Abstract
The primary obstacle to producing renewable fuels from lignocellulosic biomass is a plant's recalcitrance to releasing sugars bound in the cell wall. From a sample set of wood cores representing 1,100 individual undomesticated Populus trichocarpa trees, 47 extreme phenotypes were selected across measured lignin content and ratio of syringyl and guaiacyl units (S/G ratio). This subset was tested for total sugar release through enzymatic hydrolysis alone as well as through combined hot-water pretreatment and enzymatic hydrolysis using a high-throughput screening method. The total amount of glucan and xylan released varied widely among samples, with total sugar yields of up to 92% of the theoretical maximum. A strong negative correlation between sugar release and lignin content was only found for pretreated samples with an S/G ratio < 2.0. For higher S/G ratios, sugar release was generally higher, and the negative influence of lignin was less pronounced. When examined separately, only glucose release was correlated with lignin content and S/G ratio in this manner, whereas xylose release depended on the S/G ratio alone. For enzymatic hydrolysis without pretreatment, sugar release increased significantly with decreasing lignin content below 20%, irrespective of the S/G ratio. Furthermore, certain samples featuring average lignin content and S/G ratios exhibited exceptional sugar release. These facts suggest that factors beyond lignin and S/G ratio influence recalcitrance to sugar release and point to a critical need for deeper understanding of cell-wall structure before plants can be rationally engineered for reduced recalcitrance and efficient biofuels production.
Lignocellulosic biomass is the only sustainable resource in terms of cost, availability, and scale that can be converted into liquid fuels to reduce the prevailing role of petroleum in providing energy for the world's transportation needs (1, 2) and to decrease the emissions of fossil CO2 that damage the world's climate (3). The primary obstacle to producing liquid transportation fuels by bioconversion methods is the release of sugars in high quantities at low costs from recalcitrant lignocellulosic biomass feedstocks (4, 5). Genetic modification of plants to make them less recalcitrant is a promising path to address this challenge on the feedstock side, but the effort would be greatly aided by improving understanding of the fundamental relationship between cell-wall composition and sugar release through pretreatment and enzymatic hydrolysis.
In this paper, we focus on the influence of lignin content and the ratio of its syringyl and guaiacyl units (S/G ratio) on recalcitrance to sugar release, because these two traits were previously identified as dominant factors (6). Although it is generally perceived that low lignin contents increase the ability of cellulolytic enzymes to hydrolyze plant fibers (7–11), only a limited number of studies investigated the effect of lignin S/G ratio on sugar release through combined pretreatment and enzymatic hydrolysis. Although some found no clear trend (8, 12), Li et al. (13) demonstrated that an Arabidopsis mutant containing mainly S-lignin showed a much higher sugar yield after hot-water pretreatment and enzymatic hydrolysis compared with the wild type and the S-deficient plant. Furthermore, a high S/G ratio is known to enhance the efficiency of Kraft pulping (14–17), but it adversely affects xylose release through dilute acid hydrolysis (6). However, the mentioned studies are characterized by small population sizes or coverage of narrow ranges in lignin content and S/G ratio. Thus, we initiated an unrivaled large-scale screening program by collecting 1,100 samples of a natural population of undomesticated Populus trichocarpa trees, quantifying the lignin content and S/G ratio, and selecting 47 extreme phenotypes across the entire range of measured traits. This subset was analyzed for sugar release by using our high-throughput pretreatment and enzymatic hydrolysis pipeline (18) to address the following questions:
i) How does lignin content and lignin S/G ratio correlate with recalcitrance to monosaccharide sugar release?
ii) Do changes in pretreatment process parameters influence the sugar release from each individual in the investigated sample set in the same manner, or do subsets within the population exist that are particularly susceptible to specific processing conditions? Furthermore, are there certain samples that achieve high yields if pretreatment is eliminated entirely?
iii) Finally, can biomass materials with exceptionally high or low sugar release be identified for further investigations that permit drawing conclusions on factors impacting recalcitrance?
Results
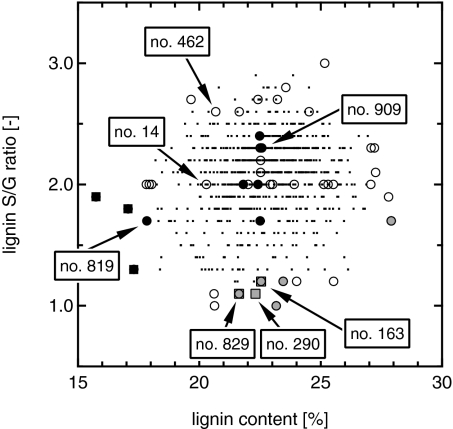
We established a large collection of biological samples from 1,100 geographically distributed, undomesticated P. trichocarpa genotypes and analyzed them for lignin content and S/G ratio. The sampled trees covered a wide span in lignin content (15.7–27.9%) and S/G ratio (1.0–3.0) (Fig. 1). A total of 47 samples were selected for in-depth analysis of recalcitrance to sugar release; 30 were selected based on their extreme values in lignin content and composition, whereas the other 17 were selected in an orthogonal manner along average S/G (2.0) and lignin (∼22.5%) values (Fig. 1). To measure sugar release, these samples were subjected to coupled pretreatment and enzymatic hydrolysis by a mixture of cellulase and xylanase using our high-throughput pretreatment and hydrolysis technique (HTPH) (18). In addition, samples were enzymatically hydrolyzed without pretreatment.
Fig. 1.
Characterization of the complete Populus association sample set, including the selected and analyzed 47 Populus samples. Relationships are shown between S/G ratios and lignin contents; 30 samples selected based on their extreme values in lignin content and composition as well as 17 additional samples selected in an orthogonal manner along an average S/G ratio (2.0) and lignin content (∼22.5%) were tested for their recalcitrance to sugar release. The dots (•) mark the complete 1,100 sample set, whereas the larger symbols mark the 47 analyzed samples. Samples exhibiting the highest (black) or lowest (gray) total sugar release from pretreatment and enzymatic hydrolysis (●) or just enzymatic hydrolysis (■) are highlighted. The labels point to interesting samples discussed in more detail in the text.
The total amount of glucan and xylan released was widely variable among the 47 tested genotypes. Sugar release ranged from 0.25 to 0.67 g glucose and xylose per gram dry raw biomass (35–91% of the theoretical sugar yield) for pretreatment at 180 °C, from 0.20 to 0.68 (28–92%) for pretreatment at 160 °C, and from 0.17 to 0.58 (23–83%) at 140 °C, showing that total sugar release generally dropped at lower pretreatment temperatures (Fig. 2 and Fig. S1). Without pretreatment, sugar yields also varied widely but were considerably lower, ranging from 0.05 to 0.40 g/g dry biomass (4–56%).
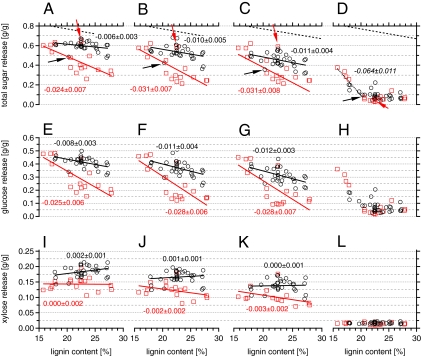
Fig. 2.
Total glucose plus xylose release for pretreatment of poplar at different temperatures followed by enzymatic hydrolysis using cellulase and xylanase and their relationship to lignin content. Samples were pretreated in just water at 180 °C for 18 min (A, E, and I), 160 °C for 28 min (B, F, and J), and 140 °C for 464 min (C, G, and K) or were directly subjected to enzymatic hydrolysis without pretreatment (D, H, and L). Each marker represents the mean value of three replicates for all pretreatment conditions and two replicates for just enzymatic hydrolysis. The sugar releases are displayed in grams sugars per grams raw biomass. The maximum theoretical sugar release based on the composition of the Populus standard is represented by the declining dotted line (A–D). The markers distinguish samples featuring S/G ratios < 2.0 (□) and ≥ 2.0 (○). The numbers denote the slope of the trend lines of the two subgroups for low and high lignin contents with the respective SDs. The black arrow points to sample number 014, and the red arrow points to sample number 152.
Total sugar release from pretreatment and enzymatic hydrolysis was negatively correlated with lignin content for all pretreatment temperatures. Furthermore, if the S/G ratio of the samples was also taken into account, the negative correlation became much stronger for samples with low S/G ratios (<2.0). This observation held for all pretreatment temperatures (Fig. 2 A–C), because the corresponding trend lines showed statistically identical slopes of −0.03 (Table S1). In contrast, samples with larger S/G ratios (≥2.0) generally showed higher sugar release, and the negative influence of lignin was less pronounced, with the slopes of regression lines approximating the slope of the theoretical maximum yield curve (−0.01), reflecting the expected tradeoff between carbohydrate and lignin contents (Fig. 2 A–C). Separate analysis of glucose and xylose release revealed that glucose followed the trends with lignin content and composition (Fig. 2 E–G), whereas xylose release was independent of lignin content but generally higher for the high S/G subset (Fig. 2 I–K and Table S1). Furthermore, we identified unusual outliers that clearly did not follow the described dependency on lignin content for the respective S/G group. For example, biomass number 014 showed a low sugar yield relative to its peers after pretreatment and hydrolysis at all temperatures, and biomass number 152 showed a comparably high sugar release for the low S/G group.
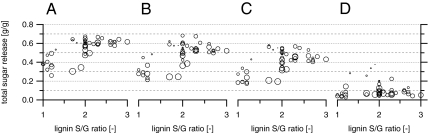
As indicated above, higher S/G ratios were beneficial to high monosaccharide release. Total sugar release tended to increase with increasing S/G ratios (Fig. 3 A–C), which was also the case for glucose and xylose release if analyzed separately (Fig. S2 A–C and E–G and Table S1).
Fig. 3.
Total sugar release from pretreatment and enzymatic hydrolysis of poplar correlated to lignin S/G ratio and lignin content. The latter is indicated by the marker size. Samples were pretreated at 180 °C (A), 160 °C (B), and 140 °C (C) or were directly subjected to enzymatic hydrolysis without pretreatment (D). Each marker represents the mean value of three replicates for all pretreatment conditions and two replicates for just enzymatic hydrolysis. The sugar releases are displayed in grams sugars per grams raw biomass.
For samples enzymatically hydrolyzed without pretreatment, sugar yields were generally low, except for individuals with lignin contents below 20%. In this subgroup, digestibility increased considerably with decreasing lignin content, yielding up to 56% of the theoretical sugar yield, higher than sugar release from most of the pretreated samples featuring an S/G value < 2.0 (Figs. 2D and 3D). Interestingly, in these samples, mainly glucose was released, whereas xylose remained virtually untouched (Fig. 2 H and L and Fig. S2 D and H).
From the 47 samples, we identified individual samples that exhibited unusually high total sugar release (i.e., the three samples with the highest sugar release for each pretreatment condition) (Table 1 and Fig. 1). Depending on pretreatment temperature, different individual biomasses ranked in the top three, with the exception of one biomass (number 909), which gave the second highest mass yields for all three pretreatment temperatures (Table 1). However, when considering a larger subset consisting of the top 20% of all tested variants, the same seven individuals ranked in the top nine for all hot-water pretreatments, and interestingly, all samples except two individuals (numbers 819 and 462) were members of the orthogonally selected control group featuring average lignin and S/G values (Fig. 1). Only two of seven well-performing pretreated samples (numbers 819 and 909) were also represented in the top nine for the no pretreatment case (Table S2). The worst nine performing biomasses were the same for all pretreatments and featured either very low S/G ratios (S/G ≤ 1.2) or very high lignin contents (≥27.8%) (Tables S2 and S3). In contrast, the S/G ratios of samples representing the lowest tier for direct enzymatic hydrolysis of not pretreated biomass covered the entire spectrum analyzed and also featured average lignin contents. Only three of nine samples (numbers 163, 290, and 829) were present on both negative lists (Tables S2 and S3).
Table 1.
Total sugar release for the top three performing Populus samples at each pretreatment condition tested (indicated by asterisk), with their performance and relative rankings listed for the other conditions applied
| Rank | Sample ID | Sugar release (g/g) and rank (number) for the other pretreatments | Lignin content (%) | S/G ratio (−) | dbh (cm) | |||
| 180 °C | 160 °C | 140 °C | No pretreatment | |||||
| 180 °C | ||||||||
| 1 | 273 | 0.672 ± 0.014* | 0.608 ± 0.006 (4) | 0.550 ± 0.010 (6) | 0.060 ± 0.030 (32) | 22.42 | 2.0 | 23 |
| 2 | 909 | 0.667 ± 0.030* | 0.675 ± 0.010 (2) | 0.568 ± 0.012 (2) | 0.224 ± 0.007 (5) | 22.57 | 2.0 | 24 |
| 3 | 349 | 0.654 ± 0.015* | 0.606 ± 0.033 (5) | 0.551 ± 0.008 (5) | 0.074 ± 0.049 (25) | 22.48 | 2.0 | 14 |
| 160 °C | ||||||||
| 1 | 833 | 0.600 ± 0.015 (23) | 0.682 ± 0.007* | 0.498 ± 0.010 (12) | 0.169 ± 0.015 (8) | 21.81 | 2.0 | 26 |
| 2 | 909 | 0.667 ± 0.030 (2) | 0.675 ± 0.010* | 0.568 ± 0.012 (2) | 0.224 ± 0.007 (5) | 22.57 | 2.0 | 24 |
| 3 | 819 | 0.634 ± 0.004 (7) | 0.622 ± 0.014* | 0.580 ± 0.019 (1) | 0.258 ± 0.002 (4) | 17.83 | 1.7 | 49 |
| 140 °C | ||||||||
| 1 | 819 | 0.634 ± 0.004 (7) | 0.622 ± 0.014 (3) | 0.580 ± 0.019* | 0.258 ± 0.002 (4) | 17.83 | 1.7 | 49 |
| 2 | 909 | 0.667 ± 0.030 (2) | 0.675 ± 0.010 (2) | 0.568 ± 0.012* | 0.224 ± 0.007 (5) | 22.57 | 2.3 | 24 |
| 3 | 152 | 0.644 ± 0.002 (5) | 0.590 ± 0.054 (9) | 0.564 ± 0.005* | 0.05 ± 0.020 (34) | 22.5 | 1.7 | 38 |
| No pretreatment | ||||||||
| 1 | 876 | 0.601 ± 0.009 (22) | 0.576 ± 0.005 (10) | 0.553 ± 0.009 (4) | 0.376 ± 0.011* | 15.74 | 1.9 | 8 |
| 2 | 081 | 0.606 ± 0.021 (19) | 0.575 ± 0.030 (11) | 0.533 ± 0.021 (8) | 0.336 ± 0.020* | 17.06 | 1.8 | 16 |
| 3 | 869 | 0.534 ± 0.008 (32) | 0.483 ± 0.030 (31) | 0.430 ± 0.017 (24) | 0.283 ± 0.013* | 17.30 | 1.3 | 92 |
Shown are the mean values from three replicates for all pretreatment conditions and two replicates for just enzymatic hydrolysis together with SEMs. The sugar releases are reported in grams sugars per grams raw biomass. dbh, diameter on breast height (i.e., the diameter of the sampled tree).
Discussion
We studied a selection of wood increment core samples from a large natural population of P. trichocarpa trees with considerable natural variation in cell-wall composition to determine fermentable sugar release from pretreatment in hot water followed by enzymatic hydrolysis. We selected pretreatment with just water at moderate temperatures to mimic pragmatic, environmental friendly, future large-scale conditions. To enhance differences in sugar release between individual samples, pretreatment times were adjusted to correspond to an overall severity of 3.6 (Eq. 1), which is below the optimum severity of 4.1 established for our internal Populus biomass standard (Fig. S3). A relatively high enzyme loading was used to overcome potential inhibition by substances released or formed during pretreatment (18), which would be washed away in the base case cellulosic ethanol process (19), and also, to ensure that changes in recalcitrance could be distinguished from limitations in enzyme performance. Despite the high enzyme loading, profound differences in sugar yield were found for different pretreatment severities (Fig. S3), allowing us to conclude that the chosen conditions were suitable to investigate the influence of S/G ratio and lignin content on recalcitrance for the selected biomasses. This judgment was indeed confirmed by the broad range of sugar release measured (Figs. 2 and 3).
We found that sugar release depended on both lignin content and lignin composition (i.e., yields tended to increase with increasing S/G ratios and decrease with lignin content). At the current state of investigations, we can only speculate on reasons for the higher reactivity of S-rich lignin. Generally, S-rich lignin features predominantly linear chains with less cross-linking than G-rich lignin because of the methoxylated and thereby, blocked C-5 position in the syringyl unit (20), resulting in fewer highly stable 5–5 and β-5 linkages (20–22). The higher occurrence of β-β units (resinols) in S-rich lignin leads to shorter chain lengths and thus, lower molecular weights (21), which potentially alter thermoplastic properties, including lowering melting points (13). The relative amount of chemically labile β-O-4 ether linkages has been shown to remain constant independent of the S/G ratio if at least some syringyl units are present (20, 21). Mainly, these linkages are cleaved not only during Kraft pulping (15) but more importantly, also during hydrothermal (23) and dilute acid pretreatment (17). Steam explosion of P. tremuloides reduced the relative amount of the remaining β-O-4 linkages from 78% to 19% when the severity (logR0) was increased from 3.2 to 4.5 (23). Furthermore, the S/G ratio has been shown to drop during dilute acid pretreatment (17), pointing to a higher reactivity of S-lignin, a trend that has also been confirmed by the faster cleavage of β-O-4 linkages in S-lignin under alkaline conditions (24). Higher pretreatment severities result in a presumably higher degree of β-O-4 cleavage that leads to higher sugar yields after enzymatic hydrolysis (Fig. S3). Thus, we deduce that the increase in digestibility from combined pretreatment and enzymatic hydrolysis with increased S/G ratios is mainly related to the more labile β-O-4 bonds in S-lignin during pretreatment.
The S/G ratio also determines the dependency of sugar release on lignin content. Although the common assumption is that high lignin content adversely affects enzymatic hydrolysis (7, 25, 26), we observed no influence of lignin content on sugar release for high S/G ratios (≥2.0) (Fig. 2 A–C). The chosen S/G ratio of 2.0 should not be considered a sharp threshold, because further analysis suggested a gradually increasing dependency of sugar release on lignin content with decreasing S/G ratio (Fig. S4); however, the sample size must be increased to statistically support this conclusion.
These results can only partly be validated by literature, because lignin composition is often not recorded (9, 10). However, Chen and Dixon (8), who investigated samples featuring very low S/G ratios (0.3–1.0), found a similar strong dependency of release on lignin content. Overall, the finding that the sugar yield does not depend on lignin content for high S/G ratios was unexpected, and it points to the possibility that pretreatment modifies lignin to such an extent that it does not impact enzymatic hydrolysis. Nevertheless, high lignin contents are not desired because of the displacement of fermentable carbohydrates in the biomass and its nonproductive binding to enzyme (27).
For enzymatic hydrolysis of Populus samples without prior pretreatment, the highest sugar release was found for lignin contents less than 20% (Fig. 2D). Furthermore, because hydrolysis was independent of the S/G ratio (Fig. 3D), as also confirmed by Li et al. (13), enzyme penetration seems to be unaltered by the above-described higher amount of cross-links in G-rich lignins. Interestingly, only glucose was released, whereas xylan was not released (Fig. 2 H and L), a remarkable result in light of the conventional perception that either hemicellulose and/or lignin needs to be removed or relocated to enable high glucose yields by enzymatic hydrolysis (7, 28–31). This raises the question as to whether these samples contained unusually high amounts of endogenous sugars, starch, or tension wood. Although samples were not routinely tested for free sugars and starch, analysis of nine random samples yielded 0.2–0.6% (wt/wt) endogenous glucose or starch based on the raw biomass (Table S4), which is in good agreement with expected values (32, 33), whereas young trees may contain higher amounts of sugars of up to 10% (34). Furthermore, the amylase activity expressed by the used enzyme mixture is low, with expected sugar yields from starch below 10% (Table S5). Taken together, it is unlikely that starch or endogenous sugar content can account for the high sugar release of up to 0.4 g/g for low lignin samples. Tension wood in Populus formed by external stimuli, such as wind or gravitropic sway (1), contains an additional inner gelatinous layer of almost pure, highly oriented, and crystalline cellulose microfibrils (35, 36) that could account for the relatively large amounts of glucose released without pretreatment. However, tension wood is also known to have an increased S/G ratio (37, 38) compared with normal wood, whereas the select samples were all below 2.0. Therefore, it is not likely that tension wood accounted for the observed high sugar yields in these samples. Attributing the high sugar release to tree age can also be ruled out, because the best performing trees covered a wide range in diameters (i.e., age) between 8 and 92 cm.
Despite the correlations between sugar release and lignin content and/or S/G ratio described above, several samples performed considerably better, although they showed average values in the analyzed cell-wall traits (Table 1). As a result, other strictures or factors must be influencing recalcitrance to sugar release, possibly including (i) the presence of incorporated p-hydroxybenzoates acylated (15, 39) or acetylated (40) monolignols, (ii) the amount of free phenolic groups in lignin (15), (iii) the amount and structural features of xylan (e.g., chain length and side-chain substitution pattern) (41), (iv) differences in cell and tissue anatomy (1), (v) insect herbivory or pathogen attack (42), and/or (vi) the proximity of the sampling point to abscised lateral branches (43). None of the above covariants were quantified in the current study, although all areas containing visual defects were avoided during sampling; however, rather extreme cell-wall phenotypes were selected and analyzed for sugar release independent of their genetic or environmentally induced origins. Thus, a deeper understanding of cell-wall structure, anatomy, and biochemistry is critically needed, because plants are being systematically engineered for reduced recalcitrance and efficient biofuels production.
Materials and Methods
Populus Association Samples.
One thousand one hundred P. trichocarpa (Torr. & Gray) trees were systematically sampled from within a 1.7° latitudinal gradient from northwest Washington to central Oregon. The sampling took place in December 2008. Each sampled tree was selected based on a range of diameters (Table 1 and Table S3), upright form, and lack of obvious physical or biological damage. We were not able to account for historical differences in microclimate or influence by external stimuli, such as gravitropic or biologic factors. The geographical location of each tree was recorded, and wood cores were extracted using a three-thread 0.17-in (4.3-mm) core and 12-in (304.8-mm) Haglöf increment borer to depth or the center of the tree. The core samples were air-dried and knife-milled along their entire length (Thomas-Wiley Mini-Mill; Thomas Scientific) to a particle size <20 mesh (0.85 mm) by Oak Ridge National Laboratory (ORNL). A homogenized subsample was obtained and then sent to the National Renewable Energy Lab (NREL) for analytical pyrolysis.
Biomass Analysis.
All biomass samples were analyzed for lignin content and S/G ratio by analytical pyrolysis as described elsewhere (43–46). Briefly, ∼4 mg ground Populus material were pyrolyzed for 2 min at 500 °C, and the pyrolysis vapors were entrained in helium flowing at 2 L/min to a mass spectrometer. Spectra were collected over an m/z range from 30 to 450 using 22.5-eV electron impact ionization. Lignin content was determined by summing the relative intensity of the major lignin peaks (m/z ratios of 120, 124, 137, 138, 150, 152, 154, 164, 167, 178, 180, 181, 182, 194, and 210) and multiplying the sum by a correction factor calculated from the mass spectrum of a standard P. deltoides (NIST 8492; National Institute of Standards and Technology) and its known absolute lignin content. S/G ratios were determined by summing the intensity of the syringyl peaks at 154, 167, 168, 182, 194, 208, and 210 and dividing by the sum of intensity of guaiacyl peaks at 124, 137, 138, 150, 164, and 178. All pyrolysis mass spectra are known to be genetically controlled and heritable (47).
Pretreatment and Enzymatic Hydrolysis.
All biomass samples were subjected to a combined high-throughput pretreatment and enzymatic hydrolysis process based on a 96-well plate format to test for sugar release as described elsewhere (18). Briefly, ∼2.6 mg Populus material were weighed into an individual Hastelloy well on a 96-well plate. Then, 247.4 μL deionized water were added to each well to produce a range of solids concentrations from 0.70% to 1.17% (wt/wt), and the biomass was incubated at room temperature for 4 h. All pretreatments were conducted at a logR0 severity of 3.6 (Eq. 1) by heating the well plate with condensing steam to temperatures of 180 °C, 160 °C, and 140 °C for 17.6, 68.1, and 464.4 min, respectively. Severity was defined as (Eq. 1)
 |
where t is in minutes and T in degrees Celsius (48).
After pretreatment, 20 μL enzyme/buffer/sodium azide mixture, as specified below, were pipetted into each well without any preceding separation or washing steps. Spezyme CP (lot number 3016295230; 116 mg protein/mL and 62 filter paper units (FPU)/mL) and Multifect Xylanase (56.6 mg protein/mL; lot number 301–04021-015; Genecore) were used as cellulolytic enzymes with a loading of 75 + 25 mg cellulase and xylanase protein, respectively, per 1 g glucan + xylan in raw biomass for the Populus standard, which had a composition of 46.2% glucan, 14.8% xylan, and 27.0% lignin. The final concentrations of citric acid buffer (pH 4.95) and sodium azide were 0.05 M and 0.01 g/L, respectively. The samples were incubated in a shaker (Multitron; Infors-HT) at 50 °C for 72 h at 150 rpm and then analyzed for sugars in the supernatant. If pretreatment was omitted, samples were only soaked for 4 h in water and then directly subjected to enzymatic hydrolysis as described.
For each independent biomass sample, three analytical replicates were performed for all pretreatment conditions, and two replicates were performed for enzymatic hydrolysis without pretreatment.
Sugar Analysis.
Cellobiose, glucose, and xylose concentrations were measured using HPLC. An Aminex HPX-87H column (BioRad) heated to 65 °C was used in a separation module (Alliance 2695; Waters) equipped with a refractive index detector (2414; Waters) using 0.005 M sulfuric acid as the eluent in an isocratic mode.
Statistical Analysis.
Linear regression of Eq. 2 using a dummy variable z with values of 0 or 1 to distinguish the respective sample sets was applied to test the null hypothesis of no statistical difference in slope and intercept (Eq. 2):
The null hypothesis was rejected at the 0.05 level. Regression analysis was performed using Igor Pro (Wavemetrics).
Supplementary Material
Acknowledgments
We thank Karen Huaying Xu (University of California Riverside) for help on the statistical analysis; Kristen Reichel, Steve Thomas, Justin Anderson, Geoffrey Turner, and Angela Ziebell [National Renewable Energy Laboratory (NREL)] for HTP screening of plant cell-wall chemistry traits; Lee Gunter, Sara Jawdy, Nancy English, and Xiaohan Yang [Oak Ridge National Laboratory (ORNL)] and Gancho Slavov and Steve DiFazio (West Virginia University) for sample collection, design, preparation, and shipping; and Susan Holladay (Oak Ridge National Laboratory) for the Laboratory Information Management System data organization. The authors would also like to extend their appreciation to Eugene Nothnagel (University of California Riverside) and Simone Brethauer [Eidgenössiche Technische Hochschule (Switzerland)] for their valuable discussions and insights. Support by the Office of Biological and Environmental Research in the Department of Energy (DOE) Office of Science for the BioEnergy Science Center (BESC) made this research possible.
Footnotes
Conflict of interest statement: C.E.W. is cofounder of Mascoma Corporation and chair of their Scientific Advisory Board. C.E.W. is also a member of the Scientific Advisory Board of Mendel Biotechnology, Inc.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009252108/-/DCSupplemental.
References
- 1.Dinus RJ, Payne P, Sewell NM, Chiang VL, Tuskan GA. Genetic modification of short rotation popular wood: Properties for ethanol fuel and fiber productions. Crit Rev Plant Sci. 2001;20:51–69. [Google Scholar]
- 2.Ragauskas AJ, et al. The path forward for biofuels and biomaterials. Science. 2006;311:484–489. doi: 10.1126/science.1114736. [DOI] [PubMed] [Google Scholar]
- 3.Tuskan GA, Walsh ME. Short-rotation woody crop systems, atmospheric carbon dioxide and carbon management: A US case study. Forest Chron. 2001;77:259–264. [Google Scholar]
- 4.Lynd LR, Cushman JH, Nichols RJ, Wyman CE. Fuel ethanol from cellulosic biomass. Science. 1991;251:1318–1323. doi: 10.1126/science.251.4999.1318. [DOI] [PubMed] [Google Scholar]
- 5.Lynd LR, Wyman CE, Gerngross TU. Biocommodity engineering. Biotechnol Prog. 1999;15:777–793. doi: 10.1021/bp990109e. [DOI] [PubMed] [Google Scholar]
- 6.Davison BH, Drescher SR, Tuskan GA, Davis MF, Nghiem NP. Variation of S/G ratio and lignin content in a Populus family influences the release of xylose by dilute acid hydrolysis. Appl Biochem Biotechnol. 2006;129-132:427–435. doi: 10.1385/abab:130:1:427. [DOI] [PubMed] [Google Scholar]
- 7.Chang VS, Holtzapple MT. Fundamental factors affecting biomass enzymatic reactivity. Appl Biochem Biotechnol. 2000;84-86:5–37. doi: 10.1385/abab:84-86:1-9:5. [DOI] [PubMed] [Google Scholar]
- 8.Chen F, Dixon RA. Lignin modification improves fermentable sugar yields for biofuel production. Nat Biotechnol. 2007;25:759–761. doi: 10.1038/nbt1316. [DOI] [PubMed] [Google Scholar]
- 9.Dien BS, et al. Improved sugar conversion and ethanol yield for forage sorghum (Sorghum bicolor L. Moench) lines with reduced lignin contents. Bioenergy Res. 2009;2:153–164. [Google Scholar]
- 10.Vermerris W, et al. Molecular breeding to enhance ethanol production from corn and sorghum stover. Crop Sci. 2007;47:S142–S153. [Google Scholar]
- 11.Wyman CE, et al. Comparative sugar recovery and fermentation data following pretreatment of poplar wood by leading technologies. Biotechnol Prog. 2009;25:333–339. doi: 10.1002/btpr.142. [DOI] [PubMed] [Google Scholar]
- 12.Jackson LA, et al. Improving saccharification efficiency of alfalfa stems through modification of the terminal stages of monolignol biosynthesis. Bioenergy Res. 2008;1:180–192. [Google Scholar]
- 13.Li X, et al. Lignin monomer composition affects Arabidopsis cell-wall degradability after liquid hot water pretreatment. Biotechnol Biofuels. 2010;3:27. doi: 10.1186/1754-6834-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huntley SK, Ellis D, Gilbert M, Chapple C, Mansfield SD. Significant increases in pulping efficiency in C4H-F5H-transformed poplars: Improved chemical savings and reduced environmental toxins. J Agric Food Chem. 2003;51:6178–6183. doi: 10.1021/jf034320o. [DOI] [PubMed] [Google Scholar]
- 15.Lapierre C, et al. Structural alterations of lignins in transgenic poplars with depressed cinnamyl alcohol dehydrogenase or caffeic acid O-methyltransferase activity have an opposite impact on the efficiency of industrial Kraft pulping. Plant Physiol. 1999;119:153–164. doi: 10.1104/pp.119.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Weng JK, Chapple C. Improvement of biomass through lignin modification. Plant J. 2008;54:569–581. doi: 10.1111/j.1365-313X.2008.03457.x. [DOI] [PubMed] [Google Scholar]
- 17.Samuel R, Pu YQ, Raman B, Ragauskas AJ. Structural characterization and comparison of switchgrass ball-milled lignin before and after dilute acid pretreatment. Appl Biochem Biotechnol. 2010;162:62–74. doi: 10.1007/s12010-009-8749-y. [DOI] [PubMed] [Google Scholar]
- 18.Studer MH, DeMartini JD, Brethauer S, McKenzie HL, Wyman CE. Engineering of a high-throughput screening system to identify cellulosic biomass, pretreatments, and enzyme formulations that enhance sugar release. Biotechnol Bioeng. 2010;105:231–238. doi: 10.1002/bit.22527. [DOI] [PubMed] [Google Scholar]
- 19.Wyman C, editor. Handbook on Bioethanol: Production and Utilization. Washington, DC: Taylor & Francis; 1996. [Google Scholar]
- 20.Stewart JJ, Akiyama T, Chapple C, Ralph J, Mansfield SD. The effects on lignin structure of overexpression of ferulate 5-hydroxylase in hybrid poplar. Plant Physiol. 2009;150:621–635. doi: 10.1104/pp.109.137059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kishimoto T, et al. Influence of syringyl to guaiacyl ratio on the structure of natural and synthetic lignins. J Agric Food Chem. 2010;58:895–901. doi: 10.1021/jf9035172. [DOI] [PubMed] [Google Scholar]
- 22.Sannigrahi P, Ragauskas AJ, Tuskan GA. Poplar as a feedstock for biofuels: A review of compositional characteristics. Biofuel Bioprod Bior. 2010;4:209–226. [Google Scholar]
- 23.Li JB, Henriksson G, Gellerstedt G. Lignin depolymerization/repolymerization and its critical role for delignification of aspen wood by steam explosion. Bioresour Technol. 2007;98:3061–3068. doi: 10.1016/j.biortech.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Tsutsumi Y, Kondo R, Sakai K, Imamura H. The difference of reactivity between syringyl lignin and guaiacyl lignin in alkaline systems. Holzforschung. 1995;49:423–428. [Google Scholar]
- 25.Dien BS, et al. Chemical composition and response to dilute-acid pretreatment and enzymatic saccharification of alfalfa, reed canarygrass, and switchgrass. Biomass Bioenergy. 2006;30:880–891. [Google Scholar]
- 26.Vinzant TB, Ehrman CI, Adney WS, Thomas SR, Himmel ME. Simultaneous saccharification and fermentation of pretreated hardwoods—effect of native lignin content. Appl Biochem Biotechnol. 1997;62:99–104. [Google Scholar]
- 27.Palonen H, Tjerneld F, Zacchi G, Tenkanen M. Adsorption of Trichoderma reesei CBH I and EG II and their catalytic domains on steam pretreated softwood and isolated lignin. J Biotechnol. 2004;107:65–72. doi: 10.1016/j.jbiotec.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Chapple C, Ladisch M, Meilan R. Loosening lignin's grip on biofuel production. Nat Biotechnol. 2007;25:746–748. doi: 10.1038/nbt0707-746. [DOI] [PubMed] [Google Scholar]
- 29.Converse AO. Substrate factors limiting enzymatic hydrolysis. In: Saddler JN, editor. Bioconversion of Forest and Agricultural Plant Residues. Wallingford, UK: CAB International; 1993. pp. 93–106. [Google Scholar]
- 30.Donohoe BS, Decker SR, Tucker MP, Himmel ME, Vinzant TB. Visualizing lignin coalescence and migration through maize cell walls following thermochemical pretreatment. Biotechnol Bioeng. 2008;101:913–925. doi: 10.1002/bit.21959. [DOI] [PubMed] [Google Scholar]
- 31.Sánchez OJ, Cardona CA. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour Technol. 2008;99:5270–5295. doi: 10.1016/j.biortech.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Essiamah S, Eschrich W. Changes of starch content in the storage tissues of deciduous trees during winter and spring. IAWA Bull. 1985;6:97–106. [Google Scholar]
- 33.Sauter JJ, Wellenkamp S. Seasonal changes in content of starch, protein and sugars in the twig wood of Salix caprea L. Holzforschung. 1998;52:255–262. [Google Scholar]
- 34.Novaes E, Kirst M, Chiang V, Winter-Sederoff H, Sederoff R. Lignin and biomass: A negative correlation for wood formation and lignin content in trees. Plant Physiol. 2010;154:555–561. doi: 10.1104/pp.110.161281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyd JD. Relationship between fiber morphology and shrinkage of wood. Wood Sci Technol. 1977;11:3–22. [Google Scholar]
- 36.Timell TE. Chemical composition of tension wood. Sven Paperstidn. 1969;72:173–181. [Google Scholar]
- 37.Pilate G, et al. Lignification and tension wood. C R Biol. 2004;327:889–901. doi: 10.1016/j.crvi.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Koehler L, Telewski FW. Biomechanics and transgenic wood. Am J Bot. 2006;93:1433–1438. doi: 10.3732/ajb.93.10.1433. [DOI] [PubMed] [Google Scholar]
- 39.Boerjan W, Ralph J, Baucher M. Lignin biosynthesis. Annu Rev Plant Biol. 2003;54:519–546. doi: 10.1146/annurev.arplant.54.031902.134938. [DOI] [PubMed] [Google Scholar]
- 40.Weng JK, Li X, Bonawitz ND, Chapple C. Emerging strategies of lignin engineering and degradation for cellulosic biofuel production. Curr Opin Biotechnol. 2008;19:166–172. doi: 10.1016/j.copbio.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 41.York WS, O'Neill MA. Biochemical control of xylan biosynthesis—which end is up? Curr Opin Plant Biol. 2008;11:258–265. doi: 10.1016/j.pbi.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Pilate G, et al. Field and pulping performances of transgenic trees with altered lignification. Nat Biotechnol. 2002;20:607–612. doi: 10.1038/nbt0602-607. [DOI] [PubMed] [Google Scholar]
- 43.Sykes R, Kodrzycki B, Tuskan G, Foutz K, Davis M. Within tree variability of lignin composition in Populus wood science and technology. Wood Sci Technol. 2008;42:649–661. [Google Scholar]
- 44.Evans RJ, Milne TA. Molecular characterization of the pyrolysis of biomass. 1. Fundamentals. Energ Fuel. 1987;1:123–137. [Google Scholar]
- 45.Sykes R, et al. In: High Throughput Screening of Plant Cell Wall Composition Using Pyrolysis Molecular Beam Mass Spectroscopy. Biofules: Methods and Protocols, Methods in Molecular Biology. Mielenz JR, editor. Totowa, NJ: Humana Press; 2010. pp. 169–183. [DOI] [PubMed] [Google Scholar]
- 46.Tuskan G, et al. Two high-throughput techniques for determining wood properties as part of a molecular genetics analysis of hybrid poplar and loblolly pine. Appl Biochem Biotechnol. 1999;77:55–65. [Google Scholar]
- 47.Yin TY, et al. Differential detection of genetic Loci underlying stem and root lignin content in Populus. PLoS One. 2010;5:e14021. doi: 10.1371/journal.pone.0014021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chum HL, Johnson DK, Black SK, Overend RP. Pretreatment catalyst effects and the combined severity parameter. Appl Biochem Biotechnol. 1990;24-5:1–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.