Abstract
Expression rates of long (L) and short (S) alleles of the serotonin (5-HT) transporter (5-HTT) gene have been shown to differ under various circumstances. We compared 5-HTT uptake (function) level and paroxetine binding (density) in platelets of alcoholics as indices of 5-HTT expression rate among LL, LS, and SS genotypes. Concentration curves of [3H]5-HT and [3H]paroxetine were used to quantify the equilibrium constant (Km) and maximum 5-HT uptake rate (Vmax) for 5-HTT uptake into intact platelets and the dissociation constant (Kd) and maximum specific binding density (Bmax) for paroxetine binding to platelet membranes, respectively. Genotypes were determined using electrophoresis with fluorescent markers. Vmax for 5-HTT uptake did not correlate with Bmax for paroxetine binding (r=−0.095, P=0.415). Means of Vmax and Bmax did not differ in a statistically significant manner among LL, LS, and SS genotypes in these alcoholic subjects. However, Vmax for LL and SS appeared to have a bimodal distribution, so the percentage of subjects with Vmax <200 fmol/min-107 platelets was statistically significantly higher in LL than in SS (51.5% vs. 22.7%, respectively), with an odds ratio of 3.6 (P<0.05). The percentage of Vmax <200 fmol/min-107 platelets for LS was 39.3% (not significant vs. LL or SS). Previous studies of healthy human controls have shown that 5-HTT density in raphe nuclei and 5-HTT uptake in platelets are higher in the LL genotype than in S carriers. Our findings in currently drinking alcoholics support the hypothesis that those with the LL genotype of the 5′-HTTLPR region of the 5-HTT gene have reduced 5-HTT function.
Keywords: Alcohol, Alcoholism, Binding, Genotype, Humans, Serotonin, Transporter, Uptake
1. Introduction
Alcoholism is a multifactorial disorder with genetic, environmental, and gene-by-environment interaction components (Enoch et al., 2003). Although twin and adoption studies suggest a substantial genetic component in alcoholism, efforts to identify specific genes that contribute to the risk of the disorder have had limited success (Kranzler et al., 2002).
The serotonergic neurotransmitter system is widely accepted as playing an important role in the pathogenesis and maintenance of alcoholism. It has been proposed that behavioral disinhibition, enhancement of anxiety, and altered alcohol response in alcoholics might be mediated in part by the serotonergic neurotransmitter system (Heinz et al., 2001). For example, alterations in synaptic serotonin (5-HT) concentration have been suggested as the underlying cause for certain behavioral changes (Grove et al., 1997; Meltzer et al., 1994; Owens and Nemeroff, 1994). 5-HT concentration in the synapse is controlled by the presynaptic 5-HT transporter (5-HTT), and, therefore, the gene encoding the 5-HTT protein (genetic locus SLC6A4 on chromosome 17q11.1–q12) is viewed as a candidate gene for alcohol dependence (Kranzler et al., 2002). The 5′-regulatory promoter region of SLC6A4 contains the only known functional polymorphism in the serotonergic system (5-HTT-linked polymorphic region (5′-HTTLPR)) (Heils et al., 1996, 1997). The polymorphism consists of two forms: a 44-base pair insertion/long (L) variant and a deletion/short (S) variant. This gene is responsible for encoding 5-HTT in all tissues where it is expressed (Esterling et al., 1998; Ramamoorthy et al., 1993). The 5-HTT has the same amino acid sequence and pharmacological sensitivity in the brain and platelets of humans (Da Prada et al., 1988; Lesch et al., 1993; Villinger et al., 1994). These findings support the use of platelets in human clinical studies as a reflection of central 5-HTT expression and function.
The L and S alleles of the 5-HTT gene have been shown to alter transcription and function of the transporter. In studies of human lymphoblast cell lines in vitro, messenger ribonucleic acid (RNA) levels and 5-HTT uptake were lower by approximately twofold in cells transected with the SS or LS alleles vs. those with LL, probably due to underexpression of 5-HTT (Lesch et al., 1996). The S variant appeared to have a dominant effect as the difference between the S homozygotes and heterozygotes was not significant. In a study of healthy humans in which [123I]2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane ([123I]β-CIT) single-photon emission computerized tomography (SPECT) imaging was employed, a significantly lower density of raphe 5-HTT protein in S carriers compared with LL homozygotes was found (Heinz et al., 2000). This finding was similar to the in vitro findings in lymphoblast cell lines. Also, Little et al. (1998) reported that messenger RNA levels and expression of 5-HTT measured by transporter binding is lower in postmortem brains of S carriers compared with LL homozygotes. Finally, using human platelets as a peripheral model for central 5-HTTs, Greenberg et al. (1999) showed that 5-HT uptake was significantly higher in LL homozygotes compared with S carriers among a group of healthy individuals, but no significant difference was observed in transporter densities as measured by paroxetine binding. Taken together, these studies suggest that the L and S alleles of the 5-HTT gene regulate the expression of the 5-HTT protein and appear to account for functional differences.
The differences in biochemical data between the L and S alleles of the 5-HTT gene may parallel behavioral data according to the results of a number of studies. The S variant is associated with heightened trait anxiety/dysphoria, exaggerated response to fear, and increased risk of depression following adverse life events and increased risk of suicide attempts (Heinz et al., 2001; Holmes et al., 2003; Lesch et al., 1996; Owens and Nemeroff, 1994; Preuss et al., 2001). 5-HTT expression and function in alcoholics suggest a potential variable susceptibility of 5-HTT to the neurotoxic effects of excessive alcohol consumption in the two allele groups. Among alcoholics, the density of raphe 5-HTT with SPECT imaging of [123I]β-CIT was significantly lower in LL homozygous individuals compared with healthy controls and was negatively correlated with their amounts of alcohol consumption, while there was no significant difference in alcoholic and healthy S carriers (Heinz et al., 2000). However, an earlier study with human postmortem brains (Little et al., 1998) revealed an increased density of S carriers in the raphe nucleus of alcoholics compared with a matched group of non-alcohol users, while values were similar in alcohol users and comparison subjects with the L allele.
Although higher platelet 5-HT uptake in alcoholics (Daoust et al., 1991; Ernouf et al., 1993) has been demonstrated in several studies, to our knowledge the impact of the 5′-HTTLPR polymorphism on 5-HTT function and expression in platelets (peripheral 5-HTT) has not been studied to date. To further investigate the biological changes occurring in alcoholism that result from a polymorphism of the 5′-HTTLPR region of the 5-HT gene, we compared 5-HT uptake and 5-HTT density in platelets of alcoholics of these three genotypes.
2. Methods
2.1. Subjects
Subjects were 115 men and women diagnosed with alcohol dependence according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (American Psychiatric Association, 1994). Other inclusion criteria were: (1) any axis II DSM-IV disorder; (2) score of ≥8 on the alcohol use disorders identification test, which assesses the personal and social harm after alcohol consumption; (3) reported drinking of ≥21 standard drinks/ week for women and ≥30 standard drinks/week for men during the 90 days before enrollment, and (4) negative urine toxicological screen for narcotics, amphetamines, or sedative hypnotics at enrollment. One standard drink was defined as 0.35 l of beer, 0.15 l of wine, or 0.04 l of 80-proof liquor. Although abstinence at study entry was not an enrollment criterion, participants were instructed to attempt drinking cessation and to participate in the medication compliance treatment. Exclusion criteria were: (1) current axis I psychiatric diagnosis other than alcohol or nicotine dependence; (2) significant alcohol withdrawal symptoms (clinical institute withdrawal assessment for alcohol-revised score >15); (3) clinically significant physical abnormalities based on physical examination, electrocardiogram recording, hematological assessment, biochemistry including serum bilirubin concentration, and urinalysis; (4) pregnant or lactating state; and (5) treatment for alcohol dependence 30 days or less prior to enrollment. The ages of the subjects ranged from 18 years to 66 years.
Ethics approval was provided by the institutional review board at The University of Texas Health Science Center at San Antonio. Participants were recruited between March 14, 2001 and November 21, 2003, by newspaper or radio advertisements. Written informed consent was obtained from all participants.
2.2. Collection of blood samples for biological testing
Ten milliliters of blood was drawn from each subject at baseline to obtain platelets for the measurement of 5-HT uptake into intact platelets and paroxetine binding to platelet membranes (performed by C.S. in San Antonio). Also, a 10-ml sample of blood was drawn for the determination of 5-HTT genotype (performed by S.E.B. in Austin).
2.3. Genotyping
Ten milliliters of blood was drawn from each subject. White blood cells were separated from plasma and resuspended, and deoxyribonucleic acid (DNA) was isolated using PUREGENE, Gentra Systems, according to the manufacturer’s protocol. The 5′-HTTLPR 44 bp promoter region repeat polymorphism was polymerase chain reaction (PCR) amplified from ~50 ng of DNA using two primers: 5′-CGT TGC CGC TCT GAA TGC CAG-3′ and 5′-GGA TTC TGG TGC CAC CTA GAC GCC-3′ in a 25-μl final volume consisting of 0.5 U of Tfl DNA polymerase (Epicentre), 1× PCR buffer, 1.5 mM MgCl2, 200 μM dNTPs, 1× enhancer, and 0.6 μM of each primer. The PCR conditions were as follows: 94 °C for 2 min (45 cycles of 94 °C for 30 s; 70 °C for 30 s, and 72 °C for 30 s); a final extension of 72 °C for 7 min and terminal hold at 4 °C. Separation by gel electrophoresis using 4% MetaPhor agarose (Cambrex, Rockland, ME) allows visualization by ethidium bromide/UV detection of the two variants (L and S: fragment sizes=464 and 420 bp) of the promoter region of the SCL4A gene (−1415 to −951) (Heils et al., 1996).
2.4. Platelet suspension and platelet membrane preparation
Blood was drawn into 60-ml polypropylene syringes containing 10 ml of acid–citrate–dextrose buffer. The blood was then centrifuged at 150×g at 22 °C for 20 min in a Beckman TJ-6 centrifuge to obtain platelet-rich plasma (PRP). Platelet count in PRP was determined with a Coulter counter model S-plus VI and adjusted to 3×108 platelets/ml with the addition of platelet buffer (137 mM KCl, 1 mM MgCl2, 5.5 mM glucose, 5 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; pH 7.4) to prepare adjusted PRP for the 5-HT uptake experiments only. Three milliliters of adjusted PRP was used for platelet 5-HT uptake experiments, which were performed on the day of the blood draw. To prepare platelet membranes for paroxetine binding experiments, the remainder of the PRP was used. One microliter of prostaglandin I2 solution (300 ng/ml) per milliliter of PRP was added to prevent loss of platelets during centrifugation, then the sample was centrifuged at 550×g. The resulting platelet pellet was resuspended in platelet buffer and then centrifuged at 35,000×g. The platelet membrane pellet was resuspended in 1 ml of platelet buffer and then stored at −80 °C until the day of the assay to measure paroxetine binding.
2.5. Platelet 5-HT uptake
Platelet 5-HT uptake experiments were performed in 110 of 115 alcoholic subjects. The adjusted PRP suspension was used to determine platelet 5-HT uptake. Assay tubes were prepared in duplicate and contained 3[H]5-HT at six different concentrations (62.5–2000 nM) and 100 μM pargyline with or without 50 μM fluoxetine. These tubes were incubated at 37 °C for 5 min; then the reaction was started by the addition of 100 μl of adjusted PRP that contained 107 platelets. The assay tubes were incubated at 37 °C for an additional 5 min; then the reaction was quenched by rapid filtering through Whatman GF/B filters using a Brandel Cell Harvester. The filters were washed three times with 5 ml of ice-cold wash buffer (50 mM Tris–HCl, 150 mM NaCl, and 20 mM ethylene diamine tetra-acetic acid (EDTA)). Filters were placed in scintillation vials containing 5 ml of Beckman Ready Protein+ scintillation counting fluid and immediately counted. Specific uptake was calculated by subtracting the uptake occurring in tubes containing fluoxetine from that occurring in tubes without fluoxetine. Maximum 5-HT uptake rate (Vmax) in platelets was expressed as fmol 5-HT/min-107 platelets, and the equilibrium constant (Km) as nM. Km and Vmax were calculated using the one-site hyperbolic function in Prism 4 software by Graph Pad.
2.6. Paroxetine binding
Paroxetine binding experiments were performed in 80 of 115 alcoholic subjects. Platelet membranes were used to determine platelet paroxetine binding. Assay tubes were prepared in duplicate and contained incubation buffer (50 mM Tris–HCl, 5 mM KCl, and 120 mM NaCl) and 3[H]paroxetine at six different concentrations (0–2 nM) with and without 150 μM fluoxetine. The actual concentration of paroxetine in each tube was determined using a 40-μl aliquot taken from each tube prior to the addition of platelet membranes. The experiment was started by the addition of 80 μg of platelet membrane protein; then the assay tubes were incubated for 1 h at 21 °C. The reaction was quenched by the addition of ice-cold wash buffer (50 mM Tris HCl, 150 mM NaCl, and 20 mM EDTA) and rapid filtering through Whatman GF/B filters treated with 0.3% polyethylenimine using a Brandel Cell Harvester. Filters were washed three times with ice-cold wash buffer, dried overnight, placed in scintillation vials containing 5 ml of Beckman Ready Protein+ scintillation counting fluid, and counted in a Beckman LS-6500 liquid scintillation counter. The counts per minute from the 40-μl aliquots were converted into nM of paroxetine to obtain the actual concentrations in each tube. Total and nonspecific binding of paroxetine was plotted against each actual concentration. Specific binding was calculated by subtracting nonspecific binding from total binding. Paroxetine binding was expressed as fmol/mg of platelet membrane protein, and the dissociation constant (Kd) as nM. Protein concentrations were measured using a SPECTRAmax PLUS Micro plate Spectrophotometer.
2.7. Statistical analysis
Platelet 5-HT uptake (Km; Vmax) and paroxetine binding (Kd; maximum specific paroxetine binding density (Bmax)) parameters were calculated using Prism 4 software by GraphPad. The differences in Vmax, Bmax, Km, and Kd among the three genotypes were analyzed by analysis of variance (ANOVA). The distribution of 5-HT uptake data was normalized by log transformations of Vmax values and examined by pair-wise comparisons between genotypes (LL and SS, LL and LS, and LS and SS). Odds ratios were used to test for differences between categorical variables.
3. Results
Among 115 alcohol-dependent subjects, 34 (29.6%) were homozygous for the L allele (LL), 23 (20%) were homozygous for the S allele (SS), and 58 (50.4%) were heterozygous (LS). Frequency was 0.45 for the S allele and 0.55 for the L allele. This distribution was similar to that observed by Lesch et al. (1996) (LL—33%, SS—18%, and LS—49%) and Preuss et al. (2000) (LL—28%, SS—14%, and LS—58%). Our data conformed to the Hardy–Weinberg equilibrium. For analyses of data from 115 alcoholics, there were 110 subjects for 5-HT uptake experiments, 80 subjects for paroxetine binding experiments, and 76 subjects with paired data for both experiments.
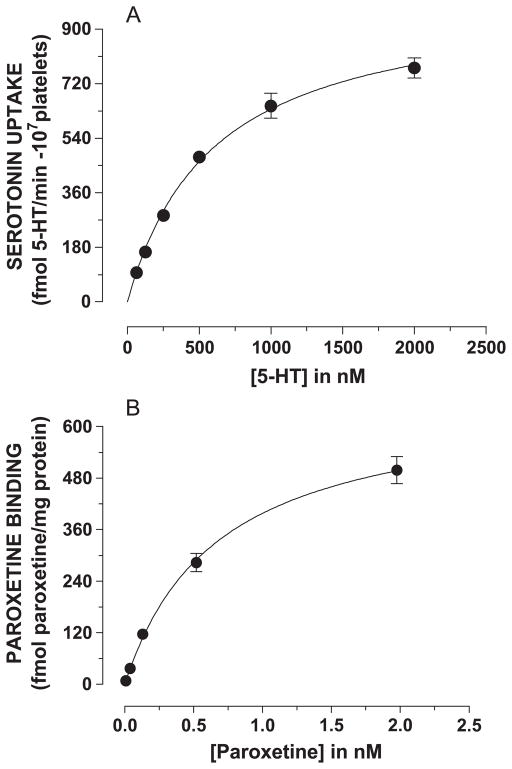
Km and Vmax values for 5-HT uptake in intact platelets and Kd and Bmax values for paroxetine binding in platelet membranes were determined using full concentration curves for 5-HT (62.5–2000 nM) and paroxetine (0.0078–2.0 nM), respectively. Specific 5-HT uptake into intact platelets and specific paroxetine binding to platelet membranes, both defined by saturating concentrations of fluoxetine, conformed to the model of a single-site hyperbolic function (Fig. 1A and B).
Fig. 1.
Specific concentration curves for 5-HT uptake and paroxetine binding to platelet membranes. (A) 5-HT uptake was performed as described in Methods in the absence and presence of 50 μM fluoxetine. Specific 5-HT uptake was determined by subtracting nonspecific uptake from total uptake. (B) [3H]paroxetine binding was performed as described in Methods in the absence and presence of fluoxetine. Specific [3H]paroxetine binding was determined by subtracting nonspecific binding from total binding. Km and Vmax values for 5-HT uptake and Kd and Bmax values for paroxetine binding were calculated using the one-site binding equation in Prism 4 software.
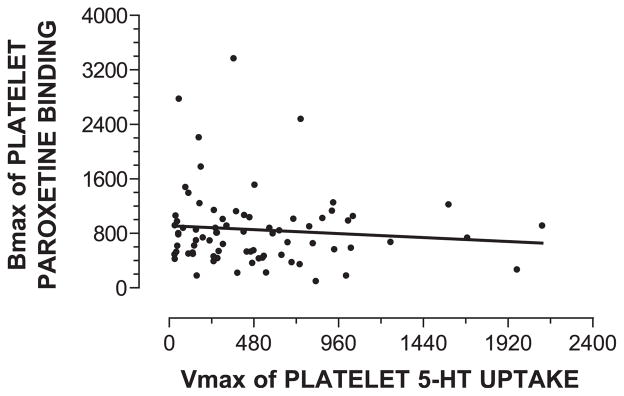
The Vmax for 5-HT uptake did not correlate with the Bmax for paroxetine binding (Pearson correlation coefficient=−0.095, P=0.415) when paired data for 79 alcoholic subjects regardless of genotype were analyzed (Fig. 2). Additionally, statistically significant correlations were not found when Vmax and Bmax were compared within the LL, LS, or SS genotype or between early- and late-onset alcoholics (data not shown).
Fig. 2.
Lack of correlation between Vmax for 5-HT uptake into intact platelets and Bmax for paroxetine binding in platelet membranes. Vmax for 5-HT uptake into intact platelets was expressed as fmol 5-HT/min-107 platelets, and Bmax for paroxetine binding to platelet membranes was expressed as fmol paroxetine/mg protein. The Spearman correlation coefficient was −0.095 (P=0.415, not significant; N=79).
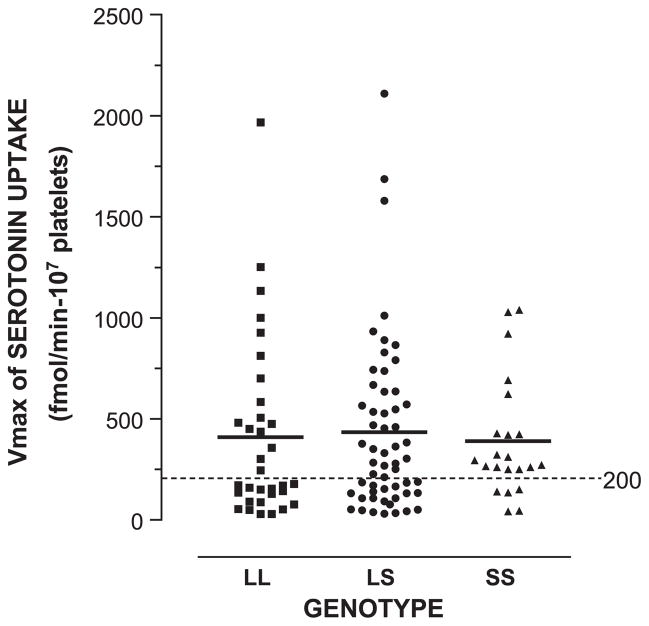
5-HT uptake experiments were performed using intact platelets of 110 of the 115 alcoholic subjects. Vmax values for 5-HT uptake were plotted according to genotype (Fig. 3). Means of Vmax values did not differ in a statistically significant manner among the LL, LS, and SS genotypes (Table 1; ANOVA). Also, pair-wise comparisons of Vmax values between the LL and LS, LS and SS, or LL and SS genotypes were not significant. Nevertheless, we noticed a bimodal distribution of subjects within the LL and LS genotypes above and below the level of 200 fmol/min-107 platelets. That is, the percentage of subjects with Vmax values below 200 fmol/min-107 platelets was statistically significantly higher in the LL genotype than in the SS genotype (51.5% vs. 22.7%, respectively), with an odds ratio of 3.6 (P<0.05). The percentage of Vmax values below 200 fmol/min-107 platelets for LS subjects was 39.3%. The differences between LL and LS and between LS and SS were not significant (LL vs. LS, P=0.3; LS vs. SS, P=0.2).
Fig. 3.
Vmax values for 5-HT uptake among genotypes for the 5-HTT. Genotype and Vmax values for 5-HT uptake were determined as described in Methods. Vmax was expressed as fmol 5-HT/min-107 platelets. Mean±S.E.M. of Vmax values for 5-HT uptake for LL, LS, and SS subjects were 409±76 (N=33), 434±57 (N=56), and 390±62 (N=22), respectively. The percentages of Vmax values below 200 fmol/min-107 platelets among the LL, LS, and SS genotypes were 51.5%, 39.3%, and 22.7%, respectively (LL vs. SS, P<0.05).
Table 1.
5-HT uptake and [3H]paroxetine binding in different genotypes
| Genotype | Mean Vmax (S.E.M.)a (fmol/min-107 platelets) | Mean Bmax (S.E.M.)a (fmol/mg protein) |
|---|---|---|
| LL | 409 (76.6) | 946 (127) |
| LS | 434 (56.9) | 826 (94.2) |
| SS | 390 (62.5) | 779 (70.9) |
ANOVA; no statistical significance among genotypes for Vmax (P=0.47) or Bmax (P=0.62).
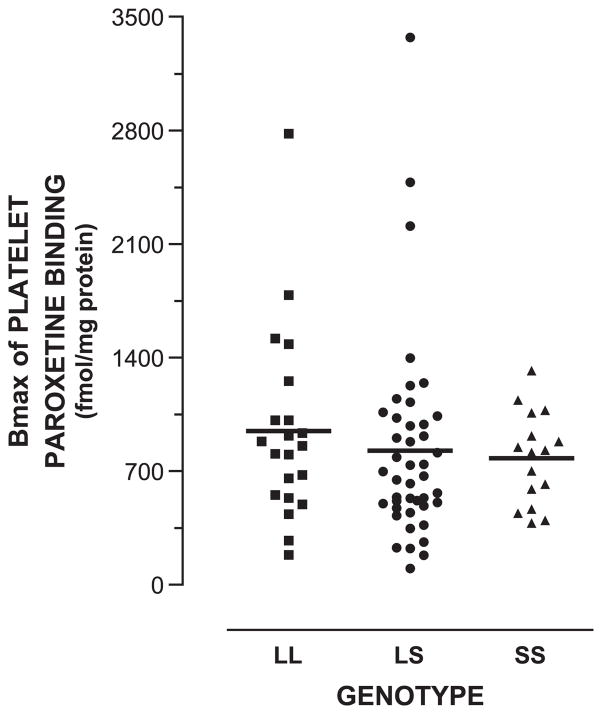
Paroxetine binding experiments were performed using platelet membranes from 76 of the 115 alcoholic subjects. Bmax values for specific paroxetine binding were plotted according to genotype (Fig. 4). Means of Bmax values did not differ in a statistically significant manner among the LL, LS, and SS genotypes (Table 1; ANOVA). Pair-wise comparisons of Bmax values between the LL and LS, LS and SS, or LL and SS genotypes were not significant. Also, binding potential (Bmax/Kd), an index of receptor bioavailability, was calculated for paroxetine binding for these subjects and compared among the genotypes (data not shown). The means were not different in the three genotypes. There was not an obvious bimodal distribution for Bmax values within subjects with the LL, LS, or SS genotype.
Fig. 4.
Bmax values for paroxetine binding among genotypes. Genotype and Bmax values for [3H]paroxetine binding to platelet membranes were determined as described in Methods. Bmax was expressed as fmol paroxetine/mg protein. Mean±S.E.M. of Bmax values for paroxetine binding for LL, LS, and SS subjects were 946±127 (N=21), 826±94 (N=43), and 779±71 (N=16), respectively.
4. Discussion
The principal finding of this study of alcoholics is that the Vmax for 5-HT uptake into platelets (5-HTT function), but not the Bmax for paroxetine binding to platelet membranes (5-HTT density), varied relative to a polymorphism of the promoter region of the 5-HTT gene. This variation is consistent with results of previous studies in which [123I]β-CIT binding to 5-HTT in the raphe of healthy control and alcoholic humans (Heinz et al., 2000) and 5-HTT uptake and paroxetine binding to platelet membranes of healthy humans (Greenberg et al., 1999) were measured. A second interesting finding was that the Vmax for 5-HTT uptake into intact platelets and the Bmax for paroxetine binding to platelet membranes, representing function and density of 5-HTTs, respectively, were not correlated.
For 5-HT uptake into intact platelets of alcoholics, the percentage of subjects below 200 fmol/min-107 platelets was higher in the LL genotype than in either the LS or SS genotype (51.5%, 40%, and 22.7%, respectively). The difference between LL and SS was statistically significant, with an odds ratio of 3.6 (P<0.05). This result suggests that alcoholics who are homozygous for the L allele may have a higher probability to have lower 5-HT uptake rates than those who have one or two S alleles. Although the percentages of subjects with Vmax values below 200 fmol/ min-107 platelets were significantly different among the genotypes, the mean of the Vmax values among genotypes did not differ. In contrast to this finding in our alcoholic subjects, Greenberg et al. (1999), using healthy control subjects, found that those with the LL genotype had significantly higher mean 5-HTT uptake rates than those with one or two S alleles. A possible explanation of this LL–SS difference between our results in alcoholics and those of healthy controls in the study by Greenberg et al. (1999) is that L allele expression may have a selective vulnerability to toxic effects of alcohol, as suggested by Heinz et al. (2000), who showed reduced density of 5-HTT in raphe nuclei of the brain of alcoholics of the LL genotype compared with healthy controls of the same genotype. Heinz et al. (2000) also showed that [123I]β-CIT binding was higher in the LL genotype than in S carriers among healthy controls. A second possible explanation is that heavy alcohol use disrupts the distribution of 5-HTT between the plasma membrane and the internal (cytosolic) compartment of the platelet. Ethanol has been shown to affect the glycosylation of proteins (Lakshman et al., 1999; Sillanaukee et al., 2001) and protein kinase C activity (Slater et al., 2003; Sultana and Babu, 2003). Both of these biochemical processes regulate the distribution of 5-HTT between the membrane and cytosol (Ramamoorthy and Blakely, 1999; Tate and Blakely, 1994).
The density of 5-HTTs, expressed as the Bmax for [3H]paroxetine binding to platelet membranes, was not significantly different among the three genotypes. This result is consistent with the results reported by Greenberg et al. (1999), who used platelets of 44 healthy human controls, and Preuss et al. (2001), who used alcoholic subjects. In these studies, [3H]paroxetine was used as the ligand. However, Lesch et al. (1996) demonstrated with in vitro experiments that both the Vmax for 5-HTT uptake into intact lymphoblasts and the Bmax for [125I]RTI binding to lymphoblast membranes were reduced in lymphoblasts transfected with the S allele vs. those with LL. In our study with platelets, the density of the 5-HTT (Bmax) was quantified using [3H]paroxetine as the ligand. It is not clear at this time whether the discrepancy that exists between the lymphoblast and platelet results is due to the ligand. It has been shown that paroxetine binds to mitochondrial and alpha granule proteins in addition to the 5-HTT (Cesura et al., 1990), which may account for this discrepancy as well as the lack of correlation between Vmax and Bmax in our study.
Finally, we did not observe a statistically significant correlation between the functional capacity of the 5-HTT (Vmax) and the number of 5-HTTs (Bmax) (Fig. 2). Since 5-HTT uptake activity (Vmax) is a measurement of cell surface expression of 5-HTT, the lack of a correlation between 5-HT uptake and paroxetine ligand binding was unexpected. We also performed correlational analyses for Vmax vs. Bmax among genotypes and between early- and late-onset alcoholics and found no significant correlations (data not shown). Several possibilities may account for this result. First, paroxetine binds to mitochondrial and alpha granule membranes in addition to its binding site on 5-HTT in the plasma membrane (Cesura et al., 1990; Laruelle et al., 1988). It is possible that binding of paroxetine to these additional sites among cellular membranes may disrupt a linear relationship between Vmax for uptake and Bmax for density of receptors. Second, the 5-HTT protein is known to be distributed between the cell surface membrane and the cytosolic compartment. This distribution is known to be affected by the exposure of surface 5-HTT to certain drugs or perhaps 5-HT. It is possible that a toxic effect of alcohol alters the distribution of the 5-HTT protein, resulting in the lack of correlation between Vmax and Bmax.
5. Conclusion
Our findings support the hypothesis that currently drinking alcoholics with the L allele of the 5′-HTTLPR region of the 5-HTT gene have a reduced 5-HTT function. These findings expand our current understanding of the role of the 5′-HTTLPR region of the 5-HTT gene in regulating the 5-HTT in alcoholism.
Acknowledgments
The authors wish to acknowledge the National Institute on Alcohol Abuse and Alcoholism for their generous support through grants U10 AA011776-07, NO1 AA01016, RO1 AA010522, and RO1 AA012964. We would also like to thank Robert H. Cormier, Jr. for his assistance with manuscript preparation.
Abbreviations
- 5-HT
serotonin
- 5-HTT
serotonin transporter
- 5′-HTTLPR
serotonin transporter-linked polymorphic region
- ANOVA
analysis of variance
- Bmax
maximum specific paroxetine binding density
- [123I]β-CIT
[123I]2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane
- DNA
deoxyribonucleic acid
- EDTA
ethylene diamine tetra-acetic acid
- Kd
dissociation constant
- Km
equilibrium constant
- L
long allelic variant
- PCR
polymerase chain reaction
- PRP
platelet-rich plasma
- RNA
ribonucleic acid
- S
short allelic variant
- SPECT
single-photon emission computerized tomography
- Vmax
maximum serotonin uptake rate
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Cesura AM, Bertocci B, Da Prada M. Binding of [3H]dihydrotetrabenazine and [125I]azidoiodoketanserin photoaffinity labeling of the monoamine transporter of platelet 5-HT organelles. Eur J Pharmacol. 1990;186:95–104. doi: 10.1016/0014-2999(90)94064-5. [DOI] [PubMed] [Google Scholar]
- Daoust M, Lhuintre JP, Ernouf D, Legrand E, Breton P, Boucly P. Ethanol intake and 3H-serotonin uptake: II. A study in alcoholic patients using platelets 3H-paroxetine binding. Life Sci. 1991;48:1977–1983. doi: 10.1016/0024-3205(91)90231-y. [DOI] [PubMed] [Google Scholar]
- Da Prada M, Cesura AM, Launay JM, Richards JG. Platelets as a model for neurons. Experientia. 1988;44:115–126. doi: 10.1007/BF01952193. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Schuckit MA, Johnson BA, Goldman D. Genetics of alcoholism using intermediate phenotypes. Alcohol, Clin Exp Res. 2003;27:169–176. doi: 10.1097/01.ALC.0000052702.77807.8C. [DOI] [PubMed] [Google Scholar]
- Ernouf D, Compagnon P, Lothion P, Narcisse G, Benard JY, Daoust M. Platelets 3H 5-HT uptake in descendants from alcoholic patients: a potential risk factor for alcohol dependence? Life Sci. 1993;52:989–995. doi: 10.1016/0024-3205(93)90190-e. [DOI] [PubMed] [Google Scholar]
- Esterling LE, Yoshikawa T, Turner G, Badner JA, Bengel D, Gershon ES, Berrettini WH, Detera-Wadleigh SD. Serotonin transporter (5-HTT) gene and bipolar affective disorder. Am J Med Genet. 1998;81:37–40. [PubMed] [Google Scholar]
- Greenberg BD, Tolliver TJ, Huang SJ, Li Q, Bengel D, Murphy DL. Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. Am J Med Genet. 1999;88:83–87. [PubMed] [Google Scholar]
- Grove G, Coplan JD, Hollander E. The neuroanatomy of 5-HT dysregulation and panic disorder. J Neuropsychiatry Clin Neurosci. 1997;9:198–207. doi: 10.1176/jnp.9.2.198. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Heils A, Mossner R, Lesch KP. The human serotonin transporter gene polymorphism—basic research and clinical implications. J Neural Transm. 1997;104:1005–1014. doi: 10.1007/BF01273314. [DOI] [PubMed] [Google Scholar]
- Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, Linnoila M, Weinberger DR. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry. 2000;47:643–649. doi: 10.1016/s0006-3223(99)00171-7. [DOI] [PubMed] [Google Scholar]
- Heinz A, Mann K, Weinberger DR, Goldman D. Serotonergic dysfunction, negative mood states, and response to alcohol. Alcohol, Clin Exp Res. 2001;25:487–495. [PubMed] [Google Scholar]
- Holmes A, Lit Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2003;2:365–380. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Kranzler H, Lappalainen J, Nellissery M, Gelernter J. Association study of alcoholism subtypes with a functional promoter polymorphism in the serotonin transporter protein gene. Alcohol, Clin Exp Res. 2002;26:1330–1335. doi: 10.1097/01.ALC.0000030840.48315.40. [DOI] [PubMed] [Google Scholar]
- Lakshman MR, Rao MN, Marmillot P. Alcohol and molecular regulation of protein glycosylation and function. Alcohol. 1999;19:239–247. doi: 10.1016/s0741-8329(99)00041-5. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Vanisberg MA, Maloteaux JM. Regional and subcellular localization in human brain of [3H]paroxetine binding, a marker of serotonin uptake sites. Biol Psychiatry. 1988;24:299–309. doi: 10.1016/0006-3223(88)90198-9. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Wolozin BL, Murphy DL, Reiderer P. Primary structure of the human platelet serotonin uptake site: identity with the brain serotonin transporter. J Neurochem. 1993;60:2319–2322. doi: 10.1111/j.1471-4159.1993.tb03522.x. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Little KY, McLaughlin DP, Zhang L, Livermore CS, Dalack GW, McFinton PR, DelProposto ZS, Hill E, Cassin BJ, Watson SJ, Cook EH. Cocaine, ethanol, and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels. Am J Psychiatry. 1998;155:207–213. doi: 10.1176/ajp.155.2.207. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Maes M, Elkis H. The biological basis of refractory depression. In: Nolen WA, editor. Refractory Depression: Current Strategies and Future Directions. Wiley; New York: 1994. pp. 177–198. [Google Scholar]
- Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem. 1994;40:288–295. [PubMed] [Google Scholar]
- Preuss UW, Soyka M, Bahlmann M, Wenzel K, Behrens S, de Jonge S, Kruger M, Bondy B. Serotonin transporter gene regulatory region polymorphism (5-HTTLPR), [3H]paroxetine binding in healthy control subjects and alcohol-dependent patients and their relationships to impulsivity. Psychiatry Res. 2000;96:51–61. doi: 10.1016/s0165-1781(00)00190-6. [DOI] [PubMed] [Google Scholar]
- Preuss UW, Koller G, Soyka M, Bondy B. Association between suicide attempts and 5-HTTLPR-S-allele in alcohol-dependent and control subjects: further evidence from a German alcohol-dependent inpatient sample. Biol Psychiatry. 2001;50:636–639. doi: 10.1016/s0006-3223(01)01196-9. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Blakely RD. Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science. 1999;285:763–766. doi: 10.1126/science.285.5428.763. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, Ganapathy V, Blakely RD. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci U S A. 1993;90:2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillanaukee P, Strid N, Allen JP, Litten RZ. Possible reasons why heavy drinking increases carbohydrate-deficient transferrin. Alcohol, Clin Exp Res. 2001;25:34–40. [PubMed] [Google Scholar]
- Slater SJ, Cook AC, Seiz JL, Malinowski SA, Stagliano BA, Stubbs CD. Effects of ethanol on protein kinase C alpha activity induced by association with Rho GTPases. Biochemistry. 2003;42:12105–12114. doi: 10.1021/bi034860e. [DOI] [PubMed] [Google Scholar]
- Sultana R, Babu PP. Ethanol-induced alteration in N-methyl-D-aspartate receptor 2A C-terminus and protein kinase C activity in rat brain. Neurosci Lett. 2003;349:45–48. doi: 10.1016/s0304-3940(03)00755-9. [DOI] [PubMed] [Google Scholar]
- Tate CG, Blakely RD. The effect of N-linked glycosylation on activity of the Na(+)- and Cl(−)-dependent serotonin transporter expressed using recombinant baculovirus in insect cells. J Biol Chem. 1994;269:26303–26310. [PubMed] [Google Scholar]
- Villinger F, Faraj BA, Olkowski ZL, Jackson RT, Ansari AA. Functional expression of the serotonin transporter in human peripheral lymphocytes. FASEB J. 1994;8:A108. [Google Scholar]