Abstract
Microorganisms infecting the dental root canal system play an unequivocal role as causative agents of apical periodontitis. Although fungi, archaea, and viruses have been found in association with some forms of apical periodontitis, bacteria are the main microbial etiologic agents of this disease. Bacteria colonizing the root canal are usually organized in communities similar to biofilm structures. Culture and molecular biology technologies have demonstrated that the endodontic bacterial communities vary in species richness and abundance depending on the different types of infection and different forms of apical periodontitis. This review paper highlights the distinctive features of the endodontic microbiota associated with diverse clinical conditions.
Keywords: endodontic infection, acute apical abscess, endodontic pathogens, biofilm
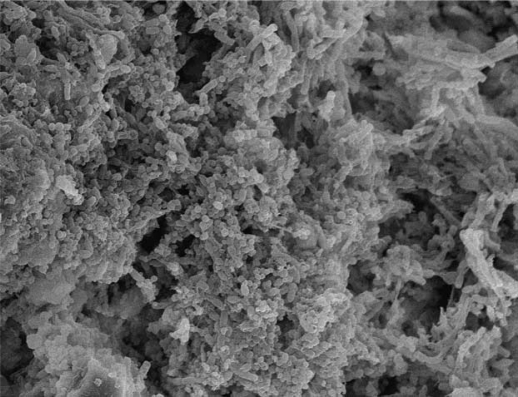
Apical periodontitis is an inflammatory disease that affects the tissues surrounding the apical portion of the dental root and is primarily caused by microorganisms infecting the root canal (1–3). The disease can manifest clinically in distinct ways and the root canal microbiota may vary accordingly. Actually, the structure of the microbiota may be responsible for the different clinical presentations of apical periodontitis. Fungi and most recently archaea and viruses have been found in association with endodontic infections (4–8), but bacteria are unarguably the major microorganisms implicated in the pathogenesis of apical periodontitis. Endodontic infections are for the most part endogenous infections that arise from the normal oral microbiota under predisposing conditions (pulp necrosis or pulp removal for treatment). In advanced stages of the infectious process, bacterial organizations resembling biofilms can be observed adhered to the dentinal root canal walls (9–11) (Fig. 1). Therefore, there is a current trend to include apical periodontitis in the group of human infectious diseases caused by bacterial biofilms.
Fig. 1.
Bacterial biofilm formed on the root canal wall (scanning electron microscopy, original magnification ×1,900).
Inevitably, the concept of endodontic infections as biofilm-arranged communities brings a more holistic view as to the causation of apical periodontitis (12). Mounting evidence indicates that while there is little specificity as to the involvement of single named species in disease etiology, specificity becomes more evident when bacterial community profiles are taken into account. In other words, while associations of any specific species with any form of apical periodontitis is seldom, if ever, observed, the bacterial community profiles seem to follow some patterns related to the different presentations of apical periodontitis (12). Community profiles are essentially determined by species richness and abundance. This review paper focuses on the distinctive features of the microbiota associated with different types of endodontic infections and different forms of apical periodontitis.
Teamwork is what counts – bacterial endodontic communities
The last years have witnessed a trend toward a more holistic concept of the etiology of human endogenous infections that considers the bacterial community as the unit of pathogenicity (12). This concept is applicable to all three major oral infectious diseases – caries, marginal periodontitis, and apical periodontitis (13–15).
Molecular methods have been largely used for profiling bacterial communities in diverse environments (16), and one of their greatest advantages in this regard reside in the ability of including as-yet-uncultivated bacteria in the analysis (17). Community profiling analyses of the endodontic microbiota have disclosed some interesting findings: (a) the different types of endodontic infections were confirmed as being composed of mixed communities (18, 19), including persistent/secondary infections associated with treated teeth (20); (b) some underrepresented uncultivated bacteria may be commonly found in infected root canals (21, 22); (c) bacterial communities may follow a specific pattern according to the clinical condition (chronic apical periodontitis, acute apical abscesses, and treated teeth) (19, 20); (d) there is a great interindividual variability in endodontic communities associated with the same clinical disease (19), i.e. each individual harbors a unique endodontic microbiota in terms of species richness and abundance; and (e) this interindividual variability is still more pronounced when individuals from different geographical locations are analyzed (18–20, 23).
The fact that the composition of the endodontic microbiota differs consistently between individuals suffering from the same disease (18–20) denotes a heterogeneous etiology for apical periodontitis, where multiple species combinations can lead to similar disease outcomes. Identification of the community members can reveal the presence of some species or group of species that may be important for the causation of some forms of disease. It is reasonable to realize that disease severity, based on intensity of signs and symptoms, or response to treatment may be related to the bacterial community composition. Therefore, specificity in causation of apical periodontitis appears to be stronger at the community level.
Features of the microbiota in different conditions
Microbial diversity in endodontic infections has been consistently assessed by anaerobic culturing and culture-independent molecular biology methods. Collectively, more than 400 different microbial taxa have been identified in endodontic samples from teeth with different forms of apical periodontitis. These taxa are usually found in combinations involving many species in primary infections and a few ones in secondary/persistent infections (24). Endodontic bacteria fall into nine of the 13 phyla that have oral representatives, namely Firmicutes, Bacteroidetes, Actinobacteria, Fusobacteria, Proteobacteria, Spirochaetes, Synergistes, TM7, and Sulphur River 1 (SR1) (25–30). In addition to bacteria, fungi and archaea have been only occasionally found in intraradicular infections (5, 31, 32), while herpesviruses and HIV have been detected in apical periodontitis lesions (4, 33–38). However, given the recognized importance of bacteria in causing apical periodontitis and the much broader species richness of bacteria when compared to fungi and archaea, this paper will limit its focus hereafter exclusively on bacteria.
Endodontic infections develop in a previously sterile place which as such does not contain a normal microbiota. Therefore, any species found in the canal has the potential to be an endodontic pathogen or at least to play a role in the ecology of the microbial community. So far, virtually all studies involved with phenotypic or genotypic identification of endodontic bacteria have followed a cross-sectional design, for obvious ethical reasons. Thus, species prevalence and consequently only species association with disease can be inferred from these studies. In addition to frequency of detection, causation may be strengthened on the basis of potential pathogenicity (in animal models or deduced from association with other human diseases). Based on cross-sectional studies, several species have emerged as candidate or putative endodontic pathogens, and the labels candidate or putative should be preserved while a definite role in disease causation is not determined. Table 1 summarizes the main features of the endodontic microbiota in different clinical conditions.
Table 1.
Distinctive features of the microbiota associated with different types of endodontic infections
| Primary infections | Persistent infections | Persistent/secondary infections | ||
|---|---|---|---|---|
| Chronic apical periontitis | Acute apical abscess | Filling stage | Treated teeth | |
 |
 |
 |
 |
|
| Community | Mixed | Mixed | Mixed, sometimes single | Mixed, sometimes single |
| No. taxa/case | 10–20 | 10–20 | 1–5 | ØAdequate treatment: 1–5 |
| ØInadequate treatment: 2–30 | ||||
| No. of cells/case | 103–108 | 104–109 | 102–105 | 103–107 |
| Uncultivated bacteria | 40–55% | 40% | 42% | 55% |
| Most prevalent groups | Gram-negative/Gram-positive anaerobes | Gram-negative anaerobes | Gram-positive facultatives/anaerobes | Gram-positive facultatives |
| Most frequent taxa | Treponema spp. | Treponema spp. | Streptococcus mitis | Enterococcus faecalis |
| Tannerela forsythia | Tannerela forsythia | Other streptococci | Candida albicans (yeast) | |
| Porphyromonas spp. | Porphyromonas spp. | Propionibacterium spp. | Streptococcus spp. | |
| Dialister spp. | Dialister spp. | Fusobacterium nucleatum | Pseudoramibacter alactolyticus | |
| Filifactor alocis | Fusobacterium nucleatum | Prevotella spp. | Propionibacterium propionicum | |
| Pseudoramibacter alactolyticus | Eikenella corrodens | Pseudoramibacter alactolyticus | Filifactor alocis | |
| Fusobacterium nucleatum | Synergistes spp. | Parvimonas micra | Dialister spp. | |
| Synergistes spp. | Prevotella spp. | Lactobacilli | Actinomyces spp. | |
| Eikenella corrodens | Olsenella spp. | Olsenella spp. | Pseudomonas aeruginosa | |
| Prevotella spp. | Parvimonas micra | Actinomyces spp. | Enteric rods | |
| Olsenella spp. | Pseudomonas aeruginosa | |||
| Parvimonas micra | Enteric rods | |||
| Peptostreptococcus spp. | ||||
| Campylobacter spp | ||||
Primary infections
Primary infections are caused by microorganisms that colonize the necrotic pulp tissue. It can also be regarded as the initial or ‘wild’ infection, in the sense that there has not been any professional intervention yet. Participating microorganisms may have been involved in the earlier stages of pulp invasion (usually via caries), which culminated in inflammation and further necrosis, or they can be latecomers that took advantage of the environmental conditions in the root canal after pulp necrosis. Primary infections are the cause of primary apical periodontitis, which can manifest itself as a chronic or acute disease. Some acute conditions may evolve to an abscess, which in some cases can spread to head and neck spaces to establish a life-threatening condition.
Chronic apical periodontitis
Primary infections are conspicuously dominated by anaerobic bacteria organized in a mixed community. Overall, the bacterial density per canal varies from 103 to 108 (2, 29, 39–41). The largest counts may be more related to large lesions and/or symptomatic cases (2). As for species richness, a mean of 10–20 species/phylotypes have been found per canal of teeth with chronic apical periodontitis as revealed by molecular biology studies (19, 24, 27, 30). Root canals of teeth with apical radiolucency associated with a draining sinus tract (chronic apical abscess or suppurative apical periodontitis) have been reported to harbor a mean number of 17 species (30). The size of the apical periodontitis lesion has been shown to be proportional to the number of bacterial species and cells in the root canal (2, 30, 42). In a molecular study using the reverse-capture checkerboard approach to assess the microbiota of teeth with chronic apical periodontitis (30), we demonstrated that the mean number of bacterial taxa per canal was in direct proportion to the lesion size – 12 taxa in teeth with small lesions (<5 mm), 16 taxa in lesions from 5 to <10 mm, 20 taxa in teeth with lesions larger than 10 mm in diameter. Some canals of teeth with large lesions were found to harbor even more than 40 taxa (30).
Bacterial named species frequently detected in primary infections, including both acute and chronic apical periodontitis, belong to diverse genera of gram-negative (Fusobacterium, Dialister, Porphyromonas, Prevotella, Tannerella, Treponema, Campylobacter, and Veillonella) and gram-positive (Parvimonas, Filifactor, Pseudoramibacter, Olsenella, Actinomyces, Peptostreptococcus, Streptococcus, Propionibacterium, and Eubacterium) bacteria (2, 24–27, 30, 32, 42–52).
Molecular biology methods have caused a great impact in the knowledge of the bacterial diversity in endodontic infections (53). Several culture-difficult species have been consistently included in the set of candidate endodontic pathogens only after the advent of molecular techniques for bacterial identification. The main examples are Tannerella forsythia, Dialister species (D. invisus and D. pneumosintes), Filifactor alocis, Prevotella baroniae, Olsenella uli, and Treponema species (27, 30, 50–52, 54–60). Strengthening of the association with apical periodontitis of some species previously recognized as candidate pathogens has also become evident by molecular findings. Examples include Fusobacterium nucleatum, Parvimonas micra (formerly Peptostreptococcus micros), Porphyromonas species (P. endodontalis and P. gingivalis), Prevotella species (P. intermedia and P. nigrescens), and Pseudoramibacter alactolyticus, all of which have been detected in higher prevalence values than previously reported by culturing studies (30, 48–50, 52, 61–64).
It is noteworthy that the most prevalent species in primary infections may vary from study to study, which can be explained by several factors: sensitivity and specificity of the identification method, sampling technique, geographic location, and accuracy or divergence in clinical diagnosis, and disease classification. Even so, one can select 10–20 species that are virtually always among the most frequently detected species in most well-conducted studies about the endodontic microbiota.
Although several known species have been associated with primary infections, the breadth of bacterial diversity has been substantially expanded by culture-independent molecular approaches to include many as-yet-uncultivated and uncharacterized bacteria. Clone library analyses of primary endodontic infections reveal that a significant proportion of the detected taxa consist of phylotypes that remain to be cultivated and phenotypically characterized (26, 27). These as-yet-uncultivated phylotypes account for approximately 55% of the taxa found in root canals of teeth with chronic apical periodontitis and in terms of abundance represent more than 38% of the clones sequenced (26). Corresponding figures for primary infections associated with acute apical abscesses are shown in the next section. Uncultivated phylotypes from several genera have been identified, including Dialister, Prevotella, Synergistes, Solobacterium, Olsenella, Fusobacterium, Eubacterium, Megasphaera, Veillonella, and Selenomonas, as well as phylotypes related to the family Lachnospiraceae or the TM7 phylum (22, 25–28, 65–67). Spirochetes comprise another bacterial group that has been shown to have as-yet-uncultivated representatives in primary infections. Sakamoto et al. (58) examined the diversity of spirochetes in primary endodontic infections and revealed that 64% of the Treponema species found have not yet been cultivated.
Synergistes clone BA121 and Bacteroidetes clone X083 are possibly the most prevalent as-yet-uncultivated phylotypes found in endodontic infections (22, 28, 30, 66, 68). There is a growing need to have these phylotypes cultivated in the laboratory in order to disclose some of their relevant phenotypic features, such as pathogenicity and susceptibility to topical and systemic antimicrobials.
Acute apical periodontitis and abscesses
Acute apical periodontitis and acute apical abscesses are typical acute forms of apical periodontitis. While an acute abscess is usually preceded by acute apical periodontitis, the latter does not necessarily evolves to the former. Therefore, the acute abscess can be regarded as an advanced stage of the acute disease. Although the transition from acute apical periodontitis to abscess can make it difficult to clinically distinguish these two conditions, in later stages of the disease process the diagnosis of acute abscesses usually does not represent a difficult task, mostly because of swelling. In symptomatic cases, the infection is located in the root canal but it may also have reached the periradicular tissues and, in abscessed cases, it has the potential to spread to other anatomical spaces of head and neck to form a cellulitis.
The microbiota involved with abscesses is mixed and dominated by anaerobic bacteria (19, 26, 46, 69, 70). Bacterial counts per abscess case have been reported to range from 104 to 109 colony forming units (46, 71, 72). The mean number of species is comparatively higher in abscesses than in canals of teeth with chronic apical periodontitis, with molecular studies revealing an average of 12–18 taxa/case in abscesses as compared to 7–12 taxa present in chronic cases (19, 26). It has been shown that as-yet-uncultivated phylotypes encompass approximately 40% of the taxa found in abscesses and collectively represent more than 30% of the clones sequenced (26).
Thus far, there is no strong evidence reporting on the specific involvement of a single species with any particular sign or symptom of apical periodontitis. Some gram-negative anaerobic bacteria have been suggested to be involved with symptomatic lesions (2, 26, 45, 73–76), but the same species may also be present in somewhat similar frequencies in asymptomatic cases (43, 47–50, 63). Therefore, factors other than the mere presence of a given putative pathogenic species may play a role in the etiology of symptomatic endodontic infections (77, 78). These factors possibly include: (a) differences in virulence ability among strains of the same species; (b) bacterial interactions resulting in additive or synergistic effects among species in mixed communities; (c) bacterial population density; (d) environment-regulated expression of virulence factors; and (e) host resistance, which may be modulated by diverse aspects including systemic diseases, concomitant virus infection, environmental factors (stress, smoking), and genetic patterns (77, 78).
The diversity of the bacterial communities has been found to differ significantly when the microbiota of chronic apical periodontitis and acute apical abscesses are compared (19). Differences are essentially represented by different dominant species in the communities and larger number of species in abscesses. A shift in the community structure is then suspected to precede the emergence of symptoms. Differences in species richness and abundance, and the resulting interactions among community members may affect virulence of the whole consortium. This is in agreement with the community-as-pathogen concept already discussed.
The apical root canal microbiota
Bacteria settled in the apical root canal are in a strategic position to induce damage to the periradicular tissues. Products released from the bacterial biofilm in the canal accumulate and reach the periradicular tissues to give rise to an inflammatory response that ultimately leads to destruction of the periodontal ligament and bone. As early as in 1894, Miller (79) emphasized the different morphology of the apical microbiota in comparison to the most coronal microbiota. Actually, the apical microbiota has been demonstrated to differ significantly from that occurring in the more coronal aspects of the canal (intraindividual analysis) in terms of predominant morphotypes (80), bacterial community profile (81), and anaerobe:facultative ratio (82). The apical root canal microbiota is predominantly anaerobic and the time of infection can influence this dominance, i.e. at late stages of infection, anaerobes comprise the large majority of isolates (82, 83).
Baumgartner and Falkler (83) investigated the cultivable microbiota of the apical 5 mm of root canals of 10 teeth with apical periodontitis and reported that the most prevalent species were P. intermedia/nigrescens, Prevotella buccae, Peptostreptococcus anaerobius, and Veillonella parvula. The total number of colony forming units in the apical 5 mm of root canals ranged from 5.6×104 to 4.3×106. Dougherty et al. (84) surveyed the apical and coronal segments of infected root canals for the occurrence of black-pigmented anaerobic bacteria and found that P. nigrescens was the most prevalent species in both coronal and apical samples. Siqueira et al. (85) evaluated samples taken from the apical segment of infected root canals associated with apical periodontitis lesions for the presence of 11 anaerobic bacterial species using species-specific polymerase chain reaction (PCR) and detected bacterial DNA in all cases. P. alactolyticus was the most prevalent species, followed by Treponema denticola, F. nucleatum, P. endodontalis, and F. alocis. In another study, Siqueira et al. (86) surveyed the same samples for the presence and levels of 28 bacterial species/phylotypes using a reverse-capture checkerboard hybridization assay. Detected taxa included P. alactolyticus (32%), Bacteroidetes clone X083 (26%), Streptococcus species (21%), O. uli (10.5%), Synergistes clone BA121 (10.5%), F. nucleatum (10.5%), P. endodontalis (10.5%), Dialister clone BS016 (5%), F. alocis (5%), P. micra (5%), and T. denticola (5%). Of these, only Bacteroidetes clone X083 and Synergistes clone BA121 were found at levels above 105.
A recent community profiling study compared the bacterial communities established at the apical and middle/coronal segments of infected root canals of teeth with apical periodontitis (81). Root fragments were cryogenically ground and used for analysis. Although the mean number of taxa in both apical and middle/coronal samples was 28, the mean of shared taxa was only 54%, ranging from 2–79%. Thus, the profile of bacterial community colonizing the apical segment of infected root canals was shown to be as diverse as that occurring at the middle/coronal segments. A high variability was observed for both interindividual (samples from the same root region but from different patients) and intraindividual (samples from different regions of the same root) comparisons. The latter can be explained by the different physico-chemical conditions and type of nutrient availability in the different regions of the canal.
Persistent and secondary infections
Secondary infections are caused by microorganisms that were not present in the primary infection, but that were introduced in the root canal at some time after professional intervention. Persistent infections are caused by microorganisms that were members of a primary or secondary infection and that persisted in the canal after antimicrobial treatment and managed to adapt to the harsh ecological conditions in instrumented and filled root canals. Persistent and secondary infections are responsible for several problems in endodontic practice, including persistent exudation, persistent symptoms, flare-ups, and treatment failure. Except for treatment failure, there are no studies in the endodontic literature that have consistently evaluated the microorganisms associated with these clinical conditions. Indeed, there are some reports that show that non-oral bacteria may be involved with secondary infections to cause persistent exudation and/or symptoms (87–90). As for flare-ups, the evidence of specificity is even weaker, although there are some reports of involvement of gram-negative anaerobes, such as black-pigmented rods and F. nucleatum (2, 91).
On the other hand, the microbiota involved with treatment failure has been extensively studied in the last decade. Microbial involvement with treatment failures is supported by two strong evidence-based arguments. First, there seems to be an increased risk of adverse treatment outcome when bacteria are present in the canal at the time of filling (92–94). Second, most (if not all) root canal-treated teeth evincing persistent apical periodontitis lesions have been demonstrated to harbor an intraradicular infection (95–102). Based on these arguments, studies have attempted to identify the microorganisms found at the root canal-filling stage, which are ‘short-term survivors’ that may put the treatment outcome at risk, and the microorganisms in root canal-treated teeth with apical periodontitis, which are ‘long-term survivors’ that are arguably the cause of post-treatment disease.
Short-term survivors: bacteria persisting immediately after treatment
Even after diligent chemomechanical preparation followed or not by intracanal medication, some canals may harbor detectable levels of cultivable bacteria. In these cases, 1–5 species can be detected per canal, with counts reaching 102–105 cells per sample (29, 41, 93, 103–105). This indicates that, even if total bacterial elimination is not the case, at least a substantial reduction in species richness and abundance is attained. No single species has been significantly found to persist after treatment procedures. Although gram-negative bacteria are common members of primary infections, they are not commonly found in post-instrumentation or post-medication samples. Gram-positive bacteria are more frequently present and include Streptococcus species, P. micra, Actinomyces species, Propionibacterium species, P. alactolyticus, Lactobacillus species, Enterococcus faecalis, and O. uli (29, 93, 103–111). Some as-yet-uncultivated phylotypes have also been found to persist after instrumentation or intracanal medication (29). Actually, approximately 40% of the taxa found in post-treatment samples are as-yet-uncultivated phylotypes (29).
Long-term survivors: bacteria in treated root canals
The microbiota in root canal-treated teeth with post-treatment apical periodontitis also exhibits a decreased diversity (both richness and abundance) in comparison to primary infections. Root canals with apparently adequate treatment usually contain 1–5 species, while the number of species in canals with inadequate treatment can reach up to 30 species, which is very similar to primary infections (20, 98–100). In terms of bacterial density, a treated canal associated with post-treatment disease can harbor 103–107 cells (39, 107, 112).
Regardless of the identification method, E. faecalis is the most frequently detected species in root canal-treated teeth, with prevalence values reaching up to 90% of the cases (97–100, 112–115). Root canal-treated teeth are about nine times more likely to harbor E. faecalis than cases of primary infections (115). The fact that E. faecalis has been commonly recovered from cases treated in multiple visits and/or in teeth left open for drainage (88) suggests that this species may be a secondary invader that succeeds in colonizing the canal and resist treatment. In other words, E. faecalis may cause a secondary infection that then becomes persistent. This species has been considered as transient in the oral cavity and its source might be food (116).
While association of E. faecalis with post-treatment disease is suggested by epidemiological studies and supported by the species attributes that allow it to survive under unfavorable environmental conditions, causation still remains unproven. The status of E. faecalis as the main causative agent of endodontic treatment failures has been recently put into question by the following arguments:
E. faecalis has not been detected in all studies evaluating the microbiota of root canal-treated teeth with post-treatment lesions (65, 117);
even when present in treated canals, E. faecalis is rarely one of the most dominant species in the bacterial community (20); and
E. faecalis is not more prevalent in root canal-treated teeth with lesions when compared to root canal-treated teeth with no lesions (114, 118).
Other bacteria found in root canal-treated teeth with apical periodontitis include Streptococcus species and some fastidious anaerobic species – P. alactolyticus, Propionibacterium propionicum, F. alocis, D. pneumosintes, and D. invisus (20, 28, 98–100, 113). As-yet-uncultivated phylotypes correspond to 55% of the taxa detected in treated canals (119), which confirms their importance in disease etiology. Bacterial community profiles in treated cases vary from individual to individual, suggesting that distinct bacterial combinations can play a role in treatment failures (20). All these findings indicate that the microbiota of root canal-treated teeth with apical periodontitis is more complex than previously anticipated by culture studies. However, it is proportionally less complex than primary infections.
Fungi are only occasionally found in primary infections, but Candida species, particularly C. albicans, have been detected in root canal-treated teeth in up to 18% of the cases (98–100, 107, 113, 117, 120, 121).
Molecular methods have strengthened the association of persistent/secondary intraradicular infections with treatment failures, i.e. bacteria have been detected in virtually all treated cases with apical periodontitis (20, 39, 97, 98). On the other hand, previous culture and microscopic studies have failed to detect microorganisms in some cases of root canal-treated teeth with persistent disease (99, 100, 113, 122). This discrepancy is better explained by the low sensitivies of culture and microscopic methods and the occurrence of as-yet-uncultivated phylotypes and strains. However, a recent study using a histobacteriologic technique managed to detect bacteria in virtually all cases of post-treatment disease (101).
Beyond the border: extraradicular infections
Apical periodontitis lesions usually represent an effective immunological barrier against spread of the infection from the root canal to the alveolar bone and other body sites. However, in some specific circumstances, microorganisms may overcome this defense barrier and establish an extraradicular infection, which can be conceivably dependent on or independent of the intraradicular infection (123).
The question as to whether the extraradicular infection is dependent on or independent of the intraradicular infections assumes special relevance from a therapeutic standpoint. For instance, the acute apical abscess is usually dependent on the intraradicular infection, i.e. once the intraradicular infection is properly controlled by root canal treatment or tooth extraction and drainage of pus is achieved, the extraradicular infection is handled by the host defenses and usually subsides.
Apical actinomycosis, which is caused by Actinomyces species or P. propionicum, has been claimed to be a form of extraradicular infection independent of the intraradicular infection in the sense that even if the treatment succeeds in eradicating intraradicular bacteria, the lesion may not heal because the causative agents are already beyond the reaches of intracanal procedures (123–125). However, clear direct evidence is lacking as to whether or not apical actinomycosis actually comprises an independent form of extraradicular infection (126).
The extraradicular occurrence of several anaerobic bacteria has also been reported in post-treatment apical periodontitis lesions (127–131). Examples include Treponema species, P. endodontalis, P. gingivalis, T. forsythia, Prevotella species, and F. nucleatum, which have been detected by culture, immunological, or molecular studies (128–134). Recent molecular studies using broad-range PCR and clone library analysis have revealed an unanticipated bacterial diversity in extraradicular infections in association with post-treatment apical periodontitis (130, 131). One study found bacteria in 85% of the lesions obtained by apicoectomy and 36% of the taxa identified were as-yet-uncultivated phylotypes (130). Another study evaluated both the resected root ends and the apical periodontitis lesions from treated teeth and reported that 54% of the taxa identified were uncultivated phylotypes (131). However, the majority of the clones (69%) belonged to cultivated or named species. As for bacterial counts, the root ends harbored significantly more bacterial cell equivalents than the lesions (mean numbers, 1×108 and 4×107, respectively) (131). Of 33 paired samples of resected root ends and apical periodontitis specimens, bacteria were always detected in the former, except for one case. In that case, bacteria were found in the associated lesion. As for the lesion specimens, bacteria were not detected in six cases (131).
Most of the cultivable named species found in extraradicular infections possess an array of virulence traits that may allow them to avoid or circumvent the host defenses in the inflamed periradicular tissues (135–138). However, there is no direct evidence showing that extraradicular bacteria were actually established as an extradicular infection independent of the intraradicular infection. Actually, findings from the Subramanian and Mickel's study (131) indicate that the large majority of cases of extraradicular infections are maintained by a concomitant apical root canal infection.
Future perspectives and challenges in endodontic microbiology research
A substantial expansion in knowledge of the diversity of the endodontic microorganisms involved with different clinical conditions has occurred over the last decade. As this knowledge is refined and incorporated into the context of community as the unit of pathogenicity (12), identification of different microbial community patterns associated with specific disease manifestations has the potential to reveal idiosyncrasies that may be used as the foundation for the establishment of evidence-based antibacterial treatment protocols. In this regard, further studies exploring a holistic view of endodontic infections are made necessary, where the species colonizing the untreated or treated canal should be seen as part of an ecosystem, in which they live in communities whose outcome of physiology and function will determine the outcome of the tissue response, i.e. emergence and severity of disease.
High-throughput methods have been used in environmental microbiology and more recently used in medical microbiology to provide massive information as to microbial identification, significantly increasing coverage of the community members. Examples include open-ended techniques, such as pyrosequencing and metagenomic approaches (139–142), and closed-ended techniques, such as DNA microarrays using hundreds to even thousands of taxon-specific probes (143–145). In addition to allowing for a more comprehensive analysis of the bacterial diversity in endodontic infections, the information brought about by these techniques has the potential to guide the development of a panel of well-selected putative pathogens to be included in closed-ended identification techniques, such as checkerboard and microarrays. This panel of selected pathogens can then be used against large numbers of clinical samples with reduced cost and time to identify associations with specific disease forms, signs, and symptoms.
Interpretation of the community behavior can be inferred by global gene expression (transcriptomics) or by comprehensive inventories of released proteins (proteomics) and metabolites (metabolomics). Information provided by these methods has the potential to disclose patterns of molecules associated with diverse clinical conditions, helping establish outcome predictors based not only in specific species combinations or communities, but perhaps even more importantly on the resultant products of these consortia. This might restrict the focus to some microbial product combinations instead of species combinations, considering the very likely possibility that the latter may reach a broader spectrum of variability than the former. This is because different species may apparently occupy the same niche in different communities as suggested by studies showing a high interindividual variability in the endodontic bacterial communities associated with the same disease condition (18, 146). Recognition of molecular patterns has the potential to allow development of chairside tests to predict outcome of the disease or treatment.
Rapid, accurate, and highly sensitive molecular assays now provide microbial identification results in a matter of minutes to a few hours. Cost reduction of devices and reagents has resulted in propagation of these methodologies to several clinical laboratories. In the rare circumstances that infections of endodontic origin are life-threatening, such as rapidly disseminating abscess/cellulitis with systemic involvement, both the clinician and the patient can significantly benefit from rapid microbiological diagnosis to adhere to proper therapy. Rapid diagnosis of bacteria involved in abscesses/cellulitis as well as of antibiotic resistance genes will allow clinicians to manage infectious diseases proactively. Unfortunately, open-ended molecular approaches for identification of bacteria in polymicrobial infections are still time-consuming but further technological advances have the potential to expedite bacterial identification by these techniques. Alternatively, identification of pathogen patterns related to disease may allow development of tests for rapid diagnosis of selected target species.
Fortunately, non-sense attitudes and absurd clinical decision-making based on the ‘focal infection theory’ are now part of the past and must not be feared in days like these, but should always be remembered as a warning to avoid that crass errors from the past century be repeated. However, while there is no reason based on solid scientific evidence to consider an infected canal as a focus of infection to distant body sites, except for systemically compromised patients, the opposite should not be disregarded either, as there is no clear evidence to consider endodontic infections as segregate events with no effect on the rest of the body. The systemic involvement of endodontic bacteria as part of the total oral infectious burden or through bacteremia following treatment or acute disease remains to be investigated in the light of current scientific concepts and technology. This is an important area of future research that has the potential to shape the future of the clinical discipline of endodontics.
The recognition that the microbiota associated with the same endodontic disease significantly differs in species richness and abundance between individuals living in different geographic locations (18, 146) raises the inevitable question as to whether the same treatment protocols, especially systemic antibiotic therapy, can be used in one-size-fits-all terms. Therefore, it is a challenge for researchers to apply sophisticated technology to identify community patterns related to geography and establish whether or not treatment protocols should be customized accordingly.
Root canal treatment approaches that predictably yield negative cultures are expected to offer a better outcome in terms of healing of apical periodontitis (93, 99, 147). Therefore, culture results have been considered as surrogate endpoints for long-term treatment outcome. However, limitations in culturing techniques, including low sensitivity and inability to detect as-yet-uncultivated bacteria, put the validity of its use as outcome predictor in question. Quantitative open-ended molecular methods that are more sensitive than culture have the potential to establish more reliable standards to predict outcome. Again, identification of specific pathogen or molecular patterns related to treatment outcome may probably be the best way to establish tests to serve as a more accurate surrogate outcome.
As one can see, many issues related to the basic and applied science of endodontic microbiology remain to be addressed. Advances in knowledge and technology open the perspectives for many questions to be answered and consequently many others to be raised in the near future. Quality of the new information and the speed of advances in science will fundamentally depend on the spread of the use of state-of-the-art technology and the ability to continually recruit qualified manpower to the research of microbiology associated with apical periodontitis.
Conflict of interest and funding
There is no conflict of interest in the present study for any of the authors.
References
- 1.Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–9. doi: 10.1016/0030-4220(65)90166-0. [DOI] [PubMed] [Google Scholar]
- 2.Sundqvist G. Odontological Dissertation no.7, University of Umea, Umea, Sweden. 1976. Bacteriological studies of necrotic dental pulps. [Google Scholar]
- 3.Bergenholtz G. Micro-organisms from necrotic pulp of traumatized teeth. Odontol Revy. 1974;25:347–58. [PubMed] [Google Scholar]
- 4.Saboia-Dantas CJ, Coutrin de Toledo LF, Sampaio-Filho HR, Siqueira JF., Jr Herpesviruses in asymptomatic apical periodontitis lesions: an immunohistochemical approach. Oral Microbiol Immunol. 2007;22:320–5. doi: 10.1111/j.1399-302X.2007.00362.x. [DOI] [PubMed] [Google Scholar]
- 5.Vianna ME, Conrads G, Gomes BPFA, Horz HP. Identification and quantification of archaea involved in primary endodontic infections. J Clin Microbiol. 2006;44:1274–82. doi: 10.1128/JCM.44.4.1274-1282.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siqueira JF, Jr, Sen BH. Fungi in endodontic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:632–41. doi: 10.1016/S1079210404000046. [DOI] [PubMed] [Google Scholar]
- 7.Slots J, Sabeti M, Simon JH. Herpesviruses in periapical pathosis: an etiopathogenic relationship? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:327–31. doi: 10.1016/s1079-2104(03)00352-4. [DOI] [PubMed] [Google Scholar]
- 8.Waltimo TM, Sen BH, Meurman JH, Orstavik D, Haapasalo MP. Yeasts in apical periodontitis. Crit Rev Oral Biol Med. 2003;14:128–37. doi: 10.1177/154411130301400206. [DOI] [PubMed] [Google Scholar]
- 9.Nair PNR. Light and electron microscopic studies of root canal flora and periapical lesions. J Endod. 1987;13:29–39. doi: 10.1016/S0099-2399(87)80089-4. [DOI] [PubMed] [Google Scholar]
- 10.Siqueira JF, Jr, Rôças IN, Lopes HP. Patterns of microbial colonization in primary root canal infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:174–8. doi: 10.1067/moe.2002.119910. [DOI] [PubMed] [Google Scholar]
- 11.Molven O, Olsen I, Kerekes K. Scanning electron microscopy of bacteria in the apical part of root canals in permanent teeth with periapical lesions. Endod Dent Traumatol. 1991;7:226–9. doi: 10.1111/j.1600-9657.1991.tb00441.x. [DOI] [PubMed] [Google Scholar]
- 12.Siqueira JF, Jr, Rôças IN. Community as the unit of pathogenicity: an emerging concept as to the microbial pathogenesis of apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:870–8. doi: 10.1016/j.tripleo.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 13.Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149:279–94. doi: 10.1099/mic.0.26082-0. [DOI] [PubMed] [Google Scholar]
- 14.Kuramitsu HK, He X, Lux R, Anderson MH, Shi W. Interspecies interactions within oral microbial communities. Microbiol Mol Biol Rev. 2007;71:653–70. doi: 10.1128/MMBR.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costerton JW. The biofilm primer. Berlin, Heidelberg: Springer-Verlag; 2007. [Google Scholar]
- 16.Nocker A, Burr M, Camper AK. Genotypic microbial community profiling: a critical technical review. Microb Ecol. 2007;54:276–89. doi: 10.1007/s00248-006-9199-5. [DOI] [PubMed] [Google Scholar]
- 17.Siqueira JF, Jr, Rôças IN, Rosado AS. Application of denaturing gradient gel electrophoresis (DGGE) to the analysis of endodontic infections. J Endod. 2005;31:775–82. doi: 10.1097/01.don.0000155221.33667.bb. [DOI] [PubMed] [Google Scholar]
- 18.Machado de Oliveira JC, Siqueira JF, Jr, Rôças IN, Baumgartner JC, Xia T, Peixoto RS, et al. Bacterial community profiles of endodontic abscesses from Brazilian and USA subjects as compared by denaturing gradient gel electrophoresis analysis. Oral Microbiol Immunol. 2007;22:14–8. doi: 10.1111/j.1399-302X.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 19.Siqueira JF, Jr, Rôças IN, Rosado AS. Investigation of bacterial communities associated with asymptomatic and symptomatic endodontic infections by denaturing gradient gel electrophoresis fingerprinting approach. Oral Microbiol Immunol. 2004;19:363–70. doi: 10.1111/j.1399-302x.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- 20.Rôças IN, Siqueira JF, Jr, Aboim MC, Rosado AS. Denaturing gradient gel electrophoresis analysis of bacterial communities associated with failed endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:741–9. doi: 10.1016/j.tripleo.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Machado de Oliveira JC, Gama TG, Siqueira JF, Jr, Rocas IN, Peixoto RS, Rosado AS. On the use of denaturing gradient gel electrophoresis approach for bacterial identification in endodontic infections. Clin Oral Investig. 2007;11:127–32. doi: 10.1007/s00784-006-0085-9. [DOI] [PubMed] [Google Scholar]
- 22.Siqueira JF, Jr, Rôças IN, Cunha CD, Rosado AS. Novel bacterial phylotypes in endodontic infections. J Dent Res. 2005;84:565–9. doi: 10.1177/154405910508400615. [DOI] [PubMed] [Google Scholar]
- 23.Siqueira JF, Jr, Rôças IN, Debelian G, Carmo FL, Paiva SS, Alves FR, et al. Profiling of root canal bacterial communities associated with chronic apical periodontitis from Brazilian and Norwegian subjects. J Endod. 2008;34:1457–61. doi: 10.1016/j.joen.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 24.Siqueira JF, Jr, Rôças IN. Exploiting molecular methods to explore endodontic infections: part 2 – redefining the endodontic microbiota. J Endod. 2005;31:488–98. doi: 10.1097/01.don.0000157990.86638.49. [DOI] [PubMed] [Google Scholar]
- 25.Saito D, de Toledo Leonardo R, Rodrigues JLM, Tsai SM, Hofling JF, Gonçalves RB. Identification of bacteria in endodontic infections by sequence analysis of 16S rDNA clone libraries. J Med Microbiol. 2006;55:101–7. doi: 10.1099/jmm.0.46212-0. [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto M, Rôças IN, Siqueira JF, Jr, Benno Y. Molecular analysis of bacteria in asymptomatic and symptomatic endodontic infections. Oral Microbiol Immunol. 2006;21:112–22. doi: 10.1111/j.1399-302X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 27.Munson MA, Pitt-Ford T, Chong B, Weightman A, Wade WG. Molecular and cultural analysis of the microflora associated with endodontic infections. J Dent Res. 2002;81:761–6. doi: 10.1177/0810761. [DOI] [PubMed] [Google Scholar]
- 28.Siqueira JF, Jr, Rôças IN. Uncultivated phylotypes and newly named species associated with primary and persistent endodontic infections. J Clin Microbiol. 2005;43:3314–9. doi: 10.1128/JCM.43.7.3314-3319.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakamoto M, Siqueira JF, Jr, Rôças IN, Benno Y. Bacterial reduction and persistence after endodontic treatment procedures. Oral Microbiol Immunol. 2007;22:19–23. doi: 10.1111/j.1399-302X.2007.00315.x. [DOI] [PubMed] [Google Scholar]
- 30.Rôças IN, Siqueira JF., Jr Root canal microbiota of teeth with chronic apical periodontitis. J Clin Microbiol. 2008;46:3599–606. doi: 10.1128/JCM.00431-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siqueira JF, Jr, Rôças IN, Moraes SR, Santos KR. Direct amplification of rRNA gene sequences for identification of selected oral pathogens in root canal infections. Int Endod J. 2002;35:345–51. doi: 10.1046/j.1365-2591.2002.00485.x. [DOI] [PubMed] [Google Scholar]
- 32.Vickerman MM, Brossard KA, Funk DB, Jesionowski AM, Gill SR. Phylogenetic analysis of bacterial and archaeal species in symptomatic and asymptomatic endodontic infections. J Med Microbiol. 2007;56:110–8. doi: 10.1099/jmm.0.46835-0. [DOI] [PubMed] [Google Scholar]
- 33.Sabeti M, Simon JH, Slots J. Cytomegalovirus and Epstein-Barr virus are associated with symptomatic periapical pathosis. Oral Microbiol Immunol. 2003;18:327–8. doi: 10.1034/j.1399-302x.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 34.Sabeti M, Valles Y, Nowzari H, Simon JH, Kermani-Arab V, Slots J. Cytomegalovirus and Epstein-Barr virus DNA transcription in endodontic symptomatic lesions. Oral Microbiol Immunol. 2003;18:104–8. doi: 10.1034/j.1399-302x.2003.00055.x. [DOI] [PubMed] [Google Scholar]
- 35.Elkins DA, Torabinejad M, Schmidt RE, Rossi JJ, Kettering JD. Polymerase chain reaction detection of human immunodeficiency virus DNA in human periradicular lesions. J Endod. 1994;20:386–8. doi: 10.1016/S0099-2399(06)80296-7. [DOI] [PubMed] [Google Scholar]
- 36.Chen V, Chen Y, Li H, Kent K, Baumgartner JC, Machida CA. Herpesviruses in abscesses and cellulitis of endodontic origin. J Endod. 2009;35:182–8. doi: 10.1016/j.joen.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H, Chen V, Chen Y, Baumgartner JC, Machida CA. Herpesviruses in endodontic pathoses: association of Epstein-Barr virus with irreversible pulpitis and apical periodontitis. J Endod. 2009;35:23–9. doi: 10.1016/j.joen.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Sunde PT, Olsen I, Enersen M, Beiske K, Grinde B. Human cytomegalovirus and Epstein-Barr virus in apical and marginal periodontitis: a role in pathology? J Med Virol. 2008;80:1007–11. doi: 10.1002/jmv.21180. [DOI] [PubMed] [Google Scholar]
- 39.Blome B, Braun A, Sobarzo V, Jepsen S. Molecular identification and quantification of bacteria from endodontic infections using real-time polymerase chain reaction. Oral Microbiol Immunol. 2008;23:384–90. doi: 10.1111/j.1399-302X.2008.00440.x. [DOI] [PubMed] [Google Scholar]
- 40.Siqueira JF, Jr, Rôças IN, Paiva SS, Guimarães-Pinto T, Magalhães KM, Lima KC. Bacteriologic investigation of the effects of sodium hypochlorite and chlorhexidine during the endodontic treatment of teeth with apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:122–30. doi: 10.1016/j.tripleo.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 41.Vianna ME, Horz HP, Gomes BP, Conrads G. In vivo evaluation of microbial reduction after chemo-mechanical preparation of human root canals containing necrotic pulp tissue. Int Endod J. 2006;39:484–92. doi: 10.1111/j.1365-2591.2006.01121.x. [DOI] [PubMed] [Google Scholar]
- 42.Siqueira JF, Jr, Rôças IN, Paiva SSM, Magalhães KM, Guimarães-Pinto T. Cultivable bacteria in infected root canals as identified by 16S rRNA gene sequencing. Oral Microbiol Immunol. 2007;22:266–71. doi: 10.1111/j.1399-302X.2007.00355.x. [DOI] [PubMed] [Google Scholar]
- 43.Haapasalo M, Ranta H, Ranta K, Shah H. Black-pigmented Bacteroides spp. in human apical periodontitis. Infect Immun. 1986;53:149–53. doi: 10.1128/iai.53.1.149-153.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sundqvist G. Associations between microbial species in dental root canal infections. Oral Microbiol Immunol. 1992;7:257–62. doi: 10.1111/j.1399-302x.1992.tb00584.x. [DOI] [PubMed] [Google Scholar]
- 45.Gomes BP, Lilley JD, Drucker DB. Clinical significance of dental root canal microflora. J Dent. 1996;24:47–55. doi: 10.1016/0300-5712(95)00042-9. [DOI] [PubMed] [Google Scholar]
- 46.Khemaleelakul S, Baumgartner JC, Pruksakorn S. Identification of bacteria in acute endodontic infections and their antimicrobial susceptibility. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:746–55. doi: 10.1067/moe.2002.129535. [DOI] [PubMed] [Google Scholar]
- 47.Baumgartner JC, Watkins BJ, Bae KS, Xia T. Association of black-pigmented bacteria with endodontic infections. J Endod. 1999;25:413–5. doi: 10.1016/S0099-2399(99)80268-4. [DOI] [PubMed] [Google Scholar]
- 48.Fouad AF, Barry J, Caimano M, Clawson M, Zhu Q, Carver R, et al. PCR-based identification of bacteria associated with endodontic infections. J Clin Microbiol. 2002;40:3223–31. doi: 10.1128/JCM.40.9.3223-3231.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siqueira JF, Jr, Rôças IN, Souto R, Uzeda M, Colombo AP. Microbiological evaluation of acute periradicular abscesses by DNA-DNA hybridization. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:451–7. doi: 10.1067/moe.2001.118620. [DOI] [PubMed] [Google Scholar]
- 50.Siqueira JF, Jr, Rôças IN, Souto R, de Uzeda M, Colombo AP. Checkerboard DNA-DNA hybridization analysis of endodontic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:744–8. doi: 10.1067/moe.2000.106576. [DOI] [PubMed] [Google Scholar]
- 51.Foschi F, Cavrini F, Montebugnoli L, Stashenko P, Sambri V, Prati C. Detection of bacteria in endodontic samples by polymerase chain reaction assays and association with defined clinical signs in Italian patients. Oral Microbiol Immunol. 2005;20:289–95. doi: 10.1111/j.1399-302X.2005.00227.x. [DOI] [PubMed] [Google Scholar]
- 52.Siqueira JF, Jr, Rôças IN. The microbiota of acute apical abscesses. J Dent Res. 2009;88:61–5. doi: 10.1177/0022034508328124. [DOI] [PubMed] [Google Scholar]
- 53.Siqueira JF, Jr, Rôças IN. Molecular analysis of endodontic infections. In: Fouad AF, editor. Endodontic microbiology. Ames, IA: Wiley-Blackwell; 2009. pp. 68–107. [Google Scholar]
- 54.Conrads G, Gharbia SE, Gulabivala K, Lampert F, Shah HN. The use of a 16S rDNA directed PCR for the detection of endodontopathogenic bacteria. J Endod. 1997;23:433–8. doi: 10.1016/S0099-2399(97)80297-X. [DOI] [PubMed] [Google Scholar]
- 55.Siqueira JF, Jr, Rôças IN. Dialister pneumosintes can be a suspected endodontic pathogen. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:494–8. doi: 10.1067/moe.2002.125202. [DOI] [PubMed] [Google Scholar]
- 56.Siqueira JF, Jr, Rôças IN. Detection of Filifactor alocis in endodontic infections associated with different forms of periradicular diseases. Oral Microbiol Immunol. 2003;18:263–5. doi: 10.1034/j.1399-302x.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 57.Siqueira JF, Jr, Rôças IN, Favieri A, Santos KR. Detection of Treponema denticola in endodontic infections by 16S rRNA gene-directed polymerase chain reaction. Oral Microbiol Immunol. 2000;15:335–7. doi: 10.1034/j.1399-302x.2000.150512.x. [DOI] [PubMed] [Google Scholar]
- 58.Sakamoto M, Siqueira JF, Jr, Rocas IN, Benno Y. Diversity of spirochetes in endodontic infections. J Clin Microbiol. 2009;47:1352–7. doi: 10.1128/JCM.02016-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gomes BP, Jacinto RC, Pinheiro ET, Sousa EL, Zaia AA, Ferraz CC, et al. Molecular analysis of Filifactor alocis, Tannerella forsythia, and Treponema denticola associated with primary endodontic infections and failed endodontic treatment. J Endod. 2006;32:937–40. doi: 10.1016/j.joen.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 60.Baumgartner JC, Khemaleelakul SU, Xia T. Identification of spirochetes (treponemes) in endodontic infections. J Endod. 2003;29:794–7. doi: 10.1097/00004770-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 61.Rôças IN, Baumgartner JC, Xia T, Siqueira JF., Jr Prevalence of selected bacterial named species and uncultivated phylotypes in endodontic abscesses from two geographic locations. J Endod. 2006;32:1135–8. doi: 10.1016/j.joen.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Baumgartner JC, Siqueira JF, Jr, Xia T, Rôças IN. Geographical differences in bacteria detected in endodontic infections using polymerase chain reaction. J Endod. 2004;30:141–4. doi: 10.1097/00004770-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 63.Jung IY, Choi BK, Kum KY, Roh BD, Lee SJ, Lee CY, et al. Molecular epidemiology and association of putative pathogens in root canal infection. J Endod. 2000;26:599–604. doi: 10.1097/00004770-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 64.Siqueira JF, Jr, Rôças IN. Pseudoramibacter alactolyticus in primary endodontic infections. J Endod. 2003;29:735–8. doi: 10.1097/00004770-200311000-00012. [DOI] [PubMed] [Google Scholar]
- 65.Rolph HJ, Lennon A, Riggio MP, Saunders WP, MacKenzie D, Coldero L, et al. Molecular identification of microorganisms from endodontic infections. J Clin Microbiol. 2001;39:3282–9. doi: 10.1128/JCM.39.9.3282-3289.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rôças IN, Siqueira JF., Jr Detection of novel oral species and phylotypes in symptomatic endodontic infections including abscesses. FEMS Microbiol Lett. 2005;250:279–85. doi: 10.1016/j.femsle.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 67.Rôças IN, Siqueira JF., Jr Characterization of Dialister species in infected root canals. J Endod. 2006;32:1057–61. doi: 10.1016/j.joen.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 68.Siqueira JF, Jr, Rôças IN. Molecular detection and identification of Synergistes phylotypes in primary endodontic infections. Oral Dis. 2007;13:398–401. doi: 10.1111/j.1601-0825.2006.01301.x. [DOI] [PubMed] [Google Scholar]
- 69.Kuriyama T, Karasawa T, Nakagawa K, Saiki Y, Yamamoto E, Nakamura S. Bacteriologic features and antimicrobial susceptibility in isolates from orofacial odontogenic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:600–8. doi: 10.1067/moe.2000.109639. [DOI] [PubMed] [Google Scholar]
- 70.de Sousa EL, Ferraz CC, Gomes BP, Pinheiro ET, Teixeira FB, de Souza-Filho FJ. Bacteriological study of root canals associated with periapical abscesses. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:332–9. doi: 10.1016/s1079-2104(03)00261-0. [DOI] [PubMed] [Google Scholar]
- 71.Lewis MA, MacFarlane TW, McGowan DA. Quantitative bacteriology of acute dento-alveolar abscesses. J Med Microbiol. 1986;21:101–4. doi: 10.1099/00222615-21-2-101. [DOI] [PubMed] [Google Scholar]
- 72.Williams BL, McCann GF, Schoenknecht FD. Bacteriology of dental abscesses of endodontic origin. J Clin Microbiol. 1983;18:770–4. doi: 10.1128/jcm.18.4.770-774.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Griffee MB, Patterson SS, Miller CH, Kafrawy AH, Newton CW. The relationship of Bacteroides melaninogenicus to symptoms associated with pulpal necrosis. Oral Surg Oral Med Oral Pathol. 1980;50:457–61. doi: 10.1016/s0030-4220(80)80015-6. [DOI] [PubMed] [Google Scholar]
- 74.Yoshida M, Fukushima H, Yamamoto K, Ogawa K, Toda T, Sagawa H. Correlation between clinical symptoms and microorganisms isolated from root canals of teeth with periapical pathosis. J Endod. 1987;13:24–8. doi: 10.1016/S0099-2399(87)80088-2. [DOI] [PubMed] [Google Scholar]
- 75.van Winkelhoff AJ, Carlee AW, de Graaff J. Bacteroides endodontalis and others black-pigmented Bacteroides species in odontogenic abscesses. Infect Immun. 1985;49:494–8. doi: 10.1128/iai.49.3.494-497.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rôças IN, Siqueira JF, Jr, Andrade AFB, Uzeda M. Identification of selected putative oral pathogens in primary root canal infections associated with symptoms. Anaerobe. 2002;8:200–8. [Google Scholar]
- 77.Siqueira JF, Jr, Barnett F. Interappointment pain: mechanisms, diagnosis, and treatment. Endod Topics. 2004;7:93–109. [Google Scholar]
- 78.Siqueira JF., Jr Microbial causes of endodontic flare-ups. Int Endod J. 2003;36:453–63. doi: 10.1046/j.1365-2591.2003.00671.x. [DOI] [PubMed] [Google Scholar]
- 79.Miller WD. An introduction to the study of the bacterio-pathology of the dental pulp. Dent Cosmos. 1894;36:505–28. [Google Scholar]
- 80.Thilo BE, Baehni P, Holz J. Dark-field observation of the bacterial distribution in root canals following pulp necrosis. J Endod. 1986;12:202–5. doi: 10.1016/S0099-2399(86)80155-8. [DOI] [PubMed] [Google Scholar]
- 81.Alves FR, Siqueira JF, Jr, Carmo FL, Santos AL, Peixoto RS, Rocas IN, et al. Bacterial community profiling of cryogenically ground samples from the apical and coronal root segments of teeth with apical periodontitis. J Endod. 2009;35:486–92. doi: 10.1016/j.joen.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 82.Fabricius L, Dahlén G, Ohman AE, Möller AJR. Predominant indigenous oral bacteria isolated from infected root canals after varied times of closure. Scand J Dent Res. 1982;90:134–44. doi: 10.1111/j.1600-0722.1982.tb01536.x. [DOI] [PubMed] [Google Scholar]
- 83.Baumgartner JC, Falkler WA., Jr Bacteria in the apical 5 mm of infected root canals. J Endod. 1991;17:380–3. doi: 10.1016/s0099-2399(06)81989-8. [DOI] [PubMed] [Google Scholar]
- 84.Dougherty WJ, Bae KS, Watkins BJ, Baumgartner JC. Black-pigmented bacteria in coronal and apical segments of infected root canals. J Endod. 1998;24:356–8. doi: 10.1016/S0099-2399(98)80134-9. [DOI] [PubMed] [Google Scholar]
- 85.Siqueira JF, Jr, Rôças IN, Alves FR, Santos KR. Selected endodontic pathogens in the apical third of infected root canals: a molecular investigation. J Endod. 2004;30:638–43. doi: 10.1097/01.don.0000125875.88377.85. [DOI] [PubMed] [Google Scholar]
- 86.Siqueira JF, Jr, Rocas IN, Alves FR, Silva MG. Bacteria in the apical root canal of teeth with primary apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:721–6. doi: 10.1016/j.tripleo.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 87.Siqueira JF, Jr, Lima KC. Staphylococcus epidermidis and Staphylococcus xylosus in a secondary root canal infection with persistent symptoms: a case report. Aust Endod J. 2002;28:61–3. doi: 10.1111/j.1747-4477.2002.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 88.Siren EK, Haapasalo MP, Ranta K, Salmi P, Kerosuo EN. Microbiological findings and clinical treatment procedures in endodontic cases selected for microbiological investigation. Int Endod J. 1997;30:91–5. [PubMed] [Google Scholar]
- 89.Ranta K, Haapasalo M, Ranta H. Monoinfection of root canal with Pseudomonas aeruginosa . Endod Dent Traumatol. 1988;4:269–72. doi: 10.1111/j.1600-9657.1988.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 90.Haapasalo M, Ranta H, Ranta KT. Facultative gram-negative enteric rods in persistent periapical infections. Acta Odontol Scand. 1983;41:19–22. doi: 10.3109/00016358309162299. [DOI] [PubMed] [Google Scholar]
- 91.Chavez de Paz LE. Fusobacterium nucleatum in endodontic flare-ups. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:179–83. doi: 10.1067/moe.2002.120803. [DOI] [PubMed] [Google Scholar]
- 92.Fabricius L, Dahlén G, Sundqvist G, Happonen RP, Möller AJR. Influence of residual bacteria on periapical tissue healing after chemomechanical treatment and root filling of experimentally infected monkey teeth. Eur J Oral Sci. 2006;114:278–85. doi: 10.1111/j.1600-0722.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 93.Sjögren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997;30:297–306. doi: 10.1046/j.1365-2591.1997.00092.x. [DOI] [PubMed] [Google Scholar]
- 94.Waltimo T, Trope M, Haapasalo M, Orstavik D. Clinical efficacy of treatment procedures in endodontic infection control and one year follow-up of periapical healing. J Endod. 2005;31:863–6. doi: 10.1097/01.don.0000164856.27920.85. [DOI] [PubMed] [Google Scholar]
- 95.Lin LM, Skribner JE, Gaengler P. Factors associated with endodontic treatment failures. J Endod. 1992;18:625–7. doi: 10.1016/S0099-2399(06)81335-X. [DOI] [PubMed] [Google Scholar]
- 96.Lin LM, Pascon EA, Skribner J, Gangler P, Langeland K. Clinical, radiographic, and histologic study of endodontic treatment failures. Oral Surg Oral Med Oral Pathol. 1991;71:603–11. doi: 10.1016/0030-4220(91)90371-i. [DOI] [PubMed] [Google Scholar]
- 97.Rôças IN, Jung IY, Lee CY, Siqueira JF., Jr Polymerase chain reaction identification of microorganisms in previously root-filled teeth in a South Korean population. J Endod. 2004;30:504–8. [PubMed] [Google Scholar]
- 98.Siqueira JF, Jr, Rôças IN. Polymerase chain reaction-based analysis of microorganisms associated with failed endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:85–94. doi: 10.1016/s1079-2104(03)00353-6. [DOI] [PubMed] [Google Scholar]
- 99.Sundqvist G, Figdor D, Persson S, Sjogren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:86–93. doi: 10.1016/s1079-2104(98)90404-8. [DOI] [PubMed] [Google Scholar]
- 100.Pinheiro ET, Gomes BP, Ferraz CC, Sousa EL, Teixeira FB, Souza-Filho FJ. Microorganisms from canals of root-filled teeth with periapical lesions. Int Endod J. 2003;36:1–11. doi: 10.1046/j.1365-2591.2003.00603.x. [DOI] [PubMed] [Google Scholar]
- 101.Ricucci D, Siqueira JF, Jr, Bate AL, Pitt Ford TR. Histologic investigation of root canal-treated teeth with apical periodontitis: a retrospective study from twenty-four patients. J Endod. 2009;35:493–502. doi: 10.1016/j.joen.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 102.Rôças IN, Hulsmann M, Siqueira JF., Jr Microorganisms in root canal-treated teeth from a German population. J Endod. 2008;34:926–31. doi: 10.1016/j.joen.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 103.Byström A, Sundqvist G. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int Endod J. 1985;18:35–40. doi: 10.1111/j.1365-2591.1985.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 104.Siqueira JF, Jr, Paiva SS, Rôças IN. Reduction in the cultivable bacterial populations in infected root canals by a chlorhexidine-based antimicrobial protocol. J Endod. 2007;33:541–7. doi: 10.1016/j.joen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 105.Siqueira JF, Jr, Magalhães KM, Rôças IN. Bacterial reduction in infected root canals treated with 2.5% NaOCl as an irrigant and calcium hydroxide/camphorated paramonochlorophenol paste as an intracanal dressing. J Endod. 2007;33:667–72. doi: 10.1016/j.joen.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 106.Gomes BP, Lilley JD, Drucker DB. Variations in the susceptibilities of components of the endodontic microflora to biomechanical procedures. Int Endod J. 1996;29:235–41. doi: 10.1111/j.1365-2591.1996.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 107.Peciuliene V, Reynaud AH, Balciuniene I, Haapasalo M. Isolation of yeasts and enteric bacteria in root-filled teeth with chronic apical periodontitis. Int Endod J. 2001;34:429–34. doi: 10.1046/j.1365-2591.2001.00411.x. [DOI] [PubMed] [Google Scholar]
- 108.Peters LB, van Winkelhoff AJ, Buijs JF, Wesselink PR. Effects of instrumentation, irrigation and dressing with calcium hydroxide on infection in pulpless teeth with periapical bone lesions. Int Endod J. 2002;35:13–21. doi: 10.1046/j.0143-2885.2001.00447.x. [DOI] [PubMed] [Google Scholar]
- 109.Chavez de Paz LE. PhD thesis, Göteborg University, Göteborg, Sweden. 2005. On bacteria persisting root canal treatment. Identification and potential mechanisms of resistance to antimicrobial measures. [Google Scholar]
- 110.Chu FC, Leung WK, Tsang PC, Chow TW, Samaranayake LP. Identification of cultivable microorganisms from root canals with apical periodontitis following two-visit endodontic treatment with antibiotics/steroid or calcium hydroxide dressings. J Endod. 2006;32:17–23. doi: 10.1016/j.joen.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 111.Siqueira JF, Jr, Guimarães-Pinto T, Rôças IN. Effects of chemomechanical preparation with 2.5% sodium hypochlorite and intracanal medication with calcium hydroxide on cultivable bacteria in infected root canals. J Endod. 2007;33:800–5. doi: 10.1016/j.joen.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 112.Sedgley C, Nagel A, Dahlen G, Reit C, Molander A. Real-time quantitative polymerase chain reaction and culture analyses of Enterococcus faecalis in root canals. J Endod. 2006;32:173–7. doi: 10.1016/j.joen.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 113.Molander A, Reit C, Dahlen G, Kvist T. Microbiological status of root-filled teeth with apical periodontitis. Int Endod J. 1998;31:1–7. [PubMed] [Google Scholar]
- 114.Zoletti GO, Siqueira JF, Jr, Santos KR. Identification of Enterococcus faecalis in root-filled teeth with or without periradicular lesions by culture-dependent and -independent approaches. J Endod. 2006;32:722–6. doi: 10.1016/j.joen.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 115.Rôças IN, Siqueira JF, Jr, Santos KR. Association of Enterococcus faecalis with different forms of periradicular diseases. J Endod. 2004;30:315–20. doi: 10.1097/00004770-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 116.Zehnder M, Guggenheim B. The mysterious appearance of enterococci in filled root canals. Int Endod J. 2009;42:277–87. doi: 10.1111/j.1365-2591.2008.01537.x. [DOI] [PubMed] [Google Scholar]
- 117.Cheung GS, Ho MW. Microbial flora of root canal-treated teeth associated with asymptomatic periapical radiolucent lesions. Oral Microbiol Immunol. 2001;16:332–7. doi: 10.1034/j.1399-302x.2001.160603.x. [DOI] [PubMed] [Google Scholar]
- 118.Kaufman B, Spangberg L, Barry J, Fouad AF. Enterococcus spp. in endodontically treated teeth with and without periradicular lesions. J Endod. 2005;31:851–6. doi: 10.1097/01.don.0000164133.04548.26. [DOI] [PubMed] [Google Scholar]
- 119.Sakamoto M, Siqueira JF, Jr, Rôças IN, Benno Y. Molecular analysis of the root canal microbiota associated with endodontic treatment failures. Oral Microbiol Immunol. 2008;23:275–81. doi: 10.1111/j.1399-302X.2007.00423.x. [DOI] [PubMed] [Google Scholar]
- 120.Möller AJR. Microbial examination of root canals and periapical tissues of human teeth. Odontol Tidskr. 1966;74:1–380. [PubMed] [Google Scholar]
- 121.Egan MW, Spratt DA, Ng YL, Lam JM, Moles DR, Gulabivala K. Prevalence of yeasts in saliva and root canals of teeth associated with apical periodontitis. Int Endod J. 2002;35:321–9. doi: 10.1046/j.1365-2591.2002.00478.x. [DOI] [PubMed] [Google Scholar]
- 122.Nair PN, Sjogren U, Krey G, Kahnberg KE, Sundqvist G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: a long-term light and electron microscopic follow-up study. J Endod. 1990;16:580–8. doi: 10.1016/S0099-2399(07)80201-9. [DOI] [PubMed] [Google Scholar]
- 123.Siqueira JF., Jr Periapical actinomycosis and infection with Propionibacterium propionicum . Endod Topics. 2003;6:78–95. [Google Scholar]
- 124.Happonen RP. Periapical actinomycosis: a follow-up study of 16 surgically treated cases. Endod Dent Traumatol. 1986;2:205–9. doi: 10.1111/j.1600-9657.1986.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 125.Sjögren U, Happonen RP, Kahnberg KE, Sundqvist G. Survival of Arachnia propionica in periapical tissue. Int Endod J. 1988;21:277–82. doi: 10.1111/j.1365-2591.1988.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 126.Ricucci D, Siqueira JF., Jr Apical actinomycosis as a continuum of intraradicular and extraradicular infection: case report and critical review on its involvement with treatment failure. J Endod. 2008;34:1124–9. doi: 10.1016/j.joen.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 127.Sunde PT, Olsen I, Gobel UB, Theegarten D, Winter S, Debelian GJ, et al. Fluorescence in situ hybridization (FISH) for direct visualization of bacteria in periapical lesions of asymptomatic root-filled teeth. Microbiology. 2003;149:1095–102. doi: 10.1099/mic.0.26077-0. [DOI] [PubMed] [Google Scholar]
- 128.Sunde PT, Tronstad L, Eribe ER, Lind PO, Olsen I. Assessment of periradicular microbiota by DNA-DNA hybridization. Endod Dent Traumatol. 2000;16:191–6. doi: 10.1034/j.1600-9657.2000.016005191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gatti JJ, Dobeck JM, Smith C, White RR, Socransky SS, Skobe Z. Bacteria of asymptomatic periradicular endodontic lesions identified by DNA-DNA hybridization. Endod Dent Traumatol. 2000;16:197–204. doi: 10.1034/j.1600-9657.2000.016005197.x. [DOI] [PubMed] [Google Scholar]
- 130.Handal T, Caugant DA, Olsen I, Sunde PT. Bacterial diversity in persistent periapical lesions on root-filled teeth. J Oral Microbiol. 2009;1:1–7. doi: 10.3402/jom.v1i0.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Subramanian K, Mickel AK. Molecular analysis of persistent periradicular lesions and root ends reveals a diverse microbial profile. J Endod. 2009;35:950–7. doi: 10.1016/j.joen.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 132.Tronstad L, Barnett F, Riso K, Slots J. Extraradicular endodontic infections. Endod Dent Traumatol. 1987;3:86–90. doi: 10.1111/j.1600-9657.1987.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 133.Barnett F, Stevens R, Tronstad L. Demonstration of Bacteroides intermedius in periapical tissue using indirect immunofluorescence microscopy. Endod Dent Traumatol. 1990;6:153–6. doi: 10.1111/j.1600-9657.1990.tb00411.x. [DOI] [PubMed] [Google Scholar]
- 134.Sjögren U, Hanstrom L, Happonen RP, Sundqvist G. Extensive bone loss associated with periapical infection with Bacteroides gingivalis: a case report. Int Endod J. 1990;23:254–62. doi: 10.1111/j.1365-2591.1990.tb00858.x. [DOI] [PubMed] [Google Scholar]
- 135.van Winkelhoff AJ, van Steenbergen TJ, de Graaff J. Porphyromonas (Bacteroides) endodontalis: its role in endodontal infections. J Endod. 1992;18:431–4. doi: 10.1016/s0099-2399(06)80843-5. [DOI] [PubMed] [Google Scholar]
- 136.Fenno JC, McBride BC. Virulence factors of oral treponemes. Anaerobe. 1998;4:1–17. doi: 10.1006/anae.1997.0131. [DOI] [PubMed] [Google Scholar]
- 137.Bolstad AI, Jensen HB, Bakken V. Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum . Clin Microbiol Rev. 1996;9:55–71. doi: 10.1128/cmr.9.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2000;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 139.Al Masalma M, Armougom F, Scheld WM, Dufour H, Roche PH, Drancourt M, et al. The expansion of the microbiological spectrum of brain abscesses with use of multiple 16S ribosomal DNA sequencing. Clin Infect Dis. 2009;48:1169–78. doi: 10.1086/597578. [DOI] [PubMed] [Google Scholar]
- 140.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55:205–11. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–9. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Palmer C, Bik EM, Digiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huyghe A, Francois P, Charbonnier Y, Tangomo-Bento M, Bonetti EJ, Paster BJ, et al. Novel microarray design strategy to study complex bacterial communities. Appl Environ Microbiol. 2008;74:1876–85. doi: 10.1128/AEM.01722-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Palmer C, Bik EM, Eisen MB, Eckburg PB, Sana TR, Wolber PK, et al. Rapid quantitative profiling of complex microbial populations. Nucleic Acids Res. 2006;34:e5. doi: 10.1093/nar/gnj007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Siqueira JF, Jr, Rocas IN, Debelian GJ, Carmo FL, Paiva SS, Alves FR, et al. Profiling of root canal bacterial communities associated with chronic apical periodontitis from Brazilian and Norwegian subjects. J Endod. 2008;34:1457–61. doi: 10.1016/j.joen.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 147.Molander A, Warfvinge J, Reit C, Kvist T. Clinical and radiographic evaluation of one- and two-visit endodontic treatment of asymptomatic necrotic teeth with apical periodontitis: a randomized clinical trial. J Endod. 2007;33:1145–8. doi: 10.1016/j.joen.2007.07.005. [DOI] [PubMed] [Google Scholar]