Abstract
Primary culture of the cancer cells from patients’ tumors can provide crucial information of individual tumors, yet the technology has not been optimized until now. We developed an innovative culture method for primary colorectal cancer cells, based on the principle that cell–cell contact of cancer cells was maintained throughout the process. When tumor tissue was dissociated into cell clusters, in which cell–cell contact was retained, they rapidly formed spheroids that we termed cancer tissue-originated spheroids (CTOSs). CTOSs of colorectal cancer consisted of highly purified and viable cancer cells, and they were prepared with high efficiency. In immunodeficient mice, CTOSs formed xenograft tumors that retained the features of the parental tumors. Moreover, CTOSs were able to be cultured and expanded in vitro using a 3D culture system and stem cell culture medium. This method allowed evaluation of chemosensitivity and signal pathway activation in cancer cells from individual patients. Easy preparation and culture of pure primary cancer cells provides an innovative platform for studying cancer biology and developing personalized medicine.
Keywords: colon, drug sensitivity
Cancer remains a leading cause of death in developed countries despite intensive research and recent progress in molecular targeted therapies. The diversity or heterogeneity of cancer presents an obstacle to the development of new therapies (1) and also makes it difficult to identify likely responders (2, 3). It is imperative to find biomarkers, which are characteristics that indicate normal biological or pathogenic processes, or pharmacological responses to a therapeutic intervention. Studying biological samples from patients provides crucial information to further elucidate the characteristics of individual cancer (2).
Most clinical cancer specimens used for assessing individual characteristics are denatured and fixed or frozen at sampling for further analysis. These samples represent a snapshot, a status of the cells at the time point when they were denatured. With these snapshot samples, it is difficult to examine the response of cancer cells to each intervention. For instance, in molecular targeting therapies, pathway activation and its inhibition by targeting molecules should be evaluated (2, 4). Although cell lines are suitable for investigating the response of cells to various stimuli with easy handling and reproducibility, cell lines acquire substantial bias and lose several characteristics of parental tumors during culture establishment and passage (5, 6). In contrast, cells in primary culture would better retain the properties of the original tumor (6, 7). However, the conditions for primary culture of cancer cells are currently not optimized. Primary culture can be laborious, with the potential for low cancer cell viability, contamination by host cells, and results that are difficult to reproduce (7). Thus, it is imperative to develop a method by which primary cancer cells can be cultured as feasibly and effectively as cell lines.
In a normal colon, cells at the villous tips undergo rapid apoptosis and shed into the lumen (8, 9). The cells in isolated crypts also undergo apoptosis (10). Thus, once detached from a matrix, epithelial cells undergo anoikis (11, 12), a particular type of apoptosis. Although resistance to anoikis is believed to be a hallmark of malignancy, most of the cancer cells in solid tumors, especially those of epithelial origin, depend on cell-matrix interactions for their survival (11, 12). It is reported that cell–cell contact rescues cancer cells (13, 14) and, partially, normal colonic epithelial cells (10) from anoikis.
Here, we report an innovative culture method for primary colorectal cancer cells, in which cell–cell contact of cancer cells is retained throughout the process. We observed that partially dissociated clusters of cells from colorectal cancer tissue rapidly formed spheroids that we termed cancer tissue-originated spheroids (CTOSs). By the CTOS method, highly purified and viable primary colorectal cancer cells were effectively prepared and cultured in vitro. CTOSs formed xenograft tumors that retained the features of the parental tumors. Easy preparation and culture of pure primary cancer cells will provide a unique preclinical model for personalized medicine.
Results
Preparation of Primary Cancer Cells from Tumor Specimens.
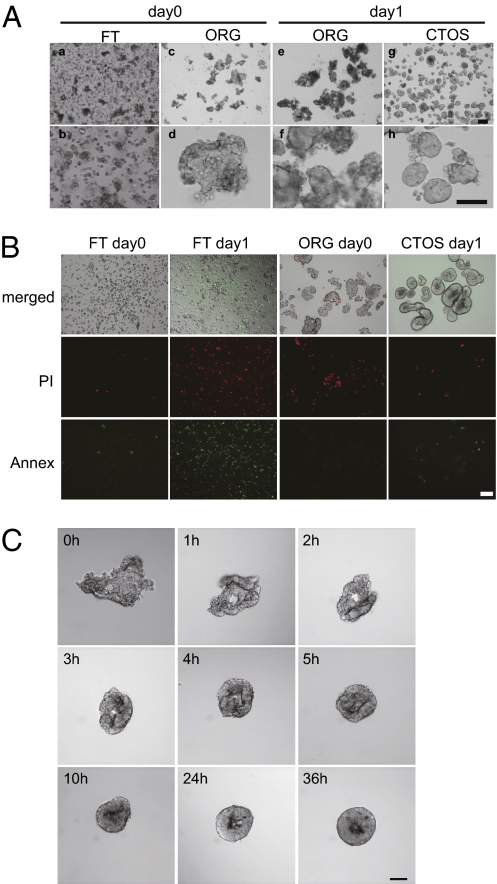
We hypothesized that if cell–cell contact is crucial for maintaining the viability of cancer cells, then these cells could best be isolated and cultured from patient samples if the cell–cell interactions were maintained throughout the process. To successfully maintain the cell–cell contact, we used Liberase DH (Roche Diagnostics) as a blend of digestion enzymes. The enzyme is used for the pancreas islet isolation in the practice of islet transplantation (15, 16). It is imperative to isolate islets as intact and functional masses for the success of transplantation. We optimized the enzyme concentration and the digestion time to avoid overdigestion of colorectal tissues. Cancer specimens from colorectal cancer patients were mechanically and enzymatically digested and separated into two fractions using a cell strainer: the organoid fraction (ORG), which was retained in the 40-μm strainer, and the flow-through fraction (FT), which passed through the strainer (Fig. 1A). The FT mainly contained single cells (Fig. 1A, a and b), and many of the cells in the FT with epithelial markers were dead, even immediately after dissociation (Fig. S1A). After overnight suspension culture, most of the epithelial cells in the FT were dead (Fig. 1B and Fig. S1A). In contrast, the ORG contained irregular sheet- or tube-like structures, which we termed organoids (Fig. 1A, c and d). After overnight culture, the organoids became spherical and bright with a smooth surface (Fig. 1A, g and h). We termed the spherical structures CTOSs. During formation, the CTOSs were draped with cellular debris (Fig. 1A, e and f) that easily detached with pipetting. The diameter of the CTOSs was ∼40–500 μm, depending on the cell strainer size. A CTOS with a diameter of 100 μm consisted of ∼100 cells. Although most of the cells in the FT fraction were single cells, we also observed tiny CTOSs, smaller than 40 μm in diameter, in the FT fraction. We were able to prepare CTOSs in most of the colorectal cancer specimens (Fig. S1B and Table 1), regardless of the disease stage and histology (Table S1). CTOSs were highly viable with only a few dead cells on the outside (Fig. 1B). TUNEL staining revealed a few apoptotic cells outside the CTOSs and, rarely, within the CTOSs (Fig. S1C). The spheroid structure was also prepared from normal colon mucosa, although the spheroids disrupted over time when cultured in the CTOS culture conditions (Fig. S1D). Next, the process of CTOS formation was monitored. Aliquots of the ORG fraction were observed over time in 96-well plates (Fig. 1C). The edge of a sheet-like organoid structure curled up within a few hours and formed a spherical CTOS within several hours.
Fig. 1.
Formation of CTOSs from colorectal cancer tissue. (A) Phase contrast images: a and b, flow-through (FT) fraction; c–f, organoid (ORG) fraction; g and h, CTOSs. (Scale bars: Upper, 200 μm; Lower, 100 μm.) (B) Dead cells in the FT, ORG, and CTOS preparations. PI (red) and annexin V-FITC staining (green) are indicated. (Upper) Merged bright-field images. (Scale bar: 100 μm.) (C) CTOS formation from a mC45 sample over time. (Scale bar: 100 μm.)
Table 1.
Success rate of CTOS formation, growth in ECM, and passage in vitro
| Formation (%) | Growth (%) | Passage (%) | |
| OP | 74/75 (98.7) | 30/38 (79.0) | 13/21 (61.9) |
| Biopsy | 29/32 (90.6) | 11/20 (55.0) | 7/9 (77.8) |
| Xeno | 10/10 (100.0) | 9/10 (90.0) | 4/5 (80.0) |
| Total | 113/117 (96.6) | 50/68 (73.5) | 24/35 (68.6) |
OP, surgically removed samples; biopsy, samples from endoscopic biopsy; Xeno, samples from the mouse tumor generated by direct transplantation of OP samples.
Sustained E-cadherin–Mediated Cell–Cell Interaction Allowed Cancer Cells to Survive.
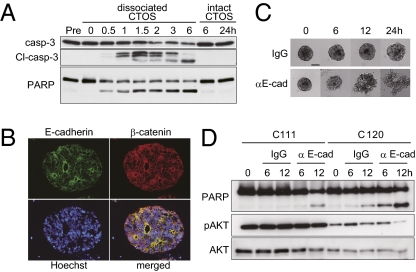
We next investigated whether cell–cell contact was necessary for survival of the CTOS-forming cells. Once CTOSs were dissociated into single cells, the amount of cleaved caspase-3 and poly(ADP-ribose) polymerase (PARP) increased over time in dissociated CTOS cells but not in nondissociated CTOS cells, even at longer culture periods (Fig. 2A). The viability of cells in CTOSs was maintained from 1 wk to several months, depending on the cases, under suspension culture conditions. In addition, when CTOSs were dissociated into single cells, the number of annexin-V single-positive cells and annexin-V and PI double-positive cells increased with time; in contrast, cells in nondissociated CTOSs remained negative (Fig. S2 A and B). The cell–cell contact through E-cadherin, accompanied by AKT activation, is crucial for the survival of epithelial cells (10, 17, 18). In CTOSs, E-cadherin expression was localized to the cell surface, which was lined by β-catenin (Fig. 2B). We investigated whether E-cadherin was necessary for maintenance of the CTOSs. When treated with a neutralizing antibody of E-cadherin, CTOS structure was destroyed over time, accompanied by massive cell death within 24 h (Fig. 2C and Fig. S2C). Western blotting analysis revealed cleavage of caspase-3 and PARP increased after treatment with the anti–E-cadherin antibody, although the sensitivity and the change in AKT phosphorylation were different between individual CTOSs (Fig. 2D and Fig. S2C). Thus, cell–cell contact mediated by E-cadherin was crucial for preparing and maintaining highly viable tumor cells in suspension culture.
Fig. 2.
Role of E-cadherin mediated cell–cell contact in the survival of tumor cells in CTOSs. (A) Immunoblot of apoptosis-related proteins after dissociation. CTOSs were dissociated into single cells (dissociated CTOS) or not dissociated (intact CTOS), and cultured for the indicated time. (B) Immunohistochemistry of C97 CTOSs. E-cadherin (green), β-catenin (red), and Hoechst 33342 (blue). (C) Phase contrast images of CTOSs treated with control IgG (Upper) or anti–E-cadherin neutralizing antibody (Lower) over time. (Scale bar, 100 μm.) (D) Immunoblot of apoptosis-related proteins from CTOSs treated with control IgG or anti–E-cadherin neutralizing antibody and cultured for the indicated periods.
CTOS Method Provides Clusters of Highly Purified Cancer Cells in High Yield.
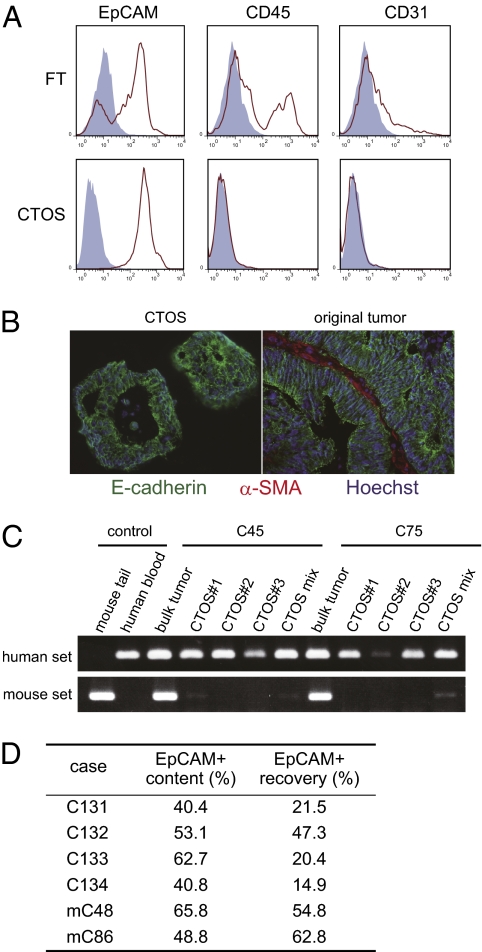
Cancer tissue contains heterogeneous cells, so we next evaluated the purity of the cells in the CTOSs. Flow cytometry analysis revealed FT cells contained a substantial number of CD45+ blood cells and CD31+ endothelial cells, in addition to EpCAM+ epithelial cells (Fig. 3A). In contrast, the cells in the CTOSs were almost entirely EpCAM+ cells (98.4 ± 0.4%; n = 5; Fig. 3A). The CTOSs, derived from the parental tumors with detectable E-cadherin expression, were subjected to immunohistochemical analysis. E-cadherin was localized to the cell membranes of all cells in CTOSs (Fig. 3B). However, α-SMA, a marker of activated fibroblasts, and CD68, a macrophage marker, were detected in the stroma of the original tumors but not at all in the CTOSs (Fig. 3B and Fig. S3A). To further evaluate the purity of CTOSs, PCR-based genome analysis was performed. CTOSs can form tumors in NOD/SCID mice as described below (Fig. 4A, Fig. S3B, and Table S2). From the same xenograft tumors, both bulk tumor-tissue fragment, which contains host cells of mouse origin, and the CTOSs were derived, and then genomic DNA were extracted from them. Using human- or mouse-specific primer sets, genomic analysis revealed that CTOSs contained only human cells, whereas the bulk xenograft tumors contained both human- and mouse-derived cells, indicating that the CTOS cells were exclusive of murine cells (Fig. 3C). Taken together, these findings indicate that CTOSs are composed of highly purified cells of epithelial linage.
Fig. 3.
High yield of purified cancer cells by the CTOS method. (A) Flow cytometric analysis of cell surface antigens (red line) or isotype controls (blue filled). (B) Immunohistochemistry of C93: E-cadherin (green), α-SMA (red), and Hoechst 33342 (blue). (C) PCR analysis of genomic DNA using human- or mouse-specific primers. CTOSs 1–3, isolated single CTOSs; CTOS mix, mixture of ∼1,000 CTOSs; bulk tumor, fragment of xenograft tumor. (D) Proportion of EpCAM+ cells. EpCAM+ content is ratio of the number of EpCAM+ cells to that of total cells in tumor specimens. EpCAM+ recovery is the ratio of the number of EpCAM+ cells in CTOSs to that of total EpCAM+ cells.
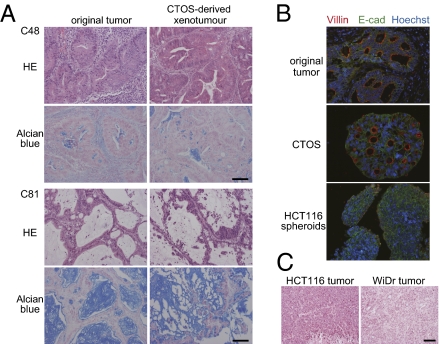
Fig. 4.
Characteristics of original tumors are preserved in CTOSs. (A) H&E staining and Alcian blue staining of tumors from the indicated patients (Left) and the derived xenotumor (Right). (B) Immunohistochemistry of the parental tumor (C45), a corresponding CTOS, and HCT116-derived spheroids. E-cadherin (green), villin (red), and Hoechst 33342 (blue). (C) H&E staining of xenograft tumors derived from indicated colorectal cancer cell lines. (Scale bar: 100 μm.)
Next we evaluated the recovery rate of CTOSs. The proportion of EpCAM+ cells in cancer tissues was 40.4–65.8% (51.9% on average), which is consistent with a previous report (19). The recovery rate of EpCAM+ cells in CTOSs was 14.9–62.8% (37.0% on average; Fig. 3D and Fig. S3C), indicating that CTOS formation is a common feature of colorectal cancers and is not likely to originate from a minor subpopulation. The expression of CD133, a putative marker of cancer stem cells, was not enriched in CTOSs (Fig. S3D). Taken together, these results indicate that the CTOS preparation method provided a high yield of purified cancer cells.
Characteristics of the Original Tumor Are Preserved in CTOSs.
Next we asked whether CTOSs had tumor-initiating capacity, using s.c. transplantation in immunodeficient mice. When 40- to 100-μm–diameter CTOSs (1 × 103 CTOSs, corresponding to ∼1 × 105 cells) from 12 patient tumors were transplanted into mice, all formed tumors in at least one injection site (Table S2). This indicated that CTOSs contain tumorigenic cancer cells. CTOS-derived tumors showed histological characteristics of adenocarcinoma, including mucus production, and also characteristics resembling the original tumors (Fig. 4 A and B). The villin expression was localized on the surface of every lumen in CTOSs (Fig. 4B). In contrast, tumors or spheroids derived from colon cancer cell lines had anaplastic features lacking glandular-like structures (Fig. 4 B and C), and those spheroids were also lacking villin expression (Fig. 4B). Next, we examined whether CTOS-derived xenograft tumors preserved the genetic characteristics of the parental tumors. About 50% of colorectal cancers have TP53 mutations (20, 21). Mutant TP53 proteins often evade degradation; thus, positive p53 staining indicates the presence of mutant p53 protein. The TP53 staining pattern of the parental tumors was preserved in the CTOSs (Fig. S4A). Thus, CTOSs had the molecular characteristics of the cancers from which they were derived. KRAS and BRAF mutations are common in colorectal cancer and are markers of resistance to EGFR-targeted therapy (22). We examined KRAS status in original tumors as well as in CTOSs (Fig. S4B). KRAS mutations were detected in 8 of 25 (32%) parental tumors, whereas in 20 of 25 (80%) cases, the KRAS status of the original tumor was the same as the corresponding CTOSs, and the type of mutation was the same if present. In four cases (16%), KRAS mutations were detected only in the CTOSs but not in the corresponding parental tumors, and in one case, the mutation was detected in the corresponding parental tumor but not in the CTOSs. The BRAF mutation was detected in 1 of 25 cases, and the same type of mutation was found in the corresponding CTOSs.
CTOSs Can Be Cultured and Expanded in Vitro.
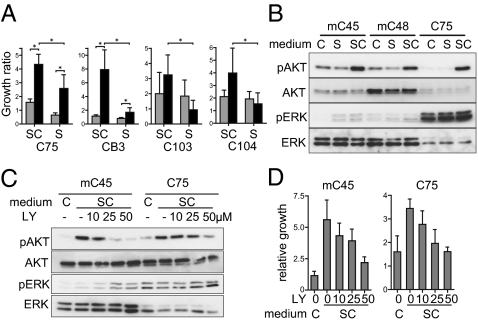
For optimization, various culture conditions were screened, and the best proliferation was accomplished with serum-free medium designed for embryonic stem cells. Extracellular matrix was also critical for favorable growth of CTOSs. A comparison of each culture condition is shown in Fig. 5A; CTOSs were cultured in stem cell medium (SC) or in conventional medium containing serum (S) using both suspension cultures and a 3D type I collagen-based culture system. The CTOSs in 3D culture grew best in SC (Fig. 5A). We applied the culture system to 68 cases, and the success rate for growth was 73.5% (Table 1). Immunohistochemical analysis of the growing CTOSs revealed that, as in the tumors, proliferating cells predominantly localized to the outer rim of the CTOSs (Fig. S5A).
Fig. 5.
CTOSs can be cultured and expanded in vitro. (A) Growth ratios of CTOSs from the indicated patients after 1 wk of culture in stem cell medium (SC) or conventional serum-containing medium (S). Suspension culture, gray bars; 3D culture, black bars; mean ± SD is shown for n = 8/group for C75 and CB3 and 12/group for C103 and C104. *P < 0.05. (B) Western blotting of AKT, ERK, and their phosphorylated forms for CTOSs cultured with SC or S. DMEM/F12 was used as control medium (indicated as C). (C) Western blotting of AKT, ERK, and their phosphorylated forms. The CTOSs cultured in SC were treated with LY294002 at the indicated doses. (D) Relative growth of CTOSs. CTOSs were treated with SC and LY294002 at the indicated doses. DMEM/F12 was used as control medium (indicated as C).
The survival and proliferation of cancer cells depend largely on activation of several signaling pathways, which is the basis of molecular targeted therapy (23–25). We further investigated the signal pathway that contributed to the advantage of SC over S. AKT and ERK, two major molecules for signal transduction that are crucial for survival and proliferation of cancer cells (23, 25), were evaluated. Western blotting analysis revealed that marked AKT phosphorylation was observed more with SC than S, whereas ERK phosphorylation did not change (Fig. 5B). We next evaluated whether the increase of AKT phosphorylation was related to the growth advantage of CTOS in SC. When AKT phosphorylation was inhibited by LY294002 (Fig. 5C), the growth of CTOSs was repressed in a dose-dependent manner (Fig. 5D). Thus, CTOS growth in vitro in stem cell culture depends, at least partially, on efficient AKT activation.
Next, we attempted to expand the primary cancer cells in vitro using CTOSs. In most of the CTOSs, the increase in size slowed down after about 14 d of culture, even if the medium was changed frequently. We found CTOSs can be passaged; when a CTOS was mechanically divided, each fragment formed a new CTOS in a short period and grew well in 3D culture. This could be repeated, allowing expansion of CTOSs in vitro (Fig. S5B). Because the success rate of CTOS passage was 68.6%, including the biopsy samples (Table 1), even a single CTOS will provide enough cancer cells for many analyses. The success rate was much higher than that of establishing cancer cell lines (11–38%; Table S3). The passage was able to be repeated in some CTOSs—up to 22 times for 19 mo in CB3, followed by 12 times for 12 mo in C75. The CTOSs, at passage 21 for CB3 and 10 for C75, formed xenograft tumors, which retained morphological characteristics of original tumors (Fig. S5C).
5-FU Sensitivity Assay with CTOSs from Colorectal Cancer.
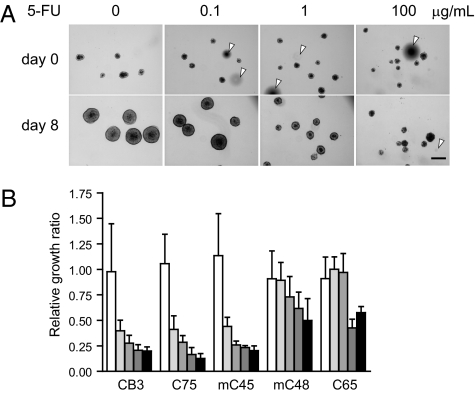
CTOSs are composed of highly purified and viable cancer cells, retain the characteristics of the parental tumors, and are stable and grow in vitro. Thus, CTOSs may be useful for personalized diagnostic applications, including chemosensitivity assays (26). When 5-FU, a key drug for treating colorectal cancer, was added to the culture medium, CTOS growth was inhibited dose dependently (Fig. 6A). Response of CTOSs to the drug differed in individual cases (Fig. 6B). In the CTOSs from three patients, growth of the CTOSs was significantly inhibited even at 0.1 μg/mL, whereas in two cases, 10 μg/mL 5-FU was not enough to inhibit growth at the same level (Fig. 6B), suggesting the potential of CTOSs for detecting individual chemosensitivity of colorectal cancer.
Fig. 6.
Response of CTOSs to a chemotherapeutic drug. (A) CTOSs of mC45 were treated as indicated. Arrowheads indicate air bubbles. (Scale bar: 100 μm.) (B) The sensitivity of CTOSs from five patients to 0.1, 0.5, 1.0, 5.0, and 10 μg/mL 5-FU (Left to Right). Values are relative growth ratios compared with 0.1% DMSO-treated control CTOSs; n = 10/group.
Discussion
In this study, we report a unique method for preparing highly purified viable colorectal cancer cells, from clinical specimens, with high yield and feasibility. Maintaining cell–cell contact during the preparation process is a key principle of the CTOS method. Cancer cells in CTOSs were highly viable even after prolonged culture, in contrast to the fragility of the single cells in the FT fraction. Once CTOSs were dissociated into single cells, massive apoptosis was observed within a short period. We showed that cell–cell contact, at least in part mediated by E-cadherin, contributed to the high viability of CTOS cells. The method was applicable to various types of cancer, including lung and bladder cancer. The success rate was 72.5% (58/80) and 70% (60/86), respectively.
When tumor specimens are digested into single cells, cancer cells are contaminated with other types of cells, such as fibroblasts, blood cells, and endothelial cells. In contrast, CTOSs are composed of pure epithelial cells with no detectable levels of nonepithelial lineage cells. There must be some cell-sorting process during CTOS formation. The purity is advantageous in biochemical studies. Mutations of KRAS and BRAF are highlighted in colorectal cancers because they are inversely correlated with the response to therapies targeting EGFR. At present, the most frequently used method to test for these mutations is direct sequencing of PCR products using formalin-fixed paraffin-embedded (FFPE) or fresh biopsy samples as templates (22, 27). This method has low sensitivity because, at least in part, the samples substantially contain normal cells. Therefore, alternative methods with higher sensitivity have been proposed, although they tend to be costly and cumbersome (27). We demonstrated here that direct sequencing using CTOSs provided higher sensitivity than using FFPE samples as templates, probably because CTOSs consisted of highly purified cancer cells. Therefore, CTOSs might provide a better template for mutation analysis.
The high rate of recovery of cancer cells from tumor tissue is another advantage of the CTOS method. The fraction of cancer cells recovered as CTOSs ranged from 14.9% to ∼62.8%. The recovery is advantageous for clinical applications from small biopsy samples. However, not all cancer cells were recovered. The loss of some cancer cells during the procedure might be a technical issue. Alternatively, the cells that failed to be included in CTOSs had different characteristics from CTOS-composing cells.
In this study, we attempted to optimize the growth conditions for CTOS cultures in vitro. Among the various culture media examined, a serum-free medium for ES cell culture provided the most favorable growth of CTOSs. We found the growth-promoting effect of StemPro was accomplished, at least in part, through significant activation of AKT. Additionally, we used type I collagen gel as a matrix, which is a major component of the stromal matrix in colorectal cancer (28). Nonetheless, the success rate of growth in culture was still 73.5%. We are investigating more appropriate conditions to increase the success rate of CTOS growth in culture. Growth factors and ECM might have to be individually optimized.
Tumorigenic capacity is a critical property of cancer cells and is discussed often in recent reports on cancer stem-like cells (29, 30). We showed here that CTOSs were able to form tumors in NOD/SCID mice, indicating that CTOSs contained tumor-initiating cells. In the stem cell theory, it is assumed that a single cell exists that is capable of forming tissue or tumor (29, 30). We demonstrated here that most of the cells from tumor tissue that dissociated into single cells died through apoptosis when they were cultured in suspension. The tumor-initiating cells might be the cells that are able to survive through single-cell status. Alternatively, because the cells were stable in CTOSs, the tumor-initiating cells are not necessarily single cells but rather a multicellular unit. The integrity of the CTOS might provide a niche for a subfraction of the cancer cells, supporting cancer stem-like cells. The inability of culturing spheroids from normal colon epithelium by the CTOS method might indicate that CTOS is the functionally autonomous unit. It is unclear whether CTOSs prepared from a biopsy specimen are genetically and functionally homogenous. Genetically, a KRAS mutation detected in the FFPE samples from the original tumor was not detected in the CTOSs in one exceptional case of the 25 matched cases examined. Although this occurred at a low frequency, it is possible that the original tumor was heterogeneous in terms of the KRAS mutation, and cells with wild-type KRAS were selected during the CTOS preparation in that particular case. E-cadherin expression is often altered in colorectal cancer (31–33). During CTOS preparation, a subpopulation of cells with high levels of E-cadherin expression might be enriched, whereas cells with reduced expression of E-cadherin in the tumor budding at the invasive front might be lost, but only when the change in E-cadherin expression levels is irreversible.
There are some reported spheroid structures that can be compared with CTOSs: the cell line-derived multicellular spheroid (MCS) (5, 26, 34), colosphere (35), and stem cell-originated spheroid (30, 36). The MCS is reported to recapitulate, in part, the characteristics of in vivo cancer compared with cultured cells on a dish (5, 26, 34). Cell lines are irreversibly changed from original tumors (5, 6). The histological characteristics of the originated tumors are preserved in xenograft tumors derived from CTOSs, which is not the case in xenograft tumors derived from cell lines. Recently, Weiswald et al. (35) reported that formation of a primary MSC was observed in colorectal cancer specimens, which was termed a colosphere. Although colospheres and CTOSs are morphologically similar, their correlation is not clear, as it was not elucidated whether the structure is generated from cell aggregation or from tumor fragments in the colosphere. In addition, the frequency of observing a colosphere is quite low compared with a CTOS: colospheres were obtained in only 47% of the tumors, whereas CTOSs were obtained in 98% of samples, including endoscopic biopsy samples, with a higher yield. Mammary organoids have been used for preparation of primary breast cancer cells, which are also generated from tissue fragments (37). Heterogenous components in mammary organoids are different from colorectal cancer CTOS (38, 39). The study of breast cancer CTOS will clarify the relation between CTOS and mammary organoids.
One of the characteristics of cancer stem cells (CSC) is the capacity to form spheroids in culture (30, 36). By definition, these spheroids must be derived from a single CSC. The CSC-derived spheroid formation was observed in low frequency with enrichment of CD133+ cells (30, 36). CTOS has markedly different characteristics from CSC-derived spheroid: (i) CTOS was derived from cell clusters but not from a single cell (Fig. 1); (ii) the levels of CD133 expression were similar in the FT and CTOSs, suggesting that CD133-expressing cells were not enriched in the CTOSs (Fig. S3D); (iii) high rate of recovery of EpCAM+ cells in CTOS indicates that CTOS did not originate from a minor subpopulation (Fig. 3D); and (iv) CTOS was tumorigenic, but a substantial number of cancer cells in CTOSs was required in general to generate tumors in NOD/SCID mice (Table S2). Therefore, CTOS might be a mixture of CSC and non-CSC in similar representation to the frequency of CSCs in the original tumor.
Personalized medicine is an emerging issue in cancer treatment. CTOSs might be useful for evaluating the response of cancer cells to various stimuli. The sensitivity assay in personalized medicine must be performed feasibly and reproducibly and reflect the clinical response of the patients. CTOSs might be useful for these necessary assays. As mentioned previously, CTOSs can be feasibly prepared with high yield and are stable in culture, and assays can be repeated. Furthermore, original cancer cell characteristics are preserved in CTOSs. We demonstrated here that CTOSs can be applied to chemosensitivity assays using a conventional key chemotherapy drug, 5-FU. The responses of CTOSs to 5-FU differed in individual cases, although clinical relevance must be studied in the future.
Evaluating pathway activation in CTOSs is a promising way to find responders (2, 4). Recently, various molecular targeting drugs emerged as clinical therapeutics. Molecular targeting drugs are directed at specific signaling pathways; therefore, assessing the changes in pathway activation by treating CTOSs with the drug will provide useful information. A prediction of the responders to each molecular targeting drug is imperative not only for the benefit of individual patients, but also for reducing the spiraling cost of those drugs. As an example, we showed here that inhibition of the PI3K/AKT pathway by a PI3K inhibitor resulted in inhibition of pathway activation, followed by inhibition of CTOS growth. CTOSs might be useful for other sensitivity assays, such as for radiation and photodynamic therapy. Studies assessing if these assays reflect the patient's response to the therapy are underway.
Materials and Methods
Tumor Cell Preparation.
A total of 117 colorectal cancer samples were obtained from 107 patients at the Osaka Medical Center for Cancer and Cardiovascular Diseases (Table S1). The study protocol was approved by the local ethics committee. The detailed method for CTOS preparation and expansion is described in SI Materials and Methods and Fig. S6. Briefly, tumor specimens were minced, washed, and digested with Liberase DH solution (Roche). The partially digested tissue was filtered through cell strainer (Becton Dickinson), and the tumor tissue retained in the strainer was collected to stem cell medium. CTOSs were cultured in suspension in stem cell medium. For 3D culture, the CTOSs were embedded in Cellmatrix type I-A (Nitta Gelatin) droplets with stem cell medium.
Animal Studies.
Animal studies were performed in compliance with the guidelines of our institutional animal studies committee. For primary colon cancer transplantation, tissue specimens were implanted s.c. in the flanks of NOD/SCID mice (Charles River). For implantation, CTOSs were injected s.c. in the flanks of the mice. The CTOSs prepared from mouse xenografts, which were generated by transplantations of primary tumor pieces, were designated with prefix of “m” in the figures and the legends.
Cell–Cell Contact Inhibition.
CTOSs were suspended in fresh medium containing anti E-cadherin neutralizing antibody (Takara) or mouse IgG isotype control (Sigma), collected at the indicated time points, and analyzed by Western blotting.
Inhibition of the PI3K/AKT Signaling Pathway.
To evaluate the influence of PI3K inhibition on growth, CTOSs were cultured in the presence or absence of LY294002 (Calbiochem) at the indicated concentrations. After 7 d of cultivation, growth ratios were calculated.
Statistical Analysis.
Data are expressed as mean ± SD. Statistical analysis was performed using Student's t test. Differences were considered significant at P < 0.05.
Detailed information can be found in SI Materials and Methods, including information on genomic analysis, flow cytometry, apoptosis detection, and immunohistochemistry.
Supplementary Material
Acknowledgments
We thank Drs. S. Noura and R. Ishihara for preparing clinical samples; J. Joyce for critical reading of the manuscript; T. Takeda and T. Egawa-Takata for helpful discussions; and M. Ito and Y. Yoshida for technical assistance. This work was supported in part by the Japan Advanced Molecular Imaging Program (J-AMP) of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
Conflict of interest statement: This study was partly supported by the REI Medical Corporation.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015938108/-/DCSupplemental.
References
- 1.Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer. 2006;6:813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 2.Bernards R. It's diagnostics, stupid. Cell. 2010;141:13–17. doi: 10.1016/j.cell.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Samson DJ, Seidenfeld J, Ziegler K, Aronson N. Chemotherapy sensitivity and resistance assays: A systematic review. J Clin Oncol. 2004;22:3618–3630. doi: 10.1200/JCO.2004.04.077. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida T, et al. Matuzumab and cetuximab activate the epidermal growth factor receptor but fail to trigger downstream signaling by Akt or Erk. Int J Cancer. 2008;122:1530–1538. doi: 10.1002/ijc.23253. [DOI] [PubMed] [Google Scholar]
- 5.Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro—a growing case for three-dimensional (3D) culture systems. Semin Cancer Biol. 2005;15:405–412. doi: 10.1016/j.semcancer.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Ochs RL, et al. Evidence for the isolation, growth, and characterization of malignant cells in primary cultures of human tumors. In Vitro Cell Dev Biol Anim. 2003;39:63–70. doi: 10.1290/1543-706X(2003)039<0063:EFTIGA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: The importance of apoptosis. J Cell Sci. 1994;107:3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 9.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofmann C, et al. Cell-cell contacts prevent anoikis in primary human colonic epithelial cells. Gastroenterology. 2007;132:587–600. doi: 10.1053/j.gastro.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiarugi P, Giannoni E. Anoikis: A necessary death program for anchorage-dependent cells. Biochem Pharmacol. 2008;76:1352–1364. doi: 10.1016/j.bcp.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Kang HG, et al. E-cadherin cell-cell adhesion in Ewing tumor cells mediates suppression of anoikis through activation of the ErbB4 tyrosine kinase. Cancer Res. 2007;67:3094–3105. doi: 10.1158/0008-5472.CAN-06-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantak SS, Kramer RH. E-cadherin regulates anchorage-independent growth and survival in oral squamous cell carcinoma cells. J Biol Chem. 1998;273:16953–16961. doi: 10.1074/jbc.273.27.16953. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro AM, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 16.Langer RM. Islet transplantation: Lessons learned since the Edmonton breakthrough. Transplant Proc. 2010;42:1421–1424. doi: 10.1016/j.transproceed.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Pece S, Chiariello M, Murga C, Gutkind JS. Activation of the protein kinase Akt/PKB by the formation of E-cadherin-mediated cell-cell junctions. Evidence for the association of phosphatidylinositol 3-kinase with the E-cadherin adhesion complex. J Biol Chem. 1999;274:19347–19351. doi: 10.1074/jbc.274.27.19347. [DOI] [PubMed] [Google Scholar]
- 18.De Santis G, Miotti S, Mazzi M, Canevari S, Tomassetti A. E-cadherin directly contributes to PI3K/AKT activation by engaging the PI3K-p85 regulatory subunit to adherens junctions of ovarian carcinoma cells. Oncogene. 2009;28:1206–1217. doi: 10.1038/onc.2008.470. [DOI] [PubMed] [Google Scholar]
- 19.West NP, et al. The proportion of tumour cells is an independent predictor for survival in colorectal cancer patients. Br J Cancer. 2010;102:1519–1523. doi: 10.1038/sj.bjc.6605674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohue M, et al. A frequent alteration of p53 gene in carcinoma in adenoma of colon. Cancer Res. 1994;54:4798–4804. [PubMed] [Google Scholar]
- 21.Iacopetta B. TP53 mutation in colorectal cancer. Hum Mutat. 2003;21:271–276. doi: 10.1002/humu.10175. [DOI] [PubMed] [Google Scholar]
- 22.Allegra CJ, et al. American Society of Clinical Oncology provisional clinical opinion: Testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091–2096. doi: 10.1200/JCO.2009.21.9170. [DOI] [PubMed] [Google Scholar]
- 23.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 24.Lawlor MA, Alessi DR. PKB/Akt: A key mediator of cell proliferation, survival and insulin responses? J Cell Sci. 2001;114:2903–2910. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- 25.Fang JY, Richardson BC. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005;6:322–327. doi: 10.1016/S1470-2045(05)70168-6. [DOI] [PubMed] [Google Scholar]
- 26.Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA. Spheroid-based drug screen: Considerations and practical approach. Nat Protoc. 2009;4:309–324. doi: 10.1038/nprot.2008.226. [DOI] [PubMed] [Google Scholar]
- 27.Soulières D, et al. KRAS mutation testing in the treatment of metastatic colorectal cancer with anti-EGFR therapies. Curr Oncol. 2010;17(Suppl 1):S31–S40. doi: 10.3747/co.v17is1.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hilska M, et al. The distribution of collagen types I, III, and IV in normal and malignant colorectal mucosa. Eur J Surg. 1998;164:457–464. doi: 10.1080/110241598750004274. [DOI] [PubMed] [Google Scholar]
- 29.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 30.Ricci-Vitiani L, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 31.Tsanou E, Peschos D, Batistatou A, Charalabopoulos A, Charalabopoulos K. The E-cadherin adhesion molecule and colorectal cancer. A global literature approach. Anticancer Res. 2008;28(6A):3815–3826. [PubMed] [Google Scholar]
- 32.Hugh TJ, et al. Cadherin-catenin expression in primary colorectal cancer: A survival analysis. Br J Cancer. 1999;80:1046–1051. doi: 10.1038/sj.bjc.6690461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ilyas M, et al. Tumour recurrence is associated with Jass grouping but not with differences in E-cadherin expression in moderately differentiated Dukes’ B colorectal cancers. J Clin Pathol. 1997;50:218–222. doi: 10.1136/jcp.50.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mueller-Klieser W. Three-dimensional cell cultures: From molecular mechanisms to clinical applications. Am J Physiol. 1997;273:C1109–C1123. doi: 10.1152/ajpcell.1997.273.4.C1109. [DOI] [PubMed] [Google Scholar]
- 35.Weiswald LB, et al. Newly characterised ex vivo colospheres as a three-dimensional colon cancer cell model of tumour aggressiveness. Br J Cancer. 2009;101:473–482. doi: 10.1038/sj.bjc.6605173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vermeulen L, et al. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci USA. 2008;105:13427–13432. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burdall SE, Hanby AM, Lansdown MR, Speirs V. Breast cancer cell lines: Friend or foe? Breast Cancer Res. 2003;5:89–95. doi: 10.1186/bcr577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gusterson BA, et al. Distribution of myoepithelial cells and basement membrane proteins in the normal breast and in benign and malignant breast diseases. Cancer Res. 1982;42:4763–4770. [PubMed] [Google Scholar]
- 39.Haslam SZ, Drolet A, Smith K, Tan M, Aupperlee M. Progestin-regulated luminal cell and myoepithelial cell-specific responses in mammary organoid culture. Endocrinology. 2008;149:2098–2107. doi: 10.1210/en.2007-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.