Abstract
Context
Biochemical disease recurrence after radical prostatectomy often prompts salvage radiotherapy, but no studies to date have had sufficient numbers of patients or follow-up to determine whether radiotherapy improves survival, and if so, the subgroup of men most likely to benefit.
Objectives
To quantify the relative improvement in prostate cancer–specific survival of salvage radiotherapy vs no therapy after biochemical recurrence following prostatectomy, and to identify subgroups for whom salvage treatment is most beneficial.
Design, Setting, and Patients
Retrospective analysis of a cohort of 635 US men undergoing prostatectomy from 1982–2004, followed up through December 28, 2007, who experienced biochemical and/or local recurrence and received no salvage treatment (n=397), salvage radiotherapy alone (n=160), or salvage radiotherapy combined with hormonal therapy (n=78).
Main Outcome Measure
Prostate cancer–specific survival defined from time of recurrence until death from disease.
Results
With a median follow-up of 6 years after recurrence and 9 years after prostatectomy, 116 men (18%) died from prostate cancer, including 89 (22%) who received no salvage treatment, 18 (11%) who received salvage radiotherapy alone, and 9 (12%) who received salvage radiotherapy and hormonal therapy. Salvage radiotherapy alone was associated with a significant 3-fold increase in prostate cancer–specific survival relative to those who received no salvage treatment (hazard ratio [HR], 0.32 [95% confidence interval {CI}, 0.19–0.54]; P<.001). Addition of hormonal therapy to salvage radiotherapy was not associated with any additional increase in prostate cancer–specific survival (HR, 0.34 [95% CI, 0.17–0.69]; P=.003). The increase in prostate cancer–specific survival associated with salvage radiotherapy was limited to men with a prostate-specific antigen doubling time of less than 6 months and remained after adjustment for pathological stage and other established prognostic factors. Salvage radiotherapy initiated more than 2 years after recurrence provided no significant increase in prostate cancer–specific survival. Men whose prostate-specific antigen level never became undetectable after salvage radiotherapy did not experience a significant increase in prostate cancer–specific survival. Salvage radiotherapy also was associated with a significant increase in overall survival.
Conclusions
Salvage radiotherapy administered within 2 years of biochemical recurrence was associated with a significant increase in prostate cancer–specific survival among men with a prostate-specific antigen doubling time of less than 6 months, independent of other prognostic features such as pathological stage or Gleason score. These preliminary findings should be validated in other settings, and ultimately, in a randomized controlled trial.
Nearly 60 000 men (27% of newly diagnosed cases) will have undergone radical prostatectomy in 2007.1 Although surgery provides excellent cancer control, approximately 15% to 40% of these men will experience cancer recurrence within 5 years,2,3 usually manifested only by elevated prostate-specific antigen (PSA) level. It is currently difficult to determine whether increasing serum PSA level after surgery represents an isolated recurrence at the surgical site or occult metastases that cannot be detected by imaging. For such men it is unknown whether salvage radiotherapy confers a survival benefit compared with observation. Furthermore, it is unknown whether the likelihood of benefit differs for immediate vs delayed salvage radiotherapy, or among subgroups of men defined by pathological attributes. These are critical questions because 65% of men with biochemical or local recurrence will develop overt metastases if left untreated and the majority of these will die from their disease.4 No studies to date have evaluated the association of salvage radiotherapy with prostate cancer–specific survival.
Recently, the need to determine the impact of salvage therapy on survival has been given a sharper focus by publication of 2 large randomized controlled trials of adjuvant radiotherapy in men with pathologically advanced (stage pT3) prostate cancer. Both trials demonstrated that adjuvant radiotherapy provides significant improvement in biochemical relapse–free survival and clinical recurrence–free survival, but no significant improvement in metastasis-free or overall survival.5,6 These results have sparked debate as to whether all patients with pT3 disease who undergo prostatectomy should receive immediate adjuvant treatment, or whether close surveillance with salvage treatment provided early upon PSA relapse can provide a similar benefit and avoid overtreating men who do not progress.7,8 To evaluate the benefit of salvage radiotherapy on prostate cancer–specific survival, we assembled a large cohort of men with biochemical or local recurrence following radical prostatectomy who received either no salvage therapy, salvage radiotherapy alone, or salvage radiotherapy combined with hormonal therapy. We evaluated their survival experience to determine the association between salvage radiotherapy and prostate cancer–specific survival and identify subgroups in which salvage radiotherapy provides the greatest survival benefit.
METHODS
Patients
Between June 1982 and August 2004, 926 men developed recurrent disease following radical retropubic prostatectomy with staging pelvic lymphad-enectomy at Johns Hopkins Hospital (Baltimore, Maryland) for clinically localized prostate cancer (clinical stage T1-T2), and either did not receive salvage therapy, received salvage radiotherapy alone, or received salvage therapy combined with hormonal therapy. The latter included hormonal therapy administered immediately prior to, during, or immediately following salvage radiotherapy, or administered after the first PSA measurement following salvage radiotherapy but before further progression or metastasis. Patients were followed up through December 2007. Clinical and self-reported demographic data from patient charts are recorded in the Brady Urological Institute Master Radical Prostatectomy database under a waiver of consent that allows use for research purposes without disclosing patient identifiers. The database is approved by the Johns Hopkins institutional review board and conforms to the Health Insurance Portability and Accountability Act guidelines.
Men who received adjuvant hormonal or radiation therapy prior to recurrence were not included, nor were men who received only salvage hormonal therapy. Excluded from analysis were 7 men who declined to participate, 6 men whose salvage treatment status could not be determined, 4 men for whom survival time could not be determined, 60 men censored with no follow-up data subsequent to prostate cancer recurrence, and 214 men lacking sufficient data to calculate PSA doubling time following recurrence. A total of 635 men (68.6%) remained for analysis of the effect of salvage radiotherapy on prostate cancer–specific survival.
Routine postoperative follow-up at Johns Hopkins Hospital generally included digital rectal examination and serum PSA level determination every 3 months in the first year, every 6 months in the second year, and annually thereafter. Because Johns Hopkins Hospital is a referral center, many men received follow-up care in their home states, so follow-up protocols were not uniform. However, such variation is likely to be random with respect to treatment. A single PSA measurement of 0.2 ng/mL or higher (to convert to μg/L, multiply by 1.0) was the criterion for biochemical recurrence. Postsurgical PSA levels of 0.2 ng/mL or higher within 24 months of biochemical recurrence and prior to salvage radiotherapy were used for the PSA doubling time, which was calculated as 0.693 divided by the slope of the linear regression of the natural log of PSA level vs time of PSA measurement in months.4 If the slope was 0 (elevated but constant PSA level) or negative (decreasing PSA level after initial increase), the PSA doubling time was arbitrarily set to 100.
Radiation Treatment
Salvage radiotherapy was defined as local radiation to the prostate bed alone or combined with hormonal therapy following biochemical or local recurrence. All patients managed at Johns Hopkins Hospital underwent simulation and were treated using conformal radiotherapy techniques; fields encompassed the prostate and seminal vesicle bed plus periprostatic tissues. No attempt was made to comprehensively irradiate pelvic lymph nodes. In general, 45.0 Gy was delivered to initial fields in 1.8 to 2.0 Gy daily fractions, after which the lateral fields were modified to reduce rectal wall irradiation. Fields were shaped to protect the small bowel, bladder, and posterior rectal wall. All men who underwent salvage radiotherapy had a negative bone scan, pelvic computerized tomographic scan, and chest radiograph. Salvage treatments were administered at the discretion of treating physicians and the timing of initiating treatment was not standardized.
Statistical Methods
Comparisons between subgroups of men were performed using χ2 tests for categorical data, and t tests or analysis of variance for continuous data. Prostate cancer–specific survival and overall survival probabilities were estimated for each treatment group and within strata of prognostic factors using the Kaplan-Meier approach. Estimated survival curves were compared using the log-rank test. Multivariable analyses of prostate cancer–specific survival and overall survival were performed using the Cox proportional hazards model. Because of the observational, retrospective nature of this study, patients who did and did not receive salvage radiotherapy may differ with respect to well-established prognostic factors (PSA doubling time, pathological stage, postoperative Gleason score, age) that were considered by physicians in their decision to administer salvage radiotherapy. Given the established importance of these variables, they were considered a priori as potential confounding factors to be controlled in multivariable models (along with time from surgery to recurrence and year of surgery), as well as potential interactions with salvage radiotherapy and stratification factors for subgroup analyses. Because salvage radiotherapy was initiated with varying time delays after diagnosis of recurrence, salvage treatment was modeled using time-dependent covariates.9 Interactions between established prognostic factors and salvage treatment were modeled as cross-product terms, and also treated as time-dependent covariates. Comparison of models was based on the likelihood ratio test, and evidence of confounding of the salvage radiotherapy effect was indicated by the change in the hazard ratio (HR). The level of significance was set at .05 in all analyses, except in analyses in which a Bonferroni adjustment for multiple comparisons was applied, in which case the significance level was .05 divided by the number of comparisons. All statistical analyses were performed using SAS version 9.1 (SAS Institute Inc, Cary, North Carolina).
RESULTS
The analysis included 397 men (63%) who did not receive salvage therapy, 160 (25%) who received salvage radiotherapy alone, and 78 (12%) who received salvage radiotherapy and hormonal therapy. Median follow-up from a diagnosis of recurrence was 6 years (range, 1–20). There was 9 or more years of follow-up for 167 men (26%). Median follow-up from the date of prostatectomy was 9 years (range, 2–23). Among men who received salvage radiotherapy, the median time from recurrence to initiating salvage radiotherapy was 1 year (range, <1–8). Radiation dose data were available for men who received salvage radiotherapy at Johns Hopkins Hospital (135/238 [57%]). Median dose was 66.5 Gy (interquartile range, 63.8–68.0) among men who received only salvage radiotherapy (n=92), and 67.2 Gy (interquartile range, 64.9–68.4) among men who received salvage radiotherapy plus hormonal therapy (n=43). Men with dose data available and those lacking dose data did not differ significantly with respect to age (P=.73), PSA level at diagnosis (P=.56), PSA level at start of salvage radiotherapy (P=.40), PSA doubling time (P=.89), time from surgery to recurrence (P = .65), pathological stage (P=.22), surgical margins (P=.71), and Gleason score (P=.34). Given the comparability of the 2 groups, it is likely that the dose data reflect the entire salvage radiotherapy cohort. A total of 116 men (18.3%) died from prostate cancer, and 49 (7.7%) died from other causes during the period of observation.
The 3 study groups differed significantly for all prognostic factors except surgical margin status (Table 1). Notably, men with no salvage therapy had a much higher prevalence of positive lymph nodes (30% vs 3%–4%; P<.001). Although no single group consistently showed a worse prognostic profile, men who received salvage radiotherapy and hormonal therapy had significantly shorter time to recurrence, shorter PSA doubling time, and higher PSA level at the time radiotherapy was initiated.
Table 1.
Characteristics of Men With Recurrence Following Radical Prostatectomy
| Characteristic | Salvage Treatment, No. (%)a |
P Value | |||
|---|---|---|---|---|---|
| None (n = 397) | Radiation Only (n = 160) | Radiation + Hormone (n = 78) | |||
| Age at surgery, mean (SD), y | 60.3 (5.80) | 58.3 (6.17) | 58.5 (6.27) | <.001 | |
| Race | |||||
| White, non-Hispanic | 345 (90.6) | 152 (95.0) | 66 (86.8) | .19 | |
| Black | 28 (7.4) | 7 (4.4) | 9 (11.8) | ||
| Other | 8 (2.1) | 1 (0.6) | 1 (1.3) | ||
| PSA level, median (range), ng/mL | |||||
| Preoperative | 9.6 (0.8–129.0) | 8.3 (0.1–57.1) | 7.7 (0.3–41.1) | .02 | |
| At start of radiation | NA | 0.70 (0.2–22.0) | 0.90 (0.3–30.0) | .03 | |
| Pathological Gleason score | |||||
| 4–6 | 63 (15.9) | 46 (28.8) | 11 (14.1) | <.001 | |
| 7 | 204 (51.4) | 89 (55.6) | 44 (56.4) | ||
| 8–10 | 130 (32.8) | 25 (15.6) | 23 (29.5) | ||
| Pathological stage | |||||
| Organ-confined | 42 (10.7) | 29 (18.2) | 6 (7.8) | <.001 | |
| Extraprostatic extension | 166 (42.1) | 104 (65.4) | 57 (74.0) | ||
| Seminal vesicle involvement | 69 (17.5) | 22 (13.8) | 11 (14.3) | ||
| Lymph node metastases | 117 (29.7) | 4 (2.5) | 3 (3.9) | ||
| Surgical margin status | |||||
| Negative | 225 (56.6) | 98 (61.3) | 42 (53.9) | .48 | |
| Positive | 172 (43.3) | 62 (38.8) | 36 (46.2) | ||
| PSA doubling time, median (range), mo | 12.0 (0.7–612.7) | 12.6 (0.9–563.4) | 7.0 (1.0–143.9) | .007 | |
| Period, y | |||||
| 1982–1988 | 90 (22.7) | 51 (31.9) | 17 (21.8) | .09 | |
| 1989–1991 | 52 (13.1) | 23 (14.4) | 7 (9.0) | ||
| 1992–2004 | 255 (64.2) | 86 (53.8) | 54 (69.2) | ||
| Time from surgery to recurrence, median (range), y | 3.0 (1.0–20.0) | 3.0 (1.0–18.0) | 2.5 (1.0–13.0) | .007 | |
| Developed metastases | 176 (45.3) | 43 (27.2) | 15 (19.5) | <.001b | |
| Death | |||||
| Prostate cancer | 89 (22.4) | 18 (11.3) | 9 (11.5) | .002b | |
| Any cause | 120 (31.1) | 30 (19.4) | 15 (19.7) | .007b | |
Abbreviations: NA, not applicable; PSA, prostate-specific antigen.
SI conversion factor: To convert PSA to μg/L, multiply by 1.0
Unless otherwise indicated.
P value is for a comparison among the crude proportions and does not reflect adjustment for survival time.
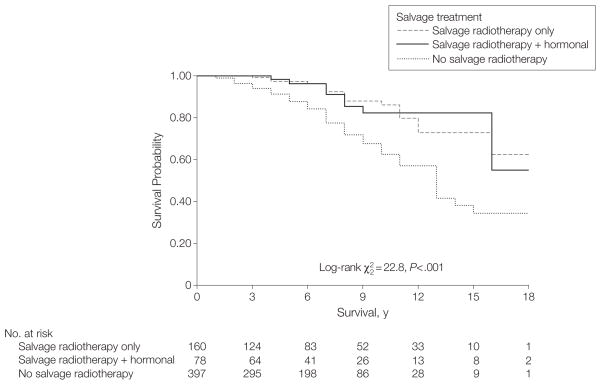
Kaplan-Meier curves demonstrate significant differences in prostate cancer–specific survival (P<.001) among the 3 groups (no salvage therapy vs salvage radiotherapy alone vs salvage radiotherapy and hormonal therapy) (Figure 1). There is no significant difference between the 2 salvage radiotherapy groups (P=.98). Five- and 10-year prostate cancer–specific survival estimates are 0.88 and 0.62 for no salvage treatment, 0.96 and 0.86 for salvage radiotherapy alone, and 0.96 and 0.82 for salvage radiotherapy plus hormonal therapy, respectively. Neither radiotherapy group has reached median survival.
Figure 1.
Prostate Cancer-Specific Survival Following Recurrence After Radical Prostatectomy (1982–2004)
Univariate proportional hazards models demonstrated significant associations with prostate cancer–specific survival for the logarithm of PSA doubling time, time from surgery to recurrence, postoperative Gleason score of 8 or greater, positive lymph nodes, and salvage radiotherapy (Table 2). Salvage radiotherapy, regardless of whether given alone or with hormonal therapy, was associated with a statistically significant decrease in the risk of death of nearly 60%.
Table 2.
Proportional Hazards Models for Risk of Prostate Cancer–Specific Mortality, 1982–2004a
| Variables | No. of Deaths/No. of Individuals in Subgroup | Univariate Model |
Multivariable Model |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |||
| Natural logarithm for PSA doubling time, mo | 0.38 (0.31–0.46)b | <.001 | 0.36 (0.28–0.46)b | <.001 | ||
| Time from surgery to recurrence, y | 0.80 (0.73–0.88)c | <.001 | 0.91 (0.82–1.00)c | .06 | ||
| Calendar year of surgery | 0.99 (0.94–1.03)c | .53 | 0.91 (0.86–0.96)c | .001 | ||
| Age, y | 1.01 (0.98–1.05) | .47 | 1.00 (0.97–1.04) | .81 | ||
| Postoperative Gleason score | ||||||
| <7 | 11/120 | 1 [Reference] | ||||
| 7 | 46/337 | 1.68 (0.87–3.25) | .13 | 1 [Reference]d | .003 | |
| 8–10 | 59/178 | 4.23 (2.21–8.09) | <.001 | 1.77 (1.21–2.60) | ||
| Surgical margins | ||||||
| Negative | 53/365 | 1 [Reference] | 1 [Reference] | .94 | ||
| Positive | 63/270 | 1.28 (0.88–1.85) | .19 | 0.99 (0.66–1.48) | ||
| Postoperative stage | ||||||
| Organ-confined | 7/77 | 1 [Reference] | 1 [Reference] | |||
| Extraprostatic extension | 38/327 | 0.98 (0.44–2.20) | .96 | 0.98 (0.42–2.29) | .97 | |
| Seminal vesicle involvement | 27/102 | 2.07 (0.90–4.76) | .09 | 1.28 (0.52–3.15) | .59 | |
| Lymph node metastases | 44/124 | 2.45 (1.10–5.46) | .03 | 1.00 (0.40–2.53) | .99 | |
| Salvage treatment | ||||||
| No salvage treatment | 89/397 | 1 [Reference] | 1 [Reference] | |||
| Radiotherapy only | 18/160 | 0.42 (0.25–0.71) | .001 | 0.32 (0.17–0.57) | <.001 | |
| Radiotherapy + hormonal | 9/78 | 0.43 (0.22–0.87) | .02 | 0.35 (0.16–0.74) | .006 | |
Abbreviations: CI, confidence interval; HR, hazard ratio; PSA, prostate-specific antigen.
Survival time measured from date of recurrence.
The HR is for an increase of 1.0 in the natural logarithm of PSA doubling time, which corresponds to a 2.72-fold increase in absolute PSA doubling time.
The HR is per 1-year increase.
The reference group is a Gleason score of 7 or less and the HR is for Gleason scores of 8 to 10 vs 7 or less.
In multivariable models, after adjusting for the logarithm of PSA doubling time, time from surgery to recurrence, year of surgery, and postoperative Gleason score, salvage radiotherapy was associated with a statistically significant reduction in risk of death of more than 65% (Table 2). Again, the association between salvage radiotherapy and prostate cancer–specific survival did not differ according to whether salvage hormonal therapy also was given. Although neither pathological stage overall nor lymph node status were statistically significant prognostic factors in the multivariable model, there is a potential for bias because men with lymph node involvement are rarely referred for salvage radiotherapy. To demonstrate that improved prostate cancer–specific survival outcomes associated with salvage radiotherapy are not simply due to confounding resulting from the large imbalance in lymph node metastases, we repeated the multivariable model from Table 2 after excluding all men with positive lymph nodes (n=124). The results were essentially unchanged for men who received salvage radiotherapy alone vs those who received no salvage therapy (HR, 0.34; 95% confidence interval [CI], 0.19–0.63) and for those who received salvage radiotherapy and hormonal therapy vs those who received no salvage treatment (HR, 0.35; 95% CI, 0.16–0.74) (model not shown).
Because salvage radiotherapy should only benefit men at high risk for disease recurring locally, we sought to distinguish clinical subgroups of men in whom salvage radiotherapy was associated with a significant increase in prostate cancer–specific survival with those in whom salvage radiotherapy demonstrated little or no association with prostate cancer–specific survival. To this end, we evaluated models with a priori interactions between salvage radiotherapy and other prognostic factors, including time from surgery to recurrence, surgical margin status, postoperative Gleason score, pathological stage, and the logarithm of PSA doubling time (continuous). Only the logarithm of PSA doubling time demonstrated a statistically significant interaction with salvage radiotherapy (P=.007; model not shown).
To identify clinically relevant subgroups with differential survival benefit from salvage radiotherapy, we evaluated interactions between salvage radiotherapy and PSA doubling time dichotomized at cut points reported in the literature (3, 6, 9, 10, and 12 months).4,10 Prostate-specific antigen doubling time dichotomized at 6 months most strongly separated men for whom salvage radiotherapy was and was not associated with an increase in prostate cancer–specific survival, and provided the greatest improvement in model fit. Among 166 men (26%) with a PSA doubling time of less than 6 months, salvage radiotherapy alone and salvage therapy with hormonal treatment were associated with a reduction in risk of prostate cancer–specific mortality by more than 75%. In contrast, salvage radiotherapy was not significantly associated with prostate cancer–specific survival among men with a PSA doubling time of 6 months or longer (Table 3). Although the cut point was chosen by data exploration among several suggested by the literature, the interaction would be statistically significant even if the significance level were set at .01 using a Bonferroni adjustment for multiple comparisons. The results were unchanged when the analysis was repeated after excluding men with positive lymph nodes (ie, when PSA doubling time <6 months, prostate cancer–specific mortality was reduced among men who received salvage radiotherapy alone) (HR, 0.12; 95%CI, 0.04–0.35)or those who received salvage radiotherapy with hormone therapy (HR, 0.18; 95% CI, 0.05–0.62), but associations were not statistically significant when PSA doubling time was 6 months or longer(HR, 0.93[95%CI, 0.46–1.89] and HR, 0.84 [95% CI, 0.33–2.11], respectively).
Table 3.
Multivariable Proportional Hazards Model of Salvage Radiotherapy and Interaction With PSA Doubling Time for Risk of Prostate Cancer–Specific Mortality, 1982–2004a
| Variables | No. of Deaths/No. of Individuals in Subgroup | HR (95% CI) | P Value |
|---|---|---|---|
| Time from surgery to recurrence, y | 0.85 (0.77–0.94)b | .001 | |
| Calendar year of surgery | 0.91 (0.86–0.95)b | <.001 | |
| Postoperative Gleason score | |||
| ≤7 | 57/457 | 1 [Reference] | |
| 8–10 | 59/178 | 2.57 (1.75–3.77) | <.001 |
| Salvage treatment | <.001c | ||
| PSA doubling time <6 mo | |||
| No salvage treatment | 51/103 | 1 [Reference] | |
| Radiotherapy only | 4/29 | 0.14 (0.05–0.39) | |
| Radiotherapy + hormonal | 3/34 | 0.24 (0.07–0.77) | |
| PSA doubling time ≥6 mo | |||
| No salvage treatment | 38/294 | 1 [Reference] | |
| Radiotherapy only | 14/131 | 0.85 (0.45–1.59) | |
| Radiotherapy + hormonal | 6/44 | 0.66 (0.28–1.58) | |
Abbreviations: CI, confidence interval; HR, hazard ratio; PSA, prostate-specific antigen.
Survival time measured from date of recurrence.
The HR is per 1-year increase.
P value represents the likelihood ratio test for adding salvage treatment (2 variables) and 2 interaction terms to the model: likelihood ratio .
We also examined whether associations with salvage radiotherapy differed according to the delay between recurrence and initiation of salvage radiotherapy, the PSA level at the time salvage radiotherapy was initiated, and PSA response to salvage radiotherapy. Because of the reduced sample size with subgroup-specific models, and the fact that salvage radiotherapy alone and salvage radiotherapy plus hormonal therapy produce similar survival benefits (Table 3), we used a single variable combining both salvage radiotherapy groups. Separate proportional hazards models were fit to compare the effect of salvage radiotherapy initiated less than 1 year vs 1 or more years after recurrence, and also for less than 2 years vs 2 or more years after recurrence. The former did not separate a time interval when salvage radiotherapy was effective vs not effective. Among men with a PSA doubling time of less than 6 months, salvage radiotherapy was significantly associated with increased prostate cancer–specific survival regardless of whether initiated less than 1 year after recurrence (HR, 0.15; 95% CI, 0.06–0.38) or 1 or more years after recurrence (HR, 0.22; 95% CI, 0.05–0.91). Furthermore, a cut point at 1 year could potentially confound the prognostic effect of delay in administering salvage radiotherapy with that associated with year of surgery because a short delay occurred primarily in men treated in recent years. In contrast, with the cut point at 2 years, the effect of salvage radiotherapy differed dramatically according to delay. In men with a delay of less than 2 years, salvage radiotherapy was associated with an increase in prostate cancer–specific survival only for men with a PSA doubling time of less than 6 months (P<.001). In contrast, salvage radiotherapy initiated 2 or more years after recurrence was not significantly associated with prostate cancer–specific survival regardless of PSA doubling time (Table 4).
Table 4.
Effect of Delay From Recurrence to Initiation of Salvage Radiotherapy on Multivariable Proportional Hazards Model for Risk of Prostate Cancer–Specific Mortality, 1982–2004a
| Delay <2 y (n = 552); (105 Deaths)b |
Delay ≥2 y (n = 480); (100 Deaths)c |
|||||
|---|---|---|---|---|---|---|
| Variables | HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Time from surgery to recurrence, y | 0.87 (0.79–0.96)d | .006 | 0.86 (0.77–0.96)d | .006 | ||
| Calendar year of surgery | 0.89 (0.85–0.94)d | <.001 | 0.93 (0.88–0.98)d | .006 | ||
| Postoperative Gleason score | ||||||
| ≤7 | 1 [Reference] | <.001 | 1 [Reference] | <.001 | ||
| 8–10 | 2.58 (1.73–3.85) | 2.67 (1.77–4.03) | ||||
| Salvage treatment | <.001e | .87f | ||||
| PSA doubling time <6 mo | ||||||
| No salvage therapy | 1 [Reference] | 1 [Reference] | ||||
| Radiotherapy (with or without hormonal therapy) | 0.14 (0.06–0.34) | 0.80 (0.11–5.93) | ||||
| PSA doubling time ≥6 mo | ||||||
| No salvage therapy | 1 [Reference] | 1 [Reference] | ||||
| Radiotherapy (with or without hormonal therapy) | 0.72 (0.36–1.46) | 0.84 (0.40–1.74) | ||||
Abbreviations: CI, confidence interval; HR, hazard ratio; PSA, prostate-specific antigen.
Survival time measured from date of recurrence.
A total of 103 received salvage radiotherapy alone and 52 received salvage radiotherapy plus hormonal therapy. Analysis included the entire cohort of 397 men who did not receive salvage therapy.
A total of 57 received salvage radiotherapy alone and 26 received salvage radiotherapy plus hormonal therapy. Analysis included the entire cohort of 397 men who did not receive salvage therapy.
The HR is per 1-year increase.
P value represents the likelihood ratio test for adding salvage treatment (1 variable) and 1 interaction term to the model: likelihood ratio .
P value represents the likelihood ratio test for adding salvage treatment (1 variable) and 1 interaction term to the model: likelihood ratio .
Similarly, salvage radiotherapy initiated in men while their PSA level was 2 ng/mL or lower was associated with a significant increase in prostate cancer–specific survival (HR, 0.27; 95% CI, 0.15–0.50), whereas the effect was smaller and no longer significant for salvage radiotherapy initiated in men while their PSA level was higher than 2 ng/mL (HR, 0.59; 95% CI, 0.32–1.10) (models not shown). However, among men with a PSA doubling time of less than 6 months, salvage radiotherapy was associated with significantly increased prostate cancer–specific survival regardless of whether initiated when their PSA level was 2 ng/mL or lower (HR, 0.10; 95% CI, 0.03–0.32) or when their PSA level was higher than 2 ng/mL (HR, 0.34; 95% CI, 0.12–0.95). Thus, the PSA level at the start of salvage radiotherapy no longer predicted differential response when the interaction between salvage radiotherapy and PSA doubling time also was in the model.
To evaluate whether PSA response to radiotherapy modified the association between salvage radiotherapy and prostate cancer–specific survival, we excluded men with hormonal therapy immediately before, during, or immediately after salvage radiotherapy (n=47) because a PSA level decrease following such treatment could be due to either radiation or hormonal treatment. Salvage radiotherapy was associated with a significant increase in prostate cancer–specific survival relative to no salvage therapy for men whose PSA level became undetectable (<0.2 ng/mL; n=100)—even if it eventually began to increase again (HR, 0.15 [95% CI, 0.06–0.42]; P<.001). Among men whose PSA level never became undetectable (n=80), the association between salvage radiotherapy and prostate cancer–specific survival was not statistically significant (HR, 0.63 [95% CI, 0.38–1.07]; P =.09) (models not shown).
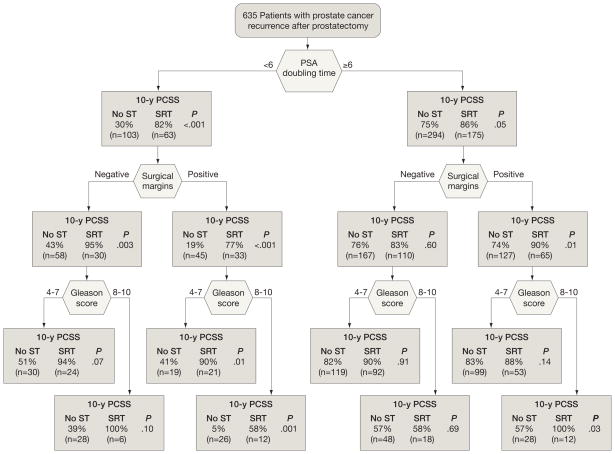
To facilitate clinical decisions for specific patient subgroups, we compared Kaplan-Meier prostate cancer–specific survival estimates between men who received no salvage therapy with those who received salvage radiotherapy(with or without hormonal therapy) within prognostic subgroups defined by successive stratification on PSA doubling time (<6 vs ≥6 months), surgical margins (negative vs positive), and postoperative Gleason score (4–7 vs 8–10). Although this analysis is not as rigorous as the multivariable time-dependent covariates model, it serves to illustrate the absolute magnitude of prostate cancer–specific survival differences associated with salvage radiotherapy within subgroups. We did not stratify by delay because it is not defined for the group with no salvage therapy. For PSA doubling time of less than 6 months, 10-year prostate cancer–specific survival rates were higher for men who received salvage radiotherapy, regardless of surgical margins or Gleason score (Figure 2). In contrast, for those with a PSA doubling time of 6 months or longer, there was no difference in prostate cancer–specific survival associated with salvage radiotherapy except in the subgroup with positive surgical margins and Gleason scores between 8 and 10.
Figure 2.
Kaplan-Meier Prostate Cancer-Specific Survival (PCSS) Estimates at 10 Years
Survival rates are following recurrence in men who received no salvage therapy (ST) vs salvage radiotherapy (SRT; with or without hormonal therapy), stratified by prostate-specific antigen doubling time, surgical margin status, and postoperative Gleason score.
Our decision to exclude men with missing PSA doubling time data was based on numerous studies demonstrating its prognostic importance in the salvage setting.11–14 In our study, men who did vs did not have available PSA doubling time data did not differ significantly with respect to median follow-up time, time from prostatectomy to recurrence, PSA level at diagnosis, pathological stage, surgical margins, and era of diagnosis (data not shown), while a Gleason score between 8 and 10 was somewhat more frequent among the former (178 [28.0%] vs 46 [21.6%]; P=.05). However, the large number of men excluded (n=214) could represent a source of bias if reasons for missing PSA doubling time data were not random. Therefore, we repeated the analysis from Table 2 but omitted the logarithm of PSA doubling time from the model and compared the results based on all 849 men with the results based on the 635 men for whom PSA doubling time was available. The results were statistically significant and similar for both groups of men. Among all men, salvage radiotherapy alone vs no salvage therapy yielded an HR of 0.59 (95% CI, 0.39–0.88) and salvage radiotherapy with hormonal therapy vs no salvage therapy yielded an HR of 0.55 (95% CI, 0.34–0.89). Among the 635 men, the associations yielded an HR of 0.47 (95% CI, 0.28–0.79) for salvage radiotherapy alone and 0.47 (95% CI, 0.23–0.94) for salvage radiotherapy with hormonal therapy (models not shown). The similarity of results suggests that excluding the men with missing PSA doubling time data from the analyses is unlikely to introduce serious bias.
Finally, to facilitate comparison of the survival experience of this salvage radiotherapy cohort with that observed with immediate adjuvant radiotherapy in 2 recently published randomized controlled trials,5,6 we also analyzed overall survival by restricting the cohort to patients with stage pT3 prostate cancer (n=424) as was done in those trials. Salvage radiotherapy was associated with a significant increase in overall survival and remained dependent on PSA doubling time (<6 months yielded an HR of 0.28 [95% CI, 0.14–0.57] and ≥6 months yielded an HR of 0.83 (95% CI, 0.49–1.40) (P=.01 for interaction; model not shown). The Kaplan-Meier estimates of 5- and 10-year overall survival for patients who received salvage radiotherapy were 98% and 89%, respectively.
COMMENT
Recurrence of prostate cancer heralded by rising PSA level after potentially curative surgery presents a difficult dilemma for patients and their physicians. For the patient, it is a sign that their initial opportunity for cure has not been successful and brings a sense of urgency to administer second-line therapy. For the physician, it brings choices that do not have clear-cut answers: should salvage therapy be administered or can therapy wait until signs of metastatic progression; if salvage therapy is chosen, should it be radiation, hormonal, or combined therapy, and should it begin immediately or can it be delayed? These choices all bring potential trade-offs between therapeutic benefit, complications of treatment, and quality of life. However, conclusive evidence has not been available to determine which approach, if any, prolongs life.
Recent retrospective studies have shown that salvage radiotherapy in men who experience cancer recurrence after prostatectomy offers a durable progression-free interval to approximately one-third of men.11–20 None of the studies evaluated prostate cancer–specific survival. All but one study20 had post-irradiation follow-up of less than 5 years. Freedom from biochemical relapse at 5 years in patients who received radiation ranged from 16% to 54%. Median radiation doses were 64 to 66.7 Gy. The most common predictors of postsalvage PSA level relapse in multivariable models were PSA level at the time of radiation (6 studies), Gleason score of 7 or less (4 studies), and seminal vesicle invasion (4 studies). Although positive surgical margins have been thought to indicate a higher likelihood of local recurrence and thus predictive of a better response to salvage radiotherapy, we are aware of only 2 studies14,18 that found such an association.
We compared prostate cancer–specific survival among men who received salvage radiotherapy vs observation in a retrospective cohort of 635 men with biochemical or local recurrence after prostatectomy. With a median follow-up of 6 years after recurrence, salvage radiotherapy was associated with a significant 3-fold improvement in prostate cancer–specific survival, regardless of whether hormonal treatment also was included, and the improvement was primarily confined to men with a PSA doubling time of less than 6 months. This effect was observed regardless of surgical margin status or Gleason score. Furthermore, salvage radiotherapy was associated with an increase in survival only if given sooner than 2 years after recurrence. Because men in this study underwent surgery during a wide period (1982–2004), the median interval between recurrence and initiating salvage radiotherapy of 1 year is longer than current practice. However, because the survival benefit appears to decrease with greater delay in initiating salvage radiotherapy, our results may actually underestimate the effect of salvage radiotherapy. Initiating salvage radiotherapy when PSA level is 2 ng/mL or lower also was associated with a larger survival benefit, although men with a PSA doubling time of less than 6 months benefited even if they had a PSA level of higher than 2 ng/mL when treatment was initiated.
This is the first study of salvage radiotherapy to compare outcomes with a group that did not receive any salvage treatment, and to evaluate associations with prostate cancer–specific survival. This provides the opportunity to evaluate factors that predict differential response to radiotherapy. In previous studies of salvage radiotherapy that lacked an untreated group, it cannot be determined whether factors associated with increased progression are prognostic factors that affect outcome regardless of treatment, or truly predictive factors that define subgroups with differential benefit from salvage radiotherapy.
The distinction between prognostic factors and those that predict response to therapy is clinically important. Men with a PSA doubling time of less than 6 months have a worse prognosis overall than those with a PSA doubling time of 6 months or longer (Figure 2), but in our data they derive more of a benefit from salvage therapy relative to untreated patients. This may indicate that many men with a PSA doubling time of 6 months or longer have biologically less aggressive disease that is less likely to result in metastatic disease or death during their lifetime.12 This is consistent with our finding that men with a PSA doubling time of 6 months or longer have prostate cancer–specific survival of 80% at 10 years after recurrence. It also is consistent with features in other cancers that predict response to postsurgery radiotherapy. An important example is the use of postmastectomy radiation for breast cancer. Women with clinical and pathological features suggestive of aggressive disease and elevated risk of local recurrence achieve a cause-specific and overall survival advantage from chest wall and regional lymph node radiation following mastectomy and axillary node dissection compared with women who underwent the same surgery but had not received radiation. This survival benefit is seen even in women with negative surgical margins. Women without such high-risk features did not derive this same benefit from postmastectomy radiation, emphasizing the importance of identifying features predictive of response.21–24
This study provides provocative evidence that even men with adverse prognostic features such as rapid PSA doubling time or high Gleason score may benefit from salvage radiotherapy. If true, this has somewhat surprising implications for the prevalence of local recurrence as a source of PSA relapse. Previous studies suggest that local recurrence is associated with relatively favorable prognostic features (eg, Gleason score <8, organ-confined disease, and slow PSA velocity).4,25 However, as suggested by Stephenson et al,12 our results indicate that local recurrence with a potentially lethal phenotype but biologically responsive to radiation can occur among men with aggressive features. This suggests that recurrent or residual disease in the pelvis may be a more frequent source or contributor to biochemical recurrence than has been believed. This is supported by data showing the majority of men with biochemical recurrence demonstrate a decrease in PSA level following radiation.13 In our data, a statistically significant improvement in prostate cancer–specific survival associated with salvage radiotherapy was confined to men whose PSA level became undetectable following radiation, even if it eventually began to increase again. Among men whose PSA level never became undetectable, a benefit of salvage radiotherapy appears less likely, but cannot be entirely ruled out due to the broad 95% CI for the HR.
Prostate cancer–specific survival was virtually identical in men who received salvage radiotherapy alone and salvage radiotherapy plus hormonal therapy, despite the latter exhibiting worse prognostic features. This is indirect evidence that hormonal therapy may provide additional benefit in the salvage radiotherapy setting for men with higher-risk features. However, this should be interpreted with caution given the small number of men who received combined therapy, and requires more rigorous study with consideration of timing and duration of hormonal therapy.
We demonstrated that salvage radiotherapy also was associated with improved overall survival among patients with stage pT3 prostate cancer, who exhibited overall survival of 98% at 5 years, similar to that observed with immediate adjuvant radiotherapy in randomized trials by Bolla et al5 (93%) and Thompson et al6 (approximately 90%). Possible reasons for somewhat higher overall survival observed in patients with stage pT3 prostate cancer who received salvage radiotherapy in our study compared with the studies by Bolla et al and Thompson et al include younger age at surgery (58 vs 64–65 years), lower percentage with seminal vesicle involvement (17% vs 25%–33%), lower preoperative PSA level (8.2 vs 10–12.3 ng/mL), and higher median radiation dose (66.6 vs 60–62 Gy).
The major limitation is the nonrandomized, retrospective nature of this study. Treatment decisions are heavily influenced by the established prognostic factors, which differ among the treatment groups in this study. Because these are well controlled in our analyses, it is unlikely that the apparent survival benefit associated with salvage radiotherapy is an artifact of imbalance in prognostic factors. We do not have data on comorbidity, which also can influence treatment decisions, but the similarity in deaths from causes unrelated to prostate cancer among the treatment groups suggests that comorbidity is an un-likely source of bias. The treatment groups may differ in unknown factors as well, and retrospective studies such as this are subject to variability in the completeness of follow-up data. Because hidden sources of bias cannot be ruled out, these results should be regarded as preliminary until validated in another setting. Another potential limitation is that the median follow-up in the study was 6 years after biochemical recurrence. Data from our institution indicate that in men with biochemical recurrence after radical prostatectomy, the median time to death is 13 years.4 Therefore, although follow-up in this study is longer than in previous studies of salvage radiotherapy, these estimates of survival benefit may change with more mature data. Finally, nonwhites comprised only 54 men (9%) in the study, so it is possible that results in a more diverse population would differ from those reported herein.
At this juncture, the potentially curative choices available for men with high risk of recurrence following prostatectomy consist of immediate adjuvant radiotherapy, salvage radiotherapy soon after evidence of biochemical recurrence, or salvage radiotherapy delayed until local recurrence. Favorable data from retrospective studies prompted recent randomized trials of adjuvant radiotherapy in patients with stage pT3 prostate cancer,5,6 the results of which strongly support a reduction in subsequent progression. The major disadvantage of immediate adjuvant radiotherapy is the need to treat 30% to 40% of men who would never develop biochemical failure, and expose them to the potential adverse effects of radiotherapy following surgery, which are frequently higher compared with radiotherapy used in the definitive setting.5,6 The question is whether careful patient follow-up with early initiation of salvage radiotherapy upon biochemical recurrence can achieve a comparable survival benefit while avoiding overtreating men who will not experience a recurrence. Although our study is not a comparison of salvage radiotherapy with adjuvant radiotherapy, our data provide the first evidence (albeit retrospective and hence, provisional) that early salvage radiotherapy is associated with improved prostate cancer–specific survival, and the magnitude of the survival benefit is similar to that observed in adjuvant radiotherapy trials. These data suggest that men for whom salvage radiotherapy is most beneficial are those with a PSA doubling time of less than 6 months, who also undergo treatment within 2 years of an increase in PSA level. If validated in other settings, these results could motivate a clinical trial comparing adjuvant with salvage radiotherapy, with prostate cancer–specific survival and overall survival as the primary end points.
Acknowledgments
Funding/Support: This study was supported in part by funds from the National Cancer Institute (grant CA58236) SPORE in Prostate Cancer and by gifts from Dr and Mrs Peter S. Bing to Dr Trock. Funding also was provided by the Department of Defense Prostate Cancer Research Program and the American Urological Association Foundation/Astellas Rising Star in Urology Award to Dr Freedland.
Role of the Sponsors: None of the sponsors or funders had any involvement in the design or conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review or approval of the manuscript.
Footnotes
Financial Disclosures: Dr Freedland reported serving on the speaker’s and advisory boards for AstraZeneca and the advisory board for GTX Inc. No other authors reported financial disclosures.
Author Contributions: Dr Trock had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Trock, Han, Freedland, Partin, Walsh.
Acquisition of data: Han, Freedland, Humphreys, Partin, Walsh.
Analysis and interpretation of data: Trock, Han, DeWeese, Walsh.
Drafting of the manuscript: Trock, Han, DeWeese, Partin.
Critical revision of the manuscript for important intellectual content: Trock, Freedland, Humphreys, DeWeese, Partin, Walsh.
Statistical expertise: Trock, Han, Humphreys.
Obtained funding: Trock, Partin, Walsh.
Administrative, technical, or material support: Partin.
Study supervision: Trock, Walsh.
References
- 1.Penson DF, Chan JM. Prostate cancer. J Urol. 2007;177(6):2020–2029. doi: 10.1016/j.juro.2007.01.121. [DOI] [PubMed] [Google Scholar]
- 2.Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy: the 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28(3):555–565. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 3.Ward JF, Moul JW. Rising prostate-specific antigen after primary prostate cancer therapy. Nat Clin Pract Urol. 2005;2(4):174–182. doi: 10.1038/ncpuro0145. [DOI] [PubMed] [Google Scholar]
- 4.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 5.Bolla M, van Poppel H, Collette L, et al. European Organization for Research and Treatment of Cancer. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005;366(9485):572–578. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 6.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296(19):2329–2335. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 7.Choo R, Kawakami J, Siemens R, et al. Two different perspectives in the management of pT3 and/or margin-positive prostate cancer after radical prostatectomy. BJU Int. 2006;98(4):773–776. doi: 10.1111/j.1464-410X.2006.06423.x. [DOI] [PubMed] [Google Scholar]
- 8.Boccon-Gibod L. pT3 prostate cancer: the case for salvage (as opposed to adjuvant) radiation therapy. Eur Urol Suppl. 2007;6(8):521–524. [Google Scholar]
- 9.Harrell FE., Jr . Logistic Regression and Survival Analysis. New York, NY: Springer Verlag; 2001. Regression Modeling Strategies With Applications to Linear Models. [Google Scholar]
- 10.Maffezzini M, Bossi A, Collette L. Implications of prostate-specific antigen doubling time as indicator of failure after surgery or radiation therapy for prostate cancer. Eur Urol. 2007;51(3):605–613. doi: 10.1016/j.eururo.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 11.Leventis AK, Shariat SF, Kattan MW, et al. Prediction of response to salvage radiation therapy in patients with prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2001;19(4):1030–1039. doi: 10.1200/JCO.2001.19.4.1030. [DOI] [PubMed] [Google Scholar]
- 12.Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291(11):1325–1332. doi: 10.1001/jama.291.11.1325. [DOI] [PubMed] [Google Scholar]
- 13.Ward JF, Zincke H, Bergstralh EJ, Slezak JM, Blute ML. Prostate specific antigen doubling time subsequent to radical prostatectomy as a prognosticator of outcome following salvage radiotherapy. J Urol. 2004;172(6 pt 1):2244–2248. doi: 10.1097/01.ju.0000145262.34748.2b. [DOI] [PubMed] [Google Scholar]
- 14.Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25(15):2035–2041. doi: 10.1200/JCO.2006.08.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anscher MS, Clough R, Dodge R. Radiotherapy for a rising prostate-specific antigen after radical prostatectomy: the first 10 years. Int J Radiat Oncol Biol Phys. 2000;48(2):369–375. doi: 10.1016/s0360-3016(00)00645-3. [DOI] [PubMed] [Google Scholar]
- 16.Song DY, Thompson TL, Ramakrishnan V, et al. Salvage radiotherapy for rising or persistent PSA after radical prostatectomy. Urology. 2002;60(2):281–287. doi: 10.1016/s0090-4295(02)01709-0. [DOI] [PubMed] [Google Scholar]
- 17.Chawla AK, Thakral HK, Zietman AL, Shipley WU. Salvage radiotherapy after radical prostatectomy for prostate adenocarcinoma: analysis of efficacy and prognostic factors. Urology. 2002;59(5):726–731. doi: 10.1016/s0090-4295(02)01540-6. [DOI] [PubMed] [Google Scholar]
- 18.Katz MS, Zelefsky MJ, Venkatraman ES, et al. Predictors of biochemical outcome with salvage conformal radiotherapy after radical prostatectomy for prostate cancer. J Clin Oncol. 2003;21(3):483–489. doi: 10.1200/JCO.2003.12.043. [DOI] [PubMed] [Google Scholar]
- 19.Liauw SL, Webster WS, Pistenmaa DA, Roehrborn CG. Salvage radiotherapy for biochemical failure of radical prostatectomy: a single-institution experience. Urology. 2003;61(6):1204–1210. doi: 10.1016/s0090-4295(03)00044-x. [DOI] [PubMed] [Google Scholar]
- 20.Buskirk SJ, Pisansky TM, Schild SE, et al. Salvage radiotherapy for isolated prostate specific antigen increase after radical prostatectomy: evaluation of prognostic factors and creation of a prognostic scoring system. J Urol. 2006;176(3):985–990. doi: 10.1016/j.juro.2006.04.083. [DOI] [PubMed] [Google Scholar]
- 21.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy: Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337(14):949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 22.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353(9165):1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 23.Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97(2):116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- 24.Clarke M, Collins R, Darby S, et al. Early Breast Cancer Trialists’ Collaborative Group. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 25.Partin AW, Pearson JD, Landis PK, et al. Evaluation of serum prostate-specific antigen velocity after radical prostatectomy to distinguish local recurrence from distant metastases. Urology. 1994;43(5):649–659. doi: 10.1016/0090-4295(94)90180-5. [DOI] [PubMed] [Google Scholar]