Abstract
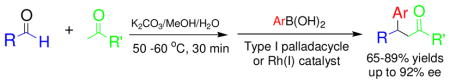
Sequential aldol condensation of aldehydes with methyl ketones followed by transition metal-catalyzed addition reactions of arylboronic acids to form β-substituted ketones is described. By using the 1,1′-spirobiindane-7,7′-diol (SPINOL)-based phosphite, an asymmetric version of this type of sequential reaction, with up to 92% ee, was also realized. Our study provided an efficient method to access β-substituted ketones and might lead to the development of other sequential/tandem reactions with transition metal-catalyzed addition reactions as the key step.
Transition metal-catalyzed addition reactions of arylboronic acids with carbonyl-containing compounds and derivatives have recently emerged as useful transformations for organic synthesis in part due to the nature of low toxicity and air/moisture stability of arylboronic acids.1, 2 One of the most noteworthy achievements in this field might be transition metal-catalyzed addition reactions of arylboronic acids with α,β-unsaturated ketones, which yield synthetically useful β-substituted ketones as the products.2,3 While good to high enantioselectivities have been achieved for this type of addition reaction, the prepurifed α, β-unsaturated ketones were used. Although α, β-unsaturated ketones can be “readily” obtained from the aldol condensation of aldehydes and/or ketones, the use of prepurified α, β-unsaturated ketones apparently posed some limits: they require an extra purification/separation step from aldehydes/ketones and are less available than aldehydes/ketones. During our study on transition metal-catalyzed addition reactions of arylboronic acids with carbonyl-containing compounds,4,5,6,7 we became interested in combining the formation of α, β-unsaturated ketones, the aldol condensation, with the addition reactions in a tandem or sequential fashion.8 We reasoned that achieving such tandem/sequential reactions will minimize the effort for the preparation of α, β-unsaturated ketones because prepurification for such α, β-unsaturated ketones is eliminated, and may also expand the α, β-unsaturated ketone substrate scope. Herein, we report our results on such new sequential reactions, including an asymmetric Rh(I)-catalyzed sequential reaction.
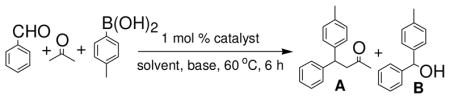
We began our study by mixing benzaldehyde, acetone and p-tolylboronic acid together with palladacycle 1 1, 9, 10,11 or [Rh(COD)Cl]2 as the catalyst. We found with toluene or THF-MeOH as the solvent, the desired reaction product (A) was the minor product and the major product was the 1,2-addition product (B) (Table 1). We speculated that this reaction outcome was likely due to the fact that transition metal-catalyzed addition of p-tolylboronic acid with benzaldehyde occurred faster than the aldol condensation of benzaldehyde with acetone under the reaction condition.
Table 1.
Tandem Aldol Condensation-Transition Metal-Catalyzed Reaction of Benzaldehyde, Acetone and p-Tolylboronic Acida
 | |||||
|---|---|---|---|---|---|
| entry | catalyst | solvent | base | conv (%)b | A/Bb |
| 1 |
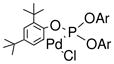 (1) (Ar = 2,4-di-t-BuC6H3) |
Toluene | K3PO4 | 99 | 1:99 |
| 2 | 1 | Toluene | K3PO4 | 63c | 12:88 |
| 3 | [Rh(COD)Cl]2 | Toluene | K3PO4 | 30 | 1:99 |
| 4 | 1 | THF-MeOH | K2CO3 | 24d | 1:99e |
| 5 | [Rh(COD)Cl]2 | THF-MeOH | K2CO3 | 87d | 1:99f |
Reaction condition: benzaldehyde (0.25 mmol), acetone (0.3 mL), p-tolylboronic acid (2.0 equiv), toluene (0.7 mL) or THF/MeOH mL/0.1 mL), base (3.0 equiv), 60 °C.
Based on GC-MS analysis.
2.0 equiv of H2O were added to the reaction system.
22 equiv of H2O were added to the reaction system.
14% of phenyl p-tolyl ketone was observed.
9% of phenyl p-tolyl ketone was observed.
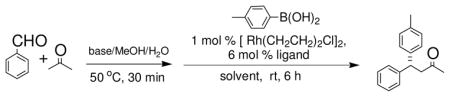
To overcome the fast 1,2-addition reaction issue, we decided to carry out the reaction of aldehydes, methyl ketones and arylboronic acids in a sequential fashion: the arylboronic acids, and the catalyst were introduced into the reaction system after the completion of the aldol condensation. We found with K2CO3 as the base and THF/MeOH as the solvent, the sequential reactions of acetone, aldehydes and arylboronic acids occurred smoothly at room temperature (Table 1, entries 1–3). Different aldehydes and arylboronic acids were tested for the sequential reaction, and good yields were observed (Table 1, entries 1–9). We also tested 2-butanone, 2-pentanone, acetophenone and 3-pentanone for the reaction. We found that 2-butanone, 2-pentanone and acetophenone were suitable ketones (Table 1, entries 10–15). On the other hand, we also found that 3-pentanone was inefficient for the sequential reaction (Table 1, entry 16), likely because the the aldol condensation between benzaldehyde and 3-pentanone occurred too slowly. We also found aliphatic aldehydes, which can also undergo aldol reactions with themselves, were suitable starting materials for the new sequential reaction (Table 1, entries 17, 18).
We next turned our attention to the asymmetric version of this sequential β-aryl ketone formation process. We selected Rh(I) complexes for our study because Rh(I)/chiral ligand-catalyzed 1,4-addition reactions of arylboronic acids with α, β-unsaturated ketones have been established.1,3 We examined four optically active ligands, 2,12 3,13 1,1′-spirobiindane-7,7′-diol (SPINOL)-based phosphite 414 and 5,15 that were available to us and our results are listed in Table 3. We found while Rh(I)/ligand 3 and Rh(I)/ligand 5 were poor catalysts for the sequential aldol condensation-addition reaction (Table 3, entries 2, 4), Rh(I)/(R)-BINAP 2 and Rh(I)/ligand 4 exhibited good catalytic activities and enantioselectivities (Table 3, entries 1, 3). Other factors that could influence the enantioselectivity of the reaction were then examined. We found that with 4 as the ligand, K2CO3 as the base and THF as the solvent, the enantioselectivity could be improved to 89% (Table 3, entries 6–11). Decreasing the reaction temperature from room temperature to 0 °C further improved the enantioselectivity to 92% (Table 3, entry 12).
Table 3.
Asymmetric Sequential Aldol Condensation-Rh(I)/Ligand-Catalyzed Addition Reaction of Benzaldehyde, Acetone and p-Tolylboronic Acida
 | ||||||
|---|---|---|---|---|---|---|
| entry | ligand | base | temp | solvent | yield (%)b | ee (%)c |
| 1 |
 (2) |
KOH | 100 °C | Toluene | 82 | 81 |
| 2 |
 (3) |
KOH | rt | Toluene | 10 | – |
| 3 |
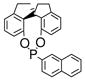 (4) |
KOH | rt | Toluene | 81 | 79 |
| 4 |
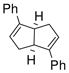 (5) |
KOH | rt | Toluene | 30 | – |
| 5 | 2 | K2CO3 | rt | THF | 80 | 80 |
| 6 | 4 | K2CO3 | rt | THF | 83 | 89 |
| 7 | 4 | K2CO3 | rt | THF | 81d | 88 |
| 8 | 4 | K2CO3 | rt | Toluene | 78 | 81 |
| 9 | 4 | K3PO4 | rt | Toluene | 70 | 53 |
| 10 | 4 | Cs2CO3 | rt | Toluene | 81 | 75 |
| 11 | 4 | K2CO3 | rt | 1,4-dioxane | 76 | 83 |
| 12 | 4 | K2CO3 | 0 °C | THF | 84e | 92 |
Reaction condition: benzaldehyde (0.25 mmol,1.0 eqiuv), p-tolylboronic acid (2.0 equiv), solvent (1 mL), acetone (0.2 mL), H2O (0.1 mL), base (1.0 equiv).
Isolated yield.
Determined by HPLC (Chiralcel OD Column).
4 mol % 4 was used.
Reaction temperature: 0 °C.
Several aldehydes, methyl ketones and arylboronic acids were examined for the asymmetric sequential aldol condensation-Rh(I)/4-catalyzed addition reaction. Optically active β-arylated ketones were obtained in good yields and good enantioselectivity (Table 3, entries 1–8). Because this sequential reaction involved α, β-unsaturated ketones, generated from the aldol condensation of aldehydes and methyl ketones, and arylboronic acids, we reasoned that optically active β-arylated ketones with opposite chiral configurations could be obtained with the same Rh(I)/4 catalyst by simply reversing the aryl groups on aldehydes and arylboronic acids. We found indeed that (R)-4-phenyl-4-p-tolylbutan-2-one, generated from benzaldehyde, acetone and p-tolylboronic acid, and (S)-4-phenyl-4-p-tolylbutan-2-one, generated from p-tolualdehyde, acetone and phenylboronic acid, were obtained in excellent enantioselectivity with the same Rh(I)/4 catalyst (Table 5, entries 1, 9).
In summary, we demonstrated that the aldol condensation of aldehydes with methyl ketones followed by transition metal-catalyzed addition reactions with arylboronic acids could occur efficiently in a sequential fashion, affording various β-arylated ketones. By using an optically active 1,1′-spirobiindane-7,7′-diol (SPINOL)-based phosphite as the ligand, a Rh(I)-catalyzed asymmetric version of such a sequential reaction has been realized and up to 92% ee was achieved. Our study provided an efficient method to access β-substituted ketones from readily available aldehydes with methyl ketones, and might lead to the development of other new sequential/tandem reactions with transition metal-catalyzed addition reactions as part of the reaction sequence.
Supplementary Material
Table 2.
Sequential Aldol Condensation-Transition Metal-Catalyzed Addition Reactions of Aldehydes, Methyl Ketones and Arylboronic Acidsa
 | |||||
|---|---|---|---|---|---|
| entry | catalyst | RCHO | R′COCH3 | Ar′B(OH)2 | yield(%)b |
| 1 | 1 | 84 | |||
| 2 | [Rh(COD)Cl]2 | 81c | |||
| 3 | 1 | 87 | |||
| 4 | 1 | 88 | |||
| 5 | [Rh(COD)Cl]2 | 82 | |||
| 6 | [Rh(COD)Cl]2 | 85 | |||
| 7 | [Rh(COD)Cl]2 | 89 | |||
| 8 | [Rh(COD)Cl]2 | 86 | |||
| 9 | [Rh(COD)Cl]2 | 84 | |||
| 10 | 1 | 86 | |||
| 11 | 1 | 74 | |||
| 12 | [Rh(COD)Cl]2 | 86 | |||
| 13 | [Rh(COD)Cl]2 | 85 | |||
| 14 | [Rh(COD)Cl]2 | 81 | |||
| 15 | [Rh(COD)Cl]2 | 84 | |||
| 16 | 1 | 0d | |||
| 17 | 1 | 65 | |||
| 18 | [Rh(COD)Cl]2 | 82 | |||
Reaction condition: aldehyde (0.25 mmol,1.0 equiv), acetone (0.1 mL), H2O (0.1 mL) and K2CO3 (1.0 equiv), 50 °C for 30 min, then 1 or [Rh(COD)Cl]2 (1 mol %), THF (1 mL) and arylboronic acids (0.5 mmol, 2.0 equiv) were added into the mixture at rt for another 6 h.
Isolated yield.
The reaction was carried out in 2.5 mmol scale.
16% of 1-Phenyl-2-methyl-1-penten-3-one was observed.
Table 4.
Asymmetric Sequential Aldol Condensation-Rh(I)-Catalyzed Addition Reactions of Aldehydes, Methyl Ketones and Arylboronic Acidsa
 | |||||
|---|---|---|---|---|---|
| entry | ArCHO |  |
Ar′B(OH)2 | yield (%)b | ee (%)c |
| 1 | 84 | 92 (R)d | |||
| 2 | 80 | 87 (R)d | |||
| 3 | 87 | 82 | |||
| 4 | 87 | 83 | |||
| 5 | 85 | 86 | |||
| 6 | 81 | 87 | |||
| 7 | 80 | 82 | |||
| 8 | 83 | 86 | |||
| 9 | 86 | 91 (S) | |||
Reaction condition: aldehyde (0.25 mmol,1.0 equiv), arylboronic acid (2.0 equiv), MeOH (0.1 mL), ketone (0.2 mL), H2O(0.1 mL), K2CO3 (3.0 equiv), 0 °C.
Isolated yield.
Determined by HPLC analysis(Chiral OD Column).
Established by comparision of the HPLC data with reported ones.
Acknowledgments
We gratefully thank the NSF (CHE0719311) and NIH (1R15 GM094709) for funding. Partial support from PSC-CUNY Research Award Programs is also acknowledged. We thank the Frontier Scientific for its generous gifts of arylboronic acids.
Footnotes
Supporting Information Available: General procedures and product characterization for sequential aldol condesation-transition metal-catalyzed addition reactions of aldehydes, methyl ketones and arylboronic acids. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For recent reviews: Glorius F. Angew Chem Int Ed. 2004;43:3364–3366. doi: 10.1002/anie.200301752.Hayashi T, Yamasaki K. Chem Rev. 2003;103:2829–2844. doi: 10.1021/cr020022z.Fagnou K, Lautens M. Chem Rev. 2003;103:169–196. doi: 10.1021/cr020007u. and references cited therein.
- 2.(a) Miyaura N. Synlett. 2009:2039–2050. [Google Scholar]; (b) Gutnov A. Eur J Org Chem. 2008:4547–4554. [Google Scholar]
- 3.For examples since 2009: Jeletic MS, Ghiviriga I, Abboud K, Veige AS. Dalton Transactions. 2010;39:6392–6394. doi: 10.1039/c0dt00268b.Lang F, Li D, Chen J, Chen J, Li L, Cun L, Zhu J, Deng J, Liao J. Adv Synth Catal. 2010;352:843–846.Hu X, Cao Z, Liu Z, Wang Y, Du H. Adv Synth Catal. 2010;352:651–655.Drinkel E, Briceno A, Dorta R, Dorta R. Organometallics. 2010;29:2503–2514.Nishimura T, Yasuhara Y, Sawano T, Hayashi T. J Am Chem Soc. 2010;132:7872–7873. doi: 10.1021/ja1034842.Lin S, Lu X. Org Lett. 2009;12:2536–2539. doi: 10.1021/ol100767u.Chen QA, Dong X, Chen MW, Wang DS, Zhou YG, Li YX. Org Lett. 2009;12:1928–1931. doi: 10.1021/ol100536e.Xu Q, Zhang R, Zhang T, Shi M. J Org Chem. 2010;75:3935–3937. doi: 10.1021/jo1006224.Chen J, Chen J-M, Lang F, Zhang XY, Cun LF, Zhu J, Deng JG, Liao J. J Am Chem Soc. 2010;132:4552–4553. doi: 10.1021/ja1005477.Hahn BT, Tewes F, Froehlich R, Glorius F. Angew Chem Int Ed. 2010;49:1143–1146. doi: 10.1002/anie.200905712.Gendrineau T, Genet JP, Darses S. Org Lett. 2009;12:308–310. doi: 10.1021/ol902646j.Brown MK, Corey EJ. Org Lett. 2009;12:172–175. doi: 10.1021/ol9025793.Chen Q, Kuriyama M, Hao X, Soeta T, Yamamoto Y, Yamada K-i, Tomioka K. Chem Pharm Bull. 2009;57:1024–1027. doi: 10.1248/cpb.57.1024.Hu X, Zhuang M, Cao Z, Du H. Org Lett. 2009;11:4744–4747. doi: 10.1021/ol901949n.Minuth T, Boysen MK. Org Lett. 2009;11:4212–4215. doi: 10.1021/ol901579g.Yuan WC, Cun LF, Mi AQ, Jiang YZ, Gong LZ. Tetrahedron. 2009;65:4130–4141.Wallace GA, Gordon TD, Hayes ME, Konopacki DB, Fix-Stenzel SR, Zhang X, Grongsaard P, Cusack KP, Schaffter LM, Henry RF, Stoffel RH. J Org Chem. 2009;74:4886–4889. doi: 10.1021/jo900376b.Kim SB, Cai C, Faust MD, Trenkle WC, Sweigart DA. Organometallics. 2009;28:2625–2628.Buergi JJ, Mariz R, Gatti M, Drinkel E, Luan X, Blumentritt S, Linden A, Dorta R. Angew Chem Int Ed. 2009;48:2768–2771. doi: 10.1002/anie.200900429.Korenaga T, Osaki K, Maenishi R, Sakai T. Org Lett. 2009;11:2325–2328. doi: 10.1021/ol900719z.Yamamoto T, Iizuka M, Takenaka H, Ohta T, Ito Y. J Organomet Chem. 2009;694:1325–1332.Iuliano A, Facchetti S, Funaioli T. Chem Commun. 2009:457–459. doi: 10.1039/b814568g.Facchetti S, Cavallini I, Funaioli T, Marchetti F, Iuliano A. Organometallics. 2009;28:4150–4158.Jana R, Tunge JA. Org Lett. 2009;11:971–974. doi: 10.1021/ol802927v.
- 4.(a) Liao YX, Xing CH, He P, Hu QS. Org Lett. 2008;10:2509–2512. doi: 10.1021/ol800774c. [DOI] [PubMed] [Google Scholar]; (b) He P, Lu Y, Dong CG, Hu QS. Org Lett. 2007;9:343–346. doi: 10.1021/ol062814b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) He P, Lu Y, Hu QS. Tetrahedron Lett. 2007;48:5283–5288. doi: 10.1016/j.tetlet.2007.05.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Xing CH, Liao YX, He P, Hu QS. Chem Commun. 2010:3010–3012. doi: 10.1039/c001104e. [DOI] [PubMed] [Google Scholar]; (b) Xing C-H, Liu T-P, Zheng JR, Ng J, Esposito M, Hu Q-S. Tetrahedron Lett. 2009;50:4953–4957. [Google Scholar]
- 6.Xing CH, Hu QS. Tetrahedron Lett. 2010;51:924–927. [Google Scholar]
- 7.Liao YX, Xing CH, Hu QS. J Org Chem. 2010;75:6986–6989. doi: 10.1021/jo101469s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.For examples of intramolecular tandem reactions initialized by Rh(I)-catalyzed addition reactions of arylboronic acids with α, β-unsaturated ketones or esters: Navarro C, Csaky AG. Synthesis. 2009:860–863.Youn SW, Song JH, Jung DI. J Org Chem. 2008;73:5658–5661. doi: 10.1021/jo800914c.Navarro C, Csakye AG. Org Lett. 2008;10:217–219. doi: 10.1021/ol702571c.Bocknack BM, Wang LC, Krische MJ. Proc Nat Acad Sci. 2004;101:5421–5424. doi: 10.1073/pnas.0307120101.Cauble DF, Gipson JD, Krische MJ. J Am Chem Soc. 2003;125:1110–1111. doi: 10.1021/ja0211095.Nishikata T, Kobayashi Y, Kobayshi K, Yamamoto Y, Miyaura N. Synlett. 2007:3055–3057.
- 9.Recent reviews of metalacycles: Dupont J, Consorti CS, Spencer J. Chem Rev. 2005;105:2527–2572. doi: 10.1021/cr030681r.Beletskaya IP, Cheprakov AV. J Organomet Chem. 2004;689:4055–4082.Bedford RB. Chem Commun. 2003:1787–1796.Newkome GR, Puckett WE, Gupta VK, Kiefer GE. Chem Rev. 1986;86:451–489.
- 10.Most Type I palladacycles are known to exist as bridged dimers and to dissociate into monomeric forms during reactions. Type I palladacycles in this paper were drawn in monomeric forms.
- 11.For other examples: Suzuma Y, Hayashi S, Yamamoto T, Oe Y, Ohta T, Ito Y. Tetrahedron: Asymmetry. 2009;20:2751–2758.Yu A, Cheng B, Wu Y, Li J, Wei K. Tetrahedron Lett. 2008;49:5405–5407.Bedford RB, Betham M, Charmant JPH, Haddow MFA, Orpen G, Pilarski LT, Coles SJ, Hursthouse MB. Organometallics. 2007;26:6346–6353.Gibson S, Foster DF, Eastham GR, Tooze RP, Cole-Hamilton DJ. Chem Commun. 2001:779–780.
- 12.(a) Takaya Y, Ogasawara M, Hayashi T, Sakai M, Miyaura N. J Am Chem Soc. 1998;120:5579–5580. [Google Scholar]; (b) Hayashi T, Takahashi M, Takaya Y, Ogasawara M. J Am Chem Soc. 2002;124:5052–5058. doi: 10.1021/ja012711i. [DOI] [PubMed] [Google Scholar]
- 13.Sakai M, Euda M, Miyaura N. Angew Chem, Int Ed. 1998;37:3279–3281. doi: 10.1002/(SICI)1521-3773(19981217)37:23<3279::AID-ANIE3279>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 14.(a) Duan HF, Xie JH, Shi WJ, Zhang Q, Zhou QL. Org Lett. 2006;8:1479–1481. doi: 10.1021/ol060360c. [DOI] [PubMed] [Google Scholar]; (b) Duan HF, Xie JH, Qiao XC, Wang LX, Zhou QL. Angew Chem Int Ed. 2008;47:4351–4353. doi: 10.1002/anie.200800423. [DOI] [PubMed] [Google Scholar]
- 15.Wang ZQ, Feng CG, Xu MH, Lin GQ. J Am Chem Soc. 2007;129:5336–5337. doi: 10.1021/ja0710914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


