Abstract
The European species Hypocrea epimyces (Hypocreales, Ascomycota, Fungi) is redescribed based on the holotype including the drawing on its envelope by Saccardo and freshly collected material. The holomorphs of two closely related species, H. alni and H. brunneoviridis, are described as new species of the genus. They are characterized with morphological and molecular methods, including culture studies and phylogenetic analyses with internal transcribed spacers 1 and 2 as a part of the ribosomal RNA gene cluster, calmodulin, endochitinase, intron 4 of the translation elongation factor 1-alpha gene, and a part of the RNA polymerase II subunit B gene as phylogenetic markers. All species described here have green ascospores. Although phylogenetically closely related to H. lixii, they form reddish brown instead of green to black stromata. Except for H. brunneoviridis, forming nearly gliocladium-like conidiophores, the anamorphs of these species are similar to each other but vary in the angles of conidiophore branches and phialides, in phenotypic arrangement of conidiation on growth plates and in growth rates of cultures.
Keywords: Ascomycetes, calmodulin, cal1, chi18-5, DNA barcode, endochitinase, Hypocreales, ITS, morphology, phylogenetic markers, rpb2, sequence analysis, tef1, Trichoderma
INTRODUCTION
Teleomorphs of Hypocrea Fr., typified by H. rufa (Pers.: Fr.) Fr. (Jaklitsch et al 2006b), are characterized by perithecia immersed in fleshy stromata and by hyaline, 2-celled ascospores, disarticulating at the septum within asci. Ascospore color long was regarded an important criterion for generic classification, therefore similar fungi, deviating from Hypocrea only by green ascospores, were segregated in genera Creopus Link or Chromocrea Seaver. Following earlier authors, Doi (1969, 1972) recognized the segregate genera as synonyms of Hypocrea. In several works by Samuels and collaborators synonymy was confirmed by molecular data. Species of Hypocrea with green ascospores eventually were monographed by Chaverri and Samuels (2003). This work provides a firm basis for future research on such species. It shows that species with green ascospores are nested firmly within the phylogenetic tree of Hypocrea. Forty species were recognized and described by the authors. Hypocrea epimyces Sacc. and Pat., originally described from Europe, also has green ascospores. The name was introduced by Patouillard (1891) to replace H. vinosa Pat., after realizing that he had created a homonym of H. vinosa Cooke. Hypocrea epimyces originally was described from France on a basidiome of Phellinus nigricans (Fr.) P. Karst. and characterized by 4–8 mm diam, at first reddish, roundish to turbinate, later discoidal, sometimes short-stipitate stromata, dark reddish brown to olivaceous, becoming partly black at maturation and having cylindrical asci containing disarticulating green-brown ascospores. An inspection of the holomorph and the drawing on its envelope by Saccardo suggested that some fresh specimens recently collected in Europe correspond well to the concept of this species. Extensive searches in Europe for Hypocrea teleomorphs revealed two additional morphologically similar species, which are described below. As will be shown these three species belong to the largest clade containing green-spored species, currently termed the Harzianum clade or Catoptron/Lixii clade according to Chaverri and Samuels (2003). Many species have been described in this clade (e.g. Bissett 1991, Chaverri and Samuels 2003); some are important with respect to biological control (Hjeljord and Tronsmo 1998) or as mushroom parasites (Samuels et al 2002, Park et al 2006, Komon-Zelazowska et al 2007). Anamorphs of most species in this cluster share a Pachybasium morphology (cf. Bissett 1991), but the range of variation includes also verticillium-, trichoderma- and gliocladium-like conidiophores. The core of this clade is represented by the ubiquitous and common H. lixii Pat./Trichoderma harzianum Rifai, a species complex with an extraordinarily high degree of phylogenetic but little morphological variation (Chaverri et al 2003, Samuels et al 2002). Conidiophores of this species are narrow and flexuous and therefore were characterized as “typical of section Trichoderma” and keyed out in both section Pachybasium and section Trichoderma (Gams and Bissett 1998). Due to nearly identical structures of conidiophores, sizes and shapes of phialides and subglobose conidia, considerable efforts are required to distinguish taxa in the Harzianum complex, as is exemplified by T. aggressivum (Samuels et al 2002).
MATERIALS AND METHODS
Isolates and specimens
Single-ascospore isolates were prepared from fresh specimens of Hypocrea stromata and maintained as described by Jaklitsch et al (2005, 2006a). Isolates investigated in this study, GenBank accession numbers for ITS, cal1, chi18-5, rpb2 and tef1 sequences of Hypocrea alni, H. brunneoviridis, H. epimyces and H. lixii are provided (Table I). Isolates given as C.P.K. are those maintained in the collection of the Institute of Chemical Engineering, Vienna University of Technology. Representative isolates have been deposited at the Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands (CBS). Specimens including types were deposited in the Herbarium of the Institute of Botany, University of Vienna, Austria (WU).
TABLE I.
Accession numbers for rpb2, tef1, cal1, chi18-5 and ITS sequences of strains of H. alni, H. brunneoviridis, H. epimyces, H. lixii isolated by the first author
| Name | Strain | rpb2 | tef1 | cal1 | chi18-5 | ITS |
|---|---|---|---|---|---|---|
| Hypocrea alni | CBS 120633 | EU498349 | EU498312 | EU498326 | EU498337 | EU518651 |
| Hypocrea alni | C.P.K. 2494 | EU498350 | EU498313 | — | — | EU518652 |
| Hypocrea alni | C.P.K. 2854 | EU498351 | EU498314 | — | — | EU518653 |
| Hypocrea alni | C.P.K. 2858 | — | EU498315 | — | — | EU518654 |
| Hypocrea alni | C.P.K. 3139 | EU498352 | — | — | — | EU518655 |
| Hypocrea alni | C.P.K. 3141 | EU498353 | — | — | — | — |
| Hypocrea alni | C.P.K. 3142 | EU498354 | — | EU498327 | EU498338 | EU518656 |
| Hypocrea alni | C.P.K. 3145 | EU498355 | — | EU498328 | EU498339 | EU518657 |
| Hypocrea alni | C.P.K. 3154 | EU498356 | — | — | — | EU518658 |
| Hypocrea brunneoviridis | CBS 121130 | EU498357 | EU498316 | EU498329 | EU498340 | EU518659 |
| Hypocrea brunneoviridis | C.P.K. 2425 | — | EU498317 | — | — | EU518660 |
| Hypocrea brunneoviridis | CBS 120928 | EU498358 | EU498318 | EU498330 | EU498341 | EU518661 |
| Hypocrea epimyces | C.P.K. 1980 | EU498359 | EU498319 | EU498331 | EU498342 | EU518662 |
| Hypocrea epimyces | CBS 120534 | EU498360 | EU498320 | EU498332 | EU498343 | EU518663 |
| Hypocrea epimyces | C.P.K. 2417 | — | EU498321 | EU498333 | EU498344 | EU518664 |
| Hypocrea epimyces | C.P.K. 2487 | EU498361 | EU498322 | — | — | EU518665 |
| Hypocrea lixii | C.P.K. 1934 | EU498362 | EU498323 | EU498334 | EU498345 | — |
| Hypocrea lixii | C.P.K. 1935 | EU498363 | EU498324 | EU498335 | EU498346 | — |
| Hypocrea lixii | C.P.K. 1940 | EU498364 | — | — | EU498347 | — |
| Hypocrea lixii | C.P.K. 1941 | EU498365 | EU498325 | EU498336 | EU498348 | — |
Note: All other sequences used in phylogenetic analyses can be retrieved from GenBank (NCBI).
Growth characterization
Experiments were carried out as described by Jaklitsch et al (2005, 2006a). Where noted malt extract agar (MEA; 2% malt extract, 2% agar-agar, both from Merck, in distilled water) also was used.
Morphological observations
Methods described by Jaklitsch et al (2005, 2006a) were used. The terms autolytic activity, noted as terminal excretions from hyphae, particularly at colony margins, and coilings of hyphae on colony surfaces were applied as illustrated in Jaklitsch et al (2005). They were observed in the compound microscope (10× objective). Quantitative measurements are reported either as maxima and minima in parentheses and the mean plus and minus the standard deviation of a number of measurements given in parentheses, or as minimum-maximum ranges. Colors were determined and cited according to Kornerup and Wanscher (1981).
DNA extraction, PCR amplifications and sequencing
All methods used are defined in Jaklitsch et al (2005, 2006a), except these modifications and additions: A 0.4 kb fragment of the calmodulin encoding gene (cal1) was amplified with the primers cal228F and cal737R (Carbone and Kohn 1999). PCR products were purified either with the QIAquick Kit (QIAGEN) according to the manufacturer’s instructions or with an enzymatic PCR cleanup (Werle et al 1994). For the latter 20 μL PCR reactions were digested with 10 units exonuclease I (Fermentas, St Leon-Rot, BRD) and 2 units calf intestine alkaline phosphatase (Fermentas) for 45 min at 37 C, followed by an enzyme deactivation step at 85 C for 15 min. DNA was cycle-sequenced with the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit v. 3.1 (Applied Biosystems, Warrington) with the same primers as in PCR or with the internal primers 5′-CCGTGA(T/C)TTCATCAAGAACATG-3 and 5′-TTGGCAGTGTCCATCTTGTTG-3′ for tef1 and an automated DNA sequencer (ABI Genetic Analyzers, Applied Biosystems).
Molecular phylogenetic analysis
DNA sequences were subjected to Hypocrea/Trichoderma DNA barcode tools implemented in www.ISTH.info. TrichOKey was used for the analysis of ITS1 and 2 (Druzhinina et al 2005) and TrichoBLAST for all genes except cal1 (Kopchinskiy et al 2005). Corresponding sequences of related species were selected and visually aligned with those obtained in this study. The final alignments made in Genedoc 2.6 (Nicholas et al 1997) were formatted into interleaved NEXUS files with PAUP*4.0b10 (Swofford 2002) and manually corrected for the MrBayes v 3.0B4. The Bayesian approach to phylogenetic reconstructions (Rannala and Yang 1996, Yang and Rannala 1997) was implemented with MrBayes 3.0B4 (Huelsenbeck and Ronquist 2001). Modeltest3-06 (http://bioag.byu.edu/zoology/crandall_lab/modeltest.htm) was used to compare the likelihood of different nested models of DNA substitution and select the best-fit model for the investigated dataset. According to the protocol of Leache and Reeder (2002), posterior probabilities (PP) lower than 0.95 were not considered significant, while values below 0.9 are not shown on phylograms. Nucleotide characteristics of individual sets of sequences, parameters of phylogenetic analysis and settings for the adopted substitution models are provided (Table II). The multiple sequence alignment file has been deposited in the TreeBASE database (http://www.treebase.org/treebase/submit.html) and is available under accession numbers S2108 and M3969.
TABLE II.
Nucleotide properties of used loci and details of phylogenetic analysis
| Phylogenetic marker |
||||
|---|---|---|---|---|
| Parameters | cal1 | chi18-5 | rpb2 | tef1 |
| Fragment characterization | exon/intron | Exon | exon | intron |
| Number of sequences | 35 | 42 | 81 | 60 |
| Number of characters | 432 | 644 | 815 | 311 |
| parsimony informative | 76 | 103 | 302 | 112 |
| constant | 314 | 497 | 477 | 168 |
| Substitution model | GTR | GTR | GTR | GTR |
| Mean nt frequencies* A/C/ G/T |
0.27/0.28/0.24/0.21 | 0.22/0.33/0.23/0.22 | 0.22/0.26/0.28/0.24 | 0.19/0.29/0.18/0.34 |
| Substitution A <–> C | 0.56 | 1.27 | 1.68 | 0.85 |
| rates* A <–> G | 4.59 | 3.12 | 8.11 | 6.46 |
| A <–> T | 0.92 | 0.6 | 0.81 | 0.67 |
| C <–> G | 0.74 | 0.65 | 0.78 | 1.41 |
| C <–> T | 3.11 | 6.45 | 8.12 | 2.85 |
| G <–> T | 1 | 1 | 1 | 1 |
| alpha* | 0.34 | 0.24 | 0.24 | 0.23 |
| MCMC generations, million | 1 | 1 | 3 | 3 |
| Number of chains/temp (λ) | 4/0.2 | 4/0.2 | 4/0.2 | 4/0.2 |
| Sampling frequency | 100 | 100 | 100 | 100 |
| Number of discarded first generations |
400 | 500 | 1000 | 600 |
| Total tree length | 0.93 | 0.8 | 3.75 | 7.33 |
As estimated after MCMC run; GTR (Tavaré 1986) general time reversible model with eight free parameters, I proportion of invariable sites and G gamma rates.
RESULTS
Phylogeny
To infer the phylogenetic position of Hypocrea alni, H. brunneoviridis, and H. epimyces we first PCR-amplified and sequenced the universal fungal barcode locus ITS1 and 2 (internal transcribed spacers of the rRNA gene cluster). Analysis by TrichOKEY, the oligonucleotide barcode program for identification of Hypocrea/Trichoderma species (Druzhinina and Kopchinskiy 2006, Druzhinina et al 2005, www.isth.info/molkey), showed the presence of all genus-specific hallmarks, but no barcodes specific for any known species within the genus were found. Similarity searches performed against the Tricho-BLAST and NCBI GenBank databases suggested that all three species possess yet unknown ITS1 and 2 alleles of species belonging to the Harzianum clade. Hypocrea alni and H. epimyces sequences were most similar to T. aggressivum (93% and 90% similarity respectively). Sequences of H. brunneoviridis are characterized by a number of unique nucleotide signatures resulting in maximum similarities with H. epimyces and T. pleurotum (90% and 89% respectively). Because no intraspecific variabilities in ITS1 and 2 sequences were detected only one set of species-specific barcodes for each of the three new species were designed and integrated in TrichOKEY.
The phylogenetic position of Hypocrea alni, H. brunneoviridis and H. epimyces was tested further by analyzing four other phylogenetic markers used in molecular taxonomy of Hypocrea/Trichoderma: the highly variable fourth (long) intron of tef1, the partial exon of chi18-5, an intron-containing fragment of cal1 and a fragment of rpb2. For the analysis of rpb2 we included all species of the Harzianum clade, representative species of all other clades of Hypocrea/Trichoderma and the majority of known lone lineages (i.e. species for which clear ancestors are lacking) (Fig. 1). The resulting tree confirms that all three species described below form statistically supported lineages within the Harzianum clade. Hypocrea alni and H. epimyces thereby belong to the same subclade as the three green mold species on mushrooms (T. aggressivum, T. pleurotum and T. pleuroticola) and H. tawa. Hypocrea brunneoviridis on the other hand forms a basal subclade to the above-mentioned clade plus H. lixii.
Fig. 1.
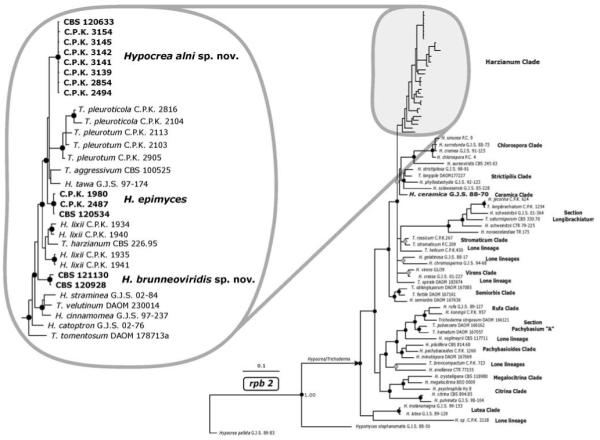
Bayesian analysis of the phylogenetic position of Hypocrea alni, H. epimyces and H. brunneoviridis, based on their rpb2 sequences. Posterior probability coefficients are given at respective nodes and shown only if the clade was highly supported (> 0.94 black circles, 0.90–0.94 gray circles). The arrow indicates the branch leading to genus Hypocrea/Trichoderma as currently recognized. GenBank accession numbers for H. alni, H. epimyces and H. brunneoviridis are provided (Table I). Accession numbers for other sequences may be retrieved from the NCBI Entrez search engine using [species strain RNA polymerase] keywords.
For the analysis of the three additional markers tef1 intron 4 (Fig. 2), cal1 and chi18-5 (both shown in Fig. 3) only members of the Harzianum clade were compared. A Bayesian analysis of the correspondingly aligned sequences reveals that H. alni and H. tawa exhibit a sister-clade relationship that is supported in the chi18-5 and tef1 tree and not rejected by cal1 and rpb2. Also H. epimyces seems to be closely related to T. aggressivum, but strong support is obtained only by the chi18-5 tree. In the tef1 and cal1 tree this sister relationship receives only low support and it is rejected in the rpb2 tree. These four species also appear to share a common ancestor because they are arising from a statistically supported branch in the tef1 and rpb2 trees that is weakly supported in the cal1 tree and not rejected in the chi18-5 tree.
Fig. 2.
Bayesian analysis of the phylogenetic position of H. alni, H. epimyces and H. brunneoviridis within the Harzianum clade based on the large intron of tef1 sequences. Posterior probability coefficients are given at respective nodes and shown only if the clade was highly supported (> 0.94 black circles, 0.90–0.94 gray circles). Arrows indicate branches leading to currently recognized species. Gray shadow indicates the poorly resolved phylogeny of H. lixii/T. harzianum species complex. GenBank accession numbers for H. alni, H. epimyces and H. brunneoviridis are provided (Table I). Accession numbers for other sequences may be retrieved from the NCBI Entrez search engine using [species strain translation elongation] keywords.
Fig. 3.
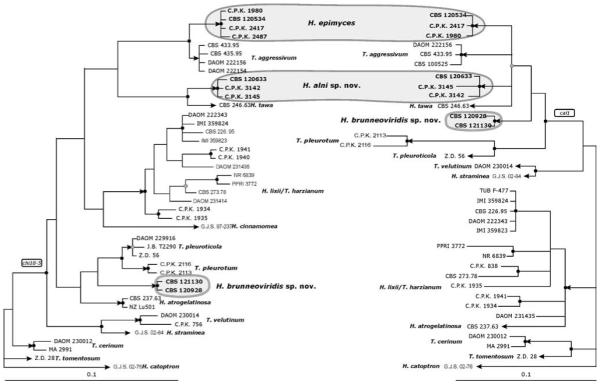
a, b. Bayesian analyses of the phylogenetic position of H. alni, H. epimyces and H. brunneoviridis within the Harzianum Clade based on the partial chi18-5 (a) and cal1 (b) sequences. Posterior probability coefficients are given at respective nodes and shown only if the clade was highly supported (> 0.94 black circles, 0.90–0.94 gray circles). Arrows indicate branches leading to currently recognized species. GenBank accession numbers for H. alni, H. epimyces and H. brunneoviridis are provided (Table I). Accession numbers for other sequences may be retrieved from the NCBI Entrez search engine using [species strain endochitinase] and [species strain calmodulin] keywords for chi18-5 and cal1, respectively.
The phylogenetic position of H. brunneoviridis could not be determined. Although it clustered within the Harzianum clade in all gene trees its position varied and none of the placements received statistical support.
DESCRIPTION OF THE SPECIES
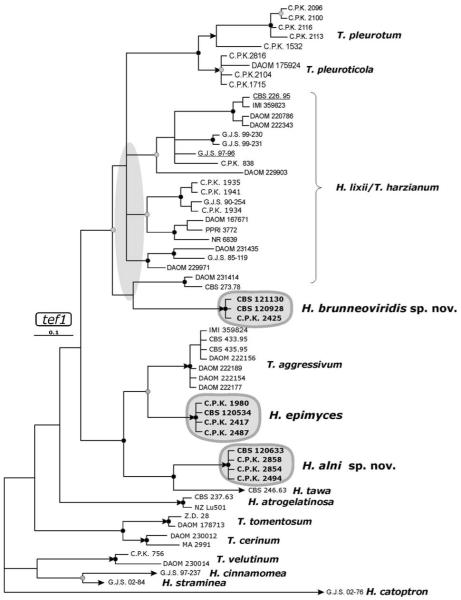
Hypocrea alni W.M. Jaklitsch sp. nov. Fig. 4a–l
Fig. 4.
Teleomorph of Hypocrea alni. a, b. Fresh stromata (all mature except rosy immature stromata on the left in (a). c, d, e. Dry stromata (c. immature, velutinous, d, e. mature). f. Surface of stroma in face view. g. Surface of mature stroma in 3% KOH after reconstitution in water. h. Perithecium in section. i. Cortical and subcortical tissue in section. j. Subperithecial tissue in section. k. Base of stroma in section. l. Asci with ascospores. a, c, f-k. WU 28224; b, e. WU 28226; d. WU 28232; l. WU 28225. Bars: a, b, e = 1.6 mm; c, d = 1 mm; f = 20 μm; g = 75 μm; h, k = 35 μm; i = 30 μm; j = 60 μm; l = 10 μm.
MycoBank MB 512089
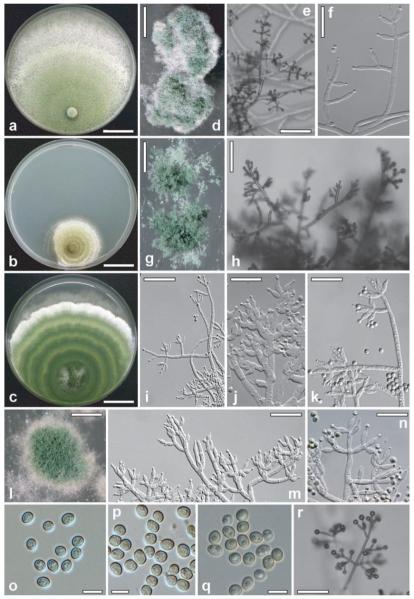
Anamorph: Trichoderma alni W.M. Jaklitsch sp. nov. Fig. 7a, d, e, f, o
Fig. 7.
Cultures and anamorph of Hypocrea alni, H. brunneoviridis and H. epimyces. a, d, e, f, o. H. alni (CBS 120633). a. Culture on PDA (7 d, 25 C). d. Tufts (CMD, 25 C). e. Conidiophores of effuse conidiation on growth plate (CMD, 25 C). f. Conidiophores from tufts (CMD, 25 C). o. Conidia (CMD, 25 C). b, g, h, j, m, p. H. brunneoviridis. b. Culture on PDA (7 d, 25 C). g. Granular pustules (CBS 121130, CMD, 25 C). h. Conidiophores on growth plate (C.P.K. 2425, CMD, 25 C). j, m. Conidiophores from pustules (CBS 121130, CMD, 25 C). p. Conidia (CBS 120928, CMD, 25 C). c, i, k, l, n, q, r. H. epimyces. c. Culture on PDA (CBS 120534, 7 d, 25 C). i, k, n. Conidiophores from pustules (C.P.K. 2487, CMD, 25 C). l. Pustule (CBS 120534, CMD, 25 C). q. Conidia (CBS 120534, CMD, 25 C). r. Conidiophore of effuse conidiation on growth plate (CBS 120534, CMD, 25 C). Bars: a, b, c = 21 mm; d = 1 mm; e = 80 μm; f = 25 μm; g = 0.5 mm; h, i, r = 50 μm; j, k, n = 20 μm; l = 0.6 mm; m = 30 μm; o, p, q = 5 μm.
MycoBank MB 512095
Etymology
reflecting the nearly exclusive occurrence on Alnus glutinosa.
Stromata typice in cortice et ligno Alni glutinosae, solitaria vel gregaria vel aggregata in fasciculis parvis, pulvinata ad semiglobosa, 1–8(−12) mm diam, 1–3(−5) mm alta, fusce rubro-brunnea. Asci cylindrici, (67–)80–96(−113) × (4.0–)4.5–5.5(−6.5) μm. Ascosporae bicellulares, verruculosae, virides, ad septum disarticulatae, pars distalis subglobosa, (3.7–)4.0–5.0(−6.0) × (3.0–)3.5–4.0(−4.5) μm, pars proxima oblonga ad cuneata, (3.5–)4.5–6.0(−7.2) × (2.7–)3.0–3.5(−4.0) μm.
Anamorphosis Trichoderma alni
Phialides in conidiophoribus effusis (8–)9–15(−18) × (2.2–)2.5–3.0(−3.5) μm, anguste lageniformes, phialides in conidiophoribus pustulatis lageniformes vel ampulliformes, (6–)7–11(−13) × (2.2–)2.5–3.5(−4.5) μm. Conidia viridia, subglobosa ad ovoidea, glabra, (2.2–)2.5–3.5(−4.7) × (2.2–)2.5–2.7(−3.0) μm.
Stromata when fresh (Fig. 4a, b) 1–8(−12) mm diam, 1–3(−5) mm thick, solitary, gregarious or aggregated in small groups, sometimes erumpent through bark fissures, pulvinate to nearly semiglobose, or undulate; broadly attached. Margin free and often wrinkled. Perithecia entirely immersed, surface smooth, tubercular to rugose, with a whitish downy covering layer or flakes when young. Ostiolar dots numerous, typically hardly visible, brown, turning green to nearly black due to spore powder. Color reddish gray, gray-brown to light reddish brown when young, becoming dark reddish brown, 9E7–8, often covered by dark green spore powder.
Stromata when dry 0.5–6.0 × 0.5–4.7 mm, 0.3–2.8 mm (n = 90) thick; pulvinate to semiglobose, sometimes discoid with sunken center, undulate or coarsely folded. Outline variable, circular, oblong to irregular. Margin thick, rounded, typically free. Sides straight and vertical or attenuated downward, velutinous, whitish to light gray or light brown when young; rarely with white basal mycelial margin. Surface when young smooth or often velutinous to tomentose or covered with whitish or greenish flakes of Trichoderma conidiophores; surface when mature smooth and iridescent or finely to coarsely tubercular. Ostiolar dots (15–)35–70(−94) μm diam (n = 80), inconspicuous, typically visible only under strong magnification, numerous, densely disposed, plane, dark brown to black or dark green from spore powder. Color when young gray to gray-brown (ca. 5DE3; Fig. 4c); when mature or old dark (gray to reddish) brown (5–7F2–4, 7F3–8, 8F3–5 to 9EF4–6; Fig. 4d, e), to black; cream or brownish inside. Consistency of stromata hard. Spore powder dark green, effuse, granular to pustulate. In 3% KOH dark blood red (Fig. 4g), ostiolar dots more distinct, hyaline.
Stroma anatomy
Ostioles (Fig. 4h) (69–)74–97(−110) μm (n = 30) long, plane or projecting to 10 μm, (17–)20–30(−36) μm (n = 30) wide at apex, cylindrical, periphysate, apical cells parallel, cylindrical, 1–3 μm wide, ends broadly rounded. Perithecia (Fig. 4h) (180–)210–260(−290) × (110–)120–200(−260) μm (n = 30), globose to flask-shape, crowded, 8–9 per mm of stroma length. Peridium (14–)17–25(−29) μm wide at base, (5–)9–17(−21) μm (n = 30) at sides, yellow to nearly orange, thickened around ostiole, of thick-walled compressed elongate cells, isodiametric at base and apex. Cortical layer (17–)19–36(−46) μm (n = 30) thick, a t. angularis of thick-walled (to ca. 1 μm), orange-brown to yellow, angular to oblong cells (5–)7–14(−18) × (3.5–)5.0–8.5(−12.0) μm (n = 60) in face view (Fig. 4f) and in vertical section (Fig. 4i); covered by a thin, noncontinuous layer of an orange, amorphous substance and collapsed or compressed cells, arranged in minute flakes. Cortical cells gradually paler yellow downward, merging into the hyaline subcortical t. angularis to t. epidermoidea (Fig. 4i) of thin-walled, roundish to oblong cells (3.5–)4.5–11(−17) × (2.0–)3.5–6.5(−7.0) μm (n = 30) and hyaline hyphae (2.5–)3.5–5.5(−6.0) μm (n = 32) wide, descending parallel between perithecia. Subperithecial tissue (Fig. 4j) consisting of a hyaline t. epidermoidea of variably thick-walled (to ca. 1.5 μm) cells (5–)10–33(−53) × (3–)7–16(−20) μm (n = 30), including some hyphal elements. Basal tissue (Fig. 4k) hyaline to yellowish-brown, consisting of a dense t. intricata of hyphae (2.5–)3.0–5.0(−6.0) μm (n = 33) wide with walls to ca. 1 μm, incorporating substrate cells. No hairs on mature stromata, no stipe seen. Asci (Fig. 4l) (67–)80–96(−113) × (4.0–)4.5–5.5(−6.5) μm (n = 80), including a stipe (2–)7–30 (−50) μm (n = 80) long, cylindrical, basally often thickened; apex truncate, thickened to ca. 1.5 μm. Ascospores (Fig. 4l) green, brown in KOH, verrucose, warts to ca. 0.5 μm diam, multiguttulate when vital, cells dimorphic, distal cell (3.7–)4.0–5.0(−6.0) × (3.0–)3.5–4.0(−4.5) μm, l/w (1.0–)1.1–1.3(−1.5) (n = 90), (sub)globose to oblong or wedge-shape, proximal cell (3.5–)4.5–6.0(−7.2) × (2.7–)3.0–3.5(−4.0) μm, l/w (1.0–)1.3–1.9(−2.4) (n = 90), oblong to wedge-shape or subglobose; some asci only with 4 ascospores.
Cultures and anamorph (strain CBS 120633 studied in detail)
Optimum growth at 25 C on all media; no growth at 35 C. On CMD 10–13 mm at 15 C, 35–40 mm at 25 C, 23–25 mm at 30 C after 72 h (colony radius measured, applies to all stated growth rates). Mycelium covering plate after 5 d at 25 C. Colony hyaline, thin; mycelium loose, hyphae narrow, radial; one or several broad concentric zones of conidiation appearing, starting at distal margin of the colony. Surface becoming downy, developing fine white floccules, fluffy tufts or pustules to 3 mm diam, confluent to amorphous masses to 10(−15) mm diam. Aerial hyphae frequent, particularly at margin of colony, erect, richly branched, several millimeters high, becoming fertile. Autolytic activity absent or inconspicuous. Coilings frequent. No diffusing pigment, no odor noted. Chlamydospores noted only after 2 wk at 30 C. Conidiation at 25 C noted after 2 d, first effuse, starting around plug, spreading across colony, later in variable cottony fluffy tufts or pustules, generally with a hairy aspect due to superposed long fertile aerial hyphae; after 5 d slowly becoming matte gray-green (26–27D4, 27E4–5). Effuse conidiation (CBS 120633, after 4d): conidiophores (Fig. 7e) loosely disposed, little branched, 2–4 μm wide; short, erect, verticillium-like, or as fertile aerial hyphae to 1–2 mm long and high, generally asymmetrical. Stipe thick-walled, to 1 mm long and 4–5 μm wide, attenuated to 2 μm toward ends; bearing unpaired branches. Branches to 250 μm long and (2.0–)2.5–3.5(−4.5) μm wide; with 1–2-fold further loose asymmetrical branching, terminating in broad or pyramidal side branches with numerous, terminal, minute, wet conidial heads < 20 μm diam, greenish under stereomicroscope. Side branches short, simple, of a 1–3-celled axis and paired or unpaired, typically distinctly divergent, often right-angled branches successively shorter toward the tip of the axis, bearing phialides solitary or divergent in whorls of mostly three, often with two paired phialides directly below terminal whorl, arising from cells 1.5–4.0(−4.5) μm wide; cell below phialide often slightly thickened. Phialides (8–)9–15(−18) × (2.2–)2.5–3.0(−3.5) μm, l/w (2.5–)3.0–5.6(−7.0), (1.5–)1.7–2.5(−2.7) μm (n = 30) wide at base, lageniform, mostly thickened above middle, straight and symmetrical, less commonly slightly curved and inaequilateral. Conidia 3.0–4.0(−4.2) × (2.2–)2.5–3.0(−3.2) μm, l/w 1.1–1.3(−1.4) (n = 32), green, smooth, subglobose to oval or ellipsoidal, with few fine guttules, scar indistinct.
Pustulate conidiation (CBS 120633, after 7–10 d): tufts/pustules (Fig. 7d) arising from a stipe 4–7 μm wide, asymmetrically branched to form a loose mesh of irregularly oriented narrow conidiophores, becoming dense within pustule upon formation of conidia; periphery remaining loose, sometimes with straight to sinuous, sterile to fertile elongations 70–130(−150) μm long. Right-angled paired branches uncommon; short, usually 1-celled branches of minute terminal side branches (Fig. 7f) often paired, bearing phialides solitary or in whorls of 2–3(−5), most commonly in cruciform whorls of three. Phialides (Fig. 7f) (6–)7–11(−13) × (2.2–)2.5–3.5(−4.5) μm, l/w (1.7–)2.5–4.2(−5.6), (1.2–)1.5–2.0(−2.2) μm (n = 32) wide at base, lageniform to ampulliform, thickened at or above middle, often attenuated at base, particularly narrow when solitary. Conidia (Fig. 7o) (2.2–)2.5–3.5(−4.7) × (2.2–)2.5–2.7(−3.0) μm, l/w 1.0–1.4(−1.8) (n = 32), green, mostly subglobose to oval, sometimes ellipsoidal, smooth, with few minute guttules. At 15 C fluffy tufts arranged in a broad downy, distal zone, remaining white. At 30 C colony zonate; coilings numerous; conidiation effuse and in greenish granules on proximal margin and tufts in a broad distal zone.
On PDA 10–13 mm at 15 C, 27–32 mm at 25 C, 17–22 mm at 30 C after 72 h; mycelium covering plate after 6–7 d at 25 C. Colony (Fig. 7a) indistinctly zonate. Mycelium dense, opaque, thick; hyphae wide, wavy and often in strands on margin. Margin thin, ill defined. Aerial hyphae numerous, several millimeters high, without a distinct orientation, adhering in strands, forming a dense white downy, fluffy or floccose mat, with large drops of fluid in aged cultures. Autolytic activity absent or inconspicuous. Coilings frequent. No diffusing pigment, no odor noted. Conidiation noted after 2–3 d, effuse, starting around plug on short aerial hyphae, spreading on lower levels of mats of aerial hyphae across plate; becoming pale to matte gray-green (27CE3–5 to 26DE4) after 5–7 d in a proximal to central area and often in several additional, indistinctly separated, flat, velutinous, concentric zones. At 15 C fluffy tufts formed in the center, becoming only pale greenish in proximal areas. At 30 C hyphae becoming yellow, conidiation scant, colorless. Autolytic excretions numerous, yellowish brown. Coilings numerous. Reverse becoming (gray-)yellow to orange (4A5–6, 4B4–8, 5B5–6).
On SNA 10–13 mm at 15 C, 30–32 mm at 25 C, 11–13 mm at 30 C after 72 h; mycelium covering plate after ca. 6 d at 25 C. Colony similar to CMD. Mycelium loose, hyphae more curly, marginal surface hyphae wide. Margin ill defined. Indistinctly separated, downy concentric zones with numerous, long, erect aerial hyphae, appearing in distal parts of the colony; developing ill defined fluffy tufts to 2.5 mm diam, confluent to 5 mm, becoming diffuse pale green (27C3–4, 27E3–6) after 4 d, starting in proximal areas of the growth plate; often margin of tufts remaining white. Autolytic activity absent or inconspicuous, stronger at 30 C. Coilings in superficial hyphae conspicuous, numerous at all temperatures. No diffusing pigment, no odor noted. Chlamydospores noted after 2 wk at 30 C, (4.5–)6.0–10.0(−12.5) × (3.5–)5.5–9.0(−11.0) μm, l/w (0.8–)0.9–1.3(−1.7) (n = 31), globose, pyriform or angular, smooth, terminal and intercalary. Conidiation similar to CMD, noted after 2 d. At 15 C conidiation effuse and in amorphous greenish tufts. At 30 C conidiation scant, simple, effuse.
Habitat
on wood and bark of Alnus glutinosa, in one instance found on Betula pendula; in wet habitats, often along rivers.
Known distribution
Europe, collected in Austria, The Netherlands, Ukraine and the UK.
Holotype
UNITED KINGDOM, Derbyshire, Peak District National Park, Baslow, Longshaw Country Park, elev. 350 m, 53°18′20″N, 01°36′18″W, on partly decorticated branches of Alnus glutinosa, 1–4 cm thick, holomorph, 11 Sep 2004, W. Jaklitsch and H. Voglmayr, WJ 2701 (WU 28224, ex-type culture CBS 120633 = C.P.K. 1982). Holotype of Trichoderma alni isolated from WU 28224 and deposited as a dry culture together with the holotype of H. alni as WU 28224a.
Other specimens examined
AUSTRIA, Niederösterreich, Mödling, Wienerwald, Kaltenleutgeben, along brook Dürre Liesing between Am Brand and Stangau, MTB (“Messtischblatt”) 7862/4, elev. 450 m, 48°06′45″N, 16°08′43″E, on partly decorticated branch of Alnus glutinosa, on wood, bark and effete immersed pyrenomycete, soc. Steccherinum ochraceum, Hypocrea moravica; holomorph, 22 Oct 2006, H. Voglmayr and W. Jaklitsch, WJ 3027 (WU 28225, culture C.P.K. 2494). ITALY, Sardinia, central western coastal area close to Capo Mannu, elev. 239 m, 40°04′06″N, 8°32′15″E, anamorph isolated from soil sample, Oct 2004, Q. Migheli (culture C.P.K. 2657 = UNISS 10–16). NETHERLANDS, Utrecht, De Uithof, small mixed deciduous forest between sport park Olympos, botanical garden and motorway crossing Rijnsweerd, elev. 0 m, on corticated, 0.5–2 cm thick branches of Alnus glutinosa, on wood and bark, holomorph, anamorph partly distorting and overgrowing basidiomata of Macrotyphula cf. contorta, 18 Nov 2006, H. Voglmayr, WJ 3044 (WU 28226, culture C.P.K. 2854). Utrecht, between Botanical Garden and University at Leuvenlaan, elev. 0 m, on branch of Alnus glutinosa, holomorph, 2 Dez 2006, H. Voglmayr, WJ 3051 (WU28227, culture C.P.K. 2858). UKRAINE, Poltava oblast, Mirgorod area, close to Velikiye Sorochintsy, 800 m W from the river Psyol, flood lands alder forest, elev. ca. 100 m, 49°58′14″N, 33°59′03″E, on branches of Alnus glutinosa, 18 Aug 2007, A. Akulov, AS 2488 (WU 28232, culture C.P.K. 3154). UNITED KINGDOM, Lancashire, Ribble Valley, Clitheroe, north from Dunsop Bridge, elev. ca. 300 m, 53°56′47″N, 02°32′24″W, on 2–3 cm thick branch of Alnus glutinosa, holomorph, anamorph soc. initials of Macrotyphula cf. contorta basidiomata, 6 Sep 2007, W. Jaklitsch and H. Voglmayr, WJ 3140 (WU 28229, culture C.P.K. 3141). —, north from and close to Bashal Eaves, elev. 200 m, 53°53′52″N, 02°28′31″W, on corticated, 2–3 cm thick twigs of Alnus glutinosa, in wet grass, holomorph, 7 Sep 2007, W. Jaklitsch and H. Voglmayr, WJ 3141 (WU 28230, culture C.P.K. 3142). Norfolk, North Wootton, close to Castle Rising, elev. ca. 10 m, 52°47′15″N, 00°26′55″E, on corticated twig of Betula pendula, 1–2.5 cm thick, lying in wet Sphagnum, holomorph, 9 Sep 2007, W. Jaklitsch and H. Voglmayr, WJ 3145 (WU 28231, culture C.P.K. 3145). North Yorkshire, Thornton le Dale, Dalby Forest, Forest Drive, shortly after Low Dalby at Dalby Beck, elev. 150 m, 54°16′58″N, 00°41′21″W, on thin corticated twigs of Alnus glutinosa lying in wet grass, mainly anamorph, 5 Sep 2007, W. Jaklitsch and H. Voglmayr, WJ 3138 (WU 28228, culture C.P.K. 3139).
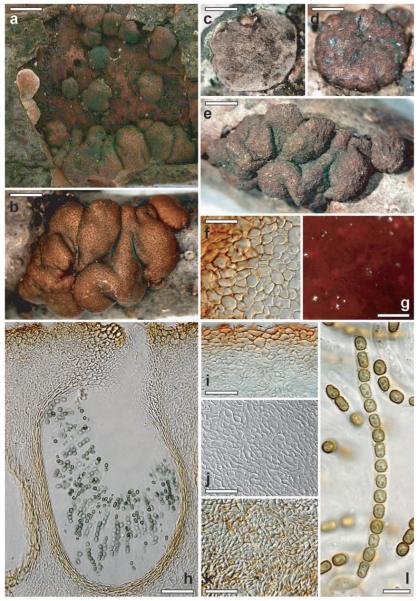
Hypocrea brunneoviridis W.M. Jaklitsch sp. nov. Fig. 5a–n
Fig. 5.
Teleomorph of Hypocrea brunneoviridis. a, b, c, e. Fresh stromata (a. stroma initial, b. stroma initial with partial pigmentation, c. immature, e. mature). d, h. Dry stromata (d. immature. h. mature). f. Surface of stroma in face view. g. Surface of mature stroma in 3% KOH after reconstitution in water. i. Perithecium in section. j. Cortical and subcortical tissue in section. k. Subperithecial tissue in section. l. Base of stroma in section. m, n. Asci with ascospores. a–c, e, n. WU 28235; d, f–m. WU 28233. Bars: a = 0.4 mm; b, e, g, h = 0.7 mm; c, d = 1 mm; f = 15 μm; i = 35 μm; j, k, l = 25 μm; m, n = 10 μm.
MycoBank MB 512097
Anamorph: Trichoderma brunneoviride W.M. Jaklitsch sp. nov. Fig. 7b, g, h, j, m, p
MycoBank MB 512098
Etymology
the epithet describes the combination of brown stroma and green ascospores.
Stromata in cortice et ligno, solitaria vel gregaria vel aggregata in fasciculis parvis, pulvinata ad undulata, 1–10 mm diam, ad 3 mm alta, fusce rubro-brunnea. Asci cylindrici, (70–)80–102(−113) μm × (4.0–)4.5–5.0(−6.0) μm. Ascosporae bicellulares, verrucosae, flavo-virides, ad septum disarticulatae, pars distalis subglobosa, (3.0–)3.5–4.7(−5.7) × (2.5–)3.5–4.0(−4.5) μm, pars proxima oblonga ad cuneata, (3.5–)4.5–6.0(−7.0) × (2.5–)3.0–3.5(−4.0) μm.
Anamorphosis Trichoderma brunneoviride
Incrementum in agaris CMD, PDA et SNA irregulare, tardum et limitatum. Conidiophora in agaris CMD et SNA in pustulis, ramis ardue ascendentis, phialidibus in fasciculis divergentibus ad prope parallelis. Phialides lageniformes, (5.5–)7.0–11.0 (−13.5) × (3.0–)3.5–4.5(−5.0) μm. Conidia viridia, subglobosa ad ellipsoidea, glabra, (3.2–)3.5–4.5(−5.5) × (2.5–) 3.0–3.5(−3.7) μm.
Stromata when fresh (Fig. 5a, b, c, e) 1–10 mm diam, to 3 mm thick, solitary, gregarious to aggregated in small numbers (2–3), thickly pulvinate. Outline circular to angular. Margin wavy. Surface smooth. Ostiolar dots distinct, numerous, densely disposed, finely papillate, becoming green. Development and color: first appearing as white mycelium, compacting (Fig. 5a), then center becoming brownish (Fig. 5b) or rosy to reddish brown with olivaceous margin or entirely rosy (Fig. 5c), gray-orange or reddish-brown (6B4 to 8E5–8); later dark reddish brown, often with olivaceous tones (Fig. 5e), finally dark brown (7F3–8); blackening or green by ejected spore powder; inside cream to light brown or with dark brown spots; often intense (gray-)green (26DE4–6) Trichoderma on and around immature rosy stromata.
Stromata when dry (Fig. 5d, h) 1–9 × 1–6 mm, 0.4–2.5 mm (n = 60) thick; typically pulvinate to semiglobose; button-like (short cylindrical) to discoidal with sunken center when young or when old and collapsed; sessile, broadly attached. Outline circular to oblong, sometimes irregular, with undulate margin. Margin thickly rounded, free. Sides rounded or straight, covered with short hairs, whitish, olivaceous, becoming smooth and brown; with whitish to cream mycelial margin at base when young. Surface granulose by slightly projecting ostioles and/or perithecia, rugose or smooth. Ostiolar dots (24–)37–67(−87) μm (n = 75) diam, numerous, densely disposed, minute; first pale, diffuse and plane, later convex to papillate; finally dark brown, dark green or black due to ascospores; sometimes visible only under strong magnification. Color first rosy brownish (Fig. 5d), brown-orange or light (yellow-)brown (5–6CD4–5, 5–6E5–8) when young, (reddish) brown (7E4–6, 7–8E6–8) to mostly dark (reddish) brown (7–8F4–8, 9F6–8; Fig. 5h), to nearly black when mature or old. Stromata often covered by dark green spore deposits; inside yellowish to brownish or light wood colored, particularly when old.
Associated anamorph effuse, powdery and dark green (26F4–8). Dry stromata after reconstitution with water brick-red, becoming reddish brown (Fig. 5g) and slowly turning black in 3% KOH.
Stroma anatomy
Ostioles (Fig. 5i) (64–)69–90(−106) μm (n = 30) long, umbilicate, plane or projecting to 10(−20) μm, (16–)20–36(−52) μm (n = 30) wide at apex, cylindrical, periphysate; apical cells cylindrical, 2–3.5 μm wide, terminally rounded. Perithecia (Fig. 5i) (130–)160–230(−290) × (90–)110–180(−245) μm (n = 30), 8–10 per millimeter length of stroma, globose to flask-shape or ellipsoidal, often laterally compressed. Peridium (12–)14–22(−30) μm wide at base, (5–)11–19(−23) μm (n = 30) wide at sides, thickened around ostioles, yellow; of elongate, thick-walled, refractive cells. Surface wavy, smooth, no hairs seen. Cortical layer (18–)20–35(−46) μm (n = 30) thick, around whole stroma except area of attachment, cells at sides more hyphal; of a yellow- to orange-brown t. angularis of thin-walled, isodiametric to oblong cells (4.5–)7–13(−17) × (3.5–)5–8(−10) μm (n = 60) in face view (Fig. 5f) and in vertical section (Fig. 5j), compressed at surface. Subcortical tissue (Fig. 5j) of a t. angularis to t. epidermoidea of subhyaline cells (3–)5–13(−17) × (2.0–)4.0–7.5(−10.0) μm (n = 30), mixed with hyaline hyphae (2.5–)3.5–6.0(−8.5) μm (n = 30) wide. Subperithecial tissue (Fig. 5k) of a t. epidermoidea to t. angularis of (sub)hyaline, angular to oblong cells (7–)12–30(−43) × (5–)9–16(−21) μm (n = 30); at base of stroma (Fig. 5l) slightly smaller and mixed with thick-walled, hyaline to yellowish hyphae (2.5–)3.5–6.5(−8.5) μm (n = 30) wide, penetrating the wood. Asci (Fig. 5m, n) (70–)80–102(−113) μm × (4.0–)4.5–5.0(−6.0) μm, including a stipe (1–)8–20(−30) μm (n = 45), cylindrical, apex thickened to ca. 1 μm, base thickened or not. Ascospores (Fig. 5m, n) yellowish-green, verrucose, two or more guttules per cell; cells dimorphic, distal cell (3.0–)3.5–4.7(−5.7) × (2.5–)3.5–4.0(−4.5) μm (n = 75), l/w (0.9–)1.1–1.3(−1.6) (n = 75), subglobose (to wedge shape), proximal cell (3.5–)4.5–6.0(−7.0) × (2.5–)3.0–3.5(−4.0) μm, l/w (1.2–)1.3–1.8(−2.3) (n = 75), oblong to wedge shape; proximal cell in ascus base often larger.
Cultures and anamorph (strains CBS 121130 and CBS 120928 studied in detail)
Mycelium covering plate only on MEA; precultures therefore on MEA. Optimum growth at 25 C on all media, no growth at 35 C. On CMD 4–6 mm at 15 C, 4–15 mm at 25 C, 3–11 mm at 30 C after 72 h. Colony hyaline, thin, circular to irregular. Margin often ill defined (e.g. torn into teeth). Margin of plug becoming green, followed by the formation of white granules or pustules (Fig. 7g) to ca. 1.5(−2) mm diam, with powdery to granulose surface, spreading across colony, becoming dark green (28EF5–8, 27F4–8, 27E4–6), to nearly black, aggregating to 3 mm. Marginal hyphae forming numerous short pegs, 15–70(−220) μm long, before termination of growth. Aerial hyphae absent to scant. No autolytic excretions, no coilings seen. No diffusing pigment, no odor noted. No true chlamydospores, only few thickenings in hyphae seen. Conidiation noted after 1–3 d, starting on and around plug in short minute shrubs with wet heads to 40(−70) μm diam, spreading, growing to pustules, becoming dry and green after 4–6 d. Pustules first loose, arising from a stipe 7–8(−11) μm wide, with radial, steeply ascending branches. Main branches 4–6 μm wide, 3–4 μm toward ends. Thick clusters formed inside tufts, often with marginal branches still sterile. Branching generally asymmetrical, except for terminal side branches. Terminal side branches (Fig. 7h, j, m) straight, narrowly pyramidal, with lower branches often paired and in right angles, others strongly inclined upward, bearing divergent phialides on several levels in whorls of 2–5, often strongly inclined upward to nearly parallel, originating on (sometimes thickened) cells (3.0–)3.5–4.5(−5.0) μm wide. Phialides (5.5–)7.0–11.0(−13.5) × (3.0–)3.5–4.5(−5.0) μm, l/w (1.3–)1.8–2.8(−4.0), (1.5–)2.0–3.0(−3.5) μm (n = 63) wide at the base, lageniform, plump, often longer and narrower in terminal position. Conidia (Fig. 7p) (3.2–)3.5–4.5(−5.5) × (2.5–)3.0–3.5(−3.7) μm, l/w (1.1–)1.2–1.4(−1.6) (n = 63), green, smooth, subglobose to ellipsoidal or oval, with minute guttules and minute, flat, often indistinct scar. At 15 C conidiation in small shrubs or pustules, dark green (27E5–6, 26–27F5–8), confluent to a continuum around plug. At 30 C hyphae finely submoniliform, conidiation absent or in small shrubs or green pustules.
On MEA conidiation in several flat, (yellow-)green, concentric zones; conidiophores (main axes) erect, in dense lawns or confluent, bearing numerous, minute, wet heads to 20(−30) μm diam; main axes (stipes) 6–9 μm wide, attenuated upward to (3–)4–5 μm, forked basally, or asymmetrically branched on few (max. four) levels, bearing one or several terminal side branches 0.2–0.3 mm diam, short and broad or pyramidal, often with paired branches. Solitary conidiophores to 0.8 mm long; generally strongly inclined upward, side branches to 150 μm long, some oriented downward; phialides arising in whorls of 2–5 on often slightly thickened cells (3.5–)4.0–5.0(−5.5) μm, divergent, but usually strongly curved upward, lageniform to ampulliform, thickened below or at the middle, becoming green when old. Phialides and conidia as described on CMD (measurements combined).
On PDA 4–6 mm at 15 C, 4–10 mm at 25 C, 1–9 mm at 30 C after 72 h. Colony (Fig. 7b) either conspicuously irregular with growth stopping after few days, or first circular, compact, dense, flat, white around plug, and margin well defined. Often growth discontinued (e.g. laterally, resulting in irregular outlines). Marginal surface hyphae finely submoniliform. Aerial hyphae inconspicuous. No autolytic excretions seen. Coilings frequent. Reverse gray-yellow (3–4C4–5), light to gray- or golden-yellow (4AC5–6) to (yellow-)brown (5–6E7–8). No odor noted. Conidiation noted after 1–2 d, green after ca. 3 d, starting around plug, spreading across colony, on short simple erect conidiophores forming dense lawns; concentrated in several alternating, gray-green and (olive-)yellow, concentric zones (29CD4–8, 28CD5–6, 3–4CD4–6). Surface of zones becoming powdery and eventually turning dull olivaceous to (yellow-, olive-)brown (3DE4–6, 4E6–8, 5E4–5). Sometimes stromata formed (e.g. at half of growth radius) after ca. 3 wk. Stromata pulvinate, to 10 mm diam, several millimeters thick, green and velutinous due to conidiation on surface, dull yellow-brown (5EF6–8), hard and compact inside, developing perithecia but remaining sterile. At 15 C conidiation on terminal branches of more regular shape than at 25 C, amassing in a floccose central zone, becoming gray-green (29–30CD4–5) to olive-brown (4CD3–4). Central surface dotted with numerous orange-brown drops, 200–400(−800) μm diam. Reverse pale yellow (2–3A3), gray-yellow (4B4–6), to yellow-brown (5E7–8). At 30 C conidiation effuse, on small, densely disposed shrubs, not becoming green.
On SNA 2–5 mm at 15 C, 3–12 mm at 25 C, 2–5 mm at 30 C after 72 h. Colony dense, mycelium radial. Margin ill defined, torn into teeth; marginal hyphae forming pegs. Aerial hyphae lacking. No autolytic excretions seen. No coilings seen. No diffusing pigment, no odor noted. Conidiation noted after 2–3 d, green after 3–6 d, starting in shrubs around margin of plug, aggregating to a matte green (28E4–7) continuum. In addition fine dark green (finally 28F5–8) pustules spreading across colony and growing to 2 mm. Conidia becoming compacted into numerous wet drops to 100(−200) μm diam. Conidiation also noted inside agar in the center of the colony. Chlamydospores (5–)6–10(−11) × (5–)6–8 μm, l/w (0.9–)1.0–1.4(−1.5) (n = 20), infrequent, in marginal hyphae of the colony, (sub)globose, terminal, rarely intercalary. At 15 C conidiation in numerous confluent, matte green (27–28E4–6), flat tufts or fine pustules to 1 mm diam. At 30 C conidiation in a green continuum around plug and in green shrubs to 1 mm diam with wet heads.
Habitat
on well rotted wood and bark of deciduous trees, collected on Fagus sylvatica and Alnus incana.
Known distribution
Europe, collected in Austria and Germany.
Holotype
GERMANY, Bavaria, Unterfranken, Landkreis Haßberge, Haßfurt, close to Mariaburghausen, left roadside heading from Knetzgau to Haßfurt, MTB 5929/3, elev. 270 m, 50°00′33″N, 10°31′10″E, on 3–5 cm thick, partly decorticated branches of Fagus sylvatica, on wood, bark and effuse basidiomycete, soc. and on stromata of Hypoxylon fragiforme, soc. Laxitextum bicolor, Pycnoporus cinnabarinus, rhizomorphs, effete pyrenomycete, Calosphaeria sp. in bark, holomorph, 4 Aug 2004, H. Voglmayr and W. Jaklitsch, WJ 2571 (WU 28233, ex-type culture CBS 121130 = C.P.K. 2014). Holotype of Trichoderma brunneoviride isolated from WU 28233 and deposited as a dry culture together with the holotype of H. brunneoviridis as WU 28233a.
Other specimens examined
AUSTRIA, Kärnten, Klagenfurt Land, St Margareten im Rosental, Zabrde, MTB 9452/4, elev. 550 m, 46°33′00″N, 14°25′17″E, on 4–5 cm thick, decorticated branch of Fagus sylvatica, on wood, soc. Polyporus brumalis, farinose Corticiaceae and moss, holomorph, teleomorph over mature, 29 Oct 2005, W. Jaklitsch and H. Voglmayr, WJ 2870 (WU 28234, culture C.P.K. 2425). Steiermark, Bruck/Mur, Gußwerk, Rotmoos bei Weichselboden, beside dried brook at south side of the Rotmoos, MTB 8356/2, elev. 690 m, 47°40′56″N 15°09′25″E, on 10 cm thick, corticated log of Alnus incana, holomorph, 27 Sep 2006, H. Voglmayr, WJ 2992 (WU 28235, culture CBS 120928 = C.P.K. 2477).
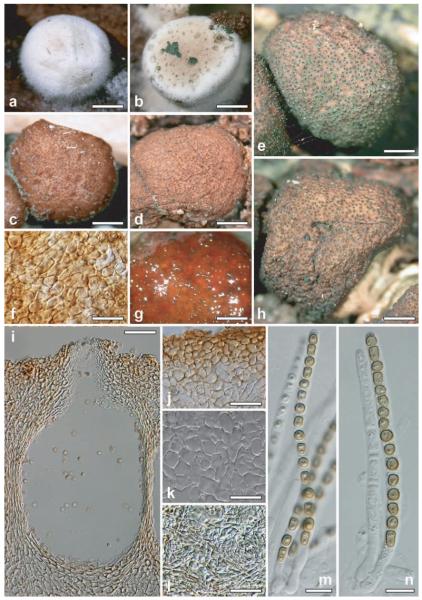
Hypocrea epimyces Sacc. and Pat., Tabulae Analyticae Fungorum 4:175, fig. 387. 1891. Fig. 6a–p ≡ Hypocrea vinosa Pat. 1881, Rev. Myc. III, fasc. 12:11 (1881), (non Cooke, Grevillea 8: 65 [1879])
Fig. 6.
Teleomorph of Hypocrea epimyces. a, g, h. Fresh stromata (a. immature, g, h. mature). b. Envelope of the holotype (drawing by Saccardo). c, e, f, j. Dry stromata (c. immature. e. mature, stipitate. f, j. mature). d. Mature stroma in 3% KOH after reconstitution in water. i. Base of stroma in section. k. Perithecium in section. l. Surface of stroma in face view. m. Cortical and subcortical tissue in section. n. Subperithecial tissue in section. o, p. Asci with ascospores. a, d–g, i, k–p. WU 28237. b, f. holotype. c. WU 28236; h, j. WU 28239. Bars: a, d, g = 1.2 mm; c = 1.7 mm; e = 0.8 mm; f = 0.5 mm; h = 2 mm; i, l = 25 μm; j = 1.4 mm; k, n = 35 μm; m = 30 μm; n = 35 μm; o, p = 10 μm.
Anamorph: Trichoderma epimyces W.M. Jaklitsch sp. nov. Fig. 7c, i, k, l, n, q, r
MycoBank MB 512096
Differt a T. harzianum, T. alni et T. brunneoviride in conspicuis zonis viridibus concentricis in agaro “PDA”, a T. harzianum, in incremento tardiore et in absentia incrementi ad 35 C, a T. alni in phialidibus dispositae in angulis arduoribus, a T. brunneoviride in incremento celeriore. In agaro “CMD” phialides in conidiophoribus effusis (5–)9–15(−17) × (2.5–)2.7–3.5(−4.0) μm, lageniformes ad subulati; phialides in conidiophoribus pustulatis (6–)7–10(−12) × (2.0–)2.5–3.0(−3.5) μm, lageniformes ad ampulliformes. Conidia (2.7–)3.0–3.7(−4.2) × (2.2–)2.7–3.0(−3.5) μm, viridia, subglobosa ad ovalia (ad ellipsoidea).
Stromata when fresh (Fig. 6a, g, h) 1–14 mm diam, 1–3 mm thick, solitary, gregarious or aggregated in small numbers, flat pulvinate, lenticular, discoidal to turbinate, sometimes undulate, attenuated to a dark base, centrally attached. Margin or large part of stroma free, sometimes lobed. Surface essentially smooth, with numerous, densely disposed, slightly darker reddish brown ostiolar dots becoming green when mature. Color first white, center becoming rosy (Fig. 6a), pale brick-red, gray-orange, -red or incarnate, often mixed with a yellowish ground tone (6AB4, 6B5–6, 6C5–7, 7B5–6, 8B4–5); turning brown-orange, reddish to olivaceous brown at maturation (5CD4–5, 9C6–7, 9D7–8; Fig. 6g, h), dark green by spore powder or with black spots when old.
Stromata when dry (Fig. 6c, e, f, j) 1–13 × 1–10 mm, 0.5–2.8 mm (n = 43) thick, flat pulvinate, discoidal, lenticular to placentiform with rounded margin, sessile or turbinate with sharp margin and short, thick stipe attenuated downward. Outline circular or somewhat elongate to irregular. Margin free, projecting to 1 mm over short central stipe, often undulate to crenate. Sides and stipe often covered with floccose, cream, rust, gray, brown to nearly black mycelium to the base. Surface often flat or concave, smooth to finely but distinctly granulose to rugose or tubercular. Ostiolar dots (24–)32–56(−71) μm (n = 43) diam, numerous, minute, conspicuously densely (ca. 8/mm) disposed, circular, flat or convex, first reddish brown, later dark to black, with circular perforation. Color matte red, gray-red, brown-orange to light (reddish) brown (8CD4–8, 7CD6–7) when immature (Fig. 6c), later dark reddish brown (7–8E5–8, 8F5–8, 9E5–6), sometimes matte red or rosy with olivaceous tone (7–8CD3–5); (over) mature stromata (Fig. 6e, f, j) dark reddish brown with black spots, or dark brown (7F4, 8F4–6), often covered by dark green (25F7–8) spore powder. In 3% KOH stromata dark reddish brown (Fig. 6d) with finely papillate, hyaline ostioles.
Associated anamorph first white, becoming green, with white margin, finally dark green (26F5–8), thickly pulvinate to effuse, powdery.
Stroma anatomy
Ostioles (Fig. 6k) (47–)55–72(−80) μm long, plane, rarely projecting to 12(−28) μm, (10–)13–25(−40) μm (n = 30) wide at apex, narrowly conical to cylindrical; apex of hyaline, narrow, cylindrical, convergent cells; no periphyses seen. Perithecia (Fig. 6k) (115–)170–230(−260) × (100–)120–180(−240) μm (n = 30), ellipsoidal to subglobose, numerous, usually crowded, peridium (10–)12–18(−22) μm at base, (9–)10–14(−16) μm (n = 30) at sides, yellowish; of thick-walled, elongated, compressed cells; thickened around ostiole. Cortical layer (16–)22–34(−38) μm(n = 36), of a t. angularis of thin-walled, isodiametric angular cells (5–)7–14(−20) × (3–)5–10(−13) μm (n = 65) in face view (Fig. 6l) and in vertical section (Fig. 6m); reddish brown in water, orange in lactic acid, extending around whole stroma except area of attachment; with a thin layer of amorphous material on the surface. No hairs seen. Subcortical tissue (Fig. 6m) of a (sub)hyaline t. epidermoidea of thin-walled cells (3–)4–10(−15) × (2.5–)3.0–6.5(−9.0) μm (n = 30), mixed with hyaline hyphae (2–)3–5(−6) μm (n = 30) wide. Subperithecial tissue (Fig. 6n) of a dense hyaline t. epidermoidea of thin-walled, globose, angular, lobed or elongate cells (4–)8–30(−55) × (4–)5–17(−24) μm (n = 32). Stipe-like base (Fig. 6i) penetrating into wood, orange-brown, inhomogeneously pigmented, dense; of globose to angular cells 4–10(−16) × 3–6(−10) μm (n = 30), mixed with pigmented hyphae (2.5–)4.0–5.0(−6.0) μm (n = 30) wide. Asci (Fig. 6o, p) (65–)75–100(−117) × (4.2–)5.0–6.0(−6.5) μm, including a stipe (2–)5–17(−28) μm (n = 46) long, cylindrical; apex thickened to ca. 1 μm, containing a flat ring; stipe slightly thickened at base. Ascospores (Fig. 6o, p) green, becoming brown in KOH, distinctly verrucose, multiguttulate when fresh; cells dimorphic, distal cell (3.5–)4.0–5.0(−5.5) × (2.7–)3.2–4.0(−4.7) μm, l/w (1.0–)1.1–1.3(−1.6) (n = 60), (sub)globose to plump wedge-shape, proximal cell (3.5–)4.2–5.5(−6.2) × (2.7–)3.0–3.7(−4.2) μm, l/w (1.1–)1.3–1.7(−1.8) (n = 60), oblong, wedge-shape to subglobose; sometimes orientation inverted.
Cultures and anamorph (strain CBS 120534 studied in detail)
Optimum growth at 25 C on all media, no growth at 35 C. On CMD 12–13 mm at 15 C, 33–35 mm at 25 C, 30–32 mm at 30 C after 72 h; mycelium covering plate after 5–6 d at 25 C. Colony hyaline, thin. Hyphae narrow, loosely disposed. Aerial hyphae inconspicuous, becoming fertile. Autolytic excretions rare. No coilings noted. No diffusing pigment, no odor noted. Chlamydospores infrequent, noted after 8–9 d. Conidiation at 25 C first noted after 1 d around plug, effuse, macroscopically invisible, on phialides sessile on surface hyphae, or on short, erect conidiophores spreading across colony to distal margin, loosely disposed, denser in downy zones. Short conidiophores simple or of a stipe with short side branches (Fig. 7r), to ca. 150 μm long, asymmetric at base. Branches often paired on higher levels, often strongly inclined upward with 0 or 1 further branch. Terminal branches of 1 to few cells, with solitary or 2–3 divergent or nearly parallel phialides; in distal and lateral areas of the colony also on aerial hyphae to 0.6 mm long and 6–9 μm wide, bearing short side branches to 100(−200) μm long and corresponding to short sessile conidiophores. Phialides (5–)9–15(−17) × (2.5–)2.7–3.5 (−4.0) μm, (1.5–)2.0–3.0(−3.5) μm wide at base, l/w (1.9–)3.0–4.8(−5.4) (n = 35), lageniform to subulate, often curved or sinuous, slightly thickened on varying levels, sometimes base strongly attenuated. Conidia held in minute wet heads to 30(−40) μm diam, green after 4–6 d, finally collapsing, conidia becoming dry.
Pustulate conidiation at 25 C noted after 6–7 d when effuse conidiation degenerated/collapsed for the greatest part. Pustules (Fig. 7l) 0.5–1.5 mm diam, circular, with granular to fluffy surface, confluent, intermingled with conidiophores of effuse conidiation, gregarious, often densely aggregated to nearly continuous at distal margin; becoming dark green (26–27F7–8) from ends of concentric zones; constructed of a transparent network with narrow branches on a stipe 5–7(−9) μm wide, with verrucose walls to nearly 2 μm thick. Branching mostly asymmetrical, right angles common, straight fertile elongations to 350 μm. Conidiophores (Fig. 7i, k, n) mostly 4–6 μm wide, 2–3 μm terminally; sometimes with thickened cells to 12 μm diam. Terminal side branches generally short, ill defined or symmetric, with mostly 1-celled branches, slightly wider downward; with phialides solitary or divergent in whorls of (2–)3–4(−5), mostly 3, often steeply inclined upward, originating on cells 1.5–3.5 μm wide. Conidia held in numerous, minute, initially wet heads < 20 μm diam. Phialides (6–)7–10(−12) × (2.0–)2.5–3.0(−3.5) μm, l/w (2.0–)2.5–3.7(−4.7), (0.9–)1.5–2.2(−2.7) μm (n = 68) wide at base, lageniform to ampulliform, often inaequilateral and curved upward, to sigmoid, sometimes repetitive; thickenings variable, neck sometimes long and thin. Conidia (Fig. 7q) (2.7–)3.0–3.7(−4.2) × (2.2–)2.7–3.0(−3.5) μm, l/w (1.0–)1.1–1.3(−1.4) (n = 100), yellowish-green, smooth, subglobose to oval, less commonly ellipsoidal; with 1 or several guttules, scar indistinct. At 15 C development slow, conidiation effuse and dense in fluffy tufts at distal and lateral margin, becoming tardily green. At 30 C effuse and in loose fluffy tufts or green confluent micropustules developing at proximal margin, and in few powdery concentric zones, becoming dark green (28EF7–8).
On PDA 10–11 mm at 15 C, 20–26 mm at 25 C, 20–23 mm at 30 C after 72 h; mycelium covering plate after 7–8 d at 25 C. Colony (Fig. 7c) more or less circular with hyaline wavy margin. Mycelium densely interwoven, little difference of width among hyphae. Aerial hyphae abundant, forming a dense mat several millimeters high, densely branched, with many strands and drops, radial only at margin. Numerous dense conidiation nests overlain by loose long aerial hyphae, causing a densely granular central surface. Center becoming homogeneously light green. Subsequently several thick, padded, velvety, alternating, green and white concentric zones formed, finally all zones green of various shades (27E4–6, 26F5–6, 27F6–8). Autolytic excretions inconspicuous. No coilings seen. No diffusing pigment, no odor noted. Conidiation at 25 C noted after 1 d, green after 2–3 d, starting around plug on short aerial hyphae, later on densely disposed, short micropustules and long aerial hyphae ascending several millimeters; spreading across colony. Conidia formed in numerous minute heads on short pyramidal branches. At 15 C colony indistinctly zonate, downy. Conidiation loose in densely disposed microtufts and on long aerial hyphae, becoming only slightly greenish in the center, or remaining hyaline. At 30 C colony with distinct, convex, green concentric zones. Conidiation abundant.
On SNA 11–13 mm at 15 C, 30–32 mm at 25 C, 22–23 mm at 30 C after 72 h; mycelium covering plate after 6 d at 25 C. Colony similar to CMD, mycelium more curly; primary hyphae thick and prominent on surface. Long and high aerial hyphae at colony margin becoming fertile. Autolytic excretions rare. Coilings moderate to frequent. No pigment, no odor noted. Chlamydospores (4–)5–8(−10) × (3–)4–6(−8) μm, l/w (0.9–)1.0–1.5(−2.1) (n = 45), noted after 7 d, numerous, but loosely disposed; variable, subglobose to pyriform when terminal, ellipsoidal to angular when intercalary. Conidiation noted after 1 d, first effuse, more abundant than on CMD; sessile or on short and simple conidiophores with 1–3 phialides, starting around plug, spreading across entire colony, concentrated in several downy to finely granular or powdery concentric zones; becoming greenish after 4–6 d. Pustulate conidiation appearing as small granules or tufts of ca. 0.5 mm diam in the concentric zones and the center; confluent to 2.5 mm. Conidia gathering in large green drops to 250 μm diam, denser and darker green (26–27F7–8) at lateral ends of concentric zones. At 15 C colony not or only indistinctly zonate, broad margin becoming downy. Conidiation effuse, nearly gliocladium-like, with heads to ca. 25 μm, and in few tufts to 1 mm diam, with long, fertile, straight to sinuous elongations. At 30 C colony circular, with several alternating broad and narrow zones. Autolytic activity and coilings conspicuous. Chlamydospores numerous, distinctly more than at 25 C, globose to angular, terminal and intercalary. Conidiation effuse.
On oatmeal agar stromata are formed (CBS 120534; CBS pers comm).
Habitat
on (medium to) strongly decomposed wood; typically on decorticated branches deeply submerged in leaf litter, usually in association with other fungi; holotype on a Phellinus basidiome, rare to uncommon.
Known distribution
Europe, collected in Austria, Germany, France (holotype).
Holotype
FRANCE, Jura, Poligny, sur Polyporus (Phellinus) nigricans, Jul 1881, N. Patouillard (PAD). Epitype designated here in order to establish the correct relationship of teleomorph, anamorph and gene sequences: AUSTRIA, Kärnten, Klagenfurt Land, St Margareten im Rosental, Zabrde, elev. 565 m, MTB 9452/4, 46°32′58″N, 14°25′11″E, on 4 cm thick, decorticated branch of Fagus sylvatica, on wood, soc. Tomentella sp, unidentified Corticiaceae, Nemania serpens, Tubeufia cerea (on effete black pyrenomycete) and old Bertia moriformis, holomorph, 19 Aug 2004, W. Jaklitsch, WJ 2608 (WU 28237, culture CBS 120534 = C.P.K. 1981). Holotype of Trichoderma epimyces isolated from WU 28237 and deposited as a dry culture together with the epitype of H. epimyces as WU 28237a.
Other specimens examined
AUSTRIA, Kärnten, Klagenfurt Land, St Margareten im Rosental, Aussicht, elev. 600 m, MTB 9452/4,46°32′50″N, 14°25′00″E, on decorticated branches of Fagus sylvatica, on strongly decomposed wood, some soc./on Steccherinum fimbriatum and soc./on a brown Corticiaceae, holomorph, 14 Oct 2006, W. Jaklitsch, WJ 3019 (WU 28239, culture C.P.K. 2487). Niederösterreich, Wien-Umgebung, Mauerbach, Friedhofstrasse, elev. 350 m, MTB 7763/1, 48°15′16″N, 16°10′33″E, on 4–5 cm thick, decorticated branch of Fagus sylvatica, covered with leaves, soc./on orange Corticiaceae and rhizomorphs, ozonium; holomorph, teleomorph immature, maturation after incubation at RT, 10 Sep 2005, W. Jaklitsch, WJ 2849 (WU 28238, culture C.P.K. 2417, from conidia). GERMANY, Bavaria, Oberbayern, Landkreis Eichstätt, Altmühltal, 2–3 km after Pfahldorf toward Eichstätt, elev. 540 m, MTB 7033/4, 48°57′00″N, 11°18′20″E, on 4.5 cm thick branch of Fagus sylvatica, on wood, soc./on ozonium, Armillaria rhizomorphs, Phanerochaete sanguinea, effete pyrenomycetes, holomorph, 5 Aug 2004, W. Jaklitsch and H. Voglmayr, WJ 2576 (WU 28236, culture C.P.K. 1980).
DISCUSSION
Of the 40 species described by Chaverri and Samuels (2003), only H. ceramica Ellis and Everh. shows reddish brown stromata. The latter is unrelated to the group of species currently termed the Harzianum clade (e.g. Fig. 1). Also its Trichoderma anamorph differs from these species by forming distinct pustules with conspicuous, straight, fertile elongations (Chaverri and Samuels 2003, and pers unpubl obs).
In contrast the three species described or redescribed here belong to the Harzianum clade. Most teleomorphic species of the Harzianum clade form yellow stromata, with the exception of H. cinnamomea Chaverri & Samuels with brown stromata, H. atrogelatinosa Dingley with translucent, brownish orange stromata, H. lixii and H. tawa Dingley (Chaverri and Samuels 2003). Stroma initials of Hypocrea lixii can be reddish but they are light green when young or dark green to black at maturity. In contrast all species described here typically form reddish brown to brown stromata although ostiolar dots can be green or black. To the unaided eye the stroma surface may appear black when over mature, but under the stereomicroscope the stroma surface is still (reddish) brown. In addition all species have KOH+ red stromata. No such reaction was reported by Chaverri and Samuels (2003) for any other species in the Harzianum clade, except H. straminea Chaverri & Samuels with initially yellow stromata becoming reddish brown in KOH. Cultures of H. lixii differ from the species described here also by faster growth and growth at 35 C. The reverse of cultures of H. alni and H. brunneoviridis on PDA respectively may turn yellow at 30 C or 25 C, but the yellow pigment in H. lixii is more conspicuous than in those species.
Based on the descriptions by Chaverri and Samuels (2003), H. tawa, known from New Zealand, Sri Lanka and Thailand, differs from H. alni, H. brunneoviridis and H. epimyces in having KOH negative stromata, slightly larger ascospores, a verticillium-like anamorph on CMD, not forming pustules or tufts, and by larger conidia. Also the Siberian Creopus testacea L.N. Vassilyeva (Vassilyeva 1998) might belong here. It was compared to H. tawa by the author. The larger ascospores (distal part 5–6 μm diam, proximal part 7–8 × 5–6 μm) given in the protolog suggest that it might be distinct from the species described here. In any case cultures of fresh collections of this species including gene sequences would be necessary to ascertain relationships.
Hypocrea epimyces is characterized by large, flat, discoidal to lenticular, sometimes stipitate stromata and numerous, fine but distinct ostiolar dots, reddish immature stromata and brown-olivaceous mature stromata. Although larger and flatter they may be difficult to distinguish from those of H. minutispora (see Lu et al 2004 for description and illustration) when immature. The anamorph is distinct from all species of the Harzianum clade (Chaverri and Samuels 2003), including H. alni and H. brunneoviridis, in well defined green concentric conidiation zones, particularly on PDA. Only H. tawa shows a comparable pattern on PDA. Stromata of the holotype were found on Phellinus nigricans, but all recent collections are on branches of Fagus submerged in leaf litter. Although identification solely based on the comparison of teleomorphs might be misleading, there are good reasons for this identification: (i) The drawing on the envelope of the holotype by Saccardo (Fig. 6b) clearly shows (a) the transition from reddish (incarnate, brick-red, rosy) to light olivaceous, (b) the discoidal to flat pulvinate, sometimes short stipitate, stromata with smooth surface and (c) the numerous densely disposed fine ostiolar dots. (ii) The holotype material, although scanty, comprises few, typical flat mature stromata on the hymenium of the polypore, with a smooth and dark reddish brown surface between dark green to black ostiolar dots. This is clearly distinct from the closely related H. lixii, also collected on polypores, which does not exhibit reddish-brown colors in mature stromata.
H. alni and H. brunneoviridis differ from H. epimyces by having thickly pulvinate stromata. Immature stromata can be rosy to reddish brown in H. brunneoviridis, similar to H. epimyces, while they are typically inconspicuous, gray-reddish to brown in H. alni. Of the currently known European species forming reddish brown stromata with green ascospores H. alni is the darkest with inconspicuous ostiolar dots when dry. The differentiation of the teleomorph from H. brunneoviridis can be difficult, but the latter can be distinguished by ecology, cultures and anamorph. H. alni is well defined ecologically by its occurrence in wet, grassy stands of Alnus glutinosa, often along brooks. In such habitats this species is common, particularly as anamorph, on mostly corticated branches lying in grass. Host specificity is generally low in wood-inhabiting species of Hypocrea, in the case of H. alni however the nearly exclusive occurrence on Alnus might be due to the co-occurrence of a Macrotyphula. The conidiation in T. alni is pale green, appearing in a pustulate form preceded by an effuse conidiation, the latter representing only a simpler form with slightly longer and narrower phialides. In both T. epimyces and T. brunneoviride branching of conidiophores and phialides are organized in steeper angles than in T. alni.
Colonies of H. brunneoviridis (all three isolates) are peculiar because they do not cover a Petri dish if grown on CMD, SNA or PDA even after 1 mo. Growth is distinctly slower than in the other species mentioned. The anamorph is distinct from most other species of the Harzianum clade by steep, nearly gliocladium-like inclination of terminal branches and phialides in dark green to nearly black pustules, reminiscent of H. thelephoricola. However T. pleurotum also has a gliocladium-like morphology, thus supporting the notion (Komon-Zelazowska et al 2007) that pachybasium-gliocladium-like morphology can occur in various monophyletic and phylogenetically unrelated Hypocrea/Trichoderma clades.
The Harzianum clade contains several species causing green mold disease in mushroom farms, such as T. aggressivum (Samuels et al 2002), T. pleurotum and T. pleuroticola (Park et al 2006, Komon-Zelazowska et al 2007). At least two of the species described here also might have mycoparasitic traits; the co-occurrence of H. alni with basidiomata of Macrotyphula cf. contorta in some specimens and growth of the anamorph on young basidiomata might suggest a mycoparasitic association with the basidiomycete. H. epimyces and H. brunneoviridis in contrast have been found on branches of mostly Fagus sylvatica partly immersed in leaf litter, but the holotype of H. epimyces was described from a basidiome of a Phellinus.
Phylogenetic analyses confirm that the three species described in this paper also match the criteria of phylogenetic species, as implied by the genealogical concordance phylogenetic species recognition (GCPSR) concept (Taylor et al 2000) (i.e. that the branches leading to the respective species clades are supported in the majority of gene trees). In fact the current data even satisfy the conservative requirements postulated by Dettman et al (2003) because the three species clades were supported in two gene trees and not rejected in any other gene tree.
As for the phylogenetic position of the three new species, a close relationship between H. alni and H. tawa received strong support whereas that between H. epimyces and T. aggressivum remains ambiguous and could not be resolved by the four phylogenetic markers used in this paper. In any case the close relationship between these four species was documented. The phylogenetic position of H. brunneoviridis on the other hand remained unresolved although it undoubtedly is located within the Harzianum clade. The reason for this is unclear. We speculate that more strains have to be collected to resolve the phylogenetic position of H. brunneoviridis on detection of an unknown close neighbor taxon.
ACKNOWLEDGMENTS
We thank Marcella Marcucci, curator of PAD, for the loan of the holotype of H. epimyces, Monika Komon-Zelazowska for providing gene sequences, and Hermann Voglmayr and Alexander Akulov for providing fresh specimens. The financial support by the Austrian Science Fund (FWF Project P19143-B17) to WMJ is gratefully acknowledged.
LITERATURE CITED
- Bissett J. A revision of the genus Trichoderma III. Section Pachybasium. Can J Bot. 1991;69:2373–2417. [Google Scholar]
- Carbone I, Kohn LM. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. [Google Scholar]
- Chaverri P, Castlebury LA, Samuels GJ, Geiser DM. Multilocus phylogenetic structure within the Trichoderma harzianum/Hypocrea lixii complex. Mol Phylog Evol. 2003;27:302–313. doi: 10.1016/s1055-7903(02)00400-1. [DOI] [PubMed] [Google Scholar]
- Chaverri P, Samuels GJ. Hypocrea/Trichoderma (Ascomycota, Hypocreales, Hypocreaceae): species with green ascospores. Stud Mycol. 2003;48:1–116. [Google Scholar]
- Dettman JR, Jacobson DJ, Taylor JW. A multilocus genealogical approach to phylogenetic species recognition in the model eukaryote Neurospora. Evolution. 2003;57:2703–1720. doi: 10.1111/j.0014-3820.2003.tb01514.x. [DOI] [PubMed] [Google Scholar]
- Doi Y. Revision of the Hypocreales with cultural observations IV. The genus Hypocrea and its allies in Japan. (1) General Part. Bull Nat Sci Mus (Tokyo) 1969;12:693–724. [Google Scholar]
- Doi Y. Revision of the Hypocreales with cultural observations IV. The genus Hypocrea and its allies in Japan. (2) Enumeration of the species. Bull Nat Sci Mus (Tokyo) 1972;15:649–751. [Google Scholar]
- Druzhinina IS, Kopchinskiy AG, Komon M, Bissett J, Szakacs G, Kubicek CP. An oligonucleotide barcode for species identification in Trichoderma and Hypocrea. Fungal Genet Biol. 2005;42:813–828. doi: 10.1016/j.fgb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Druzhinina IS, Kopchinskiy AG. TrichOKEY v. 2—a DNA oligonucleotide barcode program for the identification of multiple sequences of Hypocrea and Trichoderma; Proceedings of the 8th International Mycological Congress; Cairns, Australia. 2006. [Google Scholar]
- Gams W, Bissett J. Morphology and identification of Trichoderma. In: Kubicek CP, Harman GE, editors. Trichoderma and Gliocladium. Vol. 1. Taylor & Francis Ltd.; London: 1998. pp. 3–34. [Google Scholar]
- Hjeljord L, Tronsmo A. Trichoderma and Gliocladium in biological control: an overview. In: Harman GE, Kubicek CP, editors. Trichoderma and Gliocladium. Vol. 2. Taylor & Francis Ltd.; London: 1998. pp. 131–151. [Google Scholar]
- Huelsenbeck JP, Ronquist F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM, Komon M, Kubicek CP, Druzhinina IS. Hypocrea voglmayrii sp. nov. from the Austrian Alps represents a new phylogenetic clade in Hypocrea/Trichoderma. Mycologia. 2005;97:1365–1378. doi: 10.3852/mycologia.97.6.1365. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM, Komon M, Kubicek CP, Druzhinina IS. Hypocrea crystalligena sp. nov., a common European species with a white-spored Trichoderma anamorph. Mycologia. 2006a;98:499–513. doi: 10.3852/mycologia.98.3.499. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM, Samuels GJ, Dodd SL, Lu B-S, Druzhinina IS. Hypocrea rufa/Trichoderma viride: a reassessment, and description of five closely related species with and without warted conidia. Stud Mycol. 2006b;56:135–177. doi: 10.3114/sim.2006.56.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komon-Zelazowska M, Bissett J, Zafari D, Hatvani L, Manczinger L, Woo S, Lorito M, Kredics L, Kubicek CP, Druzhinina IS. Genetically closely related but phenotypically divergent Trichoderma species cause green mold disease in oyster mushroom farms worldwide. Appl Environm Microbiol. 2007;73:7415–7426. doi: 10.1128/AEM.01059-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopchinskiy AG, Komon M, Kubicek CP, Druzhinina IS. TrichoBLAST: a multilocus database for Trichoderma and Hypocrea identifications. Mycol Res. 2005;109:657–660. doi: 10.1017/s0953756205233397. [DOI] [PubMed] [Google Scholar]
- Kornerup A, Wanscher JH. Taschenlexikon der Farben. Muster-Schmidt Verlag; Zürich-Göttingen: 1981. [Google Scholar]
- Leache AD, Reeder TW. Molecular systematics of the eastern fence lizard Sceloporus undulatus: a comparison of parsimony, likelihood, and Bayesian approaches. Sys Biol. 2002;51:44–68. doi: 10.1080/106351502753475871. [DOI] [PubMed] [Google Scholar]
- Lu B, Druzhinina IS, Fallah P, Chaverri P, Gradinger C, Kubicek CP, Samuels GJ. Hypocrea/Trichoderma species with pachybasium-like conidiophores: teleomorphs for T. minutisporum and T. polysporum and their newly discovered relatives. Mycologia. 2004;96:310–342. [PubMed] [Google Scholar]
- Nicholas KB, Nicholas HB, Jr, Deerfield DW., II GeneDoc: analysis and visualization of genetic variation. EMBNET news. 1997;4:1–4. Program distributed by the authors. [Google Scholar]
- Park MS, Bae KS, Yu SH. Two new species of Trichoderma associated with green mold of oyster mushroom cultivation in Korea. Mycobiology. 2006;34:11–113. doi: 10.4489/MYCO.2006.34.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patouillard N. Hypocrea epimyces Sacc. et Pat. Tabulae Analyticae Fungorum. 1891;4:137–180. [Google Scholar]
- Rannala B, Yang Z. Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. J Mol Evol. 1996;43:304–311. doi: 10.1007/BF02338839. [DOI] [PubMed] [Google Scholar]
- Samuels GJ, Dodd SL, Gams W, Castlebury LA, Petrini O. Trichoderma species associated with the green mold epidemic of commercially grown Agaricus bisporus. Mycologia. 2002;94:146–170. [PubMed] [Google Scholar]
- Swofford DL. PAUP*: phylogenetic analysis using parsimony (*and other methods). Version 4.0b10. Sinauer Associates; Sunderland, Massachusetts: 2002. [Google Scholar]
- Tavaré S. Some probabilistic and statistical problems in the analysis of DNA sequences. In: Miura RM, editor. Some mathematical questions in biology—DNA sequence analysis. American Mathematical Society; Providence, Rhode Island: 1986. pp. 57–86. [Google Scholar]
- Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, Hibbett DS, Fisher MC. Phylogenetic species recognition and species concepts in Fungi. Fungal Genet Biol. 2000;31:21–31. doi: 10.1006/fgbi.2000.1228. [DOI] [PubMed] [Google Scholar]
- Vassilyeva LN. Pyrenomycetidae et Loculoascomycetidae. Nauka; St Petersburg: 1998. Plantae non vasculares, Fungi et Bryopsidae orientis extremi Rossica. Fungi 4; p. 418. In Russian. [Google Scholar]
- Werle E, Schneider C, Renner M, Völker M, Fiehn W. Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acid Res. 1994;22:4354–4355. doi: 10.1093/nar/22.20.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Rannala B. Bayesian phylogenetic inference using DNA sequences: a Markov chain Monte Carlo method. Mol Biol Evol. 1997;14:717–724. doi: 10.1093/oxfordjournals.molbev.a025811. [DOI] [PubMed] [Google Scholar]