Abstract
The power of chemical synthesis of large cysteine-free polypeptides has been significantly enhanced through the use of nonproteogenic constructs which bear strategically placed thiol groups, enabling native chemical ligation. Central to these much expanded capabilities is the specific, radical-induced, metal-free dethiolation, which can be accomplished in aqueous medium.
Keywords: alanine ligation, desulfurization, leucine ligation, peptide, valine ligation
The chemical synthesis of proteins offers the potential of solving a multitude of problems in biomedical sciences (1). Chemical synthesis can exert great control on protein composition. Moreover, chemical synthesis can facilitate the creation of new proteins with desirable properties. Historically, the chemical preparation of biotherapeutic proteins and their analogs has relied on the use of the powerful cysteine-based native chemical ligation (NCL) method of Kent and associates (2, 3). However, given the relative scarcity of cysteine residues in nature, a clear impetus arises for the realization of new NCL capabilities (4–17).
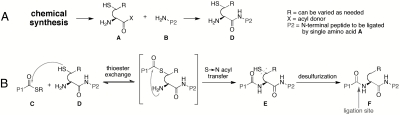
Anticipating this type of problem, our laboratory (9, 11, 14, 17) and others (5–7, 10) have developed strategies by which to accomplish ligation at a range of noncysteine amino acid residues. The logic of our approach is outlined in Fig. 1. The N-terminal peptide, D, to be ligated is equipped with an N-terminal sulfur-bearing amino acid surrogate, A, itself prepared by synthesis and coupled, by a suitable method, to the peptide B. In this way, N-terminal ligation candidate D is ready for coupling with C-terminal acyl donating peptide C (usually a thioester). Ligation product E can be maintained as such, thereby affording a specifically placed thiol group in a nonnatural context. Theoretically, de-thiolation of E will produce F wherein the "R" group had been governed by the synthetically derived A. In principle, the strategy shown in Fig. 1 is of universal scope.
Fig. 1.
Ligation at noncysteine amino acid residues. Key: (A) A single amino acid residue A has been added to peptide B to make the N-terminal ligation partner D; (B) Merger of C-terminal peptide C and N-terminal peptide D.
Studies using a variety of model peptides have demonstrated the fragment-coupling capability of the cysteine-free strategy. Here, in this paper, we seek to examine the general applicability of this strategy to the total synthesis of cysteine-poor proteins. Human Parathyroid Hormone (hPTH) is chosen to serve as a model molecule because of its representativeness in terms of amino acid composition and its therapeutic value. hPTH is a biological messenger that is secreted by the parathyroid glands as a peptide containing 84 amino acids (18, 19). Upon binding to its receptor, hPTH can enhance the concentration of calcium (Ca2+) in the blood (20). Because of their important physiological roles, hPTH and one of its fragments, hPTH (1–34), are now given (by subcutaneous injection) for the treatment of hypoparathyroidism and osteoporosis in men, as well as for postmenopausal women at high risk of fracture (21–23). Not unlike most other hormone drugs, the recombinant hPTH therapeutics have very short half-lives in the human body and need to be administered at least once a day (24, 25). The need for continuous daily subcutaneous injection is an obvious disadvantage which serves to compromise use of the hormone. Clearly, the production of more stable forms of hPTH, where “pharmacolability” is attenuated without undercutting biological activity, would be of great interest (26). It is also of interest to interrogate the consequences of employing nonproteogenic inserts (27). While this goal can be accomplished by cleverly designed recombinant methods, chemical synthesis could well be more convenient for servicing the initial production of probe structures for such structure-activity relationship (SAR) evaluations (28, 29). Previously, the chemical synthesis of hPTH required either the solid phase synthesis of an 84-mer-long peptide or the assembly of fully protected peptide segments. Such methods are not ideal for the generation of analogs (30–33). It was recognized that enhancement of the power of chemical synthesis for such objectives, including that of hPTH itself, could be accomplished by extending the reach of the underlying elegant concept of NCL (4–17). To this end, we report a pleasing and encouraging example wherein these recent findings have been pooled such that the molecule hPTH can be conveniently assembled from small synthetic peptide fragments. This technology would enable access to more proteins through chemical synthesis.
As a model for what would have to be accomplished in a typical protein synthesis, we chose to use the generally preferred convergent strategy to prepare hPTH (Fig. 2), although a more efficient synthesis might be achieved with other disconnection patterns. The primary structure of hPTH is shown in Fig. 2. On the basis of its amino acid sequence, we decided to assemble the hPTH polypeptide chain from four approximately equal sized fragments, hPTH (1–23) 1, hPTH (24–38) 2, hPTH (39–59) 3, and hPTH (60–84) 4. The peptide fragments contain 23, 15, 21 residues, and 25 amino acid residues, respectively, which are accessible through solid phase peptide synthesis (34, 35). These fragments were to be joined at the sites of three of the most abundant amino acids present in hPTH, i.e., Leu24, Ala39, and Val60 (Fig. 2).
Fig. 2.
Retrosynthetic analysis for the preparation of Human Parathyroid Hormone (hPTH) by the native chemical ligation/desulfurization strategy. The desired full-length hPTH was prepared by noncysteine native chemical ligation of 1, 2, 3, and 4 followed by desulfurization reaction.
Results and Discussion
The synthesis of hPTH is shown in Fig. 3. The fully protected peptides to be ligated were synthesized by Fmoc chemistry on a 0.05 mmol scale. The preleucine and prevaline surrogates were attached to the N termini of the fully protected peptides by 2-(1H-7-Azabenzotriazol-1-yl)-1,1,3,3-tetramethyl uronium hexafluorophosphate (HATU) coupling (17). The peptide fragments, bearing C-terminal thioesters, were prepared from the fully protected peptides using the 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI)-mediated amide formation reaction under the nonepimerizing conditions developed by Sakakibara et al. (Fig. 3A) (36). Kinetically controlled ligation (37) at leucine of fragment 1 thioester and fragment 2 alkylthioester was carried out over 9h at pH 7.5, to afford peptide 9 in 59% yield. The reaction of fragments 3 and 4 was carried out in pH 7.5 guanidine buffer for 9 h to provide peptide 10. After ligation was completed, the thiazolidine function in peptide 10 was converted into the required N-terminal cysteine by treatment with methoxylamine•HCl at pH 4.0 (86% yield over two steps, Fig. 3B). Following these syntheses, ligation of peptide 9 thioester and 11 in the presence of 200 mM 4-mercaptophenylacetic acid (MPAA) catalyst generated 12 in 63% yield (38). Not surprisingly, the modified NCL methods are operative even in the presence of internal (side chain) thiols (2). Our aqueous, metal-free desulfurization method, applied to 12, was completed in 2 h and yielded the final full-length hPTH (9). Purification by HPLC provided pure hPTH in 86% yield.
Fig. 3.
Synthesis of Human Parathyroid Hormone. Key: (A) H-Trp-SPh, EDCI, HOOBt, DIEA, DMSO, 3 h; (B) TFA∶TIS∶H2O (95∶2.5∶2.5), 45 min; (C) Boc-Leu(SSMe)-OH, HATU, DIEA, DMSO, 1 h; (D) TFE∶AcOH∶CH2Cl2 (8∶1∶1), 2 h; (E) H-Gly-SCH2CH2CO2Et, EDCI, HOOBt, DIEA, DMSO, 1 h; (F) H-Leu-SPh, EDCI, HOOBt, DIEA, DMSO, 2 h; (G) Boc-Val(SSMe)-OH, HATU, DIEA, DMSO, 1 h; (H) 6 M Gn•HCl, 100 mM Na2HPO4, and 50 mM TCEP, pH 7.5, 9 h; (I) MeONH2•HCl, pH 4, 2.5 h; (J) 6 M Gn•HCl, 300 mM Na2HPO4, 200 mM MPAA, and 20 mM TCEP, pH 7.9; and (K) VA-044, tBu-SH, TCEP, H2O, MeCN, 37 °C, 2 h.
Not surprisingly, the fully synthetic noncysteine containing hormone folded spontaneously under native conditions (39–41). Circular dichroism (CD) measurements from 190 to 250 nm served to demonstrate the folding of the fully synthetic polypeptide (Fig. 4).
Fig. 4.
Unnormalized CD spectra of hPTH. Nadirs at 208 and 222 nm are characteristic of α-helical structures. Key: (A) CD comparison of the synthetic and recombinant PTH at concentration of 14 μM; (B) CD spectra of synthetic PTH at concentration of 14 μM and 7 μM.
Remarkably, the hPTH prepared by laboratory synthesis derived from our chemistry is significantly more pure than is the recombinant hPTH obtained as a reference sample. As chemical synthesis develops further, it may well emerge as a credible alternative to “biologic“ type methods, where there is a particularly high priority for enhancing homogeneity.
In summary, we have described herein a rather interesting instance of extending the scope of native chemical ligation to the convergent synthesis of a highly active, substantially sized polypeptide, hPTH. Our method brings together readily purifiable peptide segments using the underlying logic of S → N acyl transfer inherent in NCL with the feature that the target of synthesis contains no cysteine residues.
The chemistry described above is, with some modification on a case by case basis, being extended to the synthesis of hPTH analogs, hoping to discover systems of enhanced potency and pharmacostability. The results of these efforts will be described in due course. We anticipate that the synthesis concepts demonstrated above will find broad application, and are conducting experiments toward such ends.
Materials and Methods
All commercial reagents and solvents were used without further purification. Automated peptide synthesis was performed on an Applied Biosystems Pioneer continuous flow peptide synthesizer using commercially available and synthetic building blocks. The deblock solution was a mixture of 100/5/5 of dimethylformamide/piperidine/1,8-Diazabicyclo[5.4.0]undec-7-ene. All the peptide thioesters were prepared as previously described (17). The peptide side chain cleavage solution was a mixture of 95/2.5/2.5 of trifluoroacetic acid (TFA)/Triisopropylsilane (TIS)/H2O. The purification of the peptides was achieved using a Ranin HPLC solvent delivery system equipped with a Rainin UV-1 detector and Varian Dynamax using Varian Microsorb columns at a flow rate of 16.0 mL/ min. The liquid chromatography-mass spectrometry (LC-MS) analysis of the peptide was performed using a Waters 2695 Separations Module and a Waters 996 Photodiode Array Detector equipped with Varian Microsorb columns at a flow rate of 0.2 mL/ min. The ultra performance liquid chromatography (UPLC)-MS analysis of the peptide was performed using a Waters Acquity™ UPLC system equipped with Acquity UPLC® BEH columns at a flow rate of 0.3 mL/ min. The mobile phase for HPLC analysis and separation was a mixture of 0.05% TFA (vol/vol) in water (solvent A)/0.04% TFA in acetonitrile (solvent B). All the ligation and desulfurization reactions were carried out as described previously (17). A detailed description of materials and methods is given in SI Appendix: Materials and Methods
Supplementary Material
Acknowledgments.
We thank Prof. David Elizer and Dr. Khurshida Shahidullah (Weill Cornell) for their assistance with the CD measurements. We thank Dr. Barney Yoo (Memorial Sloan-Kettering Cancer Center Organic Synthesis Core facility) and Rebecca Lambert for valuable discussions. We also thank Dr. George Sukenick, Hui Fang, and Sylvi Rusli of Sloan-Kettering Institute’s NMR core facility for mass spectral and NMR assistance and Laura Wilson for assistance with the preparation of the manuscript. Support for this research was provided by the National Institute of Health (CA28824 to S.J.D.). We dedicate this paper to the memory of Dr. Shoshanah Trachtenberg Frackman for her work in biomedical ethics.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103118108/-/DCSupplemental.
References
- 1.Reid RE. Peptide and protein drug analysis. New York: M. Dekker; 2000. pp. 1–885. [Google Scholar]
- 2.Dawson PE, Muir TW, Clark-Lewis I, Kent SB. Synthesis of proteins by native chemical ligation. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 3.Tam JP, Lu YA, Liu CF, Shao J. Peptide synthesis using unprotected peptides through orthogonal coupling methods. Proc Natl Acad Sci USA. 1995;92:12485–12489. doi: 10.1073/pnas.92.26.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tam JP, Yu QT. Methionine ligation strategy in the biomimetic synthesis of parathyroid hormones. Biopolymers. 1998;46:319–327. doi: 10.1002/(SICI)1097-0282(19981015)46:5<319::AID-BIP3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 5.Yan LZ, Dawson PE. Synthesis of peptides and proteins without cysteine residues by native chemical ligation combined with desulfurization. J Am Chem Soc. 2001;123:526–533. doi: 10.1021/ja003265m. [DOI] [PubMed] [Google Scholar]
- 6.Botti P, Tchertchian S. Side-chain extended ligation. 2006. Patent No. WO2006133962.
- 7.Crich D, Banerjee A. Native chemical ligation at phenylalanine. J Am Chem Soc. 2007;129:10064–10065. doi: 10.1021/ja072804l. [DOI] [PubMed] [Google Scholar]
- 8.Pentelute BL, Kent SB. Selective desulfurization of cysteine in the presence of Cys(Acm) in polypeptides obtained by native chemical ligation. Org Lett. 2007;9:687–690. doi: 10.1021/ol0630144. [DOI] [PubMed] [Google Scholar]
- 9.Wan Q, Danishefsky SJ. Free-radical-based, specific desulfurization of cysteine: a powerful advance in the synthesis of polypeptides and glycopolypeptides. Angewandte Chemie International Edition. 2007;46:9248–9252. doi: 10.1002/anie.200704195. [DOI] [PubMed] [Google Scholar]
- 10.Haase C, Rohde H, Seitz O. Native chemical ligation at valine. Angewandte Chemie International Edition. 2008;47:6807–6810. doi: 10.1002/anie.200801590. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Wan Q, Yuan Y, Zhu J, Danishefsky SJ. Native chemical ligation at valine: a contribution to peptide and glycopeptide synthesis. Angewandte Chemie International Edition. 2008;47:8521–8524. doi: 10.1002/anie.200803523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang R, Pasunooti KK, Li F, Liu XW, Liu CF. Dual native chemical ligation at lysine. J Am Chem Soc. 2009;131:13592–13593. doi: 10.1021/ja905491p. [DOI] [PubMed] [Google Scholar]
- 13.Kumar KSA, Haj-Yahya M, Olschewski D, Lashuel HA, Brik A. Highly efficient and chemoselective peptide ubiquitylation. Angewandte Chemie International Edition. 2009;48:8090–8094. doi: 10.1002/anie.200902936. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Wang P, Zhu JL, Wan Q, Danishefsky SJ. A program for ligation at threonine sites: application to the controlled total synthesis of glycopeptides. Tetrahedron. 2010;66:2277–2283. doi: 10.1016/j.tet.2010.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harpaz Z, Siman P, Kumar KS, Brik A. Protein synthesis assisted by native chemical ligation at leucine. Chembiochem. 2010;11:1232–1235. doi: 10.1002/cbic.201000168. [DOI] [PubMed] [Google Scholar]
- 16.Metanis N, Keinan E, Dawson PE. Traceless ligation of cysteine peptides using selective deselenization. Angewandte Chemie International Edition. 2010;49:7049–7053. doi: 10.1002/anie.201001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan Z, Shang S, Danishefsky SJ. Insights into the finer issues of native chemical ligation: an approach to cascade ligations. Angewandte Chemie International Edition. 2010;49:9500–9503. doi: 10.1002/anie.201005513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potts JT. Parathyroid hormone: past and present. J Endocrinol. 2005;187:311–325. doi: 10.1677/joe.1.06057. [DOI] [PubMed] [Google Scholar]
- 19.Potts JT, Gardella TJ. Progress, paradox, and potential: parathyroid hormone research over five decades. Ann NY Acad Sci. 2007;1117:196–208. doi: 10.1196/annals.1402.088. [DOI] [PubMed] [Google Scholar]
- 20.Talmage RV, Mobley HT. Calcium homeostasis: reassessment of the actions of parathyroid hormone. Gen Comp Endocrinol. 2008;156:1–8. doi: 10.1016/j.ygcen.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Dominguez LJ, Scalisi R, Barbagallo M. Therapeutic options in osteoporosis. Acta Biomedica. 2010;81:55–65. [PubMed] [Google Scholar]
- 22.Ellegaard M, Jorgensen NR, Schwarz P. Parathyroid hormone and bone healing. Calcif Tissue Int. 2010;87:1–13. doi: 10.1007/s00223-010-9360-5. [DOI] [PubMed] [Google Scholar]
- 23.Fraser WD. Hyperparathyroidism. Lancet. 2009;374:145–158. doi: 10.1016/S0140-6736(09)60507-9. [DOI] [PubMed] [Google Scholar]
- 24.Bieglmayer C, Prager G, Niederle B. Kinetic analyses of parathyroid hormone clearance as measured by three rapid immunoassays during parathyroidectomy. Clin Chem. 2002;48:1731–1738. [PubMed] [Google Scholar]
- 25.Abraham AK, et al. Mechanism-based pharmacokinetic/pharmacodynamic model of parathyroid hormone-calcium homeostasis in rats and humans. J Pharmacol Exp Ther. 2009;330:169–178. doi: 10.1124/jpet.109.152033. [DOI] [PubMed] [Google Scholar]
- 26.Potts JT, Jr, Gardella TJ, Juppner H, Kronenberg HM. Structure based design of parathyroid hormone analogs. J Endocrinol. 1997;154:S15–21. [PubMed] [Google Scholar]
- 27.Willick GE, Morley P, Whitfield JF. Constrained analogs of osteogenic peptides. Curr Med Chem. 2004;11:2867–2881. doi: 10.2174/0929867043364153. [DOI] [PubMed] [Google Scholar]
- 28.Voloshchuk N, Montclare JK. Incorporation of unnatural amino acids for synthetic biology. Mol Biosyst. 2010;6:65–80. doi: 10.1039/b909200p. [DOI] [PubMed] [Google Scholar]
- 29.Reissmann S, Imhof D. Development of conformationally restricted analogues of bradykinin and somatostatin using constrained amino acids and different types of cyclization. Curr Med Chem. 2004;11:2823–2844. doi: 10.2174/0929867043364135. [DOI] [PubMed] [Google Scholar]
- 30.Kimura T, Takai M, Masui Y, Morikawa T, Sakakibara S. Strategy for the synthesis of large peptides—an application to the total synthesis of Human Parathyroid-Hormone [hPTH(1–84)] Biopolymers. 1981;20:1823–1832. doi: 10.1002/bip.1981.360200907. [DOI] [PubMed] [Google Scholar]
- 31.Fairwell T, et al. Total solid-phase synthesis, purification, and characterization of Human Parathyroid Hormone-(1-84) Biochemistry. 1983;22:2691–2697. doi: 10.1021/bi00280a016. [DOI] [PubMed] [Google Scholar]
- 32.Goud NA, et al. Solid-phase synthesis and biologic activity of Human Parathyroid Hormone (1-84) J Bone Miner Res. 1991;6:781–789. doi: 10.1002/jbmr.5650060802. [DOI] [PubMed] [Google Scholar]
- 33.Fuentes G, Page K, Chantell CA, Patel H, Menakuru M. Fast conventional synthesis of Human Parathyroid Hormone 1–84. Chim Oggi. 2009;27:31–33. [Google Scholar]
- 34.Merrifield RB. Solid phase peptide synthesis. I. synthesis of a tetrapeptide. J Am Chem Soc. 1963;85:2149–2154. [Google Scholar]
- 35.Lloydwilliams P, Albericio F, Giralt E. Convergent solid-phase peptide-synthesis. Tetrahedron. 1993;49:11065–11133. [Google Scholar]
- 36.Sakakibara S. Synthesis of large peptides in solution. Biopolymers. 1995;37:17–28. doi: 10.1002/bip.360370105. [DOI] [PubMed] [Google Scholar]
- 37.Bang D, Pentelute BL, Kent SB. Kinetically controlled ligation for the convergent chemical synthesis of proteins. Angewandte Chemie International Edition. 2006;45:3985–3988. doi: 10.1002/anie.200600702. [DOI] [PubMed] [Google Scholar]
- 38.Johnson EC, Kent SB. Insights into the mechanism and catalysis of the native chemical ligation reaction. J Am Chem Soc. 2006;128:6640–6646. doi: 10.1021/ja058344i. [DOI] [PubMed] [Google Scholar]
- 39.Zull JE, Smith SK, Wiltshire R. Effect of methionine oxidation and deletion of amino-terminal residues on the conformation of parathyroid hormone: circular dichroism studies. J Biol Chem. 1990;265:5671–5676. [PubMed] [Google Scholar]
- 40.Alexander P, Orban J, Bryan P. Kinetic analysis of folding and unfolding the 56 amino acid IgG-binding domain of streptococcal protein G. Biochemistry. 1992;31:7243–7248. doi: 10.1021/bi00147a006. [DOI] [PubMed] [Google Scholar]
- 41.Engfeldt T, Renberg B, Brumer H, Nygren PA, Karlstrom AE. Chemical synthesis of triple-labeled three-helix bundle binding proteins for specific fluorescent detection of unlabelled protein. Chembiochem. 2005;6:1043–1050. doi: 10.1002/cbic.200400388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.