SUMMARY
T-cell depleted allogeneic hematopoietic stem cell transplants (TCD-HSCT) have demonstrated durable disease-free survival with a low risk of graft vs. host disease (GVHD) in patients with AML. We investigated this approach in 61 patients with primary refractory or relapsed non-Hodgkin lymphoma (NHL), who underwent TCD-HSCT from January 1992 through September 2004. Patients received myeloablative cytoreduction consisting of hyperfractionated total body irradiation, followed by either thiotepa and cyclophosphamide (45 patients) or thiotepa and fludarabine (16 patients). We determined the second-line age-adjusted International Prognostic Index score (sAAIPI) prior to transplant. Median follow-up of surviving patients is 6 years. The 10-year overall (OS) and event-free-survival (EFS) were 50% and 43%, respectively. The relapse rate at 10 years was 21% in patients with chemosensitive disease and 52% in those with resistant disease at time of HSCT. Nine of the 18 patients who relapsed entered a subsequent CR. Overall survival (p=0.01) correlated with the sAAIPI. The incidence of grade II-IV acute GVHD was 18%. We conclude that allogeneic TCD-HSCT can induce high rates of OS and EFS in advanced NHL with a low incidence of GVHD. Furthermore, the sAAIPI can predict outcomes and may be used to select the most appropriate patients for this type of transplant.
Keywords: non-Hodgkin lymphoma, allogeneic bone marrow transplantation, allogeneic, T cell-depleted, graft-vs-host disease, prognostic factors
INTRODUCTION
High-dose chemoradiotherapy and autologous hematopoietic stem cell transplantation (auto-HSCT) are currently the treatment of choice for patients with relapsed, aggressive non-Hodgkin lymphoma (NHL) without bone marrow involvement.1 In patients with relapsed disease that is sensitive to second-line chemotherapy, the 5-year event-free survival (EFS) is 40–45%. In patients with relapsed disease that is resistant to standard-dose second-line chemotherapy, however, long-term EFS with auto-HSCT is only 10–15%. This is similar to that observed with standard-dose salvage regimens.2 Previous studies have demonstrated the predictive value of the second-line age-adjusted International Prognostic Index (sAAIPI) for auto-HSCT outcomes in patients with relapsed or primary refractory NHL.3–5
An alternative approach to auto-HSCT is an allogeneic HSCT (allo-HSCT). A number of studies have demonstrated a graft-versus-lymphoma (GVL) benefit after an allo-HSCT or in response to post-transplant donor leukocyte infusion (DLI).6–15 Furthermore, allo-HSCT offers a potentially curative option for many patients who are not candidates for auto-HSCT because of marrow involvement or inadequate stem cell mobilization. Unfortunately, the risks of transplant-associated morbidity and mortality, particularly those due to graft-vs-host disease (GVHD) and its complications, have often mitigated the greater curative potential of an allo-HSCT.16 In previous reports we examined the efficacy of T-cell depleted (TCD) allogeneic stem cell transplants as post-remission therapy in patients with myeloid or lymphoid malignancies using matched related donors, and we demonstrated an almost complete elimination of acute and chronic GVHD without compromising the antileukemic efficacy of the allograft.17, 18 We now report the results of a retrospective review of 61 patients with refractory or relapsed aggressive NHL who underwent allogeneic TCD-HSCT, and we demonstrate that the second-line age-adjusted IPI (sAAIPI) is an excellent predictor of overall survival (OS).
PATIENTS AND METHODS
Patients
Sixty-one consecutive patients with relapsed or refractory NHL underwent an allogeneic TCD-HSCT at Memorial Sloan-Kettering Cancer Center (MSKCC) from January 1992 through September 2004. These patients were analyzed as of December 2008. Written informed consent for treatment was obtained from all patients and donors. Approval for this retrospective review was obtained from the Institutional Review and Privacy Board. All patients were staged according to the Cotswolds modification of the Ann Arbor staging system,19 and all representative histologic specimens were reviewed by one of three hematopathologists. Eligibility criteria for transplant included a diagnosis of relapsed or refractory NHL; age less than 60 years; availability of an HLA-identical or single-allele mismatched donor; absence of active infection; and lack of coexisting cardiac, pulmonary, hepatic, or renal dysfunction that would preclude administration of the cytoreductive regimen. All patients received allografts in lieu of autografts because of marrow involvement, failure to mobilize sufficient autologous stem cells, and the availability of an appropriately HLA-matched related or unrelated donor. Prior to 1997, HLA matching was established by serologic identity for HLA-A, B, and DR loci, and where indicated, by isoelectric focusing for subtype matching at the A and B loci and by DNA sequence specific oligonucleotide typing for HLA-DR. Thereafter, HLA matching was established by DNA sequence-specific oligonucleotide typing for HLA-A, -B, and DR-B1 loci, and additionally for C and DQ-B1 loci for unrelated donors. The primary transplant outcomes for 10 of the patients have been previously reported.18
Transplant procedure and supportive care
All patients received myeloablative cytoreduction comprising hyperfractionated total body irradiation (HFTBI), followed by thiotepa and high-dose cyclophosphamide (45 patients) or thiotepa and fludarabine (16 patients). HFTBI (total dose of 1375 cGy or 1500 cGy, with lung blocking plus chest wall and testicular boosts) was administered as previously described.17 After HFTBI, thiotepa 5mg/kg i.v. was administered daily for two days. In the 45 patients who received the first regimen, administration of thiotepa was followed by high-dose cyclophosphamide 60mg/kg i.v. daily for two days.17 In the other 16 patients, fludarabine 25 mg/m2 i.v. was administered daily for five days beginning on the first day of thiotepa.18 The two regimens were successive transplant regimens used at MSKCC. T cells were removed from bone marrow grafts by sequential soybean lectin agglutination and sheep red blood cell (sRBC)-rosette depletion.17, 20 T cell depletion of granulocyte colony stimulating factor (G-CSF)-mobilized peripheral blood stem cells (PBSC) was accomplished by positive selection of CD34+ stem cells using the ISOLEX 300i Magnetic Cell Separator and subsequent sRBC-rosette depletion.18 T-cell depleted marrow or PBSC was infused within 24–48 hours after completion of the chemotherapy. Forty-two patients received BM and 15 received PBSC transplants, all T-cell depleted as above. Four patients received combined BM and PBSC allografts. One patient received the combined product due to poor stem-cell mobilization by the donor, while the other 3 patients were on a treatment protocol in which both products were being administered. Recipients of an HLA-matched related donor who were treated with HFTBI, thiotepa, and fludarabine (n=11) and one patient under the age of 18 did not receive any rejection prophylaxis. One patient under the age of 18 received steroids alone for graft rejection prophylaxis post-transplant. Equine or rabbit anti-thymocyte globulin with short-course high-dose methylprednisolone provided graft rejection prophylaxis in all other patients.17, 18, 21 All patients received supportive care and prophylaxis against opportunistic infections according to standard guidelines. Fifty-two patients received G-CSF in the early post-transplant period. No other cytokines were administered. Myeloid engraftment was defined as an absolute neutrophil count (ANC) > 500/μl on 3 consecutive days post-transplant. Platelet engraftment was defined as an untransfused platelet count > 20,000/μl for at least 3 consecutive days. Bone marrow aspirates were obtained at regular intervals post-transplant to assess engraftment and disease status. Donor chimerism was assessed in sex mismatched donor-recipient pairs by metaphase karyotype analyses or fluorescent in situ hybridization (FISH) of the X and Y chromosomes in interphase cells. DNA polymorphisms were compared in same sex pairs. GVHD was diagnosed clinically, confirmed pathologically by biopsy whenever possible, and classified according to standard criteria.22 Patients who engrafted were evaluable for acute GVHD, and patients surviving at least 100 days were evaluable for chronic GVHD.
Determination of the second-line age-adjusted International Prognostic Index (sAAIPI)
Patients often received salvage therapy before referral for allogeneic transplantation. For consistency, the sAAIPI was determined just before transplantation and calculated as previously described.3, 4, 23 Adverse factors for the sAAIPI, which only considers patients < 60 years old, included KPS <80, LDH >normal, and stage III/IV disease. sAAIPI groups were low risk (L) with zero factors, low-intermediate risk (LI) with 1 factor, high-intermediate risk (HI) with 2 factors, and high risk (H) with all 3 factors present.
Data collection and statistical methods
Analyses were performed as of 12/31/2008. EFS and OS were assessed from the day of HSCT. An event was defined as the presence of persistent disease after HSCT, relapse, or death from any cause. The OS and EFS probabilities were calculated using the method of Kaplan and Meier, and the differences between levels within a covariate were tested using the log rank statistic.24 The relapse and non-relapse mortality probabilities were estimated using the cumulative incidence function. Differences in patient characteristics on the two conditioning regimens were analyzed using the Fisher’s Exact test. The following pre-transplant variables were assessed for their effects on overall survival: age (≤40 vs. >40), lymphoma immunophenotype (B vs. T cell), status of relapsed disease (sensitive vs. refractory), disease stage, LDH (normal vs. elevated), KPS (<80 vs. ≥80), number of extranodal sites involved (≤1 vs. >1), bone marrow involvement, number of prior regimens (<3 vs. >3), and sAAIPI (L vs. LI vs. HI vs. H). Finally, in the 42 patients with B-cell NHL, the use of pre-, post-, or pre- and post-HSCT rituximab was evaluated. Univariate analyses were performed using the log-rank test. Subsequent to the univariate analysis, a multivariate analysis using the Cox proportional hazards model was performed.
RESULTS
Patient characteristics
Pre-transplant characteristics of the 61 patients are listed in Table 1. The median age was 41 years. The median number of prior regimens was two, and 48% of patients had received more than two regimens. Thirty-six percent of patients did not have a measurable response to the chemotherapy regimen immediately preceding HSCT (Table 1). Thirty-four percent of the patients had “poor risk” disease, defined by an sAAIPI of high-intermediate or high risk. There were no statistically significant differences in the baseline patient characteristics between the two transplant regimens (data not shown).
Table 1.
Patient Characteristics
| Patient Characteristic | No. Patients |
|---|---|
| Age (y) | |
| Median [range] | 41 [5–59] |
| <20 | 8 |
| 20–29 | 4 |
| 30–39 | 16 |
| 40–59 | 33 |
| Donor | |
| Matched related | 42 |
| Mismatched related | 8 |
| Matched unrelated | 8 |
| Mismatched unrelated | 3 |
| Histologic Diagnosis (WHO classification) | |
| Diffuse large B-cell | 7 |
| Mantle Cell | 9 |
| Lymphoblastic | 10 |
| Anaplastic large cell | 7 |
| Follicular | 10 |
| Transformed | 11 |
| Othera | 7 |
| Stageb | |
| II | 9 |
| III | 11 |
| IV | 26 |
| Karnofsky Performance Status | |
| ≥ 80 | 50 |
| < 80 | 11 |
| Extranodal sites involved | |
| ≤ 1 | 51 |
| > 1 | 10 |
| LDH | |
| Median [range] | 174 [104–474] |
| ≤ 210 | 39 |
| > 210 | 22 |
| Bone marrow involvementc | |
| Yes | 14 |
| No | 47 |
| Prior chemotherapy regimens | |
| Median [range] | 2 [1–8] |
| 1–2 | 32 |
| >2 | 29 |
| Response to last therapy | |
| Complete remission | 15 |
| Partial remission | 24 |
| Stable disease | 10 |
| Refractory | 12 |
| Second-line age-adjusted IPI (sAAIPI) | |
| Low-risk | 18 |
| Low-intermediate risk (1) | 22 |
| High-intermediate risk (2) | 13 |
| High risk (3) | 8 |
Diffuse small cleaved cell (n=1), Diffuse mixed small and large cell (n=1), Small lymphocytic (n=1), HTLV-I associated Adult T cell (n=1), Angioimmunoblastic (n=1), Burkitt’s (n=1), Mycosis Fungoides (n=1);
Fifteen patients were in CR prior to allo-HSCT – highest stage since diagnosis: stage III=9, stage IV=52;
Bone marrow involvement at any time since diagnosis was present in 38 patients.
Engraftment and donor chimerism
The median cell dose of the infused T-cell depleted marrow and/or PBSC allograft was 1.8 × 107 nucleated cells/kg of recipient weight (range 0.17 – 8.59). All 61 patients achieved primary engraftment. These data are summarized in Table 2. By three months post HSCT, complete donor chimerism was observed in 45 patients and mixed chimerism in three patients. Three additional patients had complete donor chimerism at 12 months. No chimerism data were available for the remaining 10 patients, including 8 patients who died early post-transplant. There were no immune-mediated graft rejections. One patient experienced late graft failure in the setting of relapsed transformed CLL/SLL and underwent a successful non-myeloablative second transplant from the original donor 11 months after the first transplant.
Table 2.
Outcome parameters
| Engraftment | |
| Median transplant cell dose × 107/kg (range) | 1.8 (0.17 – 8.59) |
| Median days to sustaineda ANC ≥ 500 (range) | 12 (8 – 30) |
| Median days to sustainedb platelet count ≥ 20,000 (range) | 22 (10 – 170) |
| GVHD | |
| Acute, grade I | 3c |
| Acute, grade II | 7 |
| Acute, grade III | 4 |
| Acute, grade IV | 0 |
| Chronic, limited | 5c |
| Chronic, extensive | 3d |
| Causes of death | |
| Relapse | 8 |
| Infectious causes | 3 CMV pneumonitis |
| 7 othere | |
| GVHD and secondary complications | 8 |
| Interstitial pneumonitis | 2 |
| Lung cancer | 1f |
| Post-transplant EBV-LPD | 4 |
sustained denotes 3 consecutive days;
sustained denotes 3 consecutive days and platelet transfusion independent;
including one patient post-DLI;
including 2 patients post DLI;
other infections: sepsis (no identified organism) – n=2, E Coli sepsis – n=1, disseminated pseudomonas aeruginosa – n=1, disseminated adenovirus – n=1, toxoplasmosis – n=1; fever with no identified organism – n=1;
the patient who died of lung cancer was also found to have relapsed mantle cell lymphoma on a mediastinal lymph node biopsy.
Graft-vs-host disease
The median CD3+ cell dose for the 61 patients was 1 × 103/kg, which was consistent with our prior experience.18 All patients were evaluable for acute GVHD. Eleven patients (18%) developed acute grade II-III GVHD after the TCD-HSCT (Table 2). No patients developed grade IV acute GVHD. Five of the patients with acute GVHD received marrow from a matched related donor (MRD), and the other six from alternative donors (two matched unrelated donors and 4 mismatched related or unrelated donors). The rate of grade II-III acute GVHD among recipients of MRD was lower than in recipients of alternative donors (12% vs. 32%, p=0.05). An additional patient developed acute GVHD after DLI for relapsed disease.
The incidence of chronic GVHD in 43 evaluable patients was 12%. Four patients (recipient of MRD = 1, alternative donors = 3) developed limited chronic GVHD and one (recipient of MRD) developed extensive chronic GVHD following acute GVHD. No patients developed chronic GVHD de novo. An additional three patients developed acute and chronic GVHD following DLI.
Post-transplant Epstein-Barr virus lymphoproliferative disorders (EBV-LPD)
All patients were routinely monitored for EBV-LPD using standardized PCR assays. Four of the 61 patients developed a post-transplant donor-derived EBV-LPD and were treated with DLI (and with subsequent rituximab in one case). Three patients responded with resolution, but the fourth patient had progression of the EBV-LPD and subsequently died of sepsis. This fourth patient had developed EBV-LPD while on steroids for acute GVHD. A fifth patient, who died of disseminated toxoplasmosis, had evidence of EBV-LPD incidentally found at autopsy.
Causes of death
The 100-day OS was 73.8% (95% CI: 64 – 84%) and the 100-day non-relapse mortality was 19.7% (95% CI: 10 – 30%). The causes of treatment failure and other outcome parameters are summarized in Table 2. Twenty-nine of the 61 patients transplanted with advanced NHL have died, including 8 patients with relapsed disease.
Treatment outcome
Median follow-up of surviving patients is 6 years. Nineteen patients (31%) relapsed after TCD-HSCT (Table 3), with a median time to relapse of 3.6 months (range: 0.7 – 128 months). One of 15 patients in CR (a patient with transformed follicular lymphoma in CR3) before transplant relapsed, and none of the patients transplanted in CR1 (n=1) or CR2 (n=11) relapsed. Five patients who relapsed with follicular lymphoma achieved a CR after salvage therapy with rituximab (n=1); DLI (n=1); DLI with rituximab (n=1); DLI with chemotherapy (n=1); or chemotherapy, radiation, rituximab, and DLI (n=1). An additional 5 patients with other types of NHL who relapsed entered a subsequent CR after rituximab with (n=1) or without DLI (n=1); chemotherapy (n=1); surgery, radiation, and rituximab (n=1); and DLI and a second transplant (n=1).
Table 3.
Relapse based on disease status at time of transplanta.
| Response to salvage therapy |
sAAIPI |
All | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CR | PR | SD | POD | L | LI | HI | H | ||
| Diffuse large B-cell | 0/3 | 0/2 | 0/1 | 1/1 | 0/3 | 0/1 | 1/2 | 0/1 | 1/7 |
| Mantle Cell | - | 2/7 | 0/1 | 1/1 | 2/2 | 1/4 | 0/3 | - | 3/9 |
| Lymphoblastic | 0/8 | - | - | 2/2 | 0/5 | 0/3 | 1/1 | 1/1 | 2/10 |
| Anaplastic large cell | 0/1 | 1/3 | 0/1 | 1/2 | 0/2 | 2/2 | - | 0/3 | 2/7 |
| Follicular | - | 2/5 | 2/3 | 1/2 | 1/3 | 3/5 | 1/2 | - | 5/10 |
| Transformed | 1/3 | 2/3 | 0/3 | 1/2 | 1/3 | 3/5 | 0/2 | 0/1 | 4/11 |
| Otherb | - | 1/4 | 1/1 | 0/2 | - | 1/2 | 1/3 | 0/2 | 2/7 |
| Total | 1/15 (7%) | 8/24 (33%) | 3/10 (30%) | 7/12 (58%) | 4/18 (22%) | 10/22 (45%) | 4/13 (31%) | 1/8 (13%) | 19/61 (31%) |
CR: complete remission; PR: partial remission; SD: stable disease; POD: progression of disease.
Other: as per Table 1
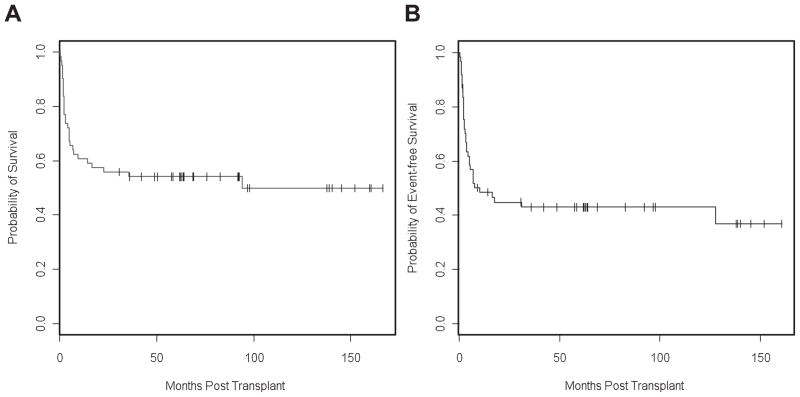
Overall and event-free survival
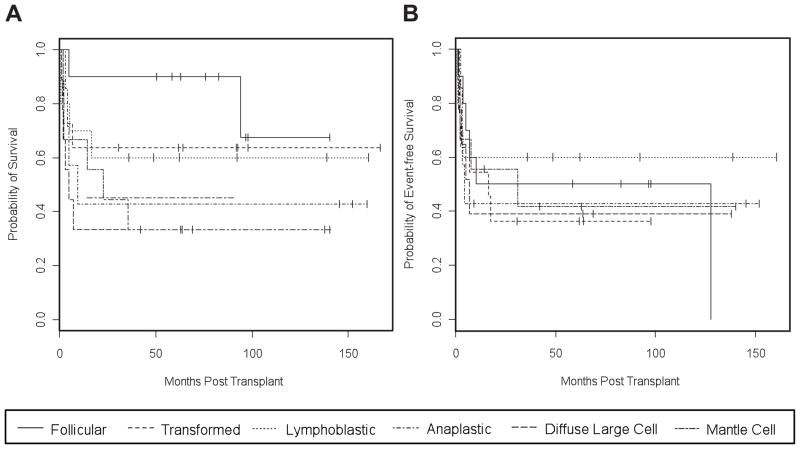
The 10-year OS for patients with NHL after TCD-HSCT was 50% (95% CI: 38 – 66%), and 10-year EFS was 43% (95% CI: 32 – 58%) (Figure 1). When analyzed by lymphoma subtype, 10-year OS was 68% (95% CI: 37 – 100%) in patients with follicular lymphoma, 64% (95% CI: 41 – 100%) in patients with transformed lymphoma, 60% (95% CI: 36 – 100%) in patients with lymphoblastic lymphoma, 43% (95% CI: 18 – 100%) in patients with anaplastic large cell lymphoma, 33% (95% CI: 13 – 84%) in patients with diffuse large cell lymphoma and 33% (95% CI: 13 – 84%) in patients with mantle cell lymphoma (Figure 2). The 10-year OS was 69% (95% CI: 49 – 96%) in patients who were in CR at the time of HSCT, 57% (95% CI: 40 – 81%) in patients with a PR, 40% (95% CI: 16 – 100%) in patients with stable but persistent disease, and 25% (95% CI: 9 – 67%) in patients with progressive disease. Five of the 10 patients with stable disease at HSCT who remain alive and free of disease had follicular lymphoma (n=2), transformed lymphoma (n=2), and mantle cell lymphoma (n=1). No differences were observed in OS or EFS when we compared outcomes for the two different regimens (TBI-thiotepa-cyclophosphamide vs. TBI-thiotepa-fludarabine) or the dose of TBI (1500 cGy vs. 1375 cGy) (data not shown).
Figure 1.
Kaplan-Meier estimates of probability of OS (A) and EFS (B) for 61 patients transplanted with TCD grafts for advanced NHL.
Figure 2.
OS (A) and EFS (B) for 61 patients transplanted with TCD grafts for advanced NHL stratified by NHL subtype. See Table 3 for details.
Analysis of prognostic factors
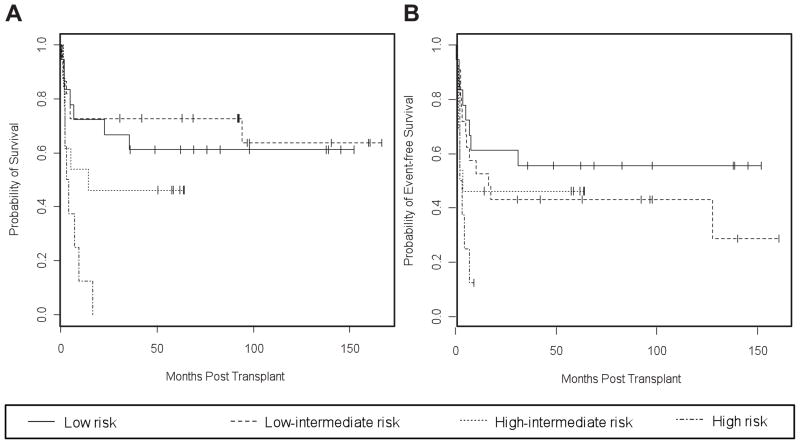
Because previous studies at our center have demonstrated the predictive value of the second-line AAIPI for auto-HSCT outcomes in patients with relapsed or primary refractory NHL,3–5 we calculated the second-line AAIPI for all patients (Table 1). Relapse rates were 17%, 55%, 31% and 13% in patients with low risk, low-intermediate risk, high-intermediate risk and high risk sAAIPI (Table 3). While the OS (p=0.01) correlated with the sAAIPI, the observed trend for EFS did not reach statistical significance (p=0.07; Table 4, Figure 3). OS at two years was 67% (95% CI: 48 – 92%) in patients with low risk sAAIPI, 73% (95% CI: 56 – 94%) with a low-intermediate risk sAAIPI, and 46% (95% CI: 26 – 83%) with a high-intermediate risk sAAIPI. The 8 patients with a high-risk sAAIPI expired. Six of the 8 patients had resistant disease and 2 achieved only a PR following pre-HSCT salvage therapy. This group included 3 patients with anaplastic large cell lymphoma (Table 3).
Table 4.
Analysis of factors predictive of EFS and OS.
| Parameter | Event-Free Survival | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysisa | Univariate analysis | Multivariate analysis | |||||
| P value | Hazard Ratio | P value | Hazard Ratio | P value | Hazard Ratio | P value | Hazard Ratio | |
| Age (<41 years) | 0.21 | 0.37 | ||||||
| No BM involvement | 0.49 | 0.39 | ||||||
| Immunophenotype | 0.31 | 0.45 | ||||||
| Stage (I, II, III, IV) | 0.15 | 0.47 | ||||||
| LDH > normal | 0.08 | 0.004 | 2.88 | 0.03 | 2.37 | |||
| > 1 extranodal site | 0.46 | 0.76 | ||||||
| < 3 prior regimens | 0.11 | 0.007 | 2.78 | 0.07 | 2.10 | |||
| Sensitive disease at BMT | 0.005 | 2.53 | 0.005 | 2.53 | 0.08 | |||
| KPS < 80 | 0.13 | 0.01 | 0.37 | |||||
| Rituximabb | 0.35, 0.16, | 0.61, 0.30, | ||||||
| 0.46 | 0.08 | |||||||
| sAAIPI (Lc, LI, HI, H) | 0.07 | 0.01 | 1.00 | |||||
| 0.81 | ||||||||
| 1.84 | ||||||||
| 4.38 | ||||||||
Multivariate analysis excludes sAAIPI;
Treatment with Rituximab (pre-, post- or pre- and post-HSCT) was analyzed in 42 patients with B-cell immunophenotype;
L: low risk; LI: low-intermediate risk; HI: high-intermediate risk; H: high risk.
Figure 3.
OS (A) and EFS (B) for patients, stratified by sAAIPI risk group: low-intermediate, intermediate, and high. Kaplan-Meier curves OS and (B) EFS in 61 transplanted patients, stratified by sAAIPI risk groups: low risk (0 factor), low-intermediate risk (1 factor), high-intermediate (2 factors), and high risk (3 factors). sAAIPI risk factors include KPS <80, LDH >normal, and stage III/IV. See Table 3 for details.
The results of the univariate analysis of factors predictive of OS and EFS are summarized in Table 4. The factors associated with improved OS were normal LDH, fewer than 3 prior regimens, and KPS ≥ 80 prior to transplant. Only the presence of treatment-sensitive disease at transplant was associated with a favorable prognosis in EFS. There was no association between survival and other factors examined, including the age of the patient, the immunophenotype, the stage of disease, the presence or absence of prior bone marrow involvement, the presence of more than one extranodal site, and the use of rituximab. There was a trend towards improved OS in recipients of matched related donors vs. non-identical donors, although this did not reach statistical significance (56% vs. 37%, p=0.08). No effect of donor type was noted on EFS. A multivariate analysis, which excluded the sAAIPI, revealed that a normal LDH and exposure to fewer than 3 prior regimens resulted in a better OS, while only the presence of sensitive disease at transplant was associated with a favorable prognosis in EFS.
DISCUSSION
We have previously shown that TCD-HSCT can result in durable disease-free survival and low rates of GVHD in patients with AML and other hematologic malignancies.17, 18 Similarly, we now report 10-year OS of 50% and 10-year EFS of 43% in patients with advanced NHL, with a low incidence of GVHD. The observed 18% grade II-III acute GVHD, while low for this patient population, is higher than previously reported by our group in TCD-BMT.17 A number of factors may explain this higher rate of GVHD, including the use of MUD and MMD vs. all MRD, the use of PBSCT and/or BM rather than exclusively BM as in the prior study, a higher median age (41 vs. 36.7), and a greater number of patients over 40 (54% vs. 32%).
A number of studies in patients with NHL have shown lower relapse rates but higher treatment-related mortality (TRM) following allo-HSCT, when compared with auto-HSCT.9, 10, 25–28 This usually resulted in a similar OS. The differences in relapse and TRM are driven in large part by immune-mediated effects of the allograft that are responsible for GVL effects as well as GVHD. Since GVL is mediated by T cells, a higher risk of relapse remains a concern after a TCD-HSCT. Two smaller series of TCD-HSCT have previously been reported. In one study of 37 patients undergoing partial TCD-HSCT, the 5-year OS was 45%, with 36% grade II-IV acute GVHD.29 Twenty-two patients, transplanted in CR or PR with grafts that were TCD using a monoclonal antibody to CD6, had an OS of 59% at 40 months with a 36% relapse rate.30 The overall relapse rate in the present study was 33%, with most of the relapses occurring in patients who were not in CR prior to transplant. As may have been predicted by the use of T cell depletion of the graft, relapses were observed primarily in patients with follicular and transformed lymphoma, consistent with the results of others demonstrating GVL effects in low-grade lymphomas.7, 8, 11, 14 It should be noted, however, that none of the patients with follicular lymphoma in the present study were in CR at the time of transplant. Furthermore, those patients who relapsed were treated successfully with DLI. The fact that relapses occurred primarily in patients with follicular, transformed, and mantle cell lymphoma may also account for the fact that we observed a higher relapse rate in patients with low-intermediate risk sAAPI. In contrast, we observed low relapse rates in patients with lymphoblastic lymphoma and DLCL, suggesting that the major benefit may be from the conditioning regimen and that the anti-lymphoma effect is preserved against these lymphoma subtypes even in the setting of TCD allografts. Furthermore, it should be noted that the rates of relapse were similar in patients with and without GVHD in the present study, suggesting that there was no direct correlation between GVHD and GVL effects.
While the present study describes the efficacy of TCD-HSCT to reduce post-transplant morbidity due to GVHD while maintaining an acceptable EFS and OS other types of allo-HSCT have been developed for specific subtypes of NHL. Reduced-intensity conditioning (RIC) has become a preferred treatment modality in patients with low-grade NHL based primarily on the decrease in TRM as well as the contribution of its GVL effect.7, 8, 11 In contrast, poor outcomes have been reported in chemoresistant and aggressive lymphoma after RIC,31 suggesting that an ablative TCD-HSCT should be considered for these patients.
In order to improve selection of patients with NHL who are most likely to benefit from an allo-HSCT, a number of investigators have tried to identify prognostic factors in these patients.15, 16, 28, 30, 32 In a recently published study of patients with NHL undergoing myeloablative transplantation, chemoresistance and prior auto-HSCT or radiotherapy were identified as adverse prognostic factors.16 In another large study of patients with follicular NHL that compared allo-HSCT (176 patients) with purged auto-HSCT (n=131) or non-purged auto-HSCT (n=597), negative prognostic factors, regardless of transplant type, included advanced age, prolonged time to transplantation, low KPS, high LDH, refractory disease, and transplant performed prior to 1993.28 No association was observed between the presence of acute or chronic GVHD and recurrence. The factors associated with improved OS in the current study were normal LDH, fewer than 3 prior regimens and KPS ≥ 80. The presence of responsive disease correlated with improved EFS. The differences noted between various studies may be attributed to small sample size and heterogeneity in lymphoma subtypes as well as transplant regimens.
The heterogeneity in NHL subtypes and the variable disease status at the time of transplant have made it difficult to compare outcomes in published reports of allo-HSCT for NHL. Survival rates ranging from 24% to 80% have been reported.6–13, 15, 16, 29, 30, 32, 33 Tools are needed to assist in comparison between studies, and in the selection of patients most likely to benefit from allo-HSCT. Investigators at our center had previously reported the utility of the sAAIPI in predicting outcomes after auto-HSCT in patients with DLCL3, 4 and peripheral T-cell lymphoma.5 We therefore investigated the use of this tool in patients undergoing TCD-HSCT, and have shown that the sAAIPI could predict OS in these patients with recurrent or refractory NHL. It should be noted that, unlike the studies of auto-HSCT where the sAAIPI was determined prior to salvage, we determined it immediately prior to transplantation. As patients who respond to salvage therapy may have an improvement in their disease staging, KPS, and LDH, this have may resulted in a more favorable sAAIPI score than one determined before salvage, as was done in patients undergoing auto-HSCT.
We conclude that myeloablative TCD-HSCT can induce high rates of OS and EFS with a low incidence of GVHD in patients with advanced NHL. Furthermore, the sAAIPI at time of transplant can predict outcomes and may be useful in selecting the appropriate patients for this type of transplant. Further prospective studies will be required to validate these findings including the use of TCD-HSCT in patients with aggressive NHL, and the predictive power of the sAAIPI, in different subtypes of NHL and using other transplant approaches such as RIC regimens for patients with low-grade lymphomas.
Acknowledgments
SUPPORT: Supported in part by P01 CA23766.
We gratefully acknowledge the expert care provided to these patients by the fellows, housestaff, and nurses of Memorial Sloan-Kettering Cancer Center.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
References
- 1.Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 2.Philip T, Armitage JO, Spitzer G, Chauvin F, Jagannath S, Cahn JY, et al. High-dose therapy and autologous bone marrow transplantation after failure of conventional chemotherapy in adults with intermediate-grade or high-grade non-Hodgkin’s lymphoma. N Engl J Med. 1987;316:1493–1498. doi: 10.1056/NEJM198706113162401. [DOI] [PubMed] [Google Scholar]
- 3.Kewalramani T, Zelenetz AD, Hedrick EE, Donnelly GB, Hunte S, Priovolos AC, et al. High-dose chemoradiotherapy and autologous stem cell transplantation for patients with primary refractory aggressive non-Hodgkin lymphoma: an intention-to-treat analysis. Blood. 2000;96:2399–2404. [PubMed] [Google Scholar]
- 4.Hamlin PA, Zelenetz AD, Kewalramani T, Qin J, Satagopan JM, Verbel D, et al. Age-adjusted International Prognostic Index predicts autologous stem cell transplantation outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2003;102:1989–1996. doi: 10.1182/blood-2002-12-3837. [DOI] [PubMed] [Google Scholar]
- 5.Kewalramani T, Zelenetz AD, Teruya-Feldstein J, Hamlin P, Yahalom J, Horwitz S, et al. Autologous transplantation for relapsed or primary refractory peripheral T-cell lymphoma. Br J Haematol. 2006;134:202–207. doi: 10.1111/j.1365-2141.2006.06164.x. [DOI] [PubMed] [Google Scholar]
- 6.van Besien K, Sobocinski KA, Rowlings PA, Murphy SC, Armitage JO, Bishop MR, et al. Allogeneic bone marrow transplantation for low-grade lymphoma. Blood. 1998;92:1832–1836. [PubMed] [Google Scholar]
- 7.Khouri IF, Saliba RM, Giralt SA, Lee MS, Okoroji GJ, Hagemeister FB, et al. Nonablative allogeneic hematopoietic transplantation as adoptive immunotherapy for indolent lymphoma: low incidence of toxicity, acute graft-versus-host disease, and treatment-related mortality. Blood. 2001;98:3595–3599. doi: 10.1182/blood.v98.13.3595. [DOI] [PubMed] [Google Scholar]
- 8.Khouri IF, Lee MS, Saliba RM, Jun G, Fayad L, Younes A, et al. Nonablative allogeneic stem-cell transplantation for advanced/recurrent mantle-cell lymphoma. J Clin Oncol. 2003;21:4407–4412. doi: 10.1200/JCO.2003.05.501. [DOI] [PubMed] [Google Scholar]
- 9.Hosing C, Saliba RM, McLaughlin P, Andersson B, Rodriguez MA, Fayad L, et al. Long-term results favor allogeneic over autologous hematopoietic stem cell transplantation in patients with refractory or recurrent indolent non-Hodgkin’s lymphoma. Ann Oncol. 2003;14:737–744. doi: 10.1093/annonc/mdg200. [DOI] [PubMed] [Google Scholar]
- 10.Levine JE, Harris RE, Loberiza FR, Jr, Armitage JO, Vose JM, Van Besien K, et al. A comparison of allogeneic and autologous bone marrow transplantation for lymphoblastic lymphoma. Blood. 2003;101:2476–2482. doi: 10.1182/blood-2002-05-1483. [DOI] [PubMed] [Google Scholar]
- 11.Maris MB, Sandmaier BM, Storer BE, Chauncey T, Stuart MJ, Maziarz RT, et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104:3535–3542. doi: 10.1182/blood-2004-06-2275. [DOI] [PubMed] [Google Scholar]
- 12.Faulkner RD, Craddock C, Byrne JL, Mahendra P, Haynes AP, Prentice HG, et al. BEAM-alemtuzumab reduced-intensity allogeneic stem cell transplantation for lymphoproliferative diseases: GVHD, toxicity, and survival in 65 patients. Blood. 2004;103:428–434. doi: 10.1182/blood-2003-05-1406. [DOI] [PubMed] [Google Scholar]
- 13.Escalon MP, Champlin RE, Saliba RM, Acholonu SA, Hosing C, Fayad L, et al. Nonmyeloablative allogeneic hematopoietic transplantation: a promising salvage therapy for patients with non-Hodgkin’s lymphoma whose disease has failed a prior autologous transplantation. J Clin Oncol. 2004;22:2419–2423. doi: 10.1200/JCO.2004.09.092. [DOI] [PubMed] [Google Scholar]
- 14.Marks DI, Lush R, Cavenagh J, Milligan DW, Schey S, Parker A, et al. The toxicity and efficacy of donor lymphocyte infusions given after reduced-intensity conditioning allogeneic stem cell transplantation. Blood. 2002;100:3108–3114. doi: 10.1182/blood-2002-02-0506. [DOI] [PubMed] [Google Scholar]
- 15.van Besien K, Carreras J, Bierman PJ, Logan BR, Molina A, King R, et al. Unrelated donor hematopoietic cell transplantation for non-hodgkin lymphoma: long-term outcomes. Biol Blood Marrow Transplant. 2009;15:554–563. doi: 10.1016/j.bbmt.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SW, Tanimoto TE, Hirabayashi N, Goto S, Kami M, Yoshioka S, et al. Myeloablative allogeneic hematopoietic stem cell transplantation for non-Hodgkin lymphoma: a nationwide survey in Japan. Blood. 2006;108:382–389. doi: 10.1182/blood-2005-02-0596. [DOI] [PubMed] [Google Scholar]
- 17.Papadopoulos EB, Carabasi MH, Castro-Malaspina H, Childs BH, Mackinnon S, Boulad F, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood. 1998;91:1083–1090. [PubMed] [Google Scholar]
- 18.Jakubowski AA, Small TN, Young JW, Kernan NA, Castro-Malaspina H, Hsu KC, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110:4552–4559. doi: 10.1182/blood-2007-06-093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630–1636. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 20.Kernan NA, Flomenberg N, Collins NH, O’Reilly RJ, Dupont B. Quantitation of T lymphocytes in human bone marrow by a limiting dilution assay. Transplantation. 1985;40:317–322. doi: 10.1097/00007890-198509000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Kernan NA, Bordignon C, Heller G, Cunningham I, Castro-Malaspina H, Shank B, et al. Graft failure after T-cell-depleted human leukocyte antigen identical marrow transplants for leukemia: I. Analysis of risk factors and results of secondary transplants. Blood. 1989;74:2227–2236. [PubMed] [Google Scholar]
- 22.Rowlings PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Henslee-Downey PJ, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 23.Shipp MA, Harrington DP, Anderson JR, Armitage JO, Bonadonna G, Brittinger G, et al. A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 24.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 25.Ratanatharathorn V, Uberti J, Karanes C, Abella E, Lum LG, Momin F, et al. Prospective comparative trial of autologous versus allogeneic bone marrow transplantation in patients with non-Hodgkin’s lymphoma. Blood. 1994;84:1050–1055. [PubMed] [Google Scholar]
- 26.Schimmer AD, Jamal S, Messner H, Keating A, Meharchand J, Huebsch L, et al. Allogeneic or autologous bone marrow transplantation (BMT) for non-Hodgkin’s lymphoma (NHL): results of a provincial strategy. Ontario BMT Network, Canada. Bone Marrow Transplant. 2000;26:859–864. doi: 10.1038/sj.bmt.1702625. [DOI] [PubMed] [Google Scholar]
- 27.Peniket AJ, Ruiz de Elvira MC, Taghipour G, Cordonnier C, Gluckman E, de Witte T, et al. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant. 2003;31:667–678. doi: 10.1038/sj.bmt.1703891. [DOI] [PubMed] [Google Scholar]
- 28.van Besien K, Loberiza FR, Jr, Bajorunaite R, Armitage JO, Bashey A, Burns LJ, et al. Comparison of autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Blood. 2003;102:3521–3529. doi: 10.1182/blood-2003-04-1205. [DOI] [PubMed] [Google Scholar]
- 29.Juckett M, Rowlings P, Hessner M, Keever-Taylor C, Burns W, Camitta B, et al. T cell-depleted allogeneic bone marrow transplantation for high-risk non-Hodgkin’s lymphoma: clinical and molecular follow-up. Bone Marrow Transplant. 1998;21:893–899. doi: 10.1038/sj.bmt.1701209. [DOI] [PubMed] [Google Scholar]
- 30.Soiffer RJ, Freedman AS, Neuberg D, Fisher DC, Alyea EP, Gribben J, et al. CD6+ T cell-depleted allogeneic bone marrow transplantation for non-Hodgkin’s lymphoma. Bone Marrow Transplant. 1998;21:1177–1181. doi: 10.1038/sj.bmt.1701271. [DOI] [PubMed] [Google Scholar]
- 31.Robinson SP, Goldstone AH, Mackinnon S, Carella A, Russell N, de Elvira CR, et al. Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood. 2002;100:4310–4316. doi: 10.1182/blood-2001-11-0107. [DOI] [PubMed] [Google Scholar]
- 32.Kiss TL, Panzarella T, Messner HA, Meharchand J, Reddy V, Schimmer AD, et al. Busulfan and cyclophosphamide as a preparative regimen for allogeneic blood and marrow transplantation in patients with non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2003;31:73–78. doi: 10.1038/sj.bmt.1703790. [DOI] [PubMed] [Google Scholar]
- 33.Dhedin N, Giraudier S, Gaulard P, Esperou H, Ifrah N, Michallet M, et al. Allogeneic bone marrow transplantation in aggressive non-Hodgkin’s lymphoma (excluding Burkitt and lymphoblastic lymphoma): a series of 73 patients from the SFGM database. Societ Francaise de Greffe de Moelle. Br J Haematol. 1999;107:154–161. doi: 10.1046/j.1365-2141.1999.01666.x. [DOI] [PubMed] [Google Scholar]