Abstract
In plant reproduction, pollination is an essential process that delivers the sperm through specialized extracellular matrices (ECM) of the pistil to the ovule. Although specific mechanisms of guidance for pollen tubes through the pistil are not known, the female tissues play a critical role in this event. Many studies have documented the existence of diffusible chemotropic factors in the lily stigma that can induce pollen tube chemotropism in vitro, but no molecules have been isolated to date. In this study, we identified a chemotropic compound from the stigma by use of biochemical methods. We purified a lily stigma protein that is active in an in vitro chemotropism assay by using cation exchange, gel filtration, and HPLC. Tryptic digestion of the protein yielded peptides that identified the protein as a plantacyanin (basic blue protein), and this was confirmed by cloning the cDNA from the lily stigma. Plantacyanins are small cell wall proteins of unknown function. The measured molecular mass by electrospray ionization ion source MS is 9,898 Da, and the molecular mass of the mature protein (calculated from the cDNA) is 9,900.2 Da. Activity of the lily plantacyanin (named chemocyanin) is enhanced in the presence of stigma/stylar cysteine-rich adhesin, previously identified as a pollen tube adhesin in the lily style.
Transport of sperm to the egg in flowering plants is a complex process involving many opportunities for signaling, including guidance (1). The extracellular matrices (ECMs) of both the pollen and the pistil are complex and involved in cell–cell signaling (2). Pollen grains land on the stigma, the receptive area of the pistil, germinate, and produce the pollen tube that contains the sperm cells. The pollen tube grows through a specialized ECM of the pistil, the transmitting tract, carrying the sperm cells at the tip of the tube cell. When the tube tip arrives at the ovary, it is guided directly to the ovule to release the sperm cells to the egg and the central cell for fertilization. There is a sizable literature on chemotropism in pollination and fertilization in flowering plants. Strong evidence points toward a universal unknown chemotropic substance in the ovule that attracts the pollen tube over a short range (2–6). Many species have chemotropic compounds in their stigmas as well (7, 8). Early reports showed that lily stigmas secreted an unidentified small molecule that could direct pollen tube growth in vitro (9, 10). On the broad lily stigma, pollen germinates and grows toward openings into the hollow stylar canal. Thus, significant directional cues are essential on the lily stigma to facilitate pollen tube entrance into the style. We have identified the chemotropic molecule in the lily stigma as chemocyanin, a small basic protein that shows sequence similarity to plantacyanins, cell wall proteins of unknown function (11). Another stigma peptide, stigma/stylar cysteine-rich adhesin (SCA) (12), potentiates the activity of chemocyanin.
Methods
Plant Materials. Plants of Lilium longiflorum (cvs. Nellie White, Snow Queen, and Eden) were grown in a greenhouse at Riverside, CA. Chemotropism assays were done with pollen from all three cultivars, and Nellie White was used as a source for other tissues. Stigmas were collected within 5 days and anthers within 2–3 days of anthesis. Tobacco (Nicotiana tabacum) plants were grown in a growth chamber (12 h light/dark cycle) at 22°C. For the expression studies, mature anthers, petals, and pistils were collected on the day of anthesis, as well as leaves and roots.
Protein Purification. The protein purification method was described previously (12) and used with some modifications. Proteins from 64 g of stigmas, ground in PBS (0.14 M NaCl/2.7 mM KCl/1.5 mM KH2PO4/8.1 mM Na2HPO4), were precipitated between 25% and 90% (NH4)2·SO4, dialyzed, applied to a CM-Sephadex C-25 cation exchange column (pH 5.6), and eluted stepwise with 0.1, 0.5, and 1 M NaCl. The chemotropic fractions were pooled and further fractionated on a Sephadex G-50 column (120 × 1 cm). Active fractions were lyophilized and dissolved in H2O (MilliQ, Millipore). Protein was estimated by the Modified Lowry Protein Assay (Pierce). At this step, a major protein band [referred to as stigma proteins (SPs)] of 7–10 kDa was detected after SDS/PAGE. SPs were further purified by capillary HPLC (Agilent 1100, Hewlett–Packard) equipped with a Jupiter C4 microbore column (2 × 150 mm, 5-μm particle diameter, 100-Å pore size, Phenomenex, Belmont, CA). SPs (55 μg), dissolved in water, were injected, and proteins were eluted with a two-step linear gradient at a flow rate of 50 μl/min by mixing mobile phases A (0.1% trifluoroacetic acid in H2O) and B (0.065% trifluoroacetic acid in CH3CN). The gradient was as follows: 5% to 45% of B for 30 min, 45% of B for 5 min, and then 45% to 90% of B for 50 min. Recovery from the column was 80% of the protein loaded. The organic solvents were evaporated, and the pellets were dissolved in 10 μl of H2O. Fractions (pooled from five runs) were used in the chemotropism assay or characterized by MS. An aliquot of 0.3 μl was diluted with 0.7 μl of H2O and used in the assay.
For the protein expression studies, tissues were ground in liquid nitrogen and suspended in PBS. The insoluble materials were removed by centrifugation.
Protein Gel Blot. SDS/PAGE (15%) and protein gel blots were done as described (12). Polyclonal Abs were made against SPs (Cocalico Biologicals, Reamstown, PA) and designed peptides, from SCA (12) and chemocyanin (Bethyl Laboratories, Montgomery, TX).
Chemotropism Assays. The first chemotropism assay was to confirm the previously reported activity of stigmatic secretions (10) (Fig. 1A). A lily stigma or a cut stem section was placed on solid 1.5-mm-thick lily pollen growth medium (GM) composed of 1% agarose, 1.27 mM Ca(NO3)2·4H2O, 0.162 mM H3BO3, 0.99 mM KNO3, and 10% sucrose at pH 5.2. The tissues were removed, and fresh pollen was applied 2–5 mm away from the imprints left by the tissues. Pollen was cultured for 6 h at room temperature. A tobacco GM (13) was supplemented with 2% sucrose but without rifampicin. The reciprocal experiments using stigmas and pollen from tobacco were also performed.
Fig. 1.
Chemotropism of lily pollen tubes toward stigma diffusates. Stigma with exudate (a dashed line indicated by an arrow shows the border of stigma exudate) (A) or a stem cross section (B) were placed on a 1% agarose growth medium for 1 h and removed before pollen grains were applied 2–5 mm from the tissue imprint. pt, pollen tubes; pg, pollen grains; sg, stigma imprint; sm, stem imprint. (Bar = 1 mm.)
In a second chemotropism assay, a central source of the protein [1-μl sample (2.5 μg/μl)] was put in a well in the center of a field of pollen cultured on an agarose GM (Fig. 2). This assay was used for the measurements in Table 1. One fresh anther was shaken in 1 ml of liquid GM, and the resulting pollen grain suspension was evenly spread on solid GM. In the field of pollen, four to five wells (≈2 mm in diameter) were made in the agarose by using a cut-end blue micropipette tip. Pollen grains in the 2- to 3-mm radius around a well were cleared by gentle vacuum. Assays were incubated 3–6 h at 25°C. When pollen tubes in the treatments reached the wells, the assays were moved to a 60°C oven to dry and stained with Coomassie blue.
Fig. 2.
Chemotropism assay in a field of lily pollen. (A) SPs. (B) BSA. (C) Proteinase K-treated SPs. In A–C, 2.5 μg/μl protein was applied to wells. Pollen tubes were stained with Coomassie blue at 6-h treatment. (D) Quantification method showing positive chemotropism. pg, pollen grain; pt, pollen tube. (Bar = 2 mm.)
Table 1. Chemotropic effect of lily SPs.
| Applications | Protein amount, μg | Activity (n)* |
|---|---|---|
| SPs (after cation exchange/gel filtration) | 2.5,5 | + (10) |
| Proteinase K-treated SPs | 5 | – (2) |
| Water | – | – (11) |
| BSA | 5 | – (4) |
| E. coli-expressed SCA | 5 | – (4) |
| SPs (after HPLC) | ||
| Peaks 2–4† +5+6+7 | ≈7 | + (2) |
| Peaks 2–4 + 5 | ≈7 | – (1) |
| Peaks 2–4 | 6.6 | – (4) |
| Peaks 2–4 + 7 | 6.6 + 0.23 | + (3) |
| Peak 7 | 0.23 (23 μM) | + (11) |
| Peak 7 | 0.69 (69 μM) | + (5) |
| Proteinase K-treated peak 7 | 0.69 (69 μM) | – (2) |
+, Activity not different from the starting material (SPs after cation exchange/gel filtration). –, Activity not different from the water control.
Chemotropic activity was quantified by using percent of pollen tubes with directional growth toward the well (see Fig. 2D for method). An average of 60 pollen tubes were measured in each experiment (n, number of experiments). Significant differences were tested with the Mann–Whitney U test (14) by using the two-tailed test at α = 0.05
Mixture of peaks 2–4, which comprised the major peak and could not be separated
A third chemotropism assay was designed to measure reorientation of pollen tubes toward a gradient of the chemotropic compound (Fig. 3). Pollen was suspended in liquid GM and pregerminated on agarose GM until the pollen tube length was 1–2 times the diameter of the pollen grain (≈100–200 μm). Individual pollen grains were transferred (0 h) by a fine needle and placed around wells to which either treatment or control sample had been added 30–40 min earlier, with the pollen tubes manually oriented away from the well. Pollen tube growth was monitored for 5 h, at which time most reoriented pollen tubes had reached the well.
Fig. 3.
Chemotropism assay showing pollen tube reorientation over time (A and B) and quantification method (C). Wells containing 1.5 μg/μl SPs (A) or water (B) were prepared 40 min before application at0hof pregerminated pollen with their tubes manually oriented away from the well. At 5 h, some pollen tubes in the control were out of the field (data not shown). A single reorienting pollen tube (indicated with arrows) monitored over time is enlarged (Inset). (C) Reorientation of a pollen tube by 90° or more was counted as positive chemotropism. (Bar = 2 mm.)
Quantification of Chemotropism. For the second chemotropism assay (Fig. 2), a line was drawn from the center of the well to the tip of the growing pollen tubes (Fig. 2D). If the direction of pollen tube growth was within ± 45° of the line toward the well, it was scored as positive chemotropism, otherwise as random growth. For the third chemotropism assay, a line was drawn from the center of the well through the center of the pollen grain; a second line was drawn perpendicular to the first line (Fig. 3C). Reorientation of the pollen tube 90° or more was counted as positive chemotropism. All pollen tubes growing in the cleared area around the well were measured.
Molecules Tested and Controls for Chemotropism Assays. Fractions from the purification procedure were tested at different aqueous dilutions. Controls included BSA (Fisher), cytochrome C (a small basic protein) (Sigma), Escherichia coli-expressed SCA (12), proteinase K (Sigma), and H2O. Fractionation on >3-kDa mass exclusion Centricon filter (Millipore) of SPs and treatment with proteinase K were also used as controls. For proteinase K treatment, the SPs (5 μg) or HPLC peak 7 (0.69 μg) were incubated with 1 μg of proteinase K (31.8 units/mg) in 10 mM Tris·Cl (pH 7.5) at 37°C for 6 h and then boiled for 15 min. Proteinase K (1 μg) in the same buffer solution was also boiled as a control. Proteins were dissolved in H2O, except for the proteinase K treatment. For additional controls, see Figs. 6 and 7 and Table 3, which are published as supporting information on the PNAS web site.
Microscopy and Capture of Images. Pollen handling and assay observations were done by using a Leica (Deerfield, IL) MZ 12 stereomicroscope. Digital images were captured with a Spot Insight Camera (Diagnostic Instruments, Sterling Heights, MI) by using the IMAGE-PRO PLUS program (Media Cybernetics, Silver Spring, MD).
MS and Amino Acid Sequencing. Fractions from HPLC were dissolved in 10 μl of 25 mM NH4HCO3. After reduction of cysteine residues by 25 μl of 10 mM DTT and alkylation by 25 μl of 55 mM iodoacetamide, proteins were digested with trypsin (modified sequencing grade, Roche Molecular Biochemicals) at 37°C for 8 h, dried, and dissolved in 10 μl of acetonitrile/0.1% formic acid in H2O (50/50) solution. This solution (0.5 μl) was mixed with 0.5 μl of α-cyano-4-hydroxycinamic acid solution [5 μg in 1 ml of acetonitrile/0.1% formic acid in H2O (50/50)] and subjected to matrix-assisted laser desorption ionization-time-offlight analysis by using a Voyager DE-STR Biospectrometry station (Applied Biosystems) with delayed extraction operated in the reflectron mode. Molecular weight of intact proteins was measured by nanoelectrospray ionization (ESI) ion source by using a quadrupole time-of-flight Ultima-Global instrument (Micromass, Manchester, UK). The MAXENT_1 deconvolution software within the instrument control software MASSLYNX was used to reconstruct the multiply charged ion mass spectra to the singly charged ion mass spectra. Sequence analyses of peptides were done by nano-ESI-tandem MS (MS/MS) with a collision energy varying from 20 to 40 eV applied in the hexapole collision cell with Ar (12 psi) as the collision gas. The QTOF was run at a capillary voltage of 1.0 KV and a cone voltage of 77 V. The source block temperature was 80°C. About 4 μl of sample was loaded into the nanoelectrospray tip (Protana, Odense, Denmark) for each ESI-MS or ESI-MS/MS analysis.
Cloning of cDNA Encoding the Lily 9.9-kDa Protein. Total RNA was extracted from stigmas on the day of anthesis by using RNeasy Mini Kit (Qiagen, Chatsworth, CA), and 1–2 μg of total RNA was used in a 20-μl reverse transcription reaction with Super-Script II RNase H- Reverse Transcriptase (20 units), ribonuclease inhibitor (10 units) (Invitrogen), and a thermal RACE primer QT (14). The resultant reverse transcription reaction product (1 μl) was used as a template in the PCR. The partial cDNA was amplified by using a QO primer and a degenerate primer based on the N-terminal peptide sequence determined by nano-ESI-MS/MS. The degenerate primer sequence was 5′-GTITTYAADTAYAAYCCNGC-3′, where I is inosine; N is A, C, G, or T; and Y is C or T. A secondary PCR was carried out by using a QI primer and a nested degenerate primer (5′-GCNGTNCAYAAYGTNGT-3′). QT, QO, and QI primer sequences have been described (15). Conditions for the PCR were as follows: 30 s at 94°C, 30 s at 42–58°C gradient (42, 42.6, 43.7, 45.5, 48, 51.2, 54.9, and 58°C), and 60 s at 72°C for 30 cycles. Two rounds of PCR amplification generated a partial cDNA of 450 bp, which was cloned into pGEM-T Easy vector (Promega) for sequencing.
To obtain the full-length cDNA of this protein, 5′-RACE was done as described (14) with the following modifications. A gene-specific primer 5′-ATCTCAAGATTTGGTAAC-3′ was used in the reverse transcription reaction for the first-strand cDNA synthesis. After tailing with terminal deoxynucleotidyl transferase (Promega), cDNAs were purified with QIAquick PCR Purification Kit (Qiagen) for the following two rounds of PCR. The first round of PCR was done with QT, QO, and LiBBP-GSP1 (5′-CACAAGAGATATCTTGGCCTAC-3′). First-round PCR products were reamplified with QI and LiBBPGSP2 (5′-AGAATCCACTCACCAGCAA-3′). The amplified DNA fragment was cloned into pGEM-T vector for sequencing. All of the primers were synthesized by Sigma–Genosys (The Woodlands, TX), and the DNA sequencing was done at the Genomics Institute DNA sequencing facility (University of California, Riverside). The deduced sequence was aligned to other proteins by using CLUSTALW multiple alignments (http://bioweb.pasteur.fr/seqanal/interfaces/clustalw-simple.html). Theoretical mass of deduced amino acid sequence from the cDNA was calculated in reduced form by using the SWISS PROT PROGRAM (http://us.expasy.org).
Results
Stigma Exudate Attracts Lily Pollen Tubes. In the crude assay (Fig. 1), lily pollen tubes grew directly toward the source of stigma secretions (Fig. 1 A) and showed no directional growth toward stem secretions (Fig. 1B). The amount of secretions was controlled for in additional experiments (Fig. 6). Tobacco pollen tubes showed no evidence of chemotropism to lily SPs or to tobacco stigma or stem exudates. Lily stigma exudates inhibited both tobacco pollen germination and tube growth (data not shown).
A Stigma Protein Fraction Shows Chemotropic Activity. Stigma protein extract fractionated over a cation exchange and eluted between 0.1 and 0.5 M NaCl showed chemotropic activity (data not shown). Further purification by gel filtration yielded a stigma extract enriched in 7- to 10-kDa proteins, SPs, that was chemotropically active (Fig. 2 A, Table 1). The active molecules were retained on a 3-kDa retentate Centricon (Millipore) filter (data not shown). The 3-kDa filtrate and other controls, including BSA (Fig. 2B) and proteinase K-treated SPs (Fig. 2C), did not induce chemotropic activity (Table 1). Diffusion of proteins into the area cleared around the well occurred within 3 h, the minimal duration of an experiment (Fig. 7). A chemotropic effect of the SPs was detected as low as 1.5 μg/μl but was lost at 0.9 μg/μl unless pollen was placed closer to the SP source (Table 3). A concentration above 5 μg/μl had no further stimulating effect. In the assay designed to show pollen tube reorientation toward a central source of attractant, live pollen tubes were monitored over time (Fig. 3). At time of transfer (0 h), pollen tube tips were oriented 180° away from the well. Within 0.5 h, pollen tubes on the SP sample began reorienting their growth, and most pollen tubes reached the well by 5 h (Fig. 3A). Dramatic reorientation was seen in 85% of the pollen tubes incubated with the SPs, whereas only 5.5% of the pollen tubes grew toward the well in the control (Fig. 3B).
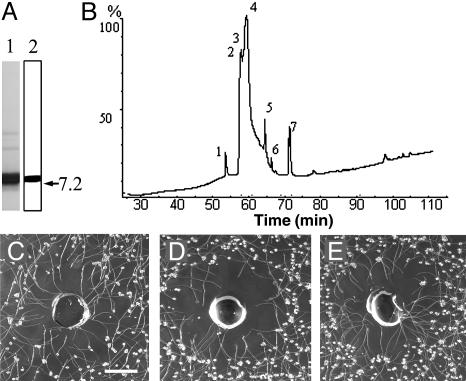
A Minor Protein Component of the SP Fraction Is Responsible for Chemotropic Activity. A major 7- to 10-kDa protein band, containing mainly SCA as shown by a protein gel blot, and several minor bands at 20–30 kDa in the SP fraction were detected by silver staining (Fig. 4A 1, 2). Further purification was done, and the SP fraction was separated by reverse-phase HPLC into seven peaks, including one major peak (eluted between 55 and 60 min) that had three components (peaks 2–4) and four minor peaks (Fig. 4B, Fig. 8A, which is published as supporting information on the PNAS web site). Peaks 1 and 7 were well separated from the major peak. Chemotropic activity of the combined fractions (peaks 2–7) (Table 1) was as strong as the starting material (i.e., SPs before HPLC), indicating that peak 1 was not involved in activity. Similarly, peaks 5 and 6 were eliminated because the combination of peaks 2–4 and 7 was sufficient to retain the chemotropic activity seen in the starting material (Fig. 4C, Table 1). Peaks 2–4 could not be separated, and peptide sequencing shows that they correspond to SCA isoforms (data not shown). A Western blot of peaks 2–4 with SCA antibodies confirmed this (data not shown). Neither SCA peaks alone nor the E. coli-expressed SCA protein showed chemotropic activity (Table 1). Peak 7 chemotropic activity was positive at 0.23 μg/μl (Fig. 4D) and markedly improved when the amount was tripled (Fig. 4E). Proteinase K treatment of peak 7 abolished its activity (Table 1).
Fig. 4.
SDS/PAGE and Western blot of SPs (A), HPLC profile of SPs (B), and chemotropism assays (C–E). (A) SDS/PAGE (lane 1) and Western blot (lane 2) of SPs. Proteins were revealed by silver staining after SDS/PAGE. The polyclonal antibody used was produced against SCA (12). (B) SPs were separated into seven peaks. (C) Peak 7 mixed with the combined SCA peaks 2–4. (D) Peak 7 alone (0.23 μg/μl). (E) Peak 7 alone (0.69 μg/μl). (Bar = 2 mm.) Note that the lower amount (0.23 μg) of peak 7 showed full activity when it was mixed with the combined SCA peaks 2–4 in C.
Chemotropic Activity of Peak 7 Increases When It Is Combined with SCA. Integration of peak 7 (MASSLYNX chromatogram integration) showed that it was ≈3.5% of the total protein of the SP fraction (Fig. 8A, which is published as supporting information on the PNAS web site). The minimum concentration of SPs to show chemotropic activity in these assays was 1.5 μg/μl, which contains ≈0.05 μg of peak 7 protein, approximately one-fourth of the amount necessary (0.23 μg/μl) for peak 7 alone to show minimal activity (Fig. 4D, Table 1). Full activity in the assay was restored when 0.23 μg of peak 7 was combined with 6.6 μg of the major SCA peaks 2–4 (Fig. 4C, Table 1).
Peak 7 Protein Is Identified as a Plantacyanin, a Member of the Blue Copper Protein Family (Phytocyanins). ESI-MS of peak 7 showed the presence of one prominent protein with a measured mass of 9,898.0 Da (Fig. 8B). The minor protein present in peak 7 is a remnant from the major peak (peaks 2–4) and corresponds to the measured mass of one isoform of SCA (Fig. 8A). Tryptic digestion of peak 7 resulted in three peptides that were analyzed by ESI-MS/MS (Table 2, Fig. 9, which is published as supporting information on the PNAS web site). Similarity searches with BLAST (http://us.expasy.org/tools/blast) and alignment with T-COFFEE (www.ch.embnet.org/software/TCoffee.html) (16) of these three peptides with other proteins showed 60–68% amino acid identity with a family of proteins called plantacyanins (basic blue proteins) (17). A cDNA that encodes a protein containing the identified peptide regions was cloned from lily stigmas by use of RT-PCR; the deduced amino acid sequence showed 71% identity with rice, 60% with Arabidopsis, and 56% with cucumber plantacyanins (Fig. 5). The calculated molecular mass for the lily protein (from the cDNA) is 9,900.2 Da, and the measured mass of the active protein is 9,898 Da (Fig. 8B). This mass difference is probably due to the reduced condition used for the calculation (2 S-H instead of one disulfide bond presumably present in the native protein). The calculated pI is 9.41.
Table 2. Peptide sequences from tryptic digest of peak 7 analyzed by ESI-MS/MS.
| No. | Sequence | Theoretical mass | Experimental mass |
|---|---|---|---|
| 1 | VVYTVGDGGGWTFGTSGWPAGK | 2,199.01 | 2,199.00 |
| 2 | AGDVL(I)VFK | 848.49 | 848.50 |
| 3 | YNPAVHNVVSVPAGGYK | 1,771.91 | 1,772.00 |
Masses (Da) were calculated and measured by using monoisotopic masses of the occurring amino acid residues and giving peptide masses as [MH]+.
Fig. 5.
Amino acid sequence alignments of lily chemocyanin with plantacyanin (basic blue protein) from other plant species. The deduced amino acid sequence was aligned with plantacyanin proteins from rice, Arabidopsis, and cucumber. The peptides identified by nano-ESI-MS/MS in chemocyanin are underlined. The arrowhead indicates the putative signal peptide cleavage site. Identical amino acids are indicated by asterisks. Colons show the conserved residues, and similar amino acids are indicated by dots. Amino acids involved in copper binding are shaded.
Chemocyanin Expression. Chemocyanin protein is expressed strongly in the stigma and style and to a lesser extent in the leaf, ovary, and petal. No signal was detected in 5 h in vitro grown pollen tubes (data not shown), mature anthers, or roots (Fig. 10, which is published as supporting information on the PNAS web site).
Discussion
There are excellent data showing that pollen tubes can be influenced in their growth direction by application of external factors in vitro (18), and studies have shown that chemotropic factors exist in the ovules of most, and the stigmas of many, species (1). Pollen tubes can respond to the signal at the ovule only if they have first passed through the stigma and style (19), which supports the premise that a hierarchy of signaling events occurs in pollination (20). Many mechanisms have evolved for pollen germination at the stigma, and they are reflected in the diversity of stigma structures, pollen grain wall types, and pollen tube germination requirements in vitro. In dry stigma types like Arabidopsis, the site of adhesion of the pollen grain to the stigma determines the point of entry of the pollen tube into the style, but evidence exists for chemotropic molecules in the style and ovary (21, 22). In wet stigmas such as lily and tobacco, ECM molecules cover the stigma surface as a copious exudate, and pollen tubes can grow to some length before entering the style. In the case of tobacco, a lipidic ECM provides a gradient of water that is thought to give a directional cue of a physical nature for pollen tube penetration into the stigma tissues (23). Indeed, we find that stigma exudates of tobacco do not induce tobacco pollen tube directional growth in vitro. In tobacco styles, there is a glycoprotein (TTS) that forms a glycosylation gradient and attracts pollen tubes in vitro (24). Genetic screens have proven harder than expected in our search for mutants in pollen tube guidance (21). Thus we used an in vitro bioassay and a biochemical/proteomics approach to identify the lily stigma chemotropic compound (chemocyanin) that was predicted to exist many years ago (7–10). Here we show that chemocyanin is a plantacyanin-like (basic blue) protein, which belongs to the ancient phytocyanin family of blue copper proteins (17). Plantacyanins display distinct spectroscopic properties that differentiate them from other blue copper proteins (11). The function of these small basic proteins in the plant cell wall is unknown, but many are capable of redox reactions (25).
Our expression data for the lily plantacyanin show its presence in other organs of the shoot. The microarray data on Arabidopsis (26, 27) and Medicago (28) as well as the EST database suggest that plantacyanins are multifunctional cell wall proteins that occur in several plant organs. The copper-binding site in blue copper proteins is formed by two histidines, one cysteine, and one methionine or a glutamine. A plantacyanin in ragweed pollen is missing these histidines and does not bind copper (29). In the lily chemocyanin, the methionine/glutamine is replaced by a leucine. So, whether this protein binds copper needs to be elucidated. The 3D structure of plantacyanin is that of a Greek key β-barrel fold, as are all of the blue copper proteins, but in the plantacyanins, the two histidine ligands are exposed to the surface, and they have two cysteines that form a disulfide bond as well as the copper-binding cysteine. Also the barrel is open, so it is more like a β-taco structure (30, 31).
When pollen tubes reorient their growth, they do so in response to a reorientation of the tip-focused calcium gradient (18), which can be artificially induced by activation of calcium channels on the flanks of the pollen tube tip. It is possible that an external signal, such as a gradient of chemocyanin and SCA, may be acting on the same channels to reorient pollen tube growth in the in vitro assay and on the stigma, either directly or indirectly. Although SCA cannot induce chemotropic activity by itself, it may have a critical role as an accessory protein, as implied by comparing the amount of lily chemocyanin (peak 7) needed for activity in the absence and presence of SCA (peaks 2–4). There is evidence that SCA binds pollen tubes (32). Such binding may physically facilitate access of the lily chemocyanin to the plasma membrane. Interestingly, in Nicotiana the S-RNases involved in self-incompatibility bind three proteins in the stylar ECM, one of which is a plantacyanin (33).
At this stage, we cannot eliminate the possibility of another factor carried by the chemocyanin that is not detected by MS. This possibility needs to be investigated by expressing chemocyanin in bacteria or another expression system.
The pollen tube is an excellent system for studying signaling in plants, because pollen grains can be cultured in vitro, and tube growth is easily manipulated (34). By using in vitro assays, we are close to reaching the limits of our understanding of this complex system of growth in the tube cell that is responsible for delivering the sperm cell to the egg. Discovery of native peptides that cause chemotropism of pollen tubes will allow us to more easily study the signaling network in the pollen tube tip, much as yeast mating factors have enabled that field to progress (35).
Supplementary Material
Acknowledgments
We thank Natasha Raikhel, Zhenbiao Yang, Julia Bailey-Serres, and Kathleen J. Eckard for critical reading of the manuscript; Patricia Springer and Linda Walling for assistance with the cDNA cloning; and Kimberly Tan for general assistance. This work was supported by National Science Foundation Grant IBN-0077886 (to E.M.L.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ECM, extracellular matrix; GM, growth medium; SP, stigma protein; SCA, stigma/stylar cysteine-rich adhesin; ESI, electrospray ionization.
Data deposition: The sequences reported in this paper have been deposited in the GenBank and Swiss-Prot databases [accession nos.: GenBank AY425323 (lily chemocyanin), Swiss-Prot Q94GZ1 (rice plantacyanin), Swiss-Prot Q8LG89 (Arabidopsis plantacyanin), and Swiss-Prot P00303 (cucumber plantacyanin)].
References
- 1.Lord, E. M. & Russell, S. D. (2002) Annu. Rev. Cell Dev. Biol. 18, 81-105. [DOI] [PubMed] [Google Scholar]
- 2.Brownlee, C. (2002) Curr. Opin. Plant Biol. 5, 396-401. [DOI] [PubMed] [Google Scholar]
- 3.Hulskamp, M., Schneitz, K. & Pruitt, R. E. (1995) Plant Cell 7, 57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ray, S., Park, S. S. & Ray, A. (1997) Development (Cambridge, U.K.) 124, 2489-2498. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu, K. K. & Okada, K. (2000) Development (Cambridge, U.K.) 127, 4511-4518. [DOI] [PubMed] [Google Scholar]
- 6.Higashiyama, T., Yabe, S., Sasaki, N., Nishimura, Y., Miyagishima, S., Kuroiwa, H. & Kuroiwa, T. (2001) Science 293, 1480-1483. [DOI] [PubMed] [Google Scholar]
- 7.Tsao, T.-H. (1949) Plant Physiol. 24, 494-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeijlemaker, F. C. J. (1956) Acta Bot. Neerl. 5, 179-186. [Google Scholar]
- 9.Miki, H. (1954) Bot. Mag. Tokyo 67, 143-147. [Google Scholar]
- 10.Welk, M., Sr., Millington, W. F. & Rosen, W. G. (1965) Am. J. Bot. 52, 774-781. [Google Scholar]
- 11.Nersissian, A. M., Immoos, C., Hill, M. C., Hart, P. J., Williams, G., Herrmann, R. G. & Valentine, J. S. (1998) Protein Sci. 7, 1915-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park, S.-Y. & Lord, E. M. (2003) Plant Mol. Biol. 51, 183-189. [DOI] [PubMed] [Google Scholar]
- 13.Read, S. M., Clarke, A. E. & Bacic, A. (1993) Protoplasma 177, 1-14. [Google Scholar]
- 14.Siegel, S. (1956) in Nonparametic Statistics for the Behavioral Sciences, ed. Harlow, H. F. (McGraw–Hill, New York), pp. 116-127.
- 15.Frohman, M. A., Dush, M. K. & Martin, G. R. (1988) Proc. Natl. Acad. Sci. USA 85, 8998-9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Notredame, C., Higgins, D. & Heringa, J. (2000) J. Mol. Biol. 302, 205-217. [DOI] [PubMed] [Google Scholar]
- 17.Ryden, L. G. & Hunt, L. T. (1993) J. Mol. Evol. 36, 41-56. [DOI] [PubMed] [Google Scholar]
- 18.Hepler, P. K., Vidali, L. & Cheung, A. Y. (2001) Annu. Rev. Cell Dev. Biol. 17, 159-587. [DOI] [PubMed] [Google Scholar]
- 19.Higashiyama, T., Kuroiwa, H., Kawano, S. & Kuroiwa, T. (1998) Plant Cell 10, 2019-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franklin-Tong, V. E. (1999) Curr. Opin. Plant Biol. 2, 490-495. [DOI] [PubMed] [Google Scholar]
- 21.Palanivelu, R. & Preuss, D. (2000) Trends Cell Biol. 10, 517-524. [DOI] [PubMed] [Google Scholar]
- 22.Palanivelu, R., Brass, L., Edlund, A. F. & Preuss, D. (2003) Cell 114, 47-59. [DOI] [PubMed] [Google Scholar]
- 23.Wolters-Arts, M., Lush, W. M. & Mariani, C. (1998) Nature 392, 818-821. [DOI] [PubMed] [Google Scholar]
- 24.Cheung, A. Y., Wang, H. & Wu, H. (1995) Cell 82, 383-393. [DOI] [PubMed] [Google Scholar]
- 25.Battistizzi, G., Borsari, M., Lodovica, L. & Sola, M. (1997) J. Biol. Inorg. Chem. 2, 350-359. [Google Scholar]
- 26.Zhao, Y., Hull, A. K., Gupta, N. R., Goss, K. A., Alonso, J., Ecker, J. R., Normanly, J., Chory, J. & Celenza, J. L. (2002) Genes Dev. 16, 3100-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitham, S. A., Quan, S., Chang, H. S., Cooper, B., Estes, B., Zhu, T., Wang, X., Hou, Y. M. (2003) Plant J. 33, 271-283. [DOI] [PubMed] [Google Scholar]
- 28.Fedorova, M., Mortel, van de J., Matsumoto, P. A., Cho, J., Town, C. D., VandenBosch, K. A., Stephen, G. & Vance, C. P. (2002) Plant Physiol. 130, 519-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt, L. T., George, D. G. & Yeh, L.-S. L. (1985) J. Mol. Evol. 21, 126-132. [DOI] [PubMed] [Google Scholar]
- 30.Guss, J. M., Merritt, E. A., Phizackerley, R. P. & Freeman, H. S. (1996) J. Mol. Biol. 262, 686-705. [DOI] [PubMed] [Google Scholar]
- 31.Einsle, O., Mehrabian, Z., Nalbandyan, R. & Messerschmidt, A. (2000) J. Biol. Inorg. Chem. 5, 666-672. [DOI] [PubMed] [Google Scholar]
- 32.Park, S.-Y., Jauh, G.-Y., Mollet, J.-C., Eckard, K. J., Nothnagel, E. A., Walling, L. L. & Lord, E. M. (2000) Plant Cell 12, 151-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClure, B. A., Cruz-Garcia, F., Beecher, B. & Sulaman, W. (2000) Ann. Bot. 85, 113-123. [Google Scholar]
- 34.Zheng, Z.-L. & Yang, Z. (2000) Trends Plant Sci. 5, 298-303. [DOI] [PubMed] [Google Scholar]
- 35.Segall, J. E. (1993) Proc. Natl. Acad. Sci. USA 90, 8332-8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.