Abstract
Purpose
To compare long-term outcome in a population-based group of children with cryptogenic vs symptomatic focal epilepsy diagnosed from 1980–2004 and to define the course of epilepsy in the cryptogenic group.
Methods
We identified all children residing in Olmsted County, MN, 1 month through 17 years with newly diagnosed, non-idiopathic focal epilepsy from 1980–2004. Children with idiopathic partial epilepsy syndromes were excluded. Medical records were reviewed to determine etiology, results of imaging and EEG studies, treatments used, and long-term outcome. Children were defined as having symptomatic epilepsy if they had a known genetic or structural/metabolic etiology, and as cryptogenic if they did not.
Key Findings
Of 359 children with newly-diagnosed epilepsy, 215 (60%) had non-idiopathic focal epilepsy. Of these, 206 (96%) were followed for more than 12 months. Ninety five children (46%) were classified as symptomatic. Median follow-up from diagnosis was similar in both groups, being 157 months (25%ile, 75%ile 89, 233) in the cryptogenic group vs 134 months (25%ile, 75%ile 78, 220) in the symptomatic group (p=0.26). Of 111 cryptogenic cases, 66% had normal cognition. Long-term outcome was significantly better in those with cryptogenic vs symptomatic etiology (intractable epilepsy at last follow-up, 7% vs 40%, p<0.001; seizure-freedom at last follow-up, 81% vs 55%, p<0.001). Of those who achieved seizure-freedom at final follow-up, 68% of the cryptogenic group versus only 46% of the symptomatic group were off antiepileptic medications (p=0.01).
One third of the cryptogenic group had a remarkably benign disorder, with no seizures seen after initiation of medication, or in those who were untreated, after the second afebrile seizure. A further 5% had seizures within the first year but remained seizure-free thereafter. With the exception of perinatal complications, which predicted against seizure remission, no other factors were found to significantly predict outcome in the cryptogenic group.
Significance
More than half of childhood non-idiopathic localization-related epilepsy is cryptogenic. This group has a significantly better long-term outcome than those with a symptomatic etiology, and should be distinguished from it.
Keywords: Focal Epilepsy, Children, Cryptogenic, Symptomatic
Focal epilepsies in children were previously subdivided by etiology into idiopathic and symptomatic forms. Several idiopathic focal epilepsy syndromes have been described, including benign epilepsy with centrotemporal spikes and Panayiotopolous syndrome. These syndromes have specific electro-clinical characteristics, usually respond well to anti-epileptic drugs, when treated, and remit with age. In contrast, symptomatic focal epilepsies are often more malignant, with higher rates of medical intractability and lower remission rates. According to the International Classification of Epilepsies and Epileptic Syndromes proposed by the International League Against Epilepsy in 1989, cryptogenic epilepsies are presumed to be symptomatic, but with an etiology that is unknown (Commission on Classification and Terminology of the International League Against Epilepsy, 1989).
However, the more recent report of the Commission on Classification and Terminology reclassified epilepsy based on 3 aspects: (i) mode of seizure onset (generalized versus focal), (ii) specific syndrome, if present, and (iii) etiology (genetic, structural/metabolic or unknown) (Berg, et al. 2010). Although distinctive constellations have been identified correlating with specific etiologies (e.g., mesial temporal lobe epilepsy with hippocampal sclerosis, Rasmussen syndrome, and gelastic seizures with hypothalamic hamartoma), a large proportion of focal epilepsy in children still does not fit into a clear syndrome and is of unknown etiology. The prognosis in these cases remains poorly understood (Reijs et al., 2006).
Several studies have attempted to address outcome in this subgroup of children. Unfortunately, previous studies have been limited by lack of a consistent definition of cryptogenic, a relatively short follow-up period, or because they were performed prior to availability of adequate neuroimaging techniques, particularly magnetic resonance imaging (Arts et al., 2004, Berg et al., 2001, Camfield et al., 1993, Camfield & Camfield, 2002, Geelhoed et al., 2005, Hauser et al., 1996, Sillanpaa et al., 1998, Verrotti et al., 2000). Those studies with longer follow-up predominantly utilized computerized tomography scans of the head, which have poor sensitivity for detecting etiologies such as focal cortical dysplasia or mesial temporal sclerosis.
The aim of this study was twofold. First, we compare long-term outcome in children with newly-diagnosed, focal epilepsy of unknown etiology, who do not fit into a clear syndrome (cryptogenic) to newly-diagnosed focal epilepsy of known genetic or structural/metabolic etiology (symptomatic). Second, we evaluate the course of epilepsy and potential predictors of outcome in the cryptogenic group. Based on the current Classification Report (Berg et al., 2010), syndromes previously classified as idiopathic focal epilepsy, including benign epilepsy with centrotemporal spikes, Panayiotopolous syndrome and benign infantile epilepsy are classified as of unknown etiology, but present with a distinct electroclinical picture. These entities were excluded from our study as they are known to have an excellent long-term prognosis.
METHODS
Case Identification
Cases were ascertained by review of the diagnostic index of the Rochester Epidemiology Project. This index includes not only inpatient diagnoses but also diagnoses at the time of outpatient and emergency room visits. Diagnostic rubrics reviewed included all seizure and convulsion codes. All charts were reviewed by a pediatric epileptologist. All children aged 1 month through 17 years, who had new onset epilepsy diagnosed while residing in Olmsted County, Minnesota, between 1980 and 2004 were identified. Epilepsy was defined as a predisposition to unprovoked seizures. Most subjects had two or more unprovoked seizures; however, patients with a single unprovoked seizure, who were determined to be at higher risk of seizure recurrence and commenced on prophylactic antiepileptic drug treatment were also included. An abnormal neurodevelopmental examination, focal abnormality on brain imaging, initial presentation in status epilepticus, or identification of EEG findings suggestive of a higher rate of recurrence (epileptiform discharge, intermittent rhythmic focal delta activity) were considered as indicative of a higher risk of recurrence. Children who were treated after a single seizure, but who lacked any of the preceding features were excluded. Children presenting with acute symptomatic seizures alone, defined as seizures in close temporal association with an acute neurological insult were excluded. Similarly, children who had only febrile seizures were excluded. Children with neonatal seizures were included only if their seizures recurred more than 28 days after birth.
For this study, only patients with cryptogenic or symptomatic focal epilepsy who were followed for a minimum of one year after diagnosis were included. Children presenting initially with spasms followed by development of focal epilepsy were included. Subjects included were defined as symptomatic if they had a known genetic or structural/metabolic etiology for their seizures, and as cryptogenic if they did not. We excluded children with generalized or indeterminate epilepsy (generalized tonic-clonic seizures by semiology, with normal EEG and imaging), as well as those whose history, seizure semiology and EEG features were consistent with idiopathic focal epilepsy, including benign epilepsy with centrotemporal spikes, Panayiotopolous syndrome, benign infantile epilepsy or benign focal genetic epilepsy syndromes including benign familial infantile epilepsy, benign familial neonatal epilepsy and autosomal dominant frontal lobe epilepsy. All cases were classified independently, based on the documented history, clinical examination, EEG and neuroimaging reports from the medical records by two epileptologists (EW and KN) and if disagreement was present, a consensus was reached by joint review.
Chart review
Medical records of children with cryptogenic or symptomatic focal epilepsy were reviewed to determine their epilepsy outcome at 1, 2, 3, 5, 10, 15 and 20 years after epilepsy diagnosis regarding both seizure control (seizure-free for the previous year or longer at each time point) and anti-epileptic drug treatment (number of current medications and number of medications failed for lack of efficacy at each time point). Additionally, epilepsy surgery procedures (resection, vagal nerve stimulator, corpus callosotomy) were documented. Cognitive function was assessed at the time of seizure diagnosis and last follow-up as normal (developmental quotient of 80 or higher), mild to moderate delay (developmental quotient of 50–79), or severely delayed (developmental quotient of less than 50). The majority of children had not undergone formalized psychometric testing. In these children, cognitive function was estimated from history of developmental milestones and school performance.
Demographic data (age and sex), significant past history (prenatal and perinatal complications, gestational age, postnatal brain injury, intracranial infection), epilepsy details (febrile seizures, seizure type, number of seizures at diagnosis, age at onset, history of status epilepticus), first degree family history of epilepsy, and neurological examination, neuroimaging and EEG results were also collected.
For the cryptogenic group, the number of remissions (defined as an extended period of seizure freedom lasting one year or longer) and relapses (defined as recurrent seizures after a remission) over the course of their epilepsy was noted. For those who had achieved at least one remission, we noted whether they had attempted to wean off antiepileptic medication.
Data Analysis
The proportion of children in both the cryptogenic and symptomatic groups who achieved seizure freedom with or without medication, or who developed intractable epilepsy were compared at each time point using chi-square tests. As not all children were followed for the entire 20 years, the total number of children followed through to each time point was used as the denominator. Intractable epilepsy was defined as having seizures more frequently than every six months during the preceding year, and failing two or more anti-epileptic drugs for lack of efficacy.
Not all children in our cryptogenic group underwent MRI imaging. Arguably, these children may have had an undetected malformation of cortical development or mesial temporal sclerosis. Thus, we also determined outcome specifically for the MRI-negative cryptogenic group.
Potential predictors of seizure-free outcome and intractable epilepsy at last follow-up in the cryptogenic group were assessed with chi-square tests for categorical variables and t test for continuous data. Factors included in the analysis included sex, age at onset, cognitive delay, abnormal neurological examination, perinatal insults, febrile seizures, complicated febrile seizures, minor non-specific changes on MRI, background slowing on EEG, epileptiform discharge on initial EEG, status epilepticus and first degree family history of epilepsy.
RESULTS
We identified 359 children with newly-diagnosed epilepsy from 1980 through 2004 and while residing in Olmsted County. All children except three were seen on at least one occasion by a child neurologist who confirmed the diagnosis of epilepsy and most were followed over the course of their epilepsy. Of the remaining three, two were diagnosed with epilepsy by a general neurologist, and one by a pediatrician.
A total of 215 (60%) met our criteria for symptomatic or cryptogenic non-idiopathic focal epilepsy. Of these children, 206 (96%) were followed for at least 12 months after diagnosis and comprise the study group (111 cryptogenic, 95 symptomatic). Median follow-up from diagnosis for these children was 157 months (25th percentile = 89 months, 75th percentile = 233 mos.) for the cryptogenic group versus 134 months (25th percentile = 78 months, 75th percentile = 220 mos) for the symptomatic group (p=0.26).
Symptomatic etiologies included prior brain insults (N=48: 20-perinatal hypoxic-ischemic brain injury, 4-stroke, 4-hydrocephalus with shunt, 3- prematurity with periventricular leukomalacia, 3-meningitis/encephalitis, 2-hypoglycemia or hyponatremia, 2-head injury, 3-postnatal hypoxic ischemic brain injury, 3-remote ischemic lesion on MRI of presumed prenatal onset, 1-prior brain tumor, 1-kernicterus, 1-calcified granuloma, 1-congenital cytomegalovirus infection), malformations of cortical development or tuberous sclerosis (N=21), mesial temporal sclerosis (N=7), vascular malformation (N=6), chromosomal abnormalities (N=5), tumor (N=3), hypothalamic hamartoma (N=1) and dual pathology of mesial temporal sclerosis with cortical dysplasia (N=4).
Descriptive information for the cryptogenic and symptomatic cohorts is given in Table 1. The median age at onset of epilepsy was younger for symptomatic cases versus cryptogenic cases (p=0.02). In addition, children with symptomatic epilepsy had higher rates of cognitive impairment (p<0.001), more frequent abnormalities on neurological examination (p<0.001), were more commonly born prematurely (p=0.012), had a higher frequency of neonatal seizures (p<0.001), had a lower likelihood of secondarily generalized seizures at the time of presentation (p=0.009), had higher numbers of seizures at treatment onset (p=0.002), exhibited higher rates of status epilepticus both at presentation (p=0.008) and ever (p<0.001), had higher rates of epileptiform abnormalities on initial EEG (p=0.02) and had a greater likelihood of background slowing on their initial EEG (p<0.001) as compared to the cryptogenic group. However, the cryptogenic group had a higher rate of febrile seizures in general (p=0.05), while the children in the symptomatic group were more likely to have complicated febrile seizures, if febrile seizures were present (p=0.004).
Table 1.
Cryptogenic and symptomatic focal epilepsy cohorts
| Cryptogenic (N = 111)a |
Symptomatic (N = 95)a |
||
|---|---|---|---|
| Characteristic | N (%) | N (%) | p value |
| Sex, male | 59 (53) | 59 (62) | 0.20 |
| Age at onset, monthsb | 65.8 (30.9, 130.9) | 52.8 (10.5, 105.0) | 0.02 |
| Length of follow-up, monthsb | 157.1 (88.7, 157.1) | 134.3 (78.4, 219.8) | 0.26 |
| Cognition at onset | |||
| Normal | 73 (66) | 40 (42) | < 0.001 |
| Mild delay | 27 (24) | 25 (26) | |
| Severe delay | 9 (8) | 27 (28) | |
| Unknown | 1 (1) | 3 (3) | |
| Cognition at follow-up | |||
| Normal | 73 (66) | 31 (33) | < 0.001 |
| Mild delay | 27 (24) | 29 (31) | |
| Severe delay | 11 (10) | 35 (37) | |
| Neurological exam, abnormal | 13 (12)c | 61 (64) | < 0.001 |
| Prenatal hospitalization | 8/110 (7) | 8/92 (9) | 0.76 |
| Gestational age < 38 weeks | 14/110 (13) | 25/94 (27) | 0.012 |
| Perinatal NICU ≥ 7 days | 9/110 (8) | 43/94 (46) | <0.001 |
| Neonatal seizures | 3/110 (3) | 19/93 (20) | < 0.001 |
| Head injury with LOC | 4/110 (4) | 2/94 (2) | 0.53 |
| Meningitis or encephalitis | 0/110 (0) | 6/93 (7) | 0.007 |
| Febrile seizures | |||
| Any | 21/110 (19) | 9 (9) | 0.05 |
| Complicated | 9/21 (43) | 9/9 (100) | 0.004 |
| 2°GTCS at presentation | 55 (50) | 30 (32) | 0.009 |
| Partial seizures at presentation | 82 (74) | 68 (72) | 0.71 |
| No. of seizures at onset of AEDsb | 3 (2, 4) | 5 (2, 10) | 0.002 |
| Status epilepticus | |||
| At presentation | 15 (14) | 27 (28) | 0.008 |
| Ever | 18 (16) | 41 (43) | < 0.001 |
| First deg. family history of epilepsy | 13/109 (12) | 11/90 (12) | 0.95 |
| Neuroimaging | |||
| MRI completed | 78 (70) | 76 (80) | |
| Mild, nonspecific changes | 7/78 (9)d | 1/76 (1) | 0.032 |
| Abnormal | 0/78 (0) | 72/76 (95) | < 0.001 |
| CT only | 28 (25) | 17 (18) | |
| Abnormal | 0/28 (0) | 12/17 (71) | < 0.001 |
| No imaging | 5 (5) | 2 (2) | |
| Initial EEG | |||
| Epileptiform abnormality | 69 (62) | 71/92 (77) | 0.02 |
| Background slowing | |||
| Generalized | 22 (20) | 51/92 (55) | < 0.001 |
| Focal | 18 (16) | 35/92 (38) | <0.001 |
Denominators are shown when some data were incomplete.
Values reported are the median and the 25th and 75th percentiles. P-values are for the Wilcoxon rank-sum test.
A total of 12 children had abnormal neurological exams including 7-motor clumsiness, 1-spasticity plus extrapyramidal signs, 1-spasticity, 3-microcephaly alone.
A total of 7 children exhibited mild non-specific MRI findings including 1-Chiari 1 malformation, 4-small T2 hyperintensity remote from interictal EEG focus, 1-small left hippocampus without signal change in child with right-sided EEG discharge, 1-minor asymmetry of sylvian fissure.
Seizure Freedom and Intractability in Cryptogenic versus Symptomatic Focal Epilepsy
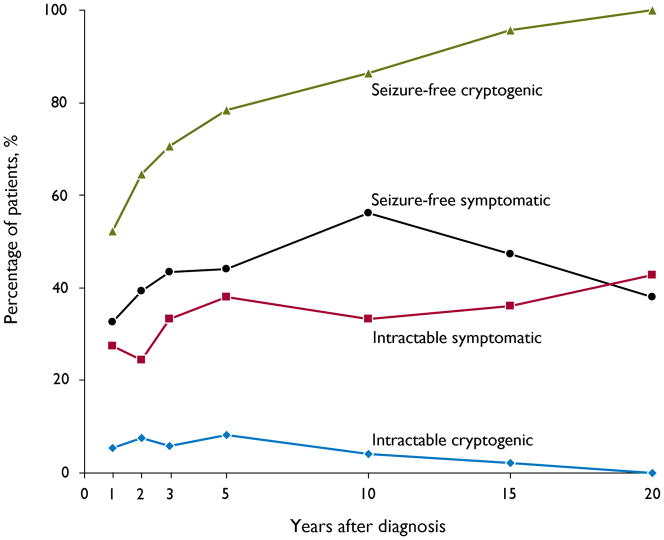
The proportion of children in the cryptogenic and symptomatic focal epilepsy groups who attained seizure-freedom or developed intractable epilepsy is shown in Figure 1. During the last year of follow-up, a significantly greater proportion of children in the cryptogenic group achieved seizure-freedom than in the symptomatic group [90/111 (81%) versus 52/95 (55%), p<0.001]. Of those who achieved seizure freedom, 61/90 (68%) of those in the cryptogenic group versus 24/52 (46%) in the symptomatic group were off antiepileptic medications (p=0.011). Similarly, significantly more children in the symptomatic group had developed medically intractable epilepsy at final follow-up than in the cryptogenic group [38/95 (40%) vs 8/111 (7%), p<0.001].
Figure 1.
Seizure-freedom and development of intractable epilepsy over time in children with cryptogenic and symptomatic focal epilepsy
Looking specifically at the MRI-negative cryptogenic group (N=78), seizure-freedom was achieved during the last year of follow-up in 60/78 (77%), and of these, 36/60 (60%) were off antiepileptic medications. Seven (9%) had developed medically intractable epilepsy at final follow-up.
Thirty three children in the cryptogenic group did not have MRI imaging. Of these, seizure freedom was achieved in the final year of follow-up in 30 (91%) and only 1 (3%) had developed intractable epilepsy.
Twenty children (21%) in the symptomatic group versus only one (1%) in the cryptogenic group underwent resective epilepsy surgery. Twelve of the 20 symptomatic patients (60%) were seizure-free postoperatively, but six continued to have intractable epilepsy at final follow-up. The only cryptogenic patient who underwent surgery had negative pathology and continued to have intractable epilepsy at final follow-up.
Course of Epilepsy and Predictors of Outcome in Cryptogenic Focal Epilepsy
The course of epilepsy for the cryptogenic group is shown in Table 2. In 36 (32%) of the cryptogenic group, epilepsy was “smooth-sailing” (Camfield & Camfield, 2002), meaning that no further seizures were seen after antiepileptic medications were commenced, or in those who were never treated, after the second afebrile seizure. A further 6 (5%) had one or more seizures within the first year after initiation of medications, but remained seizure-free thereafter. Only 13 (12%) met criteria for intractable epilepsy at any point since diagnosis.
Table 2.
Epilepsy course in cryptogenic focal epilepsy.
| Cryptogenic |
|
|---|---|
| Epilepsy Course at final follow-up | N (%) |
| Seizure freea never treated with AEDs | 6 (5) |
| Seizure free and off AEDs. Epilepsy never intractable b | 53 (48) |
| Seizure free and off AEDs. Epilepsy was intractable at some point during its course | 2 (2) |
| Seizure-free but on AEDs at final follow-up. Epilepsy never intractable | 28 (25) |
| Seizure-free but on AEDs at final follow-up. Epilepsy was intractable at some point during its course | 2 (2) |
| Not seizure-free and on AEDs at final follow-up. Epilepsy is not intractable | 11 (10) |
| Not seizure-free and on AEDs at final follow-up. Epilepsy previously was intractable but is not at last follow-up | 1 (1) |
| On AEDs at final follow-up. Epilepsy is intractable | 8 (7) |
| Off AEDs but not seizure-free at final follow-up. | 0 (0) |
Seizure-free–no seizures in final year of follow-up
Intractable–failure of 2 or more AEDs for lack of efficacy and seizures at least once every 6 months
Of the 90 subjects who were seizure-free at final follow-up, 61 (68%) were in their first remission, 20 (22%) in their second remission, and 9 (10%) in their third or higher remission. Of 29 subjects who remained on antiepileptic medication despite being seizure-free at final follow-up, 11 (38%) previously had medications weaned with seizure recurrence. Of the 21 patients who were not seizure-free at final follow-up, 11 (52%) had previously achieved a remission lasting 12 months or longer, but then had relapse of their seizures.
Potential predictors of both seizure freedom and intractable epilepsy in cryptogenic focal epilepsy are shown in Table 3. The presence of perinatal complications correlated with a lower chance of seizure freedom at follow-up (p=0.04). However, no other factor correlated significantly with either seizure freedom or intractability at final follow-up.
Table 3.
Predictors of remission of seizures or development of Intractable epilepsy in children with cryptogenic focal epilepsy
| Remission of seizures |
Intractability epilepsy |
|||||
|---|---|---|---|---|---|---|
| Yes (N=90) |
No (N=21) |
p value | Yes (N=8) |
No (N=103) |
p value | |
| N (%) | N (%) | N (%) | N (%) | |||
| Sex, male | 47 (52) | 12 (57) | 0.68 | 4 (50) | 55 (53) | 0.85 |
| Age at onset, monthsa | 64.1 (28.9, 128.8) | 74.7 (39.8, 161.4) | 0.60 | 39.8 (8.5, 145.9) | 71.9 (30.9, 130.9) | 0.61 |
| Perinatal NICU >7 daysb | 5/89 (6) | 4/21 (19) | 0.04 | 1/8 (13) | 8/102 (8) | 0.64 |
| Febrile seizuresb | ||||||
| Any | 19/89 (21) | 2/21 (10) | 0.22 | 1/8 (13) | 20/102 (20) | 0.62 |
| Complicated | 7/89 (8) | 2/21 (10) | 0.80 | 1/8 (13) | 8/102 (8) | 0.64 |
| Background slowingc | ||||||
| Generalized | 19 (21) | 3 (14) | 0.48 | 0 (0) | 22 (21) | 0.14 |
| Focal | 14 (16) | 4 (19) | 0.70 | 1 (13) | 17 (17) | 0.77 |
| Epileptiform dischargec | 54 (60) | 15 (71) | 0.33 | 6 (75) | 63 (61) | 0.44 |
| Status epilepticus | ||||||
| At presentation | 14 (16) | 1 (5) | 0.19 | 0 (0) | 15 (15) | 0.25 |
| Neurological examination | ||||||
| Abnormal | 9 (10) | 4 (19) | 0.25 | 2 (25) | 11 (11) | 0.23 |
| First degree family history of epilepsyb | 11/89 (12) | 2/20 (10) | 0.77 | 1/8 (13) | 12/101 (12) | 0.96 |
| Cognitive delay at diagnosisb | 26/89 (29) | 10/21 (48) | 0.11 | 5/8 (63) | 30/102 (30) | 0.06 |
Values reported are the median and the 25th and 75th percentiles. P-values are for the Wilcoxon rank-sum test.
Denominators are shown when some data were incomplete.
Based on the initial EEG
DISCUSSION
In our population-based study of children with new-onset epilepsy, cryptogenic focal epilepsy, as defined by (1) lack of an underlying genetic, structural or metabolic etiology and (2) not meeting criteria for a recognized idiopathic partial epilepsy syndrome, accounted for a significant proportion of new-onset cases of epilepsy in children. Nearly thirty percent of all incident cases of epilepsy, and just over half of non-idiopathic, focal epilepsies were cryptogenic. Prior reports on the proportion of cases classified as cryptogenic focal epilepsy have varied widely, from 15–49% of all cases of epilepsy, and 40–73% of all cases of non-idiopathic focal epilepsy (Akiyama et al., 2006, Berg et al., 2004, Eriksson & Koivikko, 1997, Waaler et al., 2000), as definitions have not been consistent. Some series have included all children without a known etiology, whereas others have excluded children with abnormal neurological examinations or cognitive delay, believing these findings are suggestive of a symptomatic cause, even though no identifiable etiology has been found.
We found that the prognosis for cryptogenic focal epilepsy was strikingly better than for those with a symptomatic etiology, emphasizing the importance of distinguishing these two entities. Cryptogenic patients had a significantly higher likelihood of achieving seizure freedom at final follow-up, of ultimately stopping antiepileptic medication, and a significantly lower likelihood of developing intractable epilepsy. Prior studies have also reported high remission rates in children with cryptogenic focal epilepsy. In a cohort of 132 children, all of whom had normal intelligence, a normal neurological examination, and normal imaging by computed tomography scanning, two thirds had remission of their epilepsy over a mean follow-up period of 88 months (Camfield & Camfield, 2002). In Sillanpaa’s study of 32 children followed with cryptogenic localization-related epilepsy, 20 (63%) were in remission at final follow-up. However the majority of these patients were studied prior to widespread use of neuroimaging (Sillanpaa et al., 1998). Finally, Shinnar reported that 82% of 34 children with cryptogenic partial epilepsy were in remission after a mean follow-up of 8.3 years, although this study did not comment on the cognitive status of patients (Shinnar et al.,1999). While only 55% of children with cryptogenic partial epilepsy in our study had truly remitted at final follow-up (seizure-free and off antiepileptic drugs), an additional 9% had achieved seizure-freedom but were never given a trial off medication.
Previous studies have suggested that despite a high remission rate, recurrent relapses of seizures can be problematic in a portion of children with cryptogenic localization-related epilepsy. Using a Markov process to model remission and relapse, Berg found that the cryptogenic partial group had only a slightly lower chance of being in remission at any given time compared to the idiopathic partial group. However, because of the higher relapse rate, the cryptogenic partial group was increasingly less likely over time to be in their first remission. Rather, many were subjected to a pattern of repeated remission and relapse (Berg et al., 2004). However, Camfield found that 45% of patients with cryptogenic focal epilepsy had a “remarkably” benign disorder, becoming instantly seizure-free after starting medication, discontinuing medication after a few years, and remaining seizure-free (Camfield & Camfield, 2002). Our results are similar–we found 32% became immediately seizure-free and an additional 5% had only rare seizures in the first year and then remained seizure-free thereafter. Only 12% met criteria for intractable seizures at some point in the course of their epilepsy and only 7% were medically intractable at final follow-up. A pattern of repeated remission and relapse was not seen in most of our patients. Of those who were seizure-free at final follow-up, the majority (68%) were in their first remission and only 10% had experienced three or more remissions with relapses.
These results suggest that, while the cryptogenic partial group is comprised of a large number of children with a remarkably benign epilepsy, it is clinically heterogeneous, as not all patients do well. Many of those with more resistant seizures may well have an, as yet undetected, symptomatic cause, possibly genetic or structural, as supported by our finding that children with cognitive delays tended to have poorer outcome.
The International League Against Epilepsy report from 1989 classifies cryptogenic epilepsy as probably symptomatic, but without a defined etiology. In the most recent Classification Report, the term “unknown” is used to describe etiologies that are not genetic or structural metabolic. Most child neurologists consider the severity of cryptogenic localization-related epilepsy to be intermediate between the idiopathic and symptomatic syndromes (Dunn et al., 2004). Using the Epilepsy Syndrome Severity Scores–Child, Dunn and colleagues found that neurologists rated the severity of cryptogenic partial epilepsy at a 7 on a ten point scale, significantly higher than benign epilepsy of childhood with centrotemporal spikes (rated as a 2), but lower than Landau-Kleffner syndrome (rated as an 8). Based on our results, we believe these ratings are excessively bleak. Rather than considering cryptogenic focal epilepsy as probably symptomatic, it should be considered as a separate entity, with a relatively favorable long-term outcome, particularly in children with normal imaging, normal neurological examination and normal cognition.
Our study has a number of strengths. The Rochester Epidemiology Project allowed for complete identification of a population-based incidence cohort of children with new-onset epilepsy, and allowed access to all inpatient and outpatient records in the community. Therefore, our study sample was not contaminated by selection bias, which may be the case in reports arising strictly from an epilepsy center. Secondly, our patients had ready access to high-quality health care specialists and technology. Most children were seen by a child neurologist either at the time of diagnosis or shortly thereafter, and underwent neuroimaging with magnetic resonance imaging. Thirdly, many of our patients were followed long-term, often into their adult years, allowing a more complete understanding of their risk of remission and risk of relapse.
A potential weakness of our study was that not all children in the cryptogenic group had undergone MRI imaging. These children may have had a structural abnormality which was not detected. However, this group did not have a lower rate of seizure-freedom or higher rate of intractability at final follow-up. We suspect that in most of these cases, further imaging was not pursued as these children were doing well.
In summary, recognition of cryptogenic focal epilepsy as distinct from symptomatic focal epilepsy is important, given its favorable long-term prognosis. However, its definition requires that underlying etiologies be excluded. Children should be neurologically and cognitively normal - the presence of mental handicap should be considered evidence for an underlying symptomatic cause. Furthermore, neuroimaging, preferably magnetic resonance imaging, should be performed to rule out a structural etiology such as a malformation of cortical development, prior injury, vascular abnormality, tumor or mesial temporal sclerosis. In young children, particularly those between 6 and 30 months, incomplete myelination may limit detection of a focal cortical dysplasia or appreciation of its true extent (Colombo et al., 2009). Therefore, an MRI should be repeated after 30 months of age if seizures remain problematic and the initial study was unrevealing. Further investigations of this cohort of children with cryptogenic focal epilepsy may identify specific genes responsible for some of these conditions which confer either a better or poorer prognosis. In addition, careful clinical characterization may allow descriptions of less common epilepsy syndromes with benign outcomes.
Acknowledgments
This study was supported by a CR20 Research award from the Mayo Foundation, and made possible by the Rochester Epidemiology Project (Grant # R01-AR030582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases).
Footnotes
DISCLOSURE
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
None of the authors has any conflict of interest to disclose.
Contributor Information
Elaine C Wirrell, Divisions of Child and Adolescent Neurology and Epilepsy, Department of Neurology, Mayo Clinic, Rochester, MN.
Brandon R Grossardt, Division of Biomedical Statistics and Informatics, Department of Health Sciences Research, Mayo Clinic, Rochester MN.
Elson L So, Division of Epilepsy, Department of Neurology, Mayo Clinic, Rochester, MN.
Katherine C Nickels, Divisions of Child and Adolescent Neurology and Epilepsy, Department of Neurology, Mayo Clinic, Rochester, MN.
References
- Akiyama T, Kobayashi K, Ogino T, Yoshinaga H, Oka E, Oka M, Ito M, Ohtsuka Y. A population-based survey of childhood epilepsy in Okayama Prefecture, Japan: reclassification by a newly proposed diagnostic scheme of epilepsies in 2001. Epilepsy Res. 2006;70(Suppl 1):S34–40. doi: 10.1016/j.eplepsyres.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Arts WF, Brouwer OF, Peters AC, Stroink H, Peeters EA, Schmitz PI, van Donselaar CA, Geerts AT. Course and prognosis of childhood epilepsy: 5-year follow-up of the Dutch study of epilepsy in childhood. Brain. 2004;127:1774–1784. doi: 10.1093/brain/awh200. [DOI] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshe SL, Nordli D, Plouin P, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Berg AT, Lin J, Ebrahimi N, Testa FM, Levy SR, Shinnar S. Modeling remission and relapse in pediatric epilepsy: application of a Markov process. Epilepsy Res. 2004;60:31–40. doi: 10.1016/j.eplepsyres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Berg AT, Shinnar S, Levy SR, Testa FM, Smith-Rapaport S, Beckerman B, Ebrahimi N. Two-year remission and subsequent relapse in children with newly diagnosed epilepsy. Epilepsia. 2001;42:1553–1562. doi: 10.1046/j.1528-1157.2001.21101.x. [DOI] [PubMed] [Google Scholar]
- Camfield C, Camfield P, Gordon K, Smith B, Dooley J. Outcome of childhood epilepsy: a population-based study with a simple predictive scoring system for those treated with medication. J Pediatr. 1993;122:861–868. doi: 10.1016/s0022-3476(09)90008-7. [DOI] [PubMed] [Google Scholar]
- Camfield P, Camfield C. Epileptic syndromes in childhood: clinical features, outcomes, and treatment. Epilepsia. 2002;43(Suppl 3):27–32. doi: 10.1046/j.1528-1157.43.s.3.3.x. [DOI] [PubMed] [Google Scholar]
- Colombo N, Salamon N, Raybaud C, Ozkara C, Barkovich AJ. Imaging of malformations of cortical development. Epileptic Disord. 2009;11:194–205. doi: 10.1684/epd.2009.0262. [DOI] [PubMed] [Google Scholar]
- Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- Dunn DW, Buelow JM, Austin JK, Shinnar S, Perkins SM. Development of syndrome severity scores for pediatric epilepsy. Epilepsia. 2004;45:661–666. doi: 10.1111/j.0013-9580.2004.53903.x. [DOI] [PubMed] [Google Scholar]
- Eriksson KJ, Koivikko MJ. Prevalence, classification, and severity of epilepsy and epileptic syndromes in children. Epilepsia. 1997;38:1275–1282. doi: 10.1111/j.1528-1157.1997.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Geelhoed M, Boerrigter AO, Camfield P, Geerts AT, Arts W, Smith B, Camfield C. The accuracy of outcome prediction models for childhood-onset epilepsy. Epilepsia. 2005;46:1526–1532. doi: 10.1111/j.1528-1167.2005.07405.x. [DOI] [PubMed] [Google Scholar]
- Hauser E, Freilinger M, Seidl R, Groh C. Prognosis of childhood epilepsy in newly referred patients. J Child Neurol. 1996;11:201–204. doi: 10.1177/088307389601100307. [DOI] [PubMed] [Google Scholar]
- Reijs RP, van Mil SG, van Hall MH, Arends JB, Weber JW, Renier WO, Aldenkamp AP. Cryptogenic localization-related epilepsy with childhood onset: The problem of definition and prognosis. Epilepsy Behav. 2006;8:693–702. doi: 10.1016/j.yebeh.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Shinnar S, O'Dell C, Berg AT. Distribution of epilepsy syndromes in a cohort of children prospectively monitored from the time of their first unprovoked seizure. Epilepsia. 1999;40:1378–1383. doi: 10.1111/j.1528-1157.1999.tb02008.x. [DOI] [PubMed] [Google Scholar]
- Sillanpaa M, Jalava M, Kaleva O, Shinnar S. Long-term prognosis of seizures with onset in childhood. N Engl J Med. 1998;338:1715–1722. doi: 10.1056/NEJM199806113382402. [DOI] [PubMed] [Google Scholar]
- Verrotti A, Morresi S, Basciani F, Cutarella R, Morgese G, Chiarelli F. Discontinuation of anticonvulsant therapy in children with partial epilepsy. Neurology. 2000;55:1393–1395. doi: 10.1212/wnl.55.9.1393. [DOI] [PubMed] [Google Scholar]
- Waaler PE, Blom BH, Skeidsvoll H, Mykletun A. Prevalence, classification, and severity of epilepsy in children in western Norway. Epilepsia. 2000;41:802–810. doi: 10.1111/j.1528-1157.2000.tb00246.x. [DOI] [PubMed] [Google Scholar]