Abstract
BACKGROUND AND OBJECTIVES
Inflammatory responses in the lumbar dorsal root ganglion (DRG) play a key role in pathologic pain states. Systemic administration of a common anti-inflammatory corticosteroid, triamcinolone acetonide (TA), reduces sympathetic sprouting, mechanical pain behavior, spontaneous bursting activity, cytokine and nerve growth factor production in the DRG. We hypothesized that systemic TA effects are primarily due to local effects on the DRG
METHODS
Male Sprague–Dawley rats were divided into four groups: SNL (tight ligation and transection of spinal nerves) or normal, with and without a single dose of TA injectable suspension slowly injected onto the surface of DRG and surrounding region at the time of SNL or sham surgery. Mechanical threshold was tested on postoperative days 1, 3, 5, and 7. Immunohistochemical staining examined tyrosine hydroxylase (TH) and glial fibrillary acidic protein (GFAP) in DRG, and CD11B antibody (OX-42) in spinal cord.
RESULTS
Local TA treatment attenuated mechanical sensitivity, reduced sympathetic sprouting in the DRG, and decreased satellite glia activation in the DRG and microglia activation in the spinal cord after SNL.
CONCLUSION
A single injection of corticosteroid in the vicinity of the axotomized DRG can mimic many effects of systemic TA, mitigating behavioral and cellular abnormalities induced by spinal nerve ligation. This provides a further rational for the clinical use of localized steroid injections clinically, and provides further support for the idea that localized inflammation at the level of the DRG is an important component of the spinal nerve ligation model, commonly classified as neuropathic pain model.
Introduction
Inflammatory responses in the lumbar dorsal root ganglion (DRG) play a key role in the development of a variety of pathologic pain states including chemogenic low back pain and some forms of intractable neuropathic pain. Inflammatory responses are evoked in some animal models of neuropathic pain, such as CCI (chronic constriction injury by loose ligation of the sciatic nerve), SNL (tight ligation, or ligation and transection of spinal nerves), and PSL (tight ligation of the partial sciatic nerve), even though they are classified as peripheral nerve injury models 1. Robust pain behaviors can also be induced by locally inflaming the DRG with zymosan (LID model), in the absence of any axotomy or nerve damage 2. The inflammatory response not only mediates tissue repair and regeneration, but also contributes importantly to chronic pain behaviors. Pro-inflammatory cytokines and chemokines, as well as growth factors with similar effects on neurons, have been shown to be up-regulated in these models, and genetic or pharmacologic reduction of these molecules can reduce pain behaviors and other pathologies. Macrophages are recruited from blood to the damaged nerve and DRG, where they contribute significantly to the removal of degenerating axons and to the process of regeneration. In addition, the satellite glia that surround the somata of sensory neurons proliferate and become immunoreactive for glial fibrillary acidic protein (GFAP), a marker for glial activation 3-5. In a previous study using the SNL model 6, we showed that systemic administration of a commonly used anti-inflammatory corticosteroid, triamcinolone acetonide (TA), could reduce sympathetic sprouting, mechanical pain behavior, cytokine and NGF production in the DRG, and incidence of spontaneous bursting activity. We hypothesize that observed effects of systemic TA are primarily due to local effects at the level of the DRG, since local DRG inflammation has the opposite effects in the LID model. However, this needs to be confirmed since systemic TA will have many additional effects. In clinical practice, an epidural steroid injection is a common treatment for many kinds of lower back and leg pain. These considerations led us to test the effects on local steroid injection on pain behavior, sympathetic sprouting and local inflammation indicators in the SNL model.
Methods
Animals
Male Sprague–Dawley rats (200–250 g; Harlan, Indianapolis, IN) were used for all experiments. Rats were housed two per cage under a 12-h light/dark cycle with free access to water and food, at constant temperature (22±0.5 °C). All surgical procedures and the experimental protocol were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati (Cincinnati, OH, USA). All animal experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Procedures for Spinal Nerve Ligation/transection and Local TA Injection
Rats were randomly divided into four groups: SNL, SNL+TA, Normal+TA, and Normal. Rats were anesthetized with isoflurane. For the first 3 groups, an incision was made on the back between L2 and S1. For the SNL and SNL+TA groups, the right side L5 spinal nerve was exposed, tightly ligated with 6-0 silk and cut approximately 5 mm distal to the ligature 6, 7. For SNL+TA rats, after SNL surgery, the right paraspinal muscles were separated from the mammillary process. The L5 intervertebral foramen was clearly exposed and an L-shaped 30-G needle was inserted about 2 mm into the L5 intervertebral foramen at a 30° angle with respect to the dorsal midline and 10° with respect to the vertebral horizontal line. The sharp tip of the needle was kept in the space between DRG and vertebrae to avoid damaging the L5 DRG. A single dose of 20 μl Triamcinolone Acetonide Injectable Suspension (Kenalog-10, Bristol-Myers Squibb Company Princeton, NJ 08543 USA) (10mg/ml) was slowly (in 2 minutes) injected onto the surface of DRG and the region around the DRG. This corresponds to an effective systemic dose of less than 0.001 mg/kg; for comparison systemic TA treatment in our previous study was 1.5 mg/kg for 4 days. The rats in Normal+TA group received only TA injection without nerve ligation. The muscle and skin were sutured in layers. The rats in Normal group were uninjured and no TA was injected.
Behavioral Testing for Mechanical Hypersensitivity
The testing procedure has been described in detail previously 8. Rats were inspected and tested every other day for 5 days before surgery (three testing sessions; values were averaged to give the baseline day 0 value). After surgery, behavioral testing was performed on days 1, 3, 5 and 7. To avoid potential bias, the person performing the behavioral testing was blinded as to the experimental group. Rats were placed in an acrylic glass box with a plastic mesh floor. Von Frey filaments with bending forces of 20, 40, 60, 80, 120, and160 mN, each having the same tip diameter of 0.1 mm, were applied, in the order of ascending force, to six designated loci distributed over the plantar surface of the foot for 1 s, with interstimulus interval of 10-15 s. Using a curve-fitting program (Microcal Origin 7.0, OriginLab Corp., Northampton, MA), data was fit to the Hill equation, , where P = percent withdrawal responses, Pmax = 100% withdrawal response, S = stimulus magnitude, S50 = stimulus giving 50% response of foot withdrawal, and γ = slope factor. For behavioral experiments each experimental group had 7 to 14 animals.
Immunohistochemical Staining
On postoperative day (POD) 1, POD3, POD5 or POD7, as indicated, rats were anesthetized with pentobarbital sodium (40 mg/kg, i.p.). For CD11B antibody (OX-42) staining, rats were fixed by perfusing 200-300 ml of periodate-lysine-paraformaldehyde fixative (4% paraformaldehyde in lysine (75 mM)-0.1 M phosphate buffer, and 10 mM sodium periodate, pH=7.4) through the left ventricle of the heart. For tyrosine hydroxylase (TH) and GFAP staining, the fixative was 200–300 ml of Zamboni's fixative (4% paraformaldehyde in 0.1 M phosphate buffer, pH=7.4).
In all immunohistochemical experiments, data from at least three animals were included to control for inter-animal variability. The person performing the image analysis was blinded as to the experimental group. In addition, all images were captured and analyzed by an investigator other than the one who performed immunohistostaining to avoid possible bias.
TH immunohistochemistry in DRG
The axotomized L5 DRG was removed, postfixed in the perfusion fixative for 1 h at 4°C, and embedded in gelatin overnight. The ganglia were sectioned with a Vibratome at a thickness of 40 μm, along the long axis of the DRG, parallel to the plane of the fibers in the dorsal root. Tissue sections were incubated in antibodies to TH (Pel-Freeze, Rogers, AR, USA) at a dilution of 1:1,000 for 48 h at 4°C, followed by reaction with biotinylated secondary antibody and, finally, with Vector ABC reagent (Vector Labs, Burlingame, CA, USA). Triton-X (0.3%) was used in all reaction solutions to enhance antibody penetration. Immunoreaction products were visualized by the diaminobenzidine method in the presence of H2O2 in 0.1 M phosphate buffer. Tissues were then mounted on gelatin-coated slides, air dried, dehydrated, and coverslipped for light-microscopic observation. Using ImagePro Plus software (Media Cybernetics, Inc., Silver Spring, MD, USA), images from all sections of each DRG were captured under a light microscope (20×) equipped with a SPOT Insight colored digital camera (Diagnostic Instruments, Inc., Burlingame, CA, USA) and stored in a personal computer. The numbers of neuronal somata surrounded by TH-immunoreactive basket-like structures or rings were counted from all sections of the TH-immunostained DRGs. Only DRG neurons that were encircled by TH-immunoreactive fibers for at least two thirds of the circumference of the somata and that had a clearly visible nucleus were counted. The average density of the TH-immunoreactive basket/ring within each DRG was obtained by dividing the total number of baskets/rings by the size of the total measured cellular area (mm2).
GFAP in DRG, OX-42 in spinal cord
Ipsilateral DRGs were removed. Tissue was post-fixed in the perfusion fixative for 30 min (OX-42) or 2 h (GFAP) at room temperature. The ganglia and spinal cord (L5 level) were horizontally sectioned with a cryostat at thicknesses of 8 and 30μm, respectively. DRG sections were incubated in rabbit antibody to GFAP (1:100; Immunostar, Hudson, WI, USA) and mouse anti-neuronal nuclei(NeuN) monoclonal antibody (1:500; Millipore, Billerica, MA, USA) overnight at 4 °C, followed by reaction with secondary antibody Alexa Fluor 594 goat anti-rabbit IgG (H+L) for GFAP(1:1000, Invitrogen, Carlsbad, CA, USA), and Alexa Fluor 594 goat anti-mouse IgG (H+L) for NeuN (1:500; Invitrogen, Carlsbad, CA, USA)for 1 h at room temperature. Free floating spinal cord sections were incubated in mouse anti-rat CD11B monoclonal antibody (1:500; Millipore, Billerica, MA, USA) overnight at 4 °C, followed by reaction with goat anti-mouse secondary antibody conjugated to Alexa Fluor 594 (1:1000, Invitrogen, Carlsbad, CA, USA). After drying, the sections were mounted on coverslips with Vector Hard Set mounting medium (Vector Laboratories, Inc., Burlingame, CA, USA).
Images from ~10 sections of each DRG and ~20-30 sections of each spinal cord sample were captured under a confocal microscope using Slidebook 4.1 imaging acquisition software (Intelligent Imaging Innovation, Denver, CO, USA). In the DRG sections, to measure the density of satellite glia activation, number of neurons encircled by GFAP-positive glia was counted and then normalized by the total number of neurons in the analyzed image area to give a percentage of neurons surrounded by activated SGC. In the spinal cord, the summed intensity of OX-42 signal in the dorsal horn was measured and normalized by the analyzed image area to give an intensity ratio. POD3 was chosen as the peak time point for OX-42 measurement based on previous studies 9
Data Analysis
Significance was ascribed for p<0.05. Immunohistochemical data were analyzed using one-way ANOVA, or, for data taken at multiple time points (e.g. Fig. 4 top), two-way ANOVA followed by Bonferroni post-hoc test to determine on which days groups were significantly different, if an overall significant treatment effect was observed. Statistical analysis of quantitative immunohistochemical data was performed using the averaged values from each individual animal as a single number; for immunohistochemical experiments each experimental group had 3 to 8 animals. Behavioral data was analyzed using two-way repeated measures ANOVA with Bonferroni post-hoc test. In figures, level of significance is indicated by number of symbols: *, 0.01< p<0.05; **, 0.001<p<0.01; ***, p<0.00.
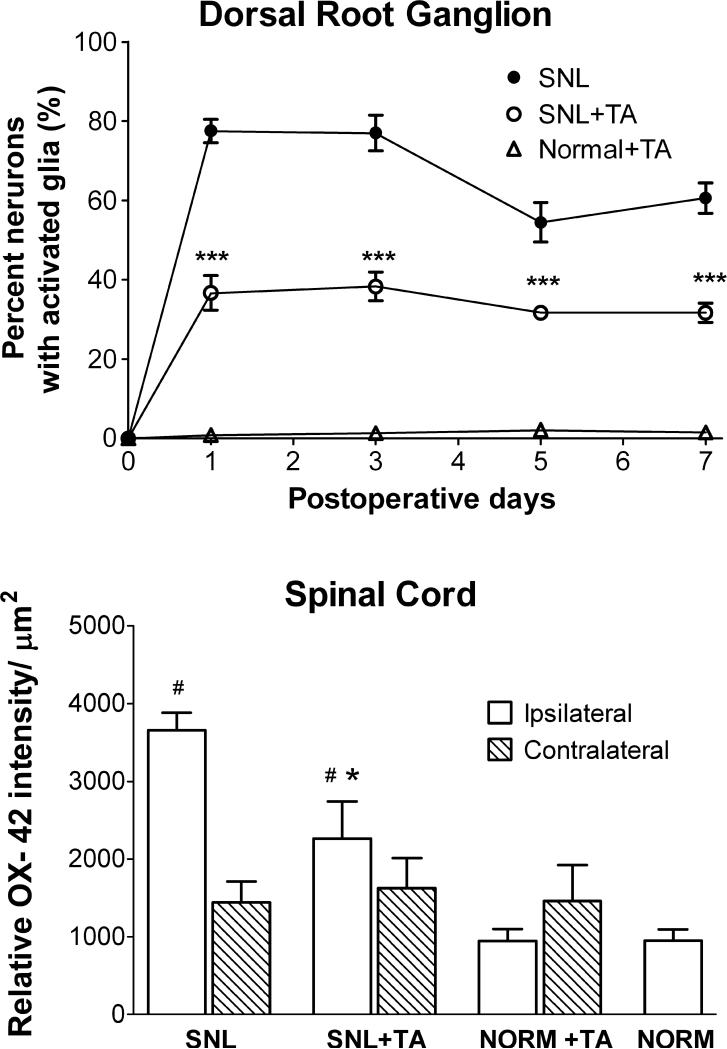
Figure 4.
Quantitative summary of glial activation data exemplified in Figs. 2 and 3. Top: Time course of effect of local TA injection on satellite glia activation in the DRG, measured as percent of neurons surrounded by GFAP staining. *, Days on which there was a significant difference between SNL+TA and SNL rats (two-way ANOVA). Both SNL and SNL+TA groups were significantly different (p<0.001) from the Normal+TA group at all postoperative time points. Bottom: Summary of relative intensity of staining for the microglia marker OX 42, examined on POD 3 in spinal cord. For the normal group, ipsilateral and contralateral data were combined to a single value. * Significantly different from SNL group. # Significantly different from Normal group
Results
Local TA Application to the DRG Attenuated Mechanical Hypersensitivity after SNL
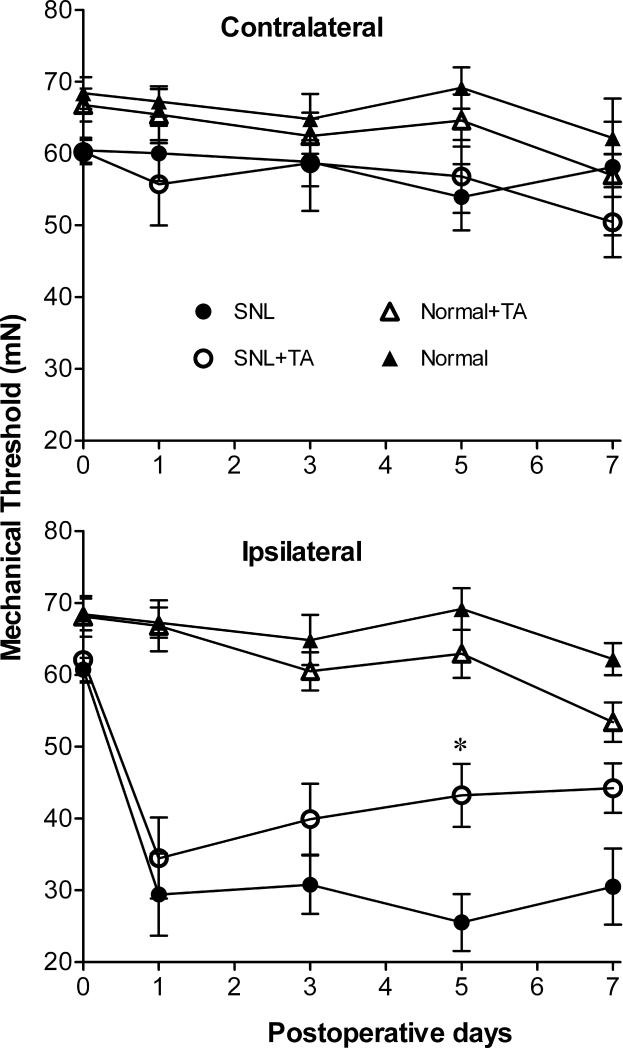
SNL increased mechanical hypersensitivity of the hind paw, an effect which was obvious 12-20h after surgery (on POD1, P<0.0001 comparing SNL or SNL+TA group to the Normal group). Treatment of animals with a single local injection of TA in the region of the ligated DRG, immediately after the spinal nerve was ligated, caused a significant decrease in the mechanical pain behaviors evoked by SNL starting on POD5 (P=0.034, n=8 each in SNL group and SNL+TA group). TA still showed a tendency to reduce the hyperalgesia on POD7, but this did not reach significance (Figure 1). TA injection had no significant effect on pain behavior in normal, uninjured rats (n=14 in Normal+TA group, n=7 in Normal group), and no contralateral effects on mechanical pain behavior were observed in any of the groups.
Figure 1.
Effect of local triamcinolone acetonide (TA) injection on mechanical pain behavior induced by spinal nerve ligation (SNL). Baseline withdrawal threshold to mechanical stimulation of the hind paws was measured in the ipsilateral paw on three measurements taken over 5 days before the surgery, and the average is plotted as the postoperative day 0 value. *, Days on which there was a significant difference between group SNL+TA and group SNL rats (one-way ANOVA). The ipsilateral reduction in threshold caused by SNL alone was significantly different from the normal group on all postoperative days; for clarity no symbols are added to indicate this.
Local TA Application to the DRG Reduced Sympathetic Basket Formation in the DRG after SNL
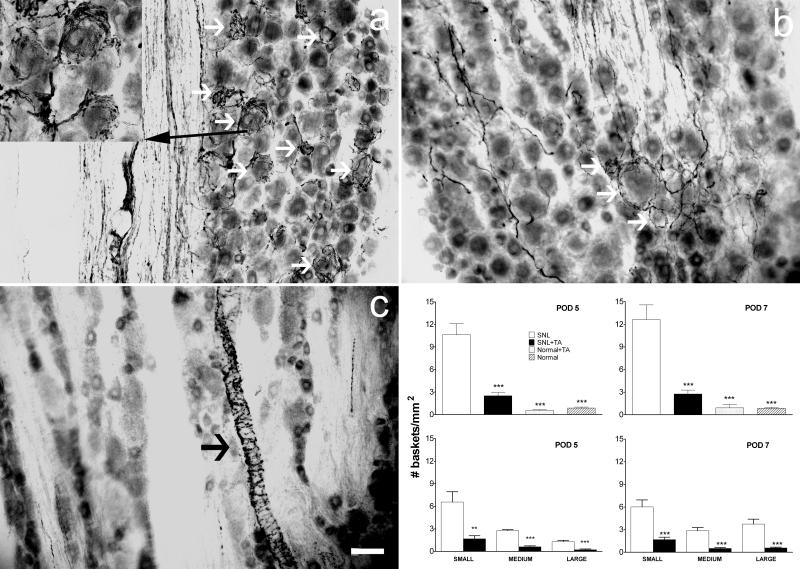
After proximal nerve lesions, sprouting of sympathetic fibers particularly around small- (early times only) and large-diameter neurons was revealed by immunostaining for TH. The fibers often formed perineuronal basket-like structures. Examples of basket formations in each group of animals on POD5 and POD7 are shown in Figure 2. We quantified sympathetic sprouting by measuring the density of these basket structures in fixed sections. In SNL animals treated with TA, the density was reduced almost four-fold on POD5 (P=0.032) or five-fold on POD7 (P=0.016) compared to the SNL group (Figure 2). Basket formations were rare in TA only animals (Figure 2c) or in normal animals.
Figure 2.
Local TA injection reduced sympathetic basket formation in the DRG after SNL on postoperative day (POD) 5 and POD 7. a – c, examples of sympathetic fiber basket formation in dorsal root ganglion sections stained for tyrosine hydroxylase on POD 5. White arrows indicate examples of neurons surrounded by basket formation. Black arrows indicate sympathetic fibers innervating vascular processes. (a) Example from a SNL rat. The inset shows a magnified view of neurons surrounded by basket formation. (b) Example from a SNL+TA rat. (c) Example from a Normal+TA rat. Scale bar = 50 μm. Graphical summary for POD 5 (left) and POD 7 (right). Upper graphs give the total density of basket formations. In the lower graphs, DRG neurons were classified by diameter as small (<30 μm), medium (30-50 μm), or large (>50 μm). *, significantly different from respective SNL group.
Local TA Application to the DRG Reduced Satellite Glia Activation in the DRG after SNL
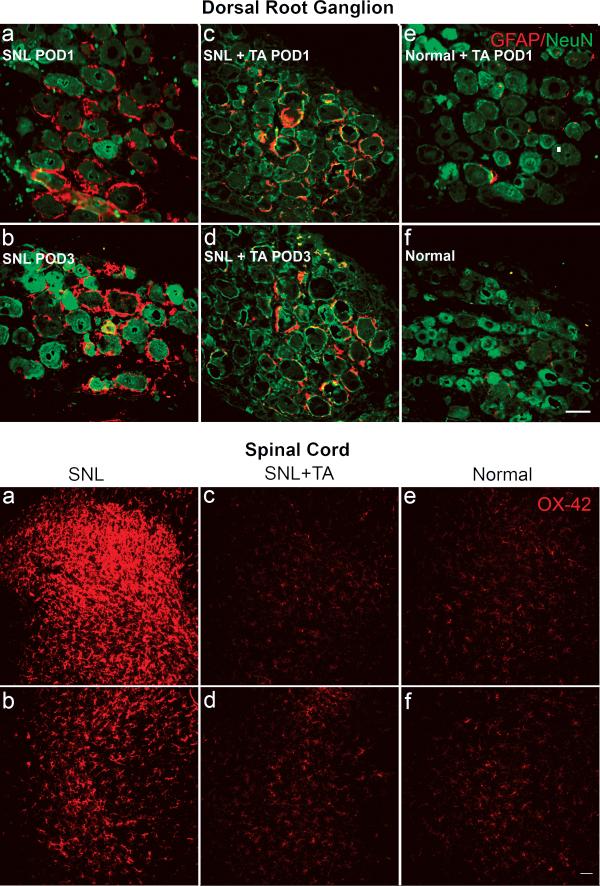
In the SNL model, marked activation of satellite glia (as measured by the percent of DRG neurons surrounded by GFAP-positive glia) in the axotomized L5 ganglion was observed as early as the first 24h (Figure 3, 4). Glia activation in SNL model peaked on POD1-3, then gradually declined but was still significantly above Normal +TA group on POD7 (Figure 4). Local injection of corticosteroid on the surface of DRG immediately after spinal nerve surgery significantly inhibited the satellite glia activation.
Figure 3.
Effect of local TA injection on glial activation. TOP: Sections of DRG stained for GFAP (red) and NeuN (Green). GFAP was barely detectable in normal rat DRG (f). Following spinal nerve injury, the expression of GFAP was dramatically increased on POD 1 (a), and remained very high on POD 3 (b). Applying TA locally to the axotomized DRG starting at the time of nerve injury reduced the nerve-injury induced GFAP expression at these time points (c, POD 1; d, POD 3). GFAP was barely detectable in examples from a Normal+TA rat at POD 1 (e) or later time points (not shown; see Fig. 4). Scale bar=50μm. BOTTOM: microglial activation on POD3 as measured by OX-42(CD11B antibody, a microglia marker) staining (red) in spinal cord. Panels on the top and bottom show dorsal horn portions of the spinal cord, the sides contra- and ipsi-lateral to nerve injury, respectively. Scale bar=100 μm. SNL caused increased OX-42 staining ipsilaterally (a) and, to a lesser degree, contralaterally (b). Reduced staining was seen in SNL animals that received a TA injection (c, d). Low levels of staining were observed in normal animals (e, f); or in normal animals treated with TA (not shown).
Local TA Application to the DRG Reduced Microglia Activation in the Spinal Cord after SNL
We also examined the effect of early injection TA onto the axotomized DRG on microglia activation in the corresponding level of the spinal cord. SNL caused a significant increase in OX-42 staining in the ipsilateral dorsal spinal cord on POD3 (Figures 3&4). The microglia activation on POD3 in the ipsilateral dorsal spinal cord was significantly increased from Normal group (P<0.0001); there was a trend for increased activation on the contralateral side as well, but this did not reach significance. Early injection of TA on the axotomized DRG immediately after injury led to a significant reduction of OX-42 staining on POD3 (P=0.044).
Discussion
Effects of spinal nerve ligation on mechanical behavior, sympathetic sprouting, and glial activation were generally similar to previous reports using this model 7, 9-11. In this study, we demonstrated that local TA injection onto the axotomized DRG at the time of spinal nerve injury attenuated mechanical hypersensitivity, reduced sympathetic basket formation and satellite glia activation in the axotomized DRG, and reduced microglia activation in the spinal cord. These findings support the idea that inflammation contributes to the development of neuropathic pain in the SNL model, and that anti-inflammatory treatment at the DRG level at the time of injury can alter the development of neuropathic pain caused by spinal nerve injury.
Systemic, intrathecal, or local corticosteroid treatments have been clinically used with various efficacies in acute or chronic pain. The efficacy and mechanism of action of corticosteroids in neuropathic pain have been much studied 12-15 using various sites of application and in various stages of development of pain. Most of these studies show corticosteroids can reduce pain behavior partially, as also demonstrated in our study. The idea that initial application of corticosteroids before the pain is well established can reduce the development of pain is also supported by our study.
The literature supports the theory that local corticosteroid application may reduce pain behavior through several mechanisms. Corticosteroids suppress proinflammatory cytokines 6 and increase anti-inflammatory cytokines 16 secreted at or near the site of a nerve injury, which are involved in the development and maintenance of central sensitization and neuropathic pain. Corticosteroids inhibit activation of nuclear factor-κB, which induces cyclooxygenase-2. Corticosteroids inhibit signal transmission in nociceptive C fibers 17; reduce the incidence of bursting pattern ectopic discharge in DRG neurons 6 and reduce ectopic discharge from traumatized nerves 18.
Compared to our previous study 6 of systematic TA (given just before surgery and once daily for the next 3 days), in this study using local treatment of TA on the axotomized DRG, TA presumably acted primarily on the level of the injured DRG. According to our direct visualization under the dissecting microscope, the single bolus of TA over the DRG did not spread even as far as the ligature site at the time of application, and the spinal cord regions to which the L5 DRG projects are about 2-3 cm away. Systemic effects of the single injection also seem unlikely, in that the effective systemic dose has been reduced to less than 0.001 mg/kg in the local TA treatment paradigm, compared to the systemic TA treatment which was 1.5 mg/kg/injection. Corticosteroids such as TA when used systemically in rodent experiments are generally given in doses on the order of mg/kg, rarely dropping below 0.1mg/kg in the lowest doses examined. For comparison, corticosterone levels in rat plasma vary over a 24 hour time course from 1 to 25 μg/dL, for an estimated plasma content varying from 0.07 to 1.75 μg in a 200 gram rat. Hence it seems quantitatively unlikely that one dose of 0.2 μg of TA, contributed by the single injection over the DRG, would have systemic effects, especially since this amount would be distributed throughout the body, not just into the plasma. However, it should be noted that an experimental group receiving a dose of 0.001 mg/kg systemically rather than locally to the DRG, was not included in the present study.
These results indicate that inflammation at the level of the injured DRG likely plays an important role in the mechanical pain induced by SNL. Lower systemic doses of corticosteroids may reduce serious side effects such as infection, appetite change, and initial weight loss. In our study of systemic TA treatment, the analgesic effect was observed as early as POD3 and lasted until POD7 (the longest time tested). However in this study, the inhibitory effect of a single local dose TA treatment on the mechanical pain behaviors evoked by SNL showed a trend on POD3 that lasted until POD7, but was only significant on POD5. Single dose TA treatment had a weaker effect than continued multi dose systemic TA treatment. In the Normal +TA group, TA treatment in normal rat showed a trend of declining mechanical pain thresholds. This might be caused by injection injury and TA itself. TA may cause pain due to its chemical irritation on normal DRG.
The main type of glia cell in the cellular regions of sensory ganglia is the satellite glial cell. The satellite glial cells respond to sensory neuron damage or inflammation by proliferating; expressing GFAP, neurotrophins and activated mitogen activated protein kinases; releasing ATP and chemokines that may contribute to the abnormal pain sensitivity; and increased gap junction coupling with each other and with neurons, all of which may contribute to enhanced pain response 5, 19-22. In this study, TA applied to the DRG in vivo reduced satellite glia activation in the DRG after SNL. This may be one mechanism by which local application of TA reduced the development of neuropathic pain. Although not examined in the present study, it is possible that local TA application may also suppress activation/recruitment of other non-neuronal cells such as macrophages, which are another source of pro-inflammatory cytokines 23.
Many studies show that activation of spinal glia (astrocytes and microglia) contributes to the pain state in various animal models of inflammatory and neuropathic pain 3, 20, 24. We observed inhibition of spinal microglia activation by local TA even though this was applied to the DRG, not the spinal cord. This effect possibly contributed to reduction of mechanical pain in our study. By affecting the DRG, the local TA might have reduced many signals coming from the DRG into the spinal cord that activate spinal microglia, such as substance P, excitatory amino acids, ATP, nitric oxide, and prostaglandins. We have previously shown that local nerve blockade in the ligated DRG can reduce activation of spinal microglia 9, 25, 26.
Previous researchers propose a mechanism for sympathetic sprouting in the DRG involving: (i) activation of satellite cells in the DRG by a factor such as LIF or IL-6, and (ii) the expression of p75-bound neurotrophins on the activated satellite cells. They also highlight the possibility that a sympathetic sprouting signal may be derived from the periphery, as NGF and LIF are produced as a result of Wallerian degeneration, and can be retrogradely transported to the DRG 11. Based on this theory, we hypothesize that TA may reduce sympathetic sprouting by suppressing activation of satellite cells in DRG and suppressing inflammation at the injury site.
In summary, this study demonstrates a single local injection of the clinically used steroid TA can mimic many of the effects of systemic TA in mitigating the behavioral and cellular abnormalities induced by spinal nerve ligation. This provides a further rational basis for the clinical use of localized steroid injections in some clinical conditions, and provides further support for the idea that localized inflammation at the level of the DRG is an important component of the spinal nerve ligation model even though this is commonly classified as neuropathic pain model. Finally, the study provides further evidence of a correlation between pain behaviors and activation of glia in the DRG and spinal cord.
Acknowledgments
Supported by: NIH grants NS55860 and NS45594 to J-M Z.
Footnotes
Conflicts of Interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Brain Res Rev. 2006;51:240–64. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang J-M. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience. 2006;142:809–822. doi: 10.1016/j.neuroscience.2006.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10:40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- 4.White FA, Bhangoo SK, Miller RJ. Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov. 2005;4:834–44. doi: 10.1038/nrd1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev. 2005;48:457–76. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Xie W, Strong JA, Zhang J-M. Systemic antiinflammatory corticosteroid reduces mechanical pain behavior, sympathetic sprouting, and elevation of proinflammatory cytokines in a rat model of neuropathic pain. Anesthesiology. 2007;107:469–77. doi: 10.1097/01.anes.0000278907.37774.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–63. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 8.Song XJ, Hu SJ, Greenquist KW, Zhang J-M, LaMotte RH. Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. J Neurophysiol. 1999;82:3347–3358. doi: 10.1152/jn.1999.82.6.3347. [DOI] [PubMed] [Google Scholar]
- 9.Xie W, Strong JA, Zhang JM. Early blockade of injured primary sensory afferents reduces glial cell activation in two rat neuropathic pain models. Neuroscience. 2009;160:847–857. doi: 10.1016/j.neuroscience.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung K, Lee BH, Yoon YW, Chung JM. Sympathetic sprouting in the dorsal root ganglia of the injured peripheral nerve in a rat neuropathic pain model. Journal of Comparative Neurology. 1996;376:241–52. doi: 10.1002/(SICI)1096-9861(19961209)376:2<241::AID-CNE6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Ramer MS, Thompson SW, McMahon SB. Causes and consequences of sympathetic basket formation in dorsal root ganglia. Pain. 1999;(Suppl 6):S111–20. doi: 10.1016/S0304-3959(99)00144-X. [DOI] [PubMed] [Google Scholar]
- 12.Johansson A, Bennett GJ. Effect of local methylprednisolone on pain in a nerve injury model. A pilot study. Reg Anesth. 1997;22:59–65. doi: 10.1016/s1098-7339(06)80057-x. [DOI] [PubMed] [Google Scholar]
- 13.Kingery WS, Agashe GS, Sawamura S, Davies MF, Clark JD, Maze M. Glucocorticoid inhibition of neuropathic hyperalgesia and spinal fos expression. Anesth Analg. 2001;92:476–482. doi: 10.1097/00000539-200102000-00037. [DOI] [PubMed] [Google Scholar]
- 14.Hashizume H, Rutkowski MD, Weinstein JN, DeLeo JA. Central administration of methotrexate reduces mechanical allodynia in an animal model of radiculopathy/sciatica. Pain. 2000;87:159–169. doi: 10.1016/S0304-3959(00)00281-5. [DOI] [PubMed] [Google Scholar]
- 15.Kingery WS, Castellote JM, Maze M. Methylprednisolone prevents the development of autotomy and neuropathic edema in rats, but has no effect on nociceptive thresholds. Pain. 1999;80:555–66. doi: 10.1016/S0304-3959(98)00251-6. [DOI] [PubMed] [Google Scholar]
- 16.Xie W, Luo S, Xuan H, Chou C, Song G, Lv R, Jin Y, Li W, Xu J. Betamethasone Affects Cerebral Expressions of NF-{kappa}B and Cytokines that Correlate with Pain Behavior in a Rat Model of Neuropathy. Ann Clin Lab Sci. 2006;36:39–46. [PubMed] [Google Scholar]
- 17.Johansson A, Hao J, Sjolund B. Local corticosteroid application blocks transmission in normal nociceptive C-fibres. Acta Anaesthesiol Scand. 1990;34:335–8. doi: 10.1111/j.1399-6576.1990.tb03097.x. [DOI] [PubMed] [Google Scholar]
- 18.Devor M, Govrin-Lippmann R, Raber P. Corticosteroids suppress ectopic neural discharge originating in experimental neuromas. Pain. 1985;22:127–37. doi: 10.1016/0304-3959(85)90173-3. [DOI] [PubMed] [Google Scholar]
- 19.Woodham P, Anderson PN, Nadim W, Turmaine M. Satellite cells surrounding axotomised rat dorsal root ganglion cells increase expression of a GFAP-like protein. Neurosci Lett. 1989;98:8–12. doi: 10.1016/0304-3940(89)90364-9. [DOI] [PubMed] [Google Scholar]
- 20.Watkins LR, Hutchinson MR, Milligan ED, Maier SF. “Listening” and “talking” to neurons: implications of immune activation for pain control and increasing the efficacy of opioids. Brain Res Rev. 2007;56:148–69. doi: 10.1016/j.brainresrev.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci U S A. 2007;104:9864–9. doi: 10.1073/pnas.0611048104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMahon SB, Cafferty WB, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol. 2005;192:444–62. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Otoshi K, Kikuchi S, Konno S, Sekiguchi M. The reactions of glial cells and endoneurial macrophages in the dorsal root ganglion and their contribution to pain-related behavior after application of nucleus pulposus onto the nerve root in rats. Spine (Phila Pa 1976) 35:264–71. doi: 10.1097/BRS.0b013e3181b8b04f. [DOI] [PubMed] [Google Scholar]
- 24.Ji RR, Kawasaki Y, Zhuang ZY, Wen YR, Decosterd I. Possible role of spinal astrocytes in maintaining chronic pain sensitization: review of current evidence with focus on bFGF/JNK pathway. Neuron Glia Biol. 2006;2:259–269. doi: 10.1017/S1740925X07000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–5. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 26.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711–23. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]