Abstract
The bone-sparing effect of estrogen is primarily mediated via estrogen receptor-α (ERα), which stimulates target gene transcription through two activation functions (AFs), AF-1 in the N-terminal and AF-2 in the ligand binding domain. To evaluate the role of ERα AF-1 and ERα AF-2 for the effects of estrogen in bone in vivo, we analyzed mouse models lacking the entire ERα protein (ERα−/−), ERα AF-1 (ERαAF-10), or ERα AF-2 (ERαAF-20). Estradiol (E2) treatment increased the amount of both trabecular and cortical bone in ovariectomized (OVX) WT mice. Neither the trabecular nor the cortical bone responded to E2 treatment in OVX ERα−/− or OVX ERαAF-20 mice. OVX ERαAF-10 mice displayed a normal E2 response in cortical bone but no E2 response in trabecular bone. Although E2 treatment increased the uterine and liver weights and reduced the thymus weight in OVX WT mice, no effect was seen on these parameters in OVX ERα−/− or OVX ERαAF-20 mice. The effect of E2 in OVX ERαAF-10 mice was tissue-dependent, with no or weak E2 response on thymus and uterine weights but a normal response on liver weight. In conclusion, ERα AF-2 is required for the estrogenic effects on all parameters evaluated, whereas the role of ERα AF-1 is tissue-specific, with a crucial role in trabecular bone and uterus but not cortical bone. Selective ER modulators stimulating ERα with minimal activation of ERα AF-1 could retain beneficial actions in cortical bone, constituting 80% of the skeleton, while minimizing effects on reproductive organs.
Estrogen is the major sex hormone involved in the regulation of bone mass in women, and several studies demonstrate that estrogen is also of importance for the male skeleton (1–5). However, estrogen treatment is associated with side effects such as breast cancer and thromboembolism (6, 7). Thus, it would be beneficial to develop a bone-specific estrogen treatment. To achieve this, it will be crucial to characterize the signaling pathways of estrogen in bone versus other tissues.
The biological effects of estradiol (E2) are mainly mediated by the nuclear estrogen receptors (ERs), ERα and ERβ, which interact with several classes of coactivators/corepressors in a ligand-dependent manner (5, 8). The bone-sparing effect of estrogen is mediated primarily via ERα (5, 9, 10), although the effect of ERα activation in bone might be slightly modulated by ERβ in female mice (11–13). In addition, some in vitro studies suggest that the membrane G protein-coupled receptor GPR30 is a functional ER, but we recently demonstrated that the E2 response on bone mass is independent of GPR30 (14).
The relative balance of receptors, coactivators, and corepressors is a critical determinant of the ability of the nuclear receptors to regulate gene transcription. As the relative concentrations of these molecules are cell type-specific, estrogen can exert vastly different effects in different tissues. Variation in the recruitment of coregulatory molecules also appears to be a mechanism by which selective ER modulators produce their tissue-specific effects (15). In vitro studies have shown that the E2-induced transactivation is mediated by AF-1 and/or AF-2 in ERα (Fig. 1A) and that this is dependent on the cell type and promoter context (16–18) and could depend on the cofactors found in the cell type evaluated. Several cofactors bind to ERα AF-1 and ERα AF-2; some are specific for either AF-1 or AF-2 and some cofactors bind to both (19). It has also been shown that the full ligand-dependent transcriptional activity of ERα is reached through a synergism between AF-1 and AF-2 (16–18, 20–22). Full-length 66-kDa ERα stimulates target gene transcription through AF-1 and AF-2, whereas another physiologically expressed, but less abundant, 46-kDa ERα isoform lacks the N-terminal A/B domains and is consequently devoid of AF-1 (Fig. 1A). Although the E2-induced interactions between AF-1 and AF-2 in ERα and coregulatory molecules are characterized in vitro, very little is known about these interactions in vivo. However, we recently developed a mouse model with a specific inactivation of AF-1 in ERα and demonstrated that AF-1 is required for the effect of E2 in uterus although it is dispensable for the vasculoprotective actions of E2 (23). The roles of AF-1 and AF-2 in ERα for the effects of E2 in bone are unknown. To evaluate the roles of ERα AF-1 and ERα AF-2 in vivo for the effects of estrogen in bone and some other major estrogen responsive tissues, mouse models with inactivation of the entire ERα protein (ERα−/−), ERα AF-1 (ERαAF-10), or ERα AF-2 (ERαAF-20) were analyzed.
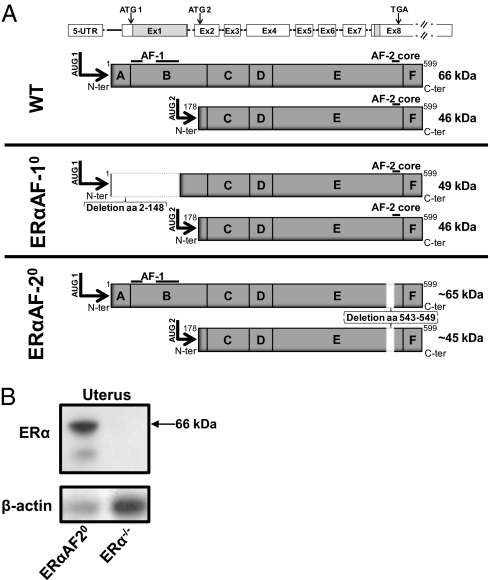
Fig. 1.
Schematic presentation of the ERα gene and proteins expressed in WT mice, mice with specific inactivation of the ERα A/B domains (ERαAF-10), and mice with specific inactivation of AF-2 in ERα (ERαAF-20). (A) Amino acids 2 to 148 are deleted in the ERαAF-10 mice and aa 543 to 549 are deleted in the ERαAF-20 mice. Both the main protein initiated by the translational initiation codon in exon 1 (ATG1) and the less abundantly expressed protein initiated by the initiation codon in exon 2 (ATG2) are shown for each genotype. (B) Western blot demonstrates ERα expression in uterus from ERαAF-20 but not from ERα−/− mice.
Results
E2 Response on Total Body Areal Bone Mineral Density Is Absent in ERαAF-20 and Attenuated in ERαAF-10 Mice.
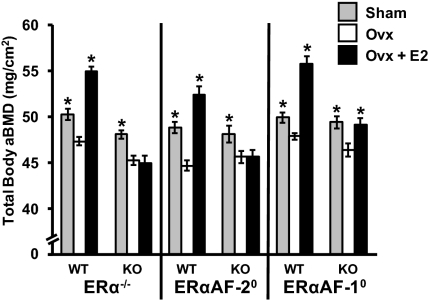
Dual energy X-ray absorptiometry (DXA) measurements showed that ovariectomy reduced total body areal bone mineral density (aBMD) in WT mice and treatment of ovariectomized (OVX) WT mice with E2 increased this parameter (Fig. 2). Although ERα is crucial for bone mass regulation, ovariectomy of ERα−/− mice reduced total body aBMD (Fig. 2). This is consistent with previous studies that demonstrated a preserved bone mass in gonadal-intact female ERα−/− mice as a result of disturbed negative feedback regulation of serum sex steroid levels, resulting in elevated levels of ovarian-derived testosterone and estradiol, which in turn preserve the bone mass via an activation of the androgen receptor and/or ERβ (9, 10). Similarly, ovariectomy of ERαAF-20 and ERαAF-10 mice resulted in a reduction of total body aBMD (Fig. 2), suggesting that the negative feedback regulation might also be disturbed in these two mouse models, resulting in an androgen-mediated preservation of the bone mass in gonadal-intact mice. To evaluate the negative feedback regulation in the ERαAF-20 and ERαAF-10 mice, analyses of serum testosterone, E2, and luteinizing hormone (LH) were performed. Not only female ERα−/− but also female ERαAF-20 and ERαAF-10 mice had elevated serum levels of testosterone, E2, and LH (Table 1), compared with their corresponding WT mice (P < 0.05). As the increased serum levels of testosterone are known to confound the interpretation of data regarding bone mass in gonadal-intact ERα−/− mice (9, 10) and probably also in ERαAF-20 and ERαAF-10 mice, we focused our further analyses to the estrogenic responses in OVX mice and, therefore, the sham groups of the three KO mouse models were not further analyzed. As expexted, E2 did not increase total body aBMD in OVX ERα−/− mice. Similarly, E2 did not increase total body aBMD in OVX ERαAF-20 mice (Fig. 2). In contrast, E2 significantly increased total body aBMD in OVX ERαAF-10 mice, although this E2 response was smaller compared with the E2 response in WT OVX mice (P < 0.01).
Fig. 2.
Role of ERα AF-1 and ERα AF-2 in the effect of E2 on total body aBMD. Total body aBMD as analyzed by DXA in ERα−/−, ERαAF-20, and ERαAF-10 and their corresponding WT mice after sham operation plus vehicle treatment (Sham), ovariectomy plus vehicle treatment (OVX), or ovariectomy plus E2 treatment (OVX + E2) (*P < 0.05, Student t test vs. OVX; values are means ± SEM; n = 7–12).
Table 1.
Role of ERα AF-1 and ERα AF-2 for the negative feedback regulation of serum sex steroids
| Steroid | ERα−/− | ERαAF-20 | ERαAF-10 | |||
| WT | KO | WT | KO | WT | KO | |
| Testosterone (ng/mL) | ND | 0.87 ± 0.10* | ND | 0.33 ± 0.07* | ND | 0.25 ± 0.08* |
| Estradiol (pg/mL) | 5.2 ± 1.1 | 42.7 ± 11.6* | 11.3 ± 1.5 | 28.6 ± 5.4* | 8.2 ± 2.0 | 17.8 ± 2.3* |
| LH (ng/mL) | 0.15 ± 0.08 | 0.60 ± 0.10* | 0.40 ± 0.08 | 1.18 ± 0.50* | 0.28 ± 0.02 | 0.43 ± 0.04* |
Measurements of testosterone, estradiol, and LH in ERα−/−, ERαAF-20 and ERαAF-10 mice. ND, not detectable.
*P < 0.05, Student t test vs WT mice (n = 5–13).
ERα AF-1 and ERα AF-2 Are Required for E2 Response in Trabecular Bone Whereas only ERα AF-2 Is Required for E2 Response in Cortical Bone.
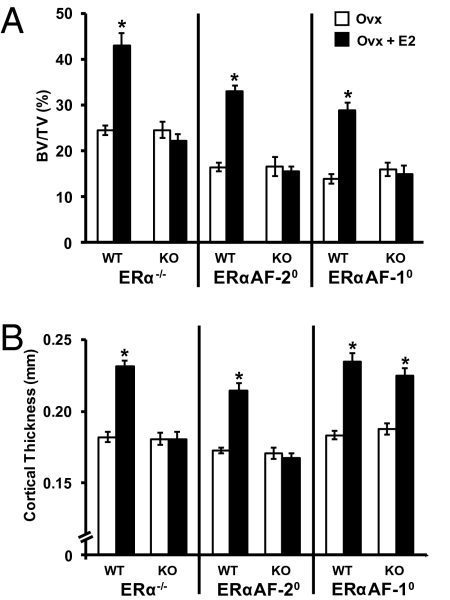
As the DXA technique cannot distinguish between the cortical and trabecular bone compartments, detailed analyses using peripheral quantitative CT (pQCT), micro-CT (μCT), and histomorphometry were performed to further characterize the intermediate estrogenic response seen on the total body aBMD in the ERαAF-10 mice. Trabecular bone analyses using μCT of L5 vertebrae demonstrated a clear estrogenic response in trabecular bone, reflected by increased bone volume/total volume (BV/TV) ratio and trabecular number, in OVX WT mice (Figs. 3A and 4 A and C and Table S1). In contrast, no E2 effect was seen on these trabecular bone parameters in OVX ERα−/−, ERαAF-20, or ERαAF-10 mice. Histomorphometric analyses of the trabecular bone in L4 vertebrae confirmed that E2 increased the trabecular BV/TV and trabecular number in OVX WT but not in OVX ERαAF-10 mice compared with vehicle-treated mice (Table S2). There was a nonsignificant trend that E2 reduced the number of osteoclasts on the bone surface of the trabecular bone in L4 vertebrae in OVX WT mice but not in OVX ERαAF-10 mice, and E2-treated OVX WT mice had significantly lower (−25%; P < 0.01) numbers of osteoclasts per bone surface than E2-treated OVX ERαAF-10 mice (Table S2).
Fig. 3.
Role of ERα AF-1 and ERα AF-2 for the effect of E2 in trabecular and cortical bone. OVX ERα−/−, ERαAF-20, and ERαAF-10 and their corresponding WT mice were treated with vehicle or E2 for 4 wk. (A) Trabecular bone (i.e., BV/TV) in L5 vertebra analyzed by using μCT. (B) Cortical thickness in femur analyzed using pQCT (*P < 0.05, Student t test vs. OVX; values are means ± SEM; n = 8–12).
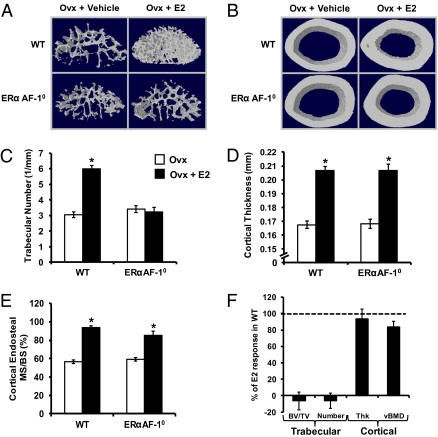
Fig. 4.
Detailed bone analyses of the effect of E2 in ERαAF-10 mice. OVX ERαAF-10 mice and WT mice were treated with vehicle or E2 for 4 wk. Analyses with μCT demonstrated that the E2 response seen on trabecular number (A and C; μCT analyses of L5 vertebra) was lost whereas the E2 response on cortical bone (cortical thickness, B and D; μCT analyses of femur diaphysis) was normal in the OVX ERαAF-10 mice compared with OVX WT mice (*P < 0.05, Student t test vs. OVX). Representative μCT figures illustrating the E2 response in (A) trabecular bone (L5 vertebra) and (B) cortical bone (femur diaphysis) in OVX WT and OVX ERαAF-10 mice. (E) Dynamic histomorphometric analyses of the endosteal surface of the femur diaphysis demonstrated that E2 increased the mineralized surface/bone surface (MS/BS) in both OVX WT and OVX ERαAF-10 mice. (F) The normal estrogenic responses for some trabecular (BV/TV and trabecular number) and cortical (cortical thickness and cortical vBMD) bone parameters in E2-treated OVX WT mice is set to 100% and the bars represent the E2 response for OVX ERαAF-10 mice in percent of E2 response in OVX WT mice. Thus, 0% means no E2 response whereas 100% is a normal E2 response. Values are means ± SEM (n = 9–11).
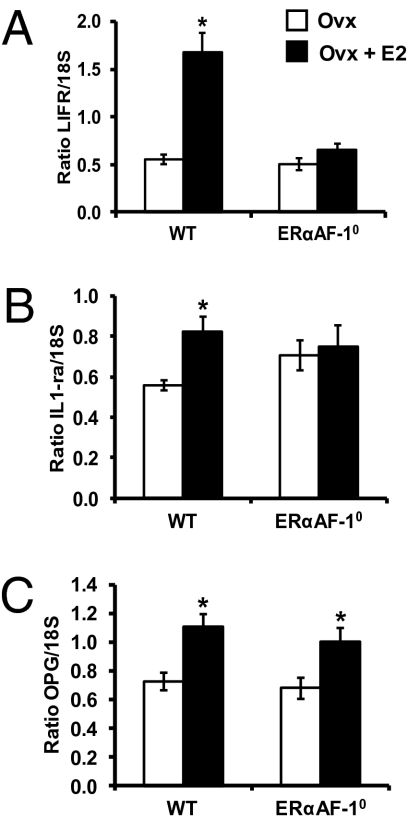
Cortical bone analyses showed that E2 treatment increased the femoral cortical mineral content in OVX WT mice, which was attributable to increased cortical bone area and cortical volumetric bone mineral density (vBMD; Table S1). The increased cortical bone area was caused by an increased cortical thickness as a result of reduced endosteal circumference but unchanged periosteal circumference (Table S1 and Fig. 3B). As expected, these cortical bone parameters were not influenced by E2 in OVX ERα−/− mice (Table S1 and Fig. 3B). Similarly, the cortical bone of ERαAF-20 mice was not responsive to E2 treatment (Table S1 and Fig. 3B). Remarkably, however, a normal E2 response was seen on the cortical bone parameters in OVX ERαAF-10 mice (Table S1 and Fig. 3B). Detailed analyses of cortical bone by using μCT confirmed that a normal E2 effect on cortical bone thickness was seen in OVX ERαAF-10 mice (94 ± 12% of the E2 response in WT mice; Fig. 4 B and D). Dynamic cortical histomorphometric analysis of the endosteal surface of the femur diaphyseal cortex demonstrated that E2 reduced the endosteal circumference by increasing the endosteal mineralized surface/bone surface and bone formation rate in OVX WT and OVX ERαAF-10 mice (Fig. 4E and Table S2). Fig. 4F summarizes the compartment-specific role of AF-1 in the skeletal effects of E2: the effect on trabecular bone parameters (trabecular BV/TV and trabecular thickness) is lost whereas the effect on cortical bone parameters (cortical thickness and cortical vBMD) is essentially normal in OVX ERαAF-10 mice. These findings demonstrate that both ERα AF-1 and ERα AF-2 are required for the E2 response in trabecular bone, whereas only ERα AF-2 is required for the E2 response in cortical bone. Gene expression analyses were performed to evaluate the role of ERα AF-1 for the effect of E2 on expression of genes previously known to be regulated by E2 in bone. We have previously, in an extensive microarray analysis, identified the leukemia inhibitory factor receptor (LIFR) and IL-1 receptor antagonist (IL-1ra) mRNA levels to be significantly increased in bone by both long-term and short-term treatment with E2 in OVX mice (24). In addition, osteoprotegerin (OPG) mRNA levels are increased by E2 treatment (25, 26). As expected, E2 treatment increased the mRNA levels of LIFR, IL-1ra, and OPG in bone from OVX WT mice (Fig. 5). No E2 effect on LIFR or IL-1ra mRNA levels but a normal E2 response on OPG mRNA levels was seen in OVX ERαAF-10 mice (Fig. 5), suggesting that the role of ERα AF-1 for mediating the E2 effects was transcript-dependent.
Fig. 5.
Estrogen-regulated transcripts in bone. Measurements of (A) LIFR, (B) IL-1ra, and (C) OPG mRNA levels in whole bone (humerus) using RT-PCR [*P < 0.05, Student t test vs. OVX mice; values are means ± SEM and are given as ratios versus 18S (n = 6–10)].
Role of ERα AF-1 Is Tissue-Dependent.
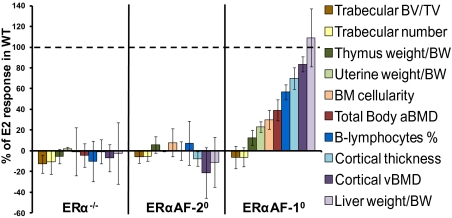
As the immune system is involved in the regulation of bone metabolism, we evaluated the role of ERα AF-1 and ERα AF-2 for the E2 response on immune cells in bone marrow and thymus. In addition, as comparison, the E2 responses on two other major E2-responsive nonbone parameters, uterine and liver weights, were evaluated. As expected, E2 treatment resulted in a significant effect on estrogen-responsive bone marrow parameters (reduced bone marrow cellularity and frequency of B lymphocytes; P < 0.01) and nonbone parameters (increased uterine weight and liver weight but reduced thymus weight; P < 0.01) in WT mice (Table S3). No effect of E2 on any of these parameters was seen in the OVX ERα−/− mice, demonstrating that the E2 effects on all these parameters are mediated via ERα (Table S3 and Fig. 6). Similarly, no E2 response on any of these parameters was seen in OVX ERαAF-20 mice, showing that an intact ERα AF-2 is required for the effects of E2 on these parameters (Fig. 6). Interestingly, the E2 response varied between the different parameters evaluated in the ERαAF-10 mice. Similarly, as seen for trabecular bone parameters, no significant E2 response was seen on thymus weight (13 ± 7% of E2 response in WT mice), and similar to that seen for cortical bone parameters, a normal E2 response was seen on liver weight (109 ± 28% of E2 response in WT mice) in OVX ERαAF-10 mice (Fig. 6). A clearly reduced and only minor E2 response was seen for the uterine weight (23 ± 5% of E2 response in WT mice; Fig. 6) and the bone marrow cellularity (30 ± 9% of E2 response in WT mice; Fig. 6), and an intermediate E2 response was seen for the frequency of B lymphocytes in the bone marrow (55 ± 7% of E2 response in WT mice; Fig. 6). Finally, we evaluated the effect of E2 on Ig secretion from bone marrow-derived B cells as an indicator of B-cell activity. E2 treatment increased IgG, IgM, and IgA secretion in OVX WT mice (Table S3). These analyses were not available for the ERα−/− mice, but in the OVX ERαAF-20 mice, no effect of E2 treatment on Ig secretion was seen, demonstrating that ERα and its AF-2 are required for these effects (Table S3). The E2 responses on IgG (43 ± 12% of E2 response in WT mice), IgM (45 ± 13% of E2 response in WT mice), and IgA (40 ± 12% of E2 response in WT mice) secretion were intermediate in OVX ERαAF-10 mice. In Fig. 6, a summary of the role of ERα AF-1 and ERα AF-2 for the effect of E2 on several different E2-responsive parameters is given, demonstrating that ERα AF-2 is required for all evaluated parameters, whereas the role of ERα AF-1 is clearly tissue-dependent (Fig. 6).
Fig. 6.
The role of ERα AF-1 is tissue-dependent. OVX ERα−/−, ERαAF-20, ERαAF-10, and their corresponding WT mice were treated with vehicle or E2 for 4 wk. As expected, E2 treatment resulted in a significant effect on several estrogen-responsive bone parameters (increased total body aBMD, cortical thickness, cortical vBMD, trabecular BV/TV, and trabecular number), bone marrow parameters (reduced bone marrow cellularity and frequency of B lymphocytes), and nonbone parameters (increased uterine weight and liver weight but reduced thymus weight) in OVX WT mice. To illustrate the role of ERα AF-1 and ERα AF-2 for the effect of E2 on these parameters, the estrogenic response in E2-treated OVX WT mice, for each parameter, is set to 100%. The bars represent the estrogenic response in percent for the E2-treated OVX ERα−/−, ERαAF-20, and ERαAF-10 mice compared with the E2 response in their OVX WT mice, respectively. Thus, 0% means no E2 response whereas 100% is a normal WT E2 response. Values are means ± SEM (n = 8–12). BM, bone marrow; BW, body weight.
Discussion
Characterization of estrogen signaling in bone versus other tissues might identify tissue-specific targets and thereby contribute to the development of a novel treatment of osteoporosis with minimal side effects in nonbone tissues. The bone-sparing effect of estrogen is primarily mediated via ERα. As the roles of AF-1 and AF-2 in ERα previously have been evaluated only in vitro, we have developed mouse models with specific deletions of AF-1 or AF-2 in ERα. These mouse models allowed us to determine the roles of ERα AF-1 and AF-2 for several bone-related parameters and for some other major estrogen-responsive parameters. Our main findings in this study, focusing on bone parameters, are that AF-2 in ERα is required for the estrogenic responses on all parameters evaluated whereas the role of AF-1 in ERα is tissue-specific, with a crucial role in trabecular bone and uterus but not cortical bone.
ERα is essential for the negative feedback regulation of serum sex steroids as reflected by elevated levels of testosterone, E2, and LH in female ERα−/− mice (27, 28). In the present study, we made the observation that not only female ERα−/− but also female ERαAF-20 and ERαAF-10 mice had elevated serum levels of testosterone, E2, and LH compared with their corresponding WT mice, demonstrating that a normal negative feedback regulation of serum sex steroids requires an intact AF-1 and an intact AF-2 in ERα. Although ERα is crucial for the effect of E2 on bone mass, it has been shown that the bone mass in gonadal-intact female ERα−/− mice is preserved as a result of their elevated ovarian-derived testosterone and estradiol levels, which in turn preserve the bone mass via an activation of the androgen receptor and/or ERβ (9, 10). As the elevated serum levels of testosterone and estradiol are known to confound the interpretation of data regarding bone mass in gonadal-intact female ERα−/− mice (10), and probably also in gonadal-intact female ERαAF-20 and ERαAF-10 mice, the sham groups of the three KO mouse models were not further analyzed.
As expected, E2 treatment increased the total body aBMD as a result of increased amount of both trabecular and cortical bone in OVX WT mice. The E2 effect in trabecular bone was mainly caused by an increased number of trabeculae whereas the effect in cortical bone was caused by an increased cortical thickness as a result of increased endosteal bone formation. In contrast, no E2 effect on any of these bone parameters was seen in OVX ERα−/− mice with complete inactivation of the ERα protein, confirming previous studies showing that ERα is the major ER with an impact on adult bone homeostasis (9, 10). Similarly, no effect on any of these bone parameters was seen in the OVX ERαAF-20 mice, clearly demonstrating that AF-2 in ERα is crucial for the effects of E2 on both the trabecular and cortical bone compartments. Interestingly, an intermediate E2 response on total body aBMD, as analyzed by DXA, was seen in the OVX ERαAF-10 mice. It is of importance to note that the DXA technique, although extremely useful clinically, cannot distinguish between the cortical and trabecular bone compartments. Further detailed analyses using CT and histomorphometry revealed a normal E2 response in cortical bone but no E2 response in trabecular bone in the OVX ERαAF-10 mice. Thus, AF-1 in ERα is dispensable for the effects of E2 in cortical but not trabecular bone. Khosla and coworkers recently proposed that the main physiological target for estrogen in bone is cortical and not trabecular bone (1). This statement is based on a number of observations. First, detailed clinical investigations using CT revealed that trabecular bone loss begins in sex hormone-replete young adults of both sexes and might be the result of cell autonomous age-related factors as suggested by Manolagas (1, 29). Second, the same studies, using CT, demonstrated that the onset of cortical bone loss in humans is closely tied to estrogen deficiency. Thus, for cortical bone, which comprises more than 80% of the skeleton and is likely the major contributor to overall fracture risk, there are data supporting the view that estrogen deficiency is the major cause of bone loss (1). The data in the present study that the effect of E2 on cortical bone mass is ERα AF-1–independent might, therefore, be useful for the development of selective ER modulators with stimulation of ERα with minimal activation of ERα AF-1, as such substances could exert beneficial actions in cortical bone while minimizing the AF-1–dependent effects on reproductive organs.
The mechanism behind the crucial role of ERα AF-1 in the trabecular but not the cortical bone is unknown. It might include different expression patterns of coregulators in trabecular versus cortical bone. In vitro studies have revealed that the steroid receptor coactivator (SRC)-1 is cooperatively recruited by AF-1 and AF-2 in ERα and thereby mediates synergism between the two AFs (30). Importantly, when OVX SRC-1 KO mice were treated with E2, the normal estrogenic response was absent in the trabecular bone and uterus whereas it was normal in cortical bone (31). Thus, these estrogenic responses in the SRC-1 KO mice show a similar pattern as our results for the ERαAF-10 mice, suggesting that SRC-1 might be involved in the AF-1–dependent E2 effects in trabecular bone and uterus but not in the AF-1–independent E2 effects in cortical bone in female mice.
Recent studies using cell-specific ERα inactivation demonstrate that osteoclast ERα is important for the bone-sparing effect of estrogen in the trabecular but not the cortical bone compartment (32, 33). In addition, it was recently demonstrated by Manolagas and coworkers that deletion of ERα in mesenchymal progenitors (using Prx1-Cre) or mature osteoblasts (using 2.3-kb Col1a1-Cre) decreases cortical bone thickness and increases osteoblast apoptosis, respectively, whereas the trabecular bone is unaffected in these mice (34). These results suggest that estrogen acting through the ERα exerts cell-autonomous effects on mesenchymal cells/osteoblasts, and that these actions are responsible for the effects of estrogens in the cortical bone compartment. Our present findings that ERα AF-1 is crucial for the estrogenic effect in trabecular but not cortical bone, together with the aforementioned findings, suggest that estrogen preserves the trabecular bone via osteoclast ERα involving AF-1 whereas it preserves the cortical bone via osteoblast/mesenchymal ERα not involving AF-1 in ERα.
We performed gene expression analyses to evaluate the role of ERα AF-1 for the effect of E2 on expression of genes previously known to be regulated by E2 in bone. Two of three analyzed transcripts were regulated by E2 in both OVX WT and OVX ERαAF-10 mice, whereas a third transcript was regulated in OVX WT mice but not in OVX ERαAF-10 mice, suggesting that the role of AF-1 in ERα for mediating the E2 effects in bone was transcript-dependent.
As it is proposed that the immune system is involved in the regulation of bone metabolism, we evaluated the role of ERα AF-1 and ERα AF-2 for the E2 response on immune cells in bone marrow and thymus. ERα and AF-2 were crucial for the E2 effect on all the evaluated immune-related parameters. Analyses of OVX ERαAF-10 mice, demonstrated that the E2 responses on the immune parameters were intermediate compared with the E2 response in WT mice. Thus, these E2 effects were facilitated by AF-1, but AF-1 is not essential for these intermediary immune-related effects. Finally, to identify estrogen signaling specific for bone, the roles of the AF-1 and AF-2 in ERα for two other major E2-responsive nonbone related parameters, uterine and liver weights, were evaluated. Similar to the results we described earlier, the normal E2-induced increase in uterine weight was dependent on ERα AF-1 (23), and we demonstrated here that the effect of E2 on uterine weight also required an intact AF-2. The effect of E2 on liver weight required a functional AF-2 whereas AF-1 was dispensable for the E2 effect on liver weight. In addition, we recently described that ERα AF-1 is dispensable for E2-induced vascular protection (23). Thus, similar to what was seen for the bone parameters, AF-2 in ERα is required for all evaluated parameters whereas the role of AF-1 is tissue-dependent (Fig. 6). This suggests that the transactivation via AF-2 is required in all examined tissues, but, for some tissues, the full estrogenic response is acquired only when both AF-2 and AF-1 are present. The role of ERα AF-1 and ERα AF-2 for other major E2-responsive tissues should be assessed in future studies.
The respective roles of the full-length ERα66 (harboring both AF-2 and AF-1) and the shorter, naturally occurring but less expressed, AF-1 deficient ERα46 could be estimated by using the ERαAF-10 mice. Our findings suggest that the effect of E2 in cortical bone and on liver weight but not in trabecular bone or on uterine weight could be mediated via ERα46. In addition, we recently provided evidence that the vasculoprotective actions of E2 could be mediated by ERα46 (23). Future work should determine if ERα46 is differentially expressed in cortical versus trabecular bone.
In vitro experiments have demonstrated that AF-1 in ERα has the capacity to exert effects in a ligand-independent manner, and we recently demonstrated in vivo, by using ERαAF-10 mice, that AF-1 in ERα exerts an atheroprotective action independently of the binding of E2 to ERα (23, 35). In the present study, we could, by comparing OVX ERαAF-20 and OVX ERαAF-10 mice with their respective OVX WT mice, evaluate the possible ligand-independent roles in bone of AF-1 and AF-2 in ERα. However, in the estrogen-deficient state, we did not see any significant difference for any evaluated trabecular or cortical bone parameters, suggesting that ligand-independent ERα AF-1– or ERα AF-2–mediated mechanisms are not crucial for adult bone homeostasis.
In conclusion, a normal negative feedback regulation of serum sex steroids requires both an intact AF-1 and AF-2 in ERα. Ligand-independent ERα AF-1– or ERα AF-2–mediated mechanisms are not crucial for adult bone homeostasis. AF-2 in ERα is required for the estrogenic responses on all parameters evaluated, whereas the role of AF-1 is tissue-specific, with a crucial role in trabecular bone and uterus but not cortical bone. Selective ER modulators stimulating ERα with minimal activation of ERα AF-1 could retain beneficial actions in cortical bone, constituting 80% of the skeleton, and on vascular protection while minimizing the effects on reproductive organs.
Materials and Methods
Generation of Mice.
All experimental procedures involving animals were approved by the ethics committee of Gothenburg University. The generation of ERα−/− and ERαAF-10 mice has previously been described (23, 36). The ERαAF-10 mice have a deletion of 441 bp of exon 1, corresponding to aa 2 to 148, with a preserved translational initiation codon in exon 1 (ATG1; Fig. 1A). The ERαAF-10 mice do not express any full-length 66-kDa protein (23). Instead they express a truncated 49-kDa ERα protein that lacks AF-1 and also the physiologically occurring but less abundantly expressed 46-kDa ERα isoform initiated by a second translational initiation codon in exon 2 (ATG2; Fig. 1A). ERαAF-20 mice were generated through the strategy outlined in Fig. 1A. Briefly, ERαAF-20 mice have a deletion of the AF-2 core, which resides within exon 8 and corresponds to aa 543 to 549 (Fig. 1A). Western blot analysis demonstrated that ERαAF-20 but not ERα−/− mice express proteins initiated from the initiation codon in exon 1 (ATG1) and the initiation codon in exon 2 (ATG2; Fig. 1B). The sizes of these proteins in ERαAF-20 mice are slightly smaller (corresponding to the 7-aa truncation located in the AF-2 region) than the WT ERα proteins of 66 kDa and 46 kDa, respectively. Further details are provided in SI Materials and Methods.
OVX or sham operation was performed on 12-wk-old female mice. The OVX mice were treated with vehicle or E2 (167 ng/mouse/d) and the sham-operated mice were treated with vehicle for 4 wk using slow-release pellets inserted s.c. (Innovative Research of America).
Western Blot.
Western Blot was essentially performed as described previously (37). Further details are provided in SI Materials and Methods.
Measurement of Serum Hormone Levels.
Commercially available RIA kits were used to assess serum concentrations of testosterone (ICN Biomedicals), E2 (Siemens Medical Solutions), and LH (Immunodiagnosticsystems).
X-Ray Analyses.
DXA analyses of total body aBMD were performed using the Lunar PIXImus mouse densitometer (Wipro GE Healthcare).
CT scans of the femur were performed by using pQCT XCT RESEARCH M (version 4.5B; Norland) as described previously (13, 38). The μCT analyses were performed on the distal femur and lumbar vertebra (L5) using a model 1072 scanner (Skyscan) (9, 39, 40). Further details are provided in SI Materials and Methods.
Histomorphometric Analyses.
Trabecular bone in L4 vertebrae and cortical bone in the middiaphyseal region of femur were evaluated by using static and dynamic histomorphometric analyses (41–43). Further details are provided in SI Materials and Methods.
Quantitative Real-Time PCR Analysis.
Total RNA from whole humerus was prepared for real-time PCR analysis. Further details are provided in SI Materials and Methods.
Bone Marrow and Thymus Cellularity and Cell Distribution.
For flow cytometry analyses, cells were stained with phycoerythrin-conjugated antibodies to CD19 for detection of B lymphocytes. The cells were then subjected to FACS analysis on a FACSCalibur device (BD Pharmingen). Further details are provided in SI Materials and Methods.
Enzyme-Linked Immunosorbent Spot Assay.
Enumeration of IgM-, IgG-, and IgA-secreting bone marrow cells was performed by using the enzyme-linked immunosorbent spot technique (44). The number of Ig-secreting cells was expressed as the frequency of spot-forming cells per 103 CD19+ cells. Further details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
This study was supported by the Swedish Research Council, COMBINE, an Avtal om Läkarutbildning och Forskning/Läkarutbildningsavtalet research grant in Gothenburg, the Lundberg Foundation, the Torsten and Ragnar Söderberg Foundation, the Novo Nordisk Foundation, Reumatikerförbundet, Gustav V 80-års Fond, and National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant DK071122.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100454108/-/DCSupplemental.
References
- 1.Khosla S, Melton LJ, 3rd, Riggs BL. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: Is a revision needed? J Bone Miner Res. 2011;26:441–451. doi: 10.1002/jbmr.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LeBlanc ES, et al. Osteoporotic Fractures in Men Study Group The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. J Clin Endocrinol Metab. 2009;94:3337–3346. doi: 10.1210/jc.2009-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mellström D, et al. Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. J Bone Miner Res. 2008;23:1552–1560. doi: 10.1359/jbmr.080518. [DOI] [PubMed] [Google Scholar]
- 4.Smith EP, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 5.Vandenput L, Ohlsson C. Estrogens as regulators of bone health in men. Nat Rev Endocrinol. 2009;5:437–443. doi: 10.1038/nrendo.2009.112. [DOI] [PubMed] [Google Scholar]
- 6.Daly E, et al. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet. 1996;348:977–980. doi: 10.1016/S0140-6736(96)07113-9. [DOI] [PubMed] [Google Scholar]
- 7.Anonymous; Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and hormone replacement therapy: Collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047–1059. [PubMed] [Google Scholar]
- 8.Mödder UI, et al. The skeletal response to estrogen is impaired in female but not in male steroid receptor coactivator (SRC)-1 knock out mice. Bone. 2008;42:414–421. doi: 10.1016/j.bone.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Movérare S, et al. Differential effects on bone of estrogen receptor alpha and androgen receptor activation in orchidectomized adult male mice. Proc Natl Acad Sci USA. 2003;100:13573–13578. doi: 10.1073/pnas.2233084100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sims NA, et al. A functional androgen receptor is not sufficient to allow estradiol to protect bone after gonadectomy in estradiol receptor-deficient mice. J Clin Invest. 2003;111:1319–1327. doi: 10.1172/JCI17246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sims NA, et al. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-beta in bone remodeling in females but not in males. Bone. 2002;30:18–25. doi: 10.1016/s8756-3282(01)00643-3. [DOI] [PubMed] [Google Scholar]
- 12.Windahl SH, et al. Female estrogen receptor beta-/- mice are partially protected against age-related trabecular bone loss. J Bone Miner Res. 2001;16:1388–1398. doi: 10.1359/jbmr.2001.16.8.1388. [DOI] [PubMed] [Google Scholar]
- 13.Windahl SH, Vidal O, Andersson G, Gustafsson JA, Ohlsson C. Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERbeta(-/-) mice. J Clin Invest. 1999;104:895–901. doi: 10.1172/JCI6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Windahl SH, et al. The role of the G protein-coupled receptor GPR30 in the effects of estrogen in ovariectomized mice. Am J Physiol Endocrinol Metab. 2009;296:E490–E496. doi: 10.1152/ajpendo.90691.2008. [DOI] [PubMed] [Google Scholar]
- 15.Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295:2465–2468. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- 16.Berry M, Metzger D, Chambon P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 1990;9:2811–2818. doi: 10.1002/j.1460-2075.1990.tb07469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metzger D, Losson R, Bornert JM, Lemoine Y, Chambon P. Promoter specificity of the two transcriptional activation functions of the human oestrogen receptor in yeast. Nucleic Acids Res. 1992;20:2813–2817. doi: 10.1093/nar/20.11.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tora L, et al. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989;59:477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- 19.McKenna NJ, Lanz RB, O'Malley BW. Nuclear receptor coregulators: Cellular and molecular biology. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 20.Kumar R, Thompson EB. The structure of the nuclear hormone receptors. Steroids. 1999;64:310–319. doi: 10.1016/s0039-128x(99)00014-8. [DOI] [PubMed] [Google Scholar]
- 21.Norris JD, Fan D, Kerner SA, McDonnell DP. Identification of a third autonomous activation domain within the human estrogen receptor. Mol Endocrinol. 1997;11:747–754. doi: 10.1210/mend.11.6.0008. [DOI] [PubMed] [Google Scholar]
- 22.Metzger D, Ali S, Bornert JM, Chambon P. Characterization of the amino-terminal transcriptional activation function of the human estrogen receptor in animal and yeast cells. J Biol Chem. 1995;270:9535–9542. doi: 10.1074/jbc.270.16.9535. [DOI] [PubMed] [Google Scholar]
- 23.Billon-Galés A, et al. The transactivating function 1 of estrogen receptor alpha is dispensable for the vasculoprotective actions of 17beta-estradiol. Proc Natl Acad Sci USA. 2009;106:2053–2058. doi: 10.1073/pnas.0808742106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindberg MK, et al. Identification of estrogen-regulated genes of potential importance for the regulation of trabecular bone mineral density. J Bone Miner Res. 2002;17:2183–2195. doi: 10.1359/jbmr.2002.17.12.2183. [DOI] [PubMed] [Google Scholar]
- 25.Hofbauer LC, et al. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology. 1999;140:4367–4370. doi: 10.1210/endo.140.9.7131. [DOI] [PubMed] [Google Scholar]
- 26.Bord S, Ireland DC, Beavan SR, Compston JE. The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone. 2003;32:136–141. doi: 10.1016/s8756-3282(02)00953-5. [DOI] [PubMed] [Google Scholar]
- 27.Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- 28.Lindberg MK, et al. Estrogen receptor specificity for the effects of estrogen in ovariectomized mice. J Endocrinol. 2002;174:167–178. doi: 10.1677/joe.0.1740167. [DOI] [PubMed] [Google Scholar]
- 29.Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31:266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Métivier R, Penot G, Flouriot G, Pakdel F. Synergism between ERalpha transactivation function 1 (AF-1) and AF-2 mediated by steroid receptor coactivator protein-1: Requirement for the AF-1 alpha-helical core and for a direct interaction between the N- and C-terminal domains. Mol Endocrinol. 2001;15:1953–1970. doi: 10.1210/mend.15.11.0727. [DOI] [PubMed] [Google Scholar]
- 31.Mödder UI, et al. Effects of loss of steroid receptor coactivator-1 on the skeletal response to estrogen in mice. Endocrinology. 2004;145:913–921. doi: 10.1210/en.2003-1089. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura T, et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130:811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 33.Martin-Millan M, et al. The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol. 2010;24:323–334. doi: 10.1210/me.2009-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almeida M, et al. ERα deletion in mesenchymal progenitors or mature osteoblasts decreases cortical bone thickness and increases apoptosis, respectively. J Bone Miner Res. 2010;25(suppl 1):1113. [Google Scholar]
- 35.Métivier R, et al. A dynamic structural model for estrogen receptor-alpha activation by ligands, emphasizing the role of interactions between distant A and E domains. Mol Cell. 2002;10:1019–1032. doi: 10.1016/s1097-2765(02)00746-3. [DOI] [PubMed] [Google Scholar]
- 36.Dupont S, et al. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 37.Börjesson AE, et al. The role of estrogen receptor α in growth plate cartilage for longitudinal bone growth. J Bone Miner Res. 2010;25:2414–2424. doi: 10.1002/jbmr.156. [DOI] [PubMed] [Google Scholar]
- 38.Vidal O, et al. Estrogen receptor specificity in the regulation of skeletal growth and maturation in male mice. Proc Natl Acad Sci USA. 2000;97:5474–5479. doi: 10.1073/pnas.97.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hildebrand T, Rüegsegger P. Quantification of bone microarchitecture with the structure model index. Comput Methods Biomech Biomed Engin. 1997;1:15–23. doi: 10.1080/01495739708936692. [DOI] [PubMed] [Google Scholar]
- 40.Waarsing JH, Day JS, Weinans H. An improved segmentation method for in vivo microCT imaging. J Bone Miner Res. 2004;19:1640–1650. doi: 10.1359/JBMR.040705. [DOI] [PubMed] [Google Scholar]
- 41.Baron RVA, Neff L, Silverglate A, Maira AS. Processing of undecalcified bone specimens for bone histomorphometry. In: Recker RR, editor. Bone Histomorphometry: Techniques and Interpretation. Boca Raton, FL: CRC Press; 1983. pp. 18–29. [Google Scholar]
- 42.Eriksen EFAD, Melsen F. Bone Histomorphometry. New York: Raven Press; 1994. [Google Scholar]
- 43.Parfitt AM, et al. Report of the ASBMR Histomorphometry Nomenclature Committee Bone histomorphometry: Standardization of nomenclature, symbols, and units. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 44.Czerkinsky CC, et al. An immunoenzyme procedure for enumerating fibronectin-secreting cells. J Immunoassay. 1984;5:291–302. doi: 10.1080/01971528408063013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.