Abstract
A key challenge in developing protein therapeutics or imaging agents that work against cytosolic targets is the intracellular delivery barrier. Here, we show that the pH-responsive, membrane-destabilizing polymer, poly (propylacrylic acid) (PPAA), can strongly enhance target cell killing through the intracellular delivery of a functional proapoptotic peptide. The Bak BH3 peptide induces apoptosis via antagonization of suppressor targets such as Bcl-2 and Bcl-xL. A genetically-engineered streptavidin that contains an N-terminal TAT peptide sequence was used to optimize the pinocytotic cell uptake of biotinylated BH3 peptide and end-biotinylated PPAA. Fluorescence microscopic analysis of DAPI-stained HELA cells was used to quantitate apoptosis. Approximately 30% of cells treated with TAT-SA:BH3 complexes revealed morphologically distinct nuclear condensation, a hallmark of apoptosis. The incorporation of biotinylated PPAA had the effect of markedly enhancing the killing effect of BH3 peptides by an additional 55% (p<0.001) to a total cell killing efficiency of 85%. Caspase-3 activity was up-regulated in a TAT-SA:BH3:PPAA dose-dependent manner. The induction of apoptosis with the TAT-SA:BH3:PPAA complex was abrogated with the L78A BH3 peptide, that had been previously shown to knock-out antagonization activity. The caspase and L78A peptide results demonstrate that the delivered BH3 is indeed working through the biologically relevant apoptosis signaling pathway. These studies establish the ability of PPAA to strongly enhance the intracellular delivery of a functional pro-apoptotic peptide. Together with the PPAA, the TAT-SA adaptor complex could prove useful as a carrier of peptide/protein cargo to cultured cells.
Keywords: protein transduction, pH-sensitive polymers, intracellular delivery, apoptosis
Introduction
The development of macromolecular drugs that function via cytosolic targets has been impeded by the intra-cellular delivery barrier. The use of the TAT protein transduction domain (PTD) has attracted considerable interest in the field of intracellular drug delivery for its ability to direct the uptake of proteins and other macromolecular cargo to which it is conjugated, complexed or fused [1–5]. Although the precise mechanism of internalization is still a matter of debate, recent studies have shown that the cationic peptide mediates a rapid electrostatic adsorption to the plasma membrane that results in subsequent cell uptake [6–8]. Despite the abundance of TAT-related research, however, relatively little work has addressed the potentially rate-limiting intracellular pharmacokinetic step of endosomal escape. Wadia et al. recently demonstrated that co-treatment of a reporter cell line with a TAT-Cre fusion protein and a fusogenic TAT-HA2 peptide resulted in enhanced TAT-Cre escape from macropinosomes and a marked dose-dependent increase in TAT-Cre-mediated recombination [9].
Additionally, we have previously shown that the pH-responsive polymer poly(propylacrylic acid) (PPAA) was capable of enhancing the cytosolic delivery of a TAT-streptavidin (TAT-SA) fusion protein [10]. The pH-responsive PPAA exhibits membrane-destabilizing activities at lowered pH values found in the endosome, and has been previously shown to enhance the cytosolic delivery of nucleic acids and an internalizing antibody [11–13]. In this study, we have investigated the ability of PPAA to enhance the functional intracellular delivery of a pro-apoptotic peptide using the TAT-SA fusion protein [10]. The Bak BH3 peptide was shown previously to exhibit a cell killing effect when fused to the Antennapedia PTD [14]. Bak BH3 peptides bind the death suppressing proteins Bcl-2 and Bcl-xL, and these binding events lead to apoptotic cell death through the release of the pro-apoptotic factor cytochrome c and the activation of caspase proteases [15]. Bcl-2 and Bcl-xL are expressed in outer mitochondrial membranes [16] and thus serve as targets for evaluating the endosomal release of peptides carried into cells via the TAT-SA carrier protein. The new streptavidin carrier construct is shown schematically in Figure 1. A strong apoptosis-inducing activity was seen only when the peptide was co-delivered in the same TAT-SA complex with the PPAA. These results show that PPAA can enhance the vesicular escape of functional peptides that act at cytosolic targets, opening the possibility that these polymers might serve as delivery agents for such peptides, proteins, or peptidomimetics.
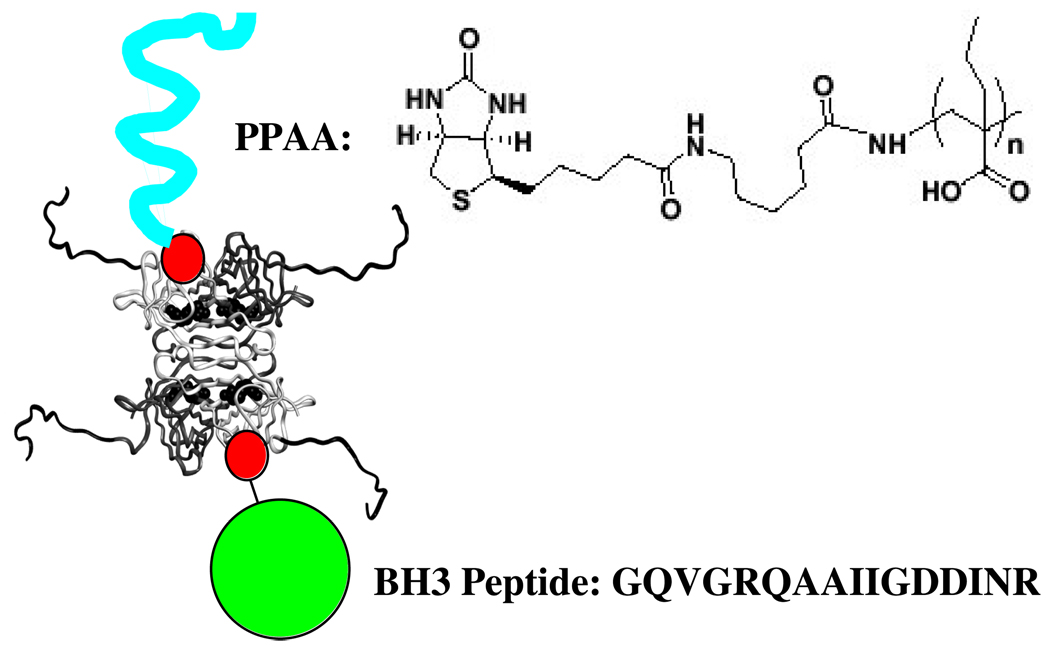
Figure 1.
Schematic representation of the TAT-SA complex with biotinylated PPAA and biotinylated BH3 peptide (biotin represented as red circle). The biotin linker and PPAA sequence are shown, together with the BH3 peptide sequence. The TAT peptide sequence has been modeled as a random coil extension onto the N-terminus of the four streptavidin subunits whose backbone folds are defined from x-ray crystallographic structures. They appear as the backbone extensions coming off the four subunits.
Experimental
Bak BH3 Peptide Synthesis
Solid phase peptide synthesis was performed with a PS3 peptide synthesizer (Rainin Instrument, Woburn, MA). The Bak BH3 (GQVGRQLAIIGDDINR) and mutant Bak BH3 (GQVGRQAAIIGDDINR) peptides, designated BH3 and BH3 (L78A) were synthesized using standard Fmoc chemistry. Both peptides were generated on Fmoc-Arg(Pbf)-Wang resin (Novabiochem, San Diego, CA) using a single 30 min coupling step for the first ten amino acids and dual 30 minute coupling steps for the final six residues. For a 100 µmole synthesis scale, all coupling steps were performed in the presence of 0.4 mmole HBTU coupling reagent. The N-terminus of both peptides was left unmodified and kept as a free amine. The cleavage of each peptide from the resin was achieved by incubation with a solution of TFA/tri-isopropylsilane(TIS)/ddH2O (95%/2.5%/2.5%) for 3 hours. After rotary evaporation of the solvent down to a volume of 2 ml, the crude peptide was precipitated with ice-cold tertbutyl methyl ether, collected by serial centrifugation at 3000rpm with 5 min steps and allowed to air dry for 24–48 hours. Dried peptide was reconstituted in water and acetonitrile and characterized by electrospray ionization mass spectrometry to verify peptide masses.
Protein Production
The TAT-SA gene construct was subcloned into a pET21a (Invitrogen) expression vector and transformed into BL21(DE3) cells (Novagen) in preparation for large-scale expression. The TAT-SA construct in pET21a was expressed and purified as previously described (10). Briefly, TAT-SA was expressed as insoluble inclusion bodies that were isolated and then dissolved in 6 M guanidine hydrochloride, followed by refolding via dilution into Tris buffer, pH 8.0. Refolded, functional protein was purified by affinity chromatography using a 2-imminobiotin agarose column (Sigma). Protein concentrations were determined using an extinction coefficient of 34,000 M−1cm−1/subunit.
Polymer Preparation
Amine-terminated poly(propylacrylic acid) (PPAA) was synthesized and end-biotinylated using Sulfosuccinimidyl-6-(biotinamido)-6-hexanoate (EZ-Link Sulfo-NHS-LC-Biotin, Pierce), as previously described [11–13]. Briefly, the conjugation reaction was performed at room temperature for 1 hour with a 5x molar excess of biotin to peptide followed by desalting on a PD-10 column (Amersham Biosciences, Piscataway, NJ) into PBS, pH 7.4. The quantity of biotinylated polymer was determined using the HABA assay.
Preparation of TAT-SA Complexes
Bak BH3 peptides were end-biotinylated with sulfosuccinimidyl-2-(biotinamido)ethyl-1,3-dithiopropionate (EZ-Link Sulfo-NHS-SS-Biotin, Pierce) using the primary amine of the N-terminus. The conjugation reaction was performed at room temperature for 1 hour with a 5x molar excess of biotin to peptide, followed by desalting on a PD-10 column (Amersham Biosciences, Piscataway, NJ) to remove the unreacted biotin and to exchange the peptide into PBS, pH 7.4. TAT-SA was complexed with biotinylated PPAA or BH3 peptide by incubation at equal molar ratios for 15 minutes at room temperature. Ternary TAT-SA:BH3:PPAA complexes were formed in serial steps by incubating TAT-SA first with 1 molar equivalent of BH3 peptide for 15 minutes, followed by a second incubation with 1 molar equivalent of b-PPAA for an additional15 minutes.
Cell Culture
HeLa cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 fetal bovine serum, 100 units/ml penicillin, 100 ug/ml streptomycin and 4 mM L-glutamine. Cells were incubated at 37° C in a 5% carbon dioxide atmosphere.
Fluorescence Microscopy
For apoptotic studies, HeLa cells were seeded in DMEM at a density of 105 cells/well in 8-well Lab-Tek II Chamber Slides (Nunc, Roskilde, Denmark). After 24 hours, cells were washed 3 times with PBS and treated with TAT-SA complexes at the indicated concentrations in serum-free DMEM for the indicated times. Apoptotic cells were determined by staining paraformaldehyde-fixed cells with DAPI nuclear stain (Sigma, St. Louis, MO) and examining fields for condensed nuclear staining and morphology. For subcellular localization studies, HeLa cells were seeded in DMEM at a density of 105 cells/well in 8-well Lab-Tek II Chamber Slides and 24 hours later incubated with 1 µM of Alexa 488-labeled TAT-SA or TAT-SA:PPAA complexes in serum-free DMEM for 4 hours. HeLa cells were washed three times, fixed with 2% paraformaldehyde, stained with DAPI and mounted with a coverslip for imaging using a Leica TCS NT/SP on a DMIRBE inverted microscope. Images were acquired using a 20× inverted objective and the appropriate filter set for Alexa-488.
Caspase-3 Activity Assay
HeLa cells were seeded in DMEM at a density of 5 × 105 cells/well in 12-well tissues culture plates. After 24 hours, cells were washed 3 times with PBS and treated with TAT-SA complexes (1–10 µM) in serum-free DMEM. After 16 hours, cells were washed 2 times, harvested with trypsin and washed an additional 2 times before resuspension in 75 µl of 1X cell lysis buffer (EnzChek Caspase-3 Assay Kit #2, Molecular Probes, Eugene, OR). Cell lysates were incubated on ice for 30 minutes with intermittent shaking and then centrifuged at 5000rpm for 5 minutes at 4° C in a microcentrifuge. After centrifugation, 50 µl of the supernatant from each sample was transferred to individual wells of a 96-well tissue culture plate. Immediately, 50 µl of 2X substrate working solution (5 mM Z-DEVD-R110) was added to each sample. In order to determine background fluorescence associated with the substrate, 50 µl of the 1X cell lysis buffer was used as a no-enzyme control. The plate was covered and incubated at room temperature for 1 hour before measuring the fluorescence emission at 520 nm.
Results
Preparation of TAT-SA, BH3 Peptides, and Biotinylated PPAA
The TAT-SA protein was produced in E. coli as previously described (10). The mass of the recombinant TAT-SA was found to be 15001 Da per monomer by MALDI mass spectrometry, in excellent agreement with the calculated theoretical mass. For the synthesized peptides, experimental peptide masses were 1728 Da for Bak BH3 and 1686 Da for Bak BH3 (L78A), in agreement with their calculated mass. Bak BH3 peptides were end-biotinylated with sulfosuccinimidyl-2-(biotinamido)ethyl-1,3-dithiopropionate (EZ-Link Sulfo-NHS-SS-Biotin, Pierce). The weight-averaged molecular weight of PPAA before biotinylation was 11 kDa as determined by organic GPC analysis. The overall streptavidin complex is represented schematically in Figure 1.
TAT-SA-Mediated Delivery of Bak BH3 Peptides
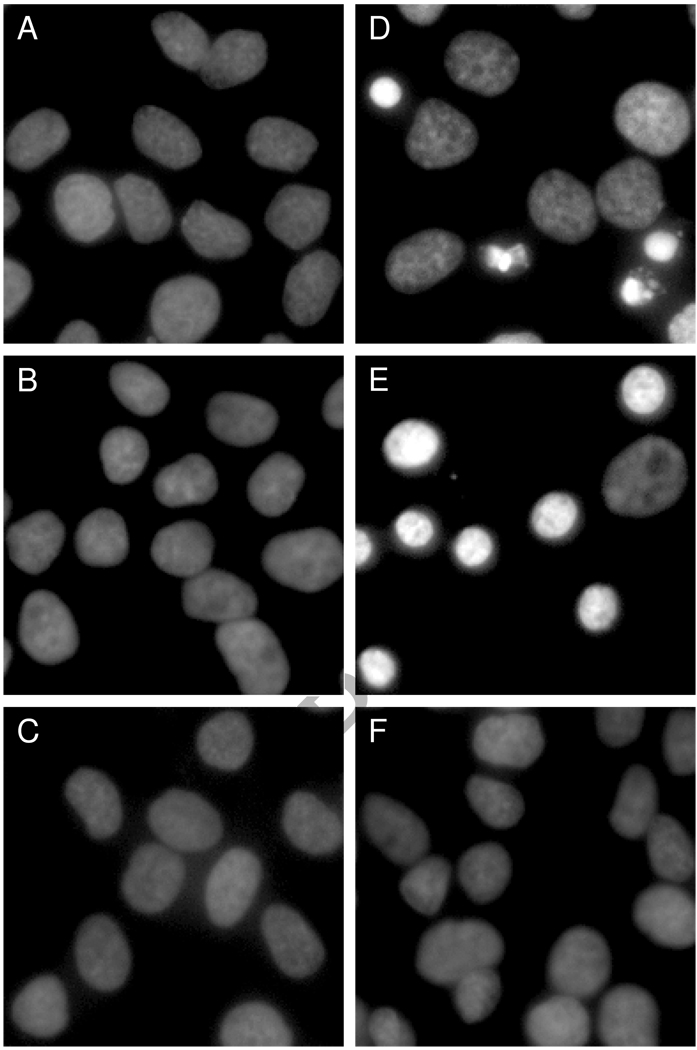
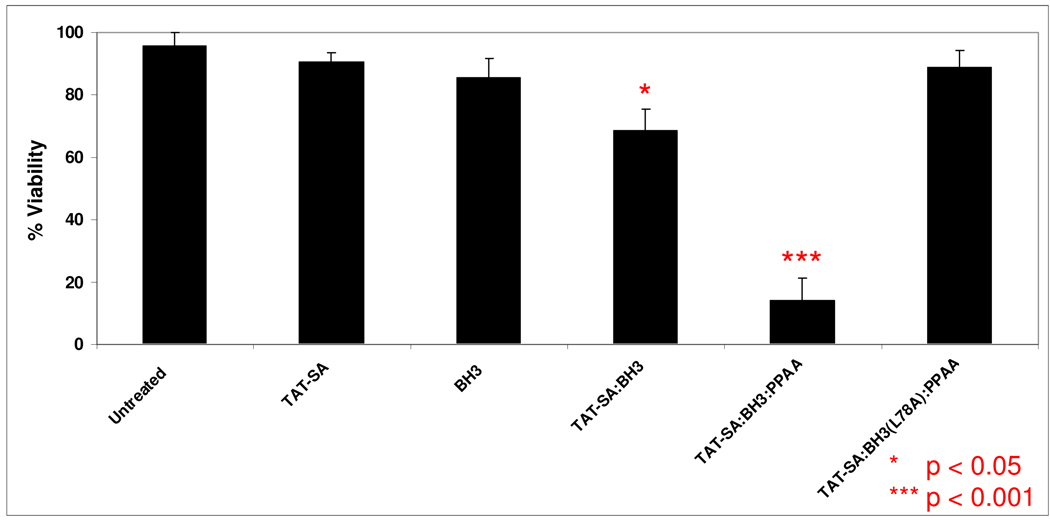
Bak BH3 peptides have previously been shown to induce substantial levels of apoptosis via their antagonization of cytosolic death-suppressing proteins, such as Bcl-2 and Bcl-xL [14, 16]. The degree of HeLa cell apoptosis was determined by staining fixed cells with DAPI nuclear stain, followed by fluorescence microscopic analysis of nuclear morphology (Figure 2). The TAT-SA:BH3 complexes displayed an approximate 30% increase in apoptosis (p < 0.05) after 24 h as compared to untreated cells (Figure 3). The number of cells displaying condensed nuclei was further increased to ~85% (p < 0.001) when the endosomal-releasing polymer PPAA was incorporated into TAT-SA:BH3 complexes, but not when cells were treated with PPAA alone. Treatment of cells with BH3 alone or TAT-SA alone also had no apparent effect on viability. When the mutant BH3(L78A) peptide was substituted for the native BH3 peptide, which is deficient in binding to Bcl-2-like proteins, no significant level of apoptosis was observed.
Figure 2.
TAT-SA-mediated delivery of BH3 peptides induces apoptotic nuclear morphology in HeLa cells. Cells were treated with the indicated reagents at 10 µM for 24 h in serum-free DMEM before fluorescence microscopic analysis of DAPI-stained nuclei. Samples were A) untreated, B) TAT-SA, C) BH3, D) TAT-SA:BH3 1:1, E) TAT-SA: BH3:PPAA 1:1:1 and F) TAT-SA:BH3-(L78A):PPAA 1:1:1. The mutant BH3-(L78A) peptide, which is deficient in binding to Bcl-2-like proteins, was used as a negative control.
Figure 3.
Quantitation of HeLa cell viability in response to TAT-SA-mediated delivery of BH3 peptides. Cells were treated with reagents at 10 µM for 24 h before fluorescence microscopy of DAPI-stained nuclei. The cell viability was defined as live or dead based on the presence or absence of condensed nuclear morphology visualized by the DAPI staining. Treatment of HeLa cells with TAT-SA:BH3 complexes resulted in an approximate 30% loss in cell viability (p<0.05, Tukey-Kramer) after 24 hours as compared to BH3-treated cells. The relative number of apoptotic to viable cells was markedly increased to approximately 85% (p<0.001, Tukey-Kramer) when the endosomal disruptive polymer PPAA was co-complexed to TAT-SA and BH3, but not when cells were treated with PPAA alone.
Caspase Pathway Activation Studies
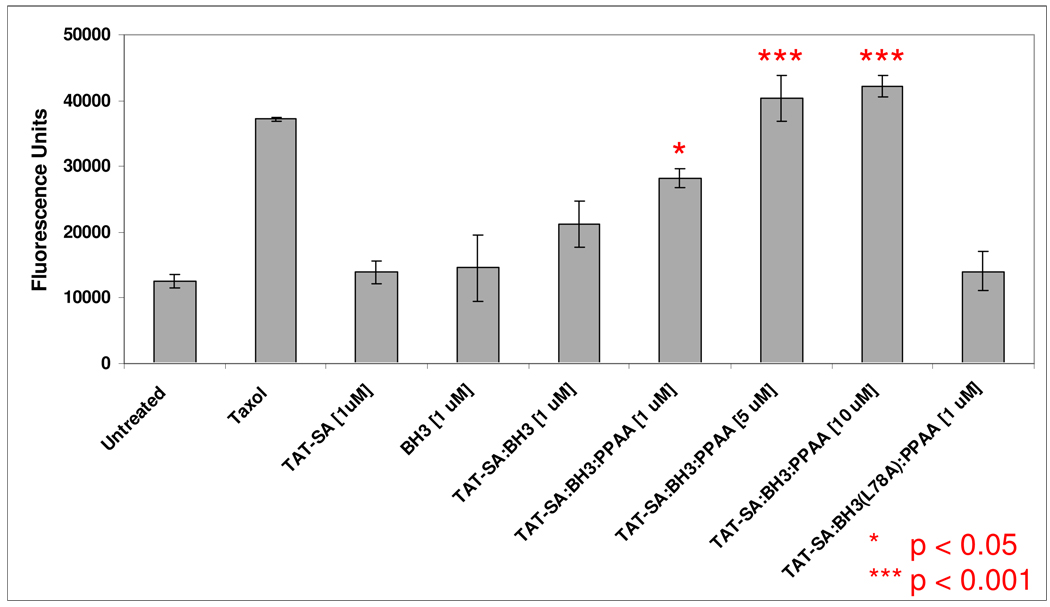
The activation of cysteine proteases such as caspase-3 is a key requirement for the induction of apoptosis via Bcl ligation. To assay for the up-regulation of caspase-3 in BH3-challenged cells, a fluorogenic caspase-3 substrate (Z-DEVD-R110) was employed to detect early induction. After 16 h of treatment with TAT-SA:BH3 complexes, HeLa cells were washed and lysed before incubation of the lysate supernatant with Z-DEVD-R110 substrate. Cells treated with TAT-SA:BH3 complexes displayed slightly elevated caspase-3 activity as compared to cells treated with TAT-SA alone or BH3 alone (p>0.05) (Figure 4), demonstrating the ability of TAT-SA alone to direct a low level of BH3 cytosolic delivery. Incorporation of the endosomal- releasing polymer PPAA resulted in strongly increased caspase-3 activity in a dose-dependent fashion (p<0.001, Tukey-Kramer). Notably, the levels of caspase-3 activity at the 5 µM and 10 µM conditions with PPAA were not statistically different (p>0.05, Tukey-Kramer), suggesting a saturation in caspase activity induction. Caspase-3 activity remained at basal levels when the BH3-(L78A) peptide was complexed to TAT-SA, confirming that specific Bcl ligation is required for apoptosis induction.
Figure 4.
TAT-SA-mediated delivery of BH3 induces upregulation of caspase-3 activity in HeLa cells. Cells were treated with the indicated reagents for 16 hours in serum-free DMEM and their lysates subsequently assayed for caspase-3-mediated cleavage of a fluorescent substrate. The microtubule-damaging agent paclitaxel (Taxol) was used as a positive control at a concentration of 0.1 µM. Cells treated with TAT-SA:BH3 displayed slightly elevated caspase-3 activity compared to cells treated with TAT-SA alone or BH3 alone (p<0.05, Tukey-Kramer), demonstrating the ability of the BH3 peptide to induce apoptosis after the enhanced uptake of TAT-SA. The co-complexation of the endosomal releasing polymer PPAA resulted in increased caspase-3 activity in a dose-dependent fashion. Notably, the level of caspase-3 activity at the 5 µM and 10 µM conditions with PPAA was not statistically different (p>0.05, Tukey-Kramer), suggesting a saturation in the level of caspase-3 activity. Furthermore, when the mutant BH3-(L78A) peptide was complexed to TAT-SA, caspase-3 activity remained at basal levels, confirming the presence of a precise intracellular target for BH3 in order to induced apoptosis.
DISCUSSION
The pharmaco-kinetics of vesicular escape and subsequent transport to subcellular targets are an important issue for the development of intracellular-acting drugs based on nucleic acids, proteins, or peptides/peptidomimetics. There are many potential peptides and peptidomimetic agents in particular that might be enabled for therapy, imaging, or basic science applications if they could be delivered more efficiently to the cytosolic compartment. Our group has been investigating the use of pH-responsive, membrane-destabilizing polymers that mimic the activities of biological fusogenic proteins to enhance the potentially rate-limiting step of vesicular escape for macromolecular delivery. These polymers transition between a water soluble, expanded coil state at physiological pH, and a collapsed, membrane active state at endosomal pH values where the carboxylate groups are protonated. The propyl segments tune the pKa of the carboxylates upward and then express their hydrophobic properties when the carboxylates are protonated by directly destabilizing membrane packing. Previous studies demonstrated that PPAA can strongly enhance the cytosolic distribution of an endocytosed antibody complex [12] as well as the TAT-streptavidin fusion protein [10]. The mechanism of TAT-streptavidin intracellular trafficking in cultured human cells has also been recently reported [20]. Model poly-arginine and TAT transduction peptides have also been recently studied by the Jones group, where the endocytosis mechanisms have been studied in detail. Their results suggest that the endosome/lysosome is indeed the major compartment for TAT peptide localization after uptake [21,22].
In this study, we have investigated the ability of PPAA to deliver a functional peptide to the cytosolic compartment, using the TAT-SA fusion protein to direct cell uptake. The mechanism of PTD internalization has been proposed to consist of a multi-step process initiated by electrostatic adsorption to the cell membrane, followed by an uptake via one or more of the internalization pathways [9]. As evidenced first by the striking induction of apoptosis that was visualized and quantitated by alterations in nuclear morphology, the PPAA strongly enhanced BH3 vesicular escape and subsequent Bcl ligation. The specificity of this interaction was probed with the mutant BH3-(L78A) peptide that has abrogated Bcl recognition. As expected, this peptide did not induce nuclear alterations or caspase activity. There was also a dose-dependent upregulation of induction of caspase activity that is consistent with the Bcl apoptosis pathway, although there are other potential branching pathways as well that could be contributing to the strong overall effect of PPAA. There was low but significant biological activity exhibited by the BH3 peptides delivered with TAT-SA alone. It was previously demonstrated that TAT-SA strongly enhances uptake of the complexed BH3 peptides, and it is likely that these high vesicular concentrations lead to low levels of leakage to the cytosolic compartment. Taken together, these results suggest that the PPAA polymer is altering the rate-limiting step of vesicular escape to enhance cytosolic delivery and subsequent Bcl target ligation.
In conclusion, these findings suggest that optimizing intracellular delivery through enhanced vesicular release could serve to expand the utility of TAT- and other PTD-based delivery systems. The TAT-SA protein was designed largely as a general reagent for transducing cultured cells with biotinylated proteins that work in the cytosolic compartment. While the effectiveness of direct TAT-SA and protein/peptide complexes appears to be low (but potentially useful for potent proteins/peptides requiring low concentrations for activity), the co-conjugates of TAT-SA and PPAA could prove useful for basic biological studies and applications requiring more robust cytosolic protein delivery. The PPAA could also allow for a reduction in the required concentration of cargo molecules to be delivered to cytosolic targets. These studies also suggest that polymeric carriers built from PPAA and alternative targeting ligands not based on PTDs could directly serve to enhance cytosolic delivery of peptide or peptido-mimetic drug candidates, though its general utility is still to be established.
Acknowledgements
The authors thank Tom Nhan for scientific discussions and technical support with fluorescence microscopy. This work was supported by NIH grants EB00252 and CA55596. The authors would also like to congratulate Prof. Kazunori Kataoka on the very special occasion of his 60th birthday. He has been a world leader in drug delivery for many years, and it is an honor for us to be invited to include our work in this special edition celebrating his 60th birthday.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fawell S, Seery J, Daikh Y, Moore C, Chen LL, Pepinsky B, Barsoum J. Tat-mediated delivery of heterologous proteins into cells. Proc. Natl. Acad. Sci. USA. 1994;91:664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- 3.Elliott G, O'Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 4.Schwarze SR, Dowdy SF. In vivo protein transduction: intracellular delivery of biologically active proteins, compounds and DNA. Trends Pharmacol. Sci. 2000;21:45–48. doi: 10.1016/s0165-6147(99)01429-7. [DOI] [PubMed] [Google Scholar]
- 5.Snyder EL, Dowdy SF. Protein/peptide transduction domains: potential to deliver large DNA molecules into cells. Curr. Opin. Mol. Ther. 2001;3:147–1452. [PubMed] [Google Scholar]
- 6.Jones SW, Christison R, Bundell K, Voyce CJ, Brockbank SM, Newham P, Lindsay MA. Characterisation of cell-penetrating peptide-mediated peptide delivery. Br. J. Pharmacol. 2005;145:1093–1102. doi: 10.1038/sj.bjp.0706279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potocky TB, Menon AK, Gellman SH. Cytoplasmic and Nuclear Delivery of a TAT-derived Peptide and a {beta}-Peptide after Endocytic Uptake into HeLa Cells. J. Biol. Chem. 2003;278:50188–50194. doi: 10.1074/jbc.M308719200. [DOI] [PubMed] [Google Scholar]
- 8.Fischer R, Kohler K, Fotin-Mleczek M, Brock R. A Stepwise Dissection of the Intracellular Fate of Cationic Cell-penetrating Peptides. J. Biol. Chem. 2004;279:12625–12635. doi: 10.1074/jbc.M311461200. [DOI] [PubMed] [Google Scholar]
- 9.Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat. Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 10.Albarran BR, To R, Stayton PS. A TAT-streptavidin fusion protein directs uptake of biotinylated cargo into mammalian cells. Prot. Eng. Des. Sel. 2005;18:147–152. doi: 10.1093/protein/gzi014. [DOI] [PubMed] [Google Scholar]
- 11.Lackey CA, Murthy N, Press OW, Tirrell DA, Hoffman AS, Stayton PS. Hemolytic activity of pH-responsive polymer-streptavidin bioconjugates. Bioconjug. Chem. 1999;10:401–405. doi: 10.1021/bc980109k. [DOI] [PubMed] [Google Scholar]
- 12.Lackey CA, Press OW, Hoffman AS, Stayton PS. Ab Biomimetic pH-responsive polymer directs endosomal release and intracellular delivery of an endocytosed antibody complex. Bioconj. Chem. 2002;13:996–1001. doi: 10.1021/bc010053l. [DOI] [PubMed] [Google Scholar]
- 13.Murthy N, Campbell J, Fausto N, Hoffman AS, Stayton PS. The design and synthesis of polymers for eukaryotic membrane disruption. J. Control. Release. 1999;61:137–143. doi: 10.1016/s0168-3659(99)00114-5. [DOI] [PubMed] [Google Scholar]
- 14.Holinger EP, Chittenden T, Lutz RJ. Bak BH3 Peptides Antagonize Bcl-xL Function and Induce Apoptosis through Cytochrome c-independent Activation of Caspases. J. Biol. Chem. 1999;274:13298–13304. doi: 10.1074/jbc.274.19.13298. [DOI] [PubMed] [Google Scholar]
- 15.Shangary S, Oliver CL, Tillman TS, Cascio M, Johnson DE. Sequence and helicity requirements for the proapoptotic activity of Bax BH3 peptides. Mol. Cancer Ther. 2004;3:1343–1354. [PubMed] [Google Scholar]
- 16.Walsh M, Lutz RJ, Cotter TG, O'Connor R. Erythrocyte survival is promoted by plasma and suppressed by a Bak-derived BH3 peptide that interacts with membrane-associated Bcl-XL. Blood. 2002;99:3439–3448. doi: 10.1182/blood.v99.9.3439. [DOI] [PubMed] [Google Scholar]
- 17.Console S, Marty C, Garcia-Echeverria C, Schwendener R, Ballmer-Hofer K. Antennapedia and HIV Transactivator of Transcription (TAT) "Protein Transduction Domains" Promote Endocytosis of High Molecular Weight Cargo upon Binding to Cell Surface Glycosaminoglycans. J. Biol. Chem. 2003;278:35109–35114. doi: 10.1074/jbc.M301726200. [DOI] [PubMed] [Google Scholar]
- 18.Ma M, Nath A. Molecular determinants for cellular uptake of Tat protein of human immunodeficiency virus type 1 in brain cells. J. Virol. 1997;71:2495–2499. doi: 10.1128/jvi.71.3.2495-2499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Truant R, Cullen BR. The arginine-rich domains present in human immunodeficiency virus type 1 Tat and Rev function as direct importin beta-dependent nuclear localization signals. Mol. Cell Biol. 1999;19:1210–1217. doi: 10.1128/mcb.19.2.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rinne J, Albarran B, Jylhava J, Ihalainen TO, Kankaanpaa P, Hytonen VP, Stayton PS, Kulomaa MS, Vihinen-Ranta M. Internalization of novel non-viral vector TAT-streptavidin into human cells. BMC Biotechnol. 2007;7:1–14. doi: 10.1186/1472-6750-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fretz MM, Penning NA, Al-Taei S, Futaki S, Takeuchi T, Nakase I, Storm G, Jones AT. Temperature-, concentration- and cholesterol-dependent translocation of L- and D-octa-arginine across the plasma and nuclear membrane of CD34+ leukaemia cells. Biochem. J. 2007;403:335–342. doi: 10.1042/BJ20061808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Taei S, Penning NA, Simpson JC, Futaki S, Takeuchi T, Nakase I, Jones AT. Intracellular traffic and fate of protein transduction domains HIV-1 TAT peptide and octaarginine. Implications for their utilization as drug delivery vectors. Bioconjug. Chem. 2006;17:90–100. doi: 10.1021/bc050274h. [DOI] [PubMed] [Google Scholar]