Abstract
Stereotactic radiosurgery and fractionated stereotactic radiotherapy represent an increasingly important option in the treatment of central nervous system disease. In this article, we discuss indications for stereotactic radiosurgery and review results reported in the medical literature.
Introduction
Stereotactic radiosurgery differs from open surgery insofar as stereotactic radiosurgery has no immediate cytoreductive role. Instead, the goal of radiosurgery is to change the biology of tumor cells so as to inhibit their proliferative potential. A successful outcome of radiosurgical treatment is therefore arrest of tumor growth, not disappearance of the tumor. Radiosurgery is therefore inappropriate for patients who are symptomatic from mass effect of tumors. Regardless of mass effect, however, another limiting aspect of radiosurgery is tumor size: Because external beam techniques can achieve only a limited degree of conformity, radiosurgical treatment of larger tumors may expose normal tissue to an unacceptably high level of radiation. Large tumors may require surgical debulking (ie, to reduce tumor volume) so that single-fraction radiosurgical treatment can be used. Fractionated treatments are another alternative for patients with large tumors.
Radiosurgery as Treatment for Benign Tumors
Radiosurgery has been used extensively for treating benign tumors of the central nervous system. The most extensively developed data for radiosurgical treatments have pertained to treatment of acoustic neuroma (vestibular schwannoma) and meningioma of the skull base. The clear margins and discrete imaging characteristics of these tumors make them ideal candidates for radiosurgical treatment.
Radiosurgical treatment eliminates risks of blood loss, infection, anesthesia complications, and other perioperative risks. In addition, radiosurgery is administered on an outpatient basis, thereby eliminating the need for hospitalization, specialized care in the intensive care unit (ICU), and rehabilitation. For these reasons, radiosurgery is a compelling treatment alternative for many patients. For patients who are medically fragile or who cannot accept the potential complications of surgery (eg, risks inherent in blood transfusion), radiosurgery may be the only feasible treatment alternative.
Radiosurgical Treatment of Acoustic Neuroma
Acoustic neuroma has been treated with radiosurgery since the 1960s. However, initial results of this technique were poor because the only imaging modalities available at the time were imprecise, methods for planning treatment were relatively primitive, and clinicians selected what we now know to be excessively high doses of radiation. Early treatment methods included angiography or contrast cisternography followed by use of two-dimensional dose-planning techniques. Such two-dimensional techniques yielded relatively nonconformal treatments that risked not only underdosing of tumor tissue but also overdosing of normal tissue. In addition, excessively high doses were used–as high as 35 Gy in a single fraction. Compared with modern methods, this treatment resulted in relatively poor tumor control and high incidence of cranial nerve injury. Nonetheless, treatment results were acceptable for some high-risk patients.
A successful outcome of radiosurgical treatment is therefore arrest of tumor growth, not disappearance of the tumor.
The advent of MRI imaging, three-dimensional computer-assisted dose planning, and modern dosing schedules have dramatically improved rates of morbidity from radiosurgery as well as overall tumor control (Figure 1). Numerous studies from various centers around the world have repeatedly shown the safety and efficacy of classical radiosurgery for treating acoustic neuroma. Five-year follow-up has shown that current techniques provide overall clinical tumor control in 97% to 98% of lesions treated.1–4 The facial nerve is preserved in approximately 99% of patients receiving this treatment, and hearing is preserved in more than 70% of treated patients. Mortality and morbidity from the procedure is extraordinarily low in comparison with contemporary series describing surgical extirpation of these tumors.
Figure 1.

Photograph shows Novalis LINAC device (BrainLAB, Heimstetten, Germany) used at the Southern California Kaiser Permanente Regional Radiation Oncology Center.
Numerous studies from various centers around the world have repeatedly shown the safety and efficacy of classical radiosurgery for treating acoustic neuroma.
From the standpoint of hearing preservation, introduction of fractionated stereotactic radiotherapy may improve upon the already superior results of radiosurgery and may allow use of radiosurgery for larger tumors not previously treatable with classical radiosurgery.5
Radiosurgery using present techniques results in outstanding cranial nerve preservation and tumor-control rates similar to those reported in the surgical literature while eliminating the risk of immediate periprocedural complications. We and others believe that radiosurgery should be firstline treatment for all acoustic tumors measuring <2.5 cm in diameter.6 Patients with larger tumors should be given the choice of receiving either fractionated stereotactic radiotherapy or surgical extirpation. The results of radiosurgical intervention for acoustic neuroma can also be applied to other types of cranial nerve schwannoma, such as trigeminal schwannoma.
Meningioma
Meningioma is a tumor that arises from arachnoidal cap cells commonly associated with arachnoid granulations at the dural venous sinuses, cranial nerve foramina, cribriform plate, and medial middle fossa. The tumor is most commonly benign but may exhibit atypical or even malignant features and behavior. The lesion may arise anywhere along the dura, including the convexity and base of the skull. Modern imaging techniques have enabled highly reliable diagnosis of this type of tumor.
Convexity and falcine meningiomas are easily treated using conventional open surgical techniques. Modern anesthesia combined with meticulous surgical techniques may result in high rates of gross total surgical resection with minimal morbidity and mortality (Figure 2). For these lesions, open surgical treatment remains the preferred treatment for patients with low medical risk.
Figure 2.

MRI scan shows response of craniopharyngioma to fractionated stereotactic radiotherapy in a boy aged eight years at time of treatment. Left, postoperative view. Right, MRI obtained nine months after the patient received fractionated stereotactic radiotherapy at the Southern California Kaiser Permanente Regional Radiation Oncology Center. Note that resolution of cystic component of tumor is accompanied by reduced brainstem compression and relief of temporal horn dilatation.
Various lesions of the skull base present substantially higher overall operative risk. Most tumors located in this region are intimately associated with critical nervous and vascular structures; therefore, attempts at total resection carry substantial risk of morbidity to these nerves. Published surgical series7,8 have shown relatively high rates of cranial nerve palsy as well as leakage of cerebrospinal fluid and high risk of tumor recurrence.
Because of these risks, radiosurgery has become an increasingly attractive alternative to microsurgical resection for lesions located at the skull base. Published series9 have described radiosurgical management of these lesions and have shown excellent overall tumor control and extremely low rates of morbidity. In fact, in patients with meningioma, tumor control with radiosurgery has been shown equivalent to that of gross total resection and produced only minimal morbidity.10
For certain types of meningioma of the skull base, such as meningioma affecting the cavernous sinus,11–15 orbital apex, clivus, and petrous bones,16–18 radiosurgery has been clearly shown to be the most preferable treatment. In addition to the data developed by numerous groups showing superior tumor control and extraordinarily low risk of cranial nerve deficits, radiosurgery and fractionated stereotactic radiotherapy clearly have improved cranial nerve function in a high percentage of patients who had functional impairment caused by tumor progression.
Pituitary Adenoma
Pituitary adenoma is a benign tumor of the anterior pituitary gland. Most of these tumors are nonfunctional from the standpoint of their endocrine activity, although others can be the proximal cause of Cushing's disease, hyperprolactinemia, acromegaly, and hyperthyroidism. Generally, the preferred means of managing these lesions is transsphenoidal excision, an approach which has been proved safe and effective. Benefits of this approach are relatively low morbidity and rapid correction of endocrinopathy. Nonetheless, subtotal resection and failure of inducing endocrine remission remain problems. The endocrine remission rate for functional adenoma remains approximately 70% among all patients who receive treatment for this tumor.19
Salvage treatments given after failed transsphenoidal exploration include reoperation and conventional fractionated external-beam radiotherapy. Conventional radiotherapy has been a time-tested option but has the disadvantage of long latency of effect before endocrine remission is established.20,21
In instances of endocrine failure or presence of gross residual disease, stereotactic radiosurgery has become an important means of salvage treatment. Of patients who had no disease remission after having surgery for Cushing's disease, 60% to 85% may have disease remission after receiving salvage stereotactic radiosurgery.22–24 Similar outcomes have resulted from using stereotactic radiosurgery to treat prolactinoma and growth hormone-secreting adenoma.25,26
Chordoma
Chordoma is a highly aggressive tumor which can arise from the skull base or from the spine. The tumor is malignant and has a high rate of recurrence after resection. Modern management of these tumors uses a multimodality approach which includes aggressive surgical resection followed by stereotactic radiosurgery, stereotactic radiotherapy, or particle-beam irradiation.27–29 Multimodality treatment results in an overall five-year survival rate of approximately 80%. Conventional external-beam techniques are difficult to use because they require use of very high radiation doses to achieve tumor control.
Patients with high-grade malignant glioma continue to have a dismal prognosis despite decades of intensive clinical and laboratory investigation.
Craniopharyngioma
Craniopharyngioma arises from remnants of the craniopharyngeal pouch. This type of tumor is histologically benign but tends to recur locally after surgical removal. Nonetheless, aggressive surgical removal of this tumor can be hazardous because it can be locally invasive of brain tissue. Common complications associated with these tumors include pituitary insufficiency (including diabetes insipidus), hypothalamic injury, and loss of vision. Surgical excision of these tumors can produce high rates of local control, but this treatment carries a substantial risk of recurrence. In cases where subtotal resection is achieved, stereotactic radiosurgery and fractionated stereotactic radiation treatment can be of great utility (Figures 3, 4), yielding high overall rates of tumor control and survival as well as low rates of morbidity.30–32
Figure 3.
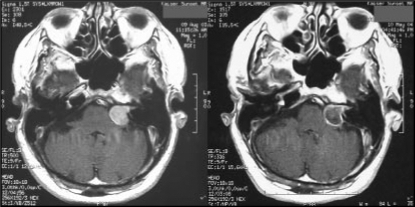
MRI scan shows early result nine months after single-fraction stereotactic radiosurgery performed at Southern California Kaiser Permanente Regional Radiation Oncology Center for left-sided acoustic neuroma. Note response to radiation as shown by loss of central contrast enhancement within tumor. Tumor size was not substantially changed in this case.
Figure 4.

Images of cranium of a 78-year-old woman who had loss of vision and sellar tumor with dural tail (tuberculum sella meningioma) treated with fractionated stereotactic radiotherapy at the Southern California Kaiser Permanente Regional Radiation Oncology Center. A, Pretreatment MRI scan shows sellar tumor; B, MRI scan shows clinically significant reduction of tumor volume nine months after treatment. Comparison of automated periphimetry scans obtained before treatment (C) and after treatment (D) shows improvement in visual fields.
Glioma
Patients with high-grade malignant glioma continue to have a dismal prognosis despite decades of intensive clinical and laboratory investigation. Current practice for management of these lesions commonly includes surgery, conventional external-beam radiotherapy, and chemotherapy.
Radiosurgery as an additional treatment modality for these tumors has been suggested to be useful in some limited circumstances.33,34 However, a recent Radiation Therapy Oncology Group phase III trial, RTOG 93-05, was unable to show any advantage of using radiosurgery for high-grade glioma.35 Thus, effective long-term control of malignant gliomas cannot be achieved by local treatment, such as radiosurgery. Effective management of this devastating disease awaits a method of treating the central nervous system as a whole.
Similarly, data regarding use of radiosurgery to treat low-grade and anaplastic-grade infiltrative glioma are weak. Use of radiosurgery to treat such lesions, therefore, cannot be considered as routine adjuvant therapy.
The advent of radiosurgery has heralded a revolution in management of metastatic lesions.
Pilocytic astrocytoma is a type of low-grade glioma that is typically well circumscribed and often amenable to surgical resection that results in long-term survival. Nonetheless, these tumors may develop in locations unfavorable for surgical management. As standalone treatment or in conjunction with conservative debulking surgery, radiosurgery for these lesions may offer important advantages over open surgery alone,36,37 although data conclusively proving this point are still unavailable.
Metastatic Disease
In contrast to glioma, where progression of disease is marked by infiltrative changes, metastases to the brain typically have discrete margins. Before radiosurgery was first introduced, metastases to the brain were best treated by surgical excision (whenever feasible) in conjunction with whole-brain radiotherapy.38
The advent of radiosurgery has heralded a revolution in management of metastatic lesions. Although external-beam radiotherapy remains an important treatment component, radiosurgery can in many instances replace surgical resection.39–41 This treatment approach results in high rates of lesion control and overall postoperative survival rates comparable to those produced by surgery with whole-brain radiotherapy. In this field, current controversy surrounds the role of radiosurgery in relation to whole-brain radiotherapy.
General selection criteria for treating metastases include Karnofsky score >70, four or fewer lesions, and lesion volume <9 mL.
Trigeminal Neuralgia
Trigeminal neuralgia is characterized by paroxysms of severe, lancinating facial pain which is sometimes caused by an arterial vessel loop compressing the trigeminal nerve in the root-entry zone. Trigeminal neuralgia typically responds well to anticonvulsant medication such as carbamazepine; in many patients, however, the condition becomes refractory to medical management. Surgical intervention may be indicated in such instances. Surgical intervention falls into two general categories: destructive techniques and microvascular decompression.
Destructive techniques include percutaneous radiofrequency rhizolysis, balloon microcompression, and glycerol injection. These procedures have the advantage of low procedural risk and have the disadvantage of precipitating facial numbness. These procedures can also be very uncomfortable for the patient.
Microvascular decompression involves craniotomy and microdissection with the goal of separating a compressing vascular loop away from the trigeminal root entry zone. Microvascular decompression offers the highest rates of long-term remission from facial pain as well as low risk of causing facial numbness. Microvascular decompression is highly invasive, however, and carries with it the risk associated with craniotomy.
Trigeminal radiosurgery is a destructive technique the target of which is the segment of the trigeminal nerve within the prepontine cistern (Figure 5). This procedure could therefore be described as a retrogasserian radiosurgical rhizolysis. Overall, it results in initially good and excellent outcomes for approximately 80% of patients who receive the procedure.42–44 Complications such as facial numbness are uncommon, and the risks of an invasive procedure are entirely eliminated. However, the risk of recurrent pain is substantial, and retreatment may become necessary.45
Figure 5.

Typical dose plan for stereotactic radiosurgery treatment of trigeminal neuralgia.
Arteriovenous Malformation
Surgical treatment of arteriovenous malformation has long represented the pinnacle of vascular neurosurgery practice. The complex anatomy of these lesions and the challenges of their surgical management have given many generations of neurosurgeons great respect for these lesions. Surgery has been a time-tested treatment that can result in complete resection of these lesions; however, rates of morbidity and mortality associated with this surgical treatment can be substantial, and great effort has been made to develop alternative methods for treating these difficult lesions. Over the past 15 years, therefore, a balanced multimodality approach has emerged that includes endovascular embolization, surgery, and stereotactic radiosurgery.
Radiosurgical treatment of these lesions has been used since the 1970s. This approach is controversial in some circles; for properly selected patients, however, we believe that radiosurgery can yield outstanding results when used alone or in a multimodality management strategy (eg, with endovascular treatment).
When used as treatment for arteriovenous malformations, radiosurgery acts by causing hyalinization within the blood vessels of an arteriovenous malformation, thereby resulting in gradual occlusion of flow through these lesions.46 Complete obliteration of the arteriovenous malformation is generally achieved two to three years after treatment. The likelihood of angiographic obliteration of the arteriovenous malformation is a function of its size, the marginal dose delivered, and the length of time since completion of the radiosurgical procedure. Radiosurgery has been shown to effectively obliterate approximately 80% of lesions with mean diameter <3 cm.47–50
The likelihood of angiographic obliteration of the arteriovenous malformation is a function of its size, the marginal dose delivered, and the length of time since completion of the radiosurgical procedure.
Radiosurgery and Fractionated Stereotactic Radiotherapy
On a typical treatment day, patients undergoing radiosurgery are admitted to the radiation clinic, where neurosurgical members of the radiosurgery team apply the stereotactic frame with the patient placed under local anesthesia (Figures 6, 7). In some cases, an anxiolytic agent is orally administered. A high-resolution CT scan is then obtained. Images from a fiducialized CT and from a previously obtained fine-cut MRI scan are then combined in a process called image fusion. This process is critical for eliminating the spatial distortion seen when MRI images are used alone in planning treatment. A protocol of dose planning and quality control is then undertaken before treatment is begun. The treatment is then delivered, typically for approximately 20 minutes to 40 minutes. When treatment is completed, the stereotactic frame is immediately removed. Most treated patients are then discharged home; in unusual instances (such as if general anesthesia is required), patients may be admitted to the hospital for overnight observation.
Figure 6.

Photograph shows patient positioned on treatment table after placement of stereotactile frame.
Figure 7.

Photograph shows patient on treatment table after placement of mask used for fractionated stereotactic radiotherapy.
Patients undergoing fractionated stereotactic radiotherapy procedures do not undergo placement of a stereotactic frame but instead are fitted with a rigid thermoplastic mask that enables precise repositioning (Figure 8). Depending on the type of pathology being treated, fractionation regimens can range from two fractions to more than 30 fractions.
Figure 8.

Photograph shows patient positioned to receive stereotactic radiosurgery.
Follow-up protocols for benign tumors and vascular conditions include serial MRI imaging done once every six months for the first two years after treatment, then annual scanning thereafter for three years. Malignant conditions warrant more frequent imaging and clinical follow-up.
For many indications, radiosurgery has proved safe and highly effective.
Conclusions
Stereotactic radiosurgery and fractionated stereotactic radiotherapy have emerged as important additions to the neurosurgical treatment armamentarium and as such have wide application. For many indications, radiosurgery has proved safe and highly effective. For some indications, radiosurgery is emerging as the preferred treatment.
References
- Petit JH, Hudes RS, Chen TT, Eisenberg HM, Simard JM, Chin LS. Reduced-dose radiosurgery for vestibular schwannomas. Neurosurgery. 2001 Dec;49(6):1299–306. doi: 10.1097/00006123-200112000-00003. discussion 1306–7. [DOI] [PubMed] [Google Scholar]
- Kondziolka D, Lunsford LD, Flickinger JC. Gamma knife radiosurgery for vestibular schwannomas. Neurosurg Clin N Am. 2000 Oct;11(4):651–8. [PubMed] [Google Scholar]
- Prasad D, Steiner M, Steiner L. Gamma surgery for vestibular schwannoma. J Neurosurg. 2000 May;92(5):745–59. doi: 10.3171/jns.2000.92.5.0745. [DOI] [PubMed] [Google Scholar]
- Noren G. Long-term complications following gamma knife radiosurgery of vestibular schwannomas. Stereotact Funct Neurosurg. 1998 Oct;70(Suppl 1):65–73. doi: 10.1159/000056408. [DOI] [PubMed] [Google Scholar]
- Andrews DW, Suarez O, Goldman HW, et al. Stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of acoustic schwannomas: comparative observations of 125 patients treated at one institution. Int J Radiat Oncol Biol Phys. 2001 Aug 1;50(5):1265–78. doi: 10.1016/s0360-3016(01)01559-0. [DOI] [PubMed] [Google Scholar]
- Pellet W, Regis J, Roche PH, Delsanti C. Relative indications for radiosurgery and microsurgery for acoustic schwannoma. Adv Tech Stand Neurosurg. 2003;28:227–82. doi: 10.1007/978-3-7091-0641-9_4. discussion 282–4. [DOI] [PubMed] [Google Scholar]
- Sekhar LN, Sen CN, Jho HD, Janecka IP. Surgical treatment of intracavernous neoplasms: a four-year experience. Neurosurgery. 1989 Jan;24(1):18–30. doi: 10.1227/00006123-198901000-00004. [DOI] [PubMed] [Google Scholar]
- De Jesus O, Sekhar LN, Parikh HK, Wright DC, Wagner DP. Long-term follow-up of patients with meningiomas involving the cavernous sinus: recurrence, progression, and quality of life. Neurosurgery. 1996 Nov;39(5):915–9. doi: 10.1097/00006123-199611000-00005. discussion 919–20. [DOI] [PubMed] [Google Scholar]
- Pollock BE, Stafford SL, Link MJ. Gamma knife radiosurgery for skull base meningiomas. Neurosurg Clin N Am. 2000 Oct;11(4):659–66. [PubMed] [Google Scholar]
- Pollock BE, Stafford SL, Utter A, Giannini C, Schreiner SA. Stereotactic radiosurgery provides equivalent tumor control to Simpson Grade 1 resection for patients with small- to medium-size meningiomas. Int J Radiat Oncol Biol Phys. 2003 Mar 15;55(4):1000–5. doi: 10.1016/s0360-3016(02)04356-0. [DOI] [PubMed] [Google Scholar]
- Chen JC, Giannotta SL, Yu C, Petrovich Z, Levy ML, Apuzzo ML. Radiosurgical management of benign cavernous sinus tumors: dose profiles and acute complications. Neurosurgery. 2001 May;48(5):1022–30. doi: 10.1097/00006123-200105000-00011. discussion 1030–2. [DOI] [PubMed] [Google Scholar]
- Duma CM, Lunsford LD, Kondziolka D, Harsh GR, 4th, Flickinger JC. Stereotactic radiosurgery of cavernous sinus meningiomas as an addition or alternative to microsurgery. Neurosurgery. 1993 May;32(5):699–704. doi: 10.1227/00006123-199305000-00001. discussion 704–5. [DOI] [PubMed] [Google Scholar]
- Lee JY, Niranjan A, McInerney J, Kondziolka D, Flickinger JC, Lunsford LD. Stereotactic radiosurgery providing long-term tumor control of cavernous sinus meningiomas. J Neurosurg. 2002 Jul;97(1):65–72. doi: 10.3171/jns.2002.97.1.0065. [DOI] [PubMed] [Google Scholar]
- Nicolato A, Foroni R, Alessandrini F, Maluta S, Bricolo A, Gerosa M. The role of Gamma Knife radiosurgery in the management of cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys. 2002 Jul;53(4):992–1000. doi: 10.1016/s0360-3016(02)02802-x. [DOI] [PubMed] [Google Scholar]
- Liscak R, Simonova G, Vymazal J, Janouskova L, Vladyka V. Gamma knife radiosurgery of meningiomas in the cavernous sinus region. Acta Neurochir (Wien) 1999;141(5):473–80. doi: 10.1007/s007010050327. [DOI] [PubMed] [Google Scholar]
- Roche PH, Pellet W, Fuentes S, Thomassin JM, Regis J. Gamma knife radiosurgical management of petroclival meningiomas: results and indications. Acta Neurochir (Wien) 2003 Oct;145(10):883–8. doi: 10.1007/s00701-003-0123-1. [DOI] [PubMed] [Google Scholar]
- Nicolato A, Foroni R, Pellegrino M, et al. Gamma knife radiosurgery in meningiomas of the posterior fossa. Experience with 62 treated lesions. Minim Invasive Neurosurg. 2001 Dec;44(4):211–7. doi: 10.1055/s-2001-19934. [DOI] [PubMed] [Google Scholar]
- Subach BR, Lunsford LD, Kondziolka D, Maitz AH, Flickinger JC. Management of petroclival meningiomas by stereotactic radiosurgery. Neurosurgery. 1998 Mar;42(3):437–43. doi: 10.1097/00006123-199803000-00001. discussion 443–5. [DOI] [PubMed] [Google Scholar]
- Thapar K, Laws ER., Jr. Pituitary tumors. In: Kaye AH, Laws ER Jr, editors. Brain tumors: an encyclopedic approach. 2nd ed. London: Churchill Livingstone; 2001. pp. 803–56. p. [Google Scholar]
- Tsang RW, Brierley JD, Panzarella T, Gospodarowicz MK, Sutcliffe SB, Simpson WJ. Role of radiation therapy in clinical hormonally-active pituitary adenomas. Radiother Oncol. 1996 Oct;41(1):45–53. doi: 10.1016/s0167-8140(96)91807-1. [DOI] [PubMed] [Google Scholar]
- Estrada J, Boronat M, Mielgo M, et al. The long-term outcome of pituitary irradiation after unsuccessful transsphenoidal surgery in Cushing' s disease. N Engl J Med. 1997 Jan 16;336(3):172–7. doi: 10.1056/NEJM199701163360303. [DOI] [PubMed] [Google Scholar]
- Sheehan JM, Vance ML, Sheehan JP, Ellegala DB, Laws ER., Jr. Radiosurgery for Cushing' s disease after failed transsphenoidal surgery. J Neurosurg. 2000 Nov;93(5):738–42. doi: 10.3171/jns.2000.93.5.0738. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Kida Y, Mori Y. Gamma knife radiosurgery in the treatment of Cushing disease: long-term results. J Neurosurg. 2002 Dec;97(5 Suppl):422–8. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- Hoybye C, Grenback E, Rahn T, Degerblad M, Thoren M, Hulting AL. Adrenocorticotropic hormone-producing pituitary tumors: 12– to 22-year follow-up after treatment with stereotactic radiosurgery. Neurosurgery. 2001 Aug;49(2):284–91. doi: 10.1097/00006123-200108000-00008. discussion 291–2. [DOI] [PubMed] [Google Scholar]
- Landolt AM, Lomax N. Gamma knife radiosurgery for prolactinomas. J Neurosurg. 2000 Dec;93(Suppl 3):14–8. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- Landolt AM, Haller D, Lomax N, et al. Stereotactic radiosurgery for recurrent surgically treated acromegaly: comparison with fractionated radiotherapy. J Neurosurg. 1998 Jun;88(6):1002–8. doi: 10.3171/jns.1998.88.6.1002. [DOI] [PubMed] [Google Scholar]
- Debus J, Schulz-Ertner D, Schad L, et al. Stereotactic fractionated radiotherapy for chordomas and chondrosarcomas of the skull base. Int J Radiat Oncol Biol Phys. 2000 Jun 1;47(3):591–6. doi: 10.1016/s0360-3016(00)00464-8. [DOI] [PubMed] [Google Scholar]
- Muthukumar N, Kondziolka D, Lunsford LD, Flickinger JC. Stereotactic radiosurgery for chordoma and chondrosarcoma: further experiences. Int J Radiat Oncol Biol Phys. 1998 May 1;41(2):387–92. doi: 10.1016/s0360-3016(98)00051-0. [DOI] [PubMed] [Google Scholar]
- Hug EB, Slater JD. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. Neurosurg Clin N Am. 2000 Oct;11(4):627–38. [PubMed] [Google Scholar]
- Schulz-Ertner D, Frank C, Herfarth KK, Rhein B, Wannenmacher M, Debus J. Fractionated stereotactic radiotherapy for craniopharyngiomas. Int J Radiat Oncol Biol Phys. 2002 Nov 15;54(4):1114–20. doi: 10.1016/s0360-3016(02)03029-8. [DOI] [PubMed] [Google Scholar]
- Selch MT, DeSalles AA, Wade M, et al. Initial clinical results of stereotactic radiotherapy for the treatment of craniopharyngiomas. Technol Cancer Res Treat. 2002 Feb;1(1):51–9. doi: 10.1177/153303460200100107. [DOI] [PubMed] [Google Scholar]
- Ulfarsson E, Lindquist C, Roberts M, et al. Gamma knife radiosurgery for craniopharyngiomas: long-term results in the first Swedish patients. J Neurosurg. 2002 Dec;97(5 Suppl):613–22. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- Nwokedi EC, DiBiase SJ, Jabbour S, Herman J, Amin P, Chin LS. Gamma knife stereotactic radiosurgery for patients with glioblastoma multiforme. Neurosurgery. 2002 Jan;50(1):41–6. doi: 10.1097/00006123-200201000-00009. discussion 46–7. [DOI] [PubMed] [Google Scholar]
- Regine WF, Patchell RA, Strottmann JM, Meigooni A, Sanders M, Young B. Combined stereotactic split-course fractionated gamma knife radiosurgery and conventional radiation therapy for unfavorable gliomas: a phase I study. J Neurosurg. 2000 Dec;93(Suppl 3):37–41. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- Roberge D, Souhami L. Stereotactic radiosurgery in the management of intracranial gliomas. Technol Cancer Res Treat. 2003 Apr;2(2):117–25. doi: 10.1177/153303460300200207. [DOI] [PubMed] [Google Scholar]
- Boethius J, Ulfarsson E, Rahn T, Lippittz B. Gamma knife radiosurgery for pilocytic astrocytomas. J Neurosurg. 2002 Dec;97(5 Suppl):677–80. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- Hadjipanayis CG, Kondziolka D, Gardner P, et al. Stereotactic radiosurgery for pilocytic astrocytomas when multimodal therapy is necessary. J Neurosurg. 2002 Jul;97(1):56–64. doi: 10.3171/jns.2002.97.1.0056. [DOI] [PubMed] [Google Scholar]
- Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998 Nov 4;280(17):1485–9. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- Chen JC, Petrovich Z, O'Day S, et al. Stereotactic radiosurgery in the treatment of metastatic disease to the brain. Neurosurgery. 2000 Aug;47(2):268–79. doi: 10.1097/00006123-200008000-00003. discussion 279–81. [DOI] [PubMed] [Google Scholar]
- Flickinger JC, Lunsford LD, Somaza S, Kondziolka D. Radiosurgery: its role in brain metastasis management. Neurosurg Clin N Am. 1996 Jul;7(3):497–504. [PubMed] [Google Scholar]
- Flickinger JC, Kondziolka D, Lunsford LD, et al. A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys. 1994 Mar 1;28(4):797–802. doi: 10.1016/0360-3016(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Pollock BE, Phuong LK, Gorman DA, Foote RL, Stafford SL. Stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg. 2002 Aug;97(2):347–53. doi: 10.3171/jns.2002.97.2.0347. [DOI] [PubMed] [Google Scholar]
- Huang CF, Kondziolka D, Flickinger JC, Lunsford LD. Stereotactic radiosurgery for trigeminal schwannomas. Neurosurgery. 1999 Jul;45(1):11–6. doi: 10.1097/00006123-199907000-00002. discussion 16. [DOI] [PubMed] [Google Scholar]
- Regis J, Manera L, Dufour H, Porcheron D, Sedan R, Peragut JC. Effect of the Gamma Knife on trigeminal neuralgia. Stereotact Funct Neurosurg. 1995;64(Suppl 1):182–92. doi: 10.1159/000098778. [DOI] [PubMed] [Google Scholar]
- Pollock BE, Foote RL, Stafford SL, Link MJ, Gorman DA, Schomberg PJ. Results of repeated gamma knife radiosurgery for medically unresponsive trigeminal neuralgia. J Neurosurg. 2000 Dec;93(Suppl 3):162–4. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- Schneider BF, Eberhard DA, Steiner LE. Histopathology of arteriovenous malformations after gamma knife radiosurgery. J Neurosurg. 1997 Sep;87(3):352–7. doi: 10.3171/jns.1997.87.3.0352. [DOI] [PubMed] [Google Scholar]
- Colombo F, Pozza F, Chierego G, Casentini L, De Luca G, Francescon P. Linear accelerator radiosurgery of cerebral arteriovenous malformations: an update. Neurosurgery. 1994 Jan;34(1):14–20. discussion 20–1. [PubMed] [Google Scholar]
- Friedman WA. Radiosurgery for arteriovenous malformations. Clin Neurosurg. 1995;42:328–47. [PubMed] [Google Scholar]
- Pollock BE. Stereotactic radiosurgery for arteriovenous malformations. Neurosurg Clin N Am. 1999 Apr;10(2):281–90. [PubMed] [Google Scholar]
- Lunsford LD, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for arteriovenous malformations of the brain. J Neurosurg. 1991 Oct;75(4):512–24. doi: 10.3171/jns.1991.75.4.0512. [DOI] [PubMed] [Google Scholar]


