Abstract
Strategies to address resistance to platin drugs are greatly needed in human epithelial cancers (e.g. ovarian, head/neck and lung) where platins are used widely and resistance occurs commonly. We found that upon ΔNp63α overexpression, AKT1 and phospho-AKT1 levels are up regulated in cancer cells. Investigations using gel-shift, chromatin immunoprecipitation and functional reporter assays implicated ΔNp63α in positive regulation of AKT1 transcription. Importantly, we found that ΔNp63α, AKT1 and phospho-AKT levels are greater in 2008CI3 CDDP-resistant ovarian cancer cells than in 2008 CDDP-sensitive cells. siRNA-mediated knockdown of ΔNp63α expression dramatically decreased AKT1 expression, whereas knockdown of either ΔNp63α or AKT1 decreased cell proliferation and increased death of ovarian and head/neck cancer cells. Conversely, enforced expression of ΔNp63α increased cancer cell proliferation and reduced apoptosis. Together, our findings define a novel ΔNp63α-dependent regulatory mechanism for AKT1 expression and its role in chemotherapeutic resistance of ovarian and head/neck cancer cells.
Keywords: ΔNp63α, AKT1, CDDP, chemoresistance
Introduction
Cis-diamminedichloroplatinum (II) (CDDP) is used for treating various human cancers (e.g. head and neck, ovarian and lung cancer), however, its efficiency is limited due to development of drug resistance by tumor cells (1). CDDP-induced programmed cell death is associated with expression of specific “cell death” genes and down regulation of “survival” genes (2). Failure of cancer cells to maintain expression of the former genes may be an important factor in chemoresistance (3-6). AKT negatively regulates many of the key cell death effector molecules, therefore rendering cancer cells CDDP resistant, while AKT inhibition reversed the CDDP-resistance phenotype (3-6).
P53 homolog p63 encodes several isoforms serving as transcription factors promoting cell death or cell survival (7-12). TAp63 isoforms consist of the long transactivation (TA)-domain, DNA-binding domain, oligomerization domain, and carboxyl-terminus domain of various length (α, β, and γ). While, ΔNp63 isoforms that lack of the TA-domain present in TAp63 isoforms are acting as pro-survival regulators (13-17). ΔNp63α is predominantly overexpressed in epithelial cancers playing an important role in the DNA damage response (8, 10, 13-19). P63 was shown to be amplified and overexpressed in all head and neck squamous cell carcinoma (HNSCC) cells derived from primary tumors tested and ΔNp63 isoforms can enhance tumor growth and activate the oncogenic β-catenin pathway (13, 14). ΔNp63α is phosphorylated by ATM-dependent mechanism following CDDP treatment functioning as a pro-survival factor in HNSCC cells (18, 19). Furthermore, ΔNp63 or ΔNp73 were shown playing a crucial role in determining the cellular chemosensitivity (8, 10, 20-26). Here, we uncover a novel molecular mechanism by which ΔNp63α positively regulates AKT1 transcription inducing cell survival and chemoresistance to CDDP.
Materials and Methods
Chemicals and antibodies
We used CDDP and β-actin antibody (Sigma); ΔNp63 antibody (Ab-1) and caspase-3 Assay kit (both from Calbiochem/EMD), TiterTACS in situ Apoptosis Detection Kit (R&D Systems); anti-p63 (4A4), anti-poly-ADP-ribosylating enzyme (PARP)-1 antibodies, anti-AKT1 antibody and the horseradish peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulins (all from Santa Cruz Biotechnology); antibodies to pan-AKT, phospho (p)-AKT (S473), p-AKT (T308) and to caspase-3 (all from Cell Signaling Technology).
Cell culture and transfection
We used HNSCC cell line JHU-022 (wild type p53, p63 amplified and ΔNp63α is overexpressed, refs. 13, 16, 17) from Head and Neck Cancer Research Division Tissue Bank (characterized, tested and provided as a gift by Dr. Joseph A. Califano, JHMI, refs. 27, 28), and human non-small cell lung carcinoma cell line H1299 (null for p53 and p63 expression at the RNA and protein levels tested by RT-PCR and immunoblotting) from the American Type Culture Collection [ATCC, according to the certificate, H1299 cell line, (#CRL-5803), was tested for multiple genetic markers and showed the p53 homozygous partial deletion and lack of p53 protein, H1299 showed no p63 expression, refs. 16, 29]. We also used isogenic CDDP-sensitive (OV2008) and its CDDP-resistant (OV2008-C13*) ovarian cancer cells (wild type p53, characterized, tested and kindly given by Prof. Stephen B. Howell, UCSD, ref. 30). The cell lines were authenticated by a short tandem repeat profiling analysis using the AmpFISTR Identifiler PCR Amplification Lit (Applied Biosystems) with the following sixteen markers: amelogenin, CSF1PO, D12S317, D16S539, D18S51, D19S433, D21S11, D2S1338, D3S1358, D5S818, D7S820, D8S1179, FGA, THO1, TPOX and VWA at the JHMI Fragment Analysis Facility. Cells were maintained in RPMI-1640 medium with 10% fetal bovine serum. Cells were transiently transfected using FuGENE HD reagent (Roche Molecular Biochemicals) for 72h. 20 nM of control siRNA, ΔNp63α siRNAs (1 and 2) and TAp63α siRNAs (all from Dharmacon) and AKT1 siRNA (Cell Signaling Technology) were introduced into cells using Lipofectamine SiRNAMAX reagent. Cells were treated with control media or 10 μg/ml CDDP for indicated time periods.
Plasmid constructs
The ΔNp63α-Flag, 6-His-ΔNp63α and TAp63α–Flag fragments were subcloned into the pcDNA3.1/Hygro vector (Invitrogen). We used the pLNCX-HA-AKT1 expression cassette (Addgene) and the AKT1 promoter-luciferase reporter construct, pGL3B-AKT1-1400 (a gift from Dr. Susanne Dihlmann, University Hospital Heidelberg, ref. 31).
Immunoblotting analysis
Cells were lysed in RIPA buffer [150mM NaCl, 100mM Tris (pH 8.0), 1% Triton X-100, 1% deoxycholic acid, 0.1% SDS, 5mM EDTA, and 10mM NaF, supplemented with 1mM PMSF and protease inhibitor cocktail (Sigma)]. Proteins were analyzed by immunoblotting as previously described (16).
Semi-quantitative and quantitative RT-PCR
Total RNA was isolated from 1 × 106 cells using RNeasy Kit (Qiagen). First-strand cDNA was synthesized using qScript™ cDNA SuperMix kit (Quanta Biosciences). PCR products amplified with semi-qPCR primers (Suppl. Table 1) were quantified by ethidium bromide staining. In tumor tissue samples from patients, the levels of AKT1 and ΔNp63α were determined by real-time qRT-PCR with Fast SYBR® Green Master Mix (Applied Biosystems) with 50nM each qPCR primer (Suppl. Table. 2) and the levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) values.
Promoter luciferase activity assays
JHU-022 cells were transfected with the pGL3B-AKT1 -1400 (31) along with 0.5μg of the ΔNp63α and TAp63α plasmids in the presence or absence of the pGL3-Basic vector and the pRL-CMV plasmid expressing Renilla luciferase (Promega). Firefly and Renilla luciferase activities were analyzed by the Dual Luciferase Activity assay Kit (Promega), monitored by luminometer, and the Firefly luciferase activity was normalized to Renilla luciferase activity. Each transfection was performed in duplicate, and all experiments were repeated in triplicate.
TUNEL assay
DNA damage was quantified with a colorimetric apoptosis detection kit (Titer TACS, R&D Systems) that uses TUNEL stain in a 96-well format according to the manufacturer's recommendations. Reaction absorbance was measured at A450nm with microplate reader along with a positive control (nuclease-treated).
Caspase-3 cleavage assay
Caspase-3 activity was measured using the Colorimetric Caspase-3 Assay Kit (Calbiochem). The 2×105 cells/10 μl supernatants were incubated with 200μM Ac-DEVD-pNA substrate at 37°C in the presence or absence of 0.1μM caspase-3 inhibitor (Ac-DEVD-CHO) for 3h and then were monitored at the A405nm. Relative increase in caspase-3 activity was determined by comparing with the untreated control.
Cellular viability assay
Cell proliferation was measured by the 3-(4, 5-dimethyl thiazol-2-yl)-2, 5-diphenyl tetrazolium bromide proliferation assay kit (MTT, ATCC) as previously described (16). 104 cells were incubated in dark for 4h at 37°C with 10μl of MTT labeling reagent (5 mg/ml) in the serum-free culture media and cell lysates were monitored at A570nm to A650nm on a Spectra Max 250 96-well plate reader (Molecular Devices) after 2h. Diagrams indicated the extent of cellular survival expressed as a percentage of control.
Chromatin immunoprecipitation (ChIP)
Flag-ΔNp63α and Flag-TAp63α constructs or siRNAs were introduced into JHU-022 or H1299 cells. A ChIP kit (Upstate Cell Signaling Solutions) along with the p63 (4A4) antibody was used for ChIP analysis as previously described (19, 32, 33). Primers used for ChIP assay shown in Suppl. Fig. 1. PCR consisted of 30 cycles of 94°C for 1min, 58°C for 1min, and 72°C for 30s using Taq polymerase (Invitrogen).
Gel-shift assay
6His–ΔNp63α protein was purified from HEK293 cells through TALON beads (BD Biosciences) confirmed by commassie staining/immunoblotting. One picomole of each DNA probe (Suppl. Fig. 1) was annealed, labeled with [γ-32P]-ATP (6000 Ci/mmol, Perkin-Elmer) using T4 kinase (New England Biolabs), purified through a QIAquick Spin Columns (QIAGEN) and counted in liquid scintillation counter LS6000IC (Beckman). 5μg of purified protein was incubated with 100fmol of radioactive probe in 10μl of 50mM Tris–HCl (pH 7.5), 50mM KCl, 5mM DTT, 10mM MgCl2, and 3% (v/v) glycerol for 20 min at room temperature followed by 20min at 4°C. The DNA-bound proteins were analyzed on a 6% native PAGE with Tris-borate buffer.
Statistical analysis
The data represent mean ± SD from 3-5 independent experiments. Statistical analysis was performed by Student's t test at a significance level of p<0.05 to <0.001. *,ψ,Ω,Λ,# symbols indicate p≤0.05 (n=5) compared with the control.
Results
CDDP exposure decreased ΔNp63α and AKT1 expression in a dose- and time-dependent manner
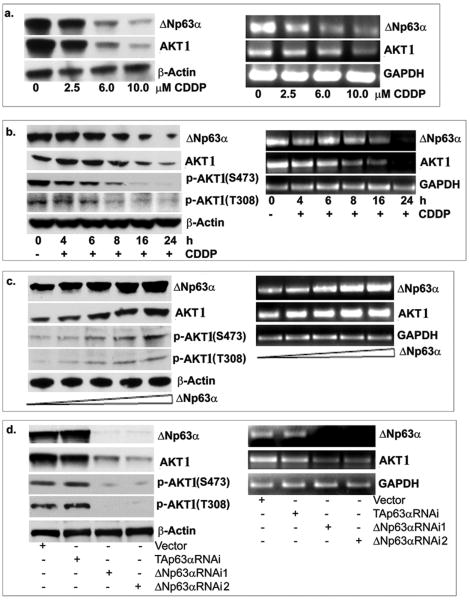
We found that with increasing concentrations (2.5-10.0μM, for 24h, Fig. 1a) and increasing time periods of CDDP exposure (10μM for 0-24h, Fig. 1b) the ΔNp63α, AKT1 and p-AKT1 endogenous levels decreased in JHU-022 cells.
Figure 1. ΔNp63α and AKT1 mRNA and protein levels in JHU-022 cells after CDDP exposure.
Levels of endogenous ΔNp63α, AKT1 and p-AKT1 in JHU-022 cells with (a) increasing dose (0-10μM, 24h) and (b) increasing time (10μM, 0 to 24h) of CDDP treatment. (c). Levels of ΔNp63α, AKT1 and p-AKT1 after transfection with increasing concentrations (0-1.5μg) of ΔNp63α. (d). Levels of ΔNp63α, AKT1 and p-AKT1 in JHU-022 cells after transfection with siRNA against ΔNp63α, TAp63α and control. Left panels are immunoblotting with indicated antibodies. Right panels are semi-qRT-PCR. We used β-actin level as a loading control for immunoblotting, and GAPDH as a loading control for semi-qRT-PCR
We then exposed H1299 cells (null for both p53 and p63 expression) transfected with an empty vector to control media or 25μM CDDP for 0-24h (Suppl. Fig. 2). We found no changes in AKT1 levels in cells without ΔNp63α transfection after CDDP exposure (Suppl. Fig. 2a). However, CDDP exposure led to a down regulation of exogenous ΔNp63α levels and concomitantly to a decrease of AKT1 level in H1299 cells (Suppl. Fig. 2b). These data suggest that the CDDP-dependent decrease in AKT1 level resulted from ΔNp63α down-regulation.
ΔNp63α modulates AKT1 expression
Supporting previous reports (13-16, 25), we showed that ΔNp63 is the predominant p63 isoform in HNSCC cells, since ΔNp63α mRNA is 100-fold more abundant than TAp63 mRNA in JHU-022 cells (Suppl. Fig. 2a).
We transfected JHU-022 cells with increasing concentrations (0, 0.25, 0.5, 1 and 1.5μg) of ΔNp63α plasmid. Using semi-qRT-PCR and immunoblotting (Fig. 1c; Suppl. Fig. 2c), we found that ΔNp63α induction caused a significant increase in AKT1 expression at the mRNA and protein levels, and p-AKT1 levels. Silencing of TAp63α or ΔNp63α in JHU-022 cells using TAp63α-siRNA and ΔNp63α-siRNA (1 and 2) led to a reduction of endogenous TAp63α and ΔNp63α levels, respectively (Fig. 1d). Intriguingly, down regulation of ΔNp63α by ΔNp63α-siRNA (1 and 2, which down regulated TAp63α and ΔNp63α, Suppl. Fig. 2d) resulted in decreased AKT1 and p-AKT1 levels in JHU-022 cell line (Fig. 1d). In contrast, TAp63α-siRNA transfection (which exclusively down regulated TAp63α) showed no change in AKT1 expression (Fig. 1d; Suppl. Fig. 2d, upper section). However, the AKT1 knockdown by AKT1-siRNA did not affect ΔNp63α expression (Suppl. Fig. 2d, lower section).
Transcriptional regulation of AKT1 expression by ΔNp63α
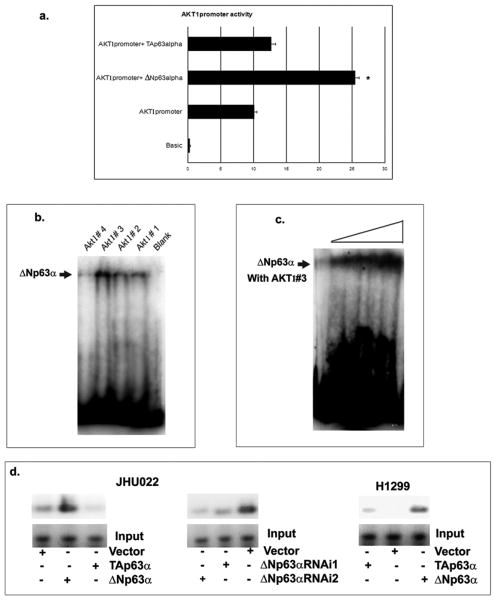
To examine the effect of ΔNp63α on the AKT1 transcription, we used the pGL3B-AKT1-1400 luciferase construct, which spans 1400 base pairs upstream of the transcription start site (31). By the luciferase reporter assay, we found that in contrast to TAp63α, ΔNp63α significantly increased the AKT1 promoter activity (Fig. 2a). We then performed gel-shift assay with the purified 6His-tagged ΔNp63α protein and four DNA probes designated as AKT1 oligos #1, #2, #3 and #4 derived from the AKT1 promoter sequence (Suppl. Fig. 1). By computer analysis of the AKT1 promoter sequence, we found a few putative p63 responsive elements (P63RE: -941 to -924, -762 to -739, -631 to -618, -499 to -477 and -214 to -189; Suppl. Fig. 1) as previously defined (33). Using gel-shift assay, we found that the ΔNp63α protein binds most strongly with AKT1 #3 compared to other oligos (Fig. 2b) in a dose-dependent manner (Fig. 2c).
Figure 2. Transcriptional regulation of ΔNp63α on AKT1 promoter.
(a). JHU-022 cells were transfected with the pGL3B-AKT1-1400 promoter-driven luciferase construct, Renilla luciferase plasmid, and with or without pcCDNA-3.1, pcDNA3.1-HA-TAp63α or pcDNA3.1-HA-ΔNp63α. 48h post transfection of the Firefly luciferase activity was determined. (b). Gel-shift assay with the 6His-tagged ΔNp63α protein along with 4 DNA probes matching the AKT1 promoter sequence. (c). Dose-dependent binding of ΔNp63α to AKT1#3 probe (100 fmol) with increasing concentration of ΔNp63α (1μg, 2μg, 5μg and 10μg). (d). The ChIP assay with JHU-022 and H1299 cells, 72h after transfection with the ΔNp63α or TAp63α expression constructs or control vector. JHU-022 cells were also transfected with the ΔNp63α siRNA or control siRNA.
We then performed ChIP analysis in JHU-022 and H1299 cells transfected with or without ΔNp63α or TAp63α (Fig. 2d). Using the anti-p63 antibody, we found that ΔNp63α displayed a strong binding to the AKT1 promoter, while TAp63 failed to do so (Fig. 2d). ΔNp63α siRNA knockdown caused a significant decrease in its binding to the AKT1 promoter (Fig. 2d). Altogether these results supported the notion that ΔNp63α directly regulates AKT1 transcription.
ΔNp63α increases cell viability through AKT1 expression
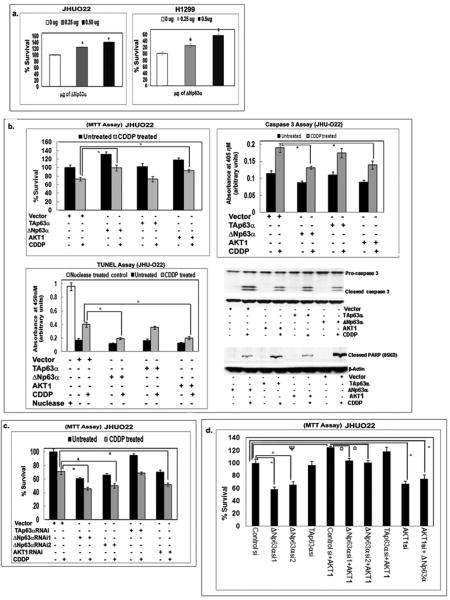
We next subjected both JHU-022 and H1299 cells to the p63 forced expression and JHU-022 cells to the p63 siRNA knockdown followed by the MTT assay. We found that the ΔNp63α overexpression led to a significant increase in basal survival (Fig. 3a), and survival upon CDDP exposure (Fig. 3b). However, no effect on survival was found in cells transfected with TAp63α (Fig. 3b). We next observed that the AKT1 overexpression led to an increased survival of cells exposed to CDDP as corroborated by MTT, TUNEL, caspase-3 and PARP1 cleavage assays (Fig. 3b).
Figure 3. Effect of ΔNp63α on cell viability.
(a) JHU-022 and H1299 cells after concentration-dependent transfection with ΔNp63α. (b). JHU-022 cells after transfection with the ΔNp63α, TAp63α and AKT1 constructs followed by 10μM CDDP treatment for 24h. (c). JHU-022 cells after transfection with the ΔNp63α, TAp63α, AKT1 or control siRNA followed by 10μM CDDP treatment for 24h. (d). JHU-022 cells after transfection with the ΔNp63α, TAp63α, AKT1 and control siRNA. 24h after siRNA transfection cells were transfected with or without 1μg of AKT1 construct. After 24h of AKT1 transfection, cells were treated with 10μM CDDP for 24h. Cell viability was evaluated by the (a-d) MTT, (b) Caspase-3 and TUNEL assays, and immunoblotting analysis of Caspase-3 and PARP1 cleavage. For various experiments, MTT values from control siRNA, empty vector, or untransfected cells were taken as 100%.
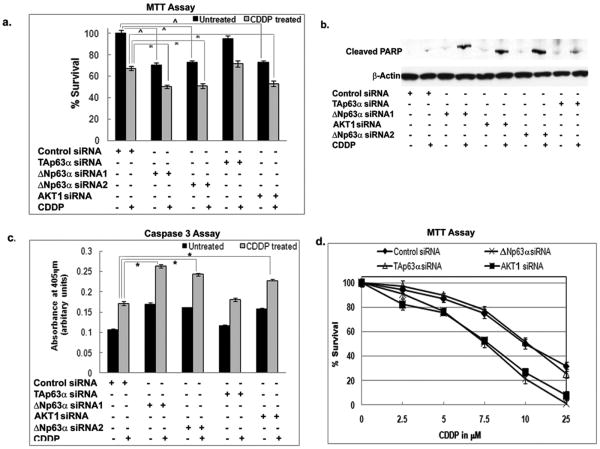
We then found that the ΔNp63α silencing significantly decreased the survival of JHU-022 cells (Fig. 3c), which was further decreased after resulting cells were treated with CDDP (Fig. 3c). However, no effect was observed with TAp63α-siRNA (Fig. 3c). Furthermore, AKT1-siRNA significantly decreased cell survival upon CDDP exposure (Fig. 3c).
JHU-022 cells were also transfected with HA-AKT1 plasmid followed by ΔNp63α-siRNA transfection after 24h. We found that the AKT1 forced expression increased the cell survival after ΔNp63α silencing (Fig. 3d). However, when cells were transfected with ΔNp63α followed by AKT1 siRNA, ΔNp63α forced expression failed to increase the cell survival after AKT1 silencing (Fig. 3d). Taken together these data support the notion that AKT1 is acting downstream of ΔNp63α and that ΔNp63α expression is a regulator of increased cell survival and CDDP chemoresistance.
ΔNp63α and AKT1 modulate the survival of CDDP sensitive/resistant ovarian cancer cells
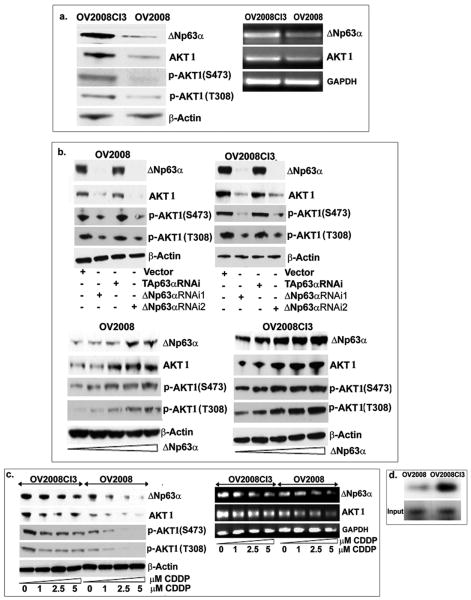
We used isogenic sensitive and resistant ovarian cancer cell lines, OV2008 and 2008CI3, respectively. ΔNp63α, AKT1 and p-AKT1 levels were found to be notably higher in OV2008CI3 than in OV2008 cells (Fig. 4a). In both OV2008 and OV2008CI3 cells, ΔNp63α siRNA effectively decreased total AKT1 and p-AKT1 levels compared to control and TAp63α knockdown (Fig. 4b, upper panel). Both OV2008 and OV2008CI3 cells also showed significant increase in AKT1 and p-AKT1 levels after increasing concentrations of exogenous ΔNp63α (Fig. 4b, lower panel). Although CDDP significantly reduced ΔNp63α and concurrently AKT1 and p-AKT1 levels in OV2008 cells, it failed to significantly affect these levels in OV2008CI3 cells (Fig. 4c). Using ChIP assay, we found that the ΔNp63α protein bound to the AKT1 promoter to a greater degree in resistant cells than in sensitive cells (Fig. 4d) suggesting the importance of the ΔNp63α/AKT1 promoter relationship in CDDP-mediated chemoresistance of ovarian cancer cells.
Figure 4. ΔNp63α and AKT1 levels affect CDDP resistance in sensitive/resistance ovarian cancer cells.
(a). Levels of ΔNp63α, AKT1 and p-AKT1 in OV2008 and OV2008C13 cells tested by immunoblotting (left panel) and semi-qRT-PCR (right panel). Levels of ΔNp63α, AKT1 and p-AKT1 in OV2008 and OV2008C13 cells after transfection with siRNA against ΔNp63α, TAp63α and control (b, upper panels), with increasing concentrations of ΔNp63α (0-1.5μg) overexpression (b, lower panels), and after dose-dependent CDDP (0-5μM) exposure for 24h (c) tested by immunoblotting (left panel) and semi-qRT-PCR (right panel). (d). Binding of ΔNp63α to the AKT promoter in OV2008 and OV2008CI3 cells was analyzed by the ChIP assay. We used β-actin level as a loading control for immunoblotting, and GAPDH as a loading control for semi-qRT-PCR
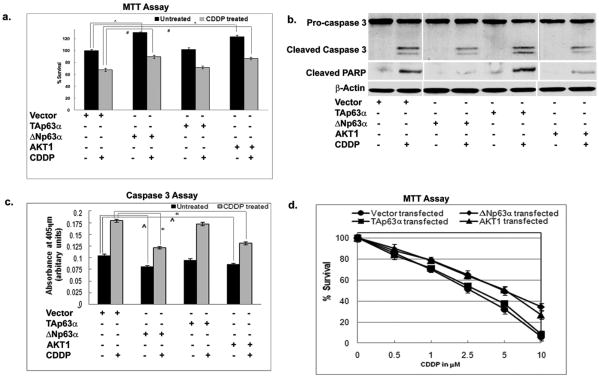
We further found that ΔNp63α and AKT1-siRNA significantly decreased the basal survival of resistant OV2008-CI3 cells (Fig. 5a, b, c, d). We then found that siRNA to both ΔNp63α and AKT1 significantly decreased the survival of OV2008CI3 cells upon CDDP exposure (Fig. 5a, b, c). The levels of PARP1 cleavage in cells transfected with the ΔNp63α or AKT1 siRNA were significantly higher than in cells with the siRNA TAp63α or control siRNA (Fig. 5b). We then observed that the overexpression of ΔNp63α or AKT1 significantly increased the viability of sensitive OV2008 cells and also OV2008 cells became more resistant to CDDP exposure tested by MTT assay, caspase-3 assay, and pro-caspase-3 maturation assay (Fig. 6).
Figure 5. ΔNp63α down regulation makes OV2008VI3 cell sensitive to CDDP exposure.
(a-c). OV2008CI3 cells were transfected with siRNA to ΔNp63α, TAp63α, AKT1 and control for 72h followed by treatment with 10μM CDDP for 24h. (a). MTT assay. (b). Immunoblotting analysis of PARP1 cleavage. (c). Caspase-3 assay. (d). OV2008VCI3 cells were transfected with siRNA to ΔNp63α, TAp63α, AKT1 and control. After 72h cells were subjected to dose-dependent CDDP exposure for 24h followed by MTT assay. (d). Values from cells transfected with an empty vector (data not shown) were taken as 100%.
Figure 6. ΔNp63α overexpression renders OV2008 cell resistant to CDDP exposure.
(a-c). OV2008 cells were transfected with the ΔNp63α, TAp63α and AKT1 expression constructs followed by 2.5μM CDDP treatment for 24h and used for (a) MTT assay, (b) immunoblotting analysis for PARP1 cleavage assay, and (c) caspase-3 assay. (d). OV2008 cells were transfected with the ΔNp63α, TAp63α and AKT1 expression constructs followed by dose-dependent CDDP exposure (0-25μM) for 24h and used for MTT assay. Values from cells transfected with an empty vector (data not shown) were taken as 100%.
Validation of ΔNp63α/AKT1 expression in vivo
Using real-time qRT-PCR, we then found that the AKT1 and ΔNp63α levels are concomitantly higher in tissue biopsies from the patients with CDDP-resistant ovarian tumors than tissue biopsies from the patients with CDDP-sensitive ovarian tumors (Suppl. Fig. 3a). By real-time qPCR, no p63 copy number variation was observed for the pair of CDDP-sensitive OV2008 and CDDP-resistant OV2008C13 ovarian cell lines (Suppl. Fig. 3b, left graph). No significant difference was also observed between similar groups of patients' samples (Suppl. Fig. 3b, right graph). Using immunohistochemistry (IHC), we further validated the ΔNp63 and AKT1 expression in tissue biopsies from HNSCC patients (tissue microarray, 48 single cores, Suppl. Fig. 3c). We observed the concomitant expression of ΔNp63 and AKT1 (Suppl. Fig. 3c), when higher ΔNp63 levels strongly correlated with higher AKT1 levels, and higher ΔNp63/AKT1 levels well-correlated with more advanced tumor stage [+++/++ in T3N0-T4N2 (18/24), ++/+ in T1N0-T2N1 (17/24)]. By IHC we further observed greater expression of ΔNp63 and AKT1 in biopsies from patients with head and neck cancer (Suppl. Fig. 4a) and ovarian tumors (Suppl. Fig. 4b) resistant to platinum therapy than in biopsies from patients with CDDP-sensitive tumors. Similarly to the HNSCC, ovarian tumor samples (tissue microarray, 24 samples in duplicate) showed a good correlation of the greater expression of ΔNp63 with greater AKT1 expression (Suppl. Fig. 5). Altogether, these data supported the notion for the important role of ΔNp63/AKT1 pathway in tumor progression and cell resistance to CDDP in vivo.
Discussion
We previously reported that an amplification and overexpression of ΔNp63α is the most common event in all (100%) primary lung SCC and HNSCC cell lines developed in our laboratory, including JHU-022 cells (13). A genome-wide microarray screen of non-small cell lung cancer revealed that the 3q26-29 locus encompassing p63 is frequently amplified in squamous cell carcinomas of the lung (11). However, no p63 amplification was found in ovarian cancer tissue samples (Suppl. Fig. 3b). By real-time PCR analysis, ΔNp63 levels were shown to dramatically increase in stage III of ovarian cancer supporting the role for ΔNp63 as a biomarker of poor patient survival outcomes and tumor progression (34). Moreover, patients with a higher ΔNp63 level demonstrated a poor response to platinum-based therapy (34).
A few studies addressed the molecular mechanisms underlying the ΔNp63α pro-survival effect (13-19, 22, 26). ΔNp63α was shown to promote tumorigenesis through direct protein-protein interactions, by direct regulation of target genes, or by inhibition of the transactivation activity of other p53 family members (13-21, 23-26). ΔNp63α was found to counteract cell death by repressing the expression of pro-apoptotic genes (7, 8). ΔNp63 was shown to induce a growth of tumor cells in vitro and in vivo, and lead to increased β-catenin accumulation and signaling (13, 14). Conversely, siRNA knockdown of ΔNp63α in malignant cells overexpressing ΔNp63α resulted in cell death, accompanied by cleavage of PARP1 (20). The ΔNp63α silencing in HNSCC cells induced the expression of pro-apoptotic genes, Puma and Noxa, suggesting the ΔNp63α role in cell survival (21-26).
Here, we provided evidence that the process of cancer cell survival under chemotherapeutic treatment is mediated by functional interplay between ΔNp63α and AKT1. We found that the ΔNp63α expression is higher in CDDP-resistant cells than in CDDP-sensitive cells and observed that higher expression of ΔNp63α led to AKT1 overexpression, which consequently made cancer cells become resistant to CDDP-induced cell death. We further found that the ΔNp63α down regulation followed by decrease in AKT1 level rendered CDDP-resistant cells to become more sensitive to CDDP. We also established the molecular mechanism of the ΔNp63α-dependent up regulation of AKT1 expression. We showed that ΔNp63α significantly activated AKT1 promoter-driven luciferase reporter functional activity. We further showed that ΔNp63α protein directly and effectively bound to AKT1 promoter in vitro. By ChIP assay, we found that ΔNp63α associated with AKT1 promoter in vivo. We believe that ΔNp63α directly regulates the AKT1 transcription by binding to the AKT1 promoter-derived p63RE using the p63 DNA-binding domain. In addition, ΔNp63α has been shown to specifically transactivate certain genes either using its short 14 residue-TA-domain (15, 35-37) or in cooperation with other transcription factors and co-activators/co-repressors (38). ΔNp63α was also shown to physically associate with the carboxyl terminus of RNA polymerase II through SRA4 regulatory protein, suggesting a certain role for ΔNp63α in the transcriptional initiation (39). Thus, it is likely that the ΔNp63α-dependent AKT1 transcriptional regulation requires other transcription factors and co-activators occupying the AKT1 promoter in vivo (40, 41). Since many fundamental questions regarding in-depth molecular mechanism of p63-dependent transcriptional regulation of AKT1 expression are beyond the scope of this work, future investigations needed to thoroughly address these issues.
The AKT pathway is known to be associated with chemoresistance in human cancers (4, 42-46). We now established a novel link between ΔNp63α–dependent transcriptional regulation of AKT1 and ΔNp63α/AKT1-induced survival of cancer cells upon cisplatin exposure. The role of the ΔNp63α/AKT1 pathway in tumor cell survival was supported by AKT1 ability to rescue JHU-022 cells from cell death induced by ΔNp63α silencing. A previous report showed that up regulation of ΔNp63α in human keratinocytes after UV exposure could lead to increase in p-AKT1 levels (S473 and T308) rescuing cells from cell death (47). Another study demonstrated that the siRNA knockdown of ΔNp63α in human squamous carcinomas led to a modulation of AKT1 phosphorylation at the same residues (48). However, both reports failed to provide the molecular mechanisms underlying these events.
Our previous observations demonstrated the ability of ΔNp63α to inhibit protein phosphatase 2A (PP2A) (14). PP2A is known to inhibit AKT1 function by dephosphorylation of S473 and T308 residues that are essential for AKT1 activity (14). ΔNp63α overexpression found in HNSCC cells would thus lead to an increase in the p-AKT levels.
Accumulated evidence demonstrated that overexpression and activation of AKT1 is common in different human malignancies (42-48). The CDDP-sensitive ovarian cancer cells transfected with constitutively active AKT1 were shown to become resistant to CDDP, whereas the dominant-negative AKT1 overexpression renders CDDP-resistant ovarian cancer cells susceptible to CDDP-induced cell death (4-6, 45). Our data obtained from cancer patients' biopsies showed that AKT1 and ΔNp63α levels are concomitantly higher in head/neck and ovarian tumors resistant to CDDP than in tumors sensitive to CDDP, further supporting our hypothesis that AKT1 is a critical mediator of ΔNp63α-dependent CDDP-resistance. Our study establishes a novel functional link between ΔNp63α and chemoresistance suggesting that the transcriptional regulation of AKT1 is one of the mechanisms through which ΔNp63α can act as a pro-survival molecule in cancer.
Supplementary Material
Acknowledgments
This research was supported by national Institutes of Health Grant R01 DE 13561-05A1 “The Role of P40 Squamous Cell Carcinoma” (D.S. and E.R.)
References
- 1.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–84. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 2.Helmbach H, Kern MA, Rossmann E, et al. Drug resistance towards etoposide and cisplatin in human melanoma cells is associated with drug-dependent apoptosis deficiency. J Invest Dermatol. 2002;118:923–32. doi: 10.1046/j.1523-1747.2002.01786.x. [DOI] [PubMed] [Google Scholar]
- 3.Dan HC, Sun M, Kaneko S, et al. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP) J Biol Chem. 2004;279:5405–12. doi: 10.1074/jbc.M312044200. [DOI] [PubMed] [Google Scholar]
- 4.Fraser M, Leung BM, Yan X, et al. p53 is a determinant of X-linked inhibitor of apoptosis protein/Akt-mediated chemoresistance in human ovarian cancer cells. Cancer Res. 2003;63:7081–8. [PubMed] [Google Scholar]
- 5.Page C, Lin HJ, Jin Y, et al. Overexpression of Akt can modulate chemotherapy-induced apoptosis. Anticancer Res. 2000;20:407–16. [PubMed] [Google Scholar]
- 6.Cheng JQ, Lindsley CW, Cheng GZ, et al. The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene. 2005;24:7482–92. doi: 10.1038/sj.onc.1209088. [DOI] [PubMed] [Google Scholar]
- 7.Carroll DK, Carroll JS, Leong CO, et al. p63 regulates an adhesion programme and cell survival in epithelial cells. Nat Cell Biol. 2006;8:551–61. doi: 10.1038/ncb1420. [DOI] [PubMed] [Google Scholar]
- 8.Flores ER, Tsai KY, Crowley D, et al. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature. 2002;416:560–4. doi: 10.1038/416560a. [DOI] [PubMed] [Google Scholar]
- 9.Mills AA. p63: oncogene or tumor suppressor? Curr Opin Genet Dev. 2006;16:38–44. doi: 10.1016/j.gde.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Murray-Zmijewski F, Lane DP, Bourdon JC. p53/p63/p73 isoforms: an orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Differ. 2006;13:962–72. doi: 10.1038/sj.cdd.4401914. [DOI] [PubMed] [Google Scholar]
- 11.Tonon G, Wong KK, Maulik G, et al. High-resolution genomic profiles of human lung cancer. Proc Natl Acad Sci U S A. 2005;102:9625–30. doi: 10.1073/pnas.0504126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang A, Kaghad M, Wang Y, et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–16. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 13.Hibi K, Trink B, Patturajan M, et al. AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad Sci U S A. 2000;97:5462–7. doi: 10.1073/pnas.97.10.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patturajan M, Nomoto S, Sommer M, et al. ΔNp63 induces β-catenin nuclear accumulation and signaling. Cancer Cell. 2002;1:369–79. doi: 10.1016/s1535-6108(02)00057-0. [DOI] [PubMed] [Google Scholar]
- 15.Wu G, Osada M, Guo Z, et al. ΔNp63α up-regulates the Hsp70 gene in human cancer. Cancer Res. 2005;65:758–66. [PubMed] [Google Scholar]
- 16.Chatterjee A, Chang X, Sen T, et al. Regulation of p53 Family Member Isoform ΔNp63α by the NF-kB Targeting Kinase IkB Kinase β. Cancer Res. 2010;70:1419–29. doi: 10.1158/0008-5472.CAN-09-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocco JW, Li D, Liggett WH, et al. p16INK4A Adenovirus-mediated Gene Therapy for Human Head and Neck Squamous Cell Cancer. Clin Cancer Res. 1998;4:1697–704. [PubMed] [Google Scholar]
- 18.Huang Y, Sen T, Nagpal J, et al. ATM kinase is a master switch for the ΔNp63α phosphorylation/degradation in human head and neck squamous cell carcinoma cells upon DNA damage. Cell Cycle. 2008;7:2846–55. doi: 10.4161/cc.7.18.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y, Chuang AY, Romano RA, et al. Phospho-ΔNp63α/NF-Y protein complex transcriptionally regulates DDIT3 expression in squamous cell carcinoma cells upon cisplatin exposure. Cell Cycle. 2010;9:328–38. doi: 10.4161/cc.9.2.10432. [DOI] [PubMed] [Google Scholar]
- 20.Casciano I, Mazzocco K, Boni L, et al. Expression of ΔNp73 is a molecular marker for adverse outcome in neuroblastoma patients. Cell Death Differ. 2002;9:246–51. doi: 10.1038/sj.cdd.4400993. [DOI] [PubMed] [Google Scholar]
- 21.DeYoung MP, Johannessen CM, Leong CO, et al. Tumor-specific p73 up-regulation mediates p63 dependence in squamous cell carcinoma. Cancer Res. 2006;66:9362–8. doi: 10.1158/0008-5472.CAN-06-1619. [DOI] [PubMed] [Google Scholar]
- 22.Gressner O, Schilling T, Lorenz K, et al. TAp63α induces apoptosis by activating signaling via death receptors and mitochondria. EMBO J. 2005;24:2458–71. doi: 10.1038/sj.emboj.7600708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leong CO, Vidnovic N, DeYoung MP, et al. The p63/p73 network mediates chemosensitivity to cisplatin in a biologically defined subset of primary breast cancers. J Clin Invest. 2007;117:1370–80. doi: 10.1172/JCI30866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melino G, Bernassola F, Ranalli M, et al. p73 Induces apoptosis via PUMA transactivation and Bax mitochondrial translocation. J Biol Chem. 2004;279:8076–83. doi: 10.1074/jbc.M307469200. [DOI] [PubMed] [Google Scholar]
- 25.Muller M, Schleithoff ES, Stremmel W, et al. One, two, three-p53, p63, p73 and chemosensitivity. Drug Resist Updat. 2006;9:288–306. doi: 10.1016/j.drup.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Rocco JW, Leong CO, Kuperwasser N, et al. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell. 2006;9:45–56. doi: 10.1016/j.ccr.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Gu X, Song X, Dong Y, et al. Vitamin E succinate induces ceramide-mediated apoptosis in head and neck squamous cell carcinoma in vitro and in vivo. Clin Cancer Res. 2008;14:1840–8. doi: 10.1158/1078-0432.CCR-07-1811. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Y, Hao Y, Ji H, et al. Combination effects of salvianolic acid B with low-dose celecoxib on inhibition of head and neck squamous cell carcinoma growth in vitro and in vivo. Cancer Prev Res (Phila) 2010;3:787–96. doi: 10.1158/1940-6207.CAPR-09-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dohn M, Zhang S, Chen X. p63α and ΔNp63α can induce cell cycle arrest and apoptosis and differentially regulate p53 target genes. Oncogene. 2001;20:3193–205. doi: 10.1038/sj.onc.1204427. [DOI] [PubMed] [Google Scholar]
- 30.Aebi S, Kurdi-Haidar B, Gordon R, et al. Loss of DNA mismatch repair in acquired resistance to cisplatin. Cancer Res. 1996;56:3087–90. [PubMed] [Google Scholar]
- 31.Dihlmann S, Kloor M, Fallsehr C, von Knebel Doeberitz M. Regulation of AKT1 expression by beta-catenin/Tcf/Lef signaling in colorectal cancer cells. Carcinogenesis. 2005;26:1503–12. doi: 10.1093/carcin/bgi120. [DOI] [PubMed] [Google Scholar]
- 32.Ortt K, Raveh E, Gat U, Sinha S. A chromatin immunoprecipitation screen in mouse keratinocytes reveals Runx1 as a direct transcriptional target of ΔNp63. J Cell Biochem. 2008;104:1204–19. doi: 10.1002/jcb.21700. [DOI] [PubMed] [Google Scholar]
- 33.Osada M, Park HL, Nagakawa Y, et al. Differential recognition of response elements determines target gene specificity for p53 and p63. Mol Cell Biol. 2005;25:6077–89. doi: 10.1128/MCB.25.14.6077-6089.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchini S, Marabese M, Marrazzo E, et al. ΔNp63 expression is associated with poor survival in ovarian cancer. Ann Oncol. 2008;19:501–7. doi: 10.1093/annonc/mdm519. [DOI] [PubMed] [Google Scholar]
- 35.King KE, Ponnamperuma RM, Yamashita T, et al. ΔNp63α functions as both a positive and a negative transcriptional regulator and blocks in vitro differentiation of murine keratinocytes. Oncogene. 2003;22:3635–44. doi: 10.1038/sj.onc.1206536. [DOI] [PubMed] [Google Scholar]
- 36.Helton ES, Zhu J, Chen X. The unique NH2-terminally deleted (ΔN) residues, the PXXP motif, and the PPXY motif are required for the transcriptional activity of the ΔN variant of p63. J Biol Chem. 2006;281:2533–42. doi: 10.1074/jbc.M507964200. [DOI] [PubMed] [Google Scholar]
- 37.Romano RA, Birkaya B, Sinha S. A functional enhancer of keratin14 is a direct transcriptional target of ΔNp63. J Invest Dermatol. 2007;127:1175–86. doi: 10.1038/sj.jid.5700652. [DOI] [PubMed] [Google Scholar]
- 38.Ma J, Meng Y, Kwiatkowski DJ, et al. Mammalian target of rapamycin regulates murine and human cell differentiation through STAT3/p63/Jagged/ Notch cascade. J Clin Invest. 2010;120:103–14. doi: 10.1172/JCI37964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang YP, Kim Y, Li Z, et al. AEC- associated p63 mutations lead to alternative splicing/protein stabilization of p63 and modulation of Notch signaling. Cell Cycle. 2005;4:1440–7. doi: 10.4161/cc.4.10.2086. [DOI] [PubMed] [Google Scholar]
- 40.Nakayama H, Ikebe T, Beppu M, Shirasuna K. High expression levels of NF-kB, IkB kinase α and Akt kinase in squamous cell carcinoma of the oral cavity. Cancer. 2001;92:3037–44. doi: 10.1002/1097-0142(20011215)92:12<3037::aid-cncr10171>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 41.Park S, Kim D, Kaneko S, et al. Molecular Cloning and Characterization of the Human AKT1 Promoter Uncovers Its Up-regulation by the Src/Stat3 Pathway. J Biol Chem. 2005;280:38932–41. doi: 10.1074/jbc.M504011200. [DOI] [PubMed] [Google Scholar]
- 42.Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–97. [PubMed] [Google Scholar]
- 43.Dan HC, Jiang K, Coppola D, et al. Phosphatidylinositol-3-OH kinase/AKT and survivin pathways as critical targets for geranylgeranyltransferase I inhibitor-induced apoptosis. Oncogene. 2004;23:706–15. doi: 10.1038/sj.onc.1207171. [DOI] [PubMed] [Google Scholar]
- 44.Kim D, Dan HC, Park S, et al. AKT/PKB signaling mechanisms in cancer and chemoresistance. Front Biosci. 2005;10:975–87. doi: 10.2741/1592. [DOI] [PubMed] [Google Scholar]
- 45.Li J, Feng Q, Kim JM, et al. Human ovarian cancer and cisplatin resistance: possible role of inhibitor of apoptosis proteins. Endocrinology. 2001;142:370–80. doi: 10.1210/endo.142.1.7897. [DOI] [PubMed] [Google Scholar]
- 46.Pommier Y, Sordet O, Antony S, et al. Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene. 2004;23:2934–49. doi: 10.1038/sj.onc.1207515. [DOI] [PubMed] [Google Scholar]
- 47.Ogawa E, Okuyama R, Ikawa S, et al. p51/p63 Inhibits ultraviolet B-induced apoptosis via Akt activation. Oncogene. 2008;27:848–56. doi: 10.1038/sj.onc.1210682. [DOI] [PubMed] [Google Scholar]
- 48.Sabbisetti V, Di Napoli A, Seeley A, et al. p63 promotes cell survival through fatty acid synthase. PLoS One. 2009;4:e5877. doi: 10.1371/journal.pone.0005877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.