Abstract
Myrmaeciella caraganae was recollected in and around Vienna, Austria and found to be morphologically different generically from the type species of Myrmaeciella, M. endoleuca. We redescribe M. caraganae in the new genus Stromatonectria. Phylogenetic analyses of LSU sequences place the genus in the Bionectriaceae, Hypocreales. S. caraganae occurs on branches of Caragana spp., Colutea arborescens and Laburnum anagyroides of the Fabaceae. It is characterized by spheroid perithecia partly or entirely immersed in a Hypocrea-like stroma, a Nectria-like centrum and bicellular hyaline ascospores. Conidia of S. caraganae are produced in compound pycnidia that are formed prior to or in association with perithecia. Sporodochia but no pycnidia are formed in culture. We discuss the genus Myrmaeciella and compare S. caraganae with species of the Nectriaceae, including Nectria balansae, N. eustromatica and N. paraguayensis.
Keywords: Ascomycetes, Bionectria, Hypocrea, Hypocreopsis, ITS, LSU, morphology, Nectria, Patellina, phylogenetic analysis, rpb2, tef1, Valsonectria
INTRODUCTION
Most Hypocreales with Hypocrea-like stromata (Rossman et al. 1999, Jaklitsch 2009) are placed in the Hypocreaceae, but some fungi with similar stromata belong to different families of the order. Examples are Nectria balansae Speg. (Samuels and Brayford 1994), N. eustromatica Jaklitsch & Voglmayr (2011), or N. paraguayensis Speg. (Samuels and Brayford 1994). Another fungus forming Hypocrea-like stromata with a nectriaceous centrum was collected recently in and around Vienna on branches of Caragana, Colutea and Laburnum, Fabaceae. Höhnel (1905) originally described this fungus as Myrmaeciella caraganae Höhn., based on material collected in the Botanical Garden of Vienna. Myrmaeciella Lindau was established as a replacement name for Myrmaecium Sacc. (1880), a later homonym of Myrmaecium Nitschke ex Fuckel (1870), which is a synonym of Valsaria Ces. & de Not. The type species of Myrmaeciella is M. endoleuca (Sacc.) Lindau. Müller & von Arx (1962), and Rogerson (1970) treated Myrmaeciella as a synonym of Hypocreopsis P. Karst. Rossman et al. (1999) argued against this synonymy due to the extensive, brightly colored, KOH+ stromata of Hypocreopsis that occur on other fungi. They suggested that Myrmaeciella is allied with the Niessliaceae, based on dark but soft-textured stromata, deliquescing paraphyses, unitunicate asci without amyloid apical ring and hyaline, equally one-septate ascospores.
Höhnel (1905) combined two other fungi, Hypocrea euphorbiae Pat. and Hypocreopsis moriformis Starbäck in Myrmaeciella, as M. euphorbiae (Pat.) Höhn. and M. moriformis (Starbäck) Höhn. With M. höhneliana Rick the genus eventually contained five species. In his description of M. caraganae (Höhnel 1905, p 54), Höhnel recognized the absence of paraphyses, but in his key to the genera of the Hypocreaceae (1905, p 97) he listed the presence of paraphyses as an important criterion of the genus. Later Höhnel (Höhnel and Weese 1910, Höhnel 1912, p 379) recognized synonymy of Myrmaeciella moriformis with Nectria paraguayensis and transferred this taxon and M. caraganae to Endothia Fr., a genus that he classified within the Hypocreaceae. He also treated Valsonectria Speg. as a synonym of Endothia (Höhnel 1905, p 55; 1909, p 19). Samuels and Brayford (1994, p 132) confirmed synonymy of H. moriformis and N. paraguayensis, a fungus occurring on bark of Celtis in temperate South America, but they accepted it as a species of Nectria. This paper serves to redescribe M. caraganae, to clarify its taxonomic and phylogenetic position and to elucidate relationships to similar fungi.
MATERIALS AND METHODS
Isolates and specimens
Representative isolates have been deposited at the Centraalbureau voor Schimmelcultures, Utrecht, the Netherlands (CBS). We deposited specimens in the Herbarium of the Institute of Botany, University of Vienna (WU).
Ascospore isolates were prepared as described by Jaklitsch (2009). Cultures were grown in 9 cm diam Petri dishes with alternating 12 h cool white fluorescent light and 12 h darkness at 25 C on 2% malt extract agar (MEA), potato dextrose agar (PDA) and cornmeal dextrose agar (CMD, Jaklitsch 2009).
Morphological observations
Conidiation structures were examined, measured and photographed on a compound microscope from cultures grown 5 d and 5 mo at room temperature in daylight on MEA after mounting in 3% KOH. Dry stromata were rehydrated overnight with water vapor in a closed glass chamber at room temperature, treated briefly with 3% KOH, embedded in Tissue-Tek O.C.T. Compound 4583 (Sakura Finetek Europe B.V., Zoeterwoude, the Netherlands) and sectioned (15–20 μm thick) with a freezing microtome. Sections were measured and photographed in lactic acid or in 50% glycerol or 3% KOH where noted. We measured asci and ascospores in separate preparations in 3% KOH. (See Jaklitsch [2009] for the terminology of stromata traits.) Measurements were reported as maxima and minima in parentheses and the mean plus and minus the standard deviation of a number of measurements given in parentheses. Nomarski differential interference contrast (DIC) was used for observations and measurements. Images were recorded with the Nikon Coolpix 4500, DS-U2 or Zeiss AxioCam ICc3 digital cameras. Measurements were made with NIS-Elements D 3.0 software.
DNA extraction, PCR amplifications and sequencing
Mycelium for DNA extraction was grown in liquid malt extract culture, harvested, freeze dried and ground according to Voglmayr and Jaklitsch (2008). We extracted genomic DNA with the modified CTAB method described in Riethmüller et al. (2002). A 1.6 kb fragment containing partial SSU, ITS1, 5.8S, ITS2 and partial LSU was amplified with the primer pair V9G (de Hoog and Gerrits van den Ende 1998) and LR5 (Vilgalys and Hester 1990). A 1.1 kb fragment of RNA polymerase II subunit B (rpb2) was amplified with the primer pair fRPB2-5f and fRPB2-7cr (Liu et al. 1999). A 1.3 kb fragment of the tef1 gene encoding translation elongation factor 1 alpha was amplified with the primer pair EF1728F (Chaverri and Samuels 2003) and TEF1LLErev (Jaklitsch et al. 2005). This fragment includes the fourth and the fifth intron and a part of the last large exon. A 1 kb fragment of the β-tub gene was amplified with primers T1 and T222 (O’Donnell and Cigelnik 1997). We purified PCR products with an enzymatic PCR cleanup (Werle et al. 1994) as described in Voglmayr and Jaklitsch (2008). DNA was cycle sequenced with the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit 3.1 (Applied Biosystems, Warrington, UK) and an automated DNA sequencer (ABI 3730xl Genetic Analyzer, Applied Biosystems) with the same primers as in PCR; for the SSU-ITS-LSU fragment primers LR3 (Vilgalys and Hester 1990), ITS4 (White et al. 1990), F5.8Sf and F5.8Sr (Jaklitsch and Voglmayr 2011) were used as internal sequencing primers, and for β-tub primer Bt2b (Glass and Donaldson 1995) was used as internal primer instead of T1. Sequences generated in this study are in the specimen information.
Molecular phylogenetic analyses
Due to the poor representation of sequences of ITS, rpb2 tef1 and β-tub of Bionectriaceae in GenBank, phylogenetic analyses were performed only on LSU rDNA; the other sequence regions are published here to provide markers for molecular identification. We selected representative LSU sequences of Bionectriaceae and Nectriaceae from GenBank according to a BLAST query that revealed a high sequence homology of LSU sequences of Myrmaeciella caraganae to the Bionectriaceae. The final matrix contained 48 sequences from 47 taxa, including 25 sequences from Nectriaceae, which we chose as outgroup to root the trees. The GenBank accession numbers of sequences downloaded for phylogenetic analyses are provided (Fig. 1) following the taxon labels. We produced all alignments with MUSCLE 3.6 (Edgar 2004). Maximum parsimony (MP) analyses were performed with PAUP* 4.0 b10 (Swofford 2002) with 1000 replicates of heuristic search with random addition of sequences and subsequent tbr branch swapping (multrees inoption in effect, collapse = maxbrlen, steepest descent option not in effect). All molecular characters were unordered and given equal weight. Analyses were performed with gaps treated as missing data. Bootstrap analysis with 1000 replicates was performed in the same way but with 10 rounds of random sequence addition and subsequent tbr branch swapping during each bootstrap replicate. For maximum likelihood (ML) and Bayesian analyses, first the appropriate model of sequence substitution was selected with Modeltest 3.6 (Posada and Crandall 1998) using the Akaike information criterion (AIC). The model of Tamura and Nei (1993) was selected, additionally assuming a proportion of invariant sites with gamma-distributed substitution rates of the remaining sites (TRN+I+G). Because this model could not be implemented in ML and Bayesian analyses the related general time reversible model was used, additionally assuming a proportion of invariant sites with gamma-distributed substitution rates of the remaining sites (GTR+I+G). For ML analyses 200 bootstrap replicates were computed with RAxML 7.0.4 (Stamatakis 2006) with GTRCAT algorithms. Bayesian analyses were performed with MrBayes 3.1.2 (Huelsenbeck and Ronquist 2001), implementing the GTR+I+G model. Three parallel runs of four incrementally heated simultaneous Markov chains were performed for 10 000 000 generations from which every 100th tree was sampled in each run. The first 2000 trees were discarded, and we computed a 90% majority rule consensus of the remaining trees to obtain estimates for the probabilities that groups are monophyletic given the sequence data (posterior probabilities). The LSU sequence alignment file was deposited in TreeBASE, which is available at http://purl.org/phylo/treebase/phylows/study/TB2:S10772 .
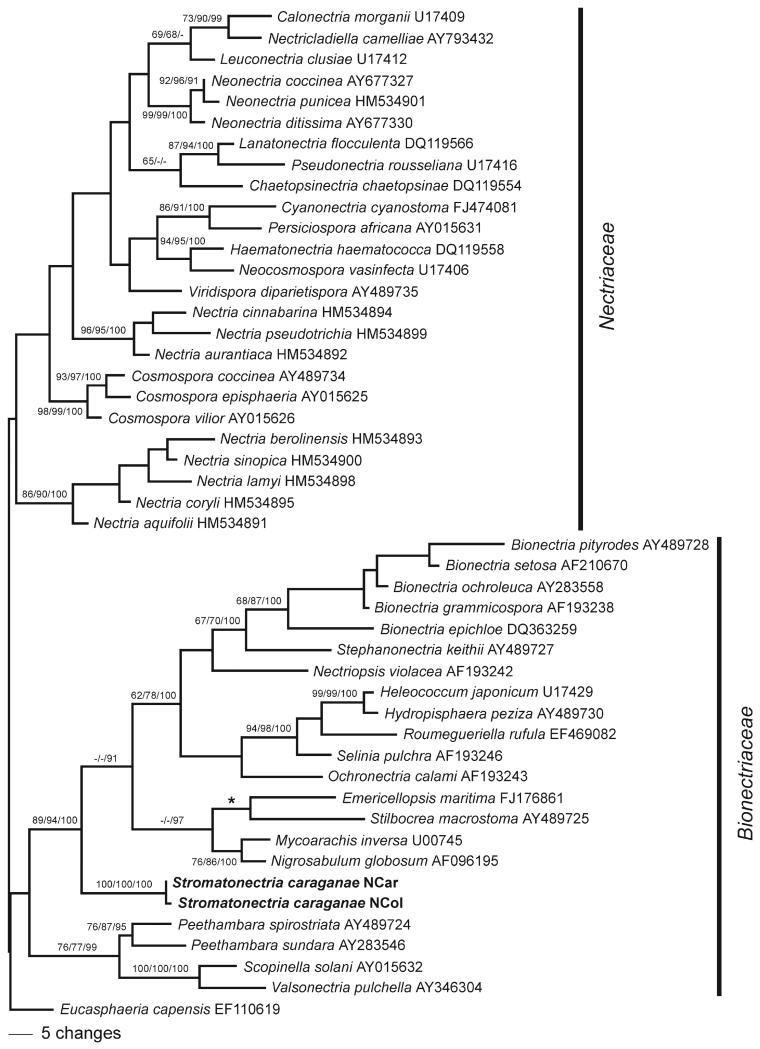
Fig. 1.
Phylogram of one of four most parsimonious trees 703 steps long revealed by PAUP from an analysis of the LSU matrix of selected Hypocreales, showing the phylogenetic position of Stromatonectria caraganae within the Bionectriaceae. MP bootstrap support above 60%, ML bootstrap support above 60% and Bayesian posterior probabilities above 90% are respectively at first, second and third position above or below the branches. The asterisk denotes a topological difference of the MP tree from ML and Bayesian trees, where Stilbocrea macrostoma is sister group of the other three taxa of the clade.
RESULTS
Molecular phylogenetic analyses
The LSU matrix contained 860 characters, of which 132 were parsimony informative. MP analysis revealed four most parsimonious trees 703 steps long, one of which is shown (Fig. 1). The four MP trees had identical topologies except within the clade containing Nectria berolinensis, N. lamyi and N. sinopica and a slightly different position of Ochronectria calami. The ML bootstrap and Bayesian trees were fully compatible with the MP trees, except for a minor topological difference concerning Stilbocrea macrostoma, which is sister taxon of a clade containing Emericellopsis, Mycoarachis and Nigrosabulum in ML and Bayesian analyses. MP and ML bootstrap values above 60% and Bayesian posterior probabilities above 90% are provided (Fig. 1).
Tree topology agreed well with the current concept of Bionectriaceae and Nectriaceae. As commonly observed in LSU phylogenies internal support was absent for deepest nodes of the tree backbone and neither Bionectriaceae nor Nectriaceae received significant support. However Myrmaeciella caraganae, combined below in the new genus Stromatonectria, was placed consistently within Bionectriaceae in a highly supported core clade, where it occupies a basal position (Fig. 1).
TAXONOMY
Stromatonectria Jaklitsch & Voglmayr, gen. nov.
MycoBank MB518755
Genus novum Bionectriacearum. Stromata pulvinata vel turbinata, flava, aurantiaca vel rubra ad purpurea, erumpentia ex cortice. Perithecia immersa in strato supremo stromatum vel superficialia, sphaeroidea, ostiolata, mollia, pallida. Hamathecium periphysibus. Asci octospori, fusoidei vel clavati, unitunicati, sine apparatu apicale. Ascosporae biseriatae, ellipsoideae, oblongae vel fusoideae, bicellulares, hyalinae ad roseae vel luteae. Pycnidia formantia in superficie stromatum, multiloculata, conidibus unicellularibus, allantoideis.
Typus generis
Stromatonectria caraganae (Höhn.) Jaklitsch & Voglmayr.
Etymology
Descriptive name for a Nectria with a true stroma.
A genus of the Bionectriaceae. Stromata pulvinate, varying from yellow over orange to red or purple, erumpent from bark. Perithecia immersed or superficial, spheroid, ostiolate, light and soft-textured. Hamathecium consisting exclusively of periphyses. Asci eight-spored, fusoid or clavate, unitunicate, lacking an apical apparatus. Ascospores biseriate, ellipsoid, oblong or fusoid, bicellular, hyaline, yellowish or rosy. Pycnidia formed on the stroma surface, multilocular, with unicellular allantoid conidia.
Stromatonectria caraganae (Höhn.) Jaklitsch & Voglmayr, comb. nov. Figs. 2, 3
-
≡
Myrmaeciella caraganae Höhn.,Österr. Bot. Z. 55:53 (1905)
-
≡
Endothia caraganae (Höhn.) Höhn., S.ber. Akad. Wiss. Wien, math. nat. Kl., I 121:380 (1912)
-
≡
Cryphonectria caraganae (Höhn.) Sacc., Syll. fung. (Abellini) 17:784 (1905)
Fig. 2.
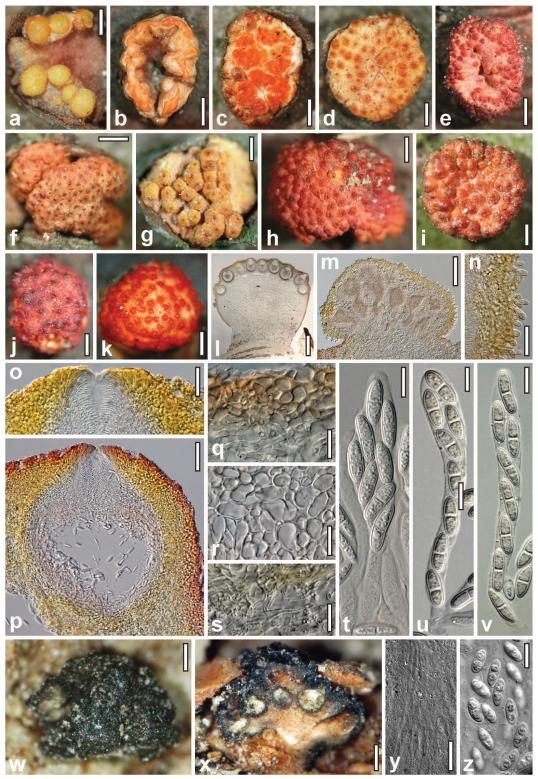
Teleomorph and conidiomata of Stromatonectria caraganae. a–c. Conidiomata (b. partly open, in ring-like configuration. c. open). d, f. Immature perithecial stromata. e, g–j. Dry perithecial stromata (e,g. with free perithecia. j. with entirely immersed perithecia). k. Rehydrated stroma in 3% KOH. l. Perithecial stroma in section. m. Closed conidioma in section. n. Conidiomatal wall with surface hairs. o. Ostiole in section. p. Perithecium in section. q. Cortical and subcortical tissue in section. r. Subperithecial tissue in section. s. Stroma base in section. t–v. Asci and ascospores. w–z. Myrmaeciella endoleuca. w. Dry stroma. x. Vertically cut stroma. y. Paraphyses exceeding immature asci. z. Ascospores. Sources: a, b, e, h, j, k–s, u, v. WU 30197; c, g, i, t. WU 30199; d, f. WU 30196. w–z. holotype from PAD. Bars: a, d, g, i, j, l = 0.4 mm; b, c, f, h, k = 0.6 mm; e = 1 mm; m = 0.15 mm; n, s, y = 20 μm; o = 30 μm; p = 50 μm; q, r = 15 μm; t–v, z = 10 μm; w = 0.1 mm; x = 0.2 mm.
Fig. 3.
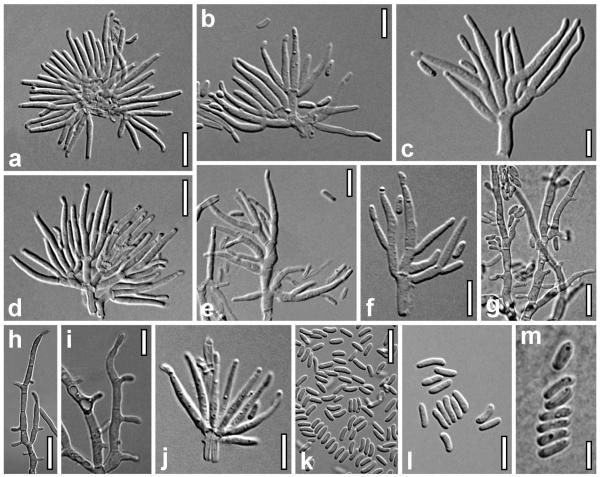
Anamorph of Stromatonectria caraganae. a–i. Conidiophores (a. subcircular fascicle of conidiophores). j. Phialide whorl. k–m. Conidia. a–f, j–l. From closed conidiomata on the natural host. g–i, m. From MEA, after 5 d at room temperature. Sources: a, d, l. WU 30197; b, c, e, f, j, k. WU 30199; g–i, m. CBS 127387. Bars: a, d, k = 10 μm. b, e, f, i, j, l = 7 μm. c, m = 5 μm. g, h = 15 μm.
MycoBank MB518756
Perithecial stromata when dry (1.0–)1.5–4(−7) × (0.8–)1.2–3.0(−6.0) mm, (0.5–)0.7–1.7(−2.3) mm thick (n = 50), solitary, less commonly in groups of 2–3, scattered, erumpent from bark, highly variable in shape and color; first appearing as a compact pseudoparenchymatous sterile hypostroma, on which pycnidia and later perithecia, often mixed on the same stroma, develop. Stromata turbinate or pulvinate; outline circular or oblong; fertile upper part often laterally projecting above the hypostroma; lower sides similar to the surface, sterile, smooth. Surface convex, flat or concave, glabrous or, particularly when young, clothed with short whitish hairs to ca. 30 μm long. Perithecia crowded in large numbers, up to more than 100, on the stroma surface, nearly free with visible outlines or partly or entirely immersed in the uppermost layer of the stroma. Ostiole openings (30–) 35–75(−120) μm diam (n = 70), plane, convex to papillate; orange, red to nearly black; darkened parts of perithecia surrounding the ostioles to ca. 250 μm diam. Stroma color variable, of various yellow tones, often mixed with red, or orange-red, less commonly dark red or purple. Stroma inside compact and dense, consisting of a whitish core and a pale to bright yellow peripheral region, bright yellow to orange also between and above perithecia. Upon rehydration ascospores ejected in rosy to cream tendrils; perithecia turning bright orange-red; unchanged in 3% KOH.
Anatomy of stromata and perithecia in vertical section
Cortical layer (22–)25–45(−60) μm thick (n = 20), consisting of a yellow t. angularis-globulosa of thick-walled cells (4–)6–11(−13) × (3.5–)5–7(−8) μm (n = 33) in section, partly perforated by thick-walled hyphae; brightly yellow in glycerol; gradually merging into subcortical tissue. Stroma surface sometimes covered by a thin, red outer layer of collapsed cells and amorphous material. Subcortical tissue between and above perithecia comprising a hyaline to pale yellowish t. intricata of thin-walled hyphae (2.5–)3–5(−6.5) μm wide (n = 30). Subperithecial tissue mostly consisting of a t. angularis of thin-walled, hyaline to pale yellowish cells (4–)8–20(−28) × (3.5–)6–12(−15) μm (n = 30), dense in lower regions, directly below perithecia looser and mixed with hyphae 4–8(−13) μm (n = 30) wide. Cells in marginal areas thicker-walled, smaller, yellow, orange to rosy in lactic acid, reddish orange or purple in glycerol or KOH. Basal tissue a dense, hyaline to yellowish t. intricata of thin-walled hyphae (1.7–)2.5–4.0(−5.0) μm(n = 33) wide. Ostioles periphysate, (74–)90–120(−135) μm long, (55–)68–112(−120) μm wide at the apex externally, (24–)28–55(−78) μm wide inside (n = 20); apex rounded or flat. Perithecia (225–)280–345(−370) μm high including ostiole, (163–)205–265(−280) μm diam (n = 20), spheroid, contents rosy in lactic acid and glycerol. Peridium (25–)32–44(−50) μm thick, of a hyaline, (10–)14–20(−25) μm thick inner layer and a yellow, (14–) 17–25(−30) μm thick (n = 20) outer layer; both layers comprising elongate, thick-walled, refractive cells, tending to be smaller and more globose outward. Asci (51–)64–88(−104) × (7–)10–14(−16) μm, including a stipe (0–)3–20(−34) μm long (n = 52), spore part in vivo mostly 60–75 μm long, oblong, narrowly clavate or fusoid, with eight ascospores partly crowded in upper parts, without a differentiated apex. Ascospores (10–)13–17(−21) × (4.0–)4.7–5.7(−7.0) μm, l/w (1.8–)2.5–3.4(−4.5) (n = 150), ellipsoid, oblong or fusoid, hyaline to faintly yellowish, with a median or slightly eccentric, not or scarcely constricted septum, rarely two septa, straight, symmetrical or slightly curved, thick-walled, smooth, inconspicuously multiguttulate.
Anamorph on the natural hosts
Compound pycnidia 0.3–0.9 mm developing atop hypostromata; semiglobose or pulvinate, Gyrostroma-like, multilocular, without a recognizable opening; pale or bright yellow, less commonly reddish; rosy or orange inside; opening by longitudinal fissures to expose several discoid conidiomata 0.2–0.7 mm diam, with bright orange, waxy, often confluent masses of conidia and conidiophores on top. Pycnidia in vertical sections mounted in 50% glycerol (220–)270–430(−550) μm high, (220–)300–600(−700) μm wide (n = 30), with circular or ellipsoid outline. Peridium (20–)27–62(−80) μm wide (n = 30), lacking at the base; two-layered, inner layer of hyaline hyphae, outer layer of a thick t. angularis of thick-walled cells (3–)5–9(−11) × (2.5–)3.5–6.5(−9.0) μm (n = 30), (sub)-hyaline in lactic acid, pale yellow in glycerol and KOH. Pycnidial surface uneven, with short hairs on the surface, particularly toward the base. Hairs (10–)11–36(−57) × (2.8–)3.5–5.5(−6.0) μm (n = 31), cylindrical, with broadly rounded ends or slightly enlarged end cell, with walls 1–1.5 μm, with warts, 2–8-celled. Locules with delicate, irregularly oriented inner walls and hyaline to rosy masses of conidia and densely packed conidiophores. Conidiophores typically formed in subcircular arrangements, originating either on a single cylindrical cell 3–12 × 2–3(−3.5) μm or on a common, often irregularly swollen hypha up to 6 μm wide; hyaline, short, variable, 1–6-celled, mostly asymmetric. Phialides formed in whorls of 2–8 or singly laterally on branches. Conidiophores in mounts including phialides ca. 20–37 μm long. After the dehiscence of conidiomata conidiophores mostly degenerated; orange masses comprising mostly conidia. Phialides (4.0–)8.5–12.7(−15.4) × (1.2–)1.4–2.0(−2.2) μm, l/w (3.5–)5.2–7.7(−8.9) (n = 63), subulate or narrowly lageniform, symmetrical or inequilateral when laterally in a whorl, straight or curved. Conidia (3.0–)4.0–5.5(−7.5) × (1.0–)1.2–1.5(−1.7) μm, l/w (2.1–)2.8–4(−5.8) (n = 125), numerous, cylindrical or allantoid with slight curvature, sigmoid in age, unicellular, hyaline, smooth.
Cultures and anamorph
Growth slow, after 10 d colonies reaching 7–8 mm on PDA, 3–5 mm radius on MEA. Colony on PDA and MEA compact, flat, with white surface and yellowish reverse. Initially conidiation starting after 3–4 d, conidia produced on hyphae, after ca. 10–16 d conidia amassing in a central, mucous, carrot or salmon mass. After 5 mo conidia produced in pink drops on top of white, internally yellow to rosy, pulvinate sporodochia up to 5 mm diam. Colony often remaining sterile after a few transfers. On MEA after 5 d conidiophores branching from growing hyphae, simple, acropleurogenous, with solitary terminal and lateral phialides or short pegs resembling phialide apices producing conidia. Phialides and conidia similar to those in conidiomata on the natural hosts, but both slightly wider and more variable in shape due to swelling soon after their formation. Phialides (5.0–)8.0–13.5(−17.0) × (1.8–)2.0–2.5(−3.2) μm, l/w (2.0–)3.4–6.3(−8.2) (n = 30), subulate or lageniform. Conidia (4.0–)4.5–6.0(−7.0) × (1.3–)1.5–2.2(−2.8) μm, l/w (2.1–)2.3–3.3(−3.9) (n = 40), unicellular, allantoid or cylindrical, hyaline, smooth; after few days swelling to irregular shapes and up to ca. 9 × 3.5 μm. Conidia produced on sporodochia identical to those on the natural hosts.
Habitat
On recently dead, standing or lying branches/trunks of Caragana spp., including C. arborescens, Colutea arborescens and Laburnum anagyroides.
Distribution
Austria, in and around Vienna.
Lectotype here designated
AUSTRIA, VIENNA, 3rd District, Botanical Garden, on corticated dead branches of Caragana arborescens, without date, V. Schiffner, Kryptogamae exsiccatae editae a Museo Palatino Vindobonensi 1612 (WU, not numbered, labeled as original specimen). Isotype: ibid., 24 Nov 1904, V. Schiffner (WU, not numbered, labeled as original specimen). Epitype, here designated to consolidate the relationship of teleomorph, anamorph, culture and gene sequences: AUSTRIA, VIENNA, 3rd District, Botanical Garden, on corticated branches of Caragana spp., 8 Apr 2010, H. Voglmayr (WU 30197, ex-epitype culture NCar = CBS 127387; ex-epitype sequences SSU-ITS1-5.8S-ITS2-LSU HQ112287, tef1 HQ112285).
Other specimens examined
AUSTRIA, NIEDERÖSTERREICH, Marchfeldkanalweg, south side, close to Gerasdorf, on branches of Colutea arborescens from several shrubs, soc. Cucurbitaria coluteae/elongata and Valsaria cf. insitiva, 6 Jun 2010, W. Jaklitsch & O. Sükösd (WU 30199). VIENNA, 10th district, at the crossing Laaerbergstrasse/Fontanastrasse, on standing trunks and branches of Laburnum anagyroides, 15 Sep 2010, W. Jaklitsch (WU 30200). 21st district, Marchfeldkanalweg, at wooden bridge and crossing with walking path to Stammersdorf, on branches of Colutea arborescens, soc. Valsaria cf. insitiva, 5 Sep 2009, W. Jaklitsch (WU 30196, culture NCol = CBS 125579; gene sequences SSU-ITS1-5.8S-ITS2-LSU HQ112288, rpb2 HQ112290, tef1 HQ112286, β-tub HQ112289). Stammersdorf, Lucken-schwemmgasse, between Dr Skoda Gasse and the primary school, on branches of Caragana arborescens attached to the shrub, 5 Jun 2010, W. Jaklitsch (WU 30198).
Notes
The original collection made by V. Schiffner was distributed as Zahlbruckner, Kryptogamae exsiccatae editae a Museo Palatino Vindobonensi 1612 (Zahlbruckner 1909) and as Rehm, Ascomycetes exsiccati 1586 (Rehm 1905). At WU two specimens of the original collection are present, one of which we selected as lectotype here. Remarkably Stromatonectria caraganae apparently had not been recorded for more than 100 y after its original description.
Stromata of Stromatonectria caraganae are equivalent to those of typical Hypocrea species. However for preparation of intact sections a thickness ca. 20 μm was necessary because the tissue between and directly below perithecia is distinctly looser than the firm subperithecial tissue, causing disintegration of the perithecial region. Asci are agglutinated in a rubber-like gel difficult to separate.
DISCUSSION
Centrum characteristics, asci and ascospores of S. caraganae are in accord with nectriaceous fungi, which currently are organized in two families, the Bionectriaceae and Nectriaceae (Rossman et al. 1999). Gene sequences and KOH-negative ascomata and stromata place Stromatonectria in the Bionectriaceae. The new generic name appeared to be necessary because two other superficially similar genera were not suitable to accommodate S. caraganae. One of these, Valsonectria, has prosenchymatous stromata and clusters with species of Peethambara and Scopinella (Fig. 1) in a different clade. The second genus is Myrmaeciella. An examination of the type specimen (USA, Texas, Corpus Christi, Apr 1869, Ravenel; PAD; Fig. 2w–z) of the generic type, M. endoleuca (Sacc.) Lindau, confirmed earlier views and descriptions (Saccardo 1880, Rossman et al. 1999, Ju et al. 1996, p 422). The fungus is characterized by black pulvinate or turbinate stromata (0.2–)0.4–1.2 mm diam, 0.2–0.3(−0.8) mm thick, erumpent from bark becoming superficial, with a smooth or tubercular surface without distinct ostiole openings, perithecia with dark brown walls immersed in a single upper layer in a soft, whitish to light brown entostroma, paraphyses that project above asci in immature ascomata, bicellular, ellipsoid, verruculose, hyaline ascospores (9.5–)10–12 × (4.5–)4.8–5.5(−6.0) μm, l/w (1.7–)1.9–2.3(−2.5) (n = 30). The dark perithecial wall is unusual for genera of the Bionectriaceae and Nectriaceae as is the presence of paraphyses in immature perithecia. Accordingly M. caraganae must be removed from Myrmaeciella. No molecular data are available for any other species currently placed in Myrmaeciella, including the type species.
As stated by Höhnel (1909, 1912) the fungus morphologically most similar to S. caraganae is Nectria paraguayensis, differing primarily by striate ascospores. Several descriptions (Starbäck 1899, p 35, Theissen 1911, p 51, Samuels and Brayford 1994, p 132) confirmed this observation. Nectria paraguayensis has similar Hypocrea-like stromata, nearly identical ascospore size, (13–)15–19(−21) × 5–6.5(−7) μm, and the discoid sporodochia of the presumed anamorph Patellina amoena Starbäck, which may be formed in pustules with perithecia, seem to be similar to the pycnidia of S. caraganae after dehiscence. In contrast to S. caraganae however the stromata of N. paraguayensis turn dark red in KOH, the cortical cells are described as having walls 3–4 μm thick, ascospores are striate, phialides are larger, 30–50 × 1–2 μm, and conidia are (4.8–)5.8–8.2(−8.8) × 1.5–2.5 μm. Due to the KOH+ stromata N. paraguayensis obviously belongs to the Nectriaceae, as do other stromatic species of Nectria, such as N. balansae or N. eustromatica. The latter lacks a pycnidial anamorph and differs from S. caraganae also in dark brown stromata with entirely immersed perithecia and much larger ascospores (Jaklitsch and Voglmayr 2011). Nectria balansae has some similarities in teleomorph morphology but differs from S. caraganae by KOH+ stromata, fewer perithecia per stroma and substantially larger, striate ascospores. The anamorph of N. balansae, as described for its putative synonym N. catalinensis C.E. Lima (Samuels and Brayford 1994), has pycnidia and unicellular micro- and macroconidia that differ substantially from the conidia of S. caraganae.
Anamorphs in both families comprising Nectria-like fungi are usually hyphomycetous. While synnemata or sporodochia occur in several genera of both families, to our knowledge only the unnamed anamorph of N. balansae, Gyrostroma and Zythiostroma anamorphs of Nectria in a restricted sense (Rossman 1989, or Pleonectria as advocated by Hirooka et al. 2009) and the Botryocrea sclerotioides anamorph of Cosmospora kurdica (Petr.) Rossman & Samuels (Rossman 1983; as Nectria kurdica) are pycnidial. We are not aware of any pycnidial anamorphs in the Bionectriaceae, which suggests that the anamorph of S. caraganae is unique in this respect. This fact supports erection of the new genus Stromatonectria in the Bionectriaceae, which parallels Nectria (or Pleonectria) in the Nectriaceae with respect to stroma formation and anamorphs. Conidiomata of S. caraganae are reminiscent of Gyrostroma such as G. austroamericanum Seeler (1940). Due to the absence of a preformed opening, multilocular architecture and lack of a basal wall they are not typical pycnidia, and therefore are termed compound pycnidia here. After dehiscence individual open conidiomata may be termed sporodochia. They may be called Patellina-like, but conidiomata are usually fully mature before dehiscence. For the original species of Patellina Speg., including P. amoena, the anamorph of Nectria paraguayensis, conidiomata were described as discoid or patellate sporodochia, not as pycnidia. Accordingly Patellina does not seem to be formally applicable to the anamorph of Stromatonectria. In conclusion a new generic name seems to be required for the anamorph of Stromatonectria, but we prefer to leave it unnamed, particularly in light of the current trend toward using a single name for an organism.
ACKNOWLEDGMENTS
We thank the curator of PAD, Rossella Marcucci, for loan of type material and the Austrian Research Fund (FWF; projects P19143, P22081) for financial support.
LITERATURE CITED
- de Hoog GS, Gerrits van den Ende AHG. Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses. 1998;41:183–189. doi: 10.1111/j.1439-0507.1998.tb00321.x. doi:10.1111/j.1439-0507.1998.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. doi:10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuckel KWGL. Symbolae mycologicae. Beiträge zur Kenntniss der Rheinischen Pilze. Jahrb Nass Ver Naturk. 1870;23–24:1–459. 1869. [Google Scholar]
- Glass NL, Donaldson GC. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirooka Y, Rossman AY, Chaverri P. Systematics of the genus Nectria based on a six-gene phylogeny [MSA meeting 2009 abstract] Inoculum. 2009;60(3):22. [Google Scholar]
- Höhnel F. Mycologisches. Österr Bot Zeitschr. 1905;55:51–55. 97–101. doi:10.1007/BF01791125. [Google Scholar]
- Höhnel F. Fragmente zur Mykologie IX. Mitteilung (No. 407 bis 467) Sitzungsber Akad Wiss Wien, mathem naturwiss Kl, Abt. I. 1909;118:1461–1552. [Google Scholar]
- Höhnel F. Fragmente zur Mykologie XIV. Mitteilung (No. 719 bis 792) Sitzungsber Akad Wiss Wien, mathem naturwiss Kl, Abt. I. 1912;121:339–424. [Google Scholar]
- Höhnel F, Weese J. Zur Synonymie in der Gattung Nectria. Annals Mycol. 1910;8:464–468. [Google Scholar]
- Huelsenbeck JP, Ronquist F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. doi:10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM. European species of Hypocrea I. The green-spored species. Stud Mycol. 2009;63:1–91. doi: 10.3114/sim.2009.63.01. doi:10.3114/sim.2009.63.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Komon M, Kubicek CP, Druzhinina IS. Hypocrea voglmayrii sp. nov. from the Austrian Alps represents a new phylogenetic clade in Hypocrea/Trichoderma. Mycologia. 2005;97:1365–1378. doi: 10.3852/mycologia.97.6.1365. doi:10.3852/mycologia.97.6.1365. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM, Voglmayr H. Nectria eustromatica sp. nov, an exceptional species with a hypocreaceous stroma. Mycologia. 2011 doi: 10.3852/10-178. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y-M, Rogers JD, Huhndorf SM. Valsaria and notes on Endoxylina, Pseudothyridaria, Pseudovalsaria and Roussoëlla. Mycotaxon. 1996;58:419–481. [Google Scholar]
- Liu YL, Whelen S, Hall BD. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol Biol Evol. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- Müller E, von Arx JA. Die Gattungen der didymosporen Pyrenomyceten. Beitr Krypt Schweiz. 1962;11:1–922. [Google Scholar]
- O’Donnell K, Cigelnik E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylogenet Evol. 1997;7:103–116. doi: 10.1006/mpev.1996.0376. doi:10.1006/mpev.1996.0376. [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA. Modeltest: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. doi:10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Rehm H. Ascomycetes exs. Fasc. 34. Annals Mycol. 1905;3:224–231. [Google Scholar]
- Riethmüller A, Voglmayr H, Göker M, Weiß M, Oberwinkler F. Phylogenetic relationships of the downy mildews (Peronosporales) and related groups based on nuclear large subunit ribosomal DNA sequences. Mycologia. 2002;94:834–849. doi: 10.1080/15572536.2003.11833177. doi:10.2307/3761698. [DOI] [PubMed] [Google Scholar]
- Rogerson CT. The Hypocrealean Fungi (Ascomycetes, Hypocreales) Mycologia. 1970;62:865–910. doi:10.2307/3757604. [PubMed] [Google Scholar]
- Rossman AY. The phragmosporous species of Nectria and related genera. Mycol Pap. 1983;150:1–164. [Google Scholar]
- Rossman AY. A synopsis of the Nectria cinnabarina-group. Mem New York Bot Gard. 1989;49:253–265. [Google Scholar]
- Rossman AY, Samuels GJ, Rogerson CT, Lowen R. Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes) Stud Mycol. 1999;42:1–248. [Google Scholar]
- Saccardo PA. Fungorum extra-Europaeorum pugillus. Michelia. 1880;2(6):138. [Google Scholar]
- Samuels GJ, Brayford D. Species of Nectria (sensu lato) with red perithecia and striate ascospores. Sydowia. 1994;46:75–161. [Google Scholar]
- Seeler EV. A monographic study of the genus Thyronectria. J Arnold Arbor. 1940;21:429–460. [Google Scholar]
- Stamatakis E. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. doi:10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Starbäck K. Ascomyceten der ersten regnellschen Expedition I. Bihang till Kungliga Svenska Vetenskaps-Akademiens Handlingar. 1899;25:1–68. [Google Scholar]
- Swofford DL. PAUP*: phylogenetic analysis using parsimony (*and other methods), Version 4.0b10. Sinauer Associates; Sunderland, Massachusetts: 2002. [Google Scholar]
- Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Theissen F. Die Hypocreaceen von Rio Grande do Sul, Südbrasilien. Ann Mycol. 1911;9:40–73. [Google Scholar]
- Vilgalys R, Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. Prosthecium species with Stegonsporium anamorphs on Acer. Mycol Res. 2008;112:885–905. doi: 10.1016/j.mycres.2008.01.020. doi:10.1016/j.mycres.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Werle E, Schneider C, Renner M, Völker M, Fiehn W. Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Res. 1994;22:4354–4355. doi: 10.1093/nar/22.20.4354. doi:10.1093/nar/22.20.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. Academic Press; San Diego: 1990. pp. 315–322. [Google Scholar]
- Zahlbruckner A. Schedae ad “Kryptogamas exsiccatas” editae a Museo Palatino Vindobonensi. Centuria XVII. Ann K.K. Naturhist Hofmus. 1909;23:213–236. [Google Scholar]