Abstract
Objective
To determine the incidence of retinal vein occlusion (RVO) in the Ocular Hypertension Treatment Study (OHTS).
Design
Retrospective analysis of data from a randomized clinical trial.
Participants
1,636 ocular hypertensive participants with a mean follow up of 9.1 years. Participants in the medication and observation groups were managed according to their original randomization assignment until June 1, 2002. At that time, the observation participants were offered ocular hypotensive treatment. Data to July 1, 2005 are included in this report.
Methods
Occurrences of RVO in study participants, categorized as branch, central or hemi-central vein occlusion, were documented. Potential RVO events were identified by a keyword search of Adverse Event Reports, the Optic Disc Reading Center database, Endpoint Committee reviews, and by response to a written request for information sent to each clinical site. To confirm a potential RVO, the complete OHTS chart was reviewed. Statistical analyses included t-tests, chi-square tests and Cox proportional hazards models.
Main Outcome Measures
Incidence of RVO
Result
s: Twenty-six RVOs-5 branch, 14 central and 7 hemi-central RVOs-were confirmed in 23 participants (15 observation and 8 medication). The 10 year cumulative incidence of RVO was 2.1 % in the observation group and 1.4% in the medication group (log-rank p-value = 0.14). At baseline, participants who later developed a RVO were significantly older (65.1 versus 55.3 years, p=0.01), and had larger horizontal cup-to-disc ratios (p=0.0004).
Conclusions
Although the incidence of RVO was higher in the observation group than the medication group, this difference did not attain statistical significance. Consistent with some previous studies, older age and larger cup-to-disc ratio were associated with the development of RVO.
The association between ocular hypertension (OHT) or glaucoma and retinal vein occlusion (RVO) has been recognized since the beginning of the 20th century.1 Most of the studies examining this association, however, have been retrospective.2–8 Case-control studies have reported that a history of glaucoma or OHT in the fellow eye was significantly more common in patients with central retinal vein occlusion (CRVO) than in controls.9, 10
In a large series of patients with CRVO and hemi-central retinal vein occlusion (HRVO), Hayreh reported the prevalences of glaucoma and OHT were approximately 10% and 16%, respectively, much higher prevalences than found in the general population. 5,8 The Eye Disease Case-Control Study found that a history of glaucoma was associated with all three types of RVO: CRVO, HRVO, and branch retinal vein occlusion (BRVO).9
In contrast, a few studies have come to a different conclusion, especially concerning the relationship between BRVO and OHT or glaucoma. The Blue Mountains Eye Study reported 9 cases of CRVO and 25 cases of BRVO during 10 years of follow up. No significant association was found between incident RVO and intraocular pressure (IOP).11 The Beaver Dam Eye Study reported 7 incident cases of CRVO and 21 of BRVO during 5 years of follow-up. A detailed risk factor analysis, which was only reported for BRVO, did not find statistically significant associations between BRVO and OHT or glaucoma.12 However, after 10 years of follow-up, the same study found that participants with incident RVOs were more likely to have had definite or probable glaucoma at baseline.13
The Ocular Hypertension Study (OHTS) provides a unique opportunity to examine the incidence of RVO in a large prospectively-followed sample of ocular hypertensive individuals.14 The OHTS participants were followed closely with IOP measurement, static automated perimetry, and optic disc examination every six months, and dilated fundus examination with optic disc photographs annually.
METHODS
Study Synopsis
The OHTS is a multicenter randomized clinical trial to determine the safety and efficacy of topical ocular hypotensive medication in delaying or preventing the onset of glaucoma in individuals with elevated IOP. All participants provided written informed consent for participation in the study. Institutional Review Board approval was obtained at all OHTS clinical sites and the study has been conducted in compliance with the HIPAA. The protocol for the OHTS is described in detail elsewhere14 and the manual of procedures for the study is available online at https://vrcc.wustl.edu/mop/mop.htm, accessed August 14, 2009.
Eligibility criteria included age between 40 to 80 years, no evidence of either glaucomatous structural or functional damage by standard clinical measures and IOP between 24 mm Hg and 32 mm Hg in one eye and between 21 mm Hg and 32 mm Hg in the other eye. Baseline medical history was by self-report. Intraocular pressure was measured by two OHTS certified study personnel, an operator and a recorder, using a calibrated Goldmann applanation tonometer. Baseline IOP was defined as the mean of 2 or 3 IOP measurements at the baseline/randomization visit as opposed to IOP measurements taken during the qualifying visits. Every effort was made to perform IOP readings at the same time of day to minimize diurnal fluctuation. The time of day of IOP readings was recorded.
From February 1994 to October 1996, participants (n=1,636) were randomly assigned to treatment with topical IOP-lowering medication or to observation. The goal of treatment with topical ocular hypotensive medication was to achieve an IOP of 24 mmHg or less and a minimum 20% reduction from the baseline IOP, except that an IOP of less than 18 mmHg was not required. Humphrey 30-2 visual fields were performed every 6 months and stereoscopic optic disc photography was performed every 12 months. The optic disc was examined every 6 months, with a dilated fundus exam performed every 12 months. After publication of results in June, 2002 showing that topical hypotensive medication was safe and effective in preventing or delaying the development of glaucoma, topical medication was offered to participants originally randomized to the observation group.15
Ascertainment of RVO
Potential cases of RVO occurring during OHTS follow-up were identified using the following methods:
Electronic searches of the OHTS database were conducted for any combination of the keywords – “central retinal vein occlusion”, “hemiretinal vein occlusion”, “branch retinal vein occlusion” in Adverse Events Forms, the Optic Disc Reading Center database, and the Endpoint Committee Forms. The Endpoint Committee, which is masked as to randomization assignment, determines whether each occurrence of confirmed visual field abnormality or optic disc change is attributable to glaucoma or not.
The Study Chairman sent letters (July, 2005) to clinic principal investigators of each of the 22 OHTS clinical sites asking for the participant identification number and date of diagnosis of any cases of RVO.
Statistical Methods
The analysis data set included data from 1,636 participants randomized between February, 1994 and October, 1996. The data set for this report closed July 1, 2005. The unit of analysis for eye-specific variables was the first affected eye among RVO cases versus one eye selected randomly among participants who were controls. The date of diagnosis of RVO was determined by the first affected eye of a participant.
The occurrence of RVO is reported as a percent of all participants randomized, either adjusted or unadjusted for variable follow-up time. The occurrences of RVO during follow-up in the observation and medication groups were plotted using Kaplan-Meier life tables and compared using the log rank statistic that adjusts for variable duration of follow-up. T-tests and chi-square statistics were used to compare baseline IOP, demographics, clinical characteristics, and self-reported health history of participants who did or did not develop RVO. A Cox proportional hazards model for the development of RVO included randomization group and baseline variables with p-values of ≤ 0.10 in univariate analyses.
Results
This report includes data from the start of randomization in February, 1994 to July 1, 2005. During this reporting period, the mean follow-up for the medication group was 9.1 ±2.7 standard deviation (SD) years, during which time these participants were treated. The mean follow-up for the observation group was 9.1 ± 2.6 SD years. The observation group participants were treated for an average of 2.9 ± 0.6 years from June 1, 2002 to July 1, 2005. The mean IOP over the entire period for the medication group was 19.0 mmHg ± 3.5. In the observation group, the mean IOP was 23.6 mmHg ± 3.8 prior to treatment and 20.0 mmHg ± 4.3 after initiation of treatment.
After identification of potential RVOs and subsequent confirmation through chart review and communication with clinical investigators (when necessary), 26 occurrences of RVO were confirmed in 23 subjects: 5 BRVO, 14 CRVO, and 7 HRVO. Two participants, both randomized to the observation group, experienced more than one RVO event. One participant was determined to have CRVO in the first eye and 58 months later to have CRVO in the fellow eye. The other participant was found to have BRVO in the right eye and HRVO in the left eye at the same visit. The left eye of this participant subsequently developed CRVO 50 months later.
Over a mean follow-up period of 9.1 years, the percent of participants who developed RVO in one or both eyes was 1.8% (15 of 819 participants) in the observation group and 1.0% (8 of 817 participants) in the medication group unadjusted for follow-up time (chi-square test, p = 0.14). The median time to RVO was 7.1 years for the medication group and 5.5 years for the observation group. Of the participants in the observation group who developed RVO, 4 (out of 15) did so after initiation of treatment. Adjusting for follow-up time including loss to follow-up and death, the cumulative proportion of participants who developed RVO in one or both eyes was 2.1% in the observation group and 1.4% in the medication group (hazard ratio, 0.53; 95% confidence interval of 0.22 to 1.24, log-rank p = 0.14) (Figure 1).
Figure 1.
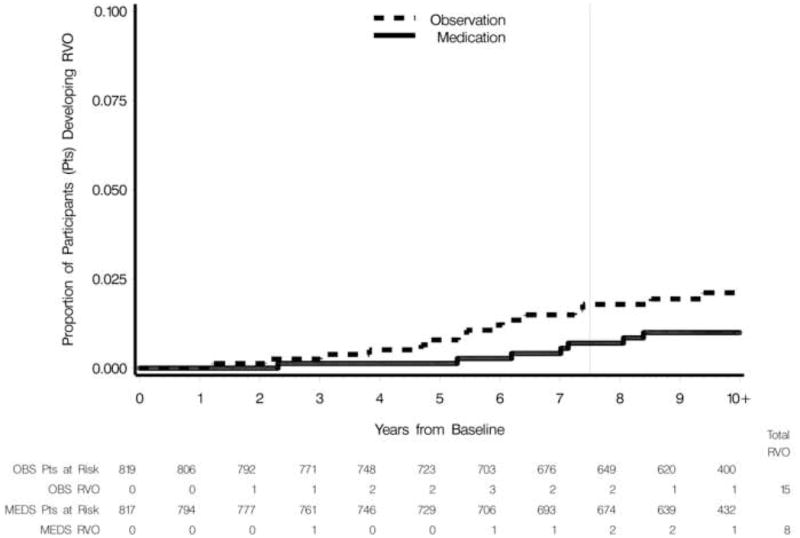
Cumulative proportion of participants in the observation (OBS) group and medication (MEDS) group who developed retinal vein occlusion (RVO). The vertical line indicates the mean follow-up (yrs) completed when hypotensive medication was offered to the observation group.
The mean baseline age of participants who later developed RVO was 65.1 ± 8.5 SD years compared to 55.3 ± 9.5 SD years among participants who did not develop RVO (t-test, p < 0.001) (Table 1). The mean age of participants at the time of RVO diagnosis was 71.6 years ± 8.7 SD. The mean baseline horizontal cup to disc ratio (CDR) was significantly higher in participants who developed RVO (0.46) than those who did not (0.36, p = 0.016). Participants who later developed RVO also had a more hyperopic baseline spherical equivalent than those who did not (+0.3 vs. −0.6 diopters, respectively), although this difference did not achieve statistical significance (p = 0.067). There was a non-significant trend (p = 0.083) for the group that developed RVO to have a lower proportion of females (39.1%) than the group that did not develop RVO (57.2%). No difference in baseline IOP was detected between participants who later developed RVO (24.9 ± 2.5 SD) and those who did not (24.9 ± 3.0 SD, t-test, p = 0.94). Analysis of the last IOPs measured prior to diagnosis of RVO found a significantly higher IOP among participants from the observation group (22.1 ± 4.0 mmHg) versus the medication group (18.4 ± 3.2 mmHg; p = 0.035). The proportion of participants who developed RVO by baseline demographic and clinical factors is reported in Table 1.
Table 1.
Baseline measures for 23 participants who developed retinal vein occlusion and 1,613 control participants.*
| RVO Status | P-Value | |||
|---|---|---|---|---|
| Control | RVO | |||
| Baseline Age | N | 1613 | 23 | <0.0001 |
| Mean | 55.3 | 65.1 | ||
| SD | 9.5 | 8.5 | ||
| Gender=Female | N | 1613 | 23 | 0.083 |
| Percent | 57.2 | 39.1 | ||
| Race=African American? | N | 1613 | 23 | 0.72 |
| Percent | 25.0 | 21.7 | ||
| Baseline Spherical Equivalent | N | 1613 | 23 | 0.067 |
| Mean | −0.6 | 0.3 | ||
| SD | 2.4 | 1.5 | ||
| Baseline ETDRS Visual Acuity (letters correct) | N | 1023 | 10 | 0.66 |
| Mean | 55.5 | 54.5 | ||
| SD | 7.2 | 3.7 | ||
| Baseline Intraocular Pressure (mmHg) | N | 1613 | 23 | 0.94 |
| Mean | 24.9 | 24.9 | ||
| SD | 3.0 | 2.5 | ||
| Baseline Horizontal Cup-to-Disc Ratio | N | 1613 | 23 | 0.016 |
| Mean | 0.36 | 0.46 | ||
| SD | 0.21 | 0.19 | ||
| Baseline Vertical Cup-to-Disc ratio | N | 1613 | 23 | 0.086 |
| Mean | 0.39 | 0.46 | ||
| SD | 0.20 | 0.23 | ||
| Baseline Mean Deviation dB | N | 1613 | 23 | 0.79 |
| Mean | 0.2 | 0.2 | ||
| SD | 1.1 | 0.9 | ||
| Central Corneal Thickness (μM) | N | 1424 | 23 | 0.41 |
| Mean | 572.5 | 565.9 | ||
| SD | 38.5 | 38.7 | ||
Eye-specific measures are reported for a randomly selected eye of controls and for the first affected eye of participants who had more than one RVO. P-values are for t-tests and chi-square tests and are not adjusted for correlation with other measures. RVO = retinal vein occlusion; ETDRS = Early Treatment Diabetic Retinopathy Study; SD = Standard deviation
The Cox multivariate proportional hazards model for the development of RVO included randomization group and baseline variables (age, horizontal cup-to-disc ratio, spherical equivalent, and gender) with p-values of ≤ 0.10 in univariate analyses (Table 1). Factors in the Cox multivariate proportional hazards regression model that were statistically significantly associated (p ≤ 0.05) with the development of RVO after adjusting for the correlation among these factors were age (hazard ratio, 3.2 per decade; 95% confidence interval of 1.9–5.4, p < 0.0001) and horizontal cup-to-disc ratio (hazard ratio, 1.35 per 0.1 units; 95% confidence interval of 1.09 to 1.67, p = 0.006).
Over the course of follow-up, the incidence of progression to primary open angle glaucoma (POAG) was higher among participants who developed RVO (26.1%, 6 of 23 participants) as compared with participants who did not develop RVO (7.4%, 120 of 1613 participants) (Fisher’s exact test, p=0.006). Of the six participants with RVO who also developed (POAG), the RVO date preceded the date of POAG in four. All four of these participants met endpoint criteria based on optic disc, as opposed to visual field changes.
Discussion
Twenty three OHTS participants had RVOs during a mean follow-up of 9.1 years for a cumulative incidence of only 1.4%. The low number of incident RVO cases limits our ability to compare the observation and medication groups and to identify predictive factors. The incidence of RVO in the OHTS (1.4% over 9.1 years) was comparable to that reported from population-based studies. The Beaver Dam Eye Study found a 5 year incidence of RVO of 0.8%, while the Blue Mountains Eye Study reported a 10 year cumulative incidence of 1.6%.11, 12 The diagnosis of RVO was aided by stereoscopic 30 degree color fundus photographs at the 5 year examination in the Beaver Dam Eye Study and at the 5 and 10 year examinations in the Blue Mountains Eye Study, with RVOs typically identified based on chronic fundus and disc changes associated with the event. Nearly all RVOs in the OHTS population were identified based on acute fundus changes, often accompanied by a change in vision. The relatively frequent follow-up employed in the OHTS study (every 6 months) with dilated fundus examinations (every 12 months), combined with the on-going documentation of adverse events, made it less likely that a visually significant RVO would go undetected in an OHTS subject. Standardized 2X optic disc photographs were taken every 12 months by certified OHTS photographers and evaluated by an independent reading center, further reducing the possibility of missing the disc changes typically associated with a central or hemicentral RVO. It remains possible that an asymptomatic RVO event, such as a small branch BRVO, which resolved fully could have gone undetected in the OHTS.
The OHTS was not designed to prospectively identify RVO and the analysis of the incidence of RVO was unplanned. No standardized diagnostic criteria for the diagnosis of RVO were employed. Instead, potential RVOs were identified and documented by OHTS investigators based on clinical findings. Unless annual study photographs were due to be taken at that time, photographic documentation of the RVO was not routinely performed as part of the study. Although many of the participants with potential RVO were ultimately referred to a retina specialist and these evaluations may have contributed to the final diagnosis provided by the OHTS clinical site, review of such documentation was outside of the scope of this analysis. Individual OHTS investigators were free to manage RVOs independently. One exception was the requirement for study approval to initiate topical ocular hypotensive treatment in an observation group participant with RVO.
A focus on the overall incidence of RVO may be misleading in that the role of IOP as a risk factor may differ across RVO subtypes. While elevated IOP has been repeatedly identified as a risk factor for CRVO and HRVO,5, 16 the results for BRVO have been less consistent. In the Beaver Dam Eye Study, for example, Klein and associates did not find an association between BRVO and glaucoma, IOP, or OHT at the 5 year follow-up.12 A higher frequency of elevated IOP in cases of CRVO as compared with BRVO has been reported in other studies.4, 17 These findings suggested the need to separate the different RVO sub-types in future analyses.18 In our analysis, the cumulative incidence of CRVO/HRVO (1.3%) was over four times that of BRVO (0.3%). This lies in stark contrast to the 5-year data from the Beaver Dam Eye Study in which this ratio was reversed: 0.6% for BRVO and 0.2% for CRVO (HRVO not identified separately).12 Similarly, in the Blue Mountains Eye Study, the 10-year incidence of BRVO was three times that of CRVO/HRVO: 1.2% BRVO and 0.4% CRVO (includes HRVO).11 Given the stronger relationship reported in the literature between elevated IOP and CRVO/HRVO, the reversal of the ratio of BRVO to CRVO/HRVO in the OHTS versus these population-based studies may be related to the requirement for elevated IOP for inclusion into the OHTS.
One consistent finding over a follow-up period of nearly 10 years was that observation group participants had a higher incidence of RVO than those in the medication group, although this difference did not achieve statistical significance. This analysis, however, is not a definitive test of the relationship between elevated IOP and risk for RVO, as there are several factors which might possibly have undermined such a relationship. The OHTS inclusion criteria limited IOP elevation to 32 mmHg in either eye at entry, with the average overall IOP at baseline being 24.9 mmHg.14 Individual OHTS investigators were allowed to request IOP lowering treatment for observation group participants with markedly elevated IOPs if deemed necessary for the safety of the participant. Observation group participants were treated with IOP lowering topical medications during roughly one-third of the analysis period (2.9 of 9.1 years), although the last measured IOP prior to RVO was still significantly higher in participants from the observation group than the medication group. Given the low number of RVOs and relative risk, the study would have needed more than 6,000 participants to detect a difference between the observation and medication groups with statistical power of 80%.
Older age at baseline was a statistically significantly predictive factor for the development of RVO in OHTS. While the average age at enrollment of all OHTS participants was 55.4 years, those developing RVO were considerably older at baseline (65.1 years versus 55.3). RVO occurred at an average of 5.9 years from entry into OHTS (range of 1.2 to 10.6 years). The Beaver Dam Eye Study enrolled subjects 43 to 86 years old and had an average age at baseline of 62.0, almost 7 years older than OHTS subjects.12 The Blue Mountains Eye Study also reported an increase in RVO incidence with age. Participants under 60 years of age had a 10 year incidence of RVO of 0.8%, those 60–69 1.6%, and patients 70 or older 2.7%.11
At baseline, OHTS participants who later developed RVO had a mean horizontal CDR of 0.46 compared to 0.36 in those who did not, a difference which reached statistical significance (p=0.016). The literature on CDR and RVO is contradictory. In one case control study, the CDR of the fellow eye of 55 CRVO patients was nearly identical to that found in the control group.19 Mansour and associates compared 45 unilateral CRVO eyes that did not have disc edema or a history of OHT or glaucoma in either eye to 27 control subjects and found a larger (0.37 vs. 0.26) but not statistically significant different horizontal CDR.20 In contrast, while controlling for baseline variables such as age and IOP, the Beaver Dam Eye Study found that participants with higher CDRs at baseline were more likely to develop RVO (predominantly BRVO) within 10 years (40% increase per 0.1 increment in CDR).13 Beaumont and Kang also reported a significant association between enlarged CDR and RVO, when the RVOs were sub-divided according to the putative site of the vascular occlusion.21
The only other baseline attribute which approached significance as a risk factor was refractive error. The mean spherical equivalent of RVO subjects was +0.3 diopters compared with −0.6 diopters for those who did not have a RVO. Several studies have associated hyperopia with BRVO17, 22, 23. Given the small number of RVOs detected in our analysis, the importance of refractive error as a baseline risk factor could not be adequately assessed.
In summary, the OHTS cohort provided an opportunity to examine prospectively the incidence of RVO in participants with the risk factor of elevated IOP. Our retrospective analysis found that development of RVO was a relatively infrequent occurrence in OHTS participants, with an incidence of 1.4% cases of RVO (23 of 1636) over a mean follow-up of 9.1 years. While RVO was more frequent in the observation as compared with the medication group, consistent with previous studies implicating elevated IOP was a risk for RVO, this difference did not achieve statistical significance. Consistent with some previous studies, older age and larger horizontal CDR at baseline were found to be statistically significant predictive factors for the development of RVO.
Acknowledgments
FUNDING
The OHTS was funded by grants from the NIH (EY09341, EY09307), the National Center on Minority Health and Health Disparities, Horncrest Foundation, Merck Research Laboratories, and Pfizer, Inc. This work was also supported by awards to the Department of Ophthalmology and Visual Sciences, Washington University from a Research to Prevent Blindness, Inc. Unrestricted grant, and the NIH Vision Core Grant P30 EY 02687.
The funding organizations had no role in the design or conduct of this research
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Verhoeff FH. The effect of chronic glaucoma on the central retinal vessels. Arch Ophthalmol. 1913;42:145–52. [Google Scholar]
- 2.Chew EY, Trope GE, Mitchell BJ. Diurnal intraocular pressure in young adults with central retinal vein occlusion. Ophthalmology. 1987;94:1545–9. doi: 10.1016/s0161-6420(87)33262-2. [DOI] [PubMed] [Google Scholar]
- 3.Dryden RM. Central retinal vein occlusions and chronic simple glaucoma. Arch Ophthalmol. 1965;73:659–63. doi: 10.1001/archopht.1965.00970030661012. [DOI] [PubMed] [Google Scholar]
- 4.Frucht J, Shapiro A, Merin S. Intraocular pressure in retinal vein occlusion. Br J Ophthalmol. 1984;68:26–8. doi: 10.1136/bjo.68.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayreh SS, Zimmerman MB, Beri M, Podhajsky P. Intraocular pressure abnormalities associated with central and hemicentral retinal vein occlusion. Ophthalmology. 2004;111:133–41. doi: 10.1016/j.ophtha.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Luntz MH, Schenker HI. Retinal vascular accidents in glaucoma and ocular hypertension. Surv Ophthalmol. 1980;25:163–7. doi: 10.1016/0039-6257(80)90093-4. [DOI] [PubMed] [Google Scholar]
- 7.Soni KG, Woodhouse DF. Retinal vascular occlusion as a presenting feature of glaucoma simplex. Br J Ophthalmol. 1971;55:192–5. doi: 10.1136/bjo.55.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vannas S, Tarkkanen A. Retinal vein occlusion and glaucoma: tonographic study of the incidence of glaucoma and of its prognostic significance. Br J Ophthalmol. 1960;44:583–9. doi: 10.1136/bjo.44.10.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eye Disease Case-Control Study Group. Risk factors for central retinal vein occlusion. Arch Ophthalmol. 1996;114:545–54. [PubMed] [Google Scholar]
- 10.Sperduto RD, Hiller R, Chew E, et al. Risk factors for hemiretinal vein occlusion: comparison with risk factors for central and branch retinal vein occlusion: the Eye Disease Case-Control Study. Ophthalmology. 1998;105:765–71. doi: 10.1016/S0161-6420(98)95012-6. [DOI] [PubMed] [Google Scholar]
- 11.Cugati S, Wang JJ, Rochtchina E, Mitchell P. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol. 2006;124:726–32. doi: 10.1001/archopht.124.5.726. [DOI] [PubMed] [Google Scholar]
- 12.Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133–41. discussion 141–3. [PMC free article] [PubMed] [Google Scholar]
- 13.Klein BE, Meuer SM, Knudtson MD, Klein R. The relationship of optic disk cupping to retinal vein occlusion: the Beaver Dam Eye Study. Am J Ophthalmol. 2006;141:859–62. doi: 10.1016/j.ajo.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Gordon MO, Kass MA Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol. 1999;117:573–83. doi: 10.1001/archopht.117.5.573. [DOI] [PubMed] [Google Scholar]
- 15.Kass MA, Heuer DK, Higginbotham EJ, et al. Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002:120–13. doi: 10.1001/archopht.120.6.701. discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 16.Shahsuvaryan ML, Melkonyan AK. Central retinal vein occlusion risk profile: a case-control study. Eur J Ophthalmol. 2003;13:445–52. doi: 10.1177/112067210301300505. [DOI] [PubMed] [Google Scholar]
- 17.Appiah AP, Trempe CL. Risk factors associated with branch vs. central retinal vein occlusion. Ann Ophthalmol. 1989;21:153–5. 157. [PubMed] [Google Scholar]
- 18.Williamson TH. Central retinal vein occlusion: what’s the story? Br J Ophthalmol. 1997;81:698–704. doi: 10.1136/bjo.81.8.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strahlman ER, Quinlan PM, Enger C, Elman MJ. The cup-to-disc ratio and central retinal vein occlusion. Arch Ophthalmol. 1989;107:524–5. doi: 10.1001/archopht.1989.01070010538026. [DOI] [PubMed] [Google Scholar]
- 20.Mansour AM, Walsh JB, Henkind P. Optic disc size in central retinal vein occlusion. Ophthalmology. 1990;97:165–6. doi: 10.1016/s0161-6420(90)32609-x. [DOI] [PubMed] [Google Scholar]
- 21.Beaumont PE, Kang HK. Cup-to-disc ratio, intraocular pressure, and primary open-angle glaucoma in retinal venous occlusion. Ophthalmology. 2002;109:282–6. doi: 10.1016/s0161-6420(01)00922-8. [DOI] [PubMed] [Google Scholar]
- 22.Johnston RL, Brucker AJ, Steinmann W, et al. Risk factors of branch retinal vein occlusion. Arch Ophthalmol. 1985;103:1831–2. doi: 10.1001/archopht.1985.01050120065021. [DOI] [PubMed] [Google Scholar]
- 23.Timmerman EA, de Lavalette VW, van den Brom HJ. Axial length as a risk factor to branch retinal vein occlusion. Retina. 1997;17:196–9. doi: 10.1097/00006982-199705000-00004. [DOI] [PubMed] [Google Scholar]


