Abstract
High endothelial venule (HEV)-like vessels have been observed in gastric B-cell lymphoma of mucosa-associated lymphoid tissue type (MALT lymphoma), as well as in its preceding lesion, chronic Helicobacter pylori gastritis. Previously we reported that glycans on HEV-like vessels in the latter lesion served as L-selectin ligands. However, the biochemical and functional nature of glycans on HEV-like vessels in gastric MALT lymphoma remained to be determined. In this study, we performed immunohistochemical analysis for sialyl Lewis X (sLeX)-related glycoepitopes using three monoclonal antibodies MECA-79, HECA-452, and NCC-ST-439, and found that MECA-79−/HECA-452+/NCC-ST-439+ HEV-like vessels preferentially appears in gastric MALT lymphoma compared to chronic H. pylori gastritis, suggesting that appearance of MECA-79−/HECA-452+/NCC-ST-439+ HEV-like vessels marks gastric MALT lymphoma. We then constructed a set of CHO cell lines expressing possible MECA-79−/HECA-452+/NCC-ST-439+ glycans, as well as other sLeX-type glycans, on CD34, and evaluated L-selectin binding to those cells using L-selectin•IgM chimera binding and lymphocyte adhesion assays. L-selectin•IgM chimeras bound to CHO cells expressing 6-sulfo sLeX attached to core 2-branched O-glycans with or without 6-sulfo sLeX attached to extended core 1 O-glycans but only marginally to other CHO cell lines. On the other hand, CHO cells expressing 6-sulfo sLeX attached to extended core 1 and/or core 2-branched O-glycans, and also non-sulfated sLeX attached to core 2-branched O-glycans showed substantial lymphocyte binding, while binding was negligible on cell lines expressing 6-sulfo and non-sulfated sLeX attached to N-glycans and non-sulfated sLeX attached to extended core 1 O-glycans. These results indicate that MECA-79−/HECA-452+/NCC-ST-439+ glycans, namely 6-sulfo and non-sulfated sLeXs attached to core 2-branched O-glycans, expressed on HEV-like vessels in gastric MALT lymphoma, function as L-selectin ligands and likely contribute to H. pylori-specific T-cell recruitment in the progression of gastric MALT lymphoma.
Keywords: stomach, mucosa-associated lymphoid tissue, lymphoma, high endothelial venule-like vessel, 6-sulfo sialyl Lewis X, non-sulfated sialyl Lewis X, core 2-branched O-glycan
Introduction
Primary gastric lymphomas account for 3% of all gastric malignancies [1]. Most are categorized as extranodal marginal zone B-cell lymphomas of mucosa-associated lymphoid tissue (MALT), or MALT lymphoma, in the WHO classification [2]. Neoplastic B cells originate from the marginal zone of secondary follicles in MALT, which are, in most cases, generated in response to Helicobacter pylori infection [3]. Proliferation of neoplastic B cells requires the presence of T-cells specifically activated by H. pylori antigens [4,5]. The importance of this stimulation is impressively demonstrated by the fact that eradication of H. pylori with antibiotics, which has become established clinical practice, results in regression of lymphoma in approximately 75% of cases [6].
MALT lymphomas are clinically indolent, requiring long-term clinical surveillance with repeated biopsies. Pathologists, cooperating closely with clinicians, play a central role in the diagnosis and management of these patients [7]. A primary diagnostic difficulty, particularly in endoscopic biopsies, is in distinguishing so-called low-grade MALT lymphoma, i.e., MALT lymphoma without transformation into diffuse large B-cell lymphoma (DLBCL), from the marked chronic inflammation that sometimes occurs in H. pylori gastritis. Such characterization may be extremely difficult, particularly in small biopsies, and repeated sampling and/or careful endoscopic follow-up is required to distinguish these conditions. Additionally, histological assessment of therapeutic effect after H. pylori eradication is also challenging. Therefore, novel markers are required to distinguish between these two pathological conditions.
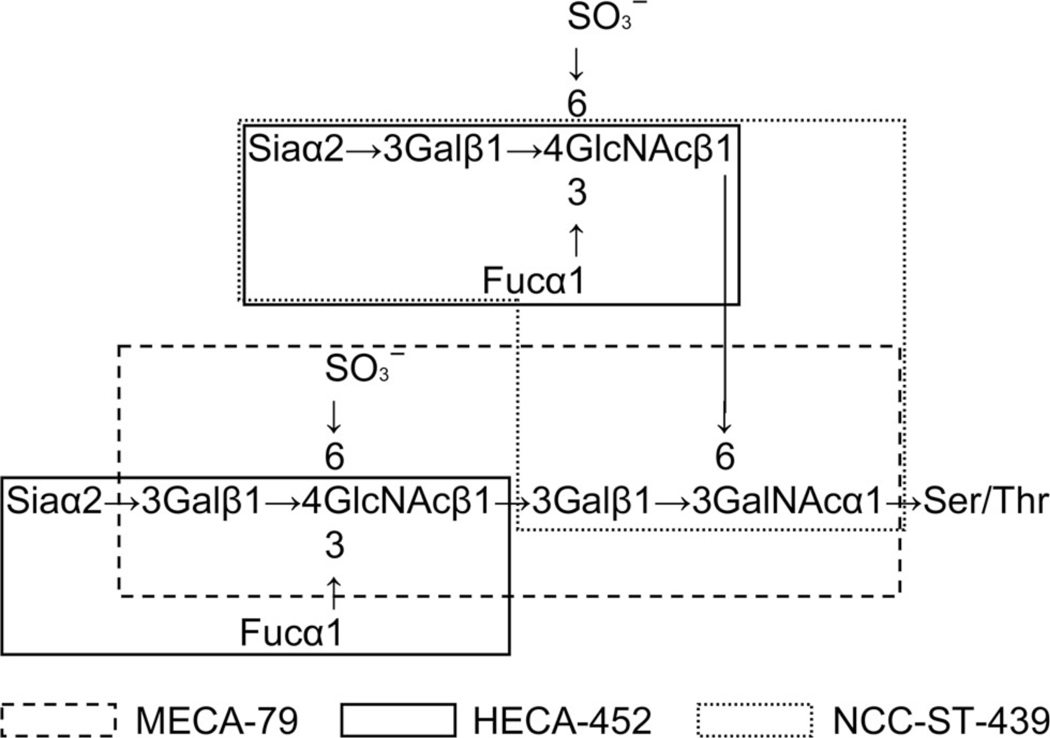
Circulating lymphocytes enter secondary lymphoid organs such as lymph nodes, tonsils, and Peyer’s patches, where they encounter foreign antigens by interacting with antigen-presenting cells [8]. This lymphocyte homing is mediated by a cascade of adhesive interactions between circulating lymphocytes and specialized venules called high endothelial venules (HEVs). Peripheral lymph node addressin (PNAd), a glycoprotein complex recognized by the MECA-79 monoclonal antibody [9], is constitutively displayed on these HEVs and bound by L-selectin expressed on lymphocytes, contributing to “tethering and rolling”, the initial step of lymphocyte homing [10]. Among PNAd family, CD34 is broadly expressed on the vascular endothelium, but limited portion of vessels, e.g., HEVs in secondary lymphoid organs and presumably HEV-like vessels in inflamed sites, express glycoforms that are L-selectin reactive [10,11]. The MECA-79 epitope has been shown to be 6-sulfo N-acetyllactosamine attached to extended core 1 O-glycans, Galβ1→4(sulfo→6)GlcNAcβ1→3Galβ1→3GalNAcα1→Ser/Thr (Figure 1), and its sialylated and fucosylated form, 6-sulfo sialyl Lewis X (sLeX) attached to extended core 1 O-glycans, Siaα2→3Galβ1→4[Fucα1→3(sulfo→6)]GlcNAcβ1→3Galβ1→3GalNAcα1→Ser/Thr (Figure 1), is also recognized by this antibody [12]. Although 6-sulfo sLeX displayed on core 2-branched O-glycans is not recognized by this antibody, it is also bound by L-selectin and plays a dominant role in lymphocyte homing [12,13]. This L-selectin ligand carbohydrate is overlapped with the epitope detected by NCC-ST-439 monoclonal antibody, which recognizes sLeX attached to core 2-branched O-glycans, Siaα2→3Galβ1→4(Fucα1→3)GlcNAcβ1→6(Galβ1→3)GalNAcα1→Ser/Thr (Figure 1), regardless of GlcNAc-6-O-sulfation [14,15]. These two L-selectin ligand carbohydrates are also detected by the HECA-452 monoclonal antibody, which recognizes sLeX-capped structures, Siaα2→3Galβ1→4(Fucα1→3)GlcNAcβ1→R (Figure 1), regardless of GlcNAc-6-O-sulfation, on both N- and O-glycans [14,16–18].
Figure 1.
Schematic representation of carbohydrate structure of L-selectin ligands. 6-sulfo sLeX attached to extended core 1 and/or core 2-branched O-glycans functions as an L-selectin ligand. Epitopes for monoclonal antibodies MECA-79 (6-sulfo N-acetyllactosamine attached to extended core 1 O-glycans), HECA-452 (sLeX, regardless of GlcNAc-6-O-sulfation), and NCC-ST-439 (sLeX attached to core 2-branched O-glycans, regardless of GlcNAc-6-O-sulfation) are shown.
Although PNAd is absent in non-lymphoid tissues under normal conditions, it is induced on HEV-like vessels in various chronic inflammatory states [10,11,14,19–21]. We previously showed that PNAd-expressing HEV-like vessels are induced in chronic H. pylori gastritis, and that progression of chronic inflammation is highly correlated with the occurrence of such vessels [14]. Moreover, we found that eradication of H. pylori with antibiotics is associated with the disappearance of these vessels and only a minimal amount of residual lymphocyte infiltrate. These results indicate that lymphocyte recruitment in chronic H. pylori gastritis is at least partly regulated by PNAd. It was reported by Dogan et al. that PNAd-expressing HEV-like vessels were also present in low-grade gastric MALT lymphoma [22]; however, biochemical and functional characteristics of L-selectin ligand carbohydrates expressed on these vessels remains to be determined.
In the present study, we demonstrate that MECA-79−/HECA-452+/NCC-ST-439+ HEV-like vessels are preferentially found in gastric MALT lymphoma compared with chronic H. pylori gastritis, a finding that should be helpful to distinguish gastric MALT lymphoma from chronic H. pylori gastritis in histological diagnosis. We also show that MECA-79−/HECA-452+/NCC-ST-439+ glycans, e.g., 6-sulfo and non-sulfated sLeX attached to core 2-branched O-glycans, function as dominant L-selectin ligands, suggesting the potential contribution of these O-glycans to H. pylori-specific T-cell recruitment in the pathogenesis of gastric MALT lymphoma.
Materials and methods
Histological samples
Formalin-fixed paraffin-embedded (FFPE) blocks of gastric biopsy specimens with MALT lymphoma without a DLBCL component (n = 22) and H. pylori gastritis with marked chronic inflammation (n = 31) as assessed by the updated Sydney system [23] were retrieved from the pathological archives of the Department of Laboratory Medicine, Shinshu University Hospital. The analysis of human stomach tissues was approved by the Ethics Committee of Shinshu University School of Medicine (reference number 191, approved on October 3rd, 2006).
Antibodies
The following monoclonal antibodies served as primary antibodies: QBEND10 recognizing human CD34, a marker for vascular endothelial cells (Immunotech, Luminy, France), MECA-79 (BD Pharmingen, San Diego, CA, USA) [9,12], HECA-452 (BD Pharmingen) [14,16–18], and NCC-ST-439 (Nippon Kayaku, Tokyo, Japan) [14,15].
Immunohistochemistry
Immunohistochemistry for CD34, MECA-79, and HECA-452 was carried out using an indirect method, and that for NCC-ST-439 was done by the labeled streptavidin-biotin (LSAB) method as described previously [20,24]. Details are given in the Supporting information, Supplementary materials and methods.
Quantification of MECA-79+, HECA-452+, and NCC-ST-439+ vessels
For each biopsy specimen, the numbers of CD34+, MECA-79+, HECA-452+, and NCC-ST-439+ vessels in 5 high-power fields of view with ×400 magnification were determined under a BX51 microscope (Olympus, Tokyo, Japan). The numbers of MECA-79+, HECA-452+, and NCC-ST-439+ vessels each were divided by the number of CD34+ vessels, yielding percentages of MECA-79+, HECA-452+, and NCC-ST-439+ vessels, respectively, as described [11,14].
Stable expression of a set of sLeX-capped glycans on CHO cells
Since CHO cells lack enzymes catalyzing core 2 branching, core 1 extension, α1,3-fucosylation, and GlcNAc-6-O-sulfation [12,13,25,26], we used this cell line to express a battery of sLeX-capped glycans by sequential transfections with cDNAs encoding enzymes functioning in one of those reactions. CD34 was introduced as a scaffold protein for such sLeX glycosylation because it has been widely accepted that it is a major scaffold protein expressed on vascular endothelial cells including HEVs [10] while contribution of this protein in gastric MALT lymphoma and chronic H. pylori gastritis has not been reported. Sequential transfections were carried out in a similar fashion as described previously [21]. Detailed transfection procedures are given in the Supporting information, Supplementary materials and methods. Each CHO cell line established was tested for CD34, MECA-79, HECA-452, and NCC-ST-439 antibody reactivity by FACS analysis using FACSort (BD Bioscience, San Jose, CA, USA) with FlowJo software (Tree Star, Ashland, OR, USA). Putative carbohydrate structures expressed on these CHO cell lines are listed in Table 1.
Table 1.
CHO cell lines expressing CD34 decorated with sLeX-type glycans
| Name | Putative sLeX-type glycans on CD34 |
|---|---|
| CHO/CD34 (control) | None |
| CHO/CD34/F7 | SLeX attached to N-glycans |
| CHO/CD34/F7/LSST | 6-sulfo sLeX attached to N-glycans |
| CHO/CD34/F7/C1 | SLeX attached to extended core 1 O-glycans and N-glycans |
| CHO/CD34/F7/C1/LSST | 6-sulfo sLeX attached to extended core 1 O-glycans and N-glycans |
| CHO/CD34/F7/C2 | SLeX attached to core 2-branched O-glycans and N-glycans |
| CHO/CD34/F7/C2/LSST | 6-sulfo sLeX attached to core 2 O-glycans and N-glycans |
| CHO/CD34/F7/C2/C1 | SLeX attached to both extended core 1 and core 2 O-glycans and N-glycans |
| CHO/CD34/F7/C2/C1/LSST | 6-sulfo sLeX attached to both extended core 1 and core 2 O-glycans and N-glycans |
Western blot analysis
Expression of a particular sLeX-type glycan on CD34 was confirmed by Western blot analysis as described previously [21]. Details are given in the Supporting information, Supplementary materials and methods.
L-selectin•IgM chimera binding assay
To obtain L-selectin•IgM chimeras, HEK 293T cells were transiently transfected with pcDNA1.1-L-selectin•IgM [14] and cultured for 4 days in Dulbecco’s Modified Eagle Medium (DMEM) (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (HyClone, South Logan, UT, USA). Conditioned medium was collected and concentrated ~40-fold using Centriprep YM-30 (Millipore, Billerica, MA, USA). CHO cell lines were dissociated into mono-dispersed cells using phosphate-buffered saline (PBS) supplemented with 0.5 mM EDTA. For control experiments, these cells were treated with 0.2 U/ml of sialidase (neuraminidase from Arthrobacter ureafaciens) (Nacalai Tesque, Kyoto, Japan) at 37°C for 60 min. After washing with DMEM, mono-dispersed cells were incubated with L-selectin•IgM chimera in the presence of Ca2+ at 4°C for 60 min with gentle rocking. After washing, cells were incubated with FITC-conjugated goat anti-human IgM Fc5µ (Pierce Biotechnology, Rockford, IL, USA) diluted 1:100 at 4°C for 15 min. After washing, stained cells were subjected to FACS analysis. Negative control experiments were done by substituting DMEM with DMEM supplemented with 1 mM EDTA throughout the procedure.
Lymphocyte adhesion assay
A lymphocyte adhesion assay was carried out as described previously [29] with modifications. 5 × 104 cells from various CHO lines were seeded onto Lab-Tech 4-well glass chamber slides (Nalge Nunc International, Naperville, IL, USA) 24 hours prior to assay, and for control experiments, CHO cells were treated with either 5,000 U/ml N-glycanase or 0.2 U/ml sialidase at 37°C for 60 min. Human peripheral blood lymphocytes were isolated from a healthy volunteer by density separation over a Ficoll gradient using Lymphoprep (Axis-Shield Poc, Oslo, Norway) according to the manufacturer’s instruction. For control experiment, lymphocytes were treated with 10 µg/ml anti-human L-selectin antibody Dreg-56 [30] at 4°C for 10 min. 500 µl of the lymphocyte suspension at a concentration of 2 × 106 cells/ml in RPMI 1640 supplemented with 20 mM HEPES were layered onto CHO cells in each well and incubated for 30 min on a rotating shaker. Non-adherent cells were washed off by rinsing three times with 1 ml of RPMI 1640 with or without 1 mM EDTA. After fixation with PBS containing 1% glutaraldehyde, slides were observed with Nomarski differential interference optics using an AX-80 microscope (Olympus). The number of each CHO cells and adherent lymphocytes in 5 fields of view with ×200 magnification was determined, and the number of lymphocytes attached per 100 CHO cells was calculated.
Cell-enzyme-linked immunosorbent assay (ELISA)
Cell-ELISA was performed essentially as described previously [31] (for details, see Supporting information, Supplementary materials and methods).
Statistical analysis
All data are expressed as means ± SEM. Differences between groups were statistically analyzed by either the two-tailed unpaired Student’s t-test or the Mann-Whitney U-test, where appropriate, using InStat 3 software (GraphPad Software, San Diego, CA, USA). p values less than 0.05 were considered significant.
Results
Preferential occurrence of MECA-79−/HECA-452+/NCC-ST-439+ HEV-like vessels in gastric MALT lymphoma
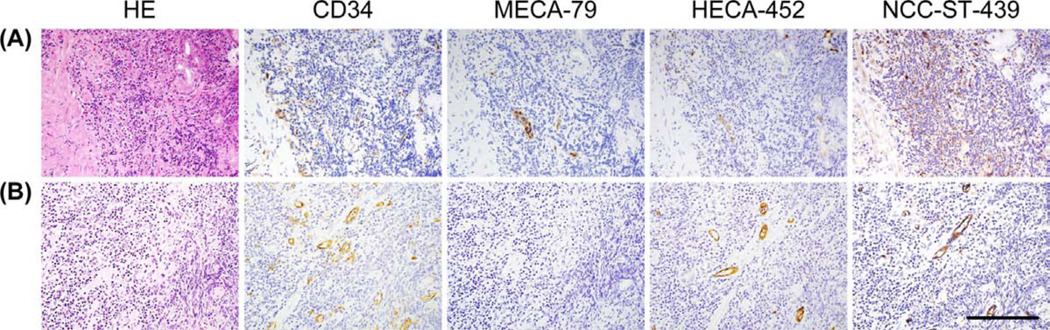
We first determined immunohistochemical profiles of HEV-like vessels in chronic H. pylori gastritis and gastric MALT lymphoma using MECA-79, HECA-452, and NCC-ST-439 antibodies. As shown in Figure 2A, HEV-like vessels induced in chronic H. pylori gastritis were robustly stained with MECA-79; however, less than half of those MECA-79+ vessels stained positively with HECA-452 and NCC-ST-439 antibodies. On the other hand, in gastric MALT lymphoma, in addition to MECA-79+ HEV-like vessels, MECA-79−/HECA-452+/NCC-ST-439+ vessels were frequently observed (Figure 2B).
Figure 2.
HEV-like vessels appear in chronic H. pylori gastritis (A) and gastric MALT lymphoma (B). Serial tissue sections were stained with hematoxylin and eosin (HE), and immunostained for CD34 as a marker of vascular endothelial cells, MECA-79, HECA-452, and NCC-ST-439. In chronic H. pylori gastritis, more than half of MECA-79+ vessels are HECA-452−/NCC-ST-439−. Conversely, in gastric MALT lymphoma, MECA-79− but HECA-452+/NCC-ST-439+ vessels are frequently observed. Bar = 200 µm.
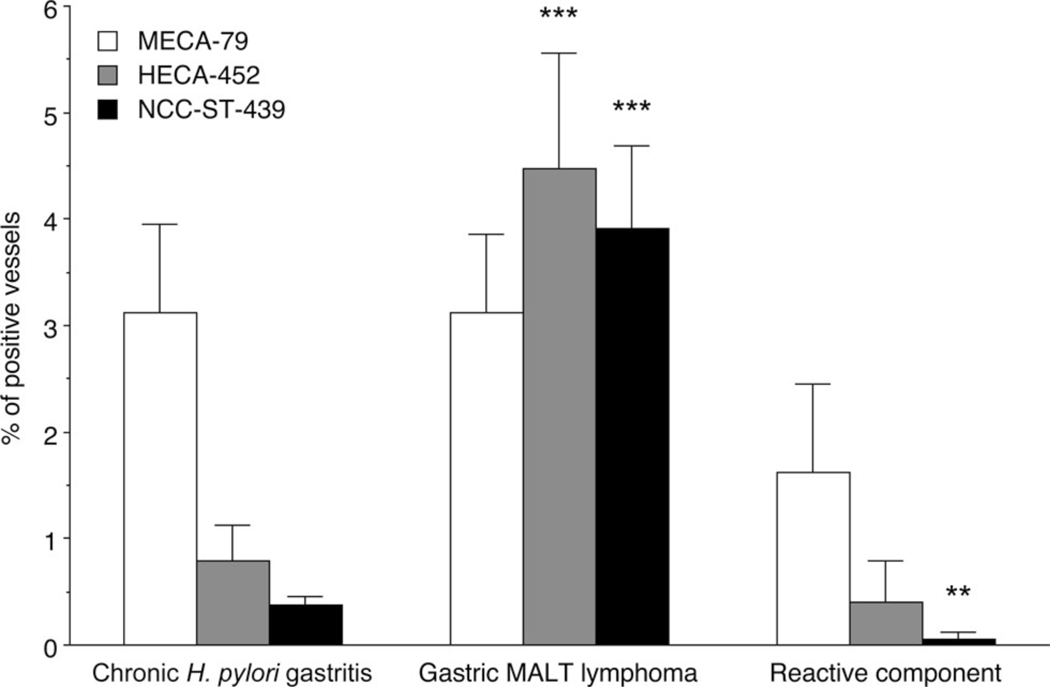
We then evaluated the proportions of MECA-79+, HECA-452+, or NCC-ST-439+ vessels among CD34+ vessels in these two pathological settings. As shown in Figure 3, in chronic H. pylori gastritis, the percentage of MECA-79+ vessels (3.12 ± 0.83%) was greater than that of HECA-452+ (0.79 ± 0.34%) and NCC-ST-439+ (0.37 ± 0.08%) vessels with high statistical significance (p < 0.001), while in gastric MALT lymphoma, the percentage of MECA-79+ (3.11 ± 0.75%), HECA-452+ (4.47 ± 1.09%), and NCC-ST-439+ (3.90 ± 0.78%) vessels did not differ significantly. Furthermore, while the percentage of MECA-79+ vessels did not differ between chronic H. pylori gastritis and gastric MALT lymphoma, the percentage of HECA-452+ and NCC-ST-439+ vessels in gastric MALT lymphoma was greater than that seen in chronic H. pylori gastritis, with high statistical significance (p < 0.001). In addition, the percentages of MECA-79+ (1.62 ± 0.40%), HECA-452+ (0.40 ± 0.40%), and NCC-ST-439+ (0.06 ± 0.06%) vessels in accompanied reactive component of gastric MALT lymphoma showed similar patterns seen in chronic H. pylori gastritis.
Figure 3.
Frequency of MECA-79+, HECA-452+, and NCC-ST-439+ HEV-like vessels in chronic H. pylori gastritis (left), gastric MALT lymphoma (middle), and its accompanied reactive component (right). In chronic H. pylori gastritis, the percentage of MECA-79+ vessels is greater than that of HECA-452+ and NCC-ST-439+ vessels, with high statistical significance (p < 0.001). Conversely, in gastric MALT lymphoma, the percentages of MECA-79+, HECA-452+, and NCC-ST-439+ vessels do not differ significantly. The percentage of HECA-452+ and NCC-ST-439+ but not MECA-79+ vessels in gastric MALT lymphoma is greater than that seen in chronic H. pylori gastritis, with statistical significance. In addition, the percentages of MECA-79+, HECA-452+, and NCC-ST-439+ vessels in accompanied reactive component of gastric MALT lymphoma shows similar patterns seen in chronic H. pylori gastritis. Data are presented as means ± SEM (n = 31 in chronic H. pylori gastritis, n = 22 in gastric MALT lymphoma, n = 3 in accompanied reactive component of gastric MALT lymphoma). ***, p < 0.001; **, p < 0.01.
These results indicate that the number of HECA-452+ and NCC-ST-439+ but not MECA-79+ HEV-like vessels increases in gastric MALT lymphoma and suggest that the presence of MECA-79−/HECA-452+/NCC-ST-439+ HEV-like vessels is a characteristic phenotype of gastric MALT lymphoma.
MECA-79−/HECA-452+/NCC-ST-439+ 6-sulfo sLeX-capped core 2-branched O-glycans are bound by an L-selectin•IgM chimera
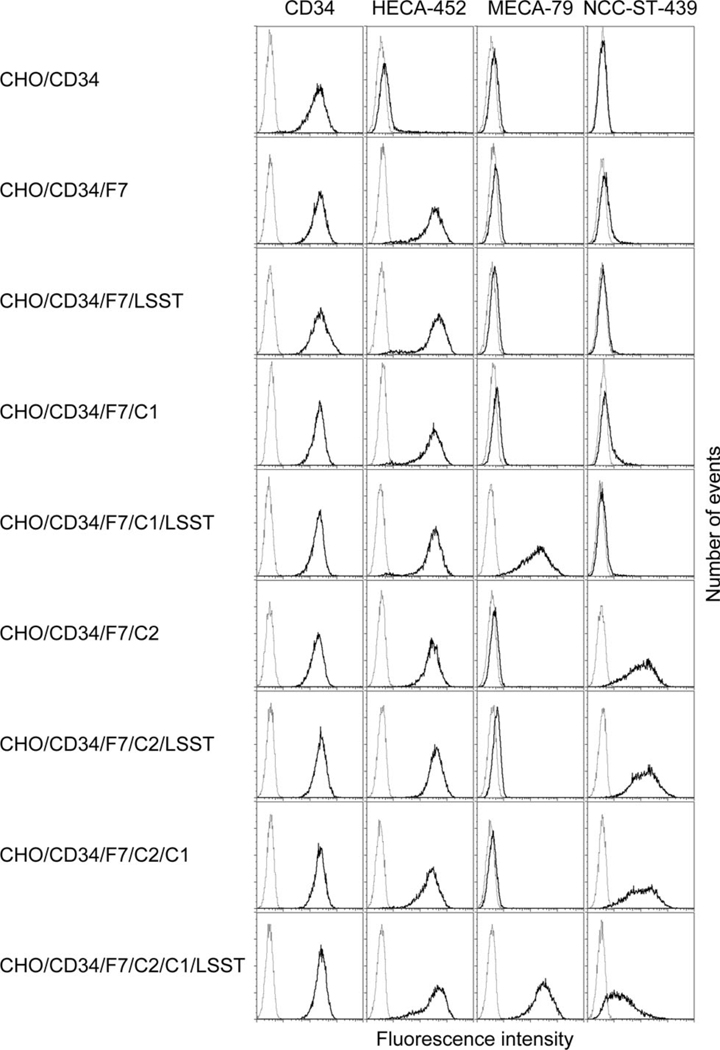
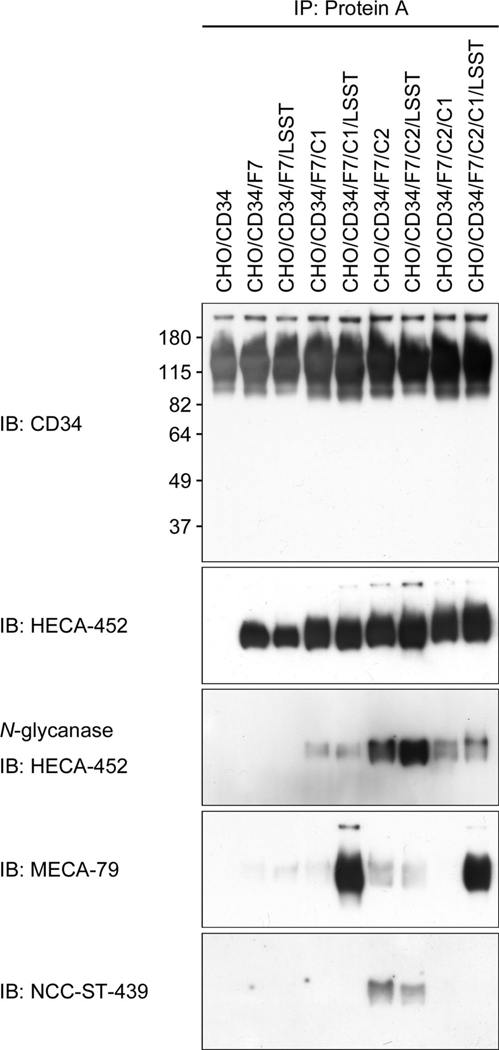
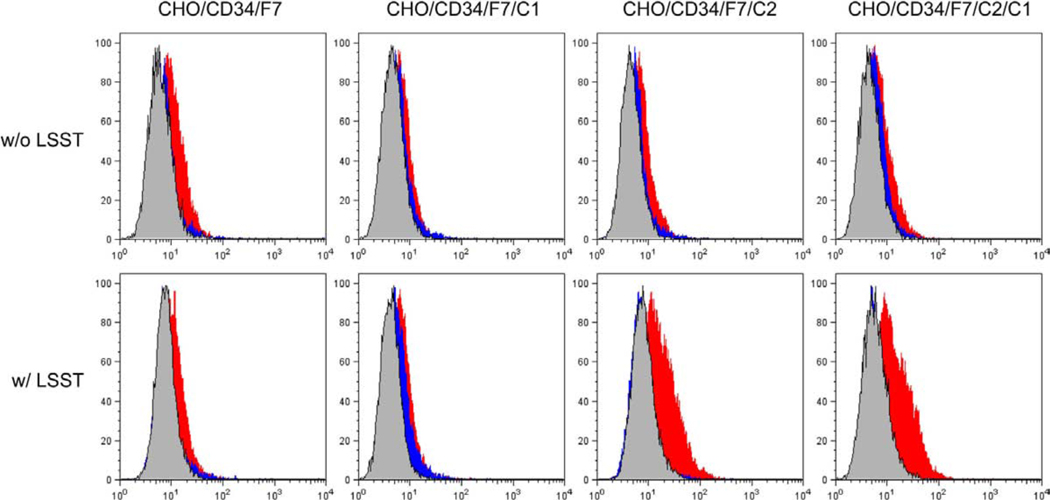
Considering the carbohydrate epitope recognized by MECA-79, HECA-452, and NCC-ST-439 antibodies (see Figure 1), MECA-79−/HECA-452+/NCC-ST-439+ HEV-like vessels observed in gastric MALT lymphoma should express at least one of the following sLeX-capped glycans: i) non-sulfated sLeX attached to core 2-branched O-glycans, ii) 6-sulfo sLeX attached to core 2-branched O-glycans, or iii) non-sulfated sLeX attached to both extended core 1 and core 2-branched O-glycans. To determine L-selectin-binding properties of these MECA-79−/HECA-452+/NCC-ST-439+ glycans, we first established CHO lines stably expressing CD34 decorated with one of the above glycans, as well as those with MECA-79−/HECA-452+/NCC-ST-439− glycans including iv) non-sulfated sLeX attached to N-glycans, v) 6-sulfo sLeX attached to N-glycans, vi) non-sulfated sLeX attached to extended core 1 O-glycans, those with MECA-79+/HECA-452+/NCC-ST-439− O-glycans vii) 6-sulfo sLeX attached to extended core 1 O-glycans, and those with MECA-79+/HECA-452+/NCC-ST-439+ O-glycans viii) 6-sulfo sLeX attached to both extended core 1 and core 2-branched O-glycans (Table 1). Expression of a particular sLeX-type carbohydrate in each line was confirmed by FACS analysis (Figure 4). As expected, all lines transfected with cDNA encoding α1,3-fucosyltransferase 7 (FucT-7) [27] were HECA-452-positive, and those transfected with cDNA encoding core 2 branching β1,6-N-acetylglucosaminyltransferase 1 (Core2GlcNAcT-1) [26] were NCC-ST-439-positive. All lines transfected with a combination of cDNAs encoding core 1 extending β1,3-N-acetylglucosaminyltransferase (Core1-β3GlcNAcT) [12] and N-acetylglucosamine-6-O-sulfotransferase 2 (GlcNAc6ST-2) [13] were MECA-79-positive. Moreover, expression of these sLeX-type carbohydrates on CD34 was confirmed by Western blot analysis (Figure 5). It revealed that these carbohydrate epitopes were displayed on CD34; however, interestingly, CD34 obtained from CHO cells expressing sLeX attached to both extended core 1 and core 2-branched O-glycans (CHO/CD34/F7/C2/C1 and CHO/CD34/F7/C2/C1/LSST) did not efficiently bound by NCC-ST-439 antibody compared to those obtained from CHO cells expressing sLeX attached to only core 2-branched O-glycans. This finding is consistent with the data obtained by FACS analysis showing less NCC-ST-439 signals of CHO/CD34/F7/C2/C1 and CHO/CD34/F7/C2/C1/LSST compared with CHO/CD34/F7/C2 and CHO/CD34/F7/C2/LSST, respectively (Figure 4). It could be postulated that the epitope for NCC-ST-439 antibody might be undergone steric hindrance by sLeX attached to extended core 1 O-glycans, particularly in SDS-PAGE.
Figure 4.
FACS analysis of CHO cell lines expressing various sugar chains. Each CHO line described in Table 1 was tested for CD34, HECA-452, MECA-79, and NCC-ST-439 reactivities. Lighter gray lines represent negative control resulting from omitting the primary antibody.
Figure 5.
Expression of a particular sLeX-type glycans on CD34 obtained from CHO cell lines described in Table 1. CD34 obtained from all lines except control CHO/CD34 cells are positive for HECA-452, and such HECA-452 glycoepitopes are eliminated after treatment with N-glycanase. The MECA-79 epitope is detected in CHO cells transfected with both Core1-β3GlcNAcT and LSST (GlcNAc6ST-2). The NCC-ST-439 epitope is detected in CHO/CD34/F7/C2 with or without transfection with LSST.
These CHO cell lines were then subjected to an L-selectin•IgM chimera binding assay. As shown in Figure 6, the L-selectin•IgM chimera bound most robustly to CHO/CD34/F7/C2/LSST and CHO/CD34/F7/C2/C1/LSST at similar levels. On the other hand, in addition to non-sulfated sLeX expressors CHO/CD34/F7, CHO/CD34/F7/C1, CHO/CD34/F7/C2, and CHO/CD34/F7/C2/C1, not only CHO/CD34/F7/LSST but also CHO/CD34/F7/C1/LSST, which should express 6-sulfo sLeX attached to extended core 1 O-glycans, were not bound efficiently by the chimera.
Figure 6.
L-selectin•IgM chimera binding assay of CHO cell lines. CHO cell lines listed in Table 1 were tested. The L-selectin•IgM chimera most robustly binds to the CHO/CD34/F7/C2/LSST and CHO/CD34/F7/C2/C1/LSST lines at almost equivalent levels. The remaining lines show only marginal L-selectin•IgM binding. Red histograms represent L-selectin•IgM chimera bindings. Blue and gray histograms indicate control experiments using cells treated with sialidase and EDTA, respectively.
This unexpected result prompted us to confirm the carbohydrate structure expressed on CHO cell lines, particularly in the CHO/CD34/F7/C1/LSST line, because it is widely accepted that 6-sulfo sLeX attached to extended core 1 O-glycans is an L-selectin ligand. Mass spectrometry analysis confirmed that 6-sulfo sLeX attached to extended core 1 O-glycans was present in CHO/CD34/F7/C1/LSST, while 6-sulfo sLeX attached to core 2-branched O-glycans was detected in CHO/CD34/F7/C2/LSST (Supporting information, Supplementary materials and methods and Figure S1). In addition, 6-sulfo sLeX on N-glycans were observed in all the lines tested (Supporting information, Figure S2).
These results collectively indicate that in an in vitro assay 6-sulfo sLeX attached to core 2-branched O-glycans is preferentially bound by L-selectin•IgM chimera compared to 6-sulfo sLeX attached to extended core 1 O-glycans and N-glycans.
MECA-79−/HECA-452+/NCC-ST-439+ 6-sulfo and non-sulfated sLeX-capped core 2-branched O-glycans are bound by L-selectin-expressing lymphocytes
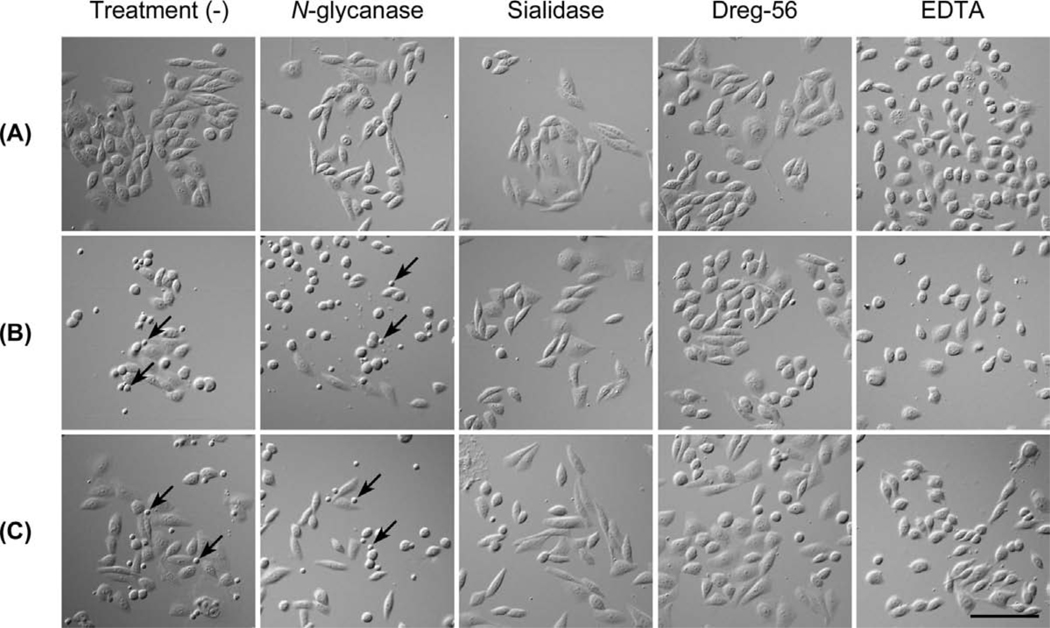
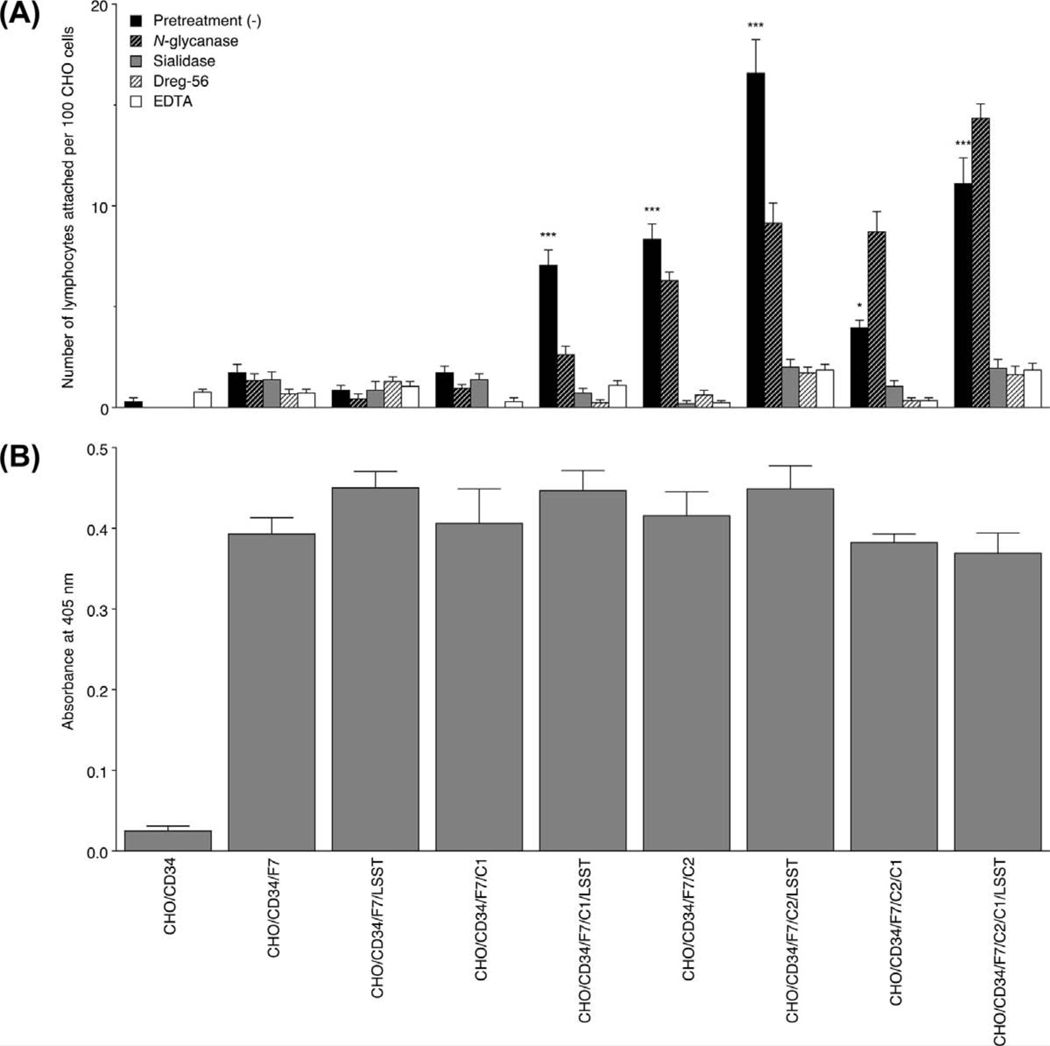
To analyze the above finding under more physiological conditions, we carried out a lymphocyte adhesion assay on the various CHO cell lines. As shown in Figure 7, lymphocytes adhered to cells expressing 6-sulfo sLeX attached to extended core 1 and/or core 2-branched O-glycans (Figure 7C), and to those expressing non-sulfated sLeX attached to core 2-branched O-glycans with or without sLeX attached to extended core 1 O-glycans (Figure 7B), but negligibly to those expressing 6-sulfo sLeX attached to N-glycans and non-sulfated sLeX attached to extended core 1 O-glycans or N-glycans, as well as control CHO/CD34 cells (Figure 7A). As shown in Figure 8A, the number of lymphocytes attached per 100 CHO cells in CHO/CD34/F7/C1/LSST, CHO/CD34/F7/C2, CHO/CD34/F7/C2/LSST, CHO/CD34/F7/C1/C2, and CHO/CD34/F7/C2/C1/LSST lines was greater than that seen in control CHO/CD34 cells with statistical significance, while that seen in the other lines did not differ significantly. Such lymphocyte adhesion was maintained after treatment with N-glycanase, and almost completely abrogated by the treatment with sialidase and Dreg-56 as well as EDTA, indicating that the lymphocyte adhesion to these CHO transfectants is L-selectin dependent. To exclude the possibility that these differences originate from differences in the absolute amount of sLeX expressed, we undertook cell-ELISA. As shown in Figure 8B, the amount of sLeX among sLeX expressors did not differ significantly, while control CHO/CD34 cells expressed minimal levels of sLeX.
Figure 7.
Lymphocyte adhesion to CHO cell lines expressing various sugar chains. CHO lines listed in Table 1 were examined. Lymphocyte adhesion to CHO/CD34 (A), CHO/CD34/F7/C2 (B), and CHO/CD34/F7/C2/LSST (C) observed with Nomarski differential interference optics is shown. Arrows indicate adhered lymphocytes. Note that treatments with sialidase, Dreg-56, and EDTA completely abolish lymphocyte adhesions. Bar = 100 µm. Data are representative of three independent experiments showing similar results.
Figure 8.
(A) The number of adherent lymphocytes per 100 CHO cells is shown. CHO cells expressing sLeX on a core 2-branched O-glycan backbone, regardless of GlcNAc-6-O-sulfation, and those expressing 6-sulfo sLeX attached to extended core 1 O-glycans are significantly bound by lymphocytes. Such lymphocyte adhesions are maintained after treatment with N-glycanase, but almost completely abrogated with treatments with sialidase, Dreg-56, or EDTA. ***, p < 0.001; *, p < 0.05. Data are representative of three independent experiments showing similar results. (B) The amount of sLeX expressed on each CHO cell line, as assessed by cell-ELISA. Expression of sLeX on CHO cell lines does not differ except for CHO/CD34, which serve as a control.
These results combined indicate that MECA-79−/HECA-452+/NCC-ST-439+ 6-sulfo and/or non-sulfated sLeX-capped core 2-branched O-glycans, which appear in gastric MALT lymphoma, as well as MECA-79+/HECA-452+/NCC-ST-439+ 6-sulfo sLeX-capped extended core 1 and core 2-branched O-glycans induced in both chronic H. pylori gastritis and gastric MALT lymphoma, function as dominant L-selectin ligands, and that 6-sulfo and/or non-sulfated sLeX-capped N-glycans, at least in this experimental context, play only marginal role as L-selectin ligands.
Discussion
The present study demonstrates preferential appearance of MECA-79−/HECA-452+/NCC-ST-439+ HEV-like vessels in gastric MALT lymphoma. Most of HEV-like vessels in chronic H. pylori gastritis showed a MECA-79+/HECA-452−/NCC-ST-439− profile, while those in gastric MALT lymphoma often showed a MECA-79−/HECA-452+/NCC-ST-439+ profile. This finding suggests that occurrence of MECA-79−/HECA-452+/NCC-ST-439+ HEV-like vessels is a hallmark of gastric MALT lymphoma, an observation that could facilitate diagnosis of these two diseases.
Presuming that HEV-like vessels are fully glycosylated and sulfated, as shown in Figure 1, such vessels should demonstrate a MECA-79+/HECA-452+/NCC-ST-439+ profile. Thus, the existence of MECA-79−/HECA-452+/NCC-ST-439+ HEV-like vessels in gastric MALT lymphoma suggests that GlcNAc-6-O-sulfation catalyzed by GlcNAc6ST-1/GlcNAc6ST-2 [13,25,28], and/or core 1 extension catalyzed by Core1-β3GlcNAcT [12], both of which are required for the MECA-79 epitope, do not always occur in those vessels. Another possibility is that expression of Core2GlcNAcT-1 [26] is increased relative to that of Core1-β3GlcNAcT in gastric MALT lymphoma. As previously reported by Drayton et al., lymphotoxin produced by T cells is the chemokine responsible for increasing GlcNAc6ST-2 transcripts [35]. Conceivably, a similar mechanism may function to GlcNAc6ST-1 and/or Core1-β3GlcNAcT; however, currently, such a mechanism remains to be clarified. It is possible that gastric MALT lymphoma cells and tumor-infiltrating T cells could not efficiently secrete lymphotoxin compared to non-neoplastic lymphocyte in chronic H. pylori gastritis. We undertook RT-PCR analysis of total RNA prepared from FFPE tissue sections but observed no apparent down-regulation of transcripts encoding GlcNAc6ST-1/GlcNAc6ST-2 or Core1-β3GlcNAcT, or increased expression of Core2GlcNAcT-1 in tissues derived from gastric MALT lymphoma relative to chronic H. pylori gastritis (data not shown and Supporting information, Supplementary materials and methods, and Figure S3). This finding might be due to “contamination” by substantial numbers of coexisting MECA-79+ vessels or by stromal and/or epithelial cells. Further study is required to compare gene expression profiles in tissue samples from these pathological conditions.
The present study also demonstrates that MECA-79−/HECA-452+/NCC-ST-439+ sLeX-capped core 2-branched O-glycans, even without GlcNAc-6-O-sulfation, play a dominant role in L-selectin-expressing lymphocyte binding. Since it is extremely difficult to isolate sufficient amounts of pure MECA-79−/HECA-452+/NCC-ST-439+ HEV-like vessels from human stomach, we could not analyze directly the carbohydrate structure and function of the glycans on these vessels. Instead, we established CHO cell lines stably expressing CD34 decorated with sLeX-capped glycans to assess L-selectin-binding properties of possible MECA-79−/HECA-452+/NCC-ST-439+ glycans in vitro. Interestingly, not only 6-sulfo sLeX but also non-sulfated sLeX attached to core 2-branched O-glycans showed demonstrable L-selectin-binding properties, as assessed by lymphocyte adhesion to CHO lines. This result is consistent with our previous study demonstrating that GlyCAM-1 derived from GlcNAc6ST-1/GlcNAc6ST-2 double-knockout mice weakly supported rolling when GlyCAM-1 was displayed at high density [36]. It has been shown that L-selectin preferentially recognizes non-sulfated sLeX on core-2 branched O-glycans when O-glycans are present at the N-terminus of P-selectin glycoprotein ligand 1 (PSGL-1), and binding to sLeX on extended core 1 O-glycans is much weaker [10,37]. Sulfated tyrosine residues present at the N-terminus of PSGL-1 are required for high affinity binding to L-selectin. These findings collectively suggest that non-sulfated sLeX attached to core 2-branched O-glycans is a critical epitope for L-selectin binding, and that GlcNAc-6-O-sulfation on this carbohydrate structure increases L-selectin-binding affinity.
Thus far, it is widely accepted that 6-sufo sLeX attached to extended core 1 O-glycans is an L-selectin ligand, and indeed, we have demonstrated its L-selectin binding using an in vitro rolling assay [12] and also in the present study by a lymphocyte adhesion assay. However, surprisingly, CHO/CD34/F7/C1/LSST cells expressing this type of O-glycan did not show detectable L-selectin binding properties in L-selectin•IgM chimera binding assay. It is tempting to speculate that L-selectin•IgM chimeras do not manifest physiologically relevant adhesion by L-selectin expressed on lymphocytes, most likely due to absence or very weak shearing force of soluble L-selectin•IgM chimera in solution. We previously reported that Core2GlcNAcT-1 single-knockout mice showed more significant reduction in lymphocyte homing compared to Core1-β3GlcNAcT single-knockout mice [38], and that CHO/CD34/F7/C1 cells showed less rolling compared to CHO/CD34/F7/C2 cells [39]. Conceivably, 6-sulfo sLeX attached to extended core 1 O-glycans may function as an L-selectin ligand, but its L-selectin-binding affinity is lower compared to that attached to core 2-branched O-glycans, which makes it difficult to detect L-selectin binding by using L-selectin•IgM chimeras. These findings, together with the present finding, indicate that 6-sulfo sLeX on core 2 branched O-glycans play a dominant role in lymphocyte rolling and subsequent homing.
Additionally, CHO/CD34/F7/LSST cells expressing 6-sulfo sLeX on N-glycans did not show apparent L-selectin binding in this study, although we previously showed that CHO cells expressing 6-sulfo sLeX only on N-glycans supported shear stress-dependent lymphocyte rolling when a flow chamber was used. [38]. In that assay, the shear force at 1.16 dynes/cm2 yielded the highest number of rolling cells, but at higher or lower shear forces resulted in negligible number of rolling lymphocytes. It is possible that the data obtained from the present in vitro lymphocyte adhesion assay do not reflect results obtained from in vitro rolling assay with optimal shear force. Also in support of our finding, Hernandez et al. recently showed that N-glycans containing potential 6-sulfo sLeX epitopes were present in L-selectin-binding human CD34, but removal of those epitopes did not abolish L-selectin binding, most likely because O-glycan-based 6-sulfo sLeX is still present [40]. These data collectively suggest that 6-sulfo sLeX attached to N-glycans does not contribute significantly to L-selectin-dependent lymphocyte rolling, at least in vitro, but that it may play a role in L-selectin-dependent lymphocyte rolling in vivo, in particular when 6-sulfo sLeX on O-glycans is absent.
It is of interest whether MECA-79−/HECA-452+/NCC-ST-439+ HEV-like vessels function in the histogenesis of gastric MALT lymphoma. Gastric MALT lymphoma develops from MALT that has already formed as a consequence of H. pylori infection. Therefore, as proposed by Dogan et al. [22], HEV-like vessels within the tumor may not play a role in lymphoma progression. However, considering that proliferation of neoplastic B cells requires the presence of H. pylori-specific T-cells [4,5], and that 30 to 95% of tumor-infiltrating T-cells in gastric MALT lymphoma express L-selectin [41], these HEV-like vessels may help recruit H. pylori-specific T-cells to the tumor site and therefore facilitate tumor progression.
In conclusion, the present study demonstrates that MECA-79−/HECA-452+/NCC-ST-439+ HEV-like vessels are preferentially found in gastric MALT lymphoma compared with chronic H. pylori gastritis, and identifies an immunohistochemical hallmark for gastric MALT lymphoma to differentiate that condition from chronic H. pylori gastritis in pathological evaluations. The present study also demonstrates that MECA-79−/HECA-452+/NCC-ST-439+ glycans, sLeXs attached to core 2-branched O-glycans, regardless of GlcNAc-6-O-sulfation, function as dominant L-selectin ligands and potentially contribute to H. pylori-specific T-cell recruitment in the histogenesis of gastric MALT lymphoma.
Supplementary Material
Acknowledgments
We thank Dr. Hiroto Kawashima for helpful discussion, Ms. Matsuko Watanabe for technical assistance, and Dr. Elise Lamar for critical reading of the manuscript. This work was supported by Grants-in-Aid for Young Scientists B-18790240 and B-22790343 from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MK), and in part by Grants-in-Aid for Scientific Research C-20570140 (JM) and B-21390104 (JN) from the Japan Society for the Promotion of Science, and grants RO1 CA33000 and PO1 CA71932 from the National Institutes of Health (MF). MS and MS/MS data were acquired at the Taiwan NRPGM Core Facilities for Proteomics and Glycomics (NSC 98-3112-B-001-023).
Footnotes
No conflicts of interest were declared.
Part of the work was presented as a poster at the annual meeting of the Society for Glycobiology, held in San Diego, California, November 12–15, 2009.
Statement of author contributions
MK and JM conceived and carried out experiments, and analyzed data, HH, SYY, YS, and KS carried out experiments, KHK carried out experiments and analyzed data, and MF and JN conceived experiments and analyzed data. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Supporting information on the internet
The following supporting information may be found in the online version of this article.
References
Note: Reference 30, 32–34 are cited in the Supporting information to this article.
- 1.Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29:252–260. doi: 10.1002/1097-0142(197201)29:1<252::aid-cncr2820290138>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 2.Isaacson PG, Huller-Hermelink HK, Paris MA, et al. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) In: Jaffe ES, Harris NL, Stein H, et al., editors. World Health Organization Classification of Tumors. Pathology and Genetics of Tumors of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001. pp. 157–160. [Google Scholar]
- 3.Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, et al. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338:1175–1176. doi: 10.1016/0140-6736(91)92035-z. [DOI] [PubMed] [Google Scholar]
- 4.Hussell T, Isaacson PG, Crabtree JE, et al. Helicobacter pylori-specific tumour-infiltrating T-cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue. J Pathol. 1996;178:122–127. doi: 10.1002/(SICI)1096-9896(199602)178:2<122::AID-PATH486>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 5.Hussell T, Isaacson PG, Crabtree JE, et al. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet. 1993;342:571–574. doi: 10.1016/0140-6736(93)91408-e. [DOI] [PubMed] [Google Scholar]
- 6.Isaacson PG, Du MQ. MALT lymphoma: from morphology to molecules. Nat Rev Cancer. 2004;4:644–653. doi: 10.1038/nrc1409. [DOI] [PubMed] [Google Scholar]
- 7.Bacon CM, Du MQ, Dogan A. Mucosa-associated lymphoid tissue (MALT) lymphoma: a practical guide for pathologists. J Clin Pathol. 2007;60:361–372. doi: 10.1136/jcp.2005.031146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 9.Streeter PR, Rouse BT, Butcher EC. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988;107:1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosen SD. Ligand for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 11.Renkonen J, Tynninen O, Hayry P, et al. Glycosylation might provide endothelial zip codes for organ-specific leukocyte traffic into inflammatory sites. Am J Pathol. 2002;161:543–550. doi: 10.1016/S0002-9440(10)64210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh JC, Hiraoka N, Petryniak B, et al. Novel sulfated lymphocyte homing receptors and their control by a core 1 extension β1,3-N-acetylglucosaminyltransferase. Cell. 2001;105:957–969. doi: 10.1016/s0092-8674(01)00394-4. [DOI] [PubMed] [Google Scholar]
- 13.Hiraoka N, Petryniak B, Nakayama J, et al. A novel, high endothelial venule-specific sulfotransferase express 6-sulfo sialyl Lewis X, an L-selectin ligand displayed by CD34. Immunity. 1999;11:79–89. doi: 10.1016/s1074-7613(00)80083-7. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi M, Mitoma J, Nakamura N, et al. Induction of peripheral lymph node addressin in human gastric mucosa infected by Helicobacter pylori. Proc Natl Acad Sci U S A. 2004;101:17807–17812. doi: 10.1073/pnas.0407503101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumamoto K, Mitsuoka C, Izawa M, et al. Specific detection of sialyl Lewis X determinant carried on the mucin GlcNAcβ1→6GalNAcα core structure as a tumor-associated antigen. Biochem Biophys Res Commun. 1998;247:514–517. doi: 10.1006/bbrc.1998.8824. [DOI] [PubMed] [Google Scholar]
- 16.Duijvestijn AM, Horst E, Pals ST, et al. High endothelial differentiation in human lymphoid and inflammatory tissues defined by monoclonal antibody HECA-452. Am J Pathol. 1988;130:147–155. [PMC free article] [PubMed] [Google Scholar]
- 17.Mitsuoka C, Kawakami-Kimura N, Kasugai-Sawada M, et al. Sulfated sialyl Lewis X, the putative L-selectin ligand, detected on endothelial cells of high endothelial venules by a distinct set of anti-sialyl Lewis X antibodies. Biochem Biophys Res Commun. 1997;230:546–551. doi: 10.1006/bbrc.1996.6012. [DOI] [PubMed] [Google Scholar]
- 18.Berg EL, Robinson MK, Mansson O, et al. A carbohydrate domain common to both sialyl Lea and sialyl LeX is recognized by the endothelial cell leukocyte adhesion molecule ELAM-1. J Biol Chem. 1991;266:14869–14872. [PubMed] [Google Scholar]
- 19.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 20.Suzawa K, Kobayashi M, Sakai Y, et al. Preferential induction of peripheral lymph node addressin on high endothelial venule-like vessels in the active phase of ulcerative colitis. Am J Gastroenterol. 2007;102:1499–1509. doi: 10.1111/j.1572-0241.2007.01189.x. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi M, Hoshino H, Masumoto J, et al. GlcNAc6ST-1-mediated decoration of MAdCAM-1 protein with L-selectin ligand carbohydrates directs disease activity of ulcerative colitis. Inflamm Bowel Dis. 2009;15:697–706. doi: 10.1002/ibd.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dogan A, Du M, Koulis A, et al. Expression of lymphocyte homing receptors and vascular addressins in low-grade gastric B-cell lymphomas of mucosa-associated lymphoid tissue. Am J Pathol. 1997;151:1361–1369. [PMC free article] [PubMed] [Google Scholar]
- 23.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney system. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi M, Nakayama J. Immunohistochemical analysis of carbohydrate antigens in chronic inflammatory gastrointestinal diseases. Methods Enzymol. 2010;479:271–289. doi: 10.1016/S0076-6879(10)79016-9. [DOI] [PubMed] [Google Scholar]
- 25.Uchimura K, Muramatsu H, Kaname T, et al. Human N-acetylglucosamine-6-O-sulfotransferase involved in the biosynthesis of 6-sulfo sialyl Lewis X: molecular cloning, chromosomal mapping, and expression in various organs and tumor cells. J Biochem. 1998;124:670–678. doi: 10.1093/oxfordjournals.jbchem.a022164. [DOI] [PubMed] [Google Scholar]
- 26.Bierhuizen MF, Fukuda M. Expression cloning of a cDNA encoding UDP-GlcNAc:Galβ1-3GalNAc-R (GlcNAc to GalNAc) β1-6GlcNAc transferase by gene transfer into CHO cells expressing polyoma large tumor antigen. Proc Natl Acad Sci U S A. 1992;89:9326–9330. doi: 10.1073/pnas.89.19.9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maly P, Thall A, Petryniak B, et al. The α(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 28.Bistrup A, Bhakta S, Lee JK, et al. Sulfotransferases of two specificities function in the reconstitution of high endothelial cell ligands for L-selectin. J Cell Biol. 1999;145:899–910. doi: 10.1083/jcb.145.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berlin C, Berg EL, Briskin MJ, et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 30.Kishimoto TK, Jutila MA, Butcher EC. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. Proc Natl Acad Sci U S A. 1990;87:2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitoma J, Miyazaki T, Sutton-Smith M, et al. The N-glycolyl form of mouse sialyl Lewis X is recognized by selectins but not by HECA-452 and FH6 antibodies that were raised against human cells. Glycoconj J. 2009;26:511–523. doi: 10.1007/s10719-008-9207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu SY, Wu SW, Khoo KH. Distinctive characteristics of MALDI-Q/TOF and TOF/TOF tandem mass spectrometry for sequencing of permethylated complex type N-glycans. Glycoconj J. 2006;23:355–369. doi: 10.1007/s10719-006-8492-3. [DOI] [PubMed] [Google Scholar]
- 33.Lee SH, Hatakeyama S, Yu SY, et al. Core 3 O-glycan synthase suppresses tumor formation and metastasis of prostate carcinoma PC3 and LNCaP cells through down-regulation of α2β1 integrin complex. J Biol Chem. 2009;284:17157–17169. doi: 10.1074/jbc.M109.010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu SY, Khoo KH, Yang Z, et al. Glycomic mapping of O- and N-linked glycans from major rat sublingual mucin. Glycoconj J. 2008;25:199–212. doi: 10.1007/s10719-007-9071-y. [DOI] [PubMed] [Google Scholar]
- 35.Drayton DL, Ying X, Lee J, et al. Ectopic LTαβ directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med. 2003;197:1153–1163. doi: 10.1084/jem.20021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawashima H, Petryniak B, Hiraoka N, et al. N-acetylglucosamine-6-O-sulfotransferase 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat Immunol. 2005;6:1069–1104. doi: 10.1038/ni1259. [DOI] [PubMed] [Google Scholar]
- 37.Leppanen A, Yago T, Otto VI, et al. Model glycosulfopeptides from P-selectin glycoprotein lingand-1 require tyrosine sulfation and a core 2-branched O-glycan to bind to L-selectin. J Biol Chem. 2003;278:26391–26400. doi: 10.1074/jbc.M303551200. [DOI] [PubMed] [Google Scholar]
- 38.Mitoma J, Bao X, Petryniak B, et al. Critical functions of N-glycans in L-selectin-mediated lymphocyte homing and recruitment. Nat Immunol. 2007;8:409–418. doi: 10.1038/ni1442. [DOI] [PubMed] [Google Scholar]
- 39.Mitoma J, Petryniak B, Hiraoka N, et al. Extended core 1 and core 2 branched O-glycans differentially modulate sialyl Lewis X-type L-selectin ligand activity. J Biol Chem. 2003;278:9953–9961. doi: 10.1074/jbc.M212756200. [DOI] [PubMed] [Google Scholar]
- 40.Hernandez Mir G, Helin J, Skarp KP, et al. Glycoforms of human endothelial CD34 that bind L-selectin carry sulfated sialyl Lewis X capped O- and N-glycans. Blood. 2009;114:733–741. doi: 10.1182/blood-2009-03-210237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koulis A, Diss T, Isaacson PG, et al. Characterization of tumor-infiltrating T lymphocytes in B-cell lymphomas of mucosa-associated lymphoid tissue. Am J Pathol. 1997;151:1353–1360. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.