Abstract
In the peripheral nervous system (PNS), damaged axons regenerate successfully, whereas axons in the CNS fail to regrow. In neurons of the dorsal root ganglia (DRG), which extend branches to both the PNS and CNS, only a PNS lesion but not a CNS lesion induces axonal growth. How this differential growth response is regulated in vivo is only incompletely understood. Here, we combine in vivo time-lapse fluorescence microscopy with genetic manipulations in mice to reveal how the transcription factor STAT3 regulates axonal regeneration. We show that selective deletion of STAT3 in DRG neurons of STAT3-floxed mice impairs regeneration of peripheral DRG branches after a nerve cut. Further, overexpression of STAT3 induced by viral gene transfer increases outgrowth and collateral sprouting of central DRG branches after a dorsal column lesion by more than 400%. Notably, repetitive in vivo imaging of individual fluorescently labeled PNS and CNS axons reveals that STAT3 selectively regulates initiation but not later perpetuation of axonal growth. With STAT3, we thus identify a phase-specific regulator of axonal outgrowth. Activating STAT3 might provide an opportunity to “jumpstart” regeneration, and thus prime axons in the injured spinal cord for application of complementary therapies that improve axonal elongation.
Keywords: in vivo microscopy, spinal cord injury, peripheral nerve lesion, intrinsic growth program
Lesioned peripheral nervous system (PNS) axons regenerate successfully, whereas lesioned CNS axons fail to regrow. This differential behavior is exemplified by neurons located in the dorsal root ganglia (DRG), which extend one branch into the PNS and another into the CNS. In these neurons, a cut in the PNS but not in the CNS is followed by neuronal outgrowth (1). If, however, the DRG neuron is “conditioned” by a transection of its peripheral branch, a subsequent central lesion can be followed by extensive outgrowth (2, 3). This suggests the existence of a common intrinsic neuronal growth program that can, in principle, support both PNS and CNS growth but is normally initiated only after a PNS lesion. In recent years, several intracellular components that might regulate this intrinsic growth program have been identified (4, 5). They include a number of transcriptional regulators such as the transcription factors cJun (6), SMAD1 (7), ATF3 (8), AKRD1 (9), NFIL3 (10), and several KLF family members (11). One particularly interesting transcriptional regulator is STAT3, which is activated as part of the JAK–STAT signaling pathway (12). The following findings make STAT3 a good candidate for regulating axon growth: first, increased levels of STAT3 expression and phosphorylation are associated with axonal regeneration (13–15) and axonal remodelling (16). Second, molecules that can affect STAT3 signaling such as the neuropoietic cytokines IL-6, ciliary neurotrophic factor, and leukemia inhibitory factor, as well as the intracellular regulator SOCS3, have been shown to influence axonal regeneration (17–20). Third, STAT3 expression promotes neuronal outgrowth in cultured CNS neurons (21) and increased STAT3 expression is directly involved in the conditioning response of DRG neurons (22).
The identification of STAT3 and other transcription factors indicates that multiple transcriptional programs exist that can, in principle, influence the neuronal growth response to injury. Whether they operate in concert or in succession, e.g., by affecting specific phases of the growth response, such as growth initiation or elongation, is not known. A direct way to elucidate how a given factor affects different phases of axonal growth is to visualize progress of regenerating axons in vivo (3, 23–25) in the presence or absence of such a factor.
Here we use in vivo imaging in combination with selective genetic manipulations to address whether and when the transcription factor STAT3 influences the divergent growth pattern of lesioned PNS and CNS axons. We show that deletion of STAT3 is sufficient to impair PNS axon regeneration. By comparing the in vivo growth pattern of regrowing STAT3-competent and STAT3-deficient axons, we discovered that STAT3 regulates the timing of growth induction but not subsequent axon elongation. In line with this finding, viral gene delivery of either STAT3 or its constitutively active version, STAT3c, to DRG neurons significantly improves terminal and collateral sprouting after a CNS lesion by promoting growth induction but not elongation. Thus, STAT3 acts as a phase-specific regulator of axonal regeneration that selectively controls the timing of growth induction after CNS and PNS lesions.
Results
STAT3 Deletion Impairs the Regeneration of PNS Axons.
To confirm that STAT3 is activated after a PNS lesion, we studied the expression of STAT3 and its active, phosphorylated form P-STAT3 in DRG neurons by immunohistochemistry at different time points after creation of bilateral lesions of the saphenous nerves, which contain the axons of the third lumbar (L3) DRGs (26). Starting within a few hours and lasting for weeks after transection, we observed a significant increase in the number of P-STAT3–positive nuclei in L3 DRG neurons (Fig. 1 A, B, and E). STAT3 expression in DRG neurons overall followed a similar time course (Fig. S1). However, at early time points, hours after the lesion, STAT3 phosphorylation appears to precede increased STAT3 expression. Together these findings indicate that, after a PNS lesion is created, STAT3 activity is regulated on both the phosphorylation and expression levels.
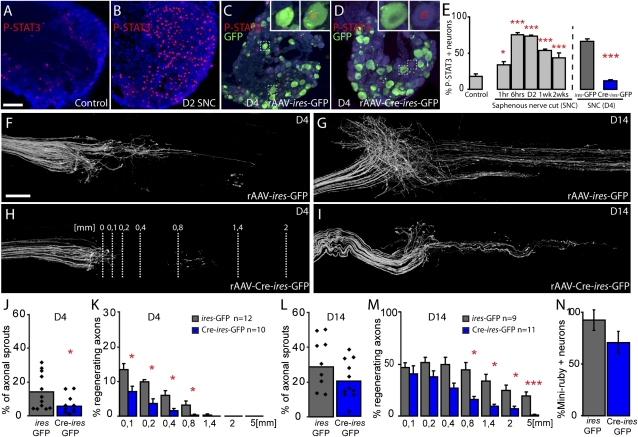
Fig. 1.
Deletion of STAT3 impairs regeneration of peripheral DRG axons after a saphenous nerve cut (SNC; A and B). Confocal images of L3 DRGs immunostained for P-STAT3 (red) and counterstained with fluorescent Nissl-like stain (NeuroTrace, cyan) in a control (unlesioned) WT mouse (A) and 2 d following an SNC (B). (C and D) L3 DRGs of STAT3fl/fl mice 4 d after SNC previously injected with rAAV-ires-GFP (C) or rAAV-Cre-ires-GFP (D; NeuroTrace, cyan; P-STAT3, red; GFP, green). GFP-positive DRG neurons (Insets, C and D) are at a magnification of ×3. (E) Quantification of the number of P-STAT3–positive DRG neurons (identified by NeuroTrace counterstaining) at different time points following SNC in WT mice and in STAT3fl/fl mice previously injected with rAAV-ires-GFP (gray column) or rAAV-Cre-ires-GFP (blue column) at 4 d following a SNC (n = 6 animals per group). (F–I) Confocal images taken at 4 d (F and H) and 14 d (G and I) after SNC display the proximal stump of STAT3fl/fl saphenous nerves that receive fibers from L3 DRGs injected with control rAAV-ires-GFP (F and G) or rAAV-Cre-ires-GFP (H and I). (J–M) Quantification of axonal sprouting at the site of lesion (J and L) and regeneration ratios (K and M) at different distances from the cut site (lines, H) of axons derived from STAT3-competent DRG neurons (ires-GFP, gray columns) and STAT3-deficient DRG neurons (Cre-ires-GFP, blue columns) at 4 d (J and K) and 14 d (L and M) after SNC. (N) Quantification of the percentage of L3 DRG neurons retrogradely labeled with the tracer Miniruby from distal STAT3-competent (ires-GFP, gray columns; n = 21 sections, n = 6 DRGs) and STAT3-deficient (Cre-ires-GFP, blue columns; n = 11 sections, n = 4 DRGs) saphenous nerves 28 d following SNC (values were normalized to the percentage of Miniruby-positive DRG neurons traced from the same anatomical localization in unlesioned mice). (Scale bars: A, 100 μm; F, 250 μm.)
To assess the contribution of STAT3 activation to PNS regeneration, we selectively deleted STAT3 expression in DRG neurons. We constructed recombinant adeno-associated viral vectors (rAAVs) expressing either a bicistronic combination of Cre recombinase and GFP (rAAV-Cre-ires-GFP) or just GFP (rAAV-ires-GFP; SI Materials and Methods). After injection of rAAV-Cre-ires-GFP into the DRGs of STAT3-floxed (STAT3fl/fl) mice (27), transduced DRG neurons become depleted of STAT3, while at the same time their axons can be readily identified by GFP expression. Injection of the control vector (rAAV-ires-GFP) labels axons without affecting STAT3 expression. The efficiency of STAT3 deletion was confirmed by analysis of P-STAT3 immunohistochemistry 4 d after lesion creation (Fig. 1 C–E and Fig. S2). We then performed bilateral saphenous nerve transections in STAT3fl/fl mice that had been injected 10 d earlier with rAAV-Cre-ires-GFP in the right L3 DRG and rAAV-ires-GFP in the left L3 DRG. We compared the growth response of STAT3-deficient and STAT3-competent GFP-positive axons over time (Fig.1 F–M). At 4 d after lesion, STAT3-competent axons showed substantial sprouting and regeneration along the nerve for as much a several hundred micrometers (Fig. 1 F, J and K). In contrast, STAT3-deficient axons showed only minimal sprouting (Fig. 1 H and J), as well as fewer and shorter regenerating axons (Fig. 1 H and K). Two weeks after lesion, long-distance axonal regeneration in STAT3-deficient axons was still impaired (Fig. 1 I and M), while many STAT3-competent axons had grown several millimeters to reenter the distal stump of the saphenous nerve (Fig. 1 G and M). However, at this time, sprouting around the lesion and axonal regeneration close to the lesion was comparable between STAT3-deficient and STAT3-competent axons (Fig. 1 L and M). This suggests that STAT3-deficient axons can still initiate the regeneration process. Indeed, retrograde labeling from the distal saphenous nerve performed at 28 d after lesion showed that similar proportions of STAT3-competent and STAT3-deficient axons had reapproached their termination zone (Fig. 1N).
STAT3 Deletion Affects Initiation but Not Perpetuation of PNS Axon Regeneration in Vivo.
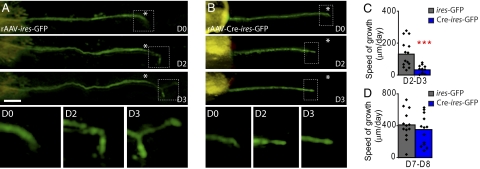
Impaired regeneration of STAT3-deficient PNS axons could be caused by delayed growth induction or reduced elongation of regenerating axons. To differentiate these possibilities, we followed the outgrowth of fluorescently labeled STAT3-competent and STAT3-deficient axons by repetitive in vivo imaging. We first imaged regrowing axons on consecutive days when axonal regeneration is induced (day 2–4 after lesion). Many STAT3-competent DRG axons initiate growth within 2 d after lesion and progress with an average speed of 132 ± 23 μm/d from day 2 to day 3 (Fig. 2 A and C). In contrast, the vast majority of STAT3-deficient axons fail to initiate growth during the first 2 d after lesion. Accordingly, the speed of regeneration is reduced more than threefold in STAT3-deficient axons in this initiation phase (33 ± 6 μm/d; Fig. 2 B and C). Notably during the axon elongation phase 7 to 8 d after lesion, when regeneration speed has increased to approximately 400 μm/d, there is no difference between axons derived from STAT3-competent and STAT3-deficient DRG neurons (411 ± 48 μm/d for axons from rAAV-ires-GFP-injected DRGs vs. 341 ± 53 μm/d for axons from rAAV-Cre-ires-GFP-injected DRGs; Fig. 2D). Taken together, these results indicate that STAT3 is crucial for the timing of growth initiation but not for subsequent elongation of PNS axons.
Fig. 2.
In vivo imaging reveals that growth of STAT3-deficient axons is reduced in the initial but not the later stages of regeneration. (A and B) In vivo time-lapse images of saphenous nerve axons (GFP, green) in sparsely labeled animals emerging from DRGs that were injected with control rAAV-ires-GFP (A) or rAAV-Cre-ires-GFP (B). Axons were imaged immediately after transection (D0) as well as 2 and 3 d after transection (asterisk indicates lesion site, orange latex beads visible on the left were used as fiduciary markers). Lower row of boxes show the boxed axon tips at magnifications of ×3 over time. (C and D) Quantification of the growth speed of axons from STAT3-competent DRG neurons (ires-GFP, gray columns) and STAT3-deficient DRG neurons (Cre-ires-GFP, blue columns) during the early (C, 2–3 d after transection) and the late phase (D, 7–8 d after transection) of the regeneration process. (Scale bar: A, 100 μm.)
STAT3 and STAT3c Gene Therapy Can Initiate but Not Perpetuate CNS Axon Outgrowth.
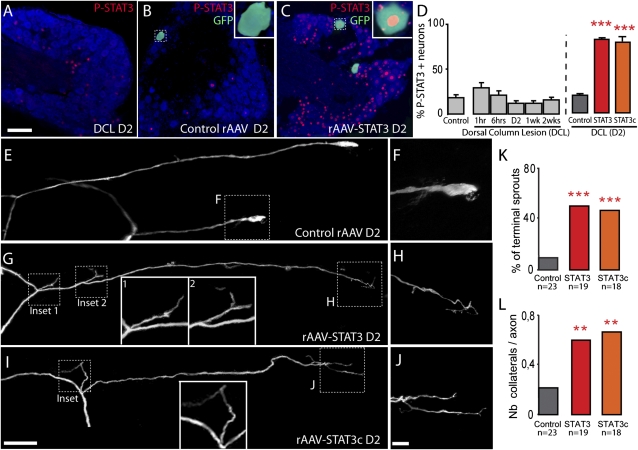
The failure to initiate growth is a key impediment to successful regeneration in the CNS. Therefore, we examined whether STAT3 overexpression is sufficient to induce outgrowth of CNS axons in vivo. We first evaluated the activation of endogenous STAT3 after a CNS lesion by P-STAT3 and STAT3 immunostaining of cervical DRGs after a bilateral dorsal column transection, which interrupts central projections from cervical DRG neurons. In contrast to the sustained activation of STAT3 after a PNS lesion (cf. Fig. 1), a dorsal column lesion induces no significant changes in the P-STAT3 and STAT3 immunoreactivity in DRG neurons (Fig. 3 A and D and Fig. S1). To exogenously increase STAT3 expression, we produced rAAVs expressing either a control protein (enhanced CFP or Cre recombinase; control rAAV), STAT3 (rAAV-STAT3), or a constitutively active variant of STAT3 (rAAV-STAT3c) and confirmed the efficiency of viral gene transfer by immunofluorescence (Fig. 3 B–D). We then injected the respective rAAVs into the DRGs of Thy1-GFPs mice, which express GFP in a subset of DRG neurons (28). Ten to 12 d later we surgically reexposed the spinal cord, lesioned individual GFP-positive axons emerging from the injected DRGs in the dorsal funiculus using a hand-held small-diameter needle, and imaged their growth response over time. As expected, 2 d after lesion, only 9% of axons emerging from DRGs injected with control rAAV had formed sprouts (Fig. 3 E, F, and K and Fig. S3). In contrast, 53% of r-AAV-STAT3–transduced axons (Fig. 3 G, H and K and Fig. S3) and 46% of the rAAV-STAT3c–transduced axons (Fig. 3 I, J, and K and Fig. S3) showed an early growth response. Interestingly, STAT3 expression not only increased terminal sprouting, but also collateral sprouting along the axon (Fig. 3 G, I, and L). The finding that a similar growth induction was observed after injection of rAAV-STAT3 and rAAV-STAT3c indicates that overexpression of STAT3 alone is sufficient to induce downstream effects on regeneration.
Fig. 3.
Viral vector gene transfer of STAT3 and STAT3c induces terminal and collateral sprouting of DRG branches after a central lesion. (A–C) Confocal images of cervical DRGs immunostained for P-STAT3 (red) and counterstained with fluorescent Nissl-like stain (NeuroTrace, cyan) in a WT mouse 2 d following a dorsal column lesion (DCL, A) and in lesioned Thy1-GFPs mice (GFP, green) injected with control rAAV (B) or rAAV-STAT3 (C). Insets: Higher-magnification (×3) of the GFP-positive neurons boxed in the images. (D) Quantification of the number of P-STAT3–positive DRG neurons (identified by NeuroTrace counterstaining) at different time points following DCL in WT mice and in mice previously injected with control rAAV (gray column), rAAV-STAT3 (red column), or rAAV-STAT3c (orange column) at 2 d after a central lesion (n = 6 animals per group). (E–J) Confocal images of lesioned spinal axon endings derived from DRGs injected with control rAAV (bulbs, E and F), rAAV-STAT3 (terminal sprout, G and H), or rAAV-STAT3c (terminal sprout, I and J). (F, H, and J) Higher-magnification views of details boxed in E, G, and I. Additional insets (G and I) show a magnification ×2 of the boxed collateral sprouts. (K and L) Quantification of terminal (K) and collateral (L) sprouting of axons derived from DRGs injected with control rAAV, rAAV-STAT3, or rAAV-STAT3c and analyzed 2 d after transection. (Scale bars: A and I, 100 μm; J, 25 μm.)
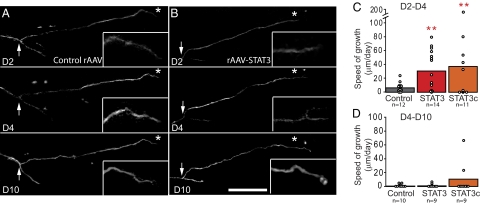
To determine how STAT3 overexpression affects different phases of axonal outgrowth, we used repetitive multiphoton imaging to follow the growth pattern of individual GFP-labeled DRG axons emerging from DRGs injected with control-rAAV, rAAV-STAT3, or rAAV-STAT3c at 2, 4, and 10 d after lesion. STAT3 and STAT3c overexpression increased the speed of axonal growth in the early phase (2–4 d) of regeneration (Fig. 4 A-C). However, this early growth cannot be sustained, and only very limited axonal extension can be observed in all groups between 4 and 10 d after lesion (Fig. 4 A, B, and D). Thus, in the CNS, as in the PNS, STAT3 regulates the initiation of axonal growth but not the elongation of regenerating axons.
Fig. 4.
In vivo imaging reveals successful initiation but not elongation of CNS axons after STAT3 and STAT3c gene therapy. (A and B) Multiphoton images of the growth pattern of spinal DRG axons emerging from DRGs injected with control rAAV (A) or rAAV-STAT3 (B) imaged 2, 4, and 10 d following a central lesion. Insets: Magnifications (×2) of boxed axon ends. (C and D) Quantification of axonal growth speed in vivo after injection of control rAAV (gray bars), rAAV-STAT3 (red bars), or rAAV-STAT3c (orange bars) analyzed early (C, 2–4 d) and late (D, 4–10 d) after transection. (Scale bar: B, 200 μm.)
Discussion
The present study identifies the transcription factor STAT3 as a phase-specific regulator of neuronal outgrowth in both the PNS and CNS. In DRG neurons, the endogenous expression of STAT3 parallels the regenerative response. By using conditional deletion of STAT3 in combination with in vivo imaging, we now also show that STAT3 expression is not only associated with axonal regeneration, but is in fact crucial for the timing of axonal growth initiation after a PNS lesion. It is interesting to note, however, that axons from STAT3-deficient neurons can still mount a growth response, albeit with a prolonged “lag” phase compared with their STAT3-competent counterparts. This suggests that in the PNS compensatory mechanisms are in place that can induce neuronal growth in the absence of STAT3. Notably, additional overexpression of STAT3 or STAT3c did not further improve PNS regeneration (Fig. S4), indicating that a PNS lesion alone is sufficient to induce optimal levels of STAT3 activation for regeneration. When regeneration has been initiated, STAT3-deficient axons grow with the same speed as STAT3-competent axons. Thus, STAT3 is primarily needed to induce a neuronal growth program. When the growth program has been initiated, STAT3 is no longer required for perpetuation of axonal outgrowth. Given this role of STAT3 in PNS regeneration, it is tempting to speculate that the failure of CNS lesions to up-regulate STAT3 expression is directly linked to the failure of CNS axons to initiate outgrowth. Indeed, we can show that overexpression of STAT3 by viral vector gene transfer alone is sufficient to initiate axonal growth initiation in more than half the lesioned CNS axons. However, as the baseline sprouting response of CNS neurons is not affected by STAT3 deletion (12% of axons with sprouts are emerging from STAT3-competent DRGs, compared with 15.4% emerging from STAT3-depleted DRG axons; n = 28–30 axons per group), it is likely that, as in the PNS, additional regulators can induce CNS outgrowth independent of STAT3.
The observation that early stages of axon growth can be initiated in many transected axons, even in the hostile CNS environment, by expression of a single intracellular molecule highlights the importance of intrinsic mediators of axonal growth. During recent years, a number of molecules that can influence the intrinsic neuronal growth response have been identified. These include c-AMP and its downstream mediators (29), the growth cone-associated proteins GAP43 and CAP23 (30), components of the PTEN/mTOR pathway (31), as well as a number of transcription factors (6–11). Further, a number of recent studies in Caenorhabditis elegans have demonstrated an essential role of the DLK-1 MAP kinase pathway for axon regeneration, and in particular growth cone formation and migration (32–34). As more and more components of the intrinsic growth response are emerging, it becomes increasingly important to understand how they act in concert to regulate the complex process of axonal outgrowth.
The present study provides evidence that, in vivo, this intrinsic growth response can be divided into at least two distinct phases: initiation and elongation. The concept of a multiphasic growth response suggests a number of conclusions. One is that the distinct phases of axonal growth are likely regulated by distinct molecular mechanisms. STAT3, for example, controls the timely initiation of axonal growth but does not affect axonal elongation. The molecular mechanisms by which STAT3 initiates this axonal growth program are currently not known. However, as STAT3 is a transcription factor, it is likely that the downstream effects are mediated by induction of gene expression. A large number of genes that are affected by STAT3 have already been identified. Some of these downstream targets like the cell cycle inhibitor P21/Cip1/Waf1 (35) or the small proline rich protein 1a (SPRR1A) (36) can directly affect neuronal outgrowth (37, 38). Notably, a recent transcriptional profiling study has identified several additional genes that are specifically regulated by STAT3 in DRG neurons. At least one of these genes, the IFN regulatory factor 1 (IRF1), is sufficient to increase neuronal outgrowth in cultured cerebellar neurons (21).
Our results further suggest that it is likely that environmental cues play key roles in shaping the distinct stages of axonal regrowth. For example, in the PNS, it is conceivable, that the transition from growth initiation to elongation is induced by the interaction of axons with Schwann cells. Schwann cells align after injury to form tubes, also called bands of Büngner, that guide the axons to their target cells (39). Axons could require STAT3 to initiate growth independently of Schwann cell guidance; however, when a regrowing axon has contacted the Schwann cell tube, it shifts to the elongation mode and no longer requires STAT3. In line with this scenario, we can show that PNS crush lesions that do not interrupt Schwann cell guidance do not induce STAT3 expression in the corresponding DRG neurons (Fig. S5). Although the reasons behind this lack of STAT induction are not yet understood and might include the induction of differential injury signals by crush and cut lesions (40), the rapid outgrowth of crushed axons (41) indicates that axonal growth along glial support structures does not require STAT3. Like in the PNS, changes in the environment may also help to explain the transition (or lack thereof) between different growth phases after a CNS lesion. Although, in the PNS, the regrowing axon can reach Schwann cell support at some point, CNS axons that initiate growth in response to STAT3 continue to encounter a growth-inhibitory CNS environment that becomes even more hostile with the development of a glial scar. These changes in the lesion environment might help to explain why STAT3-transduced axons can initiate growth early but fail to support it later. In line with this assumption, when we combined the induction of STAT3 (by viral vector gene transfer of rAAV-STAT3) with the neutralization of inhibitory scar components (by application of chondroitinase ABC) (42), the average axonal outgrowth over a period of 10 d after a CNS lesion was increased more than twofold (141 ± 53 μm for axons treated with rAAV-STAT3 and chondroitinase ABC vs. 42 ± 26 μm for axons treated with rAAV-STAT3 alone and 64 ± 28 μm for axons treated with chondroitinase alone; n = 14–20 axons per group). These results underline the importance of developing combined therapeutic strategies that target the molecularly distinct phases of the axonal growth response. In this concept, phase-specific regulators of axonal growth initiation such as STAT3 would be used to “jumpstart” the regeneration process and prime axons in the injured spinal cord for application of complementary therapies that can sustain axonal elongation in the growth-inhibitory CNS environment (43).
Materials and Methods
Mice.
Animals used in this study were adult female WT mice on a C57BL/6 background (weight 20–30 g, 6–12 wk of age) with the following exceptions: STAT3 was deleted by using STAT3fl/fl mice, which are maintained on a BL6 background (27). Further, central axon regeneration was investigated in Thy1-GFPs mice (28), which express GFP in a subset of neurons and are maintained on a mixed background. All animal experiments were performed in accordance with regulations of the animal welfare act and protocols approved by the Regierung von Oberbayern.
AAV Vector Construction, Production, and Purification.
The adeno-associated viral vectors used in this study have been cloned into the pAAV-MCS vector from Stratagene and were produced by the adenovirus-free AAV production method as detailed in SI Materials and Methods.
Tissue Processing, Immunohistochemistry, and STAT Expression Analysis.
Animals were deeply anesthetized with isoflurane and perfused transcardially with saline followed by 4% paraformaldehyde in 0.01 M phosphate buffer. DRGs were dissected out, immunostained for P-STAT3 and STAT3 and analyzed as detailed in SI Materials and Methods.
Gene Therapy with Recombinant Adeno-associated Viral Vectors.
For analysis of PNS regeneration, the left and right L3 DRGs of anesthetized STAT3fl/fl mice were surgically exposed after a dorsal laminectomy. Then, 1 μL of rAAV-ires-GFP was slowly injected into the left L3 DRG with a thinly drawn glass capillary and the same amount of rAAV-Cre-ires-GFP was injected into the right L3 DRG of the same animal. Ten days after the injection, the saphenous nerve was bilaterally transected at the midthigh level using fine iridectomy scissors as previously described (23).
For analysis of CNS regeneration, a cervical dorsal laminectomy was performed in Thy1-GFPs mice anesthetized by an i.p. injection of ketamine/xylazine (ketamine 87 mg/kg, xylazine 13 mg/kg) as previously described (24). DRGs, from which suitably labeled axons emerged, were identified by in vivo imaging (SI Materials and Methods) and surgically exposed. Then, 1 μL of rAAV-STAT3, rAAV-STAT3c, rAAV-eCFP, or rAAV-Cre (control rAAV) was slowly injected into the DRG with a thinly drawn glass capillary. Ten to 12 d later, Thy1-GFPs mice were reanesthetized, the spinal cord laminectomy site was reaccessed, and selected fluorescently labeled axons were transected with a hand-held 32-gauge hypodermic needle.
Confocal Microscopy.
We obtained confocal images of fixed tissue on a FV1000 confocal system mounted on an upright BX61 microscope (Olympus) and equipped with 20×/0.85 and 60×/1.42 oil immersion objectives. We recorded stacks of 12-bit images that were processed using MetaMorph software (Universal Imaging) or the freeware ImageJ/Fiji (http://rsbweb.nih.gov/ij).
In Situ and in Vivo Analysis of Axon Regeneration.
The regeneration of transected peripheral and central DRG axons was evaluated as detailed in SI Materials and Methods.
Statistical Analysis.
Results are given as mean ± SEM unless indicated otherwise. Statistical significance was determined using GraphPad Prism software (GraphPad). All data were analyzed by using a one-way ANOVA followed by a Tukey post-hoc test for multiple comparisons or a t test for single comparisons. For the statistical evaluation of the proportion of terminal sprouts following rAAV treatment after CNS lesion, a frequency analysis was made using a χ2 test.
Supplementary Material
Acknowledgments
We thank A. Schmalz and H. Janicki for excellent technical assistance and R. Hohlfeld, K. Dornmair, E. Meinl, and P. Williams for critical reading of the manuscript. We would also like to thank S. Akira for providing STAT3fl/fl mice and J. Sanes and I. J. Kim for help with viral production. This work was supported by a grant from the International Institute for Research in Paraplegia (to F.M.B. and M.K.). M.K. was supported by the Deutsche Forschungsgemeinschaft (DFG; Emmy-Noether Program, SFB 571 and SFB 870) and by Verein “Therapieforschung für MS-Kranke e.V.” F.M.B. was supported by DFG (SFB 870) and is the recipient of an Independent Group Leader Award from the Federal Ministry of Education and Research of Germany (BMBF). T.M. was supported by the Technische Universität München Institute for Advanced Study, Alexander von Humboldt Foundation, Center for Integrated Protein Science (Munich), and a Christopher and Dana Reeve Foundation grant. H.B. was supported by the Center for Molecular Medicine Cologne (ZMMK).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015239108/-/DCSupplemental.
References
- 1.Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- 2.Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- 3.Ylera B, et al. Chronically CNS-injured adult sensory neurons gain regenerative competence upon a lesion of their peripheral axon. Curr Biol. 2009;19:930–936. doi: 10.1016/j.cub.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Raivich G, Makwana M. The making of successful axonal regeneration: Genes, molecules and signal transduction pathways. Brain Res Brain Res Rev. 2007;53:287–311. doi: 10.1016/j.brainresrev.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Rossi F, Gianola S, Corvetti L. Regulation of intrinsic neuronal properties for axon growth and regeneration. Prog Neurobiol. 2007;81:1–28. doi: 10.1016/j.pneurobio.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Raivich G, et al. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron. 2004;43:57–67. doi: 10.1016/j.neuron.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Zou H, Ho C, Wong K, Tessier-Lavigne M. Axotomy-induced Smad1 activation promotes axonal growth in adult sensory neurons. J Neurosci. 2009;29:7116–7123. doi: 10.1523/JNEUROSCI.5397-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seijffers R, Mills CD, Woolf CJ. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J Neurosci. 2007;27:7911–7920. doi: 10.1523/JNEUROSCI.5313-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stam FJ, et al. Identification of candidate transcriptional modulators involved in successful regeneration after nerve injury. Eur J Neurosci. 2007;25:3629–3637. doi: 10.1111/j.1460-9568.2007.05597.x. [DOI] [PubMed] [Google Scholar]
- 10.MacGillavry HD, et al. NFIL3 and cAMP response element-binding protein form a transcriptional feedforward loop that controls neuronal regeneration-associated gene expression. J Neurosci. 2009;29:15542–15550. doi: 10.1523/JNEUROSCI.3938-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore DL, et al. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aaronson DS, Horvath CM. A road map for those who don't know JAK-STAT. Science. 2002;296:1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 13.Schwaiger FW, et al. Peripheral but not central axotomy induces changes in Janus kinases (JAK) and signal transducers and activators of transcription (STAT) Eur J Neurosci. 2000;12:1165–1176. doi: 10.1046/j.1460-9568.2000.00005.x. [DOI] [PubMed] [Google Scholar]
- 14.Xia XG, Hofmann HD, Deller T, Kirsch M. Induction of STAT3 signaling in activated astrocytes and sprouting septal neurons following entorhinal cortex lesion in adult rats. Mol Cell Neurosci. 2002;21:379–392. doi: 10.1006/mcne.2002.1180. [DOI] [PubMed] [Google Scholar]
- 15.Sheu JY, Kulhanek DJ, Eckenstein FP. Differential patterns of ERK and STAT3 phosphorylation after sciatic nerve transection in the rat. Exp Neurol. 2000;166:392–402. doi: 10.1006/exnr.2000.7508. [DOI] [PubMed] [Google Scholar]
- 16.Bareyre FM, Haudenschild B, Schwab ME. Long-lasting sprouting and gene expression changes induced by the monoclonal antibody IN-1 in the adult spinal cord. J Neurosci. 2002;22:7097–7110. doi: 10.1523/JNEUROSCI.22-16-07097.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cafferty WB, et al. Leukemia inhibitory factor determines the growth status of injured adult sensory neurons. J Neurosci. 2001;21:7161–7170. doi: 10.1523/JNEUROSCI.21-18-07161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cafferty WB, et al. Conditioning injury-induced spinal axon regeneration fails in interleukin-6 knock-out mice. J Neurosci. 2004;24:4432–4443. doi: 10.1523/JNEUROSCI.2245-02.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong J, Dietzel ID, Wahle P, Kopf M, Heumann R. Sensory impairments and delayed regeneration of sensory axons in interleukin-6-deficient mice. J Neurosci. 1999;19:4305–4313. doi: 10.1523/JNEUROSCI.19-11-04305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith PD, et al. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron. 2009;64:617–623. doi: 10.1016/j.neuron.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith RP, et al. Transcriptional profiling of intrinsic PNS factors in the postnatal mouse. Mol Cell Neurosci. 2011;46:32–44. doi: 10.1016/j.mcn.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu J, Cafferty WB, McMahon SB, Thompson SW. Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J Neurosci. 2005;25:1645–1653. doi: 10.1523/JNEUROSCI.3269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan YA, Misgeld T, Lichtman JW, Sanes JR. Effects of neurotoxic and neuroprotective agents on peripheral nerve regeneration assayed by time-lapse imaging in vivo. J Neurosci. 2003;23:11479–11488. doi: 10.1523/JNEUROSCI.23-36-11479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med. 2005;11:572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- 25.Dray C, Rougon G, Debarbieux F. Quantitative analysis by in vivo imaging of the dynamics of vascular and axonal networks in injured mouse spinal cord. Proc Natl Acad Sci USA. 2009;106:9459–9464. doi: 10.1073/pnas.0900222106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jankowski MP, et al. Sensitization of cutaneous nociceptors after nerve transection and regeneration: possible role of target-derived neurotrophic factor signaling. J Neurosci. 2009;29:1636–1647. doi: 10.1523/JNEUROSCI.3474-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsukawa A, et al. Aberrant inflammation and lethality to septic peritonitis in mice lacking STAT3 in macrophages and neutrophils. J Immunol. 2003;171:6198–6205. doi: 10.4049/jimmunol.171.11.6198. [DOI] [PubMed] [Google Scholar]
- 28.Feng G, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 29.Qiu J, et al. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- 30.Bomze HM, Bulsara KR, Iskandar BJ, Caroni P, Skene JHP. Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nat Neurosci. 2001;4:38–43. doi: 10.1038/82881. [DOI] [PubMed] [Google Scholar]
- 31.Park KK, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M. Axon regeneration requires a conserved MAP kinase pathway. Science. 2009;323:802–806. doi: 10.1126/science.1165527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan D, Wu Z, Chisholm AD, Jin Y. The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell. 2009;138:1005–1018. doi: 10.1016/j.cell.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghosh-Roy A, Wu Z, Goncharov A, Jin Y, Chisholm AD. Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J Neurosci. 2010;30:3175–3183. doi: 10.1523/JNEUROSCI.5464-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coqueret O, Gascan H. Functional interaction of STAT3 transcription factor with the cell cycle inhibitor p21WAF1/CIP1/SDI1. J Biol Chem. 2000;275:18794–18800. doi: 10.1074/jbc.M001601200. [DOI] [PubMed] [Google Scholar]
- 36.Pradervand S, et al. Small proline-rich protein 1A is a gp130 pathway- and stress-inducible cardioprotective protein. EMBO J. 2004;23:4517–4525. doi: 10.1038/sj.emboj.7600454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka H, et al. Cytoplasmic p21(Cip1/WAF1) regulates neurite remodeling by inhibiting Rho-kinase activity. J Cell Biol. 2002;158:321–329. doi: 10.1083/jcb.200202071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonilla IE, Tanabe K, Strittmatter SM. Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J Neurosci. 2002;22:1303–1315. doi: 10.1523/JNEUROSCI.22-04-01303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Höke A. Mechanisms of disease: What factors limit the success of peripheral nerve regeneration in humans? Nat Clin Pract Neurol. 2006;2:448–454. doi: 10.1038/ncpneuro0262. [DOI] [PubMed] [Google Scholar]
- 40.Bussmann KA, Sofroniew MV. Re-expression of p75NTR by adult motor neurons after axotomy is triggered by retrograde transport of a positive signal from axons regrowing through damaged or denervated peripheral nerve tissue. Neuroscience. 1999;91:273–281. doi: 10.1016/s0306-4522(98)00562-4. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen QT, Sanes JR, Lichtman JW. Pre-existing pathways promote precise projection patterns. Nat Neurosci. 2002;5:861–867. doi: 10.1038/nn905. [DOI] [PubMed] [Google Scholar]
- 42.Bradbury EJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 43.Kadoya K, et al. Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron. 2009;64:165–172. doi: 10.1016/j.neuron.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.