conditioned medium (CM) taken from the cell lines (BV-2, N13, and C6) will be termed BV-2/N13-CM and C6-CM, respectively, whereas medium taken from PM cultures will be referred to as PM-CM. Conditioned media from BV-2 and N13, irrespective of LPS treatment, gave very similar results and will, therefore, for sake of clarity, be treated as one.
Aggregate Cultures and Isolation of Embryonic Precursor Cells. Cortical aggregate of total CNS cells and isolation of precursor cells were performed as described (C. M. D. Berglund, S.L.B.H., J. R. Nyengaard, T. Hökfelt, K.S., J. Näslund, and M.A.A.P., unpublished data). In summary, cortical tissue from E16–E17 C57/BL mice were isolated and mechanically dissociated into single-cell suspensions, seeded in Erlenmyer flasks, and incubated on a gyratory shaker. After seeding (7 days), spherical aggregates formed, which contained the principal brain cells; astrocytes, neurons, microglia, and oligodendrocytes. After seeding (11–25 days), the culture medium was supplemented with 20 ng of epidermal growth factor (EGF)/ml (Becton Dickinson) and one-half the medium was then changed to fresh EGF-containing medium every third day.
Precursor cells were isolated from EGF-treated aggregates by incubating the aggregates in 0.25% trypsin for 5 min at 37°C followed by gently pipetting to release cells from the aggregate. The cell suspension was seeded in bacterial petridishes (Sarstedt) in NB27 supplemented with 20 ng of EGF/ml. Formed neurospheres were divided as described below for adult precursor cells.
Adult Precursor Cell Culture. Adult precursor cultures were prepared essentially as described by Johansson et al. (27). In brief, the lateral ventricles were dissected from adult female C57/BL mice (>20 weeks), and the tissue was dissociated in trypsin solution [0.25% Trypsin/EDTA/15 mM Hepes (Sigma)/0.5% glucose in 1× Hanks' balanced salt solution (Sigma)] at 37°C in 10% CO2 for 25 min, gently triturated, and incubated for 5 more min. The trypsin was quenched by the addition 0.5 vol of 2% BSA and 20 mM Hepes in 1× Hanks' balanced salt solution, and the cell solution was filtered through a 70-μm nylon mesh. Cells were washed in NB27 before seeding in bacterial petridishes in NB27 containing 20 ng of EGF/ml. One week after seeding, the cultures (now neurospheres) were divided by trypsination for 5 min and thereafter once a week.
Precursor Cell Migration. Migration from aggregates. Conditioned media were concentrated 30 times by centricon filtration (Millipore; cut-off 50 kDa). Concentrated medium was then mixed 1:1 with 4% Baculovirus agarose (Invitrogen) in Hanks' balanced salt solution at 48°C and 5-μl aliquoted to 96-well plates (Costar). Plates were then placed on ice and allowed to set before the addition of 48 μl of Matrigel (Becton Dickinson)/NB27 at a ratio of 1:3.
EGF-treated aggregates were washed extensively with fresh NB27 and cultivated for 24 h without EGF. Single aggregates were then picked and placed in the Matrigel-containing wells (on ice) close to the agarose-mixed conditioned media (Fig. 2H). Twenty-four hours later, 152 μl of NB27 was added, and after 48 h, the cultures were fixed for 15 min in 4% paraformaldehyde, washed with PBS, and kept at 4°C in 0.5% BSA/0.02% NaN3/PBS until determination.
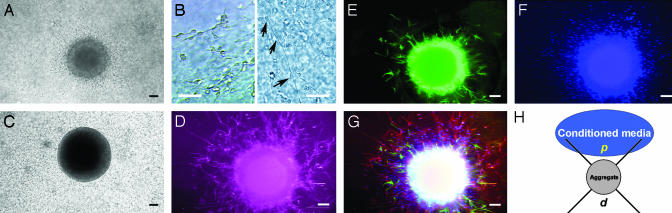
Fig. 2.
Migration of precursor cells from EGF-treated aggregates in Matrigel. (A) Precursor cells migrate radially from EGF-treated aggregate when embedded in Matrigel. (B) Cells migrated both as single cells frequently attached to long processes (arrows) and tightly packed together. (C) No cells migrated from aggregates not pretreated with EGF. (D–G) Pseudocolored immunofluorescence micrographs of the same EGF-treated aggregate 6 days after embedding in Matrigel. Aggregates were immunostained for βIII (neurons, red) (D) and GFAP (astrocytes, green) (E) and counterstained with DAPI (nuclei, blue) (F). (G) Overlay picture of D–F. (Scale bars, 50 μm.) (H) Schematic drawing showing the experimental principle and the overlaid quadrants used to quantify precursor migration from the embedded aggregate in the centre. The proximal (p) and the distal (d) quadrants relative to the conditioned media.
Fixed cells in Matrigel were either stained with Abs, Tuj-1 against β class III tubulin (βIII) (rabbit, Convance) at a dilution of 1:3,000 and anti-GFAP (mouse, Sigma) at a dilution of 1:500 for 48 h at 4°C, washed for 4 h at room temperature, and stained with secondary Abs for 24 h or only stained with 4′, 6-diamidino-2-phenylindole (DAPI, Calbiochem). Migrating cells were quantified by dividing the area around the embedded aggregate into four quadrants, as described by Wu et al. (15) (Fig. 2H).
Transwell migration. Transwell membranes (Costar, 8-μm pore size) were coated 4 h on both sides with Matrigel in NB27 (100 μg/ml), washed with NB27, and placed in 24-well plates. Below the membrane, 320 μl of cell-free conditioned medium was added and diluted in NB27 to represent a concentration of conditioned media of 0.75 × 106 cells per ml. To the upper chamber, 100 μl of precursor cells, incubated for 2 h at 37°C and 10% CO2 in NB27 (0.65 × 106 cells per ml) were added, and the plate was incubated over night at 37°C and 10% CO2. The inserts were removed and the upper surface was carefully cleansed with cotton pads. Cells on the lower surface were air dried, counterstained with Harris' hematoxylin for 20 min, and washed. Stained inserts were placed on object slides and the number of cells on the lower surface assessed at ×200 in an inverted bright-field microscope. Eight ×200 fields per insert were counted. Migration is expressed as a percentage (of cells per field) of the spontaneous migration toward NB27.
Precursor Cell Differentiation. For differentiation studies, neurospheres (passage 2–10) were dissociated to single cells in trypsin, quenched with 4% BSA, washed with NB27, and seeded as single cells on 9-mm poly(L)-ornithine (0.01%)-coated glass cover slips at a density of 35 to 50 × 103 cells per cm2. Alternatively, cells were seeded, at the same concentration, in poly(L)-lysine-coated 96-well plates (Biocat, Becton Dickinson). Cells were grown in conditioned media [diluted 1:1 in NB27 containing 4 ng of EGF/ml (only adult precursor cells)] or only NB27 with or without LPS (10 ng/ml). All experiments were performed two to four times with two to four wells per treatment.
Immunocytochemistry. Cover slips or 96 wells were fixed in 4% paraformaldehyde and washed in PBS. For identification of neurons, we used Tuj-1 (βIII) at 1:6,000 and for astrocytes anti-GFAP at 1:5,000. Microglia was identified with rabbit anti-Iba-1 at 1:500. All primary Abs were diluted in 0.3% Triton X-100, 5% normal goat serum (Vector Laboratories) in PBS. Primary Abs were present at 4°C overnight followed by wash in PBS. Secondary reagents were goat Alexa Fluor 488 and 594 (Molecular Probes) both at 1:750, diluted in 0.3% Triton X-100/PBS and incubated for 2–3 h at room temperature. Cells were counterstained with DAPI and, in the case of cover slips, mounted with Moviol (Calbiochem).
Immunoreactivity was examined at ×200 magnification in a Zeiss microscope, and images from 6–10 random fields per coverslip were captured. The numbers of DAPI+ cells were counted by the CARNOY program (www.carnoy.org), and the number of neurons and astrocytes were counted manually. All images were counted blind. Images of immunostained cells in 96-well format were automatically captured in an Image-Xpress (Axon Instruments). Cells were automatically identified by an algorithm designed to detect DAPI-stained nuclei and the presence of βIII or GFAP immunoreactivity in the cytoplasm immediately surrounding the DAPI nuclei. Five fields per well from two different wells were captured. The number of different cells is expressed as percentage of DAPI+ cells.
Statistical Analysis. Data were analyzed and tested for significance between groups by using the Student's t test assuming equal variance.
Results
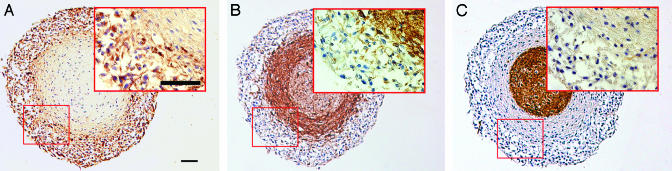
Precursor Cell Migration in Matrigel. Cortical aggregate cultures were established by cultivating mixed mouse CNS cells on a gyratory shaker. When the resulting aggregates (≈500 μm in diameter) were treated with EGF, an outer layer of proliferating cells were formed. They expressed the neuroepithelial marker nestin while lacking expression of more mature markers for astrocytes or neurons (Fig. 1 A–C; C. M. D. Berglund, J.A., S.L.B.H., J. R. Nyengaard, T. Hökfelt, K.S., J. Näslund, and M.A.A.P., unpublished data). The identity of these cells as precursor cells was previously shown by clonal analysis where clones derived from single cells gave rise to astrocytes, neurons, and oligodendrocytes (C. M. D. Berglund, J.A., S.L.B.H., J. R. Nyengaard, T. Hökfelt, K.S., J. Näslund, and M.A.A.P., unpublished data). Migration of these precursor cells was readily studied by placing the aggregates in Matrigel.
Fig. 1.
EGF-treated mixed CNS cell aggregates contain neural precursor cells. (A–C) Bright-field micrographs displaying EGF-treated aggregates, with an outer ring area of nestin-positive precursor cells (A). The outer ring of cells lack the expression of the neuronal marker βIII (B) and the astrocytic marker GFAP (C). Consecutive sections from the same aggregate are shown. (Scale bar, 50 μm.)
After being placed in Matrigel (48 h), cells were migrating out from the EGF-treated aggregates, both as single cells with one or two thin processes or as chains of cells (Fig. 2 A and B). Close to the aggregate surface, the single cells were frequently observed associated to long, thin processes extending radially from the aggregate (Fig. 2B). Cells with more extended processes and morphology were observed further away from the aggregates (not shown). In contrast, aggregates not treated with EGF, and hence lacking the outer ring of precursor cells, failed to display any migrating cells when placed in Matrigel (Fig. 2C).
When aggregates embedded in Matrigel were fixed after 6 days and immuno-stained for markers of neurons and astrocytes, most of the stained migrating cells expressed markers of early neurons (βIII), whereas very few were positive for the astrocytic marker GFAP (Fig. 2 D and E). However, a large proportion of the migrating cells were not stained by any of the used markers (Fig. 2 F and G).
In initial studies, we observed that if EGF-treated aggregates were placed in Matrigel together with microglial-conditioned media, more cells migrated out from the aggregates than when using NB27 or conditioned media from the astrosarcoma cell line C6 (C6-CM) (not shown). This suggested that microglial-conditioned media contained factors that promoted the migration of precursor cells, either by increasing their mobility (motogen) or by attracting them (attractant).
To test whether microglia cells express molecules that could direct the migration of precursor cells, EGF-treated aggregates were embedded in Matrigel close to a defined source of concentrated microglial-conditioned media. To quantify cell migration, the area surrounding the aggregates was divided into quadrants, and the number of cells in the proximal (p, closest to the concentrated media) and distal (d, opposite side from the concentrated media) quadrants were determined (see Fig. 2H).
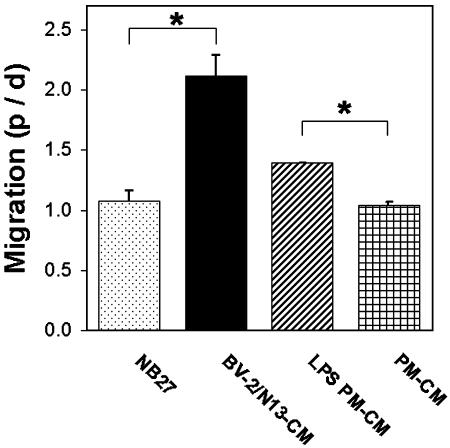
Dividing the number of cells in the proximal zone with the number of cells in the distal zone revealed that there were up to two times as many cells moving toward conditioned media (CM) from the mouse microglia cell lines BV-2 or N13 (BV-2/N13-CM), whereas no such bias was found in the control wells (NB27) (Fig. 3). A similar relationship as for BV-2/N13-CM and NB27 was found when comparing the migration of cells toward conditioned media from LPS-stimulated PM (LPS PM-CM) vs. nonstimulated PM (PM-CM) (Fig. 3). After embedding (48 h), migrating cells were observed up to 800 μm from the aggregate surface, frequently, in the case of microglial-conditioned media, migrating on top of the agarose-embedded conditioned media (data not shown).
Fig. 3.
Directed migration of precursor cells in Matrigel toward microglial-conditioned media. Ratio between number of cells in the proximal quadrant (p) and distal quadrant (d) as described in Fig. 2H. Data are the mean from 12 aggregates per treatment ± SEM. *, P < 0.05 by two-tailed t test.
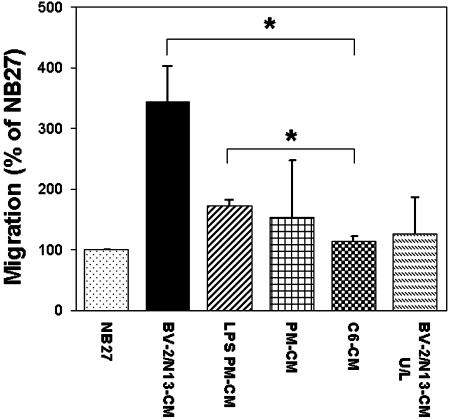
Transwell Migration of Precursor Cells. To confirm that microglia cells were capable of directing the migration of precursor cells, we also used the modified Boyden chamber where precursor cells and conditioned media are separated by a membrane containing pores large enough to allow cell passage. More than three times as many precursor cells migrated toward BV-2/N13-CM as compared to controls [NB27 and conditioned media from a astrosarcoma cell line, C6 (C6-CM)] (Fig. 4). Placing BV-2/N13-CM in both the upper and lower chamber, so as to remove any potential gradient, decreased the migration of precursor cells consistent with a chemotactic model of migration (Fig. 4). Conditioned media from LPS PM-CM or PM-CM were both able to attract more precursor cells than controls (Fig. 4).
Fig. 4.
Transwell migration of precursor cells in modified Boyden chambers. Percentage of cells per microscopic field migrating toward conditioned media, relative to the spontaneous migration toward NB27 (100%). BV-2/N13-CM U/L represent the case where conditioned media were placed in both the upper (U) and lower (L) chamber. Values are given as mean ± SEM of two to four independent experiments. *, P < 0.05 by two-tailed t test.
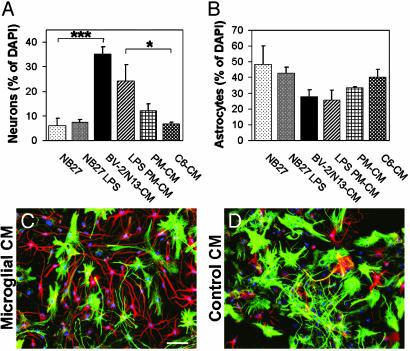
Differentiation of Embryonic Precursor Cells. To test whether microglia also affected the differentiation of embryonic precursor cells, BV-2/N13-CM and conditioned media from PM cells were prepared and added to precursor cells. In cultures treated with BV-2/N13-CM, almost six times more neurons (βIII-positive cells, βIII+) were found as compared to control cultures (C6-CM or NB27) (Fig. 5A). Similarly, a higher proportion of neurons were found in cultures cultivated in LPS PM-CM (Fig. 5A). Also, the proportion of astrocytes (GFAP+ cells) in BV-2/N13-CM and LPS PM-CM were reduced compared to control cultures (C6-CM and NB27) or precursor cells in nonstimulated PM-CM (Fig. 5B).
Fig. 5.
Increased proportion of neurons from embryonic neural precursor cells in microglial-conditioned media. (A and B) Percentages of neurons (βIII+ cells) (A) and astrocytes (GFAP+ cells) (B) of DAPI+ cells, after 7 days in conditioned media from microglial cells or controls. Data are expressed as mean ± SEM of positively stained cells per microscopic field and were counted blind from two to four independent experiments. ***, P < 0.001; *, P < 0.05 (by two-tailed t test). (C and D). Fluorescence overlay micrographs showing the morphology of neurons (red) and astrocytes (green) in BV-2/N13 (C) or C6 (D) conditioned media. Cells were counterstained with DAPI (blue). (Scale bar, 50 μm.)
We saw a morphological difference between βIII+ cells in BV-2/N13-CM or LPS PM-CM and control medium, with most of the βIII+ cells in microglial-conditioned media displaying long, frequently branched processes, whereas the βIII+ cells in control (NB27 and C6-CM) had smaller processes (Fig. 5 C and D). Furthermore, when precursor cells were treated with BV-2/N13-CM or LPS PM-CM, cells positive for both GFAP and βIII could be found (2–8% of total), whereas such double-positive cells were never observed in control cultures or cultures in nonstimulated PM-CM.
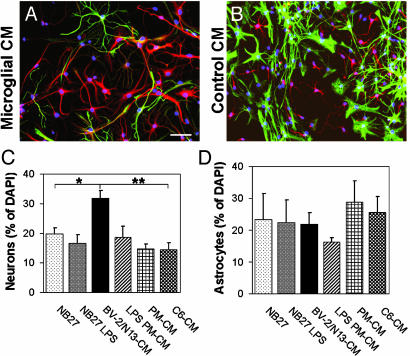
Differentiation of Adult Precursor Cells. We also asked how microglia might affect the differentiation of precursor cells isolated from adult mice, defined here as older than 20 weeks. Morphologically, day 7 BV-2/N13-CM and LPS PM-CM-treated adult precursor cells resembled embryonic precursor cells (compare Fig. 5 C and D to Fig. 6 A and B), although the astrocytes in adult cultures treated with BV-2/N13-CM or LPS PM-CM frequently projected longer processes and were not as compact as the ones in nonstimulated PM-CM or control (Fig. 6 A and B).
Fig. 6.
Differentiation of adult neural precursor cells in microglial-conditioned media. (A and B) Fluorescence overlay micrographs of adult neural precursor cells cultured for 7 days in BV-2/N13-CM (A) or NB27 (B). Neurons were identified with anti-βIII (red) and astrocytes with anti-GFAP (green). Cells were counterstained with DAPI (blue). (Scale bar, 50 μm.) (C and D) Proportions of neurons (C) and astrocytes (D) of DAPI+ cells in cultures of adult neural precursor cells treated with conditioned media from microglia or control cells. Bars represent mean ± SEM from two to four independent experiments. **, P < 0.01; *, P < 0.05 (by two-tailed t test).
The survival rate of adult cells was low, both in control cultures and in nonstimulated PM-CM-treated cultures (not shown). To increase the survival of adult precursor cells, differentiating cultures were supplemented with low amounts of EGF (2 ng/ml). When such cultures were treated with BV-2/N13-CM, >30% of the cells were positive for the neuronal marker βIII after 7 days, whereas significantly less neurons were found in control cultures (Fig. 6C). There was no significant difference in the proportion of astrocytes among the treatments, although somewhat less astrocytes were observed in LPS PM-CM (Fig. 6D). As observed in cultures of embryonic precursor cells, adult cultures treated with BV-2/N13-CM or LPS PM-CM had a small fraction of double-positive cells (1–3% of total, not shown).
Discussion
In the present paper, we have examined how mouse microglia cells affect the migration and differentiation of neural precursor cells. We report that precursor cells isolated from the embryonic brain migrated toward a gradient of microglial-conditioned media. In addition, soluble factors from microglia influenced the differentiation of precursor cells so that the proportion of neurons was increased in cultures treated with microglial-conditioned media.
Directed migration of precursor cells was observed both with precursor cells emanating from aggregate cultures embedded in a protein matrix, as well as with purified precursor cells in a modified Boyden chamber. In both experimental systems precursor cells preferentially migrated toward microglial-conditioned media, clearly showing that microglia have the capacity to direct the migration of precursor cells. Confirming this is our finding that, when purified precursor cells were placed in the modified Boyden chamber with microglial-conditioned media in both the upper and lower chamber, the migration was decreased, indicating that the observed directed migration was not merely a result of increased motility of the precursor cells (Fig. 4).
The cellular identity of migrating cells remains to be firmly established, although the presence of nestin immune reactivity and the lack of detectable expression of neuronal or astrocytic markers in the proliferating cells constituting the outer rim of the EGF-treated aggregates, makes it highly probable that these cells are true, uncommitted neural precursor cells. Such an outer rim of cells was not seen in non-EGF-treated aggregate cultures, nor did we see any migration from such aggregates (Fig. 2). Moreover, the cells migrating out from EGF-treated aggregates did acquire neuronal, and to lesser extent, astrocytic markers only when the EGF had been removed. Taken together, we reason that the migration seen from the aggregates was mainly derived from uncommitted neural precursor cells. In addition, in the experiments performed in the modified Boyden chamber, purified precursor cells were used, and the results corroborated the ones from the Matrigel assay. However, we cannot at present rule out the possibility that microglial factors attract a specific population of precursor cells.
Studying microglial effects on differentiation, we found to our surprise that precursor cell cultures, grown in conditioned media from microglia cells, contained a higher proportion of neurons than would be expected from their spontaneous differentiation alone. This effect was observed on precursor cells isolated from both the embryonic as well as the adult brain, with the strongest microglial effect on embryonic precursor cells. For adult precursor cells, such differentiation was readily induced by conditioned media from the microglia cell lines, whereas we did not observe any increased proportion of neurons in cultures treated with conditioned media from PM cells, even after LPS activation. Possibly, LPS activation may be a rather artificial way of activating microglia compared to the conditions in CNS in vivo. Accordingly, more physiological activation strategies may provide other results. In this regard, we note the consistent and highly significant effect of the microglia cells lines BV-2 and N13.
There are at least three different mechanisms that could be the basis for the increased proportion of neurons in microglial-treated cultures. First, a purely instructive role, inducing the precursor cells to be committed for a neuronal fate. Secondly, microglia may selectively promote the survival of neuronal cells e.g., by providing neurotrophins. Finally, microglia could produce factors that are toxic to astrocytes. In preliminary experiments on adult precursor cells, we have not seen any significant difference in the proportion of terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling-positive cells (apoptosis and necrosis) between the treatments (not shown), arguing against but not disproving that microglial factors increase the survival of neuronal cells. If microglia released factors were toxic to astrocytes, we would expect a more pronounced difference in the proportion of astrocytes between microglial- and control-treated cultures, a difference we do not observe (see Figs. 5 and 6). Accordingly, we currently favor an interpretation with an instructive role of the factors. Still, we would like to emphasize that the mechanism(s) responsible for the increased proportion of neurons in microglial-treated cultures remains to be formally proven.
The observation of cells stained positive for both astrocytic (GFAP) and neuronal (βIII) markers may be explained by recent findings showing that many precursor cells, although displaying all of the characteristics of neural stem cells, express GFAP (28, 29).
It is tempting to speculate that different pathological processes in the CNS may induce distinct modes of microglial activation which may result in different migration and differentiation patterns of precursor cells. For instance, several recent studies have shown that precursor cells preferentially migrate to sites of inflammation in animal models of multiple sclerosis (MS), and that these new cells preferentially differentiate into oligodendrocytes (19, 20, 30). Contrary, in experimental models of more acute damages with neuronal loss, precursor cells, both extrinsically provided as well as endogenous precursor cells, migrated to the damaged area and differentiated to neurons (7, 10–14). Microglia activation at the site of injury is firmly established in all of these experimental lesions (31, 32).
During embryonic development fetal macrophages/microglia are detected in the CNS around E8 in mice and E12 in rat (33, 34), a time-point well before the end of neurogenesis but before the onset of gliogenesis. The role of microglia cells during CNS development is generally believed to be to remove dead cells by phagocytosis. However, the strong effects of microglia cells on embryonic precursor cells, as shown here, may point to other developmental roles of microglia then just to remove dead cells and debris.
In conclusion, despite the apparent imbalance between degeneration and renewal in the CNS, several recent reports have shown migration and regeneration of neural cells to sites of injury or other pathological processes in the CNS (7, 10, 13, 14, 19, 20, 30). Microglia cells are invariably activated at such locations. The present report may add to these observations because it indicates that microglia have the capacity to direct the migration of neural precursor cells as well as effect their differentiation. A role for activated microglia in the attraction as well as differentiation of precursor cells may well represent a more general route for cell replacement in the CNS, both during development and in disease states.
Acknowledgments
We are grateful to Niklas Holmström for technical advice and Jonas Frisén, Jan Näslund, and Darius Matusevicius for fruitful discussions. The cell line N13 was kindly donated by Paola Ricciardi-Castagnoli, and Abs to Iba1 were provided by Yoshinori Imai. This work was supported by the Swedish Cancer Society, the Foundation for Strategic Research (NNN-program), and AstraZeneca R&D.
Abbreviations: βIII, β class III tubulin; CM, conditioned medium or media; DAPI, 4′, 6-diamidino-2-phenylindole; EGF, epidermal growth factor; En, embryonic day n; GFAP, glia fibrillary acidic protein; LPS, lipopolysaccharide; NB27, Neurobasal medium supplemented with B27; PM, primary microglia.
References
- 1.Alvarez-Buylla, A., Seri, B. & Doetsch, F. (2002) Brain Res. Bull. 57, 751-758. [DOI] [PubMed] [Google Scholar]
- 2.Weiss, S., Dunne, C., Hewson, J., Wohl, C., Wheatley, M., Peterson, A. C. & Reynolds, B. A. (1996) J. Neurosci. 16, 7599-7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nunes, M. C., Roy, N. S., Keyoung, H. M., Goodman, R. R., McKhann, G., II, Jiang, L., Kang, J., Nedergaard, M. & Goldman, S. A. (2003) Nat. Med. 9, 439-447. [DOI] [PubMed] [Google Scholar]
- 4.Palmer, T. D., Ray, J. & Gage, F. H. (1995) Mol. Cell. Neurosci. 6, 474-486. [DOI] [PubMed] [Google Scholar]
- 5.Doetsch, F. & Alvarez-Buylla, A. (1996) Proc. Natl. Acad. Sci. USA 93, 14895-14900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gage, F. H. (2000) Science 287, 1433-1438. [DOI] [PubMed] [Google Scholar]
- 7.Arvidsson, A., Collin, T., Kirik, D., Kokaia, Z. & Lindvall, O. (2002) Nat. Med. 8, 963-970. [DOI] [PubMed] [Google Scholar]
- 8.Parent, J. M., Yu, T. W., Leibowitz, R. T., Geschwind, D. H., Sloviter, R. S. & Lowenstein, D. H. (1997) J. Neurosci. 17, 3727-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parent, J. M., Valentin, V. V. & Lowenstein, D. H. (2002) J. Neurosci. 22, 3174-3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakatomi, H., Kuriu, T., Okabe, S., Yamamoto, S., Hatano, O., Kawahara, N., Tamura, A., Kirino, T. & Nakafuku, M. (2002) Cell 110, 429-441. [DOI] [PubMed] [Google Scholar]
- 11.Young, M. J., Ray, J., Whiteley, S. J., Klassen, H. & Gage, F. H. (2000) Mol. Cell. Neurosci. 16, 197-205. [DOI] [PubMed] [Google Scholar]
- 12.Aboody, K. S., Brown, A., Rainov, N. G., Bower, K. A., Liu, S., Yang, W., Small, J. E., Herrlinger, U., Ourednik, V., Black, P. M., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 12846-12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snyder, E. Y., Yoon, C., Flax, J. D. & Macklis, J. D. (1997) Proc. Natl. Acad. Sci. USA 94, 11663-11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svendsen, C. N., Caldwell, M. A., Shen, J., ter Borg, M. G., Rosser, A. E., Tyers, P., Karmiol, S. & Dunnett, S. B. (1997) Exp. Neurol. 148, 135-146. [DOI] [PubMed] [Google Scholar]
- 15.Wu, W., Wong, K., Chen, J., Jiang, Z., Dupuis, S., Wu, J. Y. & Rao, Y. (1999) Nature 400, 331-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu, Y., Yu, T., Zhang, X. C., Nagasawa, T., Wu, J. Y. & Rao, Y. (2002) Nat. Neurosci. 5, 719-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein, R. S., Rubin, J. B., Gibson, H. D., DeHaan, E. N., Alvarez-Hernandez, X., Segal, R. A. & Luster, A. D. (2001) Development (Cambridge, U.K.) 128, 1971-1981. [DOI] [PubMed] [Google Scholar]
- 18.Marin, O., Plump, A. S., Flames, N., Sanchez-Camacho, C., Tessier-Lavigne, M. & Rubenstein, J. L. R. (2003) Development (Cambridge, U.K.) 130, 1889-1901. [DOI] [PubMed] [Google Scholar]
- 19.Pluchino, S., Quattrini, A., Brambilla, E., Gritti, A., Salani, G., Dina, G., Galli, R., Del Carro, U., Amadio, S., Bergami, A., et al. (2003) Nature 422, 688-694. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Hur, T., Einstein, O., Mizrachi-Kol, R., Ben-Menachem, O., Reinhartz, E., Karussis, D. & Abramsky, O. (2003) Glia 41, 73-80. [DOI] [PubMed] [Google Scholar]
- 21.Suhonen, J. O., Peterson, D. A., Ray, J. & Gage, F. H. (1996) Nature 383, 624-627. [DOI] [PubMed] [Google Scholar]
- 22.Fricker, R. A., Carpenter, M. K., Winkler, C., Greco, C., Gates, M. A. & Bjorklund, A. (1999) J. Neurosci. 19, 5990-6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song, H., Stevens, C. F. & Gage, F. H. (2002) Nature 417, 39-44. [DOI] [PubMed] [Google Scholar]
- 24.Gebicke-Haerter, P. J. (2001) Microsc. Res. Tech. 54, 47-58. [DOI] [PubMed] [Google Scholar]
- 25.Streit, W. J. (2002) Glia 40, 133-139. [DOI] [PubMed] [Google Scholar]
- 26.Dobrenis, K. (1998) Methods 16, 320-344. [DOI] [PubMed] [Google Scholar]
- 27.Johansson, C. B., Momma, S., Clarke, D. L., Risling, M., Lendahl, U. & Frisen, J. (1999) Cell 96, 25-34. [DOI] [PubMed] [Google Scholar]
- 28.Doetsch, F., Caille, I., Lim, D. A., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. (1999) Cell 97, 703-716. [DOI] [PubMed] [Google Scholar]
- 29.Imura, T., Kornblum, H. I. & Sofroniew, M. V. (2003) J. Neurosci. 23, 2824-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Picard-Riera, N., Decker, L., Delarasse, C., Goude, K., Nait-Oumesmar, B., Liblau, R., Pham-Dinh, D. & Evercooren, A. B. (2002) Proc. Natl. Acad. Sci. USA 99, 13211-13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aloisi, F. (2001) Glia 36, 165-179. [DOI] [PubMed] [Google Scholar]
- 32.Streit, W. J. (2000) Toxicol. Pathol. 28, 28-30. [DOI] [PubMed] [Google Scholar]
- 33.Alliot, F., Godin, I. & Pessac, B. (1999) Brain Res. Dev. Brain Res. 117, 145-152. [DOI] [PubMed] [Google Scholar]
- 34.Sorokin, S. P., Hoyt, R. F., Jr., Blunt, D. G. & McNelly, N. A. (1992) Anat. Rec. 232, 527-550. [DOI] [PubMed] [Google Scholar]