Abstract
An increase in Ca2+ influx through L-type Ca2+ channels is thought to contribute to neuronal dysfunctions that underlie senile symptoms and Alzheimer's disease. The molecular basis of the age-dependent up-regulation in neuronal L-type Ca2+ channel activity is largely unknown. We show that phosphorylation of the L-type channel Cav1.2 by cAMP-dependent protein kinase is increased >2-fold in the hippocampus of aged rats. The hippocampus is critical for learning and is one of the first brain regions to be affected in Alzheimer's disease. Phosphorylation of Cav1.2 by cAMP-dependent protein kinase strongly enhances its activity. Therefore, increased Cav1.2 phosphorylation may account for a substantial portion of the age-related rise in neuronal Ca2+ influx and its neuropathological consequences.
Ca2+ regulates a variety of signaling pathways (1-3). Acute uncontrolled Ca2+ influx can cause neuronal death by overstimulation of the N-methyl-d-aspartate (NMDA)-type glutamate receptor (4, 5). Chronic elevation of Ca2+ influx has been implicated in age-associated neuronal loss and Alzheimer's disease (6, 7). The age-related learning impairment in rabbits is reversed by the specific L-type Ca2+ channel blocker nimodipine (8). These observations indicate that elevation of L-type channel activity causes neuronal dysfunction during aging. Ca2+ influx through L-type channels is up-regulated >2-fold in hippocampal neurons of old versus adult rats (9). The hippocampus is crucial for learning and memory; however, the molecular basis for the increase in L-type channel activity during aging is not well defined, except for an ≈20% increase in Cav1.3 protein (10, 11). We now show that cAMP-dependent protein kinase (PKA)-mediated phosphorylation of the L-type channel Cav1.2 increases >2-fold during aging and that it may underlie a substantial portion of the age-related increase in L-type channel activity.
PKA increases L-type channel activity in neurons (13-16) and in the heart, where PKA-dependent stimulation of the cardiac L-type channel is crucial for the up-regulation of the heartbeat after adrenergic stimulation (17, 18). Two different L-type channels have been identified in neurons, Cav1.2 and Cav1.3 (19-21). Cav1.2 is the prevailing L-type channel in neurons (≈80%; see ref. 20). Cav1.2 consists of a central α1-subunit (designated α11.2), which forms the ion-conducting pore (19), and the auxiliary subunits α2-δ, -β, and -γ (22). Only α11.2, and none of the auxiliary subunits, is required for the PKA-mediated increase in channel activity (23). α11.2 is phosphorylated in intact hippocampal neurons by PKA (24). The main phosphorylation site on α11.2 is serine 1928 (25, 26), which is important for the up-regulation of the channel activity by PKA (27). This site is close to the C terminus of α11.2, is present only in the full-length form of α11.2 (21), and is removed by the Ca2+-activated protease calpain after Ca2+ influx through NMDA receptors (28). Calpain cleaves α11.2 ≈300 residues upstream of the C terminus in vitro (28). Deletion of the last 300-400 residues of α11.2 increases channel activity when expressed in Xenopus oocytes (29); therefore, calpain-mediated cleavage may permanently elevate Cav1.2 activity in vivo. Because calpain has been implicated in the etiology of Alzheimer's disease (30, 31), we initially investigated whether the ratio of long-form α11.2 to short-form α11.2 is changed in old rats. The amount of long-form α11.2 was unaltered, and short-form α11.2 was slightly decreased in old rats, which suggests that calpain-mediated processing is not responsible for the increase in L-type channel activity during aging. However, we discovered that phosphorylation of α11.2 on its main and perhaps only PKA site was substantially elevated in old rats.
Experimental Procedures
Material and Antibodies. Microcystin LR and okadaic acid were from Calbiochem, and Cα, a purified catalytic subunit of PKA, was from Sigma. The CNC1 antibody was produced in rabbits against a peptide covering residues 818-835 of α11.2 (20). The antibody anti-CH1923-1932P, which specifically binds to α11.2 when phosphorylated at serine 1928, was raised against a phosphopeptide consisting of residues 1923-1932 (25). Antibodies against NMDA receptor NR1 were from R. Jahn (Max Planck Institute for Biophysical Chemistry, Goettingen, Germany); antibodies against NR2A and NR2B were from R. J. Wenthold (National Institute on Deafness and other Communication Disorders, National Institutes of Health, Bethesda) (32, 33); antibodies against PKA Cα were from C. S. Rubin (Albert Einstein College of Medicine, Bronx, NY) (26); and antibodies against protein phosphatase-1 inhibitors 1 and 2 were from A. C. Nairn (Yale University, New Haven, CT). Anti-PSD-95 (34) and anti-SAP102 correspond, respectively, to JH62092 and JH62514 in ref. 35. Antibodies against the catalytic subunit of the protein phosphatase PP2A (PP2A/C) and against microtubule-associated protein MAP2B were from Transduction Laboratories (Lexington, KY).
Immunoprecipitation and Immunoblotting. Brain tissue was collected from 12- and 26-month-old Lobund-Wistar rats. Each rat underwent a complete necropsy for pathological evaluation, including heart and kidney autopsies to exclude any pathological changes. Fifty milligrams of hippocampal tissue was homogenized in 0.5 ml of 1% Triton X-100 buffer containing protease and phosphatase inhibitors and cleared by ultracentrifugation as detailed elsewhere (26, 36). For quantification of Triton X-100-soluble and -insoluble MAP2B and NR1, 20 μl of the supernatants and pellets (resuspended in 0.5 ml of TBS) were analyzed by immunoblotting (26, 37). α11.2 was immunoprecipitated from Triton X-100 extracts with anti-CNC1 (10 μg in 500 μl; see refs. 20 and 21) before immunoblotting with anti-CH1923-1932P and subsequently with anti-CNC1 (26, 36). For quantification of PSD-95 and SAP102, 50 mg of hippocampal tissue was homogenized in 0.5 ml of 1% deoxycholate buffer before ultracentrifugation (32, 37). Supernatants were used either directly for immunoblotting (20 μl) or after immunoprecipitation (500 μl) with a mixture of anti-NR2A and anti-NR2B antibodies (32, 33, 37). All blots were exposed for increasing amounts of time (e.g., 30, 60, and 120 sec). To ensure that the signals were in the linear range, only signals doubling with correspondingly increasing exposure times were used for quantification.
The amount of PKA Cα that was associated with Cav1.2 was quantified by immunoprecipitation of Cav1.2 and immunoblotting with antibodies against Cα (26). Total amounts of PP2A/C and PP1 inhibitors 1 and 2 were determined by immunoblotting of hippocampal extracts with the corresponding antibodies. All immunoblots were quantified by densitometry (28) with IMAGE-QUANT software (Molecular Dynamics).
Phosphorylation of Cav1.2 by PKA and cAMP Measurements. For stoichiometric in vitro phosphorylation (21), Cav1.2 was immunoprecipitated, incubated in 50 μl of phosphorylation buffer (0.1% Triton X-100/50 mM Hepes-NaOH, pH 7.4/10 mM MgCl2/0.5 mM EGTA/50 μM ATP/0.5 mM DTT/protease inhibitors) containing 0.5-1 μg of catalytically active, purified PKA C subunit for 30 min at 32°C; the Cav1.2 was then washed and analyzed by immunoblotting with anti-CH1923-1932P (26). cAMP levels were determined in trichloroacetic acid extracts of hippocampal tissues according to the protocol of the manufacturer (Signal Transduction Products, San Clemente, CA).
Results
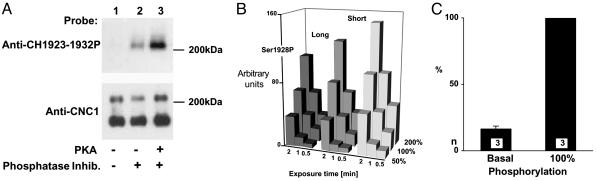
Quantification of PKA-Mediated α11.2 Phosphorylation. To determine the basal phosphorylation level of serine 1928 in adult rats, α11.2 was immunoprecipitated with anti-CNC1, which recognizes both size forms (21) (Fig. 1). α11.2 was then analyzed by immunoblotting with anti-CH1923-1932P, an antibody that specifically interacts with the serine 1928 site in its phosphorylated, but not dephosphorylated, form (26) (Fig. 1 A Upper). In some cases, immunoprecipitated α11.2 was phosphorylated on serine 1928 with purified PKA under conditions that result in stoichiometrically (i.e., near complete) phosphorylation of α11.2 (21) before immunoblotting (Fig. 1 A, lane 3). Other samples that were immunoprecipitated in parallel were left untreated (Fig. 1 A, lanes 1 and 2). Some extractions and subsequent immunoprecipitations were performed in the absence of phosphatase inhibitors, which leads to complete dephosphorylation of serine 1928 (26, 36) and, thus, the absence of an immunosignal when probed with anti-CH1923-1932P (Fig. 1 A, lane 1). These observations confirm that anti-CH1923-1932P recognizes only the phosphorylated form of α11.2. Stripping and reprobing of the immunoblots with anti-CNC1 demonstrated that comparable amounts of α11.2 were present in all samples (Fig. 1 A Lower).
Fig. 1.
Quantification of α11.2 phosphorylation on serine 1928. α11.2 was immunoprecipitated with anti-CNC1 from hippocampal Triton X-100 extracts from adult rats for immunoblotting with anti-CH1923-1932P (71). After stripping with SDS and DTT, blots were reprobed with anti-CNC1 (71). (A) Anti-CH1923-1932P recognized α11.2 when phosphatase inhibitors were present (lane 2) but not when omitted (lane 1). In vitro phosphorylation of immunoprecipitated α11.2 with purified PKA strongly increased the signal (lane 3). (B) Fifty percent (one half of one immunoprecipitate), 100%, and 200% (two immunoprecipitates combined) of immunoprecipitates were used for immunoblotting. Films were exposed for 0.5, 1, and 2 min, and immunosignals were quantified by densitometry (28, 33). Values indicated by the bars reflect the average of three samples. (C) After immunoprecipitation of α11.2, one sample but not the other was phosphorylated in vitro with purified PKA under conditions that led to near complete phosphorylation of the PKA site on α11.2, as quantified earlier (21). Immunosignals for anti-CH1923-1932P were corrected for differences in relative amounts of long-form α11.2 based on the corresponding anti-CNC1 signals. Under basal conditions 16.7 ± 1.8% (SD; n = 3, obtained in three different experiments) of long-form α11.2 is phosphorylated on serine 1928 (left bar) with 100% equaling the anti-CH1923-1932P signal obtained after in vitro phosphorylation.
We evaluated the linearity of the immunosignals for anti-CH1923-1932P and CNC1 by loading 50%, 100%, and 200% of the material of a standard immunoprecipitation onto a gel. Film exposures were taken for 0.5, 1, and 2 min, and the resulting immunosignals were quantified by densitometry (28, 33). Doubling the exposure time or the amount of sample loaded onto the gel resulted in the expected increase in the signals for anti-CH1923-2932P and the long and short forms of α11.2 as detected with anti-CNC1. Only the longest exposures of the largest amounts of antigens loaded started to show a tendency toward saturation (Fig. 1B). Accordingly, signals are largely within the linear range.
To determine the basal level of serine 1928 phosphorylation, α11.2 was immunoprecipitated with anti-CNC1 in the presence of phosphatase inhibitors; one sample of each pair was stoichiometrically phosphorylated with purified PKA to serve as 100% control before immunoblotting of both samples. The basal phosphorylation level of serine 1928 was 16.7% in hippocampal samples from 12-month-old rats (Fig. 1C).
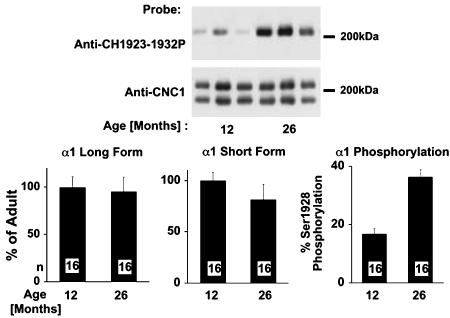
Phosphorylation of α11.2 During Aging. We used 12- and 26-month-old Lobund-Wistar rats to investigate the molecular basis for the up-regulation of L-type channel activity in aging rats. The mean lifespan of this rat strain is ≈30 months (38). Age-related primary changes should appear earlier than secondary effects. Therefore, we chose to compare rats at an early aging stage (26 months) with 12-month-old adult rats. At 26 months, several age-associated pathological changes became evident (39, 40). Three to six hippocampal samples of each age group were processed in parallel by immunoprecipitation and immunoblotting (Fig. 2). Fig. 2 A shows a representative blot of samples from three animals of each age group. Immunosignals were quantified by densitometry and normalized so that 100% equals the average of all signals from the adult rat samples for each experiment. Fig. 2B shows the results for 16 individual animals of each age group. Neither the long from nor the short form of α11.2 was significantly increased during aging. In fact, a small statistically significant decrease in the short form is detectable, which would cause a slight reduction in total L-type channel activity. Serine 1928 phosphorylation, however, was increased >2-fold. Because the basal phosphorylation level of serine 1928 was 16.7%, we used this value for normalization by setting the average of the adult sample signals in each experiment to 16.7%. The phosphorylation level was increased to 40% in old rats. Similar findings were obtained when α11.2 content and phosphorylation were compared between 12- and 25-month-old Fisher 344 rats (data not shown). Thus, phosphorylation of α11.2 on serine 1928 and, thereby, channel activity, is significantly up-regulated in the hippocampus of aging rats.
Fig. 2.
Increase in basal phosphorylation of α11.2 on serine 1928 in 26-month-old rats. α11.2 was immunoprecipitated from hippocampal extracts of 12- and 26-month-old Lobund-Wistar rats. Immunoblots were probed with anti-CH1923-1932P, stripped with SDS and DTT, and reprobed with anti-CNC1 (26). Immunosignals for long- and short-form anti-CH1923-1932P (Top) and anti-CNC1 (Middle) were quantified by densitometry. Signals for anti-CH1923-1932P corrected for differences in relative amounts of α1C based on the CNC1 signals of the long-form α1C (Bottom). Values for long- and short-form α11.2 were normalized by setting the average values for long- and short-form α11.2 in adults as 100%. The average phosphorylation level of serine 1928 is 16.7% in adult rats (see Fig. 3); therefore, we set the average of all values for adult rats from each aging experiment equal to 16.7% and normalized all measured values accordingly. Bars represent average of 16 samples ± SEM. The small decrease in total amount of short-form α11.2 in old rats to 89 ± 6% of adult is statistically significant (P < 0.05 by one-way ANOVA and Dunnett's multiple comparison test).
Evaluation of Potential Changes of Synaptic or Dendritic Structures During Aging. It is possible, although controversial, that a sizable number of neurons are lost in aging rats (41) and that the total amount of Cav1.2 channel protein is constant, because the loss on a cellular level is balanced by an increase in channel density and, thereby, activity in the surviving neurons. We determined the relative amounts of multiple neuronal marker proteins in different fractions prepared from hippocampal extracts.
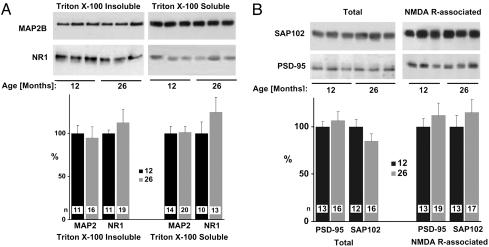
MAP2B is preferentially localized in dendrites (42). Because of its interaction with the cytoskeleton, MAP2B is not effectively extracted from dendritic locations by Triton X-100 (43). A substantial loss of dendritic structures would lead to either a reduction of total MAP2B levels or redistribution from a Triton X-100-insoluble to a Triton X-100-soluble pool. Immunoblotting after separation of soluble from insoluble MAP2B pools by ultracentrifugation did not indicate any redistribution or change of MAP2B in either pool in the 26-month-old rats (Fig. 3A).
Fig. 3.
MAP2B, NR1, PSD-95, and SAP102 are unchanged in old rats. Hippocampal samples were homogenized in 1% Triton X-100 (A) or 1% deoxycholate (B), and soluble and insoluble proteins were separated by ultracentrifugation. (A) Aliquots of comparable amounts of supernatants (Right) and pellets (Left) were analyzed by immunoblotting with antibodies against MAP2B and NR1 and quantified by densitometry. (B) Aliquots of the supernatants were analyzed either directly by immunoblotting with anti-PSD-95 and anti-SAP102 (Left) or after their coprecipitation with NMDA receptors by using a mixture of anti-NR2A and NR2B antibodies for immunoprecipitation (Right). All values were normalized with respect to the average values for each set of markers from adult rats, which corresponded to 100%. Bars represent average of n samples ± SEM (n is given in each bar).
The NR1 subunit of the NMDA receptor also exists in a Triton X-100-soluble and a Triton X-100-insoluble pool. The Triton X-100-soluble pool is strongly enriched in microsomal fractions, which are mainly derived from the endoplasmic reticulum. The majority of NR1 subunits in this pool are not associated with NR2 subunits and represent unassembled and immature receptors (44). NMDA receptors at synaptic sites are largely insoluble in Triton X-100 and require harsher ionic detergents for solubilization (37, 44-46). A loss of synaptic sites caused by a reduction in dendritic arborization or length would reduce the number of target sites for NMDA receptors. As a result, a number of surplus receptors would be unable to go to their ultimate destinations and accumulate in the endoplasmic reticulum or secretory pathways, thereby potentially increasing the Triton X-100-soluble pool. In addition, backlogged receptors may be degraded, leading to a reduction in the total number of NMDA receptors. However, neither the total level nor the distribution of NR1 between Triton X-100-soluble and -insoluble pools was changed in the 26-month-old rats (Fig. 3B).
PSD-95 is a structural protein involved in NMDA receptor clustering (46, 47) and accumulation of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors at postsynaptic sites (48, 49). NMDA receptor clusters can form in the somatic and, especially, the dendritic plasma membrane independent of PSD-95 (46, 50). Accordingly, it is thought that assembly of the fully mature NMDA receptor complex, which would include PSD-95 or one of its homologues, occurs after the NMDA receptor reaches the plasma membrane and, perhaps, the synapse (51). A reduction in the number of synapses should therefore be accompanied by a decrease in either total PSD-95 levels (caused by backlog and degradation) or the degree of its association with NMDA receptors if backlogged PSD-95 is not effectively degraded. Homologs of PSD-95 may associate with their targets in the endoplasmic reticulum (52), and such an association may be important for releasing NMDA receptor complexes from this compartment (53, 54). One candidate that may fulfill this function is SAP102 (54). SAP102 also associates with NMDA receptors and colocalizes with NMDA receptors at postsynaptic sites (35, 45, 55). SAP102 is quite abundant in the Triton X-100-soluble fraction and coimmunoprecipitates from Triton X-100 extracts with NR1 (54), which is present in this fraction mainly in an immature, unassembled form (44). In contrast, PSD-95 is hard to detect in the Triton X-100-soluble fraction. Because SAP102 contrasts with PSD-95 in its general distribution, it may change during synaptic and dendritic loss in a pattern that is different from that of PSD-95. We extracted hippocampal samples of 12- and 26-month-old rats with deoxycholate, which solubilizes Triton X-100-soluble and -insoluble pools of NMDA receptors PSD-95 and SAP102. Samples were directly used for immunoblotting with anti-PSD-95 or anti-SAP102 or after immunoprecipitation of NMDA receptor complexes. Quantification of the immunosignals showed that the total amount of PSD-95 and SAP102 and their association with NMDA receptors were unchanged in the 26-month-old rats (Fig. 3B). Collectively, these results suggest that the number and general organization of neurons are not substantially altered in the 26-month-old versus adult rats.
We investigated potential alterations of various general regulators of PKA-mediated phosphorylation, none of which showed a significant change. Levels of cAMP were slightly increased in the old rats, but changes were not statistically significant (Table 1). For efficient phosphorylation of Cav1.2, PKA has to be recruited to this channel by an A kinase anchor protein (27), one of which, MAP2B, is responsible for directly linking PKA to Cav1.2 (26, 56). As described above, neither the total amount of MAP2B nor its distribution between Triton X-100-soluble and -insoluble compartments is altered in the old rats. PKA consists of two regulatory R and two catalytic C subunits. The Cα isoform is associated with Cav1.2 (26). Cα binding to Cav1.2 is slightly elevated in the old rats, but the difference was not statistically significant (Table 1).
Table 1. General regulators of PKA-mediated protein phosphorylation are unaltered during aging.
| Regulator | % of control | n |
|---|---|---|
| cAMP | 121 ± 20 | 6 |
| PKA Cα | 126 ± 23 | 16 |
| PP2A | 101 ± 15 | 15 |
| PP1 inhibitor 1 | 82 ± 7 | 10 |
| PP1 inhibitor 2 | 127 ± 16 | 14 |
Data represent relative levels (±SEM) in hippocampal extracts (except for PKA Cα, which shows relative amounts associated with immunoprecipitated CaV1.2) from 26-month-old rats as compared with those from 12-month-old rats, which equal 100%.
We also investigated whether the increase in α11.2 phosphorylation may be caused by decreased dephosphorylation of the channel, which is mediated in part by PP2A (36). However, the total protein level of PP2A catalytic subunit was unchanged (Table 1). Finally, we investigated whether protein levels for inhibitors 1 and 2 of the okadaic acid-sensitive phosphatase PP1 were altered to a statistically significant extent, with negative results (Table 1). In summary, these data indicate that protein levels of various players that generally control PKA signaling are not significantly changed in a global or, in the case of PKA Cα, localized manner in the 26-month-old rats. Accordingly, the changes that are responsible for age-dependent up-regulation of α11.2 phosphorylation must be very subtle.
Discussion
The L-type channel antagonist nimodipine reverses learning deficits in rodents (8, 57-63). Influx of Ca2+ through L-type Ca2+ channels is robustly increased in old rodents (9, 64). L-type channels are crucial for various forms of synaptic plasticity such as long-term potentiation (LTP) (65, 66) and long-term depression (67, 68). Many forms of LTP and long-term depression depend on Ca2+ influx at postsynaptic sites, which is usually mediated by NMDA receptors (69, 70). Cav1.2 is clustered at postsynaptic sites (28, 71), where it contributes to the Ca2+ influx (72). High-frequency tetanus (200 Hz)-induced LTP in the hippocampal CA1 region is only partly inhibited by NMDA receptor blockers; the other portion is sensitive to L-type channel antagonists (65). The NMDA receptor-dependent LTP component is decreased, and the L-type channel-dependent LTP component is increased in aging rats (73). Furthermore, elevated L-type channel activity during aging is at least in part responsible for the up-regulation of afterhyperpolarization in hippocampal CA1 neurons, likely by increasing the activity of Ca2+-activated K+ channels, which leads to additional alterations in synaptic plasticity (74-76). Therefore, L-type channel-dependent dysregulation of synaptic plasticity may contribute to age-related deficits in learning and memory. However, the molecular basis for the >2-fold increase in Ca2+ influx was largely unknown. We found that the PKA-mediated phosphorylation of the L-type channel Cav1.2 is strongly up-regulated in 26-month-old Lobund-Wistar rats.
An electrophysiological characterization of the L-type channel Cav1.2 in the heart revealed that these channels exist in three different modes (77). In mode 0, Cav1.2 is not available for activation. Mode 1 is characterized by brief openings, and mode 2 is characterized by long-lasting openings and brief closings (77). The transition from mode 0 to mode 1 or 2 is regulated by PKA, which, when activated, causes a >3-fold increase in the number of functionally available L-type channels within seconds (17, 78, 79). The increase in the number of functional L-type channels in aging rats (9) can be explained by the up-regulation of PKA-mediated phosphorylation, which would bring the channel from the functionally silent mode 0 to the active mode 1 or 2. Because phosphorylation of α11.2 by PKA increases the channel activity 4- to 6-fold (23), the increase in serine 1928 phosphorylation can account to a large degree for the age-related increase in L-type channel activity (9), although additional mechanisms cannot be ruled out. In fact, α11.3 mRNA levels are increased in 24-versus 13-month-old Fisher 344 rats (12, 80). More recent work indicated that the protein level of α11.3 is up-regulated by 25% in the CA1 area without a change in α11.2 protein level (10, 11). This up-regulation of Cav1.3 expression may contribute to the increase in L-type channel activity. Nevertheless, the increase in Cav1.3 is likely not the major factor underlying the age-dependent up-regulation of L-type channel activity and the resulting neuropathological changes for two reasons: (i) Cav1.3 accounts for only 20% of the total number of L-type channels in the rat forebrain (including the hippocampus), whereas Cav1.2 represents 80%. A 25% increase in Cav1.3 would, therefore, lead to only a modest 5% increase in L-type channel activity; however, Thibault and Landfield (9) described a >2-fold increase. (ii) The sensitivity of Cav1.3 for dihydropyridines, such as nimodipine, is 20-fold lower than that of Cav1.2 (IC50 of Cav1.3 for nimodipine is ≈2.7 μM) (81, 82). Clinical doses of dihydropyridines (low- to mid-nM plasma concentrations) likely do not affect Cav1.3 in vivo but act mainly through inhibition of Cav1.2. Bradycardia, arrhythmias, and impaired hearing would be expected (see discussion in ref. 82) if Cav1.3 would be substantially inhibited by therapeutic serum concentrations of dihydropyridines, because Cav1.3 is critical for the depolarization phase in sinoatrial pacemaker cells of the heart (83), and, when knocked out, bradycardia, arrhythmia, and hearing loss caused by dysfunction and degeneration of the inner and outer hair cells result (84).
The number of synaptic contacts is reduced in the aging mammalian brain (85-88). If increased L-type channel activity impairs neuronal functions by contributing to synaptic loss during aging, up-regulation of L-type channels should occur before a significant reduction in the number of synapses. Therefore, we tested whether changes in channel phosphorylation and, thereby, activity occur before synaptic loss in Lobund-Wistar rats. We looked for alterations of various dendritic and postsynaptic marker proteins in 26-versus 12-month-old rats. Neither Triton X-100 solubility nor the total amount of MAP2B was altered in the old rats. These results indicate that the overall distribution of MAP2, which is selectively enriched in dendrites, (and, therefore, likely the length and degree of arborization of dendrites) remains unchanged during early aging. Similarly, we did not detect any changes in the total level or the Triton X-100 solubility of the NMDA receptor subunit NR1. The scaffolding protein PSD-95 mainly interacts with mature NMDA receptors at postsynaptic sites (46, 47, 89). Therefore, synaptic loss is expected to be paralleled by a decrease in total PSD-95 levels or at least in the degree of its association with NMDA receptors. However, we did not observe any significant change in total amount of PSD-95 or in its coimmunoprecipitation with NMDA receptors. These results further corroborate the hypothesis that synaptic and dendritic parameters are not dramatically changed during early aging in rats. The PSD-95 homologue SAP102 also binds to NMDA receptors and colocalizes with them at postsynaptic sites (35, 45, 55). In addition, SAP102 may interact with the NMDA receptor in the endoplasmic reticulum, and such an interaction may be necessary for the release of the NMDA receptor from this compartment (53, 54). At variance with PSD-95, a large portion of SAP102 is effectively solubilized by Triton X-100 and coimmunoprecipitates with the immature NR1 from Triton X-100 extracts. However, like PSD-95, neither the total amount of SAP102 nor its association with NMDA receptors was altered in the old rats. In summary, a multitude of neuronal marker proteins did not show any change in their distribution or protein-protein interactions, arguing against significant synaptic or dendritic changes in our aging model. Accordingly, the increase in PKA-mediated Cav1.2 phosphorylation and, thereby, channel activity occurs before significant changes in synaptic connections in Lobund-Wistar rats.
We were unable to define the molecular basis for the increase in Cav1.2 phosphorylation. General levels of cAMP, PP2A, and PP1 inhibitors 1 and 2 were unaltered. The association of PKA with Cav1.2 was also unchanged. We hypothesize that changes may be quite subtle, perhaps because they involve localized alterations in components of the corresponding signaling pathways. We recently discovered that Cav1.2 colocalizes with the β2 adrenergic receptor at postsynaptic sites in the hippocampus and assembles all components of this signaling pathway into a macromolecular signaling complex, including the β2 adrenergic receptor, the trimeric Gs protein, and an adenylyl cyclase (71) in addition to PKA and the counteracting phosphatase PP2A (26, 36). Furthermore, signaling from the β2 adrenergic receptor to Cav1.2 is locally highly restricted in hippocampal neurons (71). Hence, age-related changes in Cav1.2 phosphorylation may be caused by alterations in the association of those signaling components with Cav1.2 or localized changes in cAMP production. The determination of the physiological and molecular basis of the increase in Cav1.2 phosphorylation during aging will require investigating a multitude of such possibilities. Nevertheless, our findings provide important insight into the molecular mechanism underlying the neuropathological increase in neuronal Ca2+ influx during aging, and the findings form the foundations for further investigations. Because an increase in L-type channel activity may be involved in the etiology of senile symptoms and Alzheimer's disease, these studies may hold the potential to provide the basis for the development of therapies for patients with these and related diseases.
Acknowledgments
We thank Dr. R. Weindruch (University of Wisconsin) for sharing with us Lobund-Wistar rats from his aging rat colony; Dr. R. Jahn for antibodies against NR1; Dr. R. J. Wenthold for antibodies against NR2A and NR2B; Dr. A. C. Nairn for antibodies against PP1 inhibitors 1 and 2; Dr. C. S. Rubin for antibodies against PKA Cα; and Drs. R. Weindruch, P. J. Bertics, P. Lipton, and B. S. Ganetzky (University of Wisconsin) for critically reading the manuscript. This work was supported by National Institutes of Health Grant R01 AG17502 and Alzheimer's Association Faculty Scholar Award 94-033.
Abbreviations: NMDA, N-methyl-d-aspartate; PKA, cAMP-dependent protein kinase; LTP, long-term potentiation.
References
- 1.Carafoli, E. (2002) Proc. Natl. Acad. Sci. USA 99, 1115-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clapham, D. E. (1995) Cell 80, 259-268. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh, A. & Greenberg, M. E. (1995) Science 268, 239-247. [DOI] [PubMed] [Google Scholar]
- 4.Lee, J.-M., Zipfel, G. J. & Choi, D. W. (1999) Nature 399, Suppl., A7-A14. [DOI] [PubMed] [Google Scholar]
- 5.Rothman, S. M. & Olney, J. W. (1987) Trends Neurosci. 10, 299-302. [Google Scholar]
- 6.Disterhoft, J. F., Gispen, W. H., Traber, J. & Khachaturian, Z. S., eds. (1994) Ann. N.Y. Acad. Sci. 747, 1-482.7847664 [Google Scholar]
- 7.Porter, N. M., Thibault, O., Thibault, V., Chen, K.-C. & Landfield, P. W. (1997) J. Neurosci. 17, 5629-5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deyo, R. A., Straube, K. T. & Disterhoft, J. F. (1989) Science 243, 809-811. [DOI] [PubMed] [Google Scholar]
- 9.Thibault, O. & Landfield, P. W. (1996) Science 272, 1017-1020. [DOI] [PubMed] [Google Scholar]
- 10.Veng, L. M. & Browning, M. D. (2002) Brain Res. Mol. Brain Res. 107, 120-127. [DOI] [PubMed] [Google Scholar]
- 11.Veng, L. M., Mesches, M. H. & Browning, M. D. (2003) Brain Res. Mol. Brain Res. 110, 193-202. [DOI] [PubMed] [Google Scholar]
- 12.Herman, J. P., Chen, K. C., Booze, R. & Landfield, P. W. (1998) Neurobiol. Aging 19, 581-587. [DOI] [PubMed] [Google Scholar]
- 13.Fisher, R. & Johnston, D. (1990) J. Neurophysiol. 64, 1291-1302. [DOI] [PubMed] [Google Scholar]
- 14.Gray, R. & Johnston, D. (1987) Nature 327, 620-622. [DOI] [PubMed] [Google Scholar]
- 15.Gross, R. A., Uhler, M. D. & Macdonald, R. L. (1990) J. Physiol. 429, 483-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surmeier, D. J., Bargas, J., Hemmings, H. C., Jr., Nairn, A. C. & Greengard, P. (1995) Neuron 14, 385-397. [DOI] [PubMed] [Google Scholar]
- 17.Bean, B. P., Nowycky, M. C. & Tsien, R. W. (1984) Nature 307, 371-375. [DOI] [PubMed] [Google Scholar]
- 18.Reuter, H. (1983) Nature 301, 569-574. [DOI] [PubMed] [Google Scholar]
- 19.Ertel, E. A., Campbell, K. P., Harpold, M. M., Hofmann, F., Mori, Y., Perez-Reyes, E., Schwartz, A., Snutch, T. P., Tanabe, T., Birnbaumer, L., et al. (2000) Neuron 25, 533-535. [DOI] [PubMed] [Google Scholar]
- 20.Hell, J. W., Westenbroek, R. E., Warner, C., Ahlijanian, M. K., Prystay, W., Gilbert, M. M., Snutch, T. P. & Catterall, W. A. (1993) J. Cell Biol. 123, 949-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hell, J. W., Yokoyama, C. T., Wong, S. T., Warner, C., Snutch, T. P. & Catterall, W. A. (1993) J. Biol. Chem. 268, 19451-19457. [PubMed] [Google Scholar]
- 22.Catterall, W. A. (2000) Annu. Rev. Cell Dev. Biol. 16, 521-555. [DOI] [PubMed] [Google Scholar]
- 23.Sculptoreanu, A., Rotman, E., Takahashi, M., Scheuer, T. & Catterall, W. A. (1993) Proc. Natl. Acad. Sci. USA 90, 10135-10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hell, J. W., Yokoyama, C. T., Breeze, L. J., Chavkin, C. & Catterall, W. A. (1995) EMBO J. 14, 3036-3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Jongh, K. S., Murphy, B. J., Colvin, A. A., Hell, J. W., Takahashi, M. & Catterall, W. A. (1996) Biochemistry 35, 10392-10402. [DOI] [PubMed] [Google Scholar]
- 26.Davare, M. A., Dong, F., Rubin, C. S. & Hell, J. W. (1999) J. Biol. Chem. 274, 30280-30287. [DOI] [PubMed] [Google Scholar]
- 27.Gao, T., Yatani, A., Dell'Acqua, M. L., Sako, H., Green, S. A., Dascal, N., Scott, J. D. & Hosey, M. M. (1997) Neuron 19, 185-196. [DOI] [PubMed] [Google Scholar]
- 28.Hell, J. W., Westenbroek, R. E., Breeze, L. J., Wang, K. K. W., Chavkin, C. & Catterall, W. A. (1996) Proc. Natl. Acad. Sci. USA 93, 3362-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei, X., Neely, A., Lacerda, A. E., Olcese, R., Stefani, E., Perez-Reyes, E. & Birnbaumer, L. (1994) J. Biol. Chem. 269, 1635-1640. [PubMed] [Google Scholar]
- 30.Grynspan, F., Griffin, W. R., Cataldo, A., Katayama, S. & Nixon, R. A. (1997) Brain Res. 763, 145-158. [DOI] [PubMed] [Google Scholar]
- 31.Saito, K., Elce, J. S., Hamos, J. E. & Nixon, R. A. (1993) Proc. Natl. Acad. Sci. USA 90, 2628-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leonard, A. S. & Hell, J. W. (1997) J. Biol. Chem. 272, 12107-12115. [DOI] [PubMed] [Google Scholar]
- 33.Leonard, A. S., Lim, I. A., Hemsworth, D. E., Horne, M. C. & Hell, J. W. (1999) Proc. Natl. Acad. Sci. USA 96, 3239-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valtschanoff, J. G., Burette, A., Davare, M. A., Leonard, A. S., Hell, J. W. & Weinberg, R. J. (2000) Eur. J. Neurosci. 12, 3605-3614. [DOI] [PubMed] [Google Scholar]
- 35.Sans, N., Petralia, R. S., Wang, Y. X., Blahos, J., II, Hell, J. W. & Wenthold, R. J. (2000) J. Neurosci. 20, 1260-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davare, M. A., Horne, M. C. & Hell, J. W. (2000) J. Biol. Chem. 275, 39710-39717. [DOI] [PubMed] [Google Scholar]
- 37.Leonard, A. S., Davare, M. A., Horne, M. C., Garner, C. C. & Hell, J. W. (1998) J. Biol. Chem. 273, 19518-19524. [DOI] [PubMed] [Google Scholar]
- 38.Snyder, D. L., Pollard, M., Wostmann, B. S. & Luckert, P. (1990) J. Gerontol. Biol. Sci. 45, B52-58. [DOI] [PubMed] [Google Scholar]
- 39.Snyder, D. L. (1989) Dietary Restriction and Aging (Liss, New York).
- 40.Sanderson, J. P., Binkley, L., Roecker, E. B., Champ, J. E., Pugh, T. D., Aspnes, L. & Weindruch, R. (1997) J. Gerontol. Biol. Sci. 52, B20-B25. [DOI] [PubMed] [Google Scholar]
- 41.Geinisman, Y., Detoledo-Morrell, L., Morrell, F. & Heller, R. E. (1995) Prog. Neurobiol. 45, 223-252. [DOI] [PubMed] [Google Scholar]
- 42.Caceres, A., Banker, G., Steward, O., Binder, L. & Payne, M. (1984) Brain Res. 315, 314-318. [DOI] [PubMed] [Google Scholar]
- 43.Allison, D. W., Gelfand, V. I., Spector, I. & Craig, A. M. (1998) J. Neurosci. 18, 2423-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blahos, J., II, & Wenthold, R. J. (1996) J. Biol. Chem. 271, 15669-15674. [DOI] [PubMed] [Google Scholar]
- 45.Lau, L.-F., Mammen, A., Ehlers, M. D., Kindler, S., Chung, W. J., Garner, C. C. & Huganir, R. L. (1996) J. Biol. Chem. 271, 21622-21628. [DOI] [PubMed] [Google Scholar]
- 46.Rao, A., Kim, E., Sheng, M. & Craig, A. M. (1998) J. Neurosci. 18, 1217-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim, E., Cho, K.-O., Rothschild, A. & Scheng, M. (1996) Neuron 17, 103-113. [DOI] [PubMed] [Google Scholar]
- 48.Chen, L., Chetkovich, D. M., Petralia, R. S., Sweeney, N. T., Kawasaki, Y., Wenthold, R. J., Bredt, D. S. & Nicoll, R. A. (2000) Nature 408, 936-943. [DOI] [PubMed] [Google Scholar]
- 49.El-Husseini, A. E., Schnell, E., Chetkovich, D. M., Nicoll, R. A. & Bredt, D. S. (2000) Science 290, 1364-1368. [PubMed] [Google Scholar]
- 50.Dalva, M. B., Takasu, M. A., Lin, M. Z., Shamah, S. M., Hu, L., Gale, N. W. & Greenberg, M. E. (2000) Cell 103, 945-956. [DOI] [PubMed] [Google Scholar]
- 51.Sheng, M. & Pak, D. T. (2000) Annu. Rev. Physiol. 62, 755-778. [DOI] [PubMed] [Google Scholar]
- 52.Tiffany, A. M., Manganas, L. N., Kim, E., Hsueh, Y. P., Sheng, M. & Trimmer, J. S. (2000) J. Cell Biol. 148, 147-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scott, D. B., Blanpied, T. A., Swanson, G. T., Zhang, C. & Ehlers, M. D. (2001) J. Neurosci. 21, 3063-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Standley, S., Roche, K. W., McCallum, J., Sans, N. & Wenthold, R. J. (2000) Neuron 28, 887-898. [DOI] [PubMed] [Google Scholar]
- 55.Muller, B. M., Kistner, U., Kindler, S., Chung, W. J., Kuhlendahl, S., Fenster, S. D., Lau, L.-F., Veh, R. W., Huganir, R. L., Gundelfinger, E. D. & Garner, C. C. (1996) Neuron 17, 255-265. [DOI] [PubMed] [Google Scholar]
- 56.Vallee, R. B., DiBartolomeis, M. J. & Theurkauf, W. E. (1981) J. Cell Biol. 90, 568-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deyo, R. A., Straube, K. T., Moyer, J. R., Jr., & Disterhoft, J. F. (1989) Exp. Aging. Res. 15, 169-175. [DOI] [PubMed] [Google Scholar]
- 58.Ingram, D. K., Joseph, J. A., Spangler, E. L., Roberts, D., Hengemihle, J. & Fanelli, R. J. (1994) Neurobiol. Aging 15, 55-61. [DOI] [PubMed] [Google Scholar]
- 59.Kowalska, M. & Disterhoft, J. F. (1994) Exp. Neurol. 127, 159-166. [DOI] [PubMed] [Google Scholar]
- 60.Levere, T. E. & Walker, A. (1992) Neurobiol. Aging 13, 63-66. [DOI] [PubMed] [Google Scholar]
- 61.Meneses, A., Terron, J. A., Ibarra, M. & Hong, E. (1997) Behav. Brain Res. 85, 121-125. [DOI] [PubMed] [Google Scholar]
- 62.Sandin, M., Jasmin, S. & Levere, T. E. (1990) Neurobiol. Aging 11, 573-575. [DOI] [PubMed] [Google Scholar]
- 63.Woodruff-Pak, D. S., Chi, J., Li, Y. T., Pak, M. H. & Fanelli, R. J. (1997) Neurobiol. Aging 18, 641-649. [DOI] [PubMed] [Google Scholar]
- 64.Campbell, L. W., Hao, S.-Y., Thibault, O., Blalock, E. M. & Landfield, P. W. (1996) J. Neurosci. 16, 6286-6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grover, L. M. & Teyler, T. J. (1990) Nature 347, 477-479. [DOI] [PubMed] [Google Scholar]
- 66.Impey, S., Mark, M., Villacres, E. C., Poser, S., Chavkin, C. & Storm, D. R. (1996) Neuron 16, 973-982. [DOI] [PubMed] [Google Scholar]
- 67.Bolshakov, V. Y. & Siegelbaum, S. A. (1994) Science 264, 148-152. [DOI] [PubMed] [Google Scholar]
- 68.Christie, B. R., Schexnayder, L. K. & Johnston, D. (1997) J. Neurophysiol. 77, 1651-1655. [DOI] [PubMed] [Google Scholar]
- 69.Bliss, T. V. & Collingridge, G. L. (1993) Nature 361, 31-39. [DOI] [PubMed] [Google Scholar]
- 70.Malenka, R. C. & Nicoll, R. A. (1999) Science 285, 1870-1874. [DOI] [PubMed] [Google Scholar]
- 71.Davare, M. A., Avdonin, V., Hall, D. D., Peden, E. M., Burette, A., Weinberg, R. J., Horne, M. C., Hoshi, T. & Hell, J. W. (2001) Science 293, 98-101. [DOI] [PubMed] [Google Scholar]
- 72.Segal, M. (1995) J. Physiol. 486, 283-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shankar, S., Teyler, T. J. & Robbins, N. (1998) J. Neurophysiol. 79, 334-341. [DOI] [PubMed] [Google Scholar]
- 74.Norris, C. M., Halpain, S. & Foster, T. C. (1998) J. Neurosci. 18, 3171-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Norris, C. M., Halpain, S. & Foster, T. C. (1998) J. Neurophysiol. 80, 1567-1570. [DOI] [PubMed] [Google Scholar]
- 76.Thibault, O., Hadley, R. & Landfield, P. W. (2001) J. Neurosci. 21, 9744-9756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hess, P., Lansman, J. B. & Tsien, R. W. (1984) Nature 311, 538-544. [DOI] [PubMed] [Google Scholar]
- 78.Trautwein, W. & Hescheler, J. (1990) Annu. Rev. Physiol. 52, 257-274. [DOI] [PubMed] [Google Scholar]
- 79.Yue, D. T., Herzig, S. & Marban, E. (1990) Proc. Natl. Acad. Sci. USA 87, 753-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen, K. C., Blalock, E. M., Thibault, O., Kaminker, P. & Landfield, P. W. (2000) Proc. Natl. Acad. Sci. USA 97, 4357-4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu, W. & Lipscombe, D. (2001) J. Neurosci. 21, 5944-5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koschak, A., Reimer, D., Huber, I., Grabner, M., Glossmann, H., Engel, J. & Striessnig, J. (2001) J. Biol. Chem. 276, 22100-22106. [DOI] [PubMed] [Google Scholar]
- 83.Mangoni, M. E., Couette, B., Bourinet, E., Platzer, J., Reimer, D., Striessnig, J. & Nargeot, J. (2003) Proc. Natl. Acad. Sci. USA 100, 5543-5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Platzer, J., Engel, J., Schrott-Fischer, A., Stephan, K., Bova, S., Chen, H., Zheng, H. & Striessnig, J. (2000) Cell 102, 89-97. [DOI] [PubMed] [Google Scholar]
- 85.Bertoni-Freddari, C., Fattoretti, P., Paoloni, R., Caselli, U., Galeazzi, L. & Meier-Ruge, W. (1996) Gerontology 42, 170-180. [DOI] [PubMed] [Google Scholar]
- 86.Mrak, R. E., Griffin, W. S. T. & Graham, D. I. (1997) J. Neuropathol. Exp. Neurol. 56, 1269-1275. [DOI] [PubMed] [Google Scholar]
- 87.Saito, S., Kobayashi, S., Ohashi, Y., Igarashi, M., Komiya, Y. & Ando, S. (1994) J. Neurosci. Res. 39, 57-62. [DOI] [PubMed] [Google Scholar]
- 88.Wong, T. P., Campbell, T. M., Ribeiro-da-Silva, A. & Cuello, A. C. (1998) Neuroscience 84, 403-412. [DOI] [PubMed] [Google Scholar]
- 89.Sheng, M. & Sala, C. (2001) Annu. Rev. Neurosci. 24, 1-29. [DOI] [PubMed] [Google Scholar]