Abstract
Carcinoma-associated fibroblasts (CAFs) play a critical role in malignant progression. Loss of TGF-ϐ receptor II (TGFϐR2) in the prostate stroma is correlated with prostatic tumorigenesis. To determine the mechanisms by which stromal heterogeneity due to loss of TGFϐR2 might contribute to cancer progression, we attenuated TGF-ϐ signaling in a subpopulation of immortalized human prostate fibroblasts in a model of tumor progression. In a tissue recombination model, loss of TGFϐR2 function in 50% of the stromal cell population resulted in malignant transformation of the non-tumorigenic human prostate epithelial cell line BPH1. Mixing fibroblasts expressing the empty vector and dominant negative TGFϐR2 increased the expression of markers of myofibroblast differentiation [co-expression of vimentin and alpha smooth muscle actin (αSMA)] through elevation of TGF-ϐ1 and activation of the Akt pathway. In combination, these two populations of stromal cells recapitulated the tumor inductive activity of CAFs. TGFϐR2 activity in mixed stromal cell populations cultured in vitro caused secretion of factors that are known to promote tumor progression, including TGF-ϐ1, SDF1/CXCL12, and members of the FGF and BMP families. In vivo, tissue recombination of fibroblasts overexpressing TGF-ϐ1 and SDF1/CXCL12 not only induced transformation of BPH1 cells, but also promoted a robust growth of highly invasive cells, similar to effects produced by CAFs. While the precise nature and/or origin of the particular stromal cell populations in vivo remain unknown, these findings strongly link heterogeneity in TGF-ϐ signaling to tumor promotion by tumor stromal cells.
Keywords: stromal heterogeneity, stromal-epithelial interactions, prostate cancer
Introduction
The tumor microenvironment includes fibroblasts, smooth muscle, nerves, blood vessels and components of the inflammatory/immune system. While smooth muscle is the predominant stromal cell type associated with normal prostatic acini, fibroblasts and myofibroblasts are associated with cancer cells and are prominent modifiers of cancer progression (1–3). Cancer-associated fibroblasts (CAF) isolated from within the tumors of cancer patients, are important elements in tumor growth and progression (4). Studies suggest that these fibroblasts are recruited or activated following injury and that this “reactive stroma” provides the oncogenic signals that promote tumorigenesis (5). The role of CAF in carcinogenesis has been examined by studies in which human prostatic CAF promoted carcinogenesis in the immortalized but non-tumorigenic BPH-1 prostatic epithelial cell line (6–8).
Molecular markers of fibroblast subpopulations are poorly defined. Generally, fibroblasts are identified by their spindle-shaped morphology and the overlapping expression of various “indicators” of their fibroblastic phenotype such as vimentin and FSP1. Other markers which are not expressed in all fibroblasts have been identified (9, 10). This suggests multiple populations of fibroblasts whose specific phenotype may be conditioned by factors such as developmental stage and status (rest versus activation, as in a wound healing or cancer). Such cells may reflect different lineages or may be the result of site-specific differentiation (3).
Stroma can elicit instructive, permissive, or inductive (reactive) effects on the parenchymal epithelium (11). For example, embryonic mesenchymal cells can “instruct” epithelial cells to form functional, differentiated glands (12). In contrast, a “permissive” stroma supports a previously-induced epithelial phenotype. Tumor stroma can be considered “inductive” or “reactive” because it secretes factors that can stimulate tumor progression in suitably initiated epithelial cells (13).
Transforming growth factor beta (TGF-ϐ) can both induce and suppress differentiation and tumorigenesis in a dose and context dependant manner (14). Loss of the TGF-ϐ type II receptor (TGFϐR2) has been observed in the stroma of more than 60% of human prostate cancer patients (15). Ablation of TGFϐR2 from approximately 40% of the stromal cells in a mouse resulted in cancer of the forestomach and premalignant prostatic lesions (16). This demonstrated that changes in TGF-ϐ receptor activity in fibroblastic sub-populations can have profound effects on adjacent epithelial morphology.
The present study was designed to test whether stromal heterogeneity has direct consequences on tumor progression in a human prostatic tissue recombination model. Here we report that heterogeneous expression of TGFϐR2 in prostate stromal subpopulations elicits changes consistent with the malignant phenotype induced by CAF.
Materials and Methods
Cells
BPH-1 (a non-tumorigenic human prostate epithelial cell) was from our stocks (17). De-identified human prostatic tissue samples were obtained from the Vanderbilt Tissue Acquisition Core via the Department of Pathology consistent with Vanderbilt IRB protocols. Benign human prostate stromal cells (BHPrS1) were isolated from a benign prostate surgical sample, also used to establish the BHPrE1 cell line (18). BHPrS1 cells were immortalized using hTERT. Carcinoma Associated Fibroblasts (CAF) were isolated and bioassayed as previously described (6). Cells were maintained in RPMI-1640 (Gibco, Grand Island, NY) with 1% antibiotic/antimycotic (Life Technologies, Grand Island, NY) and 5% Cosmic Calf Serum (CCS, HyClone, Logan, Utah).
ELISA for TGF-ϐ1 and SDF1α
800,000 cells were plated in 10cm dishes, and after overnight attachment and growth, cells were washed twice with PBS and fed with serum-free RPMI-1640. CM was collected after 48 hr, centrifuged at 13,000 rpm for 15 min to pellet debris, and stored at −80°C for later use. Quantification of TGF-ϐ1 and SDF1α in the CM were assessed by ELISA according to the manufacturer’s protocol [human TGFϐ1 (DB100B) and human SDF1α (DSA00) Quantikine from R&D Systems, Inc, Minneapolis, MN]. Each experiment was performed in triplicate.
Human Prostate Tissue Array
Human prostate tissue samples from 90 patients with diagnosed prostate carcinoma representing different Gleason scores were used to generate a tissue microarray. The microarray contained 0.6 mm core samples, two from the peripheral tumor and one from the peripheral zone (PZ) away from the cancer involved area. The original diagnostic H&E stained slide as well as the Gleason score were available in all cases. Immunohistochemically stained microarray slides were reviewed by a pathologist (MPR) blinded to the Gleason score and results were expressed in a semi-quantitative manner. Percentage staining was scored on a scale of 0–4, where 0= no staining, 1= less than 25% of cells staining, 2= 25%–50% cells staining, 3=50 to 75% cells staining, 4=more than 75% cells staining. Epithelial and stromal cells were scored separately.
Tissue Recombinants and Subrenal Capsule Xenografts
Rat urogenital sinus mesenchyme (rUGM) was prepared from 18-day embryonic Sprague-Dawley rats fetuses (Harlan) as previously described (19). To prepare tissue recombinants, rUGM and CAF cells were mixed in five different ratios (100% rUGM, 75% rUGM: 25% CAF, 50% rUGM: 50% CAF, 25% rUGM: 75% CAF, and 100% CAF) for a total of 250,000 cells and recombined with 100,000 BPH1 cells. After overnight incubation at 37°C, tissue recombinants were grafted under the renal capsule of intact male CB17Icr/Hsd-severe combined immune deficient (SCID) mice (Harlan) supplemented with 5 mg testosterone pellets placed in the subcutaneous compartment. For the experiments involving engineered cells, 100,000 BPH cells were recombined with 250,000 stromal cells for BHPrS1−DNTβRII or BHPrS1−EV. The BHPrS1−DNTβRII/BHPrS1−EV mixtures were composed of 125,000 BHPrS1−DNTβRII + 125,0000 BHPrS1−EV cells to obtain a 50/50 ratio. Both BHPrS1−TGFϐ1 and BHPrS1−SDF1α cells were mixed at a density of 250,000 cells with BPH1 cells. Host mice were sacrificed after 8 weeks. The kidneys were removed, grafts cut into halves, and imaged before processing for histology. Graft dimensions were measured and the resultant tumor volume was calculated using the formula; . This formula underestimates the volume of invasive tumors, and thus tends to understate the invasive characteristics of large invasive tumors.
Results
Cancer associated fibroblasts counteract the organizational effect of embryonic mesenchymal cells on prostatic remodeling
To date, the study of stromal-epithelial interactions in vivo has assumed each tissue to have a broadly homogeneous nature. A study of stromal heterogeneity requires the presence of at least two different cell types in the stromal compartment. We examined whether rUGM could suppress the epithelial malignant phenotype resulting from exposure to CAF. To test this, we recombined CAF and rUGM cells in five different ratios with BPH1 epithelial cells.
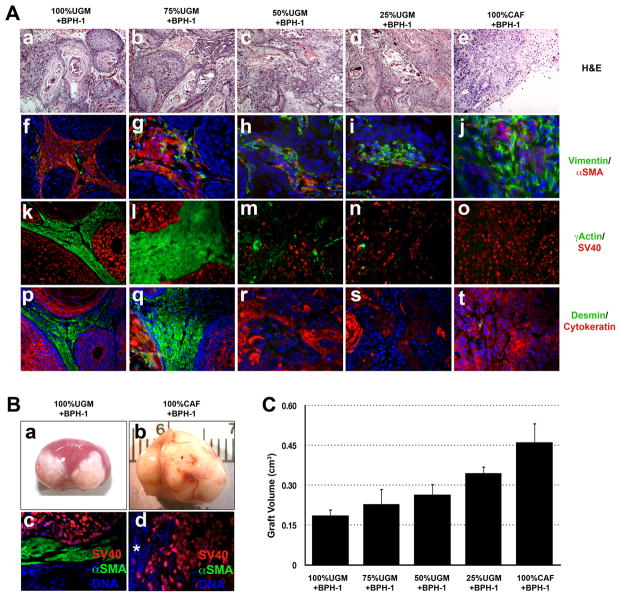
These experiments gave broadly predictable results, in that, as the proportion of CAF/rUGM cells increased, the epithelial structures in the recombinants became progressively less organized and the size of the grafts increased. Specifically, the previously-described, (20) non-malignant, well-organized epithelial cords formed under the influence of rUGM became less obvious as the proportion of CAF increased, while tumor structures and invasion became more prevalent (Fig. 1A and 1B). Concurrent with changes in epithelial cell organization, changes were also noted in the stroma. Well-organized smooth muscle surrounding the benign cords became progressively more fibroblastic in the more malignant grafts, with expression of vimentin predominating over markers of prostate stromal differentiation such as alpha and gamma smooth muscle actin (αSMA and γSMA), desmin, and calponin (Fig. 1A). These results show that the epithelial response is dictated by the overall paracrine signaling environment, and demonstrate that this response can be modified by mixing different populations of stromal cells.
Figure 1. Prostate stroma regulates the fate of prostate epithelial cells.
Heterotypic tissue recombinants of rat urogenital mesenchyme (rUGM) and carcinoma associated fibroblasts (CAF) in different ratios with BPH1 cells were sub-renal capsule grafted to male hosts mice for 8 weeks before the tissues were harvested. A.(a-e). H&E shows differentiation of BPH1 cells to benign cords when combined with 100%UGM. As the ratio of CAF cells increased, malignant transformation of BPH1 cells was observed and the epithelium became progressively less organized and more invasive. (b-e). Immunofluorescence labeling of αSMA/Vimentin (f-j), γActin/SV40 (k-o) and desmin/cytokeratin (p-t) showed a more muscular stroma surrounding areas with high percentage of rUGM (>75%). Markers of normal stromal differentiation were dramatically decreased when the ratio of CAF/rUGM increased to more than 50%. Hoescht 33258 staining of the nucleus (blue) confirmed the human nature of the stromal cells. B. Gross picture of BPH1 cells recombined with rUGM or CAF cells (a & b) showing epithelial invasion into the kidney parenchyma (*). rUGM induced benign glandular differentiation, which was separated by a thick layer of differentiated γSMA (+) fibroblasts (green) from the underlying kidney. C. Tumor volumes were quantitated, graphical representation of the mean ±SD of the grafts is shown n=6. The volume of the tumors increased with the percentage of CAF.
Human Prostate Cancer Stromal cells show heterogeneous TGF-ϐ Signaling
Elevated TGF-ϐ expression by CAF are linked to prostatic tumorigenesis (7, 21). Immunostaining for TGFBR2 has been shown to decrease in prostate stroma with increasing cancer grade (15, 22). However, the importance of TGF-ϐ-responsive versus non-responsive stromal cells has not been addressed properly. We stained for phosphorylated Smad2, as a surrogate for TGFϐ activity, in tissue recombinants composed of BPH1 with: rUGM, BHPrS1, and CAF. BHPrS1 cells were established from a benign human prostate surgical sample and characterized to express both vimentin (fibroblast) and α-SM-actin (smooth muscle) proteins without epithelial and neuroendocrine markers (Supplementary Fig 1). Recombinants composed of CAF + BPH1 showed the highest percentage of phospho-Smad2 expressing stromal cells (52%) compared to their rUGM (28.4%) and BHPrS1 (5.2%) counterparts (Fig. 2A).
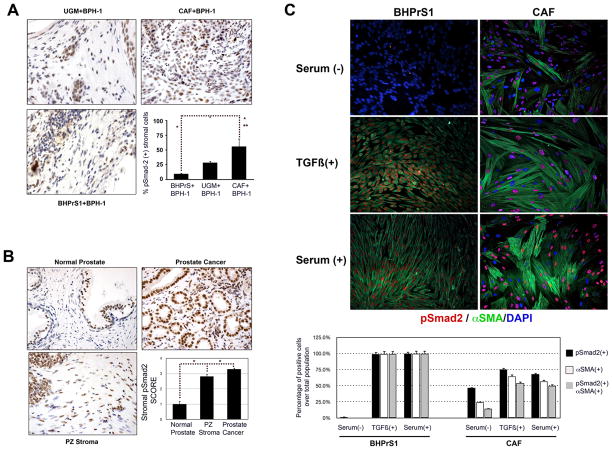
Figure 2. The prostate tumor stroma shows heterogeneous P-Smad2 expression, a surrogate marker of response to TGF-ϐ.
A. Immunohistochemical staining of tissue recombinants for phospho-Smad2. BPH1+CAF recombinants showed the highest proportion of phospho-Smad2 positive stromal cells (~50%) compared to either BPH1+BHPrS1 (<10%) or BPH1+rUGM (<25%). B. Phospho-Smad2 expression in human prostate cancer tissue array. Normal prostate epithelial cells were surrounded by cells expressing low levels of phospho-Smad2. Stromal cells in the tumors as well as those in normal looking areas in the peripheral zone of cancer patients had a phospho-Smad2 SCORE of more than 2.5 representing more than 50% of positive cells (see text for details) C. Addition of TGF-ϐ1 ligand to cultured fibroblasts induced the expression αSMA and phospho- Smad2 in a subpopulation of CAF while the BHPrS1 showed a more homogeneous pattern of expression. Note morphologic changes to CAF with serum compared to BHPrS. This phenomena may be the result of the ability of CAF to remodel collagen matrices.
We stained a prostate tissue microarray composed of ninety benign and 180 malignant samples for phospho-Smad2 and quantitated the percentage of positive/negative stromal cells. Stromal phospho-Smad2 nuclear labeling in normal appearing peripheral zone from areas distant from prostate carcinoma was elevated compared to areas of benign disease. Phospho-Smad2 positive cells were further elevated in tumor-associated PZ stroma. The phospho-Smad2 index was correlated with Gleason Score (Supplementary Table 1). Normal PZ adjacent to tumors had elevated phospho-Smad2 localization compared to uninvolved sections of prostate suggesting a possible “field effect” around tumors (Fig. 2B). Nuclear phospho-Smad2 labeling was visualized in more than 90% of epithelial cells. Heterogeneous nuclear phospho-Smad2 was observed in primary cultures of CAF isolated from prostate cancer patients (Fig. 2C). About 70% of CAF cells responded to TGF-ϐ as determined by nuclear phospho-Smad2 immunoreactivity compared to almost 100% of BHPrS1. Interestingly 71% of the TGF-ϐ responsive CAF also express αSMA suggesting that the high levels of TGF-ϐ found in the cancer stroma not only act on tumor epithelial cells, but could also exert an effect in a subset of stromal cells that have intact TGF-ϐ signaling.
Loss of TGF-ϐ responsiveness in a subpopulation of prostate stromal cells affects epithelial proliferation and induces tumorigenicity
To test whether changes in TGF-ϐ signaling in a subpopulation of normal human prostatic fibroblasts affects prostate epithelial cells, we fed BPH1 cells with CM from CAF and also from BHPrS1 expressing a dominant negative TGFϐR2 (BHPrS1−DNTβRII), a control vector (BHPrS1−EV), or a mixture of BHPrS1−DNTβRII and BHPrS1−EV in a 50/50 ratio. Consistent with previous observations in a similar system, BPH1 cells grew faster in the presence of CAF-CM compared to BHPrS1-CM (7). The combination of BHPrS1−DNTβRII and BHPrS1−EV led to faster proliferation compared to the BHPrS1−EV-CM and similar to the CAF-CM (Fig. 3A). These data suggest that factors secreted by stromal cells heterogeneous for TGFϐ-responsiveness can affect the proliferation of epithelial cells in a manner similar to CAF.
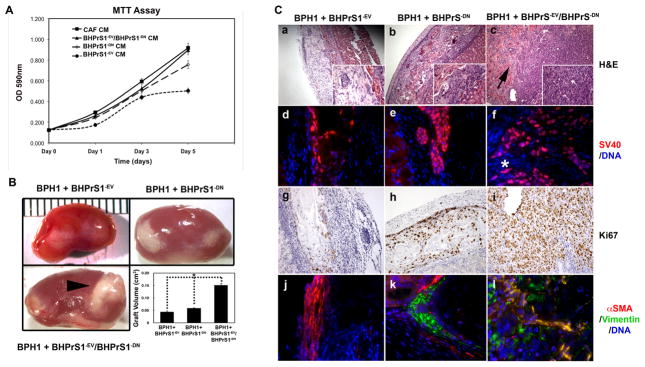
Figure 3. Prostate epithelial cell responses to a heterogeneous stroma.
A. MTT assay of BPH1 cells exposed to CAF, BHPrS1−EV, BHPrS1−DN, or BHPrS1−EV/BHPrS1−DN conditioned medium (CM). Epithelial cells proliferated faster in the presence of CAF and BHPrS1−EV/BHPrS1−DN CM as compared to medium conditioned by the other fibroblasts. B. BPH1+BHPrS1−EV/BHPrS1−DN, BHPrS1−EV, or BHPrS1−DN tissue recombinants were grafted into male SCID mice for 8 weeks before harvesting the tissues. BPH1+BHPrS1−EV/BHPrS1−DN recombinants produced the largest tumors as shown by the gross appearance (arrowhead) and quantitation of tumor volume. C. Histological examination revealed malignant transformation of BPH1 only with mixed BHPrS1−EV/BHPrS1−DN stromal cells (a-c). The adeno-squamous phenotype with invasion into the kidney resembled BPH1+CAF recombinants. The arrow indicate invasion into the host kidney. Immunofluorescence staining of SV40 confirmed the invasion of BPH1 cells into the kidney parenchyma (*) of BPH1+BHPrS1−EV/BHPrS1−DN grafts, no invasion was seen in the other recombinants (d-f). The presence of BHPrS1−DN increased the proportion of Ki67 positive cells in the BPH1+BHPrS1−EV/BHPrS1−DN and BPH1+BHPrS1−EV recombinants (g-i). Malignant transformation of BPH1 cells was accompanied by changes in the stromal compartment towards a myofibroblast phenotype, as noted by the increased expression of vimentin and αSMA (j-l).
To further define the role of the heterogeneous stroma in prostate cancer, we tested these cells in vivo. Grossly, BPH1+BHPrS1−DNTβRII/BHPrS1−EV recombinants formed larger grafts compared with BPH1+BHPrS1−DNTβRII and BPH1+BHPrS1−EV recombinants (Fig. 3B). BPH1+BHPrS1−DNTβRII/BHPrS1−EV recombinants were 2.3 times larger than BPH1+BHPrS1−DNTβRII, recombinants composed of BPH1+BHPrS1−EV showed minimal growth. The small BPH1+BHPrS1−EV grafts were primarily formed of stromal cells and a few epithelial cords without lumen formation [Fig. 3C, a]. BPH1+BHPrS1−DNTβRII grafts exhibited increased epithelium with a few sheets of stromal cells (Fig3C, b). Both BPH1+BHPrS1−EV and BPH1+BHPrS1−DNTβRII tissue recombinants had a benign appearance and did not show any sign of invasion into the host kidney (Fig 3C, d and e). In contrast, BPH1+BHPrS1−DNTβRII/BHPrS1−EV grafts (as assessed by H&E staining) resembled a poorly differentiated adenocarcinoma with areas of adenosquamous differentiation. BPH1 cells showed clear invasion into the kidney parenchyma, and there were no clear margins between the kidney and the grafts as assessed by SV40-T antigen staining [Fig. 3C, c and f (arrow)]. This is similar to previously descriptions of BPH1+CAF recombinants, although the overall tumor size here was smaller (6). The malignant BPH1+BHPrS1−DNTβRII/BHPrS1−EV recombinants had profound effects on their surrounding stroma. Vimentin and αSMA were elevated compared with BPH1+BHPrS1−EV and BPH1+BHPrS1−DNTβRII grafts (Fig. 3C, j, k, and l). BHPrS1−DNTβRII, BHPrS1−EV and BHPrS1−DNTβRII/BHPrS1−EV mixtures were grafted without BPH1 cells. All formed small non-invasive grafts composed of a thin layer of fibrous connective tissue without any sign of malignant transformation (data not shown) consistent with previous reports (6). These data suggest heterogeneous TGF-ϐ responsiveness the stroma can induce malignant transformation of the initiated prostate epithelial cell line BPH1, in a manner consistent with previous observations of CAF.
Absence of TGF-ϐ signaling in a proportion of stromal fibroblasts induces changes to phenotype and intracellular signaling
We investigated the regulation of TGF-ϐ signaling in human prostate fibroblasts on growth factor and growth factor receptor expression using low cycle number RT-PCR. Of a small initial screen of growth factors and receptors, many transcripts showed consistent patterns in the mixture (always expressed, never expressed, or intermediate between the wild-type and DN components) (Supplementary Fig. 2). Some molecules, including SDF1α and TGFϐRIII, showed patterns in which the overall expression in the BHPrS1−DNTβRII/BHPrS1−EV mixed population was clearly induced or suppressed compared with the BHPrS1−DNTβRII or BHPrS1−EV cells alone. Loss of TGF-ϐ signaling in BHPrS1−DNTβRII was correlated with increased expression of TGF-ϐ1 (Fig. 4A). We used PCR arrays to examine these cells cultured under normal serum conditions. The results revealed that members of the TGF-ϐ superfamily as well as several FGF, IGF, and interleukins were highly dysregulated in the BHPrS1−DNTβRII/BHPrS1−EV mixtures compared with the component strains (Supplementary Table 2). The data also confirmed the RT-PCR observations that TGF-ϐ expression was increased in BHPrS1−DNTβRII and BHPrS1−DNTβRII/BHPrS1−EV cells [Fig. 4B (arrow)]. This again suggests that interactions between stromal cells can modify the overall paracrine signaling environment.
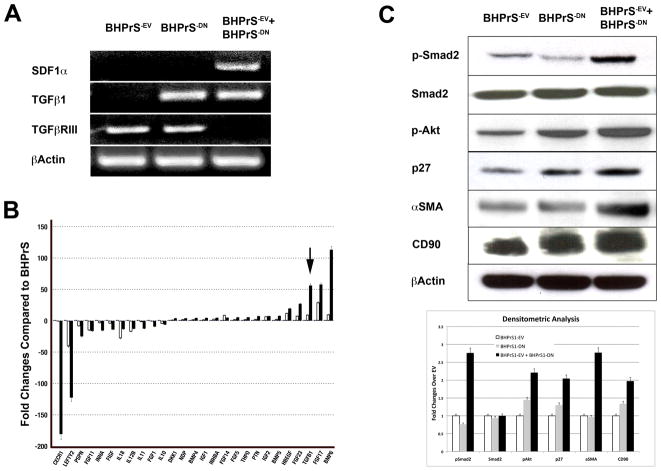
Figure 4. Molecular consequences of heterogeneous stromal cell mixes.
BHPrS1−EV, BHPrS1−DN, and BHPrS1−EV/BHPrS1−DN mixtures were cultured for 48 hr before RNA and protein isolation. A. RT-PCR analysis showed increased SDF1α expression and down- regulation of TGFϐR3 in BHPrS1−EV/BHPrS1−DN compared to the other groups. B. PCR Array analysis validated the increased expression of TGF-ϐ1 in BHPrS1−EV/BHPrS1−DN and BHPrS1−DN cells (arrow) and showed dysregulation of several genes involved in tumor progression. C. Evaluation by densitometric analysis of the protein expression of downstream signaling and potential paracrine mediators of tumor progression showed increased TGF-ϐ signaling (exemplified by phospho-Akt, αSMA, and p27) and CD90 in BHPrS1−EV/BHPrS1−DN mixtures.
To better understand the molecular mechanisms by which the mixtures of BHPrS1−DNTβRII/BHPrS1−EV cells can induce a CAF-like phenotype, we focused on several major pathways and as well as markers altered in CAF by western blot. Cells were grown under serum free conditions for 48 hrs before isolation of protein lysates. As shown in Fig 4C, expression of phospho-Smad2 was higher in the mixtures of BHPrS1−DNTβRII/BHPrS1−EV cells compared to BHPrS1−EV and even more than in BHPrS1−DNTβRII, suggesting that the high expression of TGF-ϐ1 by BHPrS1−DNTβRII in the mixtures has an effect on BHPrS1−EV with intact TGF-ϐ receptor II. These data can explain the field effect observed in patient samples in which normal prostate fibroblasts respond to the high levels of TGF-ϐ ligand secreted by tumors. Expression of phospho-Akt and p27 were higher in BHPrS1−DNTβRII/BHPrS1−EV and BHPrS1−DNTβRII compared to BHPrS1−EV. There were no significant changes in the activation of the MAPK pathway as assessed by phospho-p44/p42 expression. However, there was increased expression of the tumor stromal markers vimentin and αSMA, as well as the recently described marker of mesenchymal lineage cells, CD90, frequently found in prostate cancer stroma (23). Collectively these data suggest that changes in TGF-ϐ signaling may regulate the interactions between stromal cells that might help determine not only the CAF-phenotype, but also the overall paracrine signaling environment responsible for the tumor promoting abilities of the cancer stroma.
Over-expression of TGF-ϐ1 and SDF1α in benign human prostate fibroblasts induces in vivo malignant transformation of BPH1 cells
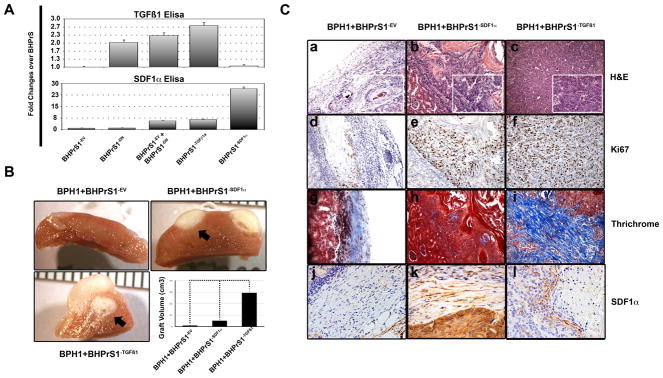
We previously reported that blocking either TGF-ϐ or SDF1α in vivo impairs the ability of CAF cells to promote tumorigenicity (7). Since these two factors were elevated in BHPrS1−DNTβRII/BHPrS1−EV, we wanted to know whether the over-expression of TGF-ϐ1 or SDF1α alone in normal prostate fibroblasts could elicit changes that mimic CAF behavior. We therefore generated BHPrS1−EV, BHPrS1−TGFϐ1, and BHPrS1−SDF1α cells using a retroviral system. Secretion of constitutively active TGF-ϐ1 and SDF1α by these cells were quantitated by ELISA and compared with BHPrS1−DNTβRII and BHPrS1−DNTβRII/BHPrS1−EV (Fig. 5A, upper). ELISA for TGF-ϐ secretion corroborated RT-PCR observations in which the BHPrS1−DNTβRII and BHPrS1−DNTβRII/BHPrS1−EV cells had higher levels compared to the BHPrS1−EV. BHPrS1−TGFϐ1 showed the highest expression with levels similar to CAF. No significant change in TGF-ϐ1 expression was elicited in BHPrS1−SDF1α (Fig. 5A, lower). To further characterize the in vivo effects, BHPrS1−EV, BHPrS1−TGFϐ1 or BHPrS1−SDF1 were each recombined with BPH1 cells and grafted under the renal capsule of SCID mice. Both BHPrS1−TGFϐ1 and BHPrS1−SDF1 formed large tumors that were highly invasive into the kidney (Fig. 5B, arrows). Notably, these grafts were smaller than typical for BPH1+CAF (data not shown). BPH1+BHPrS1−EV grafts were composed of a large bulk of stromal cells with a few solid epithelial cords (Fig. 5C, a). However, when the stroma was enriched for SDF1α, we observed large areas with adeno-squamous differentiation pushing into the kidney with minimal invasion (Fig. 5C, b). BPH1+BHPrS1−TGFϐ1 developed into poorly differentiated adenocarcinomas with a large stromal component, and were highly invasive into the kidney as noted by poor delimitation of the tumor boundaries (Fig. 5C, c). These tumors were highly proliferative measured by Ki67 staining (Fig. 5C, d, e, and f). Masson’s Trichrome staining showed increased collagen deposition in the stroma of BPH1+BHPrS1−TGFϐ1 compared to BPH1+BHPrS1−SDF1 and BPH1+BHPrS1−EV grafts, providing corroboration of TGF-ϐ1 secretion (Fig. 5C, g, h, and i). Staining for SDF1α was highest in the stroma of BPH1+BHPrS1−SDF1 recombinants followed by BPH1+BHPrS1−TGFϐ1 with little staining in BPH1+BHPrS1−EV grafts (Fig. 5C, j, k, and l). Overall our data suggest that specific stromally-secreted factors resulting from partial loss of TGFϐR2 can result in paracrine prostate cancer promotion.
Figure 5. Induction of CAF phenotype by over-expression of TGF-ϐ1 and SDF1α in normal prostate fibroblasts.
A. ELISA was used to test and corroborate the expression of the transgenes in fibroblasts. Fibroblasts expressing constitutively active TGF-ϐ1 ligand showed an increased SDF1α expression. However, over-expressing SDF1α in fibroblasts had no effect on TGF-ϐ1 levels. B. BHPrS1−TGFϐ1 and BHPrS1−SDF1α were recombined with BPH1 cells and xenografted under the kidney capsule for about 8 weeks. The volume of the tumors composed of BHPrS1−TGFϐ1+BPH1 and BHPrS1−SDF1α+BPH1 were significantly larger than the controls. Note the invasive characteristic of the tumors (arrows) C. Histological examination revealed malignant trasnformation of the epithelial cells (a-c). TGFϐ1-expressing fibroblasts had a greater impact on the proliferation of BPH1 cells as shown by Ki67 staining as compared to BHPrS1−SDF1α cells (d-f). BHPrS1−EV fibroblasts exhibited light blue stromal Trichrome staining indicative of some collagen in the stroma. In contrast recombinants composed of BHPrS1−SDF1α stained predominantly red suggesting a more muscular phenotype. Intense blue Trichrome staining in the stroma of BHPrS1−TGFϐ1+BPH1 recombinants revealed the extensive collagen deposition in these tumors. (g-i). Intense SDF1α staining corroborated the secretion of the chemokine in grafts composed of SDF1α-expressing compared to TGFϐ1-expressing fibroblasts (j-l).
Discussion
Malignant tumors are complex caricatures of developing organs in which stromal and epithelial cells are engaged in active communication. The tumor stromal cells, and the extracellular matrix which they deposit, play a key role in restraining or promoting tumorigenesis. The specific nature of the human prostate cancer stromal phenotype has been shown to be an independent clinical prognostic marker (24, 25), underlining the importance of paracrine interactions in human disease. In vivo models have been used to demonstrate paracrine mechanisms by which the stromal microenvironment acts to promote tumorigenesis (7, 21, 26). The prostate tumor microenvironment is complex, including cells of many different lineages (27–34). These include, but are not limited to, smooth muscle, various types of fibroblasts, senescent stromal cells, nerves and blood vessels and a wide variety of immune and inflammatory cell types.
The diversity in the types of cells that compose the tumor stroma makes it difficult to study the contribution of each component. In addition, the lack of stromal cell lines that can retain the tumor-inducing properties shown by CAF cells in vivo represents another important problem. In an attempt to demonstrate the impact of a heterogeneous stromal population in prostate cancer progression, we recreated a controlled tumor microenvironment by impairing TGF-ϐ signaling in about 50% of human benign prostate fibroblasts.
Mesenchyme can restrict the growth of some tumorigenic cells. For example, rat urogenital mesenchyme recombined with Dunning Prostate Adenocarcinoma epithelium resulted in the formation of organized glands and loss of tumorigenicity (35). We have also seen that rUGM decreases the invasive properties of the tumorigenic BPH1−Caftd3 cells (unpublished data). The presence of 25% CAF cells in the stroma (when mixed with 75% rUGM) was sufficient to induce small changes in the overall phenotype of epithelial cells (Fig. 1). These changes were more obvious when half of the population was composed of the tumor promoting CAF stromal cells, suggesting that the restrictive nature of the normal stroma (a feature that might prevent tumor progression) is a function determined by the overall balance of the stromal tissue. The identification of the factor(s) or the type and nature of cells involved in this restriction could be beneficial for targeting the stroma therapeutically. Changes in epithelial phenotype were accompanied with increased proportions of TGF-ϐ responsive stromal cells, as determined by the surrogate reporter of Smad2 phosphorylation, observed adjacent to areas of malignant transformation. When BPH1 cells were recombined with CAF cells, somewhat over 50% of cells showed phospho-Smad2 staining. Immmunohistochemical analysis of human prostate cancer showed around 50–75% of phospho-Smad2 positive stromal cells. We have previously shown that in prostate cancer the level of stromal TGF-ϐ receptor type II decreases with increasing tumor grade (15). This disparity could be due to the fact that Smad2 can serve as a substrate and be phosphorylated not only by TGF-ϐ action, but also by other TGF-ϐ superfamily members including the activin, nodal, BMP and GDF (36, 37).
Heterogeneity in TGF-ϐ signaling was maintained in isolated CAF cells compared to normal prostate fibroblasts in which almost all cells respond to TGF-ϐ stimulation. More than 90% of the BHPrS1 cells demonstrated by αSMA staining in response to TGF-ϐ and co-localization of phospho-Smad2/αSMA in normal fibroblasts was almost universal. In contrast only half of the CAF cells showed clear TGF-ϐ responses. Similar heterogeneity in αSMA was previously observed in a mouse model of pancreatic and breast cancer (10).
Collectively, these observations demonstrate the heterogeneous nature of TGF-ϐ signaling in the tumor microenvironment. To examine the consequences of a heterogeneous population for tumor promotion, we impaired TGF-ϐ signaling in 50% of normal prostate stromal cells. This change in the stromal composition resulted in malignant transformation of initiated epithelial cells in a minor but sufficient degree to represent some of the tumor-inductive properties CAF. PCR array was performed in normal fibroblasts and compared to those lacking TGF-ϐ responsiveness and a mix of the two populations to screen for molecular changes that might contribute to the CAF phenotype. Our results showed significant alterations in the expression of genes associated with the transition of normal fibroblasts to CAF. Several genes involved in development, cell differentiation, and angiogenesis were altered including TGF-ϐ1, which has been shown in several studies to be involved in prostate cancer progression (38). Interestingly, abrogation of TGF-ϐ signaling in fibroblasts resulted in the increase of TGF-ϐ ligand secretion. Thus, a higher BHPrS1−DNTβRII/BHPrS1−EV ratio caused more TGF-ϐ1 secretion to support the conversion to a myofibroblast phenotype
Over-expression of the chemokine SDF1α/CXCL12 can support proliferation and invasion of the prostate cancer cell line, PC3 (39). We have previously shown crosstalk between TGF-ϐ1 and CXCR4, the receptor for SDF1α, which is secreted by CAF in carcinogenesis (7). We found that overexpression of TGF-ϐ1 by normal fibroblasts was sufficient to induce neoplastic changes. Similar observations were described with fibroblasts isolated from reduction mammoplasty (40). Interestingly, SDF1α alone did not induce a robust growth of BPH1 cells, as compared to TGF-ϐ1, but had a dramatic effect in the invasiveness of the cells into the kidney parenchyma. One possible explanation is the absence of complete “activation” of fibroblasts toward a CAF phenotype, as previously reported (39). It is possible that the high amount of TGF-ϐ produced by these fibroblasts is sufficient to result in the basal alterations in the stroma required for tumor promotion, such us conversion to the myofibroblast phenotype and increased secretion of angiogenic factors (two features commonly found in cancer) (41). Once such a conversion occurs, SDF1α might support progression and invasion at a later stage. These observations indicate that an altered heterogeneous stromal environment can promote human prostate cancer formation by changes in the repertoire of secreted factors.
In this study we demonstrated that heterogeneity of human prostate stroma may be an important contributor to stromally-induced carcinogenesis. Abrogation of TGF-ϐ signaling in a subpopulation of prostate stromal cells is consistent with the previously demonstrated loss of stromal TGFϐRII in prostate cancer (22). While the biological situation is unlikely to be this simple, this model represents a step towards modeling stromal complexity. Further exploration of the intrinsic mechanisms responsible for the conversion to the CAF phenotype, and the epithelial responses to such a phenotype, are necessary for the developing of effective cancer therapies that can target both the epithelial and stromal compartments.
Supplementary Material
Acknowledgments
Financial Support: Supported by NIH grant U54-CA126505 (to SWH), DPD PCRP W81XWH-08-1-0542 (to RSJ) and DPD PCRP W81XWH-07-1-0479 (to DWS). The VUMC Institutional Flow Cytometry Core was supported by the VICC (grant P30CA68485). We also thank the Joe C. Davis Foundation for support.
References
- 1.Schor SL, Schor AM. Hypothesis: Persistent expression of fetal phenotypic characteristics by fibroblasts is associated with an increased susceptibility to neoplastic disease. Expl Cell Biol. 1987;55:11–7. doi: 10.1159/000163389. [DOI] [PubMed] [Google Scholar]
- 2.Schor SL, Schor AM, Rushton G. Fibroblasts from cancer patients display a mixture of both foetal and adult-like phenotypic characteristics. J Cell Sci. 1988;90:401–7. doi: 10.1242/jcs.90.3.401. [DOI] [PubMed] [Google Scholar]
- 3.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nature reviews. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 4.Franco OE, Shaw AK, Strand DW, Hayward SW. Cancer associated fibroblasts in cancer pathogenesis. Seminars in cell & developmental biology. 2010;21:33–9. doi: 10.1016/j.semcdb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–38. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 6.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–11. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ao M, Franco OE, Park D, Raman D, Williams K, Hayward SW. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer research. 2007;67:4244–53. doi: 10.1158/0008-5472.CAN-06-3946. [DOI] [PubMed] [Google Scholar]
- 8.He Y, Franco OE, Jiang M, et al. Tissue-specific consequences of cyclin D1 overexpression in prostate cancer progression. Cancer Res. 2007;67:8188–97. doi: 10.1158/0008-5472.CAN-07-0418. [DOI] [PubMed] [Google Scholar]
- 9.Zhao H, Peehl DM. Tumor-promoting phenotype of CD90hi prostate cancer-associated fibroblasts. Prostate. 2009;69:991–1000. doi: 10.1002/pros.20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5:1640–6. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 11.Strand DW, Franco OE, Basanta D, Anderson AR, Hayward SW. Perspectives on Tissue Interactions in Development and Disease. Curr Mol Med. 2009 doi: 10.2174/156652410791065363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunha GR, Hayward SW, Wang YZ. Role of stroma in carcinogenesis of the prostate. Differentiation. 2002;70:473–85. doi: 10.1046/j.1432-0436.2002.700902.x. [DOI] [PubMed] [Google Scholar]
- 13.Barclay WW, Woodruff RD, Hall MC, Cramer SD. A system for studying epithelial-stromal interactions reveals distinct inductive abilities of stromal cells from benign prostatic hyperplasia and prostate cancer. Endocrinology. 2005;146:13–8. doi: 10.1210/en.2004-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–20. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Placencio V, Iturregui JM, et al. Prostate tumor progression is mediated by a paracrine TGF-beta/Wnt3a signaling axis. Oncogene. 2008;27:7118–30. doi: 10.1038/onc.2008.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhowmick NA, Chytil A, Plieth D, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–51. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 17.Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N, Narayan P. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell Dev Biol Anim. 1995;31:14–24. doi: 10.1007/BF02631333. [DOI] [PubMed] [Google Scholar]
- 18.Jiang M, Strand DW, Fernandez S, et al. Functional remodeling of benign human prostatic tissues in vivo by spontaneously immortalized progenitor and intermediate cells. Stem Cells. 2010;28:344–56. doi: 10.1002/stem.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishii K, Shappell SB, Matusik RJ, Hayward SW. Use of tissue recombination to predict phenotypes of transgenic mouse models of prostate carcinoma. Lab Invest. 2005;85:1086–103. doi: 10.1038/labinvest.3700310. [DOI] [PubMed] [Google Scholar]
- 20.Hayward SW, Rosen MA, Cunha GR. Stromal-epithelial interactions in the normal and neoplastic prostate. Br J Urol. 1997;79 (Suppl 2):18–26. doi: 10.1111/j.1464-410x.1997.tb16917.x. [DOI] [PubMed] [Google Scholar]
- 21.Ao M, Williams K, Bhowmick NA, Hayward SW. Transforming growth factor-beta promotes invasion in tumorigenic but not in nontumorigenic human prostatic epithelial cells. Cancer Res. 2006;66:8007–16. doi: 10.1158/0008-5472.CAN-05-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Placencio VR, Sharif-Afshar AR, Li X, et al. Stromal transforming growth factor-beta signaling mediates prostatic response to androgen ablation by paracrine Wnt activity. Cancer Res. 2008;68:4709–18. doi: 10.1158/0008-5472.CAN-07-6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao HJ, Peehl DM. Tumor-Promoting Phenotype of CD90(hi) Prostate Cancer- Associated Fibroblasts. Prostate. 2009;69:991–1000. doi: 10.1002/pros.20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayala G, Tuxhorn JA, Wheeler TM, et al. Reactive stroma as a predictor of biochemical- free recurrence in prostate cancer. Clin Cancer Res. 2003;9:4792–801. [PubMed] [Google Scholar]
- 25.McAlhany SJ, Ayala GE, Frolov A, et al. Decreased stromal expression and increased epithelial expression of WFDC1/ps20 in prostate cancer is associated with reduced recurrence-free survival. Prostate. 2004;61:182–91. doi: 10.1002/pros.20085. [DOI] [PubMed] [Google Scholar]
- 26.Orimo A, Gupta PB, Sgroi DC, et al. Stromal Fibroblasts Present in Invasive Human Breast Carcinomas Promote Tumor Growth and Angiogenesis through Elevated SDF-1/CXCL12 Secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 27.Rowley DR. What might a stromal response mean to prostate cancer progression? Cancer Metastasis Rev. 1998;17:411–9. doi: 10.1023/a:1006129420005. [DOI] [PubMed] [Google Scholar]
- 28.Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. The Journal of urology. 2001;166:2472–83. [PubMed] [Google Scholar]
- 29.Tuxhorn JA, McAlhany SJ, Dang TD, Ayala GE, Rowley DR. Stromal cells promote angiogenesis and growth of human prostate tumors in a differential reactive stroma (DRS) xenograft model. Cancer research. 2002;62:3298–307. [PubMed] [Google Scholar]
- 30.Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8:2912–23. [PubMed] [Google Scholar]
- 31.Tuxhorn JA, McAlhany SJ, Yang F, Dang TD, Rowley DR. Inhibition of transforming growth factor-beta activity decreases angiogenesis in a human prostate cancer-reactive stroma xenograft model. Cancer research. 2002;62:6021–5. [PubMed] [Google Scholar]
- 32.Chung LW, Baseman A, Assikis V, Zhau HE. Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. The Journal of urology. 2005;173:10–20. doi: 10.1097/01.ju.0000141582.15218.10. [DOI] [PubMed] [Google Scholar]
- 33.Chung LW, Huang WC, Sung SY, et al. Stromal-epithelial interaction in prostate cancer progression. Clinical genitourinary cancer. 2006;5:162–70. doi: 10.3816/CGC.2006.n.034. [DOI] [PubMed] [Google Scholar]
- 34.Xu J, Wang R, Xie ZH, et al. Prostate cancer metastasis: role of the host microenvironment in promoting epithelial to mesenchymal transition and increased bone and adrenal gland metastasis. Prostate. 2006;66:1664–73. doi: 10.1002/pros.20488. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi N, Cunha GR. Mesenchyme-induced changes in the neoplastic characteristics of the Dunning prostatic adenocarcinoma. Cancer Res. 1991;51:4924–30. [PubMed] [Google Scholar]
- 36.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783– 810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 37.Gordon KJ, Blobe GC. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochimica et biophysica acta. 2008;1782:197–228. doi: 10.1016/j.bbadis.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Jones E, Pu H, Kyprianou N. Targeting TGF-beta in prostate cancer: therapeutic possibilities during tumor progression. Expert Opin Ther Targets. 2009;13:227–34. doi: 10.1517/14728220802705696. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Ying G, Jung Y, et al. Characterization of phosphoglycerate kinase-1 expression of stromal cells derived from tumor microenvironment in prostate cancer progression. Cancer Res. 2010;70:471–80. doi: 10.1158/0008-5472.CAN-09-2863. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Kuperwasser C, Chavarria T, Wu M, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci U S A. 2004;101:4966–71. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barcellos-Hoff MH, Ravani SA. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 2000;60:1254–60. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.