Abstract
Background
Periodontitis has been reported to be associated with coronary artery disease (CAD). Research is needed to determine if therapies that improve periodontal health also reduce systemic measures of inflammation associated with both diseases.
Methods
128 postmenopausal women with chronic periodontitis were randomly assigned to twice-daily subantimicrobial dose doxycycline (SDD) or placebo tablets for two years adjunctive to periodontal maintenance therapy. Through a supplement to the main trial investigating alveolar bone and clinical periodontal changes, inflammatory mediators and lipid profiles were assayed in baseline, one- and two-year serum samples. Data were analyzed by generalized estimating equations.
Results
In the intent-to-treat population over two years, SDD treatment reduced median high-sensitivity C-reactive protein (hs-CRP) by 18% (primary outcome; p=0.02) and reduced serum matrix metalloproteinase-9 (MMP-9; 92 kilodalton gelatinase) (difference in mean scanning units: −28.44; p<0.0001), with no significant effect on serum lipids. However, in women more than five years postmenopausal, SDD elevated high-density lipoprotein (HDL) cholesterol (difference in means [mg/dl]: 5.99; p=0.01).
Conclusions
A two-year SDD regimen in postmenopausal women significantly reduced the serum inflammatory biomarkers hs-CRP and MMP-9 and, among women more than five years postmenopausal, raised HDL cholesterol.
Clinical Implications
SDD significantly reduced the systemic inflammatory biomarkers, hs-CRP and MMP-9. More research is needed to determine whether SDD has a role in CAD risk management.
Keywords: C-reactive protein, doxycycline, inflammation, HDL cholesterol, matrix metalloproteinases, periodontitis, serum inflammatory biomarkers
INTRODUCTION
Inflammation is increasingly recognized as a significant factor in the initiation, progression and ultimate instability of atherosclerotic plaques during coronary artery disease (CAD).1,2 In this regard, chronic periodontitis is a very common chronic inflammatory disease which is increasingly being recognized as having an association with, and potential causal relationship to, CAD.3,4 Therapeutic strategies which resolve inflammation associated with both of these pathologies, and that impact CAD onset and progression, are needed.
One group in the general population that is particularly at risk for CAD is postmenopausal women.5 To date, therapeutic attempts to limit disease onset and progression in this population, such as hormone replacement therapy, have had poor outcomes.6
In atherosclerotic CAD pathogenesis, specific elements of the inflammatory process have been identified as risk factors and risk markers. For example, C-reactive protein (CRP) and matrix metalloproteinase-9 (MMP-9) have been identified as important in CAD pathogenesis and as serum markers of disease activity.7,8,9 To date, the mainstay of pharmacologic therapy to modulate these and other inflammatory mediators has been the statins,10 originally approved for their lipid-lowering effects. Previously, we showed that tetracyclines, including their chemically-modified analogs, have immunomodulatory effects independent of antimicrobial activity.11 In particular, doxycycline at a low dose (i.e., subantimicrobial dose doxycycline [SDD]) in humans modulates matrix metalloproteinase (MMP) activity and/or reduces severity of inflammatory diseases such as periodontitis,12 rheumatoid arthritis13 and lymphangioleiomyomatosis.14 Furthermore, the efficacy of non-antibiotic properties of tetracyclines in reducing risk factors for acute coronary events in patients has been suggested.15,16,17
We have investigated the effect of a two-year SDD regimen on alveolar bone loss,18 clinical periodontal measures,19 gingival crevicular fluid biomarkers of periodontitis,12 serum bone biomarkers,20 and adverse events18 including microbiologic measures of antibiotic resistance21 in a randomized, double-blind, placebo-controlled trial. The patient cohort was at risk for CAD (i.e., postmenopausal women), yet with no history of myocardial infarction, angina or stroke, and also exhibited chronic periodontitis. We now report results from a supplement to the main two-year clinical trial; the objective of this study is to determine whether long-term SDD therapy can reduce serum biomarkers of systemic inflammation and improve lipid profiles in postmenopausal women with systemic osteopenia and chronic periodontitis. To the best of our knowledge, this is the only long-term clinical trial examining systemic (not just oral) parameters of inflammation in periodontitis patients treated with a systemic pharmacological agent.
MATERIALS AND METHODS
Study Design and Eligibility Criteria
The trial design of the main study has been described in detail, following Consolidated Standards of Reporting Trials guidelines.18 This report presents the findings from a supplemental study, embedded in the main trial, focused on the effect of SDD versus placebo on the primary hs-CRP outcome measure and other secondary inflammatory biomarker and lipid levels. Briefly, this study was a two-year, double-blind randomized clinical trial with two treatment arms adjunctive to regular periodontal maintenance therapy: SDD (20 mg doxycycline hyclate) and a look-alike placebo. One hundred twenty-eight eligible subjects participated in this trial and all subjects signed University of Nebraska Medical Center and Stony Brook University Institutional Review Board-approved consent forms. Subjects were centrally randomized, through a call-in center, with stratification by study center (University of Nebraska Medical Center or Stony Brook) and current smoking status. The randomization list was computer-generated in blocks, with the size varying randomly among 4, 6 and 8. 64 eligible subjects were randomized to each treatment: SDD or placebo twice daily for two years. The treatment code identifying the SDD or placebo arms was concealed from the study investigators and the statistician doing the analyses (JAS) until all subject follow-up and outcomes measurements had been made. All subjects were provided calcium and vitamin D supplements to be taken twice daily (a total of 1200 mg calcium and 400 IU vitamin D daily). Subjects were instructed to take the supplements at least one hour after taking the study drug.
Subjects were recruited on a rolling-admission basis at the two study centers between June 2002 and October 2003. The protocol was amended in April 2004, at which point 13 subjects had withdrawn consent (placebo n=2, SDD n=10) or had withdrawn (SDD n=1) due to a serious adverse event, to allow measurement of inflammatory biomarkers in stored (−80°C) serum. Two additional SDD subjects withdrew following the protocol amendment and refused to complete an exit exam that included the addendum study consent request. The last subject completed the trial in October 2005. 113 subjects who completed the trial consented to participate in this addendum study (SDD=51; placebo=62).
Inclusion and exclusion criteria have been published.18 Briefly, subjects were 45–70 years old at telephone screening, postmenopausal, osteopenic at the lumbar spine or femoral neck (based on dual-energy x-ray absorptiometry scans), and not receiving hormone replacement therapy. Subjects had a history of generalized moderate to advanced chronic periodontitis (subjects had to have at least two sites with probing depths and clinical attachment loss ≥ 5 mm together with bleeding on probing; the two sites had to be on different posterior teeth), were undergoing periodontal maintenance therapy and had to be in good general health. Subjects were excluded if they had a tetracycline allergy or hypersensitivity, had diseases or regular drug therapy that would affect the inflammatory or immune response, had active periodontal therapy within the past year, had a diabetes history, or had osteoporosis at either the lumbar spine or femoral neck. In addition, subjects had no history of myocardial infarction, angina or stroke.
Serum Inflammatory Mediator Analyses
Specimen samples were analyzed in 3 batches by an investigator (Dr. Hsi-ming Lee) who was blinded to treatment assignment. All samples from an individual patient were analyzed in the same batch and treatment assignments were balanced among the batches.
High-sensitivity C-reactive protein
Non-fasting blood samples were drawn at baseline, 1-year and 2-year appointments. Serum was obtained by standard technique and was stored at −80°C until analysis. High-sensitivity CRP was measured by hs-Enzyme-Linked Immunosorbent Assay (ELISA) (MP Biomedicals, Diagnostic Division, Orangeburg, NY) and was the primary outcome measure in this supplemental trial. The assay had a sensitivity of 0.1 mg/L.
Cytokine analyses
Interleukin-6, interleukin-1β and tumor necrosis factor-α levels were measured by ELISA (Biosource Int., Camarillo, CA). The sensitivity to detect IL-6, interleukin-1β and tumor necrosis factor-α was as low as 2 pg/ml, 1 pg/ml, and 1.7 pg/ml, respectively.
Myeloperoxidase (MPO)
Levels were measured by ELISA (Hycult biotechnology b.v., Frontstraat 2a, 5405 PB UDEN, The Netherlands). The sensitivity to detect MPO was as low as 0.4 ng/ml.
Lipid profile
Total cholesterol, high-density lipoprotein (HDL) cholesterol and triglycerides were measured by the Stony Brook University clinical chemistry lab using standard techniques and reagents with the Roche Modular P automated analyzer. Low-density lipoprotein (LDL) cholesterol and very low-density lipoprotein (VLDL) cholesterol levels were based on calculated results: LDL cholesterol calculated as total cholesterol – (HDL cholesterol + triglycerides/5); VLDL cholesterol calculated as triglycerides /5. The sensitivity to detect lipids was as follows: assay range = 3–800 mg/dl for total cholesterol, 3–120 mg/dl for HDL cholesterol, and 4–1000 mg/dl for triglycerides.
MMP-2 and MMP-9
MMP-2 and MMP-9 levels were measured by gelatin zymography, as described previously, and the data are presented as densitometric units.17
MMP-8 and tissue inhibitor of metalloproteinases (TIMP)-1
Serum MMP-8 concentration was determined via time-resolved immunofluorometric assay22 and TIMP-1 was determined by Western Blot plus densitometric computerized quantitation, as previously described.23 The detection limit for the MMP-8 assay was 0.08 ng/ml.
Statistical Analyses
The supplemental study sample size was justified based on hs-CRP data presented by Brown et al.17 The average two-year change in hs-CRP was estimated to be a decrease of 2.5 mg/L for SDD and a decrease of 0.5 mg/L for placebo groups, with a standard deviation of the change of 3.0 mg/L. A total sample of 50 subjects per group resulted in 90% power to detect a true difference of 2 mg/L in the mean change in hs-CRP over the two-year period, assuming a standard deviation of 3.0 mg/L and a two-sided 0.05 alpha level. Analyses were performed using SAS (SAS Institute Inc., Cary, NC, USA, version 9.1.3). Repeated measurements over time were analyzed at the subject level. Linear regression models were fit using generalized estimating equations methodology to account for the correlation among repeated measurements for each subject. The follow-up biomarker measure (outcome) was modeled as a function of study drug with adjustment for the baseline biomarker measure, visit (one-year or two-year), assay batch and randomization stratification factors (independent variables).24 A visit-by-study-drug interaction was included but dropped if not significant, in which case, the SDD effect was estimated based on one-year and two-year data combined. Outcome variables whose distributions were skewed (measures of hs-CRP, myeloperoxidase, MMP-8, TIMP-1, VLDL cholesterol, and triglyceride) were log-transformed to preserve assumptions that underlie linear regression models. Regression coefficients generated from these models, when “back transformed” by exponentiation, express multiples or ratios of group medians. Because a large percentage of observations for interleukin-6 and tumor necrosis factor-α were below the level of detection, we dichotomized those outcomes as detectable or undetectable. Logistic regression models used approaches similar to those described above for linear regression models to compare the odds of detecting interleukin-6 or tumor necrosis factor-α between SDD and placebo groups. Baseline characteristic distributions were compared between treatment groups using an independent sample t-test or Chi-square test. Linear mixed effects models, with random subject and tooth terms, were used to estimate the standard deviation of baseline site-level periodontal measures.
Two formal interim outcome analyses were performed during the course of the study using the Lan-DeMets approximation to the O’Brien-Fleming procedure.25 Interim results were reported to the independent Data and Safety Monitoring Board. Final analyses are based on a two-sided 0.05 alpha level. The primary analysis was intent-to-treat, where data were analyzed from all subjects providing addendum consent according to randomized assignment (SDD=51; placebo=62). A secondary, per-protocol analysis included only measurements up to the time point at which lack of protocol adherence occurred (e.g., initiation of significant concomitant medications or pill count adherence rate18 below 80%) (SDD n=29; placebo n=25). Pre-specified subgroup analyses were performed based on smoking status, time since menopause, study medication adherence, and significant concomitant medication use, using tests of interactions.18 No formal adjustment to the alpha level was made for multiple tests.26
RESULTS
Participants
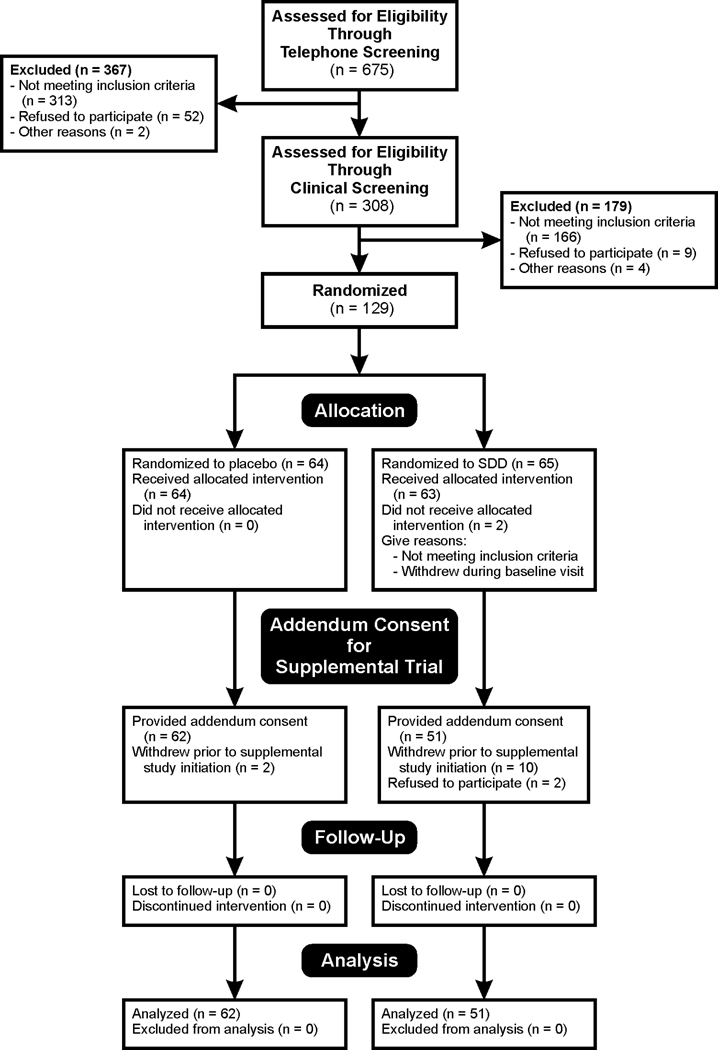
The distributions of subject baseline demographics, clinical characteristics and medication usage were similar for SDD and placebo groups (Tables 1 and 2). Figure 1 shows the flow of subjects from enrollment through intervention allocation, follow-up and data analysis.
Table 1.
Subject Demographics and Baseline Characteristics*
| Characteristic | Placebo Group (n = 62) | SDD Group (n = 51) | p-value |
|---|---|---|---|
| Age (years) | 58.06 (5.73) | 58.62 (5.96) | 0.6 |
| Ethnicity | 0.4 | ||
| Hispanic or Latino | 4 (6%) | 1 (2%) | |
| Not Hispanic or Latino | 58 (94%) | 50 (98%) | |
| Race | >0.9 | ||
| Asian | 2 (3%) | 1 (2%) | |
| African American | 1 (2%) | 1 (2%) | |
| White | 59 (95%) | 49 (96%) | |
| Years postmenopausal | 0.7 | ||
| 5 or fewer years | 22 (35%) | 20 (39%) | |
| More than 5 years | 40 (65%) | 31 (61%) | |
| Smoking status | 0.5 | ||
| Current smoker | 13 (21%) | 10 (20%) | |
| Former smoker | 17 (27%) | 19 (37%) | |
| Never smoker | 32 (52%) | 22 (43%) | |
| Hyperlipidemia† | 55 (89%) | 48 (94%) | 0.5 |
| Hypertension‡ | 12 (19%) | 8 (16%) | 0.6 |
| History of myocardial infarction, angina or stroke | 0 | 0 | |
| Diabetes | 0 | 0 | |
| Body Mass Index (kg/m2) | 28.41 (6.13) | 27.51 (4.38) | 0.4 |
| Number of teeth | 25.63 (2.64) | 25.96 (2.63) | 0.5 |
| Lumbar spine | |||
| Bone mineral density (g/cm2) | 0.91 (0.07) | 0.92 (0.09) | 0.4 |
| T-score | −1.30 (0.67) | −1.20 (0.78) | 0.5 |
| Femoral neck | |||
| Bone mineral density (g/cm2) | 0.71 (0.08) | 0.70 (0.06) | 0.5 |
| T-score | −1.29 (0.68) | −1.37 (0.52) | 0.5 |
| Alveolar bone height (mm) | 3.11 (1.29) | 3.37 (1.50) | 0.2 |
| Manual probing depth (mm) | 3.84 (1.19) | 3.81 (1.12) | 0.7 |
Data are expressed as count (%) for categorical variables and mean (standard deviation) for continuous measures. Standard deviation of alveolar bone height and probing depth was estimated using a linear mixed model.
Hyperlipidemia is defined as total cholesterol > 200 mg/dL or LDL cholesterol > 100 mg/dL or HDL cholesterol < 50 mg/dL
Hypertension was coded as present for any subject reporting use of a diuretic, calcium channel blocker, beta blocker, or angiotensin-converting enzyme inhibitor/angiotensin receptor blocker
Table 2.
Baseline Medication Use*
| Characteristic | Placebo Group (n = 62) | SDD Group (n = 51) | p-value |
|---|---|---|---|
| Aspirin (any dose) | 9 (15%) | 9 (18%) | 0.8 |
| Non-steroidal anti-inflammatory agents | 0 | 0 | |
| Steroids | 0 | 0 | |
| Antibiotics | 1 (2%) | 0 | >0.9 |
| Statins | 6 (10%) | 8 (16%) | 0.4 |
| Non-statin lipid-lowering agent | 0 | 0 | |
| Diuretics | 8 (13%) | 4 (8%) | 0.5 |
| Beta blockers | 3 (5%) | 3 (6%) | >0.9 |
| Calcium channel blockers | 4 (6%) | 1 (2%) | 0.4 |
| Angiotensin-converting enzyme inhibitor/angiotensin receptor blockers | 6 (10%) | 6 (12%) | 0.8 |
| Nitrates | 0 | 0 | |
| Thienopyridine adenosine diphosphate-receptor antagonists | 0 | 0 |
Data are expressed as count (%)
Figure 1. Flow diagram in compliance with Consolidated Standards of Reporting Trials.
Diagram shows flow of subjects through each stage of the two-year clinical trial. An additional subject, the 65th subject in the SDD group, was randomized and was deemed ineligible at the baseline visit, as she did not meet inclusion criteria.
Intent-to-treat analyses
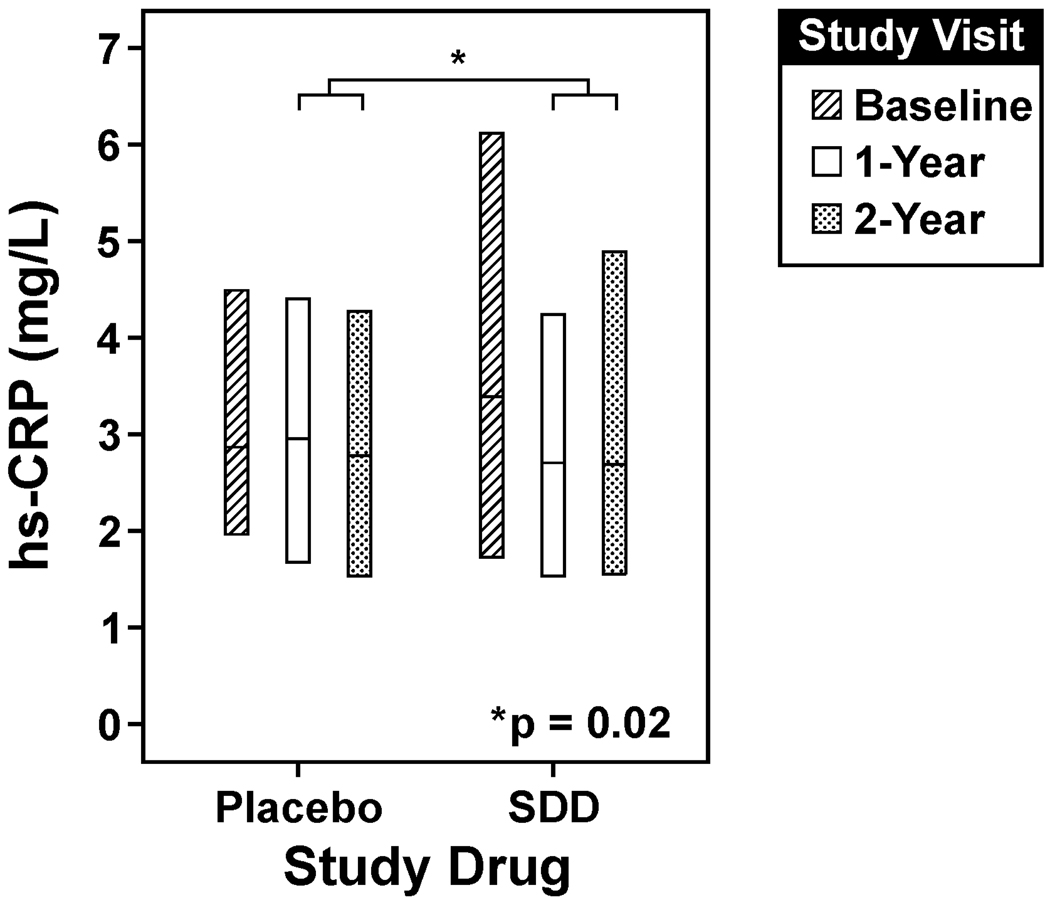
SDD reduced median hs-CRP levels by 18% compared to placebo over the two-year protocol, which was statistically significant (ratio of medians [SDD relative to placebo]: 0.82; 95% Confidence Interval (CI): 0.70 to 0.97, p=0.02) (Figure 2, Table 3). The mean hs-CRP level at baseline, although descriptively higher among SDD subjects compared to placebo subjects, did not differ significantly between treatment groups (p=0.09). When subjects with baseline hs-CRP greater than 9 mg/L (the maximum baseline level among placebo subjects) were removed from the analysis set (n=4 SDD subjects), the estimated difference in hs-CRP changes over time between the treatment groups remained essentially the same (ratio of medians: 0.83; 95%: 0.71 to 0.97, p=0.02). The estimated treatment effect did not appear to be significantly modified or confounded by statin use, diuretic use, or aspirin use (treatment by concomitant medication use interactions were not significant and the estimated SDD effect was consistent across the models with or without adjustment for concomitant medications).
Figure 2. SDD effects on serum hs-CRP over the two-year clinical trial.
SDD reduced median hs-CRP levels by 18% compared to placebo over the two-year protocol after adjustment for baseline hs-CRP level, smoking status, study center, study visit and batch effects, which was statistically significant (p=0.02, intent-to-treat analysis). The lower edge of the box represents the 25th percentile of the observed distribution, while the center line and upper edge of the box represent the 50th and 75th percentiles of the observed distribution, respectively. The number of subjects analyzed at each time point in the placebo group is as follows: 62 at baseline, 61 at the 1-year visit (serum sample not available for one subject) and 62 at the two-year visit. 51 subjects were analyzed at each time point in the SDD group. hs-CRP = high-sensitivity C-reactive protein.
Table 3.
Subantimicrobial dose doxycycline (SDD) effects on serum inflammatory biomarker levels over the two-year clinical trial
| Inflammatory Biomarker | Placebo Group (n =62 subjects) |
SDD Group (n =51 subjects) |
p-value* | |||||
|---|---|---|---|---|---|---|---|---|
| Median | Mean | SD | Median | Mean | SD | |||
| hs-CRP (mg/L) | 0.02 | |||||||
| Baseline | 2.89 | 3.33 | 1.82 | 3.41 | 4.35 | 3.92 | ||
| One Year | 2.97 | 3.45 | 2.37 | 2.72 | 3.54 | 3.21 | ||
| Two Year | 2.80 | 3.20 | 1.99 | 2.70 | 3.47 | 2.79 | ||
| Myeloperoxidase (ng/ml) | 0.2 | |||||||
| Baseline | 75.63 | 88.70 | 63.60 | 72.61 | 88.28 | 52.74 | ||
| One Year | 77.61 | 96.30 | 56.81 | 76.41 | 90.33 | 60.42 | ||
| Two Year | 85.39 | 94.17 | 44.23 | 79.04 | 91.78 | 51.90 | ||
| MMP-9 (92 kilodalton) | <0.0001 | |||||||
| Baseline | 347.84 | 344.48 | 42.31 | 333.13 | 341.15 | 45.18 | ||
| One Year | 367.23 | 355.08 | 50.64 | 327.52 | 325.31 | 46.64 | ||
| Two Year | 360.80 | 355.04 | 56.66 | 314.80 | 322.25 | 49.02 | ||
| MMP-2 (72 kilodalton) | 0.2 | |||||||
| Baseline | 201.81 | 203.66 | 32.12 | 203.33 | 204.35 | 35.81 | ||
| One Year | 199.09 | 202.99 | 35.58 | 199.11 | 201.13 | 37.49 | ||
| Two Year | 200.41 | 204.33 | 38.19 | 192.94 | 194.99 | 35.90 | ||
| MMP-8 (ng/ml) | 0.2 | |||||||
| Baseline | 21.48 | 30.01 | 24.85 | 21.78 | 28.81 | 26.60 | ||
| One Year | 21.60 | 25.02 | 17.37 | 20.59 | 25.04 | 19.47 | ||
| Two Year | 22.29 | 32.70 | 31.43 | 21.16 | 25.67 | 17.61 | ||
| TIMP-1 (scanning units) | 0.7 | |||||||
| Baseline | 3.10 | 3.59 | 2.43 | 2.90 | 3.38 | 2.37 | ||
| One Year | 3.02 | 3.51 | 2.15 | 2.58 | 3.05 | 2.14 | ||
| Two Year | 3.24 | 3.45 | 2.18 | 3.15 | 3.37 | 2.14 | ||
p-values correspond to the comparison of aggregated 1-year and 2-year mean (MMP-9 and MMP-2) or median (hs-CRP, myeloperoxidase, MMP-8, and TIMP-1) biomarker measures between SDD and placebo subjects.
SD= standard deviation
hs-CRP= high-sensitivity C-reactive protein
MMP= matrix metalloproteinase
TIMP= tissue inhibitor of metalloproteinases
Interleukin-6 was detected in 46% of samples from placebo patients and 40% of samples from SDD patients at the 2-year visit and SDD had no significant effect (ratio of odds of detectable interleukin-6 [SDD relative to placebo]: 0.72; 95% CI: 0.30 to 1.69, p=0.4). Tumor necrosis factor-α was detected in 32% of samples from placebo subjects and 40% of samples from SDD subjects at the 2-year visit, and the effect of SDD was not significant (ratio of odds of detectable tumor necrosis factor-α [SDD relative to placebo]: 1.08; 95% CI: 0.56 to 2.08, p=0.8). SDD had no significant effect on myeloperoxidase (ratio of medians [SDD relative to placebo]: 0.91; 95% CI: 0.78 to 1.06, p=0.2) (Table 3). Interleukin-1β was not detected in any serum samples. There was no significant difference between SDD and placebo with respect to mean serum lipids, or mean natural log transformed VLDL and triglyceride levels, (p≥0.2) based on intent-to-treat (Table 4).
Table 4.
Subantimicrobial dose doxycycline (SDD) effects on serum lipid levels over the two-year clinical trial
| Cholesterol Measure | Placebo Group (n =62) |
SDD Group (n =51) |
p-value* | |||||
|---|---|---|---|---|---|---|---|---|
| Median | Mean | SD | Median | Mean | SD | |||
| Total Cholesterol (mg/dl) | 0.6 | |||||||
| Baseline | 216.50 | 229.10 | 53.50 | 225.00 | 230.80 | 60.52 | ||
| One Year | 218.00 | 224.90 | 47.15 | 216.00 | 225.50 | 50.67 | ||
| Two Year | 218.00 | 216.70 | 46.44 | 223.00 | 229.10 | 46.97 | ||
| HDL Cholesterol (mg/dl) | 0.3 | |||||||
| Baseline | 58.00 | 64.77 | 27.01 | 55.00 | 57.69 | 19.83 | ||
| One Year | 57.00 | 63.82 | 24.31 | 57.00 | 60.92 | 17.81 | ||
| Two Year | 60.00 | 64.07 | 23.44 | 64.00 | 61.90 | 18.31 | ||
| LDL Cholesterol (mg/dl) | 0.4 | |||||||
| Baseline | 128.50 | 134.70 | 42.42 | 125.40 | 136.00 | 49.89 | ||
| One Year | 120.80 | 128.80 | 39.27 | 126.40 | 130.50 | 38.42 | ||
| Two Year | 128.80 | 123.69 | 37.78 | 126.40 | 132.40 | 41.93 | ||
| VLDL Cholesterol (mg/dl) | 0.2 | |||||||
| Baseline | 25.20 | 29.66 | 15.10 | 27.60 | 37.13 | 26.52 | ||
| One Year | 28.80 | 32.27 | 17.84 | 29.20 | 34.13 | 24.20 | ||
| Two Year | 24.00 | 28.97 | 17.67 | 29.40 | 34.77 | 27.18 | ||
| Triglyceride (mg/dl) | 0.2 | |||||||
| Baseline | 126.00 | 149.00 | 74.87 | 138.00 | 185.70 | 132.60 | ||
| One Year | 144.00 | 161.30 | 89.22 | 146.00 | 170.60 | 121.00 | ||
| Two Year | 120.00 | 144.80 | 88.37 | 147.00 | 173.80 | 135.90 | ||
p-values correspond to the comparison of aggregated 1-year and 2-year mean (Total, HDL, and LDL Cholesterol) or median (VLDL Cholesterol and Triglyceride) biomarker measures between SDD and placebo subjects.
SD=standard deviation
HDL = high-density lipoprotein
LDL = low-density lipoprotein
VLDL = very low-density lipoprotein
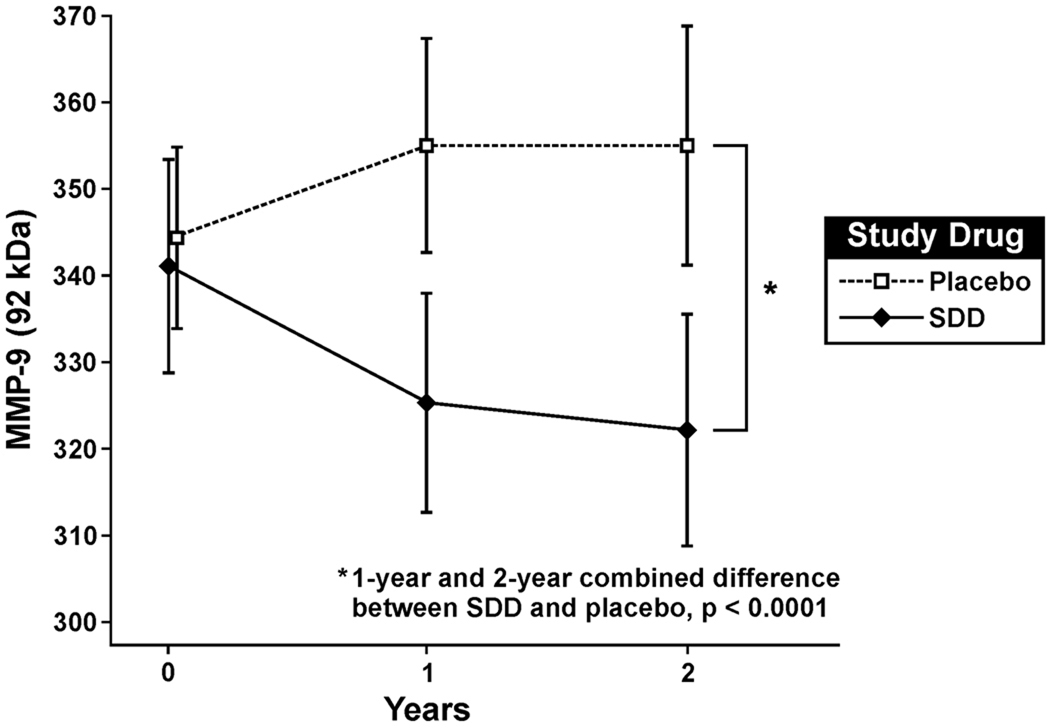
SDD significantly reduced serum MMP-9 (92 kilodalton [kDa]) relative to placebo over the two-year protocol (difference in mean scanning units [SDD minus placebo]: −28.44; 95% CI: −40.17 to −16.72, p<0.0001) (Figure 3, Table 3). There was no significant difference between groups with respect to MMP-2 (72 kDa) (difference in mean scanning units [SDD minus placebo]: −5.70; 95% CI: −13.54 to 2.14, p=0.2), MMP-8 (ratio of medians [SDD relative to placebo]: 0.85; 95% CI: 0.68 to 1.07, p=0.2) or TIMP-1 (ratio of medians [SDD relative to placebo]: 0.96, 95% CI: 0.78 to 1.18, p=0.7) (Table 3).
Figure 3. SDD effects on serum MMP-9 over the two-year clinical trial.
SDD significantly reduced mean serum MMP-9 (92 kDa) by 28.44 scanning units over the two-year protocol relative to placebo after adjustment for the baseline MMP-9 (92 kDa) level, smoking status, study center, study visit and batch effects (p<0.0001, intent-to-treat analysis). Data are expressed as mean ± 95% confidence interval for the mean. The number of subjects analyzed at each time point in the placebo group is as follows: 62 at baseline, 61 at the 1-year visit (serum sample not available for one subject) and 62 at the two-year visit. 51 subjects were analyzed at each time point in the SDD group. MMP-9 = matrix metalloproteinase-9.
Pre-specified per-protocol and subgroup analyses
In women more than five years postmenopausal, mean HDL levels were significantly higher for SDD compared to placebo subjects over two years (difference in means [mg/dl; SDD minus placebo]: 5.99; 95% CI: 1.17 to 10.81, p=0.01), while median VLDL (ratio of medians [SDD relative to placebo]: 0.87; 95% CI: 0.76 to 1.00, p=0.06) and median triglyceride levels (ratio of medians [SDD relative to placebo]: 0.87; 95% CI: 0.76 to 1.01, p=0.06) were reduced in SDD versus placebo, although not significantly at the 0.05 alpha level (Table 5).
Table 5.
Subantimicrobial dose doxycycline (SDD) effects on serum lipid levels in women more than 5 years postmenopausal over the two-year clinical trial
| Cholesterol Measure | Placebo Group (n = 40) |
SDD Group (n = 31) |
p-value* | |||||
|---|---|---|---|---|---|---|---|---|
| Median | Mean | SD | Median | Mean | SD | |||
| Total Cholesterol (mg/dl) | 0.4 | |||||||
| Baseline | 211.50 | 227.45 | 51.45 | 211.00 | 226.48 | 70.20 | ||
| One Year | 217.00 | 221.15 | 47.87 | 213.00 | 226.61 | 59.97 | ||
| Two Year | 212.00 | 216.83 | 52.49 | 220.00 | 229.13 | 54.94 | ||
| HDL Cholesterol (mg/dl) | 0.01 | |||||||
| Baseline | 56.50 | 61.00 | 25.77 | 51.00 | 52.26 | 17.79 | ||
| One Year | 52.00 | 58.28 | 22.93 | 54.00 | 59.16 | 18.31 | ||
| Two Year | 54.50 | 60.65 | 24.22 | 62.00 | 59.94 | 19.27 | ||
| LDL Cholesterol (mg/dl) | 0.4 | |||||||
| Baseline | 128.80 | 132.98 | 39.33 | 122.00 | 130.60 | 58.30 | ||
| One Year | 121.00 | 126.99 | 38.44 | 117.60 | 129.30 | 44.28 | ||
| Two Year | 127.00 | 123.95 | 41.06 | 121.20 | 129.32 | 49.18 | ||
| VLDL Cholesterol (mg/dl) | 0.06 | |||||||
| Baseline | 32.90 | 33.48 | 15.09 | 35.00 | 43.63 | 31.19 | ||
| One Year | 36.60 | 35.88 | 16.11 | 30.40 | 38.15 | 28.79 | ||
| Two Year | 28.40 | 32.23 | 15.12 | 31.80 | 39.88 | 32.84 | ||
| Triglyceride (mg/dl) | 0.06 | |||||||
| Baseline | 164.50 | 168.38 | 74.18 | 175.00 | 218.13 | 155.96 | ||
| One Year | 183.00 | 179.41 | 80.56 | 152.00 | 190.74 | 143.94 | ||
| Two Year | 142.00 | 161.13 | 75.58 | 159.00 | 199.39 | 164.18 | ||
p-values correspond to the comparison of aggregated 1-year and 2-year mean (Total, HDL, and LDL Cholesterol) or median (VLDL Cholesterol and Triglyceride) biomarker measures between SDD and placebo subjects.
SD=standard deviation
HDL = high-density lipoprotein
LDL = low-density lipoprotein
VLDL = very low-density lipoprotein
In women within five years of menopause (SDD n=18; placebo n=20), SDD significantly reduced the median MMP-8/TIMP-1 ratio by 49% at two years (ratio of medians [SDD relative to placebo]: 0.51; 95% CI: 0.31 to 0.82, p=0.006).
In protocol-adherent subjects (SDD n=29; placebo n=25), SDD significantly reduced mean serum MMP-2 levels at two years (difference in mean scanning units [SDD minus placebo]: −16.46, 95% CI: −30.95 to −1.98, p=0.03). No other subgroup or per-protocol analyses were statistically significant.
DISCUSSION
CAD represents an important clinical sequelae of menopause and a number of studies have indicated that systemic inflammation contributes to CAD pathogenesis, with hs-CRP a robust diagnostic risk marker and risk factor.7,8,10 Our two-year randomized clinical trial suggests potential benefits of SDD in improving inflammatory biomarker levels in this vulnerable population, including a reduction in serum hs-CRP and MMP-9.
High-sensitivity CRP is a systemic inflammatory biomarker that has been reported to be more predictive of cardiovascular events than elevated LDL cholesterol.8 In patients at risk for CAD due to abnormal lipid profiles, CRP may form a complex with elevated LDL cholesterol27 which has been oxidized by the inflammatory process. This complex facilitates the uptake of the modified LDL by macrophages infiltrating diseased coronary arteries, resulting in upregulation of MMP-8 and MMP-9 expression. These proteinases, MMP-8 (collagenase-2) and MMP-9 (92 kDa gelatinase), may cooperatively degrade the thin collagen/connective tissue cap covering the atherosclerotic plaque, causing plaque rupture, thrombosis and acute myocardial infarction.28 Indeed, elevated MMP-8 and MMP-9 levels in plasma and serum have been associated with increased incidence of fatal heart attacks.9,22 Furthermore, MMP-9 plasma levels were associated with total cardiovascular risk in a middle-aged population free from symptomatic CAD29 and MMP-9 serum levels were found to be the only independent predictor of plaque rupture in patients who had acute myocardial infarction and unstable angina pectoris.30 Moreover, the balance between MMPs and TIMPs is believed to be crucial in atherosclerosis development and progression22,28 and we found, in subgroup analyses, that SDD reduced the MMP-8/TIMP-1 ratio, which represents a favorable improvement in these systemic biomarkers. Finally, our randomized clinical trial is in agreement with Tuter et al.31 who, in a six-week trial, found significantly greater increases in HDL cholesterol (“good” cholesterol), and its core protein, apolipoprotein-A, in chronic periodontitis patients with CAD receiving SDD plus scaling and root planing versus placebo plus scaling and root planing.
In our six-month pilot trial on acute coronary syndrome (ACS) patients, SDD significantly reduced plasma hs-CRP, interleukin-6 and MMP-9.17 This smaller study (n=30) included aging males and females. Unlike this previous ACS study, our current two-year randomized clinical trial included only postmenopausal women in good general health with no reported history of myocardial infarction, angina or stroke and with much lower baseline serum hs-CRP levels. Nonetheless, statistically-significant reductions in hs-CRP and MMP-9 in the intent-to-treat population and a statistically-significant increase in HDL cholesterol in women more than 5 years postmenopausal were observed. The reduction in mean serum MMP-9 activity among subjects receiving SDD was approximately 6% and less than the 34% reduction in mean MMP-9 activity observed in the Brown et al. study17 in ACS patients (i.e., unhealthy patients with symptomatic CAD, unlike the subjects in good general health in the current study) treated with SDD. However, in the current trial, the 6% reduction in MMP-9 activity in the SDD group was long-term (sustained for two years) and highly statistically significant compared to the 3% increase in mean MMP-9 demonstrated by placebo subjects. Consistent with the ACS study,17 SDD reduced MMP-9 in blood (plasma) more than MMP-2. The reduced serum MMP-2 levels in protocol-adherent, non-CAD patients in this current trial is also of interest, in part, because of this proteinase’s role in cardiac myocyte dysfunction and its inhibition by doxycycline.32
The primary intent-to-treat (ITT) analysis for this clinical trial indicated that there was no statistically significant effect of SDD on alveolar bone density or height loss.18 The ITT analysis included both healthy sites and periodontal pocket sites. However, in sites with probing depths 5 mm or greater (subgroup analysis), SDD reduced alveolar bone density loss relative to placebo (p=0.003). In addition, with respect to relative clinical attachment levels, a secondary endpoint, SDD, adjunctive to periodontal maintenance, significantly decreased the odds of more progressive periodontitis by 19% relative to placebo plus adjunctive periodontal maintenance based on ITT analyses (OR = 0.81, 95% CI: 0.67 to 0.97, p = 0.03).19 Moreover, adverse event experiences were similar between SDD and placebo groups and there was no evidence of microbiologic resistance in the SDD group relative to placebo.18,21 SDD represents an attractive, adjunctive pharmacologic means to reduce systemic inflammatory biomarkers, as it is relatively inexpensive (SDD is available generically) and has been repeatedly demonstrated to be safe18,21,33: SDD was approved by the United States Food and Drug Administration in 1998 as a safe and effective adjunct to scaling and root planing in the treatment of chronic periodontitis.33
Limitations of our study include the loss of several patients to follow-up, as some subjects withdrew prior to initiation of this addendum study. However, subjects with incomplete follow-up did not differ from those with complete follow-up in terms of baseline characteristics. Also, given the multiple hypothesis tests that were performed, subgroup effects must be interpreted cautiously. Finally, inference is limited to the target population, postmenopausal women with chronic periodontitis and osteopenia, with no history of myocardial infarction, angina or stroke. Extrapolation to the general population of postmenopausal women at risk for cardiovascular disease requires further study.
The postmenopausal women were in good general health, although all had chronic periodontitis, which generates elevated levels of pro-inflammatory cytokines such as interleukin-1β,34 tumor necrosis factor-α,34 interleukin-6 35 and tissue-destructive MMPs (e.g., MMP-8 and MMP-9)36,37 in the periodontium. These aforementioned cytokines, particularly interleukin-6, are carried by the circulation to the liver, where they induce expression of acute phase proteins, notably CRP.38 In support of this pathway linking periodontitis and CAD risk, it has been reported that patients with progressive periodontitis show elevations in the same serum biomarkers (e.g., hs-CRP and biomarkers of endothelial dysfunction and dyslipidemia) as CAD patients.39 Although a causal association between CRP and CAD has recently been questioned,40 inflammation is recognized as playing a key role in CAD pathogenesis and chronic inflammatory periodontitis can potentially contribute to the systemic inflammatory burden. Furthermore, evidence indicates that tetracyclines, by non-antimicrobial mechanisms, can be effective in treating both pathogenic periodontitis and CAD pathways.11,41,42 However, a large multicenter clinical trial of longer duration would be necessary to determine whether SDD could reduce CAD risk in addition to reducing serum biomarkers of systemic inflammation.
CONCLUSIONS
In postmenopausal women in good general health, but exhibiting chronic periodontitis and systemic osteopenia, SDD (an approved treatment for chronic periodontitis) reduced serum hs-CRP and MMP-9, and, in subgroups, raised HDL cholesterol, reduced MMP-2 and reduced the MMP-8/TIMP-1 ratio. Because SDD has a favorable safety profile and is relatively inexpensive, SDD represents a potentially attractive pharmaceutical approach to manage chronic systemic inflammation.
ACKNOWLEDGEMENTS
SDD and placebo tablets were provided by CollaGenex Pharmaceuticals, Inc. (Newtown, PA).
The project was supported by Grant Number R01DE012872 from the National Institute of Dental & Craniofacial Research (Dr. Jeffrey B. Payne, PI and Dr. Lorne M. Golub, Co-PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental & Craniofacial Research or the National Institutes of Health. Dr. Sorsa was supported by grants from the Academy of Finland and the Helsinki University Central Hospital Research Foundation.
Abbreviations
- ACS
acute coronary syndrome
- CAD
coronary artery disease
- CI
Confidence Interval
- ELISA
Enzyme-Linked Immunosorbent Assay
- HDL, LDL, VLDL
high-density, low-density and very low-density lipoprotein
- hs-CRP
high-sensitivity C-reactive protein
- MMP
matrix metalloproteinase
- SDD
subantimicrobial dose doxycycline
- TIMP
tissue inhibitor of metalloproteinases
Footnotes
Clinical Trial Registration Information: Protocol registered at ClinicalTrials.gov: NCT00066027
DISCLOSURES OF INTERESTS
Dr. Golub is listed as an inventor on patents on the test medication in this clinical trial and those have been fully assigned to his institution, Stony Brook University. He is also a consultant to Galderma Research and Development (Lausanne, Switzerland) which has licensed a series of tetracycline patents from the State University of New York. No other conflicts of interest exist with the other authors.
References
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Montecucco F, Mach F. Common inflammatory mediators orchestrate pathophysiological processes in rheumatoid arthritis and atherosclerosis. Rheumatology. 2009;48(1):11–22. doi: 10.1093/rheumatology/ken395. [DOI] [PubMed] [Google Scholar]
- 3.Craig RG, Yip JK, So MK, et al. Relationship of destructive periodontal disease to the acute-phase response. J Periodontol. 2003;74(7):1007–1016. doi: 10.1902/jop.2003.74.7.1007. [DOI] [PubMed] [Google Scholar]
- 4.Friedewald VE, Kornman KS, Beck JD, et al. The American Journal of Cardiology and Journal of Periodontology Editors’ Consensus: periodontitis and atherosclerotic cardiovascular disease. Am J Cardiol. 2009;104(1):59–68. doi: 10.1016/j.amjcard.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Rees M, Stevenson J. Primary prevention of coronary heart disease in women. Menopause Int. 2008;14(1):40–45. doi: 10.1258/mi.2007.007037. [DOI] [PubMed] [Google Scholar]
- 6.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 9.Blankenberg S, Rupprecht HJ, Poirier O, et al. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107(12):1579–1585. doi: 10.1161/01.CIR.0000058700.41738.12. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 11.Golub LM, Lee HM, Ryan ME, et al. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12(2):12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- 12.Golub LM, Lee HM, Stoner JA, et al. Subantimicrobial-dose doxycycline modulates gingival crevicular fluid biomarkers of periodontitis in postmenopausal osteopenic women. J Periodontol. 2008;79(8):1409–1418. doi: 10.1902/jop.2008.070623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Dell JR, Elliott JR, Mallek JA, et al. Treatment of early seropositive rheumatoid arthritis: doxycycline plus methotrexate versus methotrexate alone. Arthritis Rheum. 2006;54(2):621–627. doi: 10.1002/art.21620. [DOI] [PubMed] [Google Scholar]
- 14.Moses MA, Harper J, Folkman J. Doxycycline treatment for lymphangioleiomyomatosis with urinary monitoring for MMPs. N Engl J Med. 2006;354(24):2621–2622. doi: 10.1056/NEJMc053410. [DOI] [PubMed] [Google Scholar]
- 15.Meier CR, Derby LE, Jick SS, et al. Antibiotics and risk of subsequent first-time acute myocardial infarction. JAMA. 1999;281(5):427–431. doi: 10.1001/jama.281.5.427. [DOI] [PubMed] [Google Scholar]
- 16.Golub LM, Greenwald RA, Thompson RW. Antibiotic use and risk of myocardial infarction. JAMA. 1999;282(21):1997–1998. [PubMed] [Google Scholar]
- 17.Brown DL, Desai KK, Vakili BA, et al. Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (MIDAS) pilot trial. Arterioscler Thromb Vasc Biol. 2004;24(4):733–738. doi: 10.1161/01.ATV.0000121571.78696.dc. [DOI] [PubMed] [Google Scholar]
- 18.Payne JB, Stoner JA, Nummikoski PV, et al. Subantimicrobial dose doxycycline effects on alveolar bone loss in post-menopausal women. J Clin Periodontol. 2007;34(9):776–787. doi: 10.1111/j.1600-051X.2007.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reinhardt RA, Stoner JA, Golub LM, et al. Efficacy of sub-antimicrobial dose doxycycline in postmenopausal women: clinical outcomes. J Clin Periodontol. 2007;34(9):768–775. doi: 10.1111/j.1600-051X.2007.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golub LM, Lee HM, Stoner JA, et al. Doxycycline effects on serum bone biomarkers in postmenopausal women. J Dent Res. 2010;89(6):644–649. doi: 10.1177/0022034510363367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker C, Puumala S, Golub LM, et al. Subantimicrobial dose doxycycline effects on osteopenic bone loss: microbiologic results. J Periodontol. 2007;78(8):1590–1601. doi: 10.1902/jop.2007.070015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuomainen AM, Nyyssönen K, Laukkanen JA, et al. Serum matrix metalloproteinase-8 concentrations are associated with cardiovascular outcome in men. Arterioscler Thromb Vasc Biol. 2007;27(12):2722–2728. doi: 10.1161/ATVBAHA.107.154831. [DOI] [PubMed] [Google Scholar]
- 23.Prikk K, Maisi P, Pirilä E, et al. Airway obstruction correlates with collagenase-2 (MMP-8) expression and activation in bronchial asthma. Lab Invest. 2002;82(11):1535–1545. doi: 10.1097/01.lab.0000035023.53893.b6. [DOI] [PubMed] [Google Scholar]
- 24.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 25.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70(3):659–663. [Google Scholar]
- 26.Pocock SJ. Clinical trials with multiple outcomes: A statistical perspective on their design, analysis and interpretation. Control Clin Trials. 1997;18(6):530–545. doi: 10.1016/s0197-2456(97)00008-1. [DOI] [PubMed] [Google Scholar]
- 27.Chang MK, Binder CJ, Torzewski M, et al. C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: Phosphorylcholine of oxidized phospholipids. Proc Natl Acad Sci USA. 2002;99(20):13043–13048. doi: 10.1073/pnas.192399699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah PK, Falk E, Badimon JJ, et al. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995;92(6):1565–1569. [PubMed] [Google Scholar]
- 29.Garvin P, Nilsson L, Carstensen J, et al. Circulating matrix metalloproteinase-9 is associated with cardiovascular risk factors in a middle-aged normal population. PLoS One. 2008;3(3):e1774. doi: 10.1371/journal.pone.0001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukuda D, Shimada K, Tanaka A, et al. Comparison of levels of serum matrix metalloproteinase-9 in patients with acute myocardial infarction versus unstable angina pectoris versus stable angina pectoris. Am J Cardiol. 2006;97(2):175–180. doi: 10.1016/j.amjcard.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 31.Tuter G, Kurtis B, Serdar M, et al. Effects of scaling and root planing and sub-antimicrobial dose doxycycline on oral and systemic biomarkers of disease in patients with both chronic periodontitis and coronary artery disease. J Clin Periodontol. 2007;34(8):673–681. doi: 10.1111/j.1600-051X.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 32.Yaras N, Sariahmetoglu M, Bilginoglu A, et al. Protective action of doxycycline against diabetic cardiomyopathy in rats. Br J Pharmacol. 2008;155(8):1174–1184. doi: 10.1038/bjp.2008.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caton JG, Ciancio SG, Blieden TM, et al. Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. J Periodontol. 2000;71(4):521–532. doi: 10.1902/jop.2000.71.4.521. [DOI] [PubMed] [Google Scholar]
- 34.Beklen A, Ainola M, Hukkanen M, et al. MMPs, IL-1 and TNF are regulated by IL-17 in periodontitis. J Dent Res. 2007;86(4):347–351. doi: 10.1177/154405910708600409. [DOI] [PubMed] [Google Scholar]
- 35.Giannopoulou C, Kamma JJ, Mombelli A. Effect of inflammation, smoking and stress on gingival crevicular fluid cytokine level. J Clin Periodontol. 2003;30(2):145–153. doi: 10.1034/j.1600-051x.2003.300201.x. [DOI] [PubMed] [Google Scholar]
- 36.Teng YT, Sodek J, McCulloch CAG. Gingival crevicular fluid gelatinase and its relationship to periodontal disease in human subjects. J Periodontal Res. 1992;27(5):544–552. doi: 10.1111/j.1600-0765.1992.tb01830.x. [DOI] [PubMed] [Google Scholar]
- 37.Sorsa T, Tervahartiala T, Leppilahti J, et al. Collagenase-2 (MMP-8) as a point-of-care biomarker in periodontitis and cardiovascular diseases. Therapeutic response to non-antimicrobial properties of tetracyclines. Pharmacol Res. 2010 doi: 10.1016/j.phrs.2010.10.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Mayer C, Gruber HJ, Landl EM, et al. Rosuvastatin reduces interleukin-6-induced expression of C-reactive protein in human hepatocytes in a STAT3- and C/EBP-dependent fashion. Int J Clin Pharmacol Ther. 2007;45(6):319–327. doi: 10.5414/cpp45319. [DOI] [PubMed] [Google Scholar]
- 39.Joshipura KJ, Wand HC, Merchant AT, et al. Periodontal disease and biomarkers related to cardiovascular disease. J Dent Res. 2004;83(2):151–155. doi: 10.1177/154405910408300213. [DOI] [PubMed] [Google Scholar]
- 40.Elliott P, Chambers JC, Zhang W, et al. Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA. 2009;302(1):37–48. doi: 10.1001/jama.2009.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Islam MM, Franco CD, Courtman DW, et al. A nonantibiotic chemically modified tetracycline (CMT-3) inhibits intimal thickening. Am J Pathol. 2003;163(4):1557–1566. doi: 10.1016/S0002-9440(10)63512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scarabelli TM, Stephanou A, Pasini E, et al. Minocycline inhibits caspase activation and reactivation, increases the ratio of XIAP to smac/DIABLO, and reduces the mitochondrial leakage of cytochrome C and smac/DIABLO. J Am Coll Cardiol. 2004;43(5):865–874. doi: 10.1016/j.jacc.2003.09.050. [DOI] [PubMed] [Google Scholar]