Abstract
Ca2+-dependent facilitation and inactivation (CDF and CDI) of Cav2.1 channels modulate presynaptic P/Q-type Ca2+ currents and contribute to activity-dependent synaptic plasticity. This dual feedback regulation by Ca2+ involves calmodulin (CaM) binding to the α1 subunit (α12.1). The molecular determinants for Ca2+-dependent modulation of Cav2.1 channels reside in CaM and in two CaM-binding sites in the C-terminal domain of α12.1, the CaM-binding domain (CBD) and the IQ-like domain. In transfected tsA-201 cells, CDF and CDI were both reduced by deletion of CBD. In contrast, alanine substitution of the first two residues of the IQ-like domain (IM-AA) completely prevented CDF but had little effect on CDI, and glutamate substitutions (IM-EE) greatly accelerated voltage-dependent inactivation but did not prevent CDI. Mutational analyses of the Ca2+ binding sites of CaM showed that both the N- and C-terminal lobes of CaM were required for full development of facilitation, but only the N-terminal lobe was essential for CDI. In biochemical assays, CaM12 and CaM34 were unable to bind CBD, whereas CaM34 but not CaM12 retained Ca2+-dependent binding to the IQ-like domain. These findings support a model in which Ca2+ binding to the C-terminal EF-hands of preassociated CaM initiates CDF via interaction with the IQ-like domain. Further Ca2+ binding to the N-terminal EF-hands promotes secondary CaM interactions with CBD, which enhance facilitation and cause a conformational change that initiates CDI. This multifaceted mechanism allows positive regulation of Cav2.1 in response to local Ca2+ increases (CDF) and negative regulation during more global Ca2+ increases (CDI).
P/Q-type Ca2+ currents conducted by presynaptic Cav2.1 channels initiate neurotransmitter release at many central synapses. In nerve terminals, activity-dependent increases in intracellular Ca2+ ions cause an initial facilitation followed by a progressive inactivation of P/Q-type Ca2+ currents, which can alter synaptic efficacy (1-3). In transfected cells, Cav2.1 channels undergo a similar dual feedback regulation by Ca2+ that is mediated by calmodulin (CaM) (4, 5). CaM binds to the poreforming α1 subunit of Cav2.1 channels (α12.1), causing an initial Ca2+-dependent facilitation (CDF) and, on a longer time scale, Ca2+-dependent inactivation (CDI) of these channels during repetitive stimuli (4, 5).
How CaM binding to α12.1 results in two opposing forms of channel regulation is not yet clear. A CaM-binding domain (CBD) in the cytoplasmic C-terminal tail of α12.1 binds CaM in a Ca2+-dependent manner, and deletion of this site diminishes but does not completely abolish CDF and CDI (4, 5). In addition, a second site on the N-terminal side of the CBD also participates in CaM regulation of Cav2.1 (6). This IQ-like domain is similar to the one that mediates CDI of L-type Ca2+ currents through Cav1.2 channels (7-9) but lacks the key Q residue at position 2 and hydrophobic residue at position 8 that characterize other IQ domains (10). Peptides corresponding to the IQ-like domain of α12.1 bind CaM in vitro, and mutations in the IQ-like domain impair CDF of Cav2.1 channels (6, 7, 11). Moreover, fluorescence resonance energy transfer experiments show that CaM mutants incapable of binding Ca2+ interact with Ca2+ channels, suggesting that apoCaM is constitutively associated with Cav2.1 (12). In contrast, biochemical experiments that require stable association of CaM show strictly Ca2+-dependent binding of CaM to the CBD and the IQ-like domain (4, 7).
To clarify the role of the CBD and IQ-like domains in CDF and CDI, we analyzed the contributions of the CBD, the IQ-like domain, and the N- and C-terminal lobes of CaM in Ca2+-dependent regulation of Cav2.1. Our results show that CDF and CDI result from a series of complex interactions between Ca2+ binding to specific lobes of CaM and distinct molecular contacts with the IQ-like domain and CBD.
Experimental Procedures
Molecular Biology. α12.1 constructs containing alterations of the CBD or IQ-like domain were generated by PCR from cDNA encoding rat α12.1 (rbA) (13). α12.1 lacking amino acids 1969-2000 (α12.1ΔCBD) has been described (5). α12.1IM-AA, α12.1IM-EE, and α12.1ΔCBD/IM-AA were constructed by subcloning EcoRV/PmlI fragments incorporating mutations at positions 1913 and 1914 into the corresponding sites of α12.1 or α12.1ΔCBD in a pBluescript SK+ shuttle vector. From this construct, a SgrAI/MluI fragment containing the mutation(s) was subcloned into rba/pMT2XS. α12.11965t was constructed by amplifying the EcoRV/PmlI fragment with primers incorporating a stop codon after position 1965 and subcloning the truncated fragment into rba/pMT2XS. cDNAs encoding CaM mutants (14), provided by John Adelman (Vollum Institute, Oregon Health and Science University, Portland, OR), were subcloned into BamHI sites of pcDNA3.1+ (Invitrogen) for mammalian cell expression or pGEX4T1 for GST-fusion protein expression. His-tagged fusion constructs were generated by subcloning α12.1 C-terminal fragments into BamHI sites of pTrcHisA (Invitrogen).
Electrophysiological Recording and Data Analysis. tsA-201 cells were grown to ≈70% confluence and transfected by the calcium phosphate method with an equimolar ratio of cDNAs encoding WT or mutant α12.1, β2a, and α2δ (15). For electrophysiological experiments, cells plated on 35-mm dishes were transfected with a total of 5 μg of DNA including 0.3 μg of a CD8 expression plasmid for detection of transfected cells. Twenty-four hours later, cells were plated at low density for electrophysiological recording.
At least 48 h after transfection, cells were incubated with CD8-antibody-coated microspheres (Dynal, Oslo) to identify transfectants and washed in extracellular solution before recording. Whole-cell Ca2+ or Ba2+ currents were recorded with a List EPC-7 patch clamp amplifier driven by PULSE software (HEKA Electronics, Lambrecht/Pfalz, Germany). Leak and capacitive transients were subtracted by using a P/-4 protocol. Extracellular recording solutions contained 150 mM Tris, 1 mM MgCl2 and 10 mM CaCl2 or BaCl2. Intracellular solutions consisted of 120 mM N-methyl-d-glucamine, 60 mM Hepes, 1 mM MgCl2, 2 mM Mg-ATP, and 0.5 mM EGTA. The pH of all solutions was adjusted to 7.3 with methanesulfonic acid. Normalized tail current-voltage curves were fit with a single Boltzmann function: A/{1 + exp[(V - V1/2)/k] + b}, where V is test pulse voltage, V1/2 is the midpoint of the activation curve, k is a slope factor, A is the amplitude, and b is the baseline. Data analysis was done with IGOR PRO (WaveMetrics, Lake Oswego, OR).
Binding Assays. GST-CaM and His-α12.1 fusion proteins were expressed in BL21 Escherichia coli and purified according to standard protocols. GST-CaM immobilized on glutathione agarose beads was incubated with 5 μg of purified His-α12.1 fusion protein for 3 h at 4°C. After extensive washing, bound proteins were eluted and subjected to SDS/PAGE and immunoblotting with monoclonal anti-His or -GST antibodies.
Results
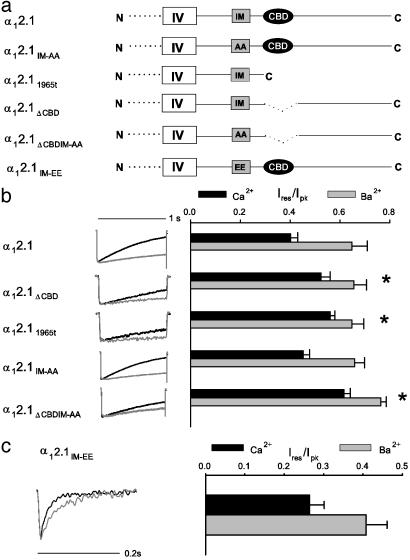
Roles of the CBD and the IQ-Like Domain in CDI. We constructed α12.1 subunits with alterations in the IQ-like domain and the CBD (Fig. 1a) and analyzed CDI of Cav2.1 channels transfected in tsA-201 cells by using Ca2+ and Ba2+ as charge carriers and a relatively low concentration of EGTA (0.5 mM) in the recording pipette to minimize Ca2+ buffering (4, 5). Inactivation of WT and mutant channels was compared quantitatively as the ratio of the residual current amplitude at 800 ms to the peak current amplitude (Ires/Ipk; Fig. 1b), and CDI was expressed as the difference between the average Ires/Ipk for Ca2+ and Ba2+. For α12.1, CDI is manifested as a faster decay of the Ca2+ current (ICa), and therefore, a significant decrease in Ires/Ipk for ICa compared to IBa (0.40 ± 0.03, n = 9 for ICa vs. 0.67 ± 0.04, n = 7 for IBa; CDI = 0.27; P < 0.01). α12.1 subunits lacking the CBD (α12.1ΔCBD) gave rise to channels with significantly reduced CDI (Ires/Ipk = 0.55 ± 0.02, n = 7 for ICa vs. 0.65 ± 0.04, n = 9 for IBa; CDI = 0.10, P < 0.01). We obtained similar results for α12.1 subunits truncated immediately before the CBD (α12.11965t; Ires/Ipk = 0.56 ± 0.02, n = 10 for ICa vs. 0.65 ± 0.05, n = 9 for IBa; CDI = 0.09, P < 0.01). Despite the significant reduction in CDI for both α12.1ΔCBD and α12.11965t, ICa still inactivated faster than IBa, suggesting that a second site such as the IQ-like domain may also be involved in CDI.
Fig. 1.
Contributions of the CBD and IQ-like domain to CDI. (a) Schematic showing constructs with alterations affecting the IQ-like domain (IM) and CBD in the C-terminal domain of α12.1; α12.1IM-AA containing AA in place of 1913I1914M; α12.11965t truncated after amino acid 1965; α12.1ΔCBD lacking amino acids 1969-2000; α12.1ΔCBDIM-AA with both mutations; and α12.1IM-EE with EE in place of1913I1914M. (b) CDI of Cav2.1 channels containing α1 subunits with altered C termini. Normalized current traces are shown for currents evoked by 1-s test pulses from a holding voltage of -80 mV to +10 or 0 mV with 10 mM extracellular Ca2+ (black trace) or Ba2+ (gray trace), respectively. Intracellular solutions contained 0.5 mM EGTA. The current amplitude was measured at 800 ms (Ires) and normalized to the peak current amplitude (Ipk). Resulting Ires/Ipk values were averaged (n = 5-10) and plotted (±SEM). Asterisks indicate significant differences between CDI [(Ires/Ipk for ICa) - (Ires/Ipk for IBa)] for mutant compared to WT channels (P < 0.05). (c) Enhanced inactivation of α12.1IM-EE. Normalized currents evoked by 0.5-s test pulses from -80 to +10 (ICa, black trace) or 0 mV (IBa, gray trace). Ires was measured at 50 ms, and Ires/Ipk was plotted as in b.
To assess the role of the IQ-like domain, alanine substitutions were made for the first two residues (IM) in this region (α12.1IM-AA). These residues are critical for CaM regulation in conventional IQ domains in many other target proteins, including Cav1.2 channels (16, 17), and the IM-AA mutation in α12.1 lowers the affinity for binding CaM by 30-fold in vitro (6). However, CDI was not significantly reduced in channels with α12.1IM-AA (Ires/Ipk = 0.45 ± 0.03, n = 10 for ICa vs. 0.66 ± 0.04 for IBa, n = 6; CDI = 0.21, P = 0.11). Double mutant channels lacking the CBD and containing the IM-AA substitution (α12.1ΔCBDIM-AA) exhibited significantly slower inactivation for ICa and IBa compared to WT (CDI = 0.12, P < 0.01; Fig. 1b), but the reduction in CDI was not greater than that caused by deletion of the CBD alone.
Substitution of negatively charged glutamate residues for IM in the IQ-like domain had substantial effects on Cav2.1 function independent of Ca2+ (α12.1IM-EE; Fig. 1c). First, the IM-EE mutation caused a nearly 5-fold reduction in current, which necessitated the use of higher extracellular Ca2+ and Ba2+ concentrations to resolve effects on CDI. Second, α12.1IM-EE inactivated rapidly, with ICa and IBa decaying completely by 200 ms, a time point at which neither ICa nor IBa through WT is strongly inactivated. This marked enhancement of inactivation of α12.1IM-EE would partially occlude CDI because it exceeds the rate at which Ca2+/CaM can induce CDI. However, comparison of the residual current amplitude at 50 ms revealed faster inactivation of ICa than IBa (Fig. 1c; Ires/Ipk = 0.26 ± 0.04, n = 5 for ICa vs. 0.41 ± 0.05, n = 5 for IBa; P = 0.06), suggesting that CDI remains at least partially intact after the IM-EE mutation. Therefore, our results support a prominent role for the CBD, but not the IQ-like domain, in CDI of Cav2.1.
Roles of the CBD and the IQ-Like Domain in CDF. To analyze CDF of Cav2.1, we used two protocols similar to those used to assess facilitation of synaptic transmission (Fig. 2). During trains of repetitive stimuli, ICa increases to a new steady level, whereas IBa remains relatively constant (Fig. 2a). During paired-pulse experiments, ICa evoked after a conditioning prepulse (P2) is greater than ICa elicited before the prepulse (P1) (Fig. 2f). These two measures of CDF provide complementary information: the paired-pulse protocol shows CDF evoked by a single episode of sustained Ca2+ influx, and the cumulative increase of ICa during trains of stimuli also reflects the stability of CDF in the interval between pulses. Our previous results show that the stability of CDF is strongly enhanced by Ca2+ and CaM (5).
Fig. 2.
Contributions of the IQ-like domain and CBD to CDF. (a-d) Facilitation of ICa during 100-Hz trains of pulses from -80 to +10 or 0 mV, as indicated in the voltage protocol shown above the panels. Test current amplitudes (± SEM, n > 4) were normalized to the first in the train, and every third point is plotted against time. (e) Averaged normalized current amplitudes between 0.5 and 1 s, (In/I1). Asterisks indicate significant difference between mutant and WT channels (P < 0.05). (f-i) CDF in paired-pulse protocols, as indicated above the panels, with extracellular 10 mM Ca2+ and intracellular 0.5 mM EGTA. Tail current amplitudes were measured on repolarization to -40 mV, normalized to the mean tail current after P1 pulses between +50 to +70 mV, and plotted against test pulse voltage. Insets show representative P1 and P2 currents. Plotted points are mean ± SEM for P1 (□) and P2 (▪) for α12.1 (n = 5), α12.1IM-AA (n = 5), α12.1ΔCBD (n = 4), and α12.1ΔCBDIM-AA (n = 7). (j) Means for ICa or IBa of P2max/P1max, where PXmax is the mean value of tails after pulses between +50 and +70 mV.
Compared to WT Cav2.1 (Fig. 2f), deletion of the CBD reduced the paired-pulse facilitation of ICa (Fig. 2 h and j). Similarly, deletion of the CBD caused a partial loss of facilitation measured in the repetitive pulse protocol (Fig. 2 c and e). By contrast, there was no significant facilitation of α12.1IM-AA or α12.1ΔCBDIM-AA in paired-pulse experiments (Fig. 2 g, i, and j) and no difference between facilitation of ICa and IBa in repetitive pulse experiments (Fig. 2 b, d, and e). These results demonstrate a primary requirement for the IQ-like domain in CDF and a modulatory role for the CBD in this process.
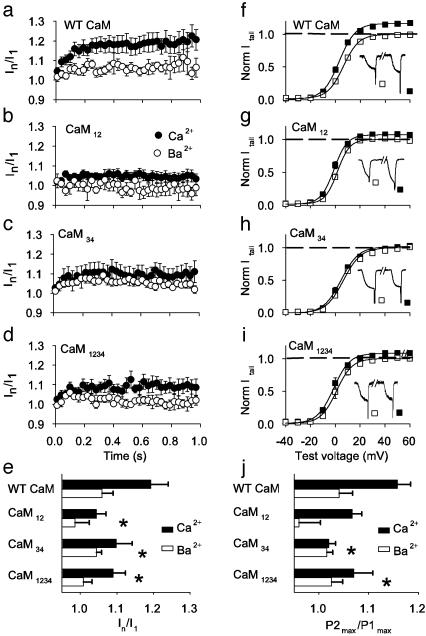
Distinct Functions of the N- and C-Terminal EF-Hands of CaM in CDI. We have shown previously that CDI but not CDF is blocked by 10 mM EGTA, a high-affinity but slow Ca2+ chelator, whereas both processes are blocked by 10 mM BAPTA, a rapid chelator (5). The distinct Ca2+ sensitivities for the two processes indicate that CDF is triggered by local Ca2+, which is intercepted rapidly by BAPTA, whereas CDI is induced by global Ca2+ elevations, which are removed more slowly by EGTA. The molecular basis for this difference between CDF and CDI could reside in the N- and C-terminal lobes of CaM, which bind Ca2+ ions with different affinities (18, 19). To test this, we used CaM constructs containing alanine substitutions for critical aspartate residues that impair Ca2+ coordination in the paired EF-hands of the N-terminal lobe (CaM12), the C-terminal lobe (CaM34), or both lobes of CaM (CaM1234) (Fig. 3) (14). Although coexpression of Cav2.1 with CaM or CaM34 did not influence CDI during 1-s depolarizing pulses, CaM12 virtually eliminated the difference in inactivation between ICa and IBa (Fig. 3). As expected, there was also substantially reduced CDI when CaM1234 was expressed, although its effect was less complete than CaM12 (Fig. 3). These results point to a specific requirement for the N-terminal EF-hands of CaM for CDI.
Fig. 3.
Requirement for N- and C-terminal EF-hands of CaM for CDI. (Upper) Schematic of CaM. (Lower) CDI for Cav2.1 channels cotransfected with CaM mutants. Normalized current traces are shown for 1-s test pulses from a holding voltage of -80 mV to +10 or 0 mV for ICa (black traces) or IBa (gray traces) with 0.5 mM EGTA intracellular. Mean Ires/Ipk values (±SEM; n = 5-12) for ICa or IBa as in Fig. 1b for the indicated CaM constructs. Asterisks indicate significant differences from WT CaM (P < 0.01).
Dual Requirement for the N- and C-Terminal Lobes of CaM in CDF. In a previous study (6), CaM12 supported CDF in paired-pulse experiments, whereas CaM34 did not, leading to the proposal that CDF was mediated primarily by the C-terminal EF-hands of CaM. We further investigated the function of the N- and C-terminal lobes of CaM by using the paired-pulse and repetitive-stimulation protocols (Fig. 4). As in the previous work, we found nearly complete loss of CDF in the paired-pulse protocol (Fig. 4 h and j) and in the repetitive-stimulation protocol (Fig. 4 c and e) with the CaM34 mutant. However, we also found a significant impact of the CaM12 mutant on facilitation in both protocols. In the paired-pulse protocol, facilitation of ICa was substantially reduced by CaM12, but facilitation of IBa was reduced to a similar extent (Fig. 4 g and j; see also ref. 6). In the repetitive pulse protocol, there was nearly complete loss of facilitation (Fig. 4 b and e), and differences between ICa and IBa with CaM12 were not significant (1.04 ± 0.02, n = 13 vs. 0.98 ± 0.04, n = 13; P = 0.22). CaM1234 also caused nearly compete loss of CDF in both protocols (Fig. 4 d, e, i, and j). Together, these results confirm an absolute requirement for Ca2+ binding to the C-terminal lobe of CaM for CDF and reveal a significant role for the N-terminal lobe as well.
Fig. 4.
Requirement for N- and C-terminal EF-hands for CDF. (a-d) CDF during 100-Hz trains of depolarizations as in Fig. 2 a-d [mean ± SEM for CaM (n = 11), CaM12 (n = 13), CaM34 (n = 9)], and CaM1234 (n = 7). (e) Averaged normalized current amplitudes between 0.5 and 1 s as in Fig. 2e. (f-i) CDF during paired-pulse protocols measured as in Fig. 2 e-i [mean ± SEM for CaM (n = 10), CaM12 (n = 6), CaM34 (n = 7), and CaM1234 (n = 5)]. (j) Mean values for P2max/P1max for ICa or IBa determined as in Fig. 2j. Asterisks indicate significant differences from WT (P < 0.05).
Differential Binding of the IQ-Like Domain and CBD to CaM. To determine whether differences in CaM-binding to the CBD and IQ-like domain contribute to their different functional effects, we measured the direct interaction of the CBD and IQ-like domains with CaM, CaM12, and CaM34 in a binding assay that detects stable, high-affinity CaM-binding interactions. Using GST-tagged CaMs immobilized on glutathione agarose beads, we observed Ca2+-dependent binding of His-tagged CBD and IQ-like domain peptides, as reported (4, 7, 11) (Fig. 5 a and b). Neither CaM12 nor CaM34 associated with the CBD, indicating that functional EF-hands in both the N- and C-terminal lobes are required for stable Ca2+-dependent CaM interaction with this site (Fig. 5c). By contrast, CaM binding to the IQ-like domain was prevented by inactivation of the N- but not C-terminal EF-hands (Fig. 5c), consistent with results of yeast-two-hybrid analyses (20). These results eliminate the possibility that blockade of CDF by CaM34 (Fig. 4) was caused by its inability to interact with the IQ-like domain. Evidently, Ca2+ binding to the N-terminal lobe is sufficient for stable CaM binding to the IQ-like domain but not for induction of CDF.
Fig. 5.
Binding of CaM to the IQ-like domain and the CBD. (a) His-tagged C-terminal fragments including the IQ-like domain (IM, amino acids 1848 - 1964), CBD (1959-2035), or an upstream sequence (1764-1848) used as a negative control. (b)Ca2+-dependent binding of His-tagged C-terminal fragments (His) to GST-tagged CaM (input, GST) immobilized on glutathione agarose in the presence of 2 μM Ca2+ or 10 mM EGTA was detected by immunoblot with anti-His antibodies. (c) Differential binding of CaM12 and CaM34 to the IQ-like domain and CBD. GST-tagged CaM12 or CaM34 was used to pull-down His-tagged α12.1 C-terminal fragments in 2 μMCa2+ as in b.(d) Model for CDI and CDF based on sequential interactions of the IQ-like domain and CBD with CaM.
Discussion
Specific Roles of the CBD and IQ-Like Domain in Ca2+-Dependent Regulation of Cav2.1. Our previous results showed that Cav2.1 channels undergo prominent CDF and CDI and identified the CBD as a CaM-binding motif that contributes to these forms of regulation (4, 5). The experiments presented here also demonstrate a significant loss of CDI in Cav2.1 channels lacking the CBD (Fig. 1) and provide the first evidence for an important role of the CBD in CDF measured in paired-pulse and repetitive stimulation paradigms (Fig. 2). Deletion of the CBD selectively in α12.1ΔCBD or by truncation of the C-terminal domain in α12.11965t caused a similar reduction in CDF and CDI. Evidently, the CBD has functionally significant interactions with CaM in both CDF and CDI, but these are not the exclusive interactions that mediate these regulatory processes because measurable CDF and CDI remain in CBD-deletion mutants.
Our results also extend the work of DeMaria and colleagues, who discovered a major role for the IQ-like domain in CDF of Cav2.1 (6). Like these authors, we find that mutation of the first two amino acid residues in the IQ-like domain to alanine (IM-AA) completely prevents CDF (Fig. 2 b and g), indicating that the IQ-like domain is necessary for CDF of Cav2.1 channels. In contrast to these results with CDF, we did not resolve a significant effect of the IM-AA mutation on CDI, in agreement with previous work (6). Because the IM-AA mutation causes a 30-fold reduction in affinity for CaM (6), these results suggest that CDF requires high-affinity binding of CaM that is prevented by the IM-AA mutation. In contrast, CDI either does not require the IQ-like domain or requires only low-affinity interactions that are retained in the IM-AA mutant.
Mutation of the same two amino acid residues in the IQ-like domain to glutamate (IM-EE) completely blocks CaM binding to the IQ-like domain and dramatically accelerates voltage-dependent inactivation of IBa (Fig. 1c and ref. 6). This result establishes an important role for these amino acid residues in control of voltage-dependent inactivation in the absence of Ca2+. Acceleration of voltage-dependent inactivation to the extent observed for the IM-EE mutation would also cause the channel to inactivate before Ca2+/CaM could initiate CDI, regardless of the role of the IQ-like domain in this process. We have shown that similar rapid, voltage-dependent inactivation of Cav2.1 caused by coexpression of auxiliary β1b rather than β2a subunits largely occludes CDI, even though these β subunits have no direct role in CDI itself (5). Despite this potential difficulty, we were able to measure residual CDI of the IM-EE mutant at an early (50 ms) time point (Fig. 1c). These results and the lack of effect of the IM-AA mutation on CDI shown here and in previous work (6) indicate that the IQ-like domain does not have a prominent role in CDI of Cav2.1 channels. Although it is possible that the IQ-like domain does have a significant role in CDI, its impact is not revealed by the IM-EE or IM-AA mutations that have been analyzed to date.
Our results differ from those of DeMaria et al. (6), who found that deletions of the CBD did not impact either CDF or CDI. These different results may in part be due to methodological differences. Our CDF protocols measure an increase in peak ICa rather than an increased rate of activation of ICa as in previous work (6), which may require more stable binding of CaM through interaction with the CBD. Our CDI experiments show that deletion of the CBD significantly reduced but did not abolish CDI (Fig. 1 and ref. 4), indicating that other regions of the channel contribute to CaM-dependent conformational changes leading to CDI. The functional roles of the CBD in CDI and CDF that we have detected by using the rat brain α12.1 (rbA isoform) may be less apparent with the human α12.1 isoform used in the previous work (6). Although the C-terminal regions, including the IQ-like domain and the CBD, are quite similar in the human and rat sequences, functionally significant differences may exist within these conserved sequences or in other parts of the channel that could influence channel modulation by CaM (21, 22).
Distinct Roles of the N- and C-Terminal Lobes of CaM. CaM mutants can disrupt Ca2+-dependent modulation of Ca2+ channels by displacing endogenous CaM binding to the channel and preventing Ca2+-dependent conformational changes leading to CDF and CDI (6-8). However, interpretation of these results is complicated by the presence of endogenous CaM at sufficient concentration to give both CDF and CDI. In this situation, the lack of effect of a mutant CaM may reflect inability to bind Cav2.1, thereby allowing endogenous CaM to function normally. Alternatively, a CaM mutant may have no apparent effect because, although it does interact with Cav2.1, it causes CDF and CDI that are indistinguishable from endogenous CaM. Moreover, some CaM mutants may have effects that are independent of their inability to bind Ca2+.
Mutation of the N-terminal EF-hands of CaM completely blocks CDI (Fig. 3 and ref. 6). These results indicate that CaM12 does interact with Cav2.1 and prevent the action of endogenous CaM but cannot cause CDI itself. Because we do not detect stable high-affinity binding of CaM12 in our biochemical experiments (Fig. 5c), reversible, low-affinity interactions of overexpressed CaM12 are apparently sufficient to disrupt CDI by endogenous CaM. By contrast, CaM34 has no effect on CDI (Fig. 3 and ref. 6), but our binding studies show that it can bind to the IQ-like domain (Fig. 5). These results indicate that CaM34 is fully active in CDI and therefore that Ca2+ binding to the N-terminal EF-hands of CaM is sufficient for CDI.
Mutation of the C-terminal lobe of CaM completely prevents CDF in both paired-pulse and repetitive-stimulation protocols (Fig. 4), as in previous work using different stimulus paradigms (6). CaM34 can bind to the IQ-like domain in the presence of Ca2+ (Fig. 5c), so the block of CDF by CaM34 evidently results from its inability to bind Ca2+ to EF-hands 3 and 4 and induce conformational changes within the IQ-like domain that favor CDF. We also found that mutation of the N-terminal lobe of CaM significantly reduces facilitation measured in the paired-pulse protocol and nearly completely prevents facilitation during trains of stimuli (Fig. 4). In contrast to the loss of CDF with CaM34, CaM12 reduces facilitation in the presence of Ca2+ but also decreases the limited facilitation observed when Ba2+ is the charge carrier. Ba2+ may bind weakly to the N-terminal EF-hands of CaM and partially support facilitation. In fact, Ba2+-dependent inactivation of Cav1.2 has been observed and proposed to be due to Ba2+ binding to CaM (23, 24). In any case, our results indicate that Ca2+ binding to EF-hands 1 and 2 is required for maximal facilitation when measured as an increase in peak ICa in paired-pulse or repetitive-stimulation protocols, perhaps because Ca2+-binding to EF-hands 1 and 2 increases the overall affinity of CaM binding to the CBD.
A Revised Molecular Model for CDF and CDI of Cav2.1. Lobe-specific regulation is a general feature of functional interactions of CaM with many targets (14, 20, 25, 26). Although a number of ion channels are modulated by direct interactions with CaM (27), Cav2.1 channels are unusual in that CaM binding mediates two opposing forms of modulation that are kinetically and mechanistically distinct (5). Our results support and extend a model (6) in which CDF and CDI result from Ca2+ binding to specific lobes of CaM and sequential changes in the interaction of Ca2+/CaM with the IQ-like domain and CBD. Although our biochemical experiments do not detect stable CaM binding to the IQ-like domain or the CBD in the absence of Ca2+ (Fig. 5b), the millisecond kinetics of CDF imply that CaM must be reversibly bound at resting Ca2+ levels in the cell. In our revised model (Fig. 5d), voltage-gated Ca2+ influx through Cav2.1 would promote local Ca2+ binding to the C-terminal lobe of preassociated CaM, which initiates or strengthens interaction with the IQ-like domain. More global increase in Ca2+ leads to binding of Ca2+ ions to the N-terminal lobe of CaM and also induces a conformational change that allows binding of fully Ca2+-liganded CaM to the CBD. Binding of Ca2+/CaM to the CBD then enhances CDF and initiates CDI. According to this model, when preassociated CaM is replaced by CaM34, Ca2+ influx initiates Ca2+ binding to the N-terminal lobe of CaM, which supports only CDI. When CaM is replaced by CaM12, Ca2+ influx initiates Ca2+ binding only to the C-terminal lobe of CaM that permits partial induction of CDF.
Considerable progress has been made toward understanding where CaM interacts with Cav2.1 and other voltage-gated Ca2+ channels and how this interaction can modulate channel function (4-6, 11, 17, 28-30). However, it is becoming increasingly clear that Ca2+-dependent regulation of Cav2.1 is complex, involving Ca2+-dependent and -independent interactions of CaM with multiple sites on the α12.1 subunit. It is possible that CaM regulation of Cav2.1 involves dynamic interactions between the CBD, IQ-like domain, and potentially other sites, as has been proposed for the multiple CaM-binding sequences in the ryanodine receptor (31, 32). Moreover, emerging evidence shows that Ca2+ sensors in addition to CaM are likely to participate in Cav2.1 regulation in neurons (33, 34). How such interactions are ultimately transduced into physiological forms of channel modulation is an intriguing question to resolve in future structural and functional analyses.
Acknowledgments
We thank Elizabeth Sharp and Hong Sun for assistance in constructing α12.1 mutants, Dr. John Adelman for his generous gift of CaM mutants, and Dr. Bertil Hille (Department of Physiology and Biophysics, University of Washington) for comments on a draft of the manuscript. This work was supported by National Institutes of Health Grants NS22625 (to W.A.C.) and NS044922 (to A.L.) and National Research Service Award NS10645 (to A.L.).
Abbreviations: CaM, calmodulin; CBD, CaM-binding domain; CDF, Ca2+-dependent facilitation; CDI, Ca2+-dependent inactivation.
References
- 1.Forsythe, I. D., Tsujimoto, T., Barnes-Davies, M., Cuttle, M. F. & Takahashi, T. (1998) Neuron 20, 797-807. [DOI] [PubMed] [Google Scholar]
- 2.Cuttle, M. F., Tsujimoto, T., Forsythe, I. D. & Takahashi, T. (1998) J. Physiol. (London) 512, 723-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borst, J. G. & Sakmann, B. (1998) J. Physiol. (London) 513, 149-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee, A., Wong, S. T., Gallagher, D., Li, B., Storm, D. R., Scheuer, T. & Catterall, W. A. (1999) Nature 339, 155-159. [DOI] [PubMed] [Google Scholar]
- 5.Lee, A., Scheuer, T. & Catterall, W. A. (2000) J. Neurosci. 20, 6830-6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeMaria, C. D., Soong, T., Alseikhan, B. A., Alvania, R. S. & Yue, D. T. (2001) Nature 411, 484-489. [DOI] [PubMed] [Google Scholar]
- 7.Peterson, B. Z., DeMaria, C. D. & Yue, D. T. (1999) Neuron 22, 549-558. [DOI] [PubMed] [Google Scholar]
- 8.Zühlke, R. G., Pitt, G. S., Deisseroth, K., Tsien, R. W. & Reuter, H. (1999) Nature 399, 159-161. [DOI] [PubMed] [Google Scholar]
- 9.Qin, N., Olcese, R., Bransby, M., Lin, T. & Birnbaumer, L. (1999) Proc. Natl. Acad. Sci. USA 96, 2435-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahler, M. & Rhoads, A. (2002) FEBS Lett. 513, 107-113. [DOI] [PubMed] [Google Scholar]
- 11.Pate, P., Mochca-Morales, J., Wu, Y., Zhang, J. Z., Rodney, G. G., Serysheva, I. I., Williams, B. Y., Anderson, M. E. & Hamilton, S. L. (2000) J. Biol. Chem. 275, 39786-39792. [DOI] [PubMed] [Google Scholar]
- 12.Erickson, M. G., Alseikhan, B. A., Peterson, B. Z. & Yue, D. T. (2001) Neuron 31, 973-985. [DOI] [PubMed] [Google Scholar]
- 13.Starr, T. V. B., Prystay, W. & Snutch, T. P. (1991) Proc. Natl. Acad. Sci. USA 88, 5621-5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keen, J. E., Khawaled, R., Farrens, D. L., Neelands, T., Rivard, A., Bond, C. T., Janowsky, A., Fakler, B., Adelman, J. P. & Maylie, J. (1999) J. Neurosci. 19, 8830-8838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stea, A., Tomlinson, W. J., Soong, T. W., Bourinet, E., Dubel, S. J., Vincent, S. R. & Snutch, T. P. (1994) Proc. Natl. Acad. Sci. USA 91, 10576-10580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuhlke, R. D., Pitt, G. S., Tsien, R. W. & Reuter, H. (2000) J. Biol. Chem. 275, 21121-21129. [DOI] [PubMed] [Google Scholar]
- 17.Erickson, M. G., Liang, H., Mori, M. X. & Yue, D. T. (2003) Neuron 39, 97-107. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, J. D., Snyder, C., Walsh, M. & Flynn, M. (1996) J. Biol. Chem. 271, 761-767. [DOI] [PubMed] [Google Scholar]
- 19.Wang, C. L. (1985) Biochem. Biophys. Res. Commun. 130, 426-430. [DOI] [PubMed] [Google Scholar]
- 20.Yus-Najera, E., Santana-Castro, I. & Villarroel, A. (2002) J. Biol. Chem. 277, 28545-28553. [DOI] [PubMed] [Google Scholar]
- 21.Ivanina, T., Blumenstein, Y., Shistik, E., Barzilai, R. & Dascal, N. (2000) J. Biol. Chem. 275, 39846-39854. [DOI] [PubMed] [Google Scholar]
- 22.Soldatov, N. M. (2003) Trends Pharmacol. Sci. 24, 167-171. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira, G., Yi, J., Rios, E. & Shirokov, R. (1997) J. Gen. Physiol. 109, 449-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun, L., Fan, J. S., Clark, J. W. & Palade, P. T. (2000) J. Physiol. (London) 529, 139-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong, L. W., Newman, R. A., Rodney, G. G., Thomas, O., Zhang, J. Z., Persechini, A., Shea, M. A. & Hamilton, S. L. (2002) J. Biol. Chem. 277, 40862-40870. [DOI] [PubMed] [Google Scholar]
- 26.Drum, C. L., Yan, S.-Z., Bard, J., Shen, Y.-Q., Lu, D., Soelaiman, S., Grabarek, Z., Bohm, A. & Tang, W.-J. (2002) Nature 415, 396-402. [DOI] [PubMed] [Google Scholar]
- 27.Saimi, Y. & Kung, C. (2002) Annu. Rev. Physiol. 64, 289-311. [DOI] [PubMed] [Google Scholar]
- 28.Pitt, G. S., Zuhlke, R. D., Hudmon, A., Schulman, H., Reuter, H. & Tsien, R. W. (2001) J. Biol. Chem. 276, 30794-30802. [DOI] [PubMed] [Google Scholar]
- 29.Romanin, C., Gamsjaeger, R., Kahr, H., Schaufler, D., Carlson, O., Abernethy, D. R. & Soldatov, N. M. (2000) FEBS Lett. 487, 301-306. [DOI] [PubMed] [Google Scholar]
- 30.Liang, H., DeMaria, C. D., Erickson, M. G., Mori, M. X., Alseikhan, B. & Yue, D. T. (2003) Neuron 39, 951-960. [DOI] [PubMed] [Google Scholar]
- 31.Rodney, G. G., Moore, C. P., Williams, B. Y., Zhang, J. Z., Krol, J., Pedersen, S. E. & Hamilton, S. L. (2001) J. Biol. Chem. 276, 2069-2074. [DOI] [PubMed] [Google Scholar]
- 32.Zhang, H., Zhang, J. Z., Danila, C. I. & Hamilton, S. L. (2003) J. Biol. Chem. 278, 8348-8355. [DOI] [PubMed] [Google Scholar]
- 33.Tsujimoto, T., Jeromin, A., Saitoh, N., Roder, J. C. & Takahashi, T. (2002) Science 295, 2276-2279. [DOI] [PubMed] [Google Scholar]
- 34.Lee, A., Westenbroek, R. E., Haeseleer, F., Palczewski, K., Scheuer, T. & Catterall, W. A. (2002) Nat. Neurosci. 5, 210-217. [DOI] [PMC free article] [PubMed] [Google Scholar]