Abstract
Individuals who are fearful of novelty have a larger hypothalamic-pituitary-adrenal axis response than do nonfearful individuals. We hypothesized that a fearful behavioral style emerging early in life would be associated with life-long altered adrenal activity. Because there is ample physiological evidence both costs and benefits of adrenal activation, we determined whether such a stable emotional-neuroendocrine trait was associated with differential morbidity and mortality. To conduct such lifespan work, we studied a relatively short-lived mammal: the Norway rat. We first established that an animal's hesitation or willingness to explore a novel environment (“neophobia” and “neophilia,” respectively) is an identifiable and stable behavioral trait in young-adult males and that neophobia, compared with neophilia, was associated with a greater glucocorticoid response to novelty. Second, we were able to detect behavioral differences among infant rats within a family, and this behavioral disposition at infancy predicted the magnitude of the glucocorticoid response in late middle age. Males identified as neophobic during infancy died sooner than their less fearful brothers. Although both types of males died with similar pathologies (tumors), neophobic males were 60% more likely to die at any point in time. This lifespan study identifies an emotional trait in infancy that predicts an early death and an associated neuroendocrine trait in adulthood that is a potential mechanism underlying the relationship between behavioral style and longevity.
Some individuals respond to novelty with fear (i.e., “neophobia”), whereas others do not. Individuals that show behavioral signs of fear also experience increased activation of certain physiological systems, such as amygdala activation, sympathetic activation of the autonomic nervous system, and activation of the hypothalamic-pituitary-adrenal axis (1-4). In humans, the neophobic behavioral/neuroendocrine response pattern (i) emerges as early as 14 mo of age (5), (ii) is stable during childhood (6, 7), and (iii) is associated with childhood adrenal activation (8-10), which can be both beneficial to and costly for health (11). We have tested the hypothesis that an individual's early propensity to respond fearfully to novelty can have cumulative effects on health and physiology over the lifespan, thereby affecting rate of aging and longevity.
In a variety of mammalian species, minimal exploration in the face of novelty is typically interpreted to indicate fear (12, 13). In humans, traits similar to neophobia include behavioral inhibition, shyness, negative affect, extraversion/surgency, and fearfulness (14-16). Because both fearful animals and fearful children show greater activation of brain areas and physiological systems associated with the classic fight/flight response (1-4), we refer to a hesitant or stilted behavioral response to novelty as neophobia across species and use “neophilia” to refer to an interactive behavioral response to the same stimulus.
Previous research on neophobia has typically measured adrenal glucocorticoid levels at a single point after a novel experience (cf. 17). However, research on neuroendocrine regulation of the adrenal axis, sleep deprivation, and aging demonstrate that there are at least three important components of adrenal axis regulation: maintenance of baseline levels, magnitude or rate of response to a stressor, and recovery rate or levels after a stressor (18-20). To determine which component(s) is associated with fearful behavior, we presented animals with a novel situation, similar to those encountered everyday, and measured glucocorticoid responses at several time points.
Because chronic stress can have negative health consequences, such as inhibition of the cell-mediated immune function (11), artherosclerosis (21, 22), glucocorticoid resistance (21, 23), ovarian dysfunction (21), and hippocampal cell loss (24), individuals with sustained corticosterone responses (elevated basal levels or slower recovery rates) could suffer from allostatic load (25, 26). Alternatively, because short-term elevations in glucocorticoid levels can have health benefits, such as enhanced inflammatory response (11), individuals with more frequent acute responses to novelty might be at reduced risk for some diseases. Thus, neophobic and neophilic individuals could experience differential aging morbidity or mortality rates.
To study long-term consequences of neophobia, and given the obvious limitations of lifespan research in humans, we developed a model by using a relatively short-lived and commonly used laboratory rodent: male Sprague-Dawley rats (Rattus norvegicus).
Study 1: Stability of Neophobia/Neophilia and Adrenal Activity in Young Adults
We hypothesized that if neophobia/neophilia is a stable trait in rats, then individual responses to novelty will be maintained in adulthood. We tested animals twice during early and middle adulthood (at 15% and 25% of maximum lifespan). In addition, we hypothesized that our behavioral measurement of neophobia would be associated with a greater adrenal response to novelty. We quantified the following aspects of the dynamic glucocorticoid response: basal, peak, and recovery levels, and the rate at which glucocorticoids increase and are removed from circulation. Because each component of the dynamic response includes a different neuroendocrine mechanism, each could differentially affect other physiological systems (e.g., chronically elevated basal glucocorticoid levels may be damaging, whereas a brief pulse may be advantageous).
Current literature suggests the following four hypotheses about differences between neophobic and neophilic glucocorticoid responses. Neophobic individuals may have (i) elevated basal glucocorticoid levels (7, 9, 27), (ii) a faster or higher rise in glucocorticoids (7), (iii) slower recovery of glucocorticoid levels due to inhibited negative feedback (19), and/or (iv) a smaller glucocorticoid response due to decreased voluntary activity (17, 28).
Experimental Protocol. Males were housed individually and tested at 4 mo of age in the “exploration arena” (described below) and retested in a similar arena at 8 mo (n = 28). Dynamics of their adrenal response to the novel arena were quantified with serial blood samples that were collected after the second test (n = 24).
Subjects. Males were purchased from Charles River Breeding Laboratories at 2 mo of age and housed singly in metal hanging cages in a colony room with other males. Food and water were available ad libitum, and the lighting schedule was 14 h of light/10 h of dark (white lights at 2000 hours and dim red lights at 1000 hours). Rats were handled gently three times a week, and their cages were changed every other week.
Test of neophobia/neophilia: The exploration arena. We developed an arena to measure an animal's willingness to explore a complex novel environment. The design is similar to that used by Einon and Morgan (13) to measure exploration and is intended to mimic the novel-room situation used with young children (15, 29). Unlike the classic “open field” arenas used to test “emotionality” (30), we designed the exploration arena to minimize anxiety-provoking aspects for the thigmotactic rat (i.e., open spaces). By minimizing fear-producing aspects that were threatening to all individuals, we sought to maximize the full range of individual responses to novelty. Animals were introduced to a home base in the environment: a ceramic bowl, providing surrounding body contact, which was located next to the high walls of the corner of the exploration arena. To provide the familiar conspecific odors that are important for this species, the floor of the exploration arena was covered with clean wood chips, which were sprinkled with a small amount of bedding that was urine-soaked by all colony animals. Of the 28 males tested, only 3 defecated in the exploration arena, indicating that it did not induce high levels of fear in the majority of males.
The 122-× 122-cm exploration arena had 46-cm walls, a Plexiglas cover, and four rat-sized objects (a bowl, an empty food hopper, a tunnel, and a brick) placed 13 cm from each corner. To ensure the novelty of the experience, objects were replaced at each trial with new ones (a food hopper, a tube, and a rock) for repeat testing. The arena was illuminated indirectly by a 90-W red lightbulb. The Plexiglas cover had a 3 × 3 grid that was superimposed to divide the arena into nine equal squares for behavioral coding.
Trials were conducted 5-8 h into the dark phase, a period of behavioral activity for these nocturnal animals. For each trial, males were transported individually from their cages and placed in the home base, which was cleaned with a dilute disinfectant solution and dried before each trial. Males remained in the arena for 5 min, and their behavior was videotaped to measure “locomotion” and “inspection.” Locomotion in the arena was quantified by the number of lines crossed on the 3 × 3 cover grid. Inspection, or willingness to engage novel stimuli, was recorded as the frequency of interactions with an object (touching with nose, touching with paws, climbing on, or climbing into). Corticosterone response to exploration arena. After the second trial in the arena (at 8 mo of age), males were transported to a separate room and blood was sampled by tail nick. Samples were collected 10, 25, 40, and 100 min after the males were first introduced to the arena. Given the 30-min time delay from increased corticotropin to peak corticosterone release (31), the 40-min sample was designed to capture maximal corticosterone release after testing. The tail scab was disturbed gently for repeat sampling, and males were placed in a clean cage between each sample. To collect a basal corticosterone measurement (comparable with levels before testing), a blood sample was collected from males at the same time of day several weeks after testing. This procedure was used to avoid nicking rats' tails before testing, which would obscure behavioral and corticosterone responses to novelty.
Blood samples were collected into microcentrifuge tubes and kept on ice until they were centrifuged 45 min later and serum was collected. Serum samples were diluted (1:200 with diluent from the assay kit) and frozen at -80°C until assayed. Corticosterone was measured by using a commercial rat and mouse corticosterone RIA kit (ICN). All diluted samples were run in duplicate on the same assay. Intraassay variability for low and high controls was 12.6% and 6.8%, respectively.
Analyses. Correlational analyses were used to test the stability of neophobia and neophilia during middle adulthood. The corticosterone responses of neophobic and neophilic males were compared with t tests on all components of the response: levels at baseline (0 min), peak (40 min), and recovery (100 min), as well as on the rates of response and recovery. Response and recovery slopes were quantified by calculating regression slopes for each male over the response phase (0, 25, and 40 min) and the recovery phase (40 and 100 min), respectively.
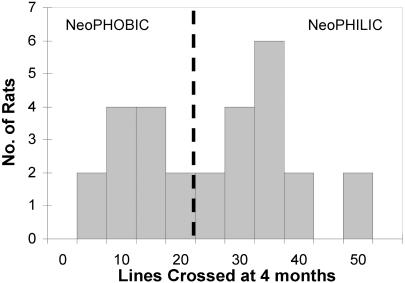
Results. Behavioral styles. Males moved either readily or very little in the exploration arena (Fig. 1). Given this bimodal distribution, males were identified as either neophobic or neophilic; males that crossed <25 lines were considered neophobic, and males that crossed >25 lines were considered neophilic. Moving in the environment (locomotion) was highly correlated with stopping to investigate objects (inspection) (r = 0.83, P < 0.0001; ref. 26). Notably, animals with low levels of locomotion also occasionally exhibited the species-typical fear posture of a hunched back and piloerection.
Fig. 1.
Locomotion in the exploration arena by 28 young-adult neophobic and neophilic males.
Behavioral stability. Males that displayed fearful behavior in the test arena (low locomotion and inspection) at 4 mo of age exhibited the same behavioral style at 8 mo (correlation across repeat trials: locomotion, r = 0.60, df = 26, P < 0.001; inspection, r = 0.66, df = 26, P < 0.0001).
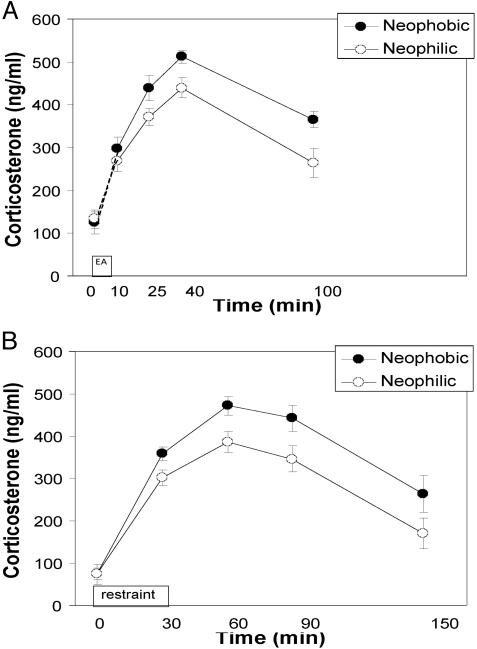
Corticosterone response to the exploration arena. Although neophobic and neophilic males had similar corticosterone levels at baseline (t = 0.37, df = 22, ns), neophobic males (n = 12) had a higher and more rapid corticosterone response to the exploration arena (Fig. 2A; peak, t = 2.67, df = 22, P < 0.05; slope, 9.5 vs. 7.4 ng/ml per min, t = 3.93, df= 22, P < 0.001). Recovery slopes were similar for the two kinds of males (-2.4 vs. -2.9 ng/ml per min; t = 0.99, df = 22, ns); however, given the greater initial corticosterone rise in the neophobic males, their circulating levels at 100 min were still ≈35% greater than those for the neophilic males (t = 2.96, df = 22, P < 0.01).
Fig. 2.
(A) Serum corticosterone response to a 5-min exploration-arena test for neophobic (•) and neophilic (○) males at 8 mo of age. Serum corticosterone values at 0 min are from several weeks after testing. (B) Plasma corticosterone response to 30-min restraint in neophobic (•) and neophilic (○) males at 15 mo of age.
Discussion. Individuals reliably adopted one of two distinct behavioral-response styles in the face of novelty: a neophobic style, characterized by cautious movements, or a neophilic style, characterized by ample locomotion and inspection. The correlation coefficient between early and middle adulthood behavior (10% of maximum lifespan) was as high as that obtained in children tested and retested for behavioral inhibition only 1 mo apart (15). In rats, the neophobic males had a more rapid and higher adrenal response to novelty compared with the neophilic males, again, similar to results with fearful children (8-10). Both types of males had similar baselines and rates of recovery associated with the new experience. The heightened glucocorticoid response for neophobic males, combined with no faster removal of glucocorticoids from circulation, may have prolonged the elevation of these steroids in circulation.
Rats are exposed routinely to comparable low-grade novel experiences even within a controlled laboratory environment (32). They are given new bedding and water (familiar conspecific odors replaced with novel ones), they are removed from their cages and exposed to novel stimuli, and they experience researchers entering the colony room at unpredictable times. Although all rats within a colony are exposed to these events, more fearful individuals may mount a greater glucocorticoid response to them than less fearful individuals, and over a lifespan this differential glucocorticoid exposure may have long-term consequences for aging.
Study 2: Lifespan Study of Neophobia and Neophilia
We conducted a second study to determine whether the neophobic and neophilic behavioral styles could be identified in infancy, as they can in children (8). We hypothesized that if this trait is stable during adulthood, a brief 10 min of behavioral testing before weaning (2% of maximum lifespan) would predict behavioral style at middle age (30% of maximum lifespan) and adrenal response dynamics at the end of middle age (45% of maximum lifespan). We hypothesized that, if this stable trait has a cumulative effect on health over the lifespan, differential morbidity or mortality would occur for neophobic and neophilic rats.
Based on prior work, we know that there are litter-specific effects on exploration-arena performance, corticosterone-response dynamics, and lifespan. To control for these family-specific effects, and because males affiliate with kin, we categorized brothers as neophobic and neophilic within the range of their litter. Groups of brothers, including one neophobic and one neophilic male, were constituted. This method of creating stable yet diverse social groups ensured life-long interactions among individuals with different emotional-neuroendocrine profiles. Finally, Sprague-Dawley rats are vulnerable to spontaneous tumors in adulthood (33). Given the time span over which tumors develop, tumor susceptibility makes these rats ideal subjects for studying how stable behavioral-response styles affect tumor growth, morbidity, and mortality (34, 35).
Experimental Protocol. We tested males for neophobia and neophilia in infancy, just before weaning (20 and 24 d of age). We selected 14 preweanling brother trios on the basis of their performance in the exploration arena (see Selection criteria at infancy). Their response to novelty was retested in middle age (11 mo), and the dynamics of their glucocorticoid response to a mild novelty stressor was measured at late middle age (15 mo). Males then lived out their natural lifespan, monitored closely in old age for external signs of disease, and necropsies was performed at the end of life. On a quarterly basis, a sentinel male from the colony room was killed, and serological analyses were used to verify the absence of 14 major rat pathogens in the colony.
Selection criteria at infancy. Brothers were selected from each of 14 litters on the basis of their mean locomotion at 20 and 24 d. The range of locomotion scores varied both within and among litters (e.g., within litter ranges for two extreme litters, 0.0-39.5 vs. 27.5-85.0). Given this variance, males were selected on the basis of their performance relative to their litter mean; the male with the highest locomotion was selected as the neophilic male, the male with the median score was the neophobic, and a third unresponsive male was selected as a third cagemate for the two study subjects. In each litter, one or more males were unresponsive during testing. This lack of response was a poor indicator of behavior later in life and was most likely a result of a slower growth trajectory, reflected by smaller body weight at infant testing: unresponsive vs. neophobic, 33.8 ± 1.3 g vs. 35.8 ± 1.1 g (unpaired t = 2.16, df = 13, P < 0.05) and unresponsive vs. neophilic, 33.8 ± 1.3 g vs. 36.1 ± 1.1 g (paired t = 3.09, df = 13, P < 0.01). Given this difference in body weight, unresponsive males were not considered neophobic, but, rather, developmentally delayed, and were not subjects in this study other than to provide a third cagemate for the neophobic/neophilic brother pairs.
Housing. Each pair of brothers lived in the same housing condition for their entire lives. From birth to 22 d of age, males lived with their mother and littermates, and from 22 to 27 d they lived in same-sex littermate groups. As part of a larger longitudinal study on early social experiences, five of the pairs were temporarily housed individually during 28-46 d, whereas nine pairs remained together with their third brother. This early social manipulation was the same for each of the two brothers in a pair and had no detectable impact on their behavior (control vs. isolation locomotion at 60 d: 51 ± 6 vs. 59 ± 5, t = 0.99, df = 26, ns), body weight at 43 d (221 ± 5.3 g vs. 222 ± 3.7 g, t = 0.18, df = 26, ns), corticosterone recovery levels at 15 mo (202 ± 34 ng/ml vs. 236 ± 51 ng/ml, t = 0.57, df = 25, ns), and/or lifespan [median lifespan: 654 vs. 675 d (Mantel-Cox log-rank test, χ2 = 0.009, df = 1, ns)]. Because brother pairs were balanced across housing conditions, and because the conditions did not affect behavior, etc., we combined data from the two housing conditions to compare neophobic with neophilic brothers. Husbandry protocols were the same as in Study 1, except that males were housed in solid-bottom plastic cages that were changed twice a week. Finally, all males lived in a colony room, into which researchers and husbandry staff entered frequently, producing daily novel experiences (e.g., collection of rats for protocols, performance of health checks in the room, visits from a veterinarian, etc.).
Repeated exploration-arena testing. Behavioral testing at infancy (20 and 24 d) and adulthood (11 mo) was the same as in Study 1, except that in infancy the test arena was smaller (92 × 92 cm, with walls of 23 cm) to accommodate the smaller animals. Corticosterone response to restraint stressor at late middle age. The corticosterone response to a commonly used psychological stressor (brief restraint in a tube) was measured at 15 mo. This novel stressor may be thought of as the equivalent of a collapsed tunnel for these burrow-dwelling animals. Tube diameters were adjusted for individual male body weights, such that no males were constricted in the tubes. Males were removed from their home cage in a predetermined randomized order, carried to an adjacent room where blood sampling was conducted, placed into a restraint tube, and bled within 4 min of removal from the home cage. After the first sample, the rats remained in the tube for 30 min, at which point a second sample was collected and the rats were removed from the tube and placed in a clean cage. Third, fourth, and fifth samples were collected at 60, 90, and 150 min, respectively, from the initial sample. The 60-min sample, collected 30 min from the end of physical restraint, was timed to capture the near-maximal corticosterone response to this challenge. Males were processed on sequential evenings at the end of the rats' active period (2000-2300 hours). Sampling day and time were balanced across neophobic and neophilic brothers.
Blood samples were collected into Microtainer EDTA tubes (Becton Dickinson). Plasma was collected, diluted, and stored as in Study 1. Samples from brother pairs were included on the same assay. Interassay and intraassay variability for a low and high control were 9.3% and 10.5% and 8.5% and 7.7%, respectively.
Lifespan. Most males were allowed to live their natural lifespans. Necropsies were performed to identify gross morphological abnormalities (e.g., tumors and enlarged or abnormally small organs) indicating disease processes at the time of death. We looked for signs of infectious disease, cardiac failure, urinary tract blockage, chronic nephrosis, periarteritis, and radiculoneuropathy (36). Because cause of death in aging rats involves a complex interaction of multiple pathologies (36), determining specific cause of death was beyond the scope of this study.
To preclude suffering, 10 of the 28 males were killed within ≈1 week of the end of life, according to the following criteria. In a previous analysis of 41 aging males that died of natural causes, we identified a subset of symptoms that increased 3-fold 1 week compared with 2 weeks before death. If any two of these symptoms (inability to ambulate and reach food and water, half-closed eyes, shallowness or constriction of breath, chromodacryorrhea, and ascension of testes) were present, males were killed. Decisions to kill were made independently by a veterinarian who made weekly health checks and an observer who was unaware of the behavioral styles of the rats. Equal numbers of neophobic and neophilic males were killed.
Analyses. Because the behavioral variables were not normally distributed, nonparametric sign tests were used to determine whether neophobic brothers, identified during infancy, showed less locomotion and inspection than their neophilic brothers during adulthood (11 mo). To compare neophobic and neophilic brothers' corticosterone values (baseline, peak and recovery values, and response and recovery-phase slopes), paired t tests were used. Response and recovery slopes were quantified for each male by calculating the slopes as in Study 1. Response slopes were calculated by using values from 0 through 60 min and recovery slopes by using values from 60 through 150 min. One male died before the second behavioral test and blood collection, so analyses of these data are limited to 13 brother pairs. Lifespan was compared between neophobic and neophilic males by using a log survival analysis; the Mantel-Cox χ2 test was used because this statistic assumes no difference in rates of death over time. Because of the prevalence of tumors in this rat strain and the difficulty in identifying a major cause of death for aged rats, we focused our analyses on tumor burden.
Results. Behavior. Behavioral response to novelty in infancy predicted behavioral responses in middle age. Neophobic infant males crossed 60% fewer lines in the exploration arena compared with the neophilic males, and these neophobic males continued to move and explore less than their neophilic brothers as adults, as determined by sign test for locomotion (z = 1.66, df = 13, P < 0.05) and inspection (z = 1.66, df = 13, P < 0.05). At middle age, only 3 of the 13 neophobic brothers were more active and exploratory during testing than their neophilic brothers.
Corticosterone response to restraint stressor. Neophobic and neophilic males did not differ in their baseline corticosterone levels at the circadian nadir (i.e., the end of the active period, paired t = 0.03, df = 12, ns). However, neophobic males mounted a greater corticosterone response to physical restraint than neophilics (Fig. 2b); peak levels in neophobic males were ≈20% higher (paired t = 3.074, df = 12, P < 0.01) with steeper response-phase slopes (6.6 vs. 5.2 ng/ml per min, paired t = 2.60, df = 12, P < 0.05). As with the males in the previous study, recovery-phase slopes were no different between neophobic and neophilic males (-2.5 vs. -2.5 ng/ml per min, paired t = 0.02, df = 12, ns), but given the greater corticosterone rise in neophobic males, their corticosterone levels at 150 min were still much higher than corticosterone levels in neophilic males (paired t = 2.97, df = 12, P < 0.05). During the latter half of the response phase (60, 90, and 150 min), neophobic males had circulating corticosterone levels ≈30% greater than neophilic males, and their parallel recovery rates suggest that neophobic males may have returned to baseline levels later than neophilic males.
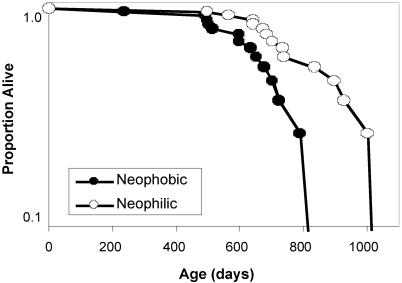
Lifespan. Neophobic males died earlier than neophilic males (Fig. 3), as determined by Mantel-Cox log-rank test (χ2 = 5.20, df = 1, P < 0.05). At any time point over the lifespan, the neophobic males were ≈60% more likely to die than the neophilic males (hazard ratio = 2.62). Median lifespan for the neophobic males was 599 d compared with 701 d for the neophilic males. All of the neophobic males were dead by 840 d of age, whereas the maximum lifespan for the neophilic males was 1,026 d, a difference of 6 mo. This finding represents ≈20% reduction in maximum lifespan for the neophobic males. (Results were similar if males killed at the end of life were included as censored data points in the survival analysis.)
Fig. 3.
Lifespan of neophobic (•) and neophilic (○) males.
Although neophobic males died earlier than neophilic ones, end-of-life pathology was the same: tumors or urinary tract blockage (79% and 7%, respectively, in neophobic males vs. 79% and 14%, respectively, in neophilic males). The three remaining males (two neophobic and one neophilic) had no signs of any of the five most common nonneoplastic lesions at death for rats (36). Moreover, no rats had clinical signs of other disease, such as infectious disease (e.g., pneumonia) or cardiac failure. All major organs were within normal limits for aged rats, except for splenic enlargement in animals with an ulcerated or necrotic tumor. No infectious pathogens were detected in sentinel animals.
Pituitary tumors were most common, typically accompanied by cachexia and neurological signs, such as weakness in forelimbs and poor balance. Fourteen of the 22 males with tumors had an enlarged pituitary gland (ranging from 0.076 to 0.550 g, relative to a normal weight of 0.025 g). The same number of neophobic and neophilic males had an enlarged pituitary at death. The weight of the pituitary gland (including tumor) was slightly but not significantly larger in the seven neophilic males than in the seven neophobic males (0.366 ± 0.048 g vs. 0.276 ± 0.045 g, t = 1.36, df = 12, P = 0.198). Males with a pituitary tumor were the first to die in the study, as determined by Mantel-Cox log-rank test (r = 8.15, P < 0.01), and the symptoms of this disease indicate that these tumors led to a rapid decline in health, precluding the growth of other tumors that develop later in life. Males that did not develop a pituitary tumor developed tumors in various other organs, such as lungs, intestines, skin, and bone. Of the remaining eight males with tumors, there were equal numbers of neophobic and neophilic males. However, the nonpituitary tumors were, on average, 25 times larger in neophilic than neophobic males (mean ± SD: 45.7 ± 20.0 g vs. 4.9 ± 3.3 g, t = 5.57, df = 6, P < 0.01, using log tumor weights).
Discussion. Preweanling male rats could be distinguished within litters according to their willingness to explore a novel environment. This early-identified behavioral trait predicted their middle-age behavioral and glucocorticoid responses to novelty. Males identified as neophobic in infancy had a greater rise in circulating corticosterone levels at late middle age after novelty, and their levels remained higher than the neophilic males for at least 90 min during recovery. The differential behavioral and glucocorticoid-response profiles were found much later in the lifespan than they were in Study 1, indicating that the behavioral-response profile that emerges early in life is likely accompanied by life-long differences in behavioral and adrenal reactivity and glucocorticoid exposure. Most strikingly, males identified as neophobic during infancy died significantly earlier than males identified as neophilic during infancy. However, pathology at the end of life was similar between the two kinds of males (primarily pituitary and other tumors), indicating that the emotional-neuroendocrine profile was not associated with differential morbidity but, rather, with differential vulnerability to the same aging trajectory. Indeed, neophilic males were able to sustain a larger tumor burden before death than were neophobic males.
General Discussion
Male Sprague-Dawley rats differ in their emotional response to a novel environment, and this difference is detectable in infancy, as is true for behavioral inhibition (shyness) in children (7). Neophobic males responded fearfully to a new environment by moving and inspecting objects only a little, whereas neophilic males moved throughout the arena inspecting objects. Neophobia was associated with a greater glucocorticoid response to novel experiences, similar to the elevations found in behaviorally inhibited, shy, and fearful children (7, 9, 37). This finding provides strong support for using the natural variance found in Sprague-Dawley rats as a model of fear-associated temperamental differences in humans.
Such behavioral and glucocorticoid profiles have been found in other species with either similar or slightly different results. Overall, however, the results across species suggest that the neophobic/neophilic trait has been relatively well conserved over evolutionary time. The elevated glucocorticoid response in the neophobic rats is analogous to “high-responder” male rats identified by Piazza and colleagues (17, 38), to “low-resistance” female pigs identified by Ruis et al. (2) and to “inhibited, fearful” capuchin monkeys identified by Byrne and Suomi (1). In Piazza's studies, high-glucocorticoid (high responder) males were the most active males tested. In contrast to Piazza's studies, the high-glucocorticoid (neophobic) males in the present study were the least active males, indicating that neophobic individuals experienced greater fear in the face of novelty, because physical activity normally leads to a greater adrenal response (17, 28). This discrepancy probably reflects a difference in testing environments, with the current study using a complex arena and the studies of Piazza and colleagues using a simple arena without objects (38), providing less stimulation and fewer surfaces to explore. Thus, Piazza's rat model is analogous to human sensation-seeking or hyperactivity in the face of environmental poverty (39), whereas we believe our model is analogous to behavioral inhibition, or fear, in an unfamiliar complex environment.
In rats, we pinpointed the component of adrenal activation associated with fearful behavior: increased rate and level of glucocorticoid secretion after encountering a mild stressor, rather than increased recovery rates or baseline levels. This finding supports prior research that fearfulness is linked with increased corticotropin-releasing hormone production (40, 41) and with the initial feed-forward response of the hypothalamic-pituitary-adrenal axis, as opposed to the negative feedback system known to decline with age (20). Future research will be needed to determine whether basal levels are similar between males at nonnadir times of the day when glucocorticoid variance among individuals is greater and therefore differences among individuals more easily detected. The higher glucocorticoid levels of neophobic males during recovery may predict elevated basal levels the following day (42), suggesting that these fearful males experience longer periods of elevated glucocorticoids than their less fearful brothers. In addition, rats routinely experience low-grade novelty in a laboratory colony, similar to the daily challenges accompanied by acute glucocorticoid elevations in humans (32, 43, 44). Given these regular novel experiences, neophobic individuals, with their greater, more frequent, or longer glucocorticoid responses, are likely exposed to more circulating glucocorticoids over the lifespan than neophilic individuals. Given this greater exposure, as the hypothalamic-pituitary-adrenal axis ages and glucocorticoid receptors are lost in the hippocampus and other regulatory brain areas (45), neophobic individuals may develop delayed glucocorticoid recovery more quickly, thus promoting premature aging.
This study demonstrates a link between an emotional-neuroendocrine trait and a shortened lifespan based on a brief (10-min) behavioral test during infancy. Neophobic individuals, identified during infancy, were 60% more likely to die at any point in time than neophilic individuals. Although differential longevity occurred, there was no evidence for differential disease processes between neophobic and neophilic males. Almost all males died with a tumor. The incidence of tumors was no different between the two kinds of males, but neophilic males were able to sustain a larger tumor burden in late life, whereas neophobic males died at an earlier stage of tumor progression. The smaller tumor size in neophobic males may result from their elevated glucocorticoid levels (46), although this seemingly protective factor does not make them more resilient.
At present, we cannot identify the specific physiological mechanisms underlying this reduction in longevity for neophobic individuals. Several hypotheses can be proposed. The elevated glucocorticoid response may itself mediate the shortened lifespan by altering apoptosis rates (47-49); altering immune function (11) and, thus, susceptibility to tumor development (50); or inducing oxidative stress (47). Alternatively, the glucocorticoid profile may be associated with other physiological systems (e.g., sympathetic nervous system activation, amygdala, or dopaminergic activity) that may either enhance pituitary tumorgenesis (51) or, more generally, reduce the ability to handle a tumor load in neophobic individuals (e.g., increased heart rate, blood pressure, etc.). The animal model that we have developed provides an ideal system in which to conduct longitudinal and experimental studies to identify the neuroendocrine, molecular, and genetic mechanisms underlying early death in neophobic individuals.
It must be noted that differential corticosterone responses and lifespans were evident between brother pairs in this longitudinal study. Brothers in this study came from the same litter, which only one male had sired. Thus, the genetic similarity among brothers is relatively high. Given this high similarity, we suggest that behavioral, adrenal, and lifespan variance within litters arises from different early environments and experiences (cf. 52). Examples of these differences include prenatal hormonal exposure generated by the sex of adjacent siblings in utero (53); postnatal maternal behavior, such as differential grooming (54); and postnatal social roles and dominance relationships formed among males within a litter. Any of these factors, or any combination of them, may explain why brothers differ in their response to novel situations. In the long run, mothers may be best served by nurturing emotionally diverse litters to maximize offspring survival when future environmental and social conditions are variable and unpredictable.
The results of this study support the allostatic-load hypothesis in that long-term alterations in glucocorticoid levels can result in the accumulation of damage to various physiological systems, thereby accelerating aging (25, 26). We have found decreased longevity in individuals that naturally produce more glucocorticoids in response to novelty. It should be noted, however, that this end-of-life consequence of neophobia may not predict life-long decreased health or fitness in neophobic individuals but perhaps a negative consequence primarily at the very end of the lifespan. There may exist antagonistic pleiotropic effects (55) in this behavioral differentiation, such that the neophobic males may be more healthy or show more adaptive behavioral traits during their prime adult years (e.g., avoidance of potentially dangerous circumstances) but that this benefit is associated with a cost during the aging process.
Acknowledgments
We thank T. Brawn, J. Clarke, K. Eisenman, J. Hoffman, A. Lindner, P. Schumm, E. Shaw-Taylor, M. Tsakalis, T. Whitney, J. Yee, and H. You for expert assistance. This research was supported by National Institute of Child Health and Human Development Grant F32 HD08693 (to S.A.C.), National Institute on Aging Grant PO1 AG018911 (to M.K.M.), and National Institute of Mental Health Grant R37 MH41788 (to M.K.M.).
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Byrne, G. & Suomi, S. J. (2002) Psychoneuroendocrinology 27, 139-154. [DOI] [PubMed] [Google Scholar]
- 2.Ruis, M. A. W., te Brake, J. H. A., van de Burgwal, J. A., de Jong, I. C., Blokhuis, H. J. & Koolhaas, J. M. (2000) Appl. Anim. Behav. Sci. 66, 31-47. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt, L. A., Fox, N. A., Schulkin, J. & Gold, P. W. (1999) Dev. Psychobiol. 35, 119-135. [PubMed] [Google Scholar]
- 4.Schwartz, C. E., Wright, C. I., Shin, L. M., Kagan, J. & Rauch, S. L. (2003) Science 300, 1952-1953. [DOI] [PubMed] [Google Scholar]
- 5.Kagan, J. & Snidman, N. (1991) Psychol. Sci. 2, 40-44. [Google Scholar]
- 6.Reznick, J. S., Kagan, J., Snidman, N., Gersten, M., Baak, K. & Rosenberg, A. (1986) Child Dev. 57, 660-680. [Google Scholar]
- 7.Kagan, J., Reznick, J. S. & Snidman, N. (1987) Child Dev. 58, 1459-1473. [PubMed] [Google Scholar]
- 8.Kagan, J., Reznick, J. S. & Snidman, N. (1988) Science 240, 167-171. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt, L. A., Fox, N. A., Rubin, K. H., Sternberg, E. M., Gold, P. W., Smith, C. C. & Schulkin, J. (1997) Dev. Psychobiol. 30, 127-140. [DOI] [PubMed] [Google Scholar]
- 10.Tennes, K., Downey, K. & Vernadakis, A. (1977) Psychosom. Med. 39, 178-187. [DOI] [PubMed] [Google Scholar]
- 11.Dhabhar, F. S. (1997) Brain Behav. Immun. 11, 286-306. [DOI] [PubMed] [Google Scholar]
- 12.Buss, A. H. & Plomin, R. (1984) Temperament: Early Developing Personality Traits (Lawrence Erlbaum Associates, Hillsdale, NJ).
- 13.Einon, D. F. & Morgan, M. (1976) Anim. Behav. 24, 415-420. [Google Scholar]
- 14.Fox, N. A., Calkins, S. D. & Bell, M. A. (1994) Dev. Psychopathol. 6, 677-696. [Google Scholar]
- 15.Garcia-Coll, C., Kagan, J. & Reznick, J. S. (1984) Child Dev. 55, 1005-1019. [Google Scholar]
- 16.Rothbart, M. K., Ahadi, S. A., Hershey, K. L. & Fisher, P. (2001) Child Dev. 72, 1394-1408. [DOI] [PubMed] [Google Scholar]
- 17.Dellu, F., Mayo, W., Vallée, M., Maccari, S., Piazza, P. V., Le Moal, M. & Simon, H. (1996) Psychoneuroendocrinology 21, 441-453. [DOI] [PubMed] [Google Scholar]
- 18.Leproult, R., Copinschi, G., Buxton, O. & Van Cauter, E. (1997) Sleep 20, 865-870. [PubMed] [Google Scholar]
- 19.Sapolsky, R. M. (1983) Endocrinology 113, 2263-2267. [DOI] [PubMed] [Google Scholar]
- 20.Sapolsky, R. M., Krey, L. C. & McEwen, B. S. (1983) Exp. Gerontol. 18, 55-64. [DOI] [PubMed] [Google Scholar]
- 21.Shively, C. A., Laber-Laird, K. & Anton, R. F. (1997) Biol. Psychiatry 41, 871-882. [DOI] [PubMed] [Google Scholar]
- 22.Brindley, D. N. & Rolland, Y. (1989) Clin. Sci. 77, 453-461. [DOI] [PubMed] [Google Scholar]
- 23.Avitsur, R., Stark, J. L., Dhabhar, F. S., Padgett, D. A. & Sheridan, J. F. (2002) J. Neuroimmunol. 124, 54-61. [DOI] [PubMed] [Google Scholar]
- 24.Sapolsky, R. M. (1985) Brain Res. 359, 300-305. [DOI] [PubMed] [Google Scholar]
- 25.Sterling, P. & Eyer, J. (1988) in Handbook of Life Stress, Cognition and Health, eds. Fisher, S. & Reason, J. (Wiley, Chichester, U.K.), pp. 631-651.
- 26.McEwen, B. (1991) Ann. N.Y. Acad. Sci. 840, 33-44. [DOI] [PubMed] [Google Scholar]
- 27.Kalin, N. H., Shelton, S. E., Rickman, M. & Davidson, R. J. (1998) Behav. Neurosci. 122, 251-254. [DOI] [PubMed] [Google Scholar]
- 28.Borer, K. T., Bestervelt, L. L., Mannheim, M., Brosamer, M. B., Thompson, M., Swamy, U. & Piper, W. N. (1992) Physiol. Behav. 51, 713-718. [DOI] [PubMed] [Google Scholar]
- 29.Rheingold, H. L. (1969) in Determinants of Infant Behaviour, ed. Foss, B. M. (Methuen, London), Vol. 4, pp. 137-166. [Google Scholar]
- 30.Hall, C. S. (1934) J. Comp. Psychol. 18, 385-403. [Google Scholar]
- 31.Hinson, J. P., Vinson, G. P., Whitehouse, B. J. & Price, G. (1985) J. Endocrinol. 104, 387-395. [DOI] [PubMed] [Google Scholar]
- 32.Sharp, J. L., Zammit, T. G., Azar, T. A. & Lawson, D. M. (2002) Contemp. Top. Lab. Anim. Sci. 41, 8-14. [PubMed] [Google Scholar]
- 33.Löhrke, H., Hesse, B. & Goerttler, K. (1982) Z. Versuchstierk. 24, 225-230. [PubMed] [Google Scholar]
- 34.Sapolsky, R. M. & Donnelly, T. M. (1985) Endocrinology 117, 662-666. [DOI] [PubMed] [Google Scholar]
- 35.Segerstrom, S. C. (2003) Brain Behav. Immun. 17, S92-S97. [DOI] [PubMed] [Google Scholar]
- 36.Simms, H. S. (1967) in Pathology of Laboratory Rats and Mice, eds. Cotchin, E. & Roe, F. J. C. (Blackwell Scientific, Oxford), pp. 733-747.
- 37.Dettling, A. C., Parker, S. W., Lane, S., Sebanc, A. & Gunnar, M. R. (2000) Psychoneuroendocrinology 25, 819-836. [DOI] [PubMed] [Google Scholar]
- 38.Piazza, P. V., Maccari, S., Deminiere, J.-M., Le Moal, M., Mormède, P. & Simon, H. (1991) Neurobiology 88, 2088-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dellu, F., Piazza, P. V., Mayo, W., Le Moal, M. & Simon, H. (1996) Neuropsychobiology 34, 136-145. [DOI] [PubMed] [Google Scholar]
- 40.Sutton, R. E., Koob, G. F., LeMoal, M., Rivier, J. & Vale, W. W. (1982) Nature 297, 331-333. [DOI] [PubMed] [Google Scholar]
- 41.Kalin, N. H., Shelton, S. E. & Davidson, R. J. (2000) Biol. Psychiatry 47, 579-585. [DOI] [PubMed] [Google Scholar]
- 42.García, A. & Armario, A. (2001) Psychoneuroendocrinology 26, 363-374. [DOI] [PubMed] [Google Scholar]
- 43.Brantley, P. J., McKnight, G. T., Jones, G. N., Dietz, L. S. & Tulley, R. (1988) J. Consult. Clin. Psych. 56, 549-551. [DOI] [PubMed] [Google Scholar]
- 44.Kanner, A. D., Coyne, J. C., Schaefer, C. & Lazarus, R. S. (1981) J. Behav. Med. 4, 1-39. [DOI] [PubMed] [Google Scholar]
- 45.Sapolsky, R. M., Krey, L. C. & McEwen, B. S. (1984) Proc. Natl. Acad. Sci. USA 81, 6174-6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu, Z. J., Jiang, W. Q. & Thompson, H. J. (2003) Carcinogenesis 24, 1225-1231. [DOI] [PubMed] [Google Scholar]
- 47.Behl, C., Lezoualch, F., Trapp, T., Widmann, M., Skutella, T. & Holsboer, F. (1997) Endocrinology 138, 101-106. [DOI] [PubMed] [Google Scholar]
- 48.Moran, T. J., Gray, S., Mikosz, C. A. & Conzen, S. D. (2000) Cancer Res. 60, 867-872. [PubMed] [Google Scholar]
- 49.Tonomura, N., McLaughlin, K., Grimm, L., Goldsby, R. A. & Osborne, B. A. (2003) J. Immunol. 170, 2469-2478. [DOI] [PubMed] [Google Scholar]
- 50.Malaguarnera, L., Ferlito, L., Di Mauro, S., Imbesi, R. M., Scalia, G. & Malaguarnera, M. (2001) Arch. Gerontol. Geriatr. 32, 77-93. [DOI] [PubMed] [Google Scholar]
- 51.Xu, R. K., Wu, X. M., Di, A. K., Xu, J. N., Pang, C. S. & Pang, S. F. (2000) Biol. Signals Recept. 9, 1-20. [DOI] [PubMed] [Google Scholar]
- 52.Gilad, G. M. & Gilad, V. H. (1995) Mech. Ageing Dev. 78, 75-83. [DOI] [PubMed] [Google Scholar]
- 53.vom Saal, F. S. (1979) Horm. Behav. 12, 1-11. [DOI] [PubMed] [Google Scholar]
- 54.Moore, C. & Power, K. L. Dev. Psychobiol. 25, 165-182. [DOI] [PubMed]
- 55.Williams, G. C. (1957) Evolution (Lawrence, Kans.) 11, 398-411. [Google Scholar]