Abstract
Altered patterns of malaria endemicity reflect, in part, changes in feeding behavior and climate adaptation of mosquito vectors. Aquaporin (AQP) water channels are found throughout nature and confer high-capacity water flow through cell membranes. The genome of the major malaria vector mosquito Anopheles gambiae contains at least seven putative AQP sequences. Anticipating that transmembrane water movements are important during the life cycle of A. gambiae, we identified and characterized the A. gambiae aquaporin 1 (AgAQP1) protein that is homologous to AQPs known in humans, Drosophila, and sap-sucking insects. When expressed in Xenopus laevis oocytes, AgAQP1 transports water but not glycerol. Similar to mammalian AQPs, water permeation of AgAQP1 is inhibited by HgCl2 and tetraethylammonium, with Tyr185 conferring tetraethylammonium sensitivity. AgAQP1 is more highly expressed in adult female A. gambiae mosquitoes than in males. Expression is high in gut, ovaries, and Malpighian tubules where immunofluorescence microscopy reveals that AgAQP1 resides in stellate cells but not principal cells. AgAQP1 expression is up-regulated in fat body and ovary by blood feeding but not by sugar feeding, and it is reduced by exposure to a dehydrating environment (42% relative humidity). RNA interference reduces AgAQP1 mRNA and protein levels. In a desiccating environment (<20% relative humidity), mosquitoes with reduced AgAQP1 protein survive significantly longer than controls. These studies support a role for AgAQP1 in water homeostasis during blood feeding and humidity adaptation of A. gambiae, a major mosquito vector of human malaria in sub-Saharan Africa.
Keywords: desiccation resistance, water homeostasis, Plasmodium vector-borne disease
Malaria is responsible for almost one million deaths of African children annually. This disease is spread through the bites of infected Anopheles mosquitoes, whose habitats are affected by changes in climate (http://www.cdc.gov/malaria). Anopheles gambiae, the major mosquito vector of the malaria parasite Plasmodium falciparum , thrives in sub-Saharan Africa during the humid rainy season but also survives long annual dry seasons. In recent years, the geographical distribution of this mosquito has expanded toward more arid regions where considerable fluctuation in temperature and relative humidity occur (1, 2).
Under different environmental conditions, A. gambiae and other species of mosquitoes retain or excrete body water during development. Living in fresh water, A. gambiae larvae absorb water continuously because of the osmotic gradient and therefore must excrete excess water (3). After emergence, adults live in the terrestrial habitat, where water availability is limited by climate. Adults must prevent unnecessary water loss due to excretion and respiration using both biological and behavioral methods. During and after blood feeding, mosquitoes must eliminate excess water from the blood meal to concentrate nutrients and reduce weight for flight. For example, Aedes aegypti discharges over 40% of the water from ingested blood in the first hour post–blood engorgement, expelling the first drops of urine within minutes (4–6). This suggests the existence of a regulated mechanism in mosquitoes for high-capacity water movements.
Aquaporins (AQPs) are water-selective channels in cell membranes and are found throughout nature in diverse taxa including prokaryotes, eukaryotes, and even archaea. The role of AQPs in water homeostasis has been well studied in mammals. Approximately 180 L of plasma is filtered by the human kidney every day, and 99% of the water is reabsorbed. Human AQP1 is expressed in the proximal tubules and descending thin limbs of the loop of Henle and constitutively transports water from the primary urine in the tubular lumen to the vascular space. In the presence of the antidiuretic hormone arginine vasopressin, human AQP2 is relocated by exocytosis from intracellular vesicles to the apical cell membrane of principal cells in renal collecting ducts, thereby maximizing water reabsorption (7).
Functionally analogous to the mammalian kidney, Malpighian tubules (MTs) are the primary organs for excretion by insects. The distal MT portion is close-ended and embedded in the hemolymph, whereas the proximal portion is connected to the hindgut. The MT epithelium is a single layer of cells, consisting of two types—larger principal cells (PCs) and smaller stellate cells (SCs). PCs account for about 80% of the total cells, whereas SCs account for about 20% and are evenly distributed among PCs in the first two-thirds of MT at the distal end (8, 9). The different functions of PCs and SCs in excretion are not fully understood.
In Drosophila melanogaster, PCs express V-type ATPase and cation channels, whereas SCs express chloride channel and a water-selective AQP, DRIP (10), predicting different functions for the two types of cells. Controlled by diuretic hormones, V-type ATPases in PCs pump sodium and potassium ions from the hemolymph into the distal Malpighian tubule lumen. Chloride ions and water are driven into the lumen through their respective channels in SCs along electrostatic or osmotic gradients. As a result, primary urine forms in the distal Malpighian tubule lumen and flows toward the proximal Malpighian tubule and hindgut under hydrostatic pressure. Potassium and other ions may be reabsorbed by hindgut to maintain salt balance while excess fluid is excreted (7). The major function of the Malpighian tubule as an excretory organ suggests that the water-selective AQP may play an important role in its physiology.
Several AQPs have been described in insects. The Drosophila melanogaster protein Big Brain (BIB) is required for normal brain development (10). An electron microscopic study revealed AQPcic, a water-specific AQP in the xylem sap-sucking insect Cicadella viridis, on microvilli and basal membrane folds of filter chamber epithelia (11). The dengue virus mosquito vector, Aedes aegypti, expresses AeaAQP in the tracheolar cells, which may contribute to water movement in the mosquito’s respiratory system (12). However, the physiological functions of these AQPs are not completely defined.
In this report, we identified AQPs from the major malaria vector Anopheles gambiae. The sentinel member, designated AgAQP1, was characterized in vitro and in vivo. When expressed in Xenopus laevis oocytes, AgAQP1 exhibits specific transport activity for water. AgAQP mRNA was detected in multiple mosquito tissues including the Malpighian tubules, gut, ovary, and fat body. By immunofluorescence microscopy, we detected AgAQP protein in the stellate cells of the excretory Malpighian tubules. AgAQP1 expression is regulated during mosquito development and under certain physiological conditions. Adults display higher expression of AgAQP1 than larvae or pupae, and females displayed higher expression than males. AgAQP1 expression is increased after blood feeding and reduced after exposure to a dehydrating environment. A. gambiae with reduced expression of AgAQP1 in gut and Malpighian tubules survive significantly longer than controls in extreme desiccation conditions. These studies indicate that expression of AgAQP1 is regulated during reproduction and environmental adaptation, processes essential to the transmission of malaria to humans by A. gambiae.
Results
Anopheles gambiae AQP Sequences
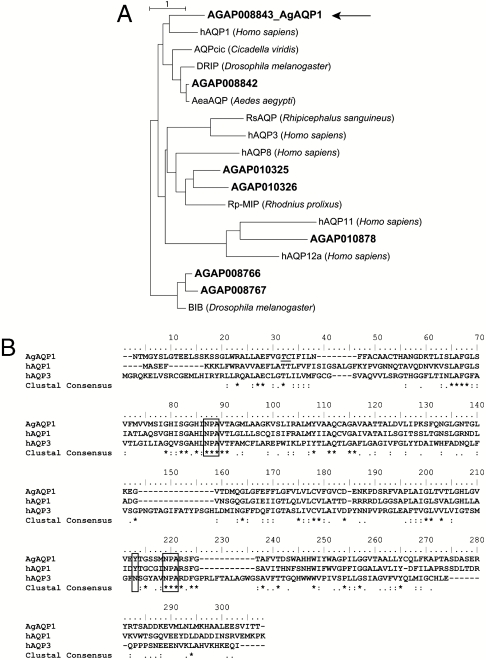
At least seven putative AQPs in A. gambiae mosquitoes (AgAQPs) have been annotated (http://www.angaged.bio.uci.edu). The gene numbers are AgAP008766, 008767, 008843, 010325, 010326, 010878, and 008842. We designed sequence-specific primers and successfully cloned six: AgAP008766, 008843, 010325, 010326, 010878, and 008842. Phylogenetic analysis revealed the existence of evolutionary clusters of AgAQPs, human AQPs, and other invertebrate orthologs (Fig. 1A), from which AgAP008843, hereafter referred to as AgAQP1, was selected for further analysis.
Fig. 1.
Maximum-likelihood phylogenetic analysis of A. gambiae AQPs and sequence alignment between AgAQP1 and selected orthologs. (A) Phylogenetic tree based on deduced protein sequences. A. gambiae AQPs are referred by their VectorBase ID in bold. For AgAQP1, the actual sequence was used; for other AgAQPs, annotated sequences were used. Selected human and arthropod AQPs are human AQP1, 3, 8, 11, and 12a; AQPcic from Cicadella viridis; Aea AQP from Aedes aegypti; DRIP and BIB from Drosophila melanogaster; Rp-MIP from Rhodnius prolixus; and Rs-AQP1 from Rhipicephalus sanguineus. AgAQP8843, later referred to as AgAQP1 in this report, is indicated with an arrow. Unit, 1 amino acid substitution. (B) Protein sequence alignment of AgAQP1, human AQP1 (hAQP1), and human AQP3 (hAQP3). Conserved NPA motifs and the tyrosines conferring TEA-sensitivity in hAQP1 and AgAQP1 are highlighted with boxes, and an asparagine is at the corresponding position in TEA-resistant hAQP3. Our clone does not contain the residues Thr30 and Cys31 annotated by VectorBase, which are depicted with underline.
The deduced amino acid sequence of AgAQP1 shares 35–38% identity with previously recognized insect AQPs: DRIP (Drosophila melanogaster), AQPcic (Cicadella viridis) and AeaAQP (Aedes aegypti). AgAQP1 contains specific features of aquaporin sequences including the two signature Asn-Pro-Ala (NPA) motifs that are known to restrict proton conductance in the channel (13) (Fig. 1B). Inhibition of human AQP1 (hAQP1) by tetraethylammonium (TEA) occurs through Tyr186 (14), and AgAQP1 contains a Tyr at the corresponding position (Fig. 1B). No Tyr occurs in the corresponding site of TEA-resistant human AQP3 (14).
Our cloned AgAQP1 sequence differs slightly from AgAP008843 as annotated in the database (http://www.angaged.bio.uci.edu). AgAP008843 contains two amino acids, Thr31 and Cys32, missing from AgAQP1 (Fig. 1B). Another AQP sequence from A. gambiae was recently deposited in the National Center for Biotechnology Information’s GenBank (accession number AB523397.1), with a deduced amino acid sequence 95% identical to AgAQP1 but lacking the first two amino acids at the N-terminus and containing a different C-terminus, and may be an alternate isoform of the gene.
Functional Characterization of AgAQP1 in Vitro
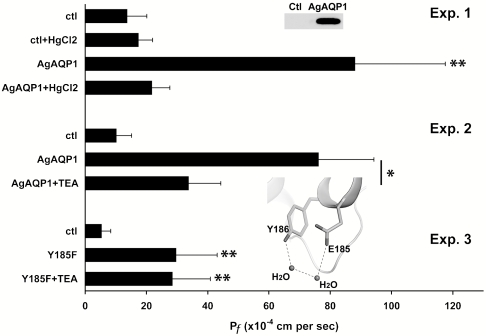
In vitro transcribed RNA of myc-tagged AgAQP1 was injected into X. laevis oocytes. AgAQP1 protein expression was confirmed on the oocyte plasma membrane by Western blot (Fig. 2, Inset of Exp. 1). Similar to known AQPs, oocytes expressing AgAQP1 swelled five times faster than control oocytes in hypoosmotic solution. Similar to most known AQPs, AgAQP1 water permeation was inhibited by 1.0 mM HgCl2, whereas water permeability of control oocytes was unaffected (Fig. 2, Exp. 1).
Fig. 2.
Functional characterization of AgAQP1 expressed in X. laevis oocytes. (Exp. 1) AgAQP1-expressing oocytes specifically facilitated water movement across cell membranes, and the transport was inhibited by 1.0 mM HgCl2. (Inset) Overexpression of myc-tagged AgAQP1 on oocyte plasma membrane (0.2 oocyte equivalents) was verified by Western blot with anti-myc antibody. (Exp. 2) 1.0 mM TEA inhibited AgAQP1-mediated water transport but did not affect control oocytes. (Exp. 3) Oocytes expressing AgAQP1-Y185F mutant were not sensitive to TEA. They exhibited similar Pf values when incubated with or without 1.0 mM TEA. (Inset) The modeled partial structure of AgAQP1. H bonds formed between Y186, E185, and nearby water molecules are presented as dashed lines. Protein backbone is presented as a ribbon cartoon. The x axis is the coefficient of osmotic water permeability (Pf). Unit, 10-4 cm/s. * P < 0.05; ** P < 0.02.
Also similar to certain known AQPs, incubation of AgAQP1 oocytes in 1.0 mM TEA reduced water permeability approximately 2-fold (Fig. 2, Exp. 2). The residue Tyr186 confers TEA sensitivity in hAQP1, and inhibition disappeared after this residue was mutated to Phe (14). In our study, the corresponding Tyr185 residue of AgAQP1 was changed to Phe by site-directed mutagenesis, and the Y185F mutant was no longer sensitive to TEA (Fig. 2, Exp. 3). Homology modeling between AgAQP1 and Pichia pastoris AQY1 (Protein Data Bank code 2W2E) revealed that Tyr185 forms H bonds with nearby water molecules and Gln184 in AgAQP1 (Fig. 2, Inset of Exp. 3). These H bonds may be important for the structural stability and activity of AgAQP1 and would be impaired by TEA binding. Unlike members of the aquaglyceroporin subfamily, the coefficient of osmotic glycerol permeability (Ps) of oocytes expressing AgAQP1 was not above baseline values (AgAQP1, 2.0 ± 1.7 × 10-6 cm/s vs. control, 0.7 ± 1.8 × 10-6 cm/s).
AgAQP1 Expression in Vivo
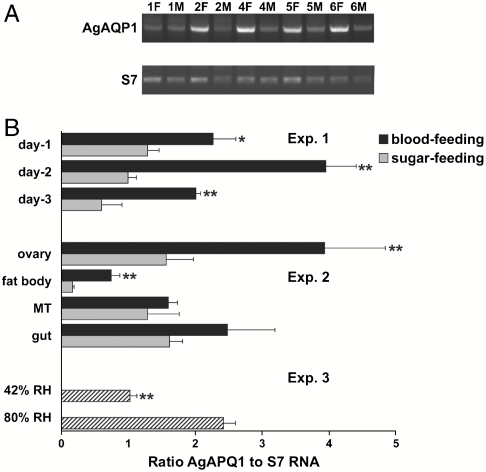
Expression of AgAQP1 mRNA was quantified in mosquitoes during development and under specific physiological conditions. Expression was higher in female adults than males (Fig. 3A), fourth instar larvae, or pupae (Fig. S1). When expression was measured in females 3 d post–blood feeding, AgAQP1 mRNA levels were two to four times higher in blood-fed females than sugar-fed counterparts (Fig. 3B, Exp. 1). Organ-specific real-time RT-PCR revealed low levels of AgAQP1 mRNA in fat body and high levels in gut, Malpighian tubules, and ovary. Blood-feeding increased AgAQP1 expression in all organs detected, but most strikingly increased expression occurred in fat body and ovary, where mRNA levels increased 4- and 2-fold, respectively (Fig. 3B, Exp. 2). Atmospheric humidity was found to significantly influence AgAQP1 mRNA expression. Adult females residing at 80% relative humidity had AgAQP1 mRNA expression 2.5 times the level found in mosquitoes residing at 42% relative humidity (Fig. 3B, Exp. 3).
Fig. 3.
Expression profiles of AgAQP1 in vivo. (A) Expression levels of AgAQP1 at different development stages were determined by semiquantitative RT-PCR. The female or male adults were 1-, 2-, 4-, 5-, or 6-d old postemergence. The upper gel shows AgAQP1 specific amplification, and the lower gel shows the amplification of the single-copy S7 control gene. (B, Exp. 1) Ratio AgAQP1 to S7 RNA on 1-, 2-, and 3-d post– blood feeding or sugar feeding in 6-d old adults determined by real-time quantitative RT-PCR. (B, Exp. 2) Ratio AgAQP1 to S7 RNA in ovary, fat body, Malpighian tubules, and gut on day 2 post–blood feeding by quantitative real-time RT-PCR. * P < 0.05; ** P < 0.02. (B, Exp. 3) Ratio AgAQP1 to S7 RNA at 42% vs. 80% relative humidity in whole female adults determined by quantitative real-time RT-PCR. ** P < 0.02.
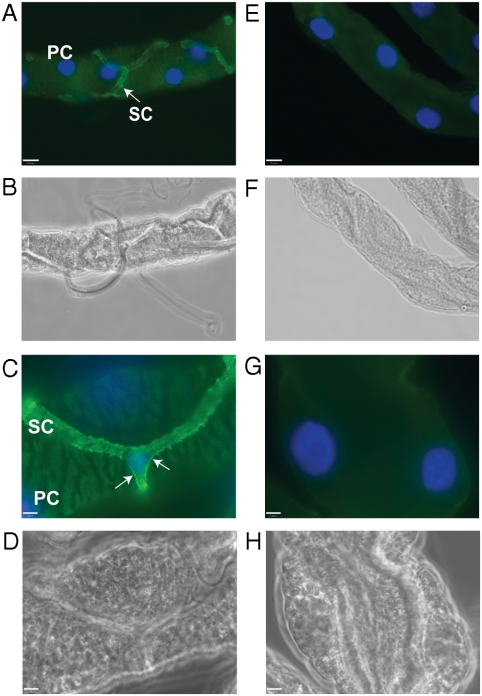
Subcellular distribution of AgAQP1 was evaluated in Malpighian tubules using a specific antibody raised against the unique N-terminus of AgAQP1. Clear immunofluorescence signal was detected in the star-shaped SCs but not in the PCs of the mosquito Malpighian tubule (Fig. 4 A and C). Except for background fluorescence, no signal was observed in samples incubated with preimmune serum (Fig. 4 E and G). In addition to the intracellular immunofluorescence, strong signal was observed on the SC surface (Fig. 4C, arrows), indicating that significant AgAQP1 is localized on the plasma membrane.
Fig. 4.
AgAQP1 localization in SCs of Malpighian tubules. AgAQP1 protein expression (green) was observed in Malpighian tubules incubated with AgAQP1-specific antibody (A and C, under a 40× objective lens and a 100× objective lens, respectively), whereas there was no signal detected in Malpighian tubules incubated with preimmune rabbit serum (E and G, under 40× and 100× lenses, respectively). A, C, E, and G are fluorescence microscopy, and B, D, F, and H are phase contrast microscopy of the same samples, respectively. A representative of each cell type, PC or SC, is labeled in A and C. AgAQP1 expression was observed on the surface of SC, as shown by arrows in C. Green, AgAQP1; blue, DAPI stained nucleus. Scale bar, 16 μm in A, B, E, and F; 6 μm in C, D, G, and H.
Reduction of AgAQP1 Expression in Vivo
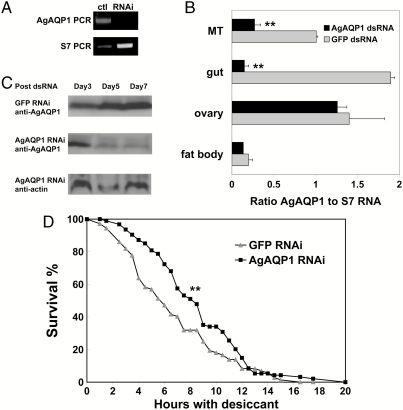
RNA interference (RNAi) was employed to reduce AgAQP1 mRNA and protein levels in adult female A. gambiae mosquitoes. Two days after dsRNA injection, reduction of AgAQP1 mRNA expression in whole female mosquitoes was detected by semiquantitative RT-PCR (Fig. 5A). Real-time RT-PCR showed that AgAQP1 mRNA was reduced 92% in gut and 73% in Malpighian tubules, whereas expression in ovary was essentially unchanged (Fig. 5B). By 7 d post–dsRNA injection, AgAQP1 protein reduction was demonstrated by Western blot (Fig. 5C) and confirmed by immunofluorescence microscopy (Fig. S2).
Fig. 5.
Down-regulation of AgAQP1 contributes to A. gambiae desiccation resistance. (A) On day 2 post–dsRNA injection, AgAQP1 mRNA was knocked down in female A. gambiae, determined by semiquantitative RT-PCR. (B) The knockdown efficiency of AgAQP1 mRNA on day 2 post–dsRNA injection in different organs determined by quantitative real-time RT-PCR. The x axis is the ratio of AgAQP1 to S7 RNA. ** P < 0.02. (C) On days 5 and 7 post–dsRNA injection, Western blot using AgAQP1-specific antibody showed that AgAQP1 protein was reduced in whole females compared to day 3 post–dsRNA injection. For each sample, total protein amount was determined by bicinchoninic acid assay, and equal amount of protein was loaded for SDS-PAGE separation. Western blot with anti-actin antibody served as another loading control. (D) A. gambiae with reduced expression of AgAQP1 in MTs and gut survived significantly longer than GFP dsRNA injected controls. The x axis is hours with desiccant; y axis is percent survival. ** P < 0.02.
A desiccation assay was employed to investigate mosquito viability under conditions of extremely low relative humidity. To simulate the aridity in the dry season, A. gambiae mosquitoes were placed in a desiccating environment (relative humidity <20%) on the seventh day after dsRNA injection as described in Methods. Living mosquitoes were counted at regular time intervals until 20 h, when all mosquitoes had died. Survival analysis showed that A. gambiae with reduced AgAQP1 expression survived significantly longer than controls (Fig. 5D). To confirm that mosquitoes in the desiccation assay died of dehydration, we incubated wild-type mosquitoes in tubes with or without desiccant. In the presence of desiccant (<20% relative humidity), median survival was 6–8.5 h. In contrast, median survival was approximately 17 h in the absence of desiccant (relative humidity 45 ± 8%).
Discussion
Although active during the humid rainy season, A. gambiae must survive the months-long dry season, suggesting a protective mechanism against aridity. Our data suggests that the regulation of AgAQP1 participates in this mechanism and contributes in part to A. gambiae adaptation to arid environments. Under dry conditions, AgAQP1 expression was significantly down-regulated (Fig. 3, Exp. 3). A. gambiae with reduced expression of AgAQP1 survived significantly longer than controls in the desiccation assay (Fig. 5D). These data indicate that by suppressing AgAQP1 expression in Malpighian tubules, A. gambiae reduces unnecessary water loss via excretion to achieve improved survival under dry conditions. Parallel to our studies, Drake et al. noted that diuresis in Aedes aegypti was impaired when three water-selective AQPs were reduced in Malpighian tubules (15).
Other pathways leading to desiccation resistance of mosquitoes have been described at the genetic and molecular level. A. gambiae mosquitoes possessing the 2La inversion are more resistant to desiccation than wild-type (16). However, the Keele strain used in our study was determined to be polymorphic for this inversion by PCR method (Fig. S3). Additionally, AgAQP1 is located on chromosome 3 rather than chromosome 2. Therefore, the AgAQP1-involved antidesiccation mechanism is most likely independent of 2La inversion. Heat shock protein 70 (HSP70), a molecular chaperone, is related to dehydration tolerance in Aedes aegypti (17), but it is unknown if AQP is a direct target of HSP70.
AgAQP1 expression in Malpighian tubules is specifically localized in SCs, which compose only 20% of the total Malpighian tubule cells. Therefore, AgAQP1 can serve as a molecular marker for SCs, paving the road for further studies of SCs. Previously, only one marker of SCs was known, the transcription factor Teashirt, which is expressed only in the nucleus (18). In contrast, AgAQP1 is expressed both intracellularly and on the plasma membrane, which can outline SCs more effectively. Besides the expression in SCs of Malpighian tubules, expression pattern of AgAQP1 in other organs, such as gut or ovary, warrants further investigation.
Our studies support a role for AgAQP1 during A. gambiae oocyte development. After a blood meal, vitellogenin is synthesized in the fat body and secreted into the hemolymph, where it is taken up by oocytes through receptor-mediated endocytosis during mosquito ovary maturation. In this study, AgAQP1 expression was detected in ovary, and the expression level was increased for several days post–blood feeding (Fig. 3, Exp. 1 and 2). This suggests that AgAQP1-mediated water transport may be critical for oocyte development. In gilthead sea bream, a water-selective AQP is responsible for oocyte hydration, where it allows fast water influx driven by osmotic gradient created by hydrolysis of yolk protein precursor and ion uptake (19). It is likely that AgAQP1 may play a similar role in A. gambiae ovaries.
Together, our studies have shown that down-regulation of AgAQP1 contributes to desiccation resistance of female A. gambiae adults. Water homeostasis is important during all stages of the A. gambiae life cycle. For example, water uptake by oocytes occurs in mosquitoes during egg development. The weight of an Aedes aegypti egg can increase from 5 to 12 μg due to the water influx. Although the whole process lasts 10–16 h, the majority of water uptake occurs within the first 2 h postoviposition (5). In another example, A. gambiae larvae live in fresh water and have a continuous water intake due to osmotic forces and drinking. Excretion of excess water through Malpighian tubules and rectum is needed to maintain normal physiology (5). Water-selective AQPs may be key players in these processes, and further investigations into these functions are needed. Moreover, the roles that all of the A. gambiae AQPs may play in malaria transmission should be explored.
Methods
A detailed description of the methods is available in SI Methods. Briefly, A. gambiae AQPs were annotated by the University of California, Irvine, expression database (http://www.angaged.bio.uci.edu). Deduced protein sequences were aligned using ClustalW2 (http://www.ebi.ac.uk/Tools/clustalw2/index.html). Sequences of representative AQPs from other arthropods were obtained from GenBank (http://www.ncbi.nlm.nih.gov/genbank). Arthropod AQP names, species of origin, accession numbers, and relevant references are listed in Table S1. A maximum-likelihood phylogeny of AQP orthologs was constructed using SeaView (20). Selected human AQPs are included for comparison; their names and accession numbers are listed in Table S2. Total RNA was isolated from mosquitoes (Keele strain) or dissected tissues using TRIzol Reagent (Invitrogen) with a motorizing pestle according to the manufacturer's instructions. Sequence-specific primers were designed for cloning, semiquantitative RT-PCR, quantitative real-time RT-PCR, Y185F mutagenesis, and RNAi (Table S3).
Polyclonal rabbit antibodies were commercially raised against the unique N- and C-terminal sequences of AgAQP1. Both peptide sequences have negligible similarity to other AgAQPs (Fig. S4). The antibody against the N-terminal sequence of AgAQP1 (YSLGTEELSSKSSGC) showed sufficient specificity and was used in this study. To detect myc-tagged AgAQP1 expressed in X. laevis oocytes, an antimyc antibody (Invitrogen) was used for Western blot. To detect A. gambiae actin, we purchased an antiactin rabbit antibody (Sigma no. A2066) for Western blot.
pXβG-myc-AgAQP1 was constructed for AgAQP1 expression in X. laevis oocytes. Five nanograms (60 nL) of AgAQP1cRNA was injected into each oocyte. Control oocytes were injected with the same volume of nuclease-free water. After growing in media for 3 d, oocytes were tested using a swelling assay, and the coefficient of osmotic water permeability (Pf) determined. Significances were determined using the Kruskal–Wallis test with the Dwass procedure for pairwise comparisons. Data are presented as mean ± SD.
Relative humidity was determined using a Fisher Science Education Thermometer/Clock/Humidity Monitor (no. S66279) with the detection range of 20–90% ± 8%. For RNAi, 400 ng of dsRNA targeting a 520-bp fragment of AgAQP1 cDNA was injected into the thorax of A. gambiae mosquitoes 4–6 d postemergence. Controls were mosquitoes injected with dsRNA targeting GFP. On day 7 postinjection, 10 mosquitoes were placed into a 50-mL tube (BD Biosciences) containing 16.5 g of Drierite desiccant (Fisher Scientific, Inc.) with a cotton ball on top of the Drierite for mosquitoes to rest on. The tubes were capped, sealed with parafilm, and incubated at room temperature. Living mosquitoes were counted every 1 or 1.5 h until 20 h, when all mosquitoes were dead. Survival analysis including Kaplan–Meier estimation of survival curves and log-rank test to calculate statistical significance were performed using software R (http://www.r-project.org).
For immunofluorescence microscopy, organs of A. gambiae were dissected and incubated with anti-AgAQP1 antibody and Alexa Fluor 488-conjugated secondary antibody. Controls were organs incubated with rabbit preimmune serum. DAPI (Invitrogen) was added to stain nucleus. Images were obtained by using a Nikon 90i upright microscope connected to a digital camera using Volocity imaging software (Improvision).
Supplementary Material
Acknowledgments.
We thank Drs. Yuemei Dong, Grant Hughes, Xiaoxia Ren, and Ms. Xiaoyan Huang at Johns Hopkins Malaria Research Institute (JHMRI) for technical support. We also appreciate helpful discussions with Drs. Guiyun Yan, Immo Hansen, David Kozono, and Brian Foy. We are grateful to the Insectary, Parasitology, Imaging and Microscopy, and Gene Array Core Facilities at JHMRI for help in data collection and supply of materials. Antibodies were produced by GenScript, Inc. This work was supported by National Institutes of Health Grant R01HL48268 to P.A. and a JHMRI pilot grant to J.L.R.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database, http://www.ncbi.nlm.nih.gov/genbank/ (accession no. JF342682).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102629108/-/DCSupplemental.
References
- 1.Minakawa N, Githure JI, Beier JC, Yan G. Anopheline mosquito survival strategies during the dry period in western Kenya. J Med Entomol. 2001;38:388–392. doi: 10.1603/0022-2585-38.3.388. [DOI] [PubMed] [Google Scholar]
- 2.Gray EM, Bradley TJ. Physiology of desiccation resistance in Anopheles gambiae and Anopheles arabiensis. Am J Trop Med Hyg. 2005;73:553–559. [PubMed] [Google Scholar]
- 3.Beyenbach KW. Transport mechanisms of diuresis in Malpighian tubules of insects. J Exp Biol. 2003;206:3845–3856. doi: 10.1242/jeb.00639. [DOI] [PubMed] [Google Scholar]
- 4.Patrick ML, Aimanova K, Sanders HR, Gill SS. P-type Na+/K+-ATPase and V-type H+-ATPase expression patterns in the osmoregulatory organs of larval and adult mosquito Aedes aegypti. J Exp Biol. 2006;209:4638–4651. doi: 10.1242/jeb.02551. [DOI] [PubMed] [Google Scholar]
- 5.Clements AN. The Biology of Mosquitoes. New York: CABI Publishing; 2000. [Google Scholar]
- 6.Bradley TJ. Physiology of osmoregulation in mosquitoes. Annu Rev Entomol. 1987;32:439–462. doi: 10.1146/annurev.en.32.010187.002255. [DOI] [PubMed] [Google Scholar]
- 7.Agre P. Nobel Lecture. Aquaporin water channels. Biosci Rep. 2004;24:127–163. doi: 10.1007/s10540-005-2577-2. [DOI] [PubMed] [Google Scholar]
- 8.Cabrero P, Pollock VP, Davies SA, Dow JA. A conserved domain of alkaline phosphatase expression in the Malpighian tubules of dipteran insects. J Exp Biol. 2004;207:3299–3305. doi: 10.1242/jeb.01156. [DOI] [PubMed] [Google Scholar]
- 9.Beyenbach KW, Skaer H, Dow JA. The developmental, molecular, and transport biology of Malpighian tubules. Annu Rev Entomol. 2010;55:351–374. doi: 10.1146/annurev-ento-112408-085512. [DOI] [PubMed] [Google Scholar]
- 10.Rao Y, Jan LY, Jan YN. Similarity of the product of the Drosophila neurogenic gene big brain to transmembrane channel proteins. Nature. 1990;345:163–167. doi: 10.1038/345163a0. [DOI] [PubMed] [Google Scholar]
- 11.Thomas D, Cavalier A. Observation of membrane proteins in situ: AQPcic, the insect aquaporin example. Methods Mol Biol. 2010;654:171–185. doi: 10.1007/978-1-60761-762-4_9. [DOI] [PubMed] [Google Scholar]
- 12.Duchesne L, Hubert JF, Verbavatz JM, Thomas D, Pietrantonio PV. Mosquito (Aedes aegypti ) aquaporin, present in tracheolar cells, transports water, not glycerol, and forms orthogonal arrays in Xenopus oocyte membranes. Eur J Biochem. 2003;270:422–429. doi: 10.1046/j.1432-1033.2003.03389.x. [DOI] [PubMed] [Google Scholar]
- 13.Murata K, et al. Structural determinants of water permeation through aquaporin-1. Nature. 2000;407:599–605. doi: 10.1038/35036519. [DOI] [PubMed] [Google Scholar]
- 14.Detmers FJ, et al. Quaternary ammonium compounds as water channel blockers. Specificity, potency, and site of action. J Biol Chem. 2006;281:14207–14214. doi: 10.1074/jbc.M513072200. [DOI] [PubMed] [Google Scholar]
- 15.Drake LL, et al. The aquaporin gene family of the yellow fever mosquito, Aedes aegypti. PLoS ONE. 2010;5:e15578. doi: 10.1371/journal.pone.0015578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray EM, Rocca KA, Costantini C, Besansky NJ. Inversion 2La is associated with enhanced desiccation resistance in Anopheles gambiae. Malaria J . 2009;8:215. doi: 10.1186/1475-2875-8-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benoit JB, Lopez-Martinez G, Phillips ZP, Patrick KR, Denlinger DL. Heat shock proteins contribute to mosquito dehydration tolerance. J Insect Physiol. 2010;56:151–156. doi: 10.1016/j.jinsphys.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denholm B, et al. Dual origin of the renal tubules in Drosophila: Mesodermal cells integrate and polarize to establish secretory function. Curr Biol. 2003;13:1052–1057. doi: 10.1016/s0960-9822(03)00375-0. [DOI] [PubMed] [Google Scholar]
- 19.Tingaud-Sequeira A, et al. Structural and functional divergence of two fish aquaporin-1 water channels following teleost-specific gene duplication. BMC Evol Biol. 2008;8:259. doi: 10.1186/1471-2148-8-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gouy M, Guindon S, Gascuel O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol. 2010;27:221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.