Abstract
Cortical dendritic spines are highly motile postsynaptic structures onto which most excitatory synapses are formed. It has been postulated that spine dynamics might reflect synaptic plasticity of cortical neurons. To test this hypothesis, we have investigated spine dynamics during the critical period in mouse visual cortex in vivo with and without sensory deprivation. The motility of spines on apical dendrites of layer 5 neurons was assayed by time-lapse two-photon microscopy. Spines were motile at the ages examined, postnatal days (P)21–P42, although motility decreased between P21 and P28 and then remained stable through P42. Binocular deprivation from before the time of eye-opening up-regulated spine motility during the peak of the critical period (P28), without affecting average spine length, class distribution, or density. Deprivation at the start of the critical period had no effect on spine motility, whereas continued deprivation through the end of the critical period appeared to reduce spine motility slightly. We conclude that spine motility might be involved in critical-period plasticity and that reduction of activity during the critical period enhances spine dynamics.
In the primary visual cortex, connections to and between neurons responding to visual information from each eye undergo activity-dependent remodeling during a well defined plastic period of development called the critical period. During this time, suturing one eye causes visual cortical cells to be driven predominantly by the open eye (1–3). Suturing both eyes, particularly over a prolonged period, causes a reduction in driving of cortical cells by either eye (4, 5). The shifts in physiological activity, at least during monocular suture, are accompanied by a remodeling of both thalamocortical axon arbors [on a slower time scale (6)] and of intracortical horizontal connections [on a faster time scale (7)]. The effect of visual deprivation and of altering activity on postsynaptic structures, however, has remained largely unstudied.
Dendritic spines are the postsynaptic sites of most excitatory synapses in the CNS (8–10), making them likely substrates for structural plasticity. Furthermore, spines have a unique morphology that allows them to act as calcium compartments (11–13). Calcium compartmentalization may be the basis of synapse-specific plasticity by allowing different biochemical environments to exist at nearby synapses. The shape and size of the spine, along with the expression of calcium permeable channels and pumps, regulate the time course of calcium signals that regulate plasticity (14). Spines also undergo rapid (on a time scale of minutes) motility that may change their ability to act as calcium compartments (15–18). The modulation of spine structural dynamics may thus be the basis for functional plasticity during the critical period.
Changes in spine shape or spine motility have been documented during development (16, 19) and during developmental deprivation protocols (20). Studies using electron microscopy have shown that spines increase in size as soon as 4 min after stimuli known to elicit long-term potentiation (LTP) (21, 22). Protocols that reduce synaptic activity, such as deafferentation in a slice preparation (23) or activity blockade in slices (24, 25), cause an increase in spine number. It has also been shown that activation of an identified subset of synapses with an LTP-like stimulus causes the outgrowth of new spines (26–28).
In this study, we have examined spine dynamics of visual cortex neurons in vivo during development and after visual deprivation. Specifically, we used time-lapse two-photon laser scanning microscopy to quantify spine motility in the intact mouse primary visual cortex at different ages during and after the critical period for ocular dominance plasticity, a period that spans postnatal days (P)19–P35 (2). In addition, we examined the effects of binocular lid suture (started before eye opening) on spine motility at the beginning, during, and after the critical period. We examined spines on apical dendrites of layer 5 neurons to specifically examine a substrate for intracortical plasticity. Our results show that spine motility is up-regulated during the critical period in mice that are binocularly deprived. Deprivation at the beginning or continuing after the critical period, however, has a small effect on spine motility. These results suggest that spine motility is involved in critical-period plasticity and may be a structural substrate for changes in synaptic strength and connectivity that occur during the critical period.
Methods
Surgical Preparation for in Vivo Imaging. Mice (C57/Bl6) expressing GFP in a subset of cortical layer 5 neurons were used (transgenic line M; ref. 29). Mice aged 21–42 days were killed by using avertin (16 μl/g of body weight; Sigma). The skull was exposed, scrubbed, and cleaned with ethanol. The skull was then glued to a thin metal plate (30). Primary visual cortex was identified based on stereotaxic coordinates (2). The skull over the visual cortex was thinned by using a high-speed dental drill (Fine Science Tools, Belmont, CA) and then removed with forceps to create a small (1 × 1 mm) craniotomy. The skull was periodically bathed in saline to ensure the underlying cortex did not experience damage due to excessive heat during drilling. Neurons could be imaged for up to 10 h postsurgery with no apparent structural abnormalities. To ensure stability while imaging, the craniotomy was filled with 2–4% agar (Sigma) and sealed with a coverslip. The skull plate was then fastened into a metal base that connected directly into the microscope stage for imaging. During imaging, anesthesia was maintained with periodic injections of avertin.
For visual deprivation, lids were sutured shut at P13, before eye opening, by scoring the eyelids and then sealing them together using tissue adhesive (Vetbond, WPI, Saratoga, FL). Mice were checked periodically to ensure that the lid suture remained patent. Compromised mice were not used further.
Two-Photon Imaging. A custom-made two-photon laser scanning microscope (31) was used for in vivo imaging. The microscope consists of a modified Fluoview confocal scanhead (Olympus, Melville, NY) and a Ti:S laser providing 100-fs pulses at 80 MHz at a wavelength of 920 nm (Tsunami, Spectra-physics, Menlo Park, CA) pumped by a 10-W solid-state source (Millenia, Spectra-physics). Fluorescence was detected by using photomultiplier tubes (HCl25-02, Hamamatsu, Ichinocho, Japan) in whole-field detection mode. The craniotomy over visual cortex was initially identified under low power (×10 air lens; Olympus), and areas with superficial dendrites were identified by using a ×60, 0.9 N.A. lens (IR2; Olympus) under whole-field fluorescence illumination. The ×60 lens was used for further identification of spiny dendrites under digital zoom (×5–10) by using two-photon imaging and spines 50–200 μm below the pial surface were studied. Image acquisition was accomplished by using fluoview software. Z stacks taken 0.5–1 μm apart were acquired every 5 min for 2 h. Mice were perfused with 4% paraformaldehyde postimaging and imaged neurons were identified in coronal sections stained with bisbenzamide to label cell bodies and to reveal the position of primary visual cortex. In some cases, injections of cholera toxin subunit B-Alexa Fluor conjugate 594 (Molecular Probes) were performed in the imaged area before perfusion to facilitate the recovery of imaged cells in fixed sections.
Data Analysis. Images were exported to matlab where they were processed with custom-written algorithms for image enhancement and alignment of the time series. Spines were analyzed on two-dimensional projections containing between 5 and 30 individual images, therefore movements in the z dimension were not analyzed. Spine motility was defined as the average change in length per unit time (μm/min). Lengths were measured from the base of the protrusion to its tip. This method has the advantage of being insensitive to drift in the images over time and therefore does not require precise alignment of the images. Only frames with good signal-to-noise ratio where spines on all parts of the dendrite were clearly visible were used for analysis, in some cases, dim frames (caused by movement artifact due to inadequate anesthesia or loss of meniscus on the objective lens) were discarded. No obvious rotational drift was observed. Dendrites remained stable over the time course of imaging and the x-y distances between dendritic sections located in different z planes were analyzed to control for rotation. Additionally, motile spines were often located next to stable spines. Spines were characterized into classes based on their lengths, spine head diameters, and neck diameters (32). Values are presented as mean ± SE. Analysis was carried out blind with respect to mouse age and manipulation.
Results
Spine Motility During the Critical Period. We studied the motility of dendritic spines during the critical period for activity-dependent plasticity in the mouse visual cortex (2). We concentrated on protrusions located on the apical dendrite of layer 5 neurons and tracked their structure over a period of 2 h with in vivo two-photon time lapse microscopy. We examined spines in young mice at the start of the critical period (P21), during the peak of the critical period (P28), and after the end of the critical period (P42). The position of imaged dendrites in the visual cortex was confirmed in some cases by fixing the brain postimaging, finding the imaged neuron, and determining its location in primary visual cortex by using Nissl staining (Fig. 1). To compare our results with those previously obtained in the somatosensory cortex (20), we decided to quantify spine motility as the absolute change in length of the protrusion per unit time, where length is defined as the distance in two dimensions between the dendrite and the tip of the protrusion (Fig. 2C). Rapid motility (on a time scale of minutes) at these ages (P21–P42) was severely decreased as compared with studies of earlier ages in in vitro systems (16, 17, 33). Changes in spine structure, however, were still evident on a slower time scale over the period studied. Types of motility observed between P21 and P42 included elongation and retraction of spines, changes in the position and shape of the spine head, and the appearance of filopodia (Fig. 2 A; see also Movie 1, which is published as supporting information on the PNAS web site), and were similar to those described in earlier in vitro studies. In vitro experiments carried out at these ages show similar amounts of motility to that observed in vivo, showing that decreased motility during later development is common to in vivo and in vitro preparations (data not shown). There was a significant tendency for longer spines to be more motile (Fig. 2B). The extent of spine motility at P21 observed in this study (see Fig. 2C) corresponds very well to that measured by using a similar technique in somatosensory cortex (20), suggesting that the developmental time course of spine motility is conserved across different brain regions. Spine motility was down-regulated during the critical period (Fig. 3A; P21 - 0.028 ± 0.002 μm/min, n = 61 spines, three mice; P28 - 0.021 ± 0.0006 μm/min, n = 149 spines, four mice; and P42 - 0.020 ± 0.0008 μm/min, n = 59 spines, three mice). Motility was significantly higher at P21 compared with P42 (P < 0.001, t test, comparing individual spines; P < 0.05, Mann–Whitney U test, comparing individual cells or mice) or P28 (P < 0.001, t test; P < 0.1 Mann–Whitney U test). These data suggest that spine motility is developmentally shaped during the critical period and might be involved in critical-period plasticity.
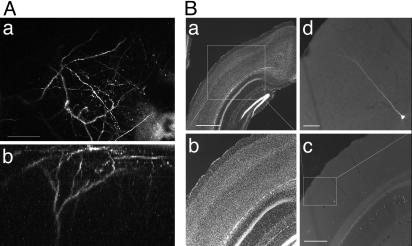
Fig. 1.
Imaging dendrites of layer 5 pyramidal neurons in the visual cortex in vivo.(A) Two-photon images of the apical tuft of a layer 5 pyramidal neuron from a P21 mouse. (Aa) A collapsed z stack showing the dendritic arbor. (Ab) Three-dimensional reconstruction showing the dendritic arbor from the side. (Scale bar, 50 μm.) (B) Examining the location of imaged neurons in histological sections. (Ba). Nissl stain of coronal section of cortex. Notice the thickening of layer 4 characteristic of primary visual cortex. (Scale bar, 1 mm.) (Bb) A higher-power image of the boxed area in Ba. (Bc) Fluorescence image of the area in Bb showing position of imaged GFP neuron. (Scale bar, 500 μm.) (Bd) Higher magnification of imaged neuron. (Scale bar, 50 μm.)
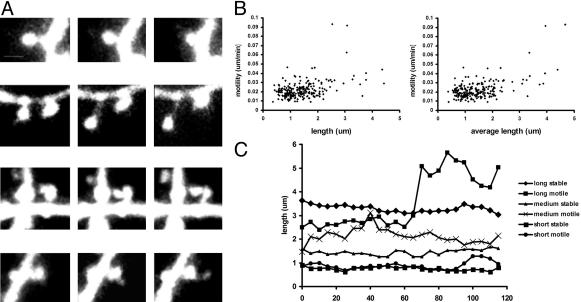
Fig. 2.
Spine motility during and beyond the critical period in mouse visual cortex in vivo.(A) Dendritic spines were motile over the time periods studied. Spines exhibited different kinds of motility such as (from top to bottom): retraction, elongation, spine head displacement and shape change, and growth of filopodia from the spine head. (Scale bar, 1 μm.) Movie 1 shows motile spines. (B) Motility of dendritic protrusions correlates with protrusion length or average length over the time course imaged (linear regression analysis; P < 0.001, n = 235 protrusions, P21–P42). (C) Time courses showing examples of changes in length of protrusions observed at P21. At any length, both stable and motile protrusions could be found.
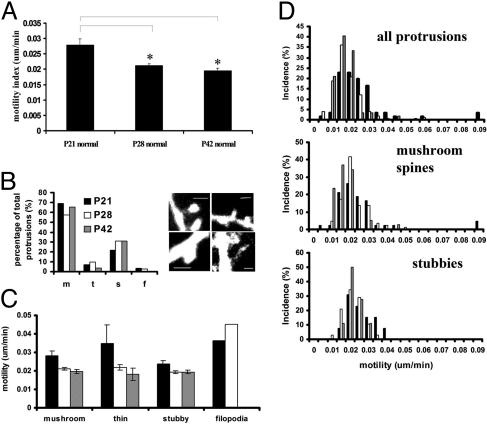
Fig. 3.
Regulation of spine motility during the critical period in vivo. (A) Spine motility significantly declines during the critical period and remains at a basal level into early adulthood. *, P < 0.001. (B) The structure of dendritic spines in visual cortex does not change during the critical period. Mushroom spines were the most common type of protrusion at all ages studied. Filopodia were uncommon at these ages and were absent at P42. (Right) Examples of protrusions and classification; clockwise from top left, mushroom, thin, filopodia, and stubby. (Scale bars, 1 μm.) (C) The motility of all classes of spines is down-regulated during the critical period. (D) Distribution of motilities at the three ages studied. Mushroom spines were most common and comprised the least and most motile protrusions.
Characterization of Spine Motility in Visual Cortex. We wondered whether a switch in the types of spines present on dendrites or in the motility of specific spine classes could be responsible for the down-regulation in spine motility during the critical period. At the ages studied, most of the protrusions observed appeared to be well developed spines, although a few filopodia were observed during the critical period (P21 and P28) and were among the most motile protrusions at these ages (Fig. 3 B and C). Spines were characterized into classes based on previously defined criteria (ref. 32 and Fig. 3B). At these ages most spines (>50% of all protrusions at each age) had mushroom-like morphologies, although stubby spines and a few thin spines were also present. Apart from the lack of filopodia after the critical period, there was no significant difference in the spine class composition of protrusions at the different ages. Between P21 and P28 motility was down-regulated in spines of all classes (mushroom, thin and stubby), whereas filopodia remained highly motile (Fig. 3C). The reduced level of motility was maintained in all protrusions through P42, suggesting that this basal motility might be maintained into adulthood. At each age, the most stable protrusions were large mushroom and stubby spines (Fig. 3D Middle and Bottom). Mushroom spines with small heads and thin spines tended to be most motile (Fig. 3D Top and Middle). On average, longer protrusions tended to be most motile, especially long filopodia-like structures (Fig. 2B). At any length, both stable (<0.15 μm/min) and motile (>0.3 μm/min) spines could be found (Fig. 2C). Additionally, spine density remained constant during the period studied (P > 0.05, t test, for all pairwise comparisons; average density = 0.6 spines per micrometer, P21–P42, n = 13 dendritic sections).
The Effect of Binocular Deprivation on Dendritic Spine Motility. To examine the involvement of spine motility in the plasticity of binocular connections in visual cortex during the critical period, we examined motility in mice that were binocularly lid-sutured from P13 (eye opening) and had never experienced normal visual activity. Binocular deprivation for a short period allows development to proceed normally (2, 4), whereas prolonged deprivation (including binocular deprivation through dark-rearing) leads to a substantial reduction of driving by either eye (5, 34, 35). In fact, we found that binocular lid suture increased spine motility at P28, during the height of the critical period, but not at the beginning (P21) or after (P42) the critical period. At P28, spine motility was up-regulated by 60% as compared with control (P28 - 0.021 ± 0.0006 μm/min, n = 149 spines, six cells, four mice; P28-deprived - 0.034 ± 0.0015 μm/min, n = 191 spines, five cells, four mice; Fig. 4A). Motility was significantly higher in deprived mice at this age than in nondeprived mice (P < 0.0001, t test comparing individual spines; P < 0.05, Mann–Whitney U test comparing individual cells or animals). Indeed, spine motility was retained at or above P21 levels, suggesting that the lack of activity may preserve a developmentally younger state at least with respect to this parameter. After the critical period, at P42, binocular lid suture reduced spine motility slightly (by 15% with respect to P42 normal, P < 0.05, t test comparing individual spines; P > 0.05, Mann–Whitney U test, comparing individual cells or animals).
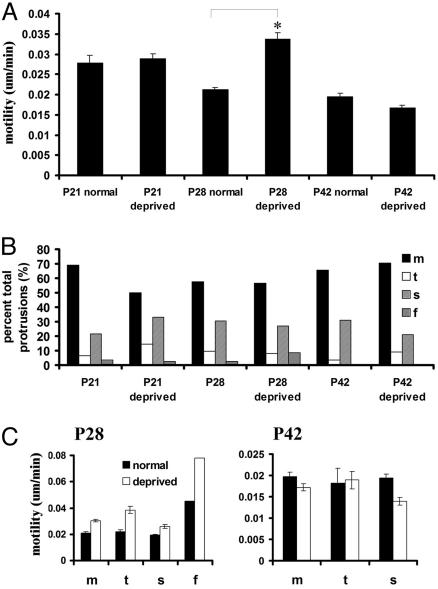
Fig. 4.
The effect of binocular deprivation on spine motility in vivo. (A) Binocular deprivation from P14 significantly increases spine motility at P28 by ≈60%. *, P < 0.001. There is no change in motility with deprivation at P21 and a slight reduction in motility in deprived mice at P42. (B) Deprivation does not alter the structure of protrusions in visual cortex at any age. (C) The increase in overall motility caused by deprivation at P28 is evident in all types of protrusions (Left). At P42, however, the deprivation-induced reduction in spine motility is mediated by a stabilization of stubby spines. m, mushroom; t, thin; s, stubby; f, filopodia.
The up-regulation of spine motility during binocular lid suture at P28 occurred due to an overall increase in motility of all classes of protrusions. In deprived mice, a small number of filopodia were present and were among the most motile protrusions. Although filpodia also appeared to be more motile in deprived mice, this effect was not significant due to the few filopodia observed at these ages. In deprived mice of all ages, the predominant class of protrusion was the mushroom spine (Fig. 4B). Stubby spines and a few thin spines were also present. The distribution of protrusions between the spine classes was not significantly different in deprived mice as compared with nondeprived mice. Although more filopodia were observed in deprived mice, this effect was not significant. At P28 in deprived mice, the most motile protrusions were again small mushroom spines and thin spines (Fig. 4C). At P42, the decrease in the average spine motility in deprived mice was caused by a decrease in the motility of stubby spines (Fig. 4C). Mushroom and thin spines showed no apparent decrease in motility due to deprivation at this age. At P42, filopodia were not observed. Deprivation did not affect the average length of protrusions (P28 normal - 1.24 ± 0.043 μm, n = 118 spines, P28-deprived - 1.31 ± 0.069 μm, n = 83 spines, P > 0.05, t test; P42 normal - 1.21 ± 0.063 μm, n = 57 spines, and P42-deprived - 1.15 ± 0.051 μm, n = 100 spines; P > 0.05, t test), or spine density (P > 0.05, t test, mean density at P28-deprived = 0.56 spines per micrometer, n = 6 dendritic sections; P42-deprived = 0.54 spines per micrometer, n = 6 dendritic sections) at either age.
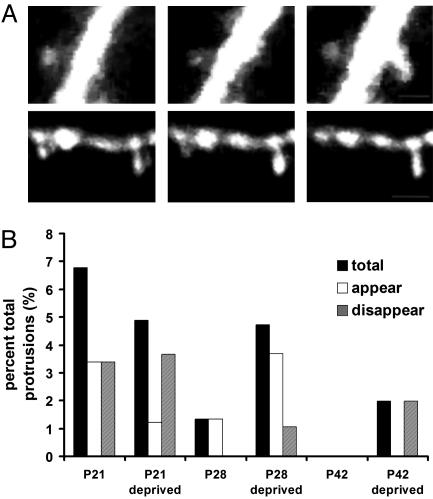
Spine Turnover During the Critical Period. Recently, two studies have explored the time course of spine turnover in the adult cortex (36, 37). We wondered whether, in the 2-h observation period in this study, we could examine spine formation and retraction. We reasoned that spine turnover rates might be high in this plastic period of development and that spine turnover may be influenced by deprivation during the critical period. Very few spines were observed to appear and disappear over the developmental period studied, however, and although there appeared to be slightly more turnover in P28-deprived mice (mostly due to the appearance and disappearance of filopodia), there was no statistically significant effect of deprivation on spine turnover at any age (Fig. 5). This observation is in agreement with the recent finding (37) that spines in nondeprived mice in visual cortex are very stable in early and late adulthood.
Fig. 5.
Spine turnover during the critical period. (A)(Upper) An example of spine outgrowth from the dendrite during the imaging period. (Scale bar, 0.5 μm.) (Lower) An example of spine retraction into the dendrite during the imaging interval. (Scale bar, 1 μm.) Images are collapsed z stacks. (B) The abscissa plots the percent of total protrusions that were added or removed during the observation period of 2 h. Low rates of spine turnover were observed at all ages. Turnover rates were highest in young mice and were not visibly affected by binocular deprivation.
Discussion
To determine whether spine motility is involved in the plasticity observed in the visual cortex during the critical period, we examined spine motility on the apical dendrite of layer 5 neurons in the primary visual cortex in vivo. We found that spine motility declined during the critical period to a basal level that was maintained after the end of the critical period. This decrease in motility was exhibited by all spine classes. Binocular lid suture resulted in an increase in spine motility at the peak of the critical period. The up-regulation was specific to this time window and did not occur at the beginning or after the end of the critical period. The up-regulation was present in all spine classes. After the end of the critical period, deprivation resulted in a slight down-regulation of spine motility in stubby spines.
Physiological Bases for Spine Motility and Critical-Period Plasticity. Longitudinal studies of spine motility in vivo (20) and in vitro (15, 16) have shown that spine motility is developmentally regulated. Motility is highest in young mice around the time of synaptogenesis and decreases systematically afterward. These studies show that spines are most motile at a time when they are either not receiving input or receiving immature input. This result, in turn, suggests that spine motility might be down-regulated by synaptic activity. Although the motility of spines on cerebellar Purkinje cells was shown not to correlate with the presence of synaptic input (38), mechanistic studies in cultured neurons have suggested that this might in fact be the case (17, 33). Spines on cultured hippocampal neurons are stabilized by the influx of calcium into the spine head that occurs after the activation of synaptic receptors and channels (17): bath application of AMPA and NMDA decreases spine motility by activating voltage-sensitive calcium channels. Short calcium pulses elicited by action potential-mediated calcium influx also affect spine structure, further suggesting that synaptic activity can affect spine dynamics by engaging calcium signaling pathways (39).
Our study suggests that synaptic activity due to sensory stimulation in vivo significantly affects spine motility. We chose binocular lid suture as a sensory deprivation protocol because pattern deprivation such as by monocular and binocular lid suture appears to have more deleterious consequences for cortical connections than a complete absence of vision or visual activity such as by dark rearing (5) or intraocular injection of tetrodotoxin (40). Prolonged binocular deprivation reduces cortical responsiveness to either eye (4, 5, 41), and prevents changes in NMDA receptor composition (42), but alters synaptic calcium signaling and plasticity (43). Because motility declines as activity-dependent development proceeds, and binocular deprivation causes motility to be preserved through much of the critical period, it is likely that calcium signaling plays a role in regulating spine motility in vivo similar to that described in vitro.
Multiple factors might operate during the critical period to cause an up-regulation of spine motility following visual deprivation. In addition to changes in receptors and channels that affect synaptic signaling and calcium fluxes during the critical period (42), a broad range of molecules influenced by intracellular calcium are also influenced by visual activity (44–47), and some are known to be developmentally regulated during the critical period (44). Levels of neuronal activity and calcium influx might also be affected by the balance of excitatory and inhibitory activity in the local cortical network. Inhibitory drive has been shown to be tightly regulated with respect to the critical period and is crucial for deprivation-induced changes during this time (48). Patterns of excitatory and inhibitory synaptic activity, as well as the resting state of the neuron, might prime the molecular machinery at the synapse for structural remodeling.
Spine motility could also be regulated by the extracellular matrix (ECM). ECM components, such as proteoglycans, are up-regulated during the critical period and likely play a role in the lack of plasticity in adult cortex (49). The stabilization of the ECM could also be responsible for the developmental down-regulation of spine motility. Dark-rearing delays the up-regulation of proteoglycans (49), suggesting that increased spine motility in binocularly deprived cortex may rely on proteoglycan expression. Additionally, molecules that digest the ECM, such as metalloproteinases and serine proteases, are developmentally regulated (50), and can be up-regulated during periods of structural remodeling (51), as well as during deprivation-induced remodeling during the critical period (52). These proteins might restructure the ECM allowing spines to become more motile. That is, the ECM might control the ability of spines to respond to calcium signaling with dynamic changes in their structure.
Effects of Deprivation on Spine Motility in Different Brain Regions. Earlier studies showed that deprivation of somatosensory cortex through whisker trimming reduced spine motility in layer 2/3 neurons (20). This effect was specific to a short developmental window, which was shown to correspond to a period of receptive field plasticity. The magnitude of the change in spine motility observed was comparable to the magnitude of the motility change observed in this study (≈50%). Additionally, both studies found no effects on the lengths, class composition, and densities of dendritic spines. The difference between the up-regulation observed in visual cortex during deprivation in the critical period, and the reduction observed in somatosensory cortex, may lie in the different cortices studied. Spines in visual cortex are more stable in adulthood than those in somatosensory cortex (36, 37) and may therefore have different rules for the regulation of their motility and turnover. Additionally, there may be differences in the behavior of spines on different cell types and in different layers (layer 5 vs. layer 2/3 or layer 4; see below). It is possible, however, that the reduction and enhancement of motility are separate phenomena that might be used by both areas of the brain in different kinds of synaptic remodeling. The motility reduction in somatosensory cortex was observed when the cortex was deprived of activity during a period of intense synaptogenesis. Our studies were carried out at a time when synapses are already established and activity-dependent remodeling occurs. These two developmental time periods might put dendritic spines in different states that are then differentially affected by activity deprivation.
Spine Turnover. The issue of how synapse and spine turnover contributes to neuronal computation has received a great deal of attention recently. Studies in visual cortex have shown that superficial layer 5 spines are highly stable in number in the adult and even as early as the tail end of the critical period (37). This same spine population in somatosensory cortex, however, appears to be much more transient (36), with more than half of the spines turning over in a period of a month. In this system, deprivation up-regulates spine turnover, further decreasing the number of stable spines (36). Examining apical layer 5 spines in visual cortex, we found an up-regulation in spine dynamics during sensory deprivation, and we wondered whether this increase in spine dynamics translated to an increase in spine turnover with deprivation. Although our imaging periods are quite brief, the fact that we rarely observe spines to appear or disappear argues that spines in visual cortex are indeed stable in number during the critical period. Spine turnover appears to be a little higher before the critical period (at P21) but then remains low during and after the critical period (P28–P42). Additionally, we see no up-regulation of spine turnover in deprived mice at any age. Although we cannot rule out small effects on turnover rate, we conclude that activity deprivation during the critical period does not induce large-scale synaptic growth or elimination.
Effects of Deprivation on Different Populations of Dendritic Spines in Visual Cortex. Our study focused on dendritic spines on the apical tuft of layer 5 neurons. Studies in acute brain slices of dark-reared mice, however, have shown that deprivation results in a small down-regulation of spine motility in basal spines on layer 3 neurons (53). This spine population receives input directly from the thalamus, and its dynamics should mirror those of thalamocortical axons. Axonal rearrangements from the thalamus occur on a slow time scale (6, 54), however, and the procession of plasticity from thalamocortical synapses to connections in the surrounding layer has recently been challenged. In fact, slice studies of both somatosensory and visual cortex have shown that corticocortical synapses display greater plasticity than thalamocortical synapses (55, 56), and brief periods of monocular deprivation cause changes in response properties of cells in deep and superficial layers, without affecting responses in layer 4 (57). Additionally, fast remodeling of intracortical horizontal connections occurs in superficial layers after the induction of artificial strabismus (7), a condition where activity from the two eyes becomes mismatched, suggesting that dynamic changes in structural organization of synapses occur in intracortical synapses before spreading to thalamocortical ones. Our findings agree with this hypothesis, by showing that deep layer neurons show structural synaptic plasticity at spines in superficial layers during manipulations of sensory input during the critical period.
Supplementary Material
Acknowledgments
We thank S. Oray and C. Leamey for assistance and useful discussions. This work was funded by grants from the National Institutes of Health (M.S.). A.M. was funded by a Whiteman Science Fellowship.
Abbreviations: Pn, posnatal day n; ECM, extracellular matrix.
References
- 1.Wiesel, T. & Hubel, D. (1965) J. Neurophysiol. 28, 1029-1040. [DOI] [PubMed] [Google Scholar]
- 2.Gordon, J. A. & Stryker, M. P. (1996) J. Neurosci. 16, 3274-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz, L. C. & Shatz, C. J. (1996) Science 274, 1133-1138. [DOI] [PubMed] [Google Scholar]
- 4.Crair, M. C., Gillespie, D. C. & Stryker, M. P. (1998) Science 279, 566-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White, L. E., Coppola, D. M. & Fitzpatrick, D. (2001) Nature 411, 1049-1052. [DOI] [PubMed] [Google Scholar]
- 6.Antonini, A. & Stryker, M. P. (1993) Science 260, 1819-1821. [DOI] [PubMed] [Google Scholar]
- 7.Trachtenberg, J. T. & Stryker, M. P. (2001) J. Neurosci. 21, 3476-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramón y Cajal, S. (1904) in La Textura del Sistema Nerviosa del Hombre y los Vertebrados (Moya, Madrid).
- 9.DeRobertis, E. D. P. & Bennett, H. (1955) J. Biophys. Biochem. Cytol. 1, 47-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palay, S. (1965) J. Biophys. Biochem. Cytol. 2, 193-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuste, R. & Denk, W. (1995) Nature 375, 682-684. [DOI] [PubMed] [Google Scholar]
- 12.Koester, H. J. & Sakmann, B. (1998) Proc. Natl. Acad. Sci. USA 95, 9596-9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emptage, N., Bliss, T. V. & Fine, A. (1999) Neuron 22, 115-124. [DOI] [PubMed] [Google Scholar]
- 14.Majewska, A., Brown, E., Ross, J. & Yuste, R. (2000) J. Neurosci. 20, 1722-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer, M., Kaech, S., Knutti, D. & Matus, A. (1998) Neuron 20, 847-854. [DOI] [PubMed] [Google Scholar]
- 16.Dunaevsky, A., Tashiro, A., Majewska, A., Mason, C. & Yuste, R. (1999) Proc. Natl. Acad. Sci. USA 96, 13438-13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer, M., Kaech, S., Wagner, U., Brinkhaus, H. & Matus, A. (2000) Nat. Neurosci. 3, 887-894. [DOI] [PubMed] [Google Scholar]
- 18.Majewska, A., Tashiro, A. & Yuste, R. (2000) J. Neurosci. 20, 8262-8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziv, N. E. & Smith, S. J. (1996) Neuron 17, 91-102. [DOI] [PubMed] [Google Scholar]
- 20.Lendvai, B., Stern, A., Chen, B. & Svoboda, K. (2000) Nature 404, 876-881. [DOI] [PubMed] [Google Scholar]
- 21.Fifkova, E. & Van Harreveld, A. (1977) J. Neurocytol. 6, 211-230. [DOI] [PubMed] [Google Scholar]
- 22.Fifkova, E., Anderson, C. L., Young, S. J. & Van Harreveld, A. (1982) J. Neurocytol. 11, 183-210. [DOI] [PubMed] [Google Scholar]
- 23.Kirov, S. A., Sorra, K. E. & Harris, K. M. (1999) J. Neurosci. 19, 2876-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocha, M. & Sur, M. (1995) Proc. Natl. Acad. Sci. USA 92, 8026-8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirov, S. A. & Harris, K. M. (1999) Nat. Neurosci. 2, 878-883. [DOI] [PubMed] [Google Scholar]
- 26.Maletic-Savatic, M., Malinow, R. & Svoboda, K. (1999) Science 283, 1923-1927. [DOI] [PubMed] [Google Scholar]
- 27.Engert, F. & Bonhoeffer, T. (1999) Nature 399, 66-70. [DOI] [PubMed] [Google Scholar]
- 28.Toni, N., Buchs, P. A., Nikonenko, I., Bron, C. R. & Muller, D. (1999) Nature 402, 421-425. [DOI] [PubMed] [Google Scholar]
- 29.Feng, G., Mellor, R. H., Bernstein, M., Keller-Peck, C., Nguyen, Q. T., Wallace, M., Nerbonne, J. M., Lichtman, J. W. & Sanes, J. R. (2000) Neuron 28, 41-51. [DOI] [PubMed] [Google Scholar]
- 30.Svoboda, K., Tank, D., Stepnoski, R. & Denk, W. (2000) in Imaging Neurons: A Laboratory Manual, eds. Yuste, R., Lanni, F. & Konnerth, A. (Cold Spring Harbor Lab. Press, Plainview, NY).
- 31.Majewska, A., Yiu, G. & Yuste, R. (2000) Pflügers Arch. 441, 398-408. [DOI] [PubMed] [Google Scholar]
- 32.Harris, K. M., Jensen, F. E. & Tsao, B. (1992) J. Neurosci. 12, 2685-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korkotian, E. & Segal, M. (2001) J. Neurosci. 21, 6115-6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherman, S. M. & Spear, P. D. (1982) Physiol. Rev. 62, 738-855. [DOI] [PubMed] [Google Scholar]
- 35.Fagiolini, M., Pizzorusso, T., Berardi, N., Domenici, L. & Maffei, L. (1994) Vision Res. 34, 709-720. [DOI] [PubMed] [Google Scholar]
- 36.Trachtenberg, J. T., Chen, B. E., Knott, G. W., Feng, G., Sanes, J. R., Welker, E. & Svoboda, K. (2002) Nature 420, 788-794. [DOI] [PubMed] [Google Scholar]
- 37.Grutzendler, J., Kasthuri, N. & Gan, W. B. (2002) Nature 420, 812-816. [DOI] [PubMed] [Google Scholar]
- 38.Dunaevsky, A., Blazeski, R., Yuste, R. & Mason, C. (2001) Nat. Neurosci. 4, 685-686. [DOI] [PubMed] [Google Scholar]
- 39.Korkotian, E. & Segal, M. (2001) Neuron 30, 751-758. [DOI] [PubMed] [Google Scholar]
- 40.Rittenhouse, C. D., Shouval, H. Z., Paradiso, M. A. & Bear, M. F. (1999) Nature 397, 347-350. [DOI] [PubMed] [Google Scholar]
- 41.Freeman, R. D., Mallach, R. & Hartley, S. (1981) J. Neurophysiol. 45, 1074-1084. [DOI] [PubMed] [Google Scholar]
- 42.Philpot, B. D., Sekhar, A. K., Shouval, H. Z. & Bear, M. F. (2001) Neuron 29, 157-169. [DOI] [PubMed] [Google Scholar]
- 43.Kirkwood, A., Rioult, M. C. & Bear, M. F. (1996) Nature 381, 526-528. [DOI] [PubMed] [Google Scholar]
- 44.Pham, A., Impey, S., Storm, D. & Stryker, M. (1999) Neuron 22, 63-72. [DOI] [PubMed] [Google Scholar]
- 45.Cancedda, L., Putignano, E., Impey, S., Maffey, L., Ratto, G. & Pissorusso, T. (2003) J. Neurosci. 23, 7012-7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mower, A., Liao, D., Nestler, E., Neve, R. & Ramoa, R. (2002) J. Neurosci. 22, 2237-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taha, S., Hanover, J., Silva, A. & Stryker, M. (2002) Neuron 36, 483-491. [DOI] [PubMed] [Google Scholar]
- 48.Fagiolini, M. & Hensch, T. (2000) Nature 404, 183-186. [DOI] [PubMed] [Google Scholar]
- 49.Pizzorusso, T., Medini, P., Berardi, N., Chierzi, S., Fawcett, J. W. & Maffei, L. (2002) Science 298, 1248-1251. [DOI] [PubMed] [Google Scholar]
- 50.Vaillant, C., Didier-Bazes, M., Hutter, A., Belin, M. & Thomasset, N. (1999) J. Neurosci. 19, 4994-5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szklarczyk, A., Lapinska, J., Rylski, M., McKay, R. & Kaczmarek, L. (2002) J. Neurosci. 22, 920-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mataga, N., Nagai, N. & Hensch, T. (2002) Proc. Natl. Acad. Sci. USA 99, 7717-7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Konur, S. & Yuste, R. (2002) J. Neurobiol., in press.
- 54.Antonini, A. & Stryker, M. P. (1996) J. Comp. Neurol. 369, 64-82. [DOI] [PubMed] [Google Scholar]
- 55.Feldman, D. E., Nicoll, R. A. & Malenka, R. C. (1999) J. Neurobiol. 41, 92-101. [PubMed] [Google Scholar]
- 56.Bear, M. F. & Rittenhouse, C. D. (1999) J. Neurobiol. 41, 83-91. [DOI] [PubMed] [Google Scholar]
- 57.Trachtenberg, J. T., Trepel, C. & Stryker, M. P. (2000) Science 287, 2029-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.