Abstract
The powerful, long-lasting association between the rewarding effects of a drug and contextual cues associated with drug administration can be studied using conditioned place preference (CPP). The GABAB receptor agonist baclofen facilitates the extinction of morphine-induced CPP in mice. The current study extended this work by determining if baclofen could enhance the extinction of methamphetamine (Meth) CPP. CPP was established using a six day conditioning protocol wherein Meth-pairings were alternated with saline-pairings. Rats were subsequently administered baclofen (2mg/kg i.p. or vehicle) immediately after each daily forced extinction session, which consisted of a saline injection immediately prior to being placed into the previously Meth- or saline-paired chamber. One extinction training cycle, consisted of six once-daily forced extinction sessions, mimicking the alternating procedure established during conditioning, followed by a test for preference (Ext test). CPP persisted for at least four extinction cycles in vehicle-treated rats. In contrast, CPP was inhibited following a single extinction training cycle. These data indicate that Meth-induced CPP was resistant to extinction, but extinction training was rendered effective when the training was combined with baclofen. These findings converge with the prior demonstration of baclofen facilitating the extinction of morphine-induced CPP indicating that GABAB receptor actions are independent of the primary (unconditioned) stimulus (i.e., the opiate or the stimulant) and likely reflect mechanisms engaged by extinction learning processes per se. Thus, baclofen administered in conjunction with extinction training may be of value for addiction therapy regardless of the class of drug being abused.
Keywords: conditioned place preference, rat, methamphetamine, GABAB receptor, baclofen
Methamphetamine (Meth) is a highly abused psychostimulant. Even after long periods of abstinence, cues associated with the rewarding properties of psychostimulants can elicit drug-craving and seeking (Ehrman et al., 1992; Hartz et al., 2001; O'Brien et al., 1992). Thus, relapse to drug use remains a major challenge for psychostimulant-addicted individuals. These responses are attributed at least in part to the robust associative learning that occurs between contextual cues (conditioned stimulus) and the rewarding effects of abused substances (unconditioned stimulus) as well as the enduring nature of the drug-context memory. This long-lasting association can be demonstrated in the laboratory with conditioned place preference (CPP) in humans and laboratory rodents (Childs and deWit H., 2009; O'Brien et al., 1998; Tzschentke, 1998; Tzschentke, 2007), where the preference for the drug-paired context are robust and can persist for long periods of time. Unwanted associative memories can be disrupted by employing extinction learning procedures. For example, extinction procedures can reduce cue-induced anxiety associated with post traumatic stress disorder in humans (Brunet et al., 2008; McCleery and Harvey, 2004). However, extinction of reward-related memories is not particularly efficacious in reducing relapse in abstinent drug-dependent humans (Conklin and Tiffany, 2002) or in rodent models of addiction (Crombag and Shaham, 2002; Di Ciano P. and Everitt, 2004) which may be the consequence of studies not achieving optimal extinction procedures (e.g., number of extinction sessions or the proper cues used); but there is evidence that combining extinction therapy with a pharmacotherapy (e.g., ligands which target the GABAB receptor) can reduce cue-elicited relapse in humans (O'Brien et al., 1990) and mice (Heinrichs et al., 2010).
Currently there are no FDA-approved pharmacotherapies for Meth addiction; however, the GABAB receptor has received considerable attention as a potential therapeutic target (Brebner et al., 2002; Rose and Grant, 2008; Xi and Gardner, 2008). GABAB receptors negatively regulate neurotransmitter systems important for reward- mediated behaviors and mnemonic processes, including glutamate (Harte and O'Connor, 2005; Lei and McBain, 2003; Porter and Nieves, 2004; Yamada et al., 1999) and dopamine (Santiago et al., 1993a; Santiago et al., 1993b; Smolders et al., 1995; Westerink et al., 1996). Cues associated with drug reward activate limbic brain regions in drug-addicted humans (Childress et al., 1999; Childress et al., 2008) and rodents (Brown et al., 1992; Ciccocioppo et al., 2001; Franklin and Druhan, 2000; Rhodes et al., 2005; Zombeck et al., 2008). This activation is attributed to the hyper-responsiveness of glutamatergic (Bell et al., 2000; Hotsenpiller et al., 2001) and dopaminergic (Lin et al., 2007) systems in response to drug-associated cues. Imaging studies indicate that baclofen, a GABAB receptor agonist, blunts the limbic activation associated with visual drug cues in drug-addicted humans (Brebner et al., 2002). Baclofen also inhibits the expression of many psychostimulant-induced behaviors in rodents including CPP (Li et al., 2001), motor sensitization (Bartoletti et al., 2004; Frankowska et al., 2009; Hotsenpiller and Wolf, 2003; Lhuillier et al., 2007) and conditioned motor sensitization, and self-administration (Brebner et al., 2005; Campbell et al., 1999; Filip et al., 2007; Ranaldi and Poeggel, 2002; Roberts and Andrews, 1997; Smith et al., 2004; Weerts et al., 2007). It is clear that baclofen can alter learning and memory process. Particularly relevant to the current study is the recent demonstration that baclofen administered immediately after daily forced extinction training sessions facilitated the extinction of morphine-induced CPP (Heinrichs et al., 2010). As baclofen modulates neurotransmitter systems that are critical for the expression of psychostimulant-induced behaviors as well as the extinction of opiate-induced CPP, we sought to determine the efficacy of baclofen to facilitate the extinction of Meth-induced associative learning. This experimental endeavor also determined if the baclofen effect seen with morphine-induced CPP generalized to the psychostimulant Meth.
Methods
Animals & Housing
Forty male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 250–300g at the start of the study were acclimated to the vivarium (a climate-controlled environment on a 12hr light/dark cycle), for at least one week prior to the onset of the experiments. Rats were housed in pairs and allowed ad libitum access to food and water. Cage mates were given identical pharmacological treatments. Rush University Medical Center housing facilities are accredited through the Association for Assessment and Accreditation of Laboratory Animal Care, and all experiments were carried out in accordance with the conditions set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and with the approval of the Rush University Medical Center Institutional Animal Care and Use Committee.
Drugs
(+)Methamphetamine HCl (Sigma, St. Louis, MO) was dissolved in 0.9% sterile saline, and the dose, 1mg/ml/kg, was administered as the base. Baclofen was also dissolved in 0.9% saline and administered at a dose of 2mg/ml/kg (Sigma, St Louis, MO). Saline vehicle injections 1ml/kg. All injections were given intraperitoneally (i.p.).
The baclofen dose of 2mg/kg is within the range of doses used in laboratory rats to successfully attenuate psychostimulant-induced behaviors, including Meth-induced CPP (1.25, 2.5, 5mg/kg, i.p. were tested) (Li et al., 2001), cocaine self-administration (2.5mg/kg, i.p.) (Smith et al., 2004), amphetamine self-administration break point (1.8, 3.2, 5.6mg/kg, i.p.) (Brebner et al., 2005), and amphetamine-induced motor sensitization (2mg/kg, i.p.) (Bartoletti et al., 2004). In addition, we determined in pilot studies that while motivated motor behavior assessed on the rotarod (San Diego Instruments, San Diego, CA) was inhibited by 4mg/kg baclofen and spontaneous motor activity was decreased by 3mg/kg baclofen, 2mg/kg did not alter motor function (unpublished data).
Apparatus for Assessing Behavior
The test room was dimly lit (54–108 lx) with white noise (San Diego Instruments, San Diego, CA) continuously present. The CPP apparatus (63cm × 30cm × 30cm) consisted of three chambers divided by Plexiglas sliding doors (AccuScan Instruments, Inc., Columbus, OH); two large conditioning chambers (25cm × 30cm × 30cm) separated by a small center chamber (13cm × 30cm × 30cm). Each chamber had distinct, yet neutral, visual and tactile cues. One conditioning chamber had vertical stripes on the walls and the other had horizontal wall stripes. Each chamber contained either a textured floor with a smooth plastic rectangle glued to the center of the floor or an alternately textured floor with a six-well overturned paint dish glued to the center of the floor. The two types of floors were randomly assigned to each conditioning chamber. The center chamber contained solid white walls and a smooth slightly raised platform floor. Time spent in each chamber and motor activity was monitored via two sets of photobeams (24 in the horizontal plane and 12 vertical).
Conditioned Place Preference
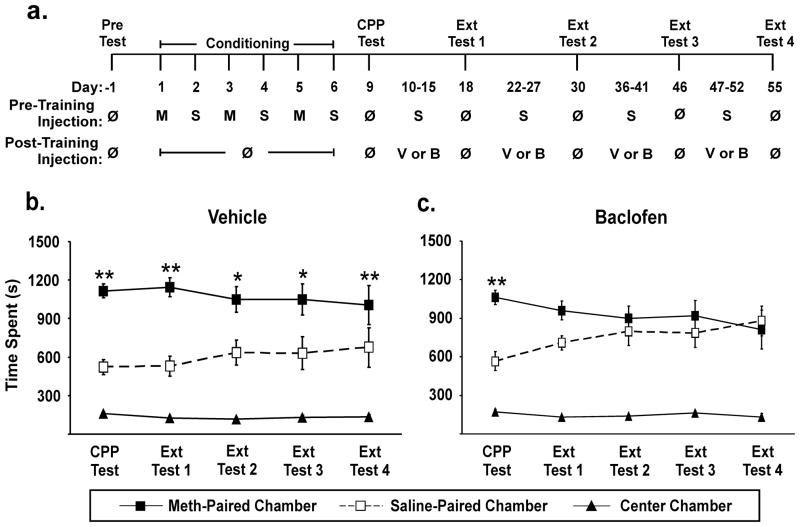
The rats were transported from the animal housing room to the adjacent test room at least 30min prior to the start of the experiment for habituation. Rats were subjected to a 30min pretest (refer to the timeline in Fig 1a) which verified that there was not a significant ‘preference’ for either chamber (data presented in Results); however, individual rats tended to spend more time in one chamber compared to the other. Thus, for conditioning, rats were administered Meth (1mg/kg) in the chamber in which they spent the least amount of time during the pre-test (i.e., individual rats spent an average of 65% and 28% of time in each chamber). The dose of 1mg/kg has been used to reliably produce Meth-induced CPP in a variety of conditioning paradigms (DeMarco et al., 2009; Herrold et al., 2009; Voigt et al., 2011; Xu et al., 2006; Yang et al., 2008). For the current study, conditioning occurred over six days. We previously determined that the order of the Meth vs. saline pairings does not influence CPP outcomes (Voigt et al., 2011); thus, Meth was paired with a unique context (i.e., chamber) on days 1, 3, and 5 and a saline (1ml/kg) was paired with a different context on days 2, 4, and 6. Pairing occurred immediately after each injection and lasted for 45min. In order to confirm that the preference developed, a drug-free CPP test was conducted on day 9 (termed CPP Test; Fig 1a). For this test, rats were placed into the center chamber and the sliding doors were immediately removed allowing free access to the entire CPP box. The test session lasted 30min and time spent in each chamber was determined. Rats that did not increase time spent in the Meth-paired chamber on the CPP Test compared to the same chamber during the pre-test by at least 10% (180s) were excluded from the studies. Culling rats based on the “robustness” of the preference has been used previously (Brenhouse and Andersen, 2008; Guo et al., 2008; Paolone et al., 2009) to assure that only those rats who clearly acquired the task were subsequently examined for the ability of various interventions to modify the extinction of previously established preference. Thus, this approach was used to determine the potential of the GABAB agonist baclofen to facilitate the extinction of previously established, and robust, CPP. Rats were assigned to either the baclofen or baclofen vehicle treatment group such that the magnitude of the preference during the initial CPP Test was approximately equal between the two groups. Each extinction cycle consisted of six consecutive once-daily forced extinction sessions (45min) followed by a CPP test three days later (referred to as the “Extinction Test”, Ext Test; Fig 1a). The once-daily forced extinction sessions consisted of pairing a saline (1ml/kg) treatment with each chamber for 45min (termed “Pre-Training Injection”; Fig 1a), alternating between the previously Meth- or saline-paired chamber (as done during conditioning), a commonly used approach for forced extinction training (Heinrichs et al., 2010; Mueller and Stewart, 2000; Schroeder and Packard, 2004). Immediately after each daily extinction session, baclofen vehicle (1ml/kg) or baclofen (2mg/kg) was administered (termed “Post-Training Injection”; Fig 1a) and rats were returned to the home cage. Four extinction cycles (each including six once-daily forced extinction sessions and the Ext test) were conducted (Fig 1a).
Fig 1.
Extinction of Meth-induced CPP was facilitated by baclofen. a. Illustration of treatment protocol. A drug-free pre-test was conducted and rats were assigned to receive Meth in the chamber in which the least amount of time was spent during the pre-test. Conditioning occurred on days 1–6. A drug-free CPP test was conducted on day 9 to verify the development of CPP. Daily extinction sessions consisted of a saline injection prior to being placed into either the previously saline- or Meth-paired chamber. Immediately following each extinction training session, baclofen vehicle or baclofen was administered, and the rats were returned to the home cage. Each six-day extinction cycle was followed three-days later by a drug-free test for preference (denoted Ext Test 1–4, on protocol days 18, 30, 46 and 55). M, methamphetamine (1mg/kg); S, saline (1mg/kg); Veh, vehicle (0.9% saline, 1ml/kg); Bac, baclofen (2mg/kg); ø, no drug. b. Meth-induced CPP (expressed on protocol day 9) was not altered by extinction sessions accompanied by vehicle administration; significant CPP was expressed on the CPP Test and through Ext Test 4 (Vehicle, n=10). c. The initial CPP Test validated that rats expressed a preference prior to initiating extinction training. The preference evident during the CPP Test was not present after the first extinction cycle with baclofen (Baclofen, n=11). Post-hoc Newman-Keuls was used to determine between chamber differences ** p<0.01. Solid line, time spent in the Meth-paired chamber; dashed line, time spent in the saline-paired chamber; grey line, time spent in the center chamber.
Statistical Analysis
A two-way repeated measures ANOVA was employed using the within group factor of chamber and the repeated measure of test. Post-hoc Newman-Keuls was used to identify between chamber differences; and significant preference was achieved when time spent in the Meth-paired chamber was significantly greater than time spent in the saline-paired chamber for any CPP/Ext Test. This approach has been used by Stewart and colleagues for similar evaluations (Botreau et al., 2006; Paolone et al., 2009). All data are presented as mean ± standard error of the mean (SEM). Statistical outliers were determined as those rats that spent greater than two standard deviations above or below the mean time spent in any chamber during any of the CPP or Ext tests (NIST/SEMATECH, 2011; Voigt et al., 2011).
Results
Results of the 30min pre-test demonstrated that, before conditioning, as a group rats spent approximately equal amount of time in each chamber (48% and 45% in each chamber; paired t-test, p=0.652; n=40). After conditioning (CPP Test, day 9), rats expressed a significant preference for the Meth-paired chamber compared to the saline-paired chamber (time spent in the Meth-paired chamber, 958±41s vs. time spent saline chamber 672±43s; paired t-test, p=0.0003; center chamber time was 170±8sec; n=40). Of the 40 total rats, 28 met the learning criteria detailed in the methods, and these rats with a strong preference for the Meth-chamber (i.e., robust learners) were subsequently assigned to receive either baclofen or baclofen vehicle in order to assess the ability of baclofen to facilitate the extinction of Meth-induced CPP. Rats that were outliers for any of the tests (CPP Test-Ext Test 4) were excluded for all tests (n=7); thus, a total of 10 and 11 rats were included in the baclofen vehicle and baclofen groups, respectively.
Significant preference was expressed on CPP Test and it persisted through four extinction cycles when vehicle was administered in conjunction with extinction training (n=10, Fig 1b). A two-way repeated measures ANOVA revealed a significant effect of chamber (F(1,18)=15.966, p=0.001) but no effect of Test (F(4,72)=0.022, p=0.999) and no chamber x test interaction (F(4,72)=1.011, p=0.407). A post-hoc Newman-Keuls test revealed significant place preference (significantly greater amount of time spent in the Meth-paired than the saline-paired chamber) for the CPP test and all subsequent extinction tests (Fig 1b; p<0.05 or p<0.01). In contrast, baclofen administered immediately after each daily extinction session (cycles 1–4), nullified previously acquired preference for the Meth-paired chamber (n=11; Fig 1c, Ext Test 1–4). A two-way repeated measures ANOVA of data obtained during the CPP Test through Extinction Test 4 revealed a significant chamber x test interaction (F(4,80)=2.799, p=0.031) with no effect of chamber (F(1,20)=2.950, p=0.101) or test (F(4,80)=0.057, p=0.994). A post-hoc Newman-Keuls test revealed significant preference for the Meth-paired chamber compared to the saline-paired chamber only during the CPP Test (p<0.01). Although time spent in the center chamber was not included in the statistical analyses reported above, we verified that this did not significantly change for either treatment group during any of the preference tests (CPP Test through Ext 4) (one-way ANOVA; vehicle treated rats F(4,45)=1.300, p=0.284, baclofen treated rats F(4,50)=1.185, p=0.329).
Motor activity was monitored during each of the preference tests (CPP and Ext Tests). Examination of these data revealed no significant between group differences during any test for horizontal or vertical activity (movements in the horizontal and vertical plane, respectively, as measured by photobeam breaks) (Student’s t-test, p>0.05; Table 1).
Table 1.
Motor activity recorded during testing revealed no significant differences between vehicle- (n=10) and baclofen- (n=11) treated rats.
| CPP Test | Ext Test 1 | Ext Test 2 | Ext Test 3 | Ext Test 4 | |
|---|---|---|---|---|---|
| Horizontal Activity | |||||
| Vehicle | 4242±226 | 3306±115 | 3098±198 | 3296±356 | 2876±152 |
| Baclofen | 4003±259 | 3291±252 | 3239±263 | 2899±201 | 2773±126 |
| Vertical Activity | |||||
| Vehicle | 746±51 | 528±44 | 519±49 | 446±33 | 406±32 |
| Baclofen | 757±82 | 569±62 | 527±60 | 380±32 | 398±26 |
Discussion
The Meth-induced preference observed in the current study was highly resistant to extinction; repeated re-exposure to the chambers over four extinction cycles did not extinguish the Meth-induced preference in vehicle treated rats. We contend that this likely reflects the robust rewarding effects of Meth, and the strength of the learned association between Meth-reward and the Meth-paired context. As Meth conditioning was conducted in the initially “non-preferred” chamber, the idea that this preference may also reflect a Meth-induced reduction in anxiety cannot be definitively ruled out. However, when treatment is sufficiently robust to alter anxiety-like behaviors, stimulants are anxiogenic (Cancela et al., 2001; Olausson et al., 2000) and we have determined in a separate study, that the Meth dose and treatment paradigm employed here does not alter anxiety as measured by the elevated plus maze (unpublished results). Thus, while we postulate that CPP generated in the current paradigm largely reflects transference of Meth-reward salience to the Meth-paired context, future studies that more fully characterize the contribution of anxiety will aid in this interpretation.
Baclofen administration immediately after chamber re-exposure inhibited the preference for the Meth-paired chamber after only one extinction cycle (Extinction Test 1). This inhibitory effect was produced even in those rats which demonstrated robust preference during the initial CPP Test. Motor activity was not significantly altered by repeated baclofen treatment; thus, the place preference results do not reflect a change in the capacity of rats to successfully execute the task. These findings indicate that augmenting GABAB receptor signaling facilitates the extinction of Meth-induced CPP.
These results corroborate the recent publication by Heinrichs and colleagues which demonstrates that baclofen facilitates the extinction of morphine-induced CPP in mice (Heinrichs et al., 2010). This suggests that baclofen is working via mechanisms that are mutually engaged during the extinction of morphine- and Meth-induced CPP. During extinction training, rats were re-exposed to contextual cues (i.e., the conditioning chambers) that were previously associated with Meth- as well as those paired with saline. Re-exposure to cues associated with Meth (Chiang et al., 2009; Rhodes et al., 2005) and morphine (Guo et al., 2008; Schroeder et al., 2000; Schroeder et al., 2003; Schroeder and Kelley, 2002) increases neuronal activity which may reflect hyper-responsive glutamatergic (Bell et al., 2000; Hotsenpiller et al., 2001) and/or dopaminergic (Lin et al., 2007) neurotransmission. GABAB receptors blunt glutamatergic (Lei and McBain, 2003; Porter and Nieves, 2004; Yamada et al., 1999) and dopaminergic (Santiago et al., 1993a; Santiago et al., 1993b; Smolders et al., 1995; Westerink et al., 1996) neurotransmission. Thus, GABAB receptor activation is a credible mechanism by which baclofen may facilitate the extinction of Meth-induced CPP (current study) and for the extinction of opiate-induced CPP (Heinrichs et al., 2010).
Extinction of a memory is believed to involve new learning; the new memory becomes stronger than the previously established memory resulting in a different conditioned response (Quirk and Mueller, 2008). Forming new memories requires memory consolidation, a process which can be facilitated by augmenting glutamatergic neurotransmission (Ungerer et al., 1998); the extinction of cocaine-induced CPP is enhanced by administering a glutamate receptor (mGluR5 receptor) positive allosteric modulator prior to extinction training (Gass et al., 2009) or a NMDA receptor agonist immediately after extinction training (Botreau et al., 2006). In the current study, baclofen was administered immediately after each daily extinction session (i.e., after re-exposure to the saline- or Meth-paired chamber), thus the effects of baclofen may have enhanced the consolidation of the extinction memory. Although not typically thought of as having beneficial effects on memory, the GABAB receptor agonist baclofen improves passive avoidance learning when administered immediately after training (Georgiev et al., 1988; Saha et al., 1993) and recognition memory deficits induced by repeated Meth administration when administered prior to training (Arai et al., 2009). Counter to this interpretation are reports that GABAB receptor activation blunts glutamatergic (Lei and McBain, 2003; Porter and Nieves, 2004; Yamada et al., 1999) and dopaminergic (Santiago et al., 1993a; Santiago et al., 1993b; Smolders et al., 1995; Westerink et al., 1996) neurotransmission. This would serve to inhibit rather than facilitate memory consolidation. Antagonism of NMDA glutamate receptors impairs the extinction of fear conditioning (Liu et al., 2009) as well as the extinction of cocaine self-administration (Feltenstein and See, 2007) and dopamine receptor antagonists impair extinction of fear conditioning (Hikind and Maroun, 2008; Holtzman-Assif et al., 2010). Therefore, while baclofen has been reported to have positive influences on memory consolidation, the negative regulation of glutamate and dopamine does not account for the extinction facilitating effects observed for this agonist in the current study.
Extinction of a previously established memory can occur when a memory is recalled (which makes the memory labile and sensitive to disruption) but not successfully reconsolidated (Bevilaqua et al., 2008; McGaugh, 2000; Taylor et al., 2009; Tronson and Taylor, 2007). Inhibiting reconsolidation as a practical means to reduce cue-induced cocaine seeking has been successfully demonstrated (Lee et al., 2005; Lee et al., 2006). GABAB receptor-induced decrease in glutamatergic neurotransmission (Harte and O'Connor, 2005; Lei and McBain, 2003; Porter and Nieves, 2004; Yamada et al., 1999) likely blunts memory reconsolidation, as administration of an NMDA receptor antagonist inhibits reconsolidation of cocaine-induced CPP memory (Brown et al., 2008; Itzhak, 2008). No effect on memory extinction has been observed with dopamine antagonism (Yim et al., 2009) however, such antagonism has been reported to facilitate the extinction of conditioned fear (an effect that may be attributed to reconsolidation) (Ponnusamy et al., 2005). In the current protocol, we cannot determine if baclofen is enhancing extinction learning or inhibiting memory re-consolidation, an issue made more difficult by the fact that these processes have many overlapping mechanisms (Alberini, 2005), these avenues need further exploration to determine how baclofen is facilitating the extinction of Meth-induced CPP.
In summary, we have found that baclofen administered in conjunction with extinction training resulted in rapid and complete extinction of Meth-induced CPP. This exciting study provides insight into the role of the GABAB receptor in memory processes engaged after re-exposure to salient drug-associated contextual cues and may be of value as an addiction therapy for abstinent, Meth-addicted individuals.
Acknowledgments
This work was supported by USPHSGs DA015760 to TCN, DA021475 to RMV & TCN, and DA023306 to AAH and TCN.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
Reference List
- 1.Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Arai S, Takuma K, Mizoguchi H, Ibi D, Nagai T, Kamei H, Kim HC, Yamada K. GABAB receptor agonist baclofen improves methamphetamine-induced cognitive deficit in mice. Eur J Pharmacol. 2009;602:101–104. doi: 10.1016/j.ejphar.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 3.Bartoletti M, Gubellini C, Ricci F, Gaiardi M. The GABAB agonist baclofen blocks the expression of sensitisation to the locomotor stimulant effect of amphetamine. Behav Pharmacol. 2004;15:397–401. doi: 10.1097/00008877-200409000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Bell K, Duffy P, Kalivas PW. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology. 2000;23:335–344. doi: 10.1016/S0893-133X(00)00100-7. [DOI] [PubMed] [Google Scholar]
- 5.Bevilaqua LR, Medina JH, Izquierdo I, Cammarota M. Reconsolidation and the fate of consolidated memories. Neurotox Res. 2008;14:353–358. doi: 10.1007/BF03033859. [DOI] [PubMed] [Google Scholar]
- 6.Botreau F, Paolone G, Stewart J. d-Cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res. 2006;172:173–178. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Brebner K, Ahn S, Phillips AG. Attenuation of d-amphetamine self-administration by baclofen in the rat: behavioral and neurochemical correlates. Psychopharmacology (Berl) 2005;177:409–417. doi: 10.1007/s00213-004-1968-6. [DOI] [PubMed] [Google Scholar]
- 8.Brebner K, Childress AR, Roberts DC. A potential role for GABA(B) agonists in the treatment of psychostimulant addiction. Alcohol Alcohol. 2002;37:478–484. doi: 10.1093/alcalc/37.5.478. [DOI] [PubMed] [Google Scholar]
- 9.Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122:460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown EE, Robertson GS, Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci. 1992;12:4112. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown TE, Lee BR, Sorg BA. The NMDA antagonist MK-801 disrupts reconsolidation of a cocaine-associated memory for conditioned place preference but not for self-administration in rats. Learn Mem. 2008;15:857–865. doi: 10.1101/lm.1152808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res. 2008;42:503–506. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Campbell UC, Lac ST, Carroll ME. Effects of baclofen on maintenance and reinstatement of intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1999;143:209–214. doi: 10.1007/s002130050937. [DOI] [PubMed] [Google Scholar]
- 14.Cancela LM, Basso AM, Martijena ID, Capriles NR, Molina VA. A dopaminergic mechanism is involved in the 'anxiogenic-like' response induced by chronic amphetamine treatment: a behavioral and neurochemical study. Brain Res. 2001;909:179–186. doi: 10.1016/s0006-8993(01)02680-4. [DOI] [PubMed] [Google Scholar]
- 15.Chiang CY, Cherng CG, Lai YT, Fan HY, Chuang JY, Kao GS, Chang WT, Yu L. Medial prefrontal cortex and nucleus accumbens core are involved in retrieval of the methamphetamine-associated memory. Behav Brain Res. 2009;197:24–30. doi: 10.1016/j.bbr.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 16.Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, Jens W, Suh J, Listerud J, Marquez K, Franklin T, Langleben D, Detre J, O'Brien CP. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS ONE. 2008;3:e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Childs E, deWit H. Amphetamine-induced place preference in humans. Biol Psychiatry. 2009;65:900–904. doi: 10.1016/j.biopsych.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci U S A. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- 21.Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- 22.DeMarco A, Dalal RM, Pai J, Aquilina SD, Mullapudi U, Hammel C, Kothari SK, Kahanda M, Liebling CN, Patel V, Schiffer WK, Brodie JD, Dewey SL. Racemic gamma vinyl-GABA (R,S-GVG) blocks methamphetamine-triggered reinstatement of conditioned place preference. Synapse. 2009;63:87–94. doi: 10.1002/syn.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology. 2004;47(Suppl 1):202–213. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Ehrman RN, Robbins SJ, Childress AR, O'Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- 25.Feltenstein MW, See RE. NMDA receptor blockade in the basolateral amygdala disrupts consolidation of stimulus-reward memory and extinction learning during reinstatement of cocaine-seeking in an animal model of relapse. Neurobiol Learn Mem. 2007;88:435–444. doi: 10.1016/j.nlm.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filip M, Frankowska M, Przegalinski E. Effects of GABA(B) receptor antagonist, agonists and allosteric positive modulator on the cocaine-induced self-administration and drug discrimination. Eur J Pharmacol. 2007;574:148–157. doi: 10.1016/j.ejphar.2007.07.048. [DOI] [PubMed] [Google Scholar]
- 27.Franklin TR, Druhan JP. Expression of Fos-related antigens in the nucleus accumbens and associated regions following exposure to a cocaine-paired environment. Eur J Neurosci. 2000;12:2097–2106. doi: 10.1046/j.1460-9568.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- 28.Frankowska M, Nowak E, Filip M. Effects of GABAB receptor agonists on cocaine hyperlocomotor and sensitizing effects in rats. Pharmacol Rep. 2009;61:1042–1049. doi: 10.1016/s1734-1140(09)70166-5. [DOI] [PubMed] [Google Scholar]
- 29.Gass JT, Osborne MP, Watson NL, Brown JL, Olive MF. mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology. 2009;34:820–833. doi: 10.1038/npp.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Georgiev VP, Yonkov DI, Kambourova TS. Interactions between angiotensin II and baclofen in shuttle-box and passive avoidance performance. Neuropeptides. 1988;12:155–158. doi: 10.1016/0143-4179(88)90047-9. [DOI] [PubMed] [Google Scholar]
- 31.Guo N, Garcia MM, Harlan RE. A morphine-paired environment alters c-Fos expression in the forebrain of rats displaying conditioned place preference or aversion. Behav Neurosci. 2008;122:1078–1086. doi: 10.1037/a0012595. [DOI] [PubMed] [Google Scholar]
- 32.Harte M, O'Connor WT. Evidence for a selective prefrontal cortical GABA(B) receptor-mediated inhibition of glutamate release in the ventral tegmental area: a dual probe microdialysis study in the awake rat. Neuroscience. 2005;130:215–222. doi: 10.1016/j.neuroscience.2004.08.045. [DOI] [PubMed] [Google Scholar]
- 33.Hartz DT, Frederick-Osborne SL, Galloway GP. Craving predicts use during treatment for methamphetamine dependence: a prospective, repeated-measures, within-subject analysis. Drug Alcohol Depend. 2001;63:269–276. doi: 10.1016/s0376-8716(00)00217-9. [DOI] [PubMed] [Google Scholar]
- 34.Heinrichs SC, Leite-Morris KA, Carey RJ, Kaplan GB. Baclofen enhances extinction of opiate conditioned place preference. Behav Brain Res. 2010;207:353–359. doi: 10.1016/j.bbr.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Herrold AA, Shen F, Graham MP, Harper LK, Specio SE, Tedford CE, Napier TC. Mirtazapine treatment after conditioning with methamphetamine alters subsequent expression of place preference. Drug Alcohol Depend. 2009;99:231–239. doi: 10.1016/j.drugalcdep.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Hikind N, Maroun M. Microinfusion of the D1 receptor antagonist, SCH23390 into the IL but not the BLA impairs consolidation of extinction of auditory fear conditioning. Neurobiol Learn Mem. 2008;90:217–222. doi: 10.1016/j.nlm.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Holtzman-Assif O, Laurent V, Westbrook RF. Blockade of dopamine activity in the nucleus accumbens impairs learning extinction of conditioned fear. Learn Mem. 2010;17:71–75. doi: 10.1101/lm.1668310. [DOI] [PubMed] [Google Scholar]
- 38.Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci. 2001;14:1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- 39.Hotsenpiller G, Wolf ME. Baclofen attenuates conditioned locomotion to cues associated with cocaine administration and stabilizes extracellular glutamate levels in rat nucleus accumbens. Neuroscience. 2003;118:123–134. doi: 10.1016/s0306-4522(02)00951-x. [DOI] [PubMed] [Google Scholar]
- 40.Itzhak Y. Role of the NMDA receptor and nitric oxide in memory reconsolidation of cocaine-induced conditioned place preference in mice. Ann N Y Acad Sci. 2008;1139:350–357. doi: 10.1196/annals.1432.051. [DOI] [PubMed] [Google Scholar]
- 41.Lee JL, Di CP, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lei S, McBain CJ. GABA B receptor modulation of excitatory and inhibitory synaptic transmission onto rat CA3 hippocampal interneurons. J Physiol. 2003;546:439–453. doi: 10.1113/jphysiol.2002.034017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lhuillier L, Mombereau C, Cryan JF, Kaupmann K. GABA(B) receptor-positive modulation decreases selective molecular and behavioral effects of cocaine. Neuropsychopharmacology. 2007;32:388–398. doi: 10.1038/sj.npp.1301102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li SM, Yin LL, Ren YH, Pan LS, Zheng JW. GABA(B) receptor agonist baclofen attenuates the development and expression of d-methamphetamine-induced place preference in rats. Life Sci. 2001;70:349–356. doi: 10.1016/s0024-3205(01)01397-2. [DOI] [PubMed] [Google Scholar]
- 46.Lin SK, Pan WH, Yeh PH. Prefrontal dopamine efflux during exposure to drug-associated contextual cues in rats with prior repeated methamphetamine. Brain Res Bull. 2007;71:365–371. doi: 10.1016/j.brainresbull.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Liu JL, Li M, Dang XR, Wang ZH, Rao ZR, Wu SX, Li YQ, Wang W. A NMDA receptor antagonist, MK-801 impairs consolidating extinction of auditory conditioned fear responses in a Pavlovian model. PLoS ONE. 2009;4:e7548. doi: 10.1371/journal.pone.0007548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCleery JM, Harvey AG. Integration of psychological and biological approaches to trauma memory: implications for pharmacological prevention of PTSD. J Trauma Stress. 2004;17:485–496. doi: 10.1007/s10960-004-5797-5. [DOI] [PubMed] [Google Scholar]
- 49.McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 50.Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- 51.NIST/SEMATECH. e-Handbook of Statistical Methods. 2011 http://itl.nist.gov/div898/handbook.
- 52.O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 53.O'Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- 54.O'Brien CP, Childress AR, McLellan T, Ehrman R. Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addict Behav. 1990;15:355–365. doi: 10.1016/0306-4603(90)90045-y. [DOI] [PubMed] [Google Scholar]
- 55.Olausson P, Engel JA, Soderpalm B. Effects of serotonergic manipulations on the behavioral sensitization and disinhibition associated with repeated amphetamine treatment. Pharmacol Biochem Behav. 2000;66:211–220. doi: 10.1016/s0091-3057(00)00228-8. [DOI] [PubMed] [Google Scholar]
- 56.Paolone G, Botreau F, Stewart J. The facilitative effects of D-cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology (Berl) 2009;202:403–409. doi: 10.1007/s00213-008-1280-y. [DOI] [PubMed] [Google Scholar]
- 57.Ponnusamy R, Nissim HA, Barad M. Systemic blockade of D2-like dopamine receptors facilitates extinction of conditioned fear in mice. Learn Mem. 2005;12:399–406. doi: 10.1101/lm.96605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Porter JT, Nieves D. Presynaptic GABAB receptors modulate thalamic excitation of inhibitory and excitatory neurons in the mouse barrel cortex. J Neurophysiol. 2004;92:2762–2770. doi: 10.1152/jn.00196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ranaldi R, Poeggel K. Baclofen decreases methamphetamine self-administration in rats. Neuroreport. 2002;13:1107–1110. doi: 10.1097/00001756-200207020-00007. [DOI] [PubMed] [Google Scholar]
- 61.Rhodes JS, Ryabinin AE, Crabbe JC. Patterns of brain activation associated with contextual conditioning to methamphetamine in mice. Behav Neurosci. 2005;119:759–771. doi: 10.1037/0735-7044.119.3.759. [DOI] [PubMed] [Google Scholar]
- 62.Roberts DC, Andrews MM. Baclofen suppression of cocaine self-administration: Demonstration using a discrete trials procedure. Psychopharmacology. 1997;131:271–277. doi: 10.1007/s002130050293. [DOI] [PubMed] [Google Scholar]
- 63.Rose ME, Grant JE. Pharmacotherapy for methamphetamine dependence: a review of the pathophysiology of methamphetamine addiction and the theoretical basis and efficacy of pharmacotherapeutic interventions. Ann Clin Psychiatry. 2008;20:145–155. doi: 10.1080/10401230802177656. [DOI] [PubMed] [Google Scholar]
- 64.Saha N, Chugh Y, Sankaranaryanan A, Sharma PL. Effects of post-training administration of (-)-baclofen and chlordiazepoxide on memory retention in ICRC Swiss mice: interactions with GABAA and GABAB receptor antagonists. Pharmacol Toxicol. 1993;72:159–162. doi: 10.1111/j.1600-0773.1993.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 65.Santiago M, Machado A, Cano J. In vivo release of dopamine from rat striatum, substantia nigra and prefrontal cortex: differential modulation by baclofen. Br J Pharmacol. 1993a;109:814–818. doi: 10.1111/j.1476-5381.1993.tb13647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Santiago M, Machado A, Cano J. Regulation of the prefrontal cortical dopamine release by GABAA and GABAB receptor agonists and antagonists. Brain Res. 1993b;630:28–31. doi: 10.1016/0006-8993(93)90638-4. [DOI] [PubMed] [Google Scholar]
- 67.Schroeder BE, Holahan MR, Landry CF, Kelley AE. Morphine-associated environmental cues elicit conditioned gene expression. Synapse. 2000;37:146–158. doi: 10.1002/1098-2396(200008)37:2<146::AID-SYN8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 68.Schroeder BE, Kelley AE. Conditioned Fos expression following morphine-paired contextual cue exposure is environment specific. Behav Neurosci. 2002;116:727–732. doi: 10.1037//0735-7044.116.4.727. [DOI] [PubMed] [Google Scholar]
- 69.Schroeder BE, Schiltz CA, Kelley AE. Neural activation profile elicited by cues associated with the anxiogenic drug yohimbine differs from that observed for reward-paired cues. Neuropsychopharmacology. 2003;28:14–21. doi: 10.1038/sj.npp.1300007. [DOI] [PubMed] [Google Scholar]
- 70.Schroeder JP, Packard MG. Facilitation of memory for extinction of drug-induced conditioned reward: role of amygdala and acetylcholine. Learn Mem. 2004;11:641–647. doi: 10.1101/lm.78504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith MA, Yancey DL, Morgan D, Liu Y, Froestl W, Roberts DC. Effects of positive allosteric modulators of the GABAB receptor on cocaine self-administration in rats. Psychopharmacology (Berl) 2004;173:105–111. doi: 10.1007/s00213-003-1706-5. [DOI] [PubMed] [Google Scholar]
- 72.Smolders I, De Klippel N, Sarre S, Ebinger G, Michotte Y. Tonic GABA-ergic modulation of striatal dopamine release studied by in vivo microdialysis in the freely moving rat. Eur J Pharmacol. 1995;284:83–91. doi: 10.1016/0014-2999(95)00369-v. [DOI] [PubMed] [Google Scholar]
- 73.Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;56(Suppl 1):186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- 75.Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 76.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 77.Ungerer A, Mathis C, Melan C. Are glutamate receptors specifically implicated in some forms of memory processes? Exp Brain Res. 1998;123:45–51. doi: 10.1007/s002210050543. [DOI] [PubMed] [Google Scholar]
- 78.Voigt RM, Herrold AA, Riddle JL, Napier TC. Administration of GABA(B) receptor positive allosteric modulators inhibit the expression of previously established methamphetamine-induced conditioned place preference. Behav Brain Res. 2011;216:419–423. doi: 10.1016/j.bbr.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weerts EM, Froestl W, Kaminski BJ, Griffiths RR. Attenuation of cocaine-seeking by GABA B receptor agonists baclofen and CGP44532 but not the GABA reuptake inhibitor tiagabine in baboons. Drug Alcohol Depend. 2007;89:206–213. doi: 10.1016/j.drugalcdep.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Westerink BHC, Kwint HF, DeVries JB. The pharmacology of mesolimbic dopamine neurons: A dual- probe microdialysis study in the ventral tegmental area and nucleus accumbens of the rat brain. J Neurosci. 1996;16:2605–2611. doi: 10.1523/JNEUROSCI.16-08-02605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xi ZX, Gardner EL. Hypothesis-driven medication discovery for the treatment of psychostimulant addiction. Curr Drug Abuse Rev. 2008;1:303–327. doi: 10.2174/1874473710801030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu DD, Mo ZX, Yung KK, Yang Y, Leung AW. Individual and combined effects of methamphetamine and ketamine on conditioned place preference and NR1 receptor phosphorylation in rats. Neurosignals. 2006;15:322–331. doi: 10.1159/000127492. [DOI] [PubMed] [Google Scholar]
- 83.Yamada J, Saitow F, Satake S, Kiyohara T, Konishi S. GABA(B) receptor-mediated presynaptic inhibition of glutamatergic and GABAergic transmission in the basolateral amygdala. Neuropharmacology. 1999;38:1743–1753. doi: 10.1016/s0028-3908(99)00126-4. [DOI] [PubMed] [Google Scholar]
- 84.Yang MH, Kim S, Jung MS, Shim JH, Ryu NK, Yook YJ, Jang CG, Bahk YY, Kim KW, Park JH. Proteomic analysis of methamphetamine-induced reinforcement processes within the mesolimbic dopamine system. Addict Biol. 2008;13:287–294. doi: 10.1111/j.1369-1600.2007.00090.x. [DOI] [PubMed] [Google Scholar]
- 85.Yim AJ, Andersen ML, Soeiro AC, Tufik S, Oliveira MG. Acute systemic blockade of D2 receptors does not accelerate the extinction of cocaine-associated place preference. Brain Res. 2009;1304:122–128. doi: 10.1016/j.brainres.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 86.Zombeck JA, Chen GT, Johnson ZV, Rosenberg DM, Craig AB, Rhodes JS. Neuroanatomical specificity of conditioned responses to cocaine versus food in mice. Physiol Behav. 2008;93:637–650. doi: 10.1016/j.physbeh.2007.11.004. [DOI] [PubMed] [Google Scholar]