Abstract
Pain is the hallmark of sickle cell disease in children and adolescents. Many children seek relief from their pain in the emergency department. These visits have historically been characterized by undertreatment, bias and distrust. Through compassionate care, aggressive pain management, and the development of clinical pathways or care guidelines, better analgesia and a better experience can be assured for the child with sickle cell disease in need of emergency care.
Keywords: Sickle cell disease, pain, vasoocclusive episode, opioids
Sickle cell disease is the most common genetic disease of African Americans. In its most common form it is an autosomal recessive disorder containing 2 abnormal copies of the gene coding for the hemoglobin beta chain. 0.2% (1/600) of African-Americans have sickle cell disease while 8% of African-Americans are heterozygotes for the abnormal gene and have sickle cell trait.
Sickle cell disease is also seen in the United States in those of Hispanic origin and those of Mediterranean extraction, albeit in much lower numbers. While the most common form of the disease is found in those mentioned above with homozygous hemoglobin SS disease, the disease also occurs if a patient has one sickle cell gene accompanied by either a hemoglobin C gene (hemoglobin SC) or beta-thalasemmia gene (sickle beta thal + or sickle beta thal 0). These variants usually have milder disease than those with hemoglobin SS however they can present with similar manifestations of the disease.
Children with sickle cell disease frequently present to the emergency department (ED) with pain. Pain is the hallmark of sickle cell disease and the dominant feature of children’s medical lives;1,2 about 70% of hospitalizations in patients presenting to the ED with sickle cell disease are for uncontrolled pain.3 Despite the feeling that patients with sickle cell disease are often in the ED for pain, most painful episodes in children and adolescents with sickle cell disease do not reach medical attention. In fact, 90% of pain episodes are treated in the home.4
The intensity and complexity of pain is sickle cell disease is often underestimated and misunderstood by providers. Children with vasoocclusive episodes experience complex pain, which has features that are consistent with acute, recurrent, and chronic pain states.4,5 Sickle cell pain is worse than postoperative pain and is as intense as terminal cancer pain.6,7 Other pediatric pain states have considerably lower mean pain intensities than sickle cell disease.8,9 Increased frequency and severity of pain episodes is associated with shortened survival.2
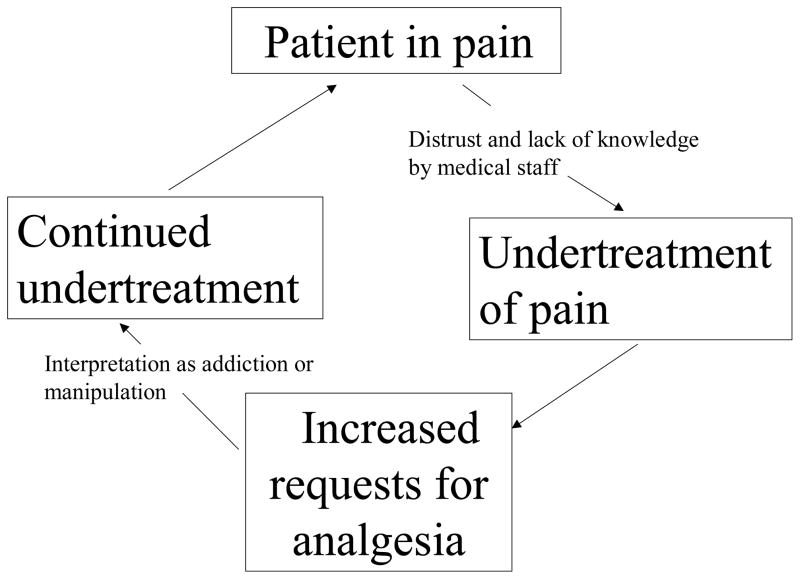
Evidence of inadequate management of sickle cell pain abounds in the literature and is likely related to caregivers’ preoccupation with the pathophysiologic causes of vasoocclusion and a profound fear of creating opioid dependence, as well as a negative attitude towards patients with sickle cell disease in urban hospitals and EDs.5,11,12 Schechter describes a cycle of under treatment (Figure 1) in these patients which persists in many care settings.1
Figure 1.
Cycle of pain under-treatment in sickle cell disease.
Epidemiology
There are somewhere between 80,000 and 100,000 individuals with sickle cell disease in the United States. In a retrospective cohort of over 20,000 children and adults with sickle cell disease, a staggering 94% of the cohort had an ED visit in a single year.13 Over 70% of ED visits are for pain.3,13 Ninety per cent of children with sickle cell disease who visit the ED are African American and only 20%–30% have private insurance.3 Almost 50% of patients come from families that are below the poverty line.14 Almost 40% of ED visits in children with sickle cell disease result in hospitalization.3
Pathogenesis
The pathogenic process in sickle cell disease is more complicated than previously believed. Polymerization of the sickle cell hemoglobin causes the red blood cells to become rigid, sticky and formed into shapes which sometime have the appearance of a sickle (thus the name).15 These sickled cells subsequently will cause obstruction in both the micro- and macro-vasculature. While this is a key event in the vasoocclusive process, it does not completely explain the pathogenesis.16,17 It is probable that a multitude of factors contribute to vasoocclusive episodes including cell deformability, blood viscosity, red cell function, adherence of the sickled cells to vascular endothelium, endothelial, hemostatic, white cell and platelet activation, and vasoregulatory and environmental factors. 16,17 This confluence of factors is called the multiple pathway model of vasoocclusion. In this model the predominant mechanism leading to vasoocclusion may vary depending on the clinical circumstance. Patients may live in a homeostatic balance with circulating sickle cells but a seemingly minor event such as a viral illness or exercise may tip this balance resulting in a full blown vasoocclusive episode.
Course of Vasoocclusive Episodes
Pain from a vasoocclusive episode (VOE) can begin early in life when levels of fetal hemoglobin decrease. Dactylitis may be the first clinical manifestation of sickle cell disease. Infarctions in the metacarpals and metatarsals result in episodes of pain and swelling involving the hands and feet. Infants and toddlers with dactylitis may become irritable, refuse to walk, or cry when they are touched or held. Prior to neonatal testing, the diagnosis of sickle cell disease was often precipitated when a child presented to the emergency department with swollen, painful hands or feet. As children with sickle cell disease get older, sites of involvement shift to the arms, legs, back and pelvis, while adolescents may also complain of involvement of the chest and abdomen.
VOEs typically last from 3–9 days. It is not atypical for those patients with longer episodes to continue to have patterns where their episodes remain prolonged. Beyer describes the chronology of VOE as an 8 phase phenomenon.18 In the first phase, at baseline, the child has no pain, but often children and caregivers can point to a specific event occurring during phase 1 that precipitated the VOE. These can include change in weather, dehydration, or overexertion. In phase 2, no pain is present but the child can exhibit some prodromal symptoms such as fatigue or scleral icterus.
During phases 3–5, the child begins having mild pain of an achy quality which is usually restricted to one area of the body. Pain intensity accelerates in phase 4 and may spread to other areas of the body. Pain peaks in phase 5 and often reaches a severe level. The child may describe it as pounding, banging, or throbbing. This is the phase where patients will often abandon home management and present to the ED or clinic. Often the pain will stay at this peak intensity for 2–3 days. Pain begins to decrease in phase 6, and steadily declines in phase 7. By phase 8 the pain intensity is much more tolerable and the child looks and acts more like him or herself.
A recent study documented significantly reduced health related quality of life (HRQL) compared to historical controls at the time of presentation for VOE on both physical and psychosocial health scales.19 We have noted a correlation between mood and pain in our inpatient population.20
Pain Management Challenges in Sickle Cell Disease
There is a long history of under-treatment of pain in patients presenting to the ED with a VOE. Perceived discrepancies between patient behavior and pain score have been documented in the care of patients with sickle cell disease and lead to mistrust between providers and their patients.21–23 While verbal pain report is used to determine the severity of vasoocclusive pain, over 85% of physician respondents felt it was not a reliable indicator of the existence or intensity of pain.21 Almost 60% of nurses report that the greatest barrier to the management of sickle cell pain episodes is the lack of an adequate pain assessment tool.23 Health care providers may take the reports of pain in patients with sickle cell disease less seriously because of attitudes and beliefs about addiction and concerns regarding drug seeking behavior.23
Patients are frustrated by the lack of consideration that they receive for their pain. Patients report insensitivity of hospital staff, inadequate analgesia administration, staff preoccupation with concerns of drug addiction, and an overall lack of sympathy and trust as contributors to their negative feelings toward medical care of sickle cell disease.24–26
Pain Assessment
Pain assessment in children is often complex and challenging. Developmental and cognitive changes that occur throughout childhood impact the ability of children to understand and describe pain.27 Difficulty in assessing pain in this population leads to problems with pain management as well.27,28
Guidelines for the management of acute pain in general, and sickle cell pain specifically, recommend the use of standard pain measures, and advocate for regular documented pain assessment as an essential component of pain management.29–31
While self report scales are the norm for the assessment of acute pain intensity in older children and adults, they cannot be used in infants and toddlers and must be modified for use in children under the age of 7.27,32 The visual analogue scale 0–10 or 0–100 (VAS) is a valid tool for assessing acute pain in children over the age of 7,27 but the verbally administered numerical pain scale (NRS), commonly used in hospitalized patients, has been validated on a more limited basis.33,34 It is important to remember, however, that pain intensity is just one component of the pain experience for children with sickle cell disease.
The acute but also recurrent and sometimes chronic nature of pain in sickle cell disease makes the assessment of pain in these children complex. Children with sickle cell disease often report high levels of pain without outward behavioral or physiologic signs of pain. This is likely due to a constant awareness of discomfort, which leads to an inability to identify improvement or deterioration. Recurrent bouts of severe pain may result in central sensitization, which in turn can permanently alter a child’s perception of pain.4,35 Additionally, previous experiences with inadequate pain relief may increase anxiety, which in itself can amplify pain.36–39 Given their previous experience with undertreatment, children with sickle cell disease may also alter the way in which they report pain to secure more opiates.5
Ratings of pain intensity, which are the primary measure used to assess acute pain, are often insensitive to clinical improvement or deterioration.40 Data from our center and others confirm that self-reported pain intensity scores remain at high levels throughout hospitalization, despite the use of potent opiates.41,42 Patients are routinely discharged despite the persistence of high pain scores. This underscores the need for assessment tools that are more sensitive to changes in clinical status than those currently employed, and that coincide with the indicators used by caregivers when making clinical decisions.
The Adolescent Pediatric Pain Tool (APPT) has been demonstrated to detect change in patients hospitalized for vasoocclusive pain, although it has not been validated for this use.43 While this tool is helpful, it paints only a narrow picture of the pain experience and does not address the functional change in patient status that is likely used by clinicians when making therapeutic decisions.
The emergency clinician must be cognizant of difficulties associated with pain assessment in the sickle cell population. It is crucial to recognize the intensity of pain in sickle cell disease and avoid allowing bias about patient report alter treatment plans or the clinician patient relationship.
Pain Management
Management of pain in sickle cell disease should take advantage of a multidisciplinary approach that would include pharmacologic, behavioral and complementary interventions. Currently the paradigm under which this pain has been treated focuses almost solely on acute pharmacologic management of recurrent episodes and does not treat sickle cell disease with the modalities that have typically been brought to bear on a chronic pain syndrome.
Pharmacologic Management
While much has been written regarding the use of pharmacologic agents in sickle cell disease, there is a lack of data supporting the use of any of the agents that will be discussed in this section.
Acetaminophen (APAP) and non-steroid anti-inflammatory agents (NSAIDs) are typically the first line therapy for patients with sickle cell disease who have mild to moderate pain. These agents should be combined with opioids in patients whose pain is not reduced with these therapies alone.
Acetaminophen, while a relatively weak analgesic, can be effective for some pain episodes in sickle cell disease. The effects of APAP are mostly central and it does not have much anti-inflammatory action. While the adverse effects of APAP are limited in sickle cell disease compared to NSAIDS, patients should be reminded to avoid exceeding recommended daily doses as this can result in hepatic toxicity. The use of combination products which contain APAP along with an opioid are especially problematic and can lead to inadvertent overdose in this population.
NSAIDs are commonly used for sickle cell patients with mild, moderate or severe acute pain either in the outpatient or inpatient setting. These agents do provide peripheral anti-inflammatory activity. They have a ceiling effect, meaning that there is a maximum dose beyond which no further benefit is derived. The present data on the efficacy of these agents in sickle cell disease is contradictory. A recent study showed no benefit in a group of hospitalized adults with sickle cell disease in a RCT of ketoprofen in regards to duration of pain episode or opiate consumption.44 One pediatric study showed no added benefit with the addition of ketorolac regarding opiate utilization or pain relief.45 Another pediatric trial demonstrated that over 50% of ED vasoocclusive episodes resolved after treatment with ketorolac and intravenous fluids.46 It may be that ketorolac is effective in milder episodes of sickle cell pain but does not provide additional benefit for more severe or longer lasting pain. The answer to this question is an important one given that NSAIDs are not without risks including gastritis, nephropathy and antiplatelet activity.
Opioids are the mainstay in the treatment of moderate to severe pain in sickle cell disease. Currently available opioids traditionally provide their analgesic action through the mu opioid receptor, although other agents such as agonist-antagonists are utilized in this population. The advantages of opioids include their potent centrally mediated analgesic action, the availability of many routes for delivery, a variety of available agents, as well as a lack of a ceiling effect, thus allowing for continued drug titration if there is lack of analgesia at lower doses.
Side effects and adverse events can limit the efficacy of opioids in children with sickle cell disease. These include nausea, vomiting, pruritis, constipation and urinary retention. Other side effects can present more serious challenges such as respiratory depression, oversedation, or delirium. Children with sickle cell disease often exhibit tolerance to opioids due to repeated use of these agents. This results in the need for higher and higher doses of opioids to provide the same level of analgesia. A recent study demonstrated that the clearance of morphine is three times faster in adults with sickle cell disease than in normals.47 Patients with sickle cell disease can also develop opioid dependence and addiction. There are many misconceptions about these issues and their prevalence in this population. Opioid dependence is a physiologic phenomenon such that abrupt discontinuation of opioid treatment will lead to symptoms of withdrawal. Patients who are on chronic opioids are opioid dependent. In contrast, opioid addiction is a psychological craving for these drugs.
Clearly concerns regarding over-reporting of pain and addiction have major impact on the administration of care to patients with sickle cell disease. Fear of drug abuse, reluctance to prescribe opioids, and disbelief in patients’ report of pain severity are three of the top five barriers to optimal sickle cell pain management reported by clinicians.21 Surveys have documented that 53% of emergency physicians and 23% of hematologists feel that > 20% of patients with sickle cell disease are addicted to opiates, while 63% of nurses feel that drug addiction frequently develops in the treatment of sickle cell pain episodes.21,23
The truth regarding opiate addiction in patients with sickle cell disease is markedly less dramatic than popular opinion would suggest.48 Prevalence estimates for opiate addiction among patients with sickle cell disease range from 0.5–8%.48 Elander et al take a unique approach to this issue separating Diagnostic and Statistical Manual of Mental Disorders (DSM IV) substance abuse and dependence symptoms into pain related symptoms and non-pain related symptoms.49 When pain related symptoms are included 31% of sickle cell patients met the DSM IV criteria, but when only non-pain related symptoms are included the percentage of patients meeting these criteria drops to 2%.49
Elander also points out that concern raising behaviors inside the hospital, i.e. asking for a certain dose or delivery mode for an opiate, are considerably less suspicious for potential abuse than behaviors such as illicit drug use, or using opiates for other symptoms other than pain which typically occur outside the hospital setting. Since most care providers in the ED or other areas of the hospital are dealing with the former not the latter behaviors, substance abuse should not be presumed. It may in fact be that pain related behaviors are normative in patients within populations of medically ill patients. 49 This “pseudoaddiction” is compounded in patients with sickle cell disease due to inadequate effect of current analgesic regimens even in the face of what is seemingly appropriate treatment.
Opioids can be administered orally, transmucosally, subcutaneously, intravenously and transdermally, or intramuscularly. For acute, severe pain intravenously administered opioids are the drug of choice. At presentation the patient should receive intermittent boluses of opioid with frequent (q 15 minute) reasessesment. The child who is going to stay in the hospital more than 2 or 3 hours should be transitioned to patient controlled analgesia (PCA).
PCA is a well tolerated and effective mode of analgesia in children over the age of 7. It allows for the child to control the titration of analgesia, and reduces the delay inherent in nurse-delivered analgesia. Due to the severity of their pain at the time of presentation, children and adolescents with sickle cell disease hospitalized for pain should receive PCA with both continuous infusion and bolus dosing. Implementation of PCA in the ED especially for those patients who are going to be hospitalized should be encouraged. Initiation of PCA in the ED will facilitate aggressive management of pain and lessen the gap between bolus dosing and PCA initiation which often occurs when the patient is transferred to the floor.
For outpatient management of pain, either short-acting oral opioids or a combination of long-acting and short-acting opioids is appropriate depending on the severity and the duration of pain. As stressed earlier, avoid the use of combination products to avoid the risk of APAP toxicity.
Adjuvants and Other Analgesics
Patients with sickle cell disease demonstrate aspects of neuropathic as well as nociceptive pain. In addition, it is likely that recurrent episodes of severe pain experienced by this patient group leads to augmentation of pain processing and centrally mediated pain. These types of pain may respond to adjuvant analgesics such antidepressants (amitryptiline and duloxitine) or anticonvulsants (gabapentin and pregabalin). There is limited evidence for the efficacy of these drugs for chronic pain syndromes in children, nor any data for these drug classes on sickle cell pain. While these drugs are used for the management of chronic pain in sickle cell disease, further study is warranted to define their utility in this patient group.
Sickle cell patients with acute and chronic pain also develop a number of other issues that may need pharmacologic intervention. Sleep disorders are common in this population and can lead to increased pain complaints as well as decreased pain coping ability. We have used a variety of sleep medications in this population including zolpidem, trazodone, melatonin, and amitryptiline. None of these drugs have been well studied in this population.
For patients with sedation secondary to opioids, a stimulant such as methylphenidate in low dose may be appropriate. It is important to place patients on opioids on a daily bowel regimen. Treatment of mood disorders such as anxiety and depression are also essential in the overall care of the patient with a recurrent or chronic pain disorder.
Topical preparations may also be helpful in the treatment of pain in sickle cell disease. For those with chronic pain in areas such as the lower back, topical lidocaine patches can be used. Our patients report relief from capsacin patches applied during VOE. Further evaluation of these modalities as well as topical NSAIDs is indicated.
Behavioral Treatment
There is ample evidence for behavioral treatments for pain associated with sickle cell disease.50–52 Many patients with pain suffer from comorbid mood disorders. Patients with sickle cell disease can also benefit from help with coping and relaxation.
Physical Treatments
For patients with recurrent and chronic pain, physical therapy to develop better functional ability and speed functional recovery from painful episodes is helpful. Modalities such as graded exercise, heat, and ultrasound can all be used to good effect. Many of our patients report relief using transcutaneous electrical nerve stimulation (TENS) for both acute VOE and chronic pain. TENS allows the patient to have some control of their pain therapy and can be used in a variety of settings including the classroom or the workplace.
Complementary Therapies
There is limited data to support the use of complementary therapies in sickle cell disease although they are clearly used by those who suffer from sickle cell pain.53,54 Anecdotally, our patients have benefited from a range of treatments such as acupuncture and massage. The limited availability and expense of these modalities is problematic. Further research on the efficacy of these techniques is warranted.
Approach to Care in The ED
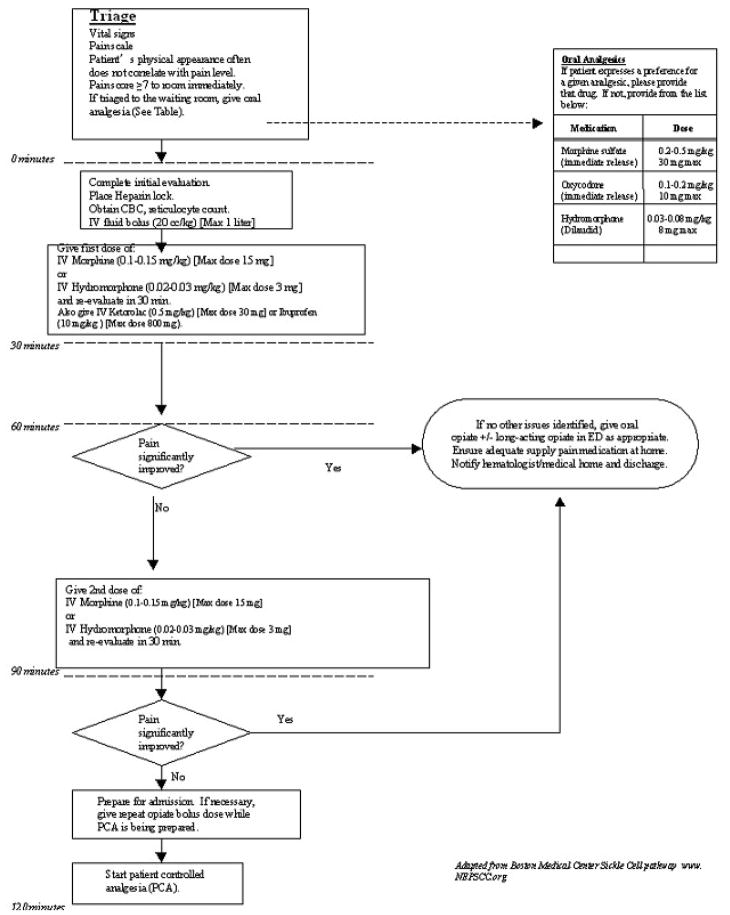
When a child with sickle cell disease presents to the ED with pain, they should be rapidly assessed. (Figure 2) Those with a pain score of 7 or above are in severe pain and should be immediately triaged into the ED for further assessment and analgesic administration. Analgesia should not be delayed. While analgesia is being administered, a full history and physical exam as well as laboratory evaluation should proceed.
Figure 2.
Management of uncomplicated vasoocclusive pain.
History should focus on precipitating factors for pain, its location, intensity, and how it compares to usual episodes. Changes in the usual characteristics of pain should trigger the clinician to look for other causes of pain other than straightforward VOE. The presence of fever, cough, chest pain, or shortness of breath should also be elicited as this may indicate the presence of an infection including pneumonia or acute chest syndrome.
Physical exam should focus on the areas of pain, but a careful examination for signs of infection should occur. Attention should be paid to evaluate for signs of pneumonia, osteomyelitis or sepsis. The examination should also evaluate for splenomegaly which may indicate splenic sequestration. Males should be evaluated for signs of priapism, as they may be reluctant to report this symptom.
Laboratory evaluation will typically include a complete blood count with a reticulocyte count. Other laboratory studies should be considered if fever or other findings are present which would indicate the need for an expanded evaluation.
Aggressive pain management is the key to the appropriate care of vasooclusive pain. Ideally, information regarding the patient’s acute pain plan would be available to the ED physician either in the form of a pain passport held by the patient, a hospital database or direct communication with the hematology staff. The patient and their family should be considered as a partner in care. Their knowledge of appropriate dosing and medication should not be disregarded and is not evidence of drug seeking or addiction.
Starting doses of opioids for many patients will be higher than that in a patient who is opioid naïve. Starting doses of intravenous morphine in the 10–15 milligram range are not unusual. Following the first IV opioid dose, reassessment should take place in 30 minutes. If the patient’s pain remains severe, a second dose of opioid should be given. Therapy for vasoocclusive pain in the ED with our current understanding of the literature should also include either an IV or an oral dose of an NSAID. A bolus of isotonic fluid at 20 cc/kg (maximum 1 liter) should also be given over an hour. Patients who fail to improve after 2 doses of opioids will likely need hospitalization. Ideally PCA should be initiated at this point but if this is unavailable or delayed, another dose of opioids should be given.
For those patients who do show improvement, discharge preparations should be made. These patients should receive oral opioids. Where appropriate, a long-acting opioid should be given (with a short-acting opioid available for breakthrough pain). Again the availability of an individualized pain plan would assist in these decisions. Early follow-up with the hematologist or primary care giver should be arranged as this has been shown to reduce return visits to the ED.
Improving Care and Reducing Barriers
Improvement in the ED care of patients with sickle cell disease should start with the development and implementation of a clinical practice guideline or pathway. These should be developed in conjunction with the institution’s hematologists and should include: strategies for avoiding ED visitation and decreasing hospitalization, ensuring ED follow-up in a primary care or hematology office setting, as well as recommending appropriate pain assessment. Rapid triage, timely and adequate opioid dosing, and utilization of PCA should all be components of a pathway. These guidelines can impact a variety of benchmarks including time to analgesia, return visits, totals ED visits, and the use of PCA in the ED for patients with sickle cell disease. 55,56
In some centers where the volume of patients permits, day hospital programs for those with vasoocclusive pain allows for patients to be treated by providers who know them well. As mentioned previously, individualized acute pain plans are helpful in improving patient care as well. A pain passport would allow the patient’s pain plan to be given to the provider in the acute setting and would validate the patient’s requests. Mandating the use of pain protocols and clinical guidelines, may be needed to help reduce the bias that has typified the care of this patient group.
Bidirectional communication between ED providers and the sickle cell community should be encouraged. This can be achieved by reaching out to local community based organizations or to hospital-based family advisory boards. Specific champions within the ED with knowledge and interest in sickle cell disease can act as a resource for both providers and patients.
Summary
The care of pain in sickle cell disease is challenging and historically has been under-recognized and undertreated. Rapid assessment and treatment of children and adolescents presenting to the ED with VOE using a carefully crafted pathway or guideline is essential for improving the care of this patient group and reducing the bias and distrust that has characterized their care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ballas SK. Pain management of sickle cell disease. Hematol Oncol Clin North Am. 2005;19:785–802. doi: 10.1016/j.hoc.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325:11–6. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf HR, Atrah HK, Grosse SD, et al. Emergency department visits made by patients with sickle cell disease: A descriptive study, 1999–2007. Am J Prev Med. 2010;38:S536–41. doi: 10.1016/j.amepre.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, Shah A, Watson M, Mankad V. Comparison of costs to the health sector of comprehensive and episodic health care for sickle cell disease patients. Pub Health Rep. 1997;110:80–6. [PMC free article] [PubMed] [Google Scholar]
- 5.Ballas SK. Sickle cell pain. Seattle, WA: IASP Press; 1998. [Google Scholar]
- 6.Schechter NL. The management of pain in sickle cell disease. In: McGrath PJ, Finley GA, editors. Chronic and recurrent pain in children and adolescents. Seattle, WA: IASP Press; 1999. pp. 99–114. [Google Scholar]
- 7.Dampier CD, Shapiro BS. Management of pain in sickle cell disease. In: Schechter NL, Berde CB, Yaster M, editors. Pain in infants, children, and adolescents. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. pp. 489–516. [Google Scholar]
- 8.Conner-Warren RL. Pain intensity and home pain management of children with sickle cell disease. Issues Compr Pediatr Nurs. 1996;19:183–95. doi: 10.3109/01460869609026860. [DOI] [PubMed] [Google Scholar]
- 9.Walco GA, Dampier CD. Pain in children and adolescents with sickle cell disease: a descriptive study. J Pediatr Psychol. 1990;15:643–58. doi: 10.1093/jpepsy/15.5.643. [DOI] [PubMed] [Google Scholar]
- 10.Varni JW, Walco CA. Chronic and recurrent pain associated with pediatric chronic diseases. Issues Compr Pediatr Nurs. 1988;11:73–86. doi: 10.3109/01460868809038010. [DOI] [PubMed] [Google Scholar]
- 11.Smith LA, Oyeku SO, Homer C, Zuckerman B. Sickle cell disease: a question of equity and quality. Pediatrics. 2006;117:1763–70. doi: 10.1542/peds.2005-1611. [DOI] [PubMed] [Google Scholar]
- 12.Todd KH, Green C, Bonham VL, et al. Sickle cell disease related pain: crisis and conflict. J Pain. 2006;7:453–8. doi: 10.1016/j.jpain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilizations and rehospitalizations for sickle cell disease. JAMA. 2010;303:1288–94. doi: 10.1001/jama.2010.378. [DOI] [PubMed] [Google Scholar]
- 14.Boulet SL, Yanni EA, Creary MS, Olney RS. Health status and healthcare use in a national sample of children with sickle cell disease. Am J Prev Med. 2010;38:S528–35. doi: 10.1016/j.amepre.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Eton WA, Hofrichter J. Sickle cell hemoglobin polymerization. Adv Protein Chem. 1990;40:63–279. doi: 10.1016/s0065-3233(08)60287-9. [DOI] [PubMed] [Google Scholar]
- 16.Embury SH. The not so simple process of sickle cell vasoocclusion. Microcirculation. 2004;11:101–13. doi: 10.1080/10739680490278277. [DOI] [PubMed] [Google Scholar]
- 17.Vekilov PG. Sickle-Cell haemoglobin polymerization: is it the primary pathogenic event of sickle cell aneamia? Br J Haemotol. 2007;139:173–84. doi: 10.1111/j.1365-2141.2007.06794.x. [DOI] [PubMed] [Google Scholar]
- 18.Beyer JE, Simmons LE, Woods GM, Woods PM. A chronology of pain and comfort in children with sickle cell disease. Arch Pediatr Adolesc Med. 1999;153:913–20. doi: 10.1001/archpedi.153.9.913. [DOI] [PubMed] [Google Scholar]
- 19.Brandow AM, Brousseau D, Pajewski NM, Panepinto JA. Vaso-occlusive events in sickle cell disease: impact on child well-being. Pediatr Blood Cancer. 2009;54:92–7. doi: 10.1002/pbc.22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zempsky WT, Corsi JM, Loiselle KA, et al. Functional assessment for children hospitalized for sickle cell pain: a new paradigm. Blood. 2009;114:2561. [Google Scholar]
- 21.Pack-Mabien A, Labbé E, Herbert D, Haynes J. Nurses’ attitudes and practices in sickle cell pain management. Appl Nurs Res. 2001;14:187–92. doi: 10.1053/apnr.2001.26783. [DOI] [PubMed] [Google Scholar]
- 22.Labbé E. Physicians’ attitude and practices in sickle cell disease pain management. J Palliat Care. 2005;21:246–51. [PubMed] [Google Scholar]
- 23.Shapiro BS, Benjamin LJ, Payne R, Heidrich G. Sickle cell-related pain: perceptions of medical practitioners. J Pain Symptom Manage. 1997;14:168–74. doi: 10.1016/S0885-3924(97)00019-5. [DOI] [PubMed] [Google Scholar]
- 24.Alleyne J, Thomas VJ. The management of sickle cell crisis pain as experienced by patients and their carers. J Adv Nurs. 1993;19:725–32. doi: 10.1111/j.1365-2648.1994.tb01144.x. [DOI] [PubMed] [Google Scholar]
- 25.Harris A, Parker N, Barker C. Adults with sickle cell disease: psychological impact and experience of hospital services. Psychol Health Med. 1998;3:171–9. [Google Scholar]
- 26.Maxwell K, Streetly A, Bevan D. Experiences of hospital care and treatment seeking for pain from sickle cell disease: qualitative study. BMJ. 1999;318:1585–90. doi: 10.1136/bmj.318.7198.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGrath PJ, Turk DC, Dworkin, et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. Pain. 2008;9(9):771–83. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Finley GA, McGrath PJ, editors. Measurement of pain in infants and children. Seattle, WA: IASP Press; 1998. [Google Scholar]
- 29.Platt A, Eckman JR, Beasley J, Miller G. Treating sickle cell pain: an update from the Georgia Comprehensive Sickle Cell Center. J Emerg Nurs. 2002;28:297–303. doi: 10.1067/men.2002.125268. [DOI] [PubMed] [Google Scholar]
- 30.Rees DC, Olujohungbe AD, Parker NE. Guidelines for the management of the acute painful crisis in sickle cell disease. Br J Haematol. 2003;120:744–52. doi: 10.1046/j.1365-2141.2003.04193.x. [DOI] [PubMed] [Google Scholar]
- 31.Benjamin LJ, Dampier CD, Jacox AK, et al. Guideline for the management of acute and chronic pain in sickle cell disease. Glenview, IL: American Pain Society; 1999. [Google Scholar]
- 32.Johnston CC. Psychometric issues in the measurement of pain. In: Finley GA, McGrath PJ, editors. Measurement of pain in infants and children. Seattle, WA: IASP Press; 1998. pp. 5–20. [Google Scholar]
- 33.Stinson JN, Kavanagh T, Yamada J, et al. Systematic review of the psychometric properties interpretability and feasibility of self-report pain intensity measures for use in clinical trials in children and adolescents. Pain. 2006;125:143–57. doi: 10.1016/j.pain.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 34.von Baeyer CL, Spagrud LJ, McCormick JC, et al. Three new datasets supporting use of the numerical rating scale (NRS-11) for children’s self-reports of pain intensity. Pain. 2009;143:223–7. doi: 10.1016/j.pain.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartate acid receptor activation: Implication for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–9. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 36.Gil KM, Thompson RJ, Keith BR, et al. Sickle cell disease pain in children and adolescents: change in pain frequency and coping strategies over time. J Pediatr Psychol. 1993;18:621–37. doi: 10.1093/jpepsy/18.5.621. [DOI] [PubMed] [Google Scholar]
- 37.McCrae JD, Lumley MA. Health status in sickle cell disease: examining the roles of pain coping strategies, somatic awareness, and negative affectivity. J Behav Med. 1998;21:35–55. doi: 10.1023/a:1018763404868. [DOI] [PubMed] [Google Scholar]
- 38.Varni JW, Rapoff MA, Waldron SA, et al. Chronic pain and emotional distress in children and adolescents. J Dev Behav Pediatr. 1996;17:154–61. [PubMed] [Google Scholar]
- 39.Varni JW, Rapoff MA, Waldron SA, et al. Effects of perceived stress on pediatric chronic pain. J Behav Med. 1996;19:515–28. doi: 10.1007/BF01904901. [DOI] [PubMed] [Google Scholar]
- 40.Palermo TM. Impact of recurrent and chronic pain on child and family daily functioning: a critical review of the literature. J Dev Behav Pediatr. 2000;21:58–69. doi: 10.1097/00004703-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Jacob E, Miaskowski C, Savedra M, et al. Management of vaso-occlusive pain in children with sickle cell disease. J Pediatr Hematol Oncol. 2003;25:307–11. doi: 10.1097/00043426-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Zempsky WT, Loiselle KA, McKay K, et al. Retrospective evaluation of pain assessment and treatment for vasoocclusive episodes in children with sickle cell disease. Pediatr Blood Cancer. 2008;51(2):265–8. doi: 10.1002/pbc.21572. [DOI] [PubMed] [Google Scholar]
- 43.Jacob E, Miaskowski C, Savedra M, et al. Changes in intensity, location, and quality of vaso-occlusive pain in children with sickle cell disease. Pain. 2003;102:187–93. doi: 10.1016/s0304-3959(02)00374-3. [DOI] [PubMed] [Google Scholar]
- 44.Bartolucci P, El Murr E, Roudot-Thoroval F, et al. A randomized, controlled clinical trial of ketoprofen for sickle-cell disease vaso-occlusive crises in adults. Blood. 2009;114(18):3742–7. doi: 10.1182/blood-2009-06-227330. [DOI] [PubMed] [Google Scholar]
- 45.Hardwick WE, Givens TG, Monroe KW, et al. Effect of ketorolac in pediatric sickle cell vaso-oclusive pain crisis. Pediatr Emerg Care. 1999;15:179–82. doi: 10.1097/00006565-199906000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Bieter JL, Simon HK. Intravenous ketorolac in the emergency department management of sickle cell pain and predictors of its effectiveness. Arch Pedatri Adol Med. 2001;155(4):496–500. doi: 10.1001/archpedi.155.4.496. [DOI] [PubMed] [Google Scholar]
- 47.Darbari DS, Neely M, VandenAnker J, Rana SR. Morphine pharmacokinetics in sickle cell disease: implications for pain management. Blood. 2009;114:2574. [Google Scholar]
- 48.Zempsky WT. Treatment of sickle cell pain: fostering trust and justice. JAMA. 2009;302:2479–80. doi: 10.1001/jama.2009.1811. [DOI] [PubMed] [Google Scholar]
- 49.Elander J, Lusher J, Bevan D, et al. Understanding the causes of problematic pain management in sickle cell disease: Evidence that psuedo-addiction plays a more important role than genuine analgesic dependence. J Pain Symptom Manage. 2004;27:156–69. doi: 10.1016/j.jpainsymman.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 50.Thomas V. Cognitive behavioral therapy in pain management for sickle cell disease. Int J Palliat Nurs. 2000;6:434–42. doi: 10.12968/ijpn.2000.6.9.9055. [DOI] [PubMed] [Google Scholar]
- 51.Broome ME, Maikler V, Kelber S, et al. An intervention to increase coping and reduce health care utilization for school-age children and adolescents with sickle cell disease. J Natl Black Nurses Assoc. 2001;12:6–14. [PubMed] [Google Scholar]
- 52.Chen E, Cole SW, Kato PM. A review of empirically supported psychosocial interventions for pain and adherence outcomes in sickle cell disease. J Pediatr Psychol. 2004;29:197–209. doi: 10.1093/jpepsy/jsh021. [DOI] [PubMed] [Google Scholar]
- 53.Lemanek KL, Ranalli M, Lukens C. A randomized controlled trial of massage therapy in children with sickle cell disease. J Pediatr Psychol. 2009;34:1091–6. doi: 10.1093/jpepsy/jsp015. [DOI] [PubMed] [Google Scholar]
- 54.Sebigna EM, Shindell DL, Casella JF, et al. Pediatric patients with sickle cell disease: use of complementary and alternative therapies. J Altern Complement Med. 2006;12:291–8. doi: 10.1089/acm.2006.12.291. [DOI] [PubMed] [Google Scholar]
- 55.Morrisey LK, Shea JO, Kalish LA, et al. Clinical practice guidelines improve the treatment of sickle cell vasoocclusive pain. Pediatr Blood Cancer. 2009;52:369–72. doi: 10.1002/pbc.21847. [DOI] [PubMed] [Google Scholar]
- 56.Givens M, Rutherford C, Joshi G, Delaney K. Impact of an emergency department pain management protocol on the pattern of visits by patients with sickle cell disease. J Emerg Med. 2007;32:239–43. doi: 10.1016/j.jemermed.2006.07.022. [DOI] [PubMed] [Google Scholar]