Abstract
Pyruvate dehydrogenase plays a critical role in the regulation of hepatic glucose and fatty acid oxidation, however surprisingly little is known about its regulation in vivo. In this study we examined the individual effects of insulin and substrate availability on the regulation of pyruvate dehydrogenase flux (VPDH) to tricarboxylic acid flux (VTCA) in livers of awake rats with lipid-induced hepatic insulin resistance. VPDH/VTCA flux was estimated from the [4-13C]glutamate/[3-13C]alanine enrichments in liver extracts and assessed under conditions of fasting and during a hyperinsulinemic-euglycemic clamp, while the effects of increased plasma glucose concentration on VPDH/VTCA flux was assessed during a hyperinsulinemic-hyperglycemic clamp. The effect of an acute increase of plasma fatty acid concentration on VPDH/VTCA was determined by infusing Liposyn II during a hyperinsulinemic-euglycemic clamp. The effects of chronic lipid-induced hepatic insulin resistance on this flux were also examined by performing these measurements in rats fed a high-fat diet for three weeks. Using this approach we found that fasting VPDH/VTCA was reduced by 95% in rats with hepatic insulin resistance (from 17.2±1.5% to 1.3±0.7%, P<0.00001). Surprisingly neither hyperinsulinemia per se or hyperglycemia per se were sufficient to increase VPDH/VTCA flux. Only under conditions of combined hyperglycemia and hyperinsulinemia did VPDH/VTCA flux increase (44.6±3.2%, P<0.0001 vs. basal) in low-fat fed animals but not in rats with chronic-lipid induced hepatic insulin resistance. In conclusion these studies demonstrate that the combination of both hyperinsulinemia and hyperglycemia are required to increase VPDH/VTCA flux in vivo and that this flux is severely diminished in rats with chronic lipid-induced hepatic insulin resistance.
Pyruvate dehydrogenase (PDH) is a multienzyme complex, located in the mitochondrial matrix, and is responsible for the oxidative decarboxylation of pyruvate into acetyl-CoA with concomitant reduction of NAD+ to NADH. This reaction has a particularly important role in the regulation of fuel selection since it controls the entry of glucose carbons into the tricarboxylic acid (TCA) cycle. PDH is allosterically inhibited by high [acetyl-CoA]/[CoA] and [NADH]/[NAD+] and activated by high pyruvate concentrations. Regulation of PDH is also accomplished by a cycle of phosphorylation (inhibition) and dephosphorylation (activation) catalyzed by PDH kinase (PDK) and PDH phosphatase (PDP), respectively (1,2).
Hepatic PDH activity is thought to be reduced during fasting due to the increased availability and oxidation of fatty acids (3,4). In contrast, during the fed state, insulin is thought to increase PDH activity (3,4) thereby promoting more glucose oxidation, and sparing fatty acids for re-esterification into triglycerides (5).
To the best of our knowledge, however, the relative effects of insulin, glucose and fatty acids on the regulation of hepatic VPDH/VTCA cycle flux have never been assessed in vivo. Therefore the aim of this study was to apply a Proton-Observed Carbon-Edited (POCE)-NMR method in combination with glucose-insulin clamps to directly estimate hepatic VPDH/VTCA flux in vivo under several conditions of altered insulin and substrate concentrations. VPDH/VTCA flux was assessed under conditions of fasting and during a hyperinsulinemic-euglycemic clamp while the effects of increased plasma glucose concentration on VPDH/VTCA flux was assessed during a hyperinsulinemic-hyperglycemic clamp. The effect of an acute increase of plasma fatty acid concentration on VPDH/VTCA was determined by infusing liposyn during a hyperinsulinemic-euglycemic clamp.
Finally, given the potentially important role of dysregulated hepatic fatty acid oxidation in the pathogenesis of non alcoholic fatty liver disease and lipid-induced hepatic insulin resistance we also examined VPDH/VTCA flux in a chronically high-fat fed rat model of hepatic insulin resistance.
Experimental Procedures
Animals
All experiments were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and all protocols were approved by the Yale Animal Care and Use Committee. Male Sprague-Dawley rats (Charles River, Wilmington, MA) were housed in an environmentally controlled room with a 12h light/dark cycle and free access to water and food. Animals were fed either with a low-fat diet - Purina 5008 chow: 60% carbohydrate calories, 10% fat calories and 30% protein calories (Ralston-Purina, St. Louis, MO)–or with a high fat diet–TD.97070: 21% carbohydrate calories, 60% fat calories and 19% protein calories (Harlan Teklad, Madison, WI) for a 3-week period. One week before the end of the study, catheters were implanted and externalized as previously described (6).
Infusion experiments
After an overnight fast, animals from each diet were divided into different groups according to the type of infusion (6–8 animals per group). Fasting VPDH/VTCA was studied during an infusion of [1-13C]glucose (99% 13C enriched) for a 2h period at a rate of 1 mg/(kg-min). During the hyperinsulinemic-euglycemic clamp a continuous infusion of human insulin (Humulin; Eli Lilly, Indianapolis, IN) was used for 2h at a rate of 4 mU/(kg-min) to raise plasma insulin concentrations to postprandial levels. At the same time a variable infusion of [1-13C]glucose (25% 13C enriched, 20 g/dL) was used to maintain glucose at 100–110 mg/dL. The effect of plasma glucose concentrations on the insulin-stimulated VPDH/VTCA was assessed during a hyperinsulinemic hyperglycemic clamp. Plasma glucose levels were maintained at 200 mg/dL using a variable infusion of 25% [1-13C]glucose (20 g/dL). To assess the effects of fatty acids on the insulin stimulated VPDH/VTCA, an infusion of a fat emulsion (Liposyn II 20%, Hospira, Lake Forest, IL) plus heparin (APP Pharmaceuticals, Schaumburg, IL) at a rate of 5.56μL/(kg-min) was performed during a hyperinsulinemic euglycemic clamp.
Blood samples were taken every 15 min to estimate plasma glucose and insulin concentrations and [1-13C]glucose enrichments. At the end of the experiments, animals were euthanized with sodium pentobarbital and the livers were quickly excised. Each liver was frozen immediately using liquid N2-cooled aluminium blocks and stored at −80°C until further analysis.
Model assumptions and calculation of VPDH/VTCA flux
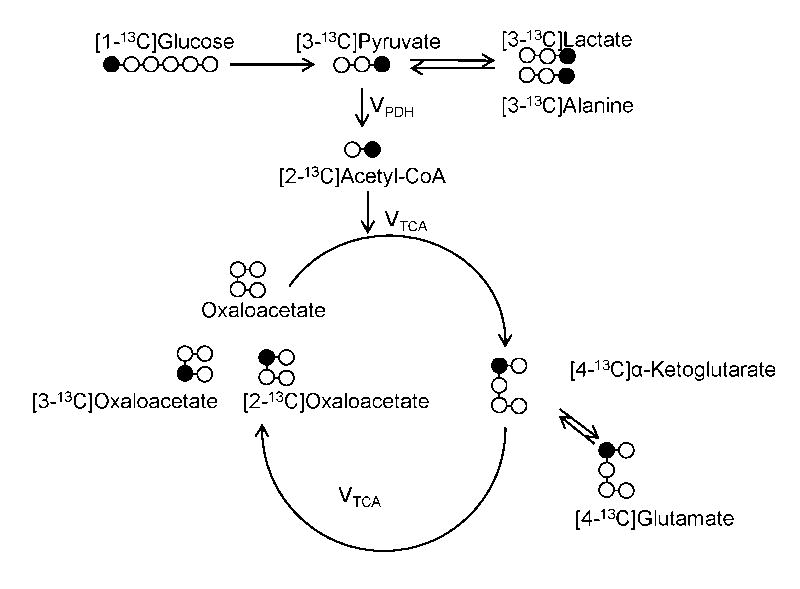
Once in the cells, [1-13C]glucose is metabolized to [3-13C]pyruvate in the glycolytic pathway. Through the action of PDH, [3-13C]pyruvate is then converted to [2-13C]acetyl-CoA and shuttled into the TCA cycle where, through a series of reactions, it forms α-[4-13C]ketoglutarate which is in equilibrium with its isotopic equivalent pool ([4-13C]glutamate) (Figure 1).
Figure 1.
Schematic of metabolite labeling pattern following [1-13C]glucose infusion. VPDH and VTCA represent the fluxes through pyruvate dehydrogenase (PDH) and tricarboxylic acid cycle (TCA), respectively.
As previously shown (7), the ratio of [2-13C]acetyl-CoA to [3-13C]pyruvate equals VPDH/VTCA at steady state. However the small size of these two pools complicates their analysis by NMR. Therefore, the following assumptions were made: 1) since glucose is the only source of 13C labeling, the enrichment of [4-13C]glutamate can be used as a surrogate of [2-13C]acetyl-CoA; 2) due to the fast exchange between lactate, alanine and pyruvate, [3-13C]alanine can be used as a surrogate of [3-13C]pyruvate enrichment at steady state (7). Therefore the relative contribution of glucose oxidation to TCA cycle flux can be calculated using equation 1.
| (Equation 1) |
NMR analysis
Spectra from tissue extracts were recorded using a Bruker 5-mm broadband NMR probe in an 11.7-T vertical magnet. 13C enrichments of alanine, lactate and glutamate were determined by (POCE)-NMR (8). This methodology requires two different experiments (Online Appendix). In the first experiment (S1) a 90° pulse is used to excite all the protons present in the sample. When the excitation power is turned off the proton magnetization starts dephasing. At the end of half of the echo time (TE/2), an 180° pulse is applied to reverse the direction of the magnetization evolution, along the XY plane, so that the frequencies are refocused at the end of the echo time. In a second experiment (S2) the sequence is modified to include a nonselective 180° pulse on the 13C channel during the proton refocusing pulse. When TE is chosen as 1/J13C-H, where J13C-H is the heteronuclear scalar coupling constant (125–140Hz), the proton resonances from the 13C-labeled metabolites appear inverted relative to the proton resonances attached to 12C-nuclei. The difference of the two spectra obtained from these two experiments represents the protons directly bound to 13C. The atom percent excess (APE) for each of the metabolites of interest was calculated according to equation 2.
| (Equation 2) |
The water signal was suppressed by a series of six 20 ms adiabatic full passage pulses and 1ms magnetic field crusher gradients were applied. A composite decoupling pulse was used during the acquisition to collapse satellite resonances into single lines. The free induction decay (FID) obtained (under fully relaxed conditions, with a repetition time of 10 sec) were subjected to an exponential multiplication of 1.0 Hz before the Fourier transformation and phase corrected manually. The areas of interest were determined by line fitting using NUTS software (Acorn NMR, Amherst, MA).
Analytical methods
Plasma glucose was measured by a glucose oxidase method (Analyzer II; Beckman Instruments, Fullerton, CA). Plasma [1-13C]glucose enrichment was determined, after derivatization, by GC/MS as previously described (9). Circulating levels of insulin were measured using double-antibody RIA kits (Linco, St. Louis, MO). Liver extractions were performed using 7% perchloric acid followed by neutralization. The supernatant was lyophilized and resuspended in 50 mM KH2PO4 buffer containing 50% of 2H2O.
Akt/PKB activity
Insulin signaling was determined by measuring Akt/PKB activity in protein extracts using the Akt/PKB Kinase Activity Assay Kit (Assay Designs, Ann Arbor, MI) and the protocol provided by the supplier.
Extraction of diacylglycerol
The extraction procedure for diacylglycerol (DAG) species was performed on 50–100 mg of liver as described previously (10).
Western Blot analysis
Protein levels of PDK2, PDK4, peroxisome proliferator-activated receptor α (PPARα), total PDH and phosphorylation of Ser232 of PDH were detected by western blot analysis after separating 50μg of total protein lysate on a 4–12% tris-glycine gel (Invitrogen, Carlsbad, CA) and transferring to a polyvinylidene difluoride membrane (Immobilon-P 0.45μm, Millipore, Billerica, MA). Primary antibodies anti-PDK2 (Epitomics, Burlingame, CA), anti-PDK4 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-PPARα (Abcam, Cambridge, MA), anti-phospho-PDH Ser232 (MitoSciences, Eugene, OR) and anti-PDH-E1α (MitoSciences, Eugene, OR) as well as the secondary antibodies (Sigma Aldrich, St Louis, MO) were used in a BSA 5% solution. The intensity of the bands obtained was quantified using ImageJ software (version 1.42q, NIH, USA).
Glycogen Concentrations
Tissues were treated with perchloric acid 0.6 N followed by neutralization. A sample of this extract was taken to measure free glucose and the remaining was incubated with acetate buffer, 0.40M, pH 4.8, containing 2 mg/mL of amyloglucosidase (Sigma Aldrich, St Louis, MO) for 3h at 37°C. Glycogen concentration was given by the difference between the amount of glucose hydrolysed by the incubation with amyloglucosidase and the amount of free glucose before the digestion.
Statistical analysis
All data are reported as means ± SE. Unpaired two-tailed Student’s t tests were used for single measurements; those containing multiple comparisons were analysed using ANOVA. All differences were considered statistically significant at P<0.05.
Results
Effect of high-fat feeding on whole body and hepatic insulin sensitivity
High fat feeding promoted a ~25% increase of body weight paralleled by a mild increase in fasting plasma glucose and insulin concentrations (Table 1). The development of whole-body insulin resistance was further supported by a 70% and a 30% reduction in the glucose infusion rate (GIR) required to maintain plasma glucose concentrations during the hyperinsulinemic-euglycemic and hyperinsulinemic-hyperglycemic clamps, respectively (Table 1). Hepatic insulin resistance in the high-fat fed rats was documented by a higher rate of hepatic glucose production in the high-fat fed rats (7.0±1.6 mg/(kg-min) compared to the low-fat fed rats (3.2±1.1 mg/(kg-min), P<0.04 unpaired (1-tailed) t-test], which was associated with a 25% reduction in insulin-stimulated Akt/PKB activity (0.14±0.01 AU/μg protein versus 0.11±0.01 AU/μg protein, P<0.01). Hepatic insulin resistance in the high-fat fed rats was associated with a ~30% increase in hepatic DAG content (430±32nmol/g versus 560±30nmol/g, P<0.05), which has previously been shown to induce defects in insulin signaling through activation of protein kinase Cε (PKCε) (11,12).
Table 1.
Key variables pertaining to the fasting and clamp experiments in rats fed with either a low-fat or a high-fat diet and fasted 12 h prior to experiments
| Fasting | Hyperinsulinemic Euglycemic Clamp | Hyperinsulinemic Hyperglycemic Clamp | Hyperinsulinemic Euglycemic Clamp + Liposyn | ||
|---|---|---|---|---|---|
| Low-Fat Diet | Body Weight (g) | 400±30 | 401±22 | 362±8 | 390±18 |
| Basal Plasma Glucose (mg/dL) | 127±5 | 137±4 | 138±4 | 128±3 | |
| Clamp Plasma Glucose (mg/dL) | - | 100±3 | 196±9 | 97±3 | |
| GINF (mg/min/Kg) | 1 | 28±3 | 63±2 | 27±2 | |
| Basal Plasma Insulin (μU/mL) | 18±7 | 29±6 | 22±4 | 30±7 | |
| Clamp Plasma Insulin (μU/mL) | - | 103±10 | 125±5 | 102±13 | |
| High-Fat Diet | Body Weight (g) | 505±19* | 477±27 | 461±15* | |
| Basal Plasma Glucose (mg/dL) | 148±4* | 147±8 | 140±4 | ||
| Clamp Plasma Glucose (mg/dL) | - | 97±7 | 206±8 | ||
| GINF (mg/min/Kg) | 1 | 7.5±1.4** | 45.4±3.8** | ||
| Basal Plasma Insulin (μU/mL) | 38±13* | 34±11 | 52±7* | ||
| Clamp Plasma Insulin (μU/mL) | - | 100±12 | 161±14 | ||
P<0.05,
P<0.005 compared to the same group from the low-fat fed animals
Effect of insulin on VPDH/VTCA on insulin-sensitive and insulin-resistant livers
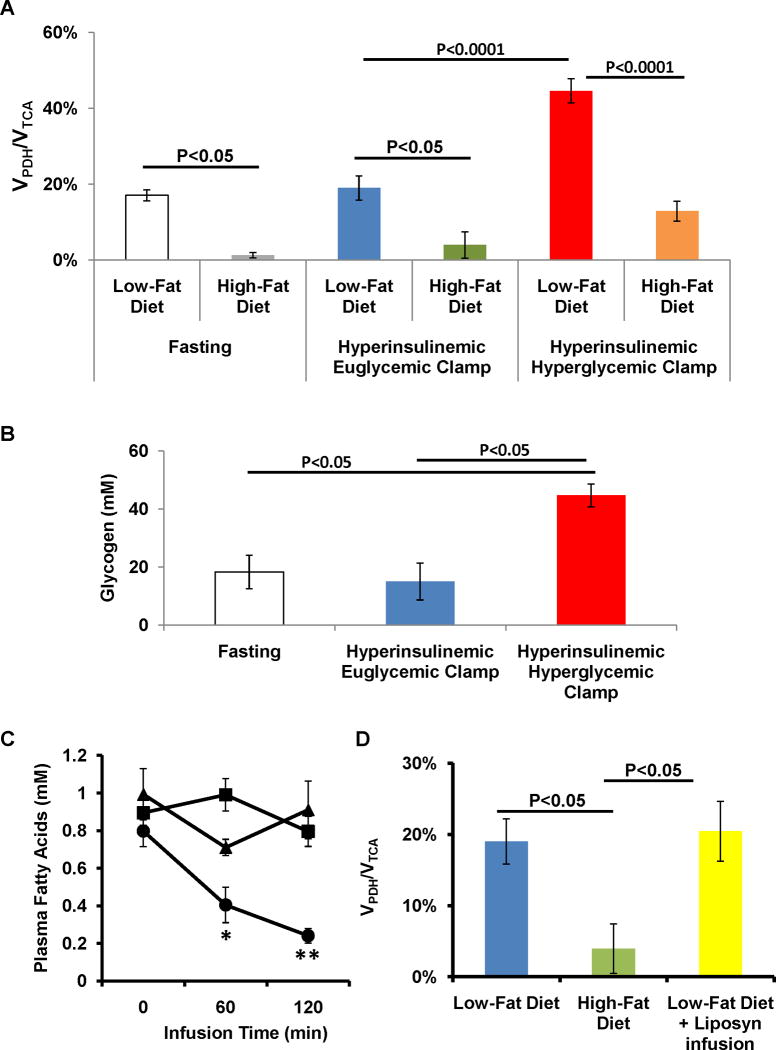
In the low-fat diet, insulin had no significant effect in the fraction of glycolytic flux supported by plasma glucose during the hyperinsulinemic-euglycemic clamp when compared with fasting (Table 2). In the high-fat diet a similar result was observed (Table 2). In the low-fat fed animals, VPDH/VTCA was not affected by insulin stimulation: 17.2±1.5% during fasting and 19.0±3.2% during the hyperinsulinemic euglycemic clamp (Figure 2A). In the high-fat fed animals, fasting VPDH/VTCA was reduced by ~90% when compared to the low-fat fed animals (Figure 2A). Similarly to the above observed, VPDH/VTCA was not affect by the stimulation with insulin (Figure 2A).
Table 2.
Fraction of hepatic glycolytic flux supported by plasma glucose measured as the ratio of [3-13C]lactate and [3-13C]alanine to plasma [1-13C]glucose from low-fat and high-fat fed animals during fasting, hyperinsulinemic euglycemic clamp with and without a concomitant liposyn infusion and hyperinsulinemic hyperglycemic clamp
| Fasting | Hyperinsulinemic Euglycemic Clamp | Hyperinsulinemic Hyperglycemic Clamp | Hyperinsulinemic Euglycemic Clamp + Liposyn | ||
|---|---|---|---|---|---|
| Low Fat Diet | [1-13C]Alanine | 10.2±1.3% | 18.4±0.9% | 31.1±3.4%**† | 11.3±1.6%¥ |
| [1-13C]Lactate | 12.8±1.1% | 20.5±1.0% | 31.3±4.1%**† | 13.1±1.7%¥ | |
| High Fat Diet | [1-13C]Alanine | 14.6±2.1% | 6.3±2.3%‡ | 23.8±1.5%*† | |
| [1-13C]Lactate | 12.8±2.8% | 7.0±1.6%‡ | 24.6±0.8%*† | ||
P<0.05,
P<0.0001 compared to Fasting
P<0.0001 compared to Hyperinsulinemic Euglycemic Clamp
P<0.0001 compared to Hyperinsulinemic Hyperglycemic Clamp
P<0.05 compared to the same group from the low-fat fed animals
Figure 2.
Effect of insulin and substrate on the hepatic contribution of pyruvate dehydrogenase flux (VPDH) to total TCA cycle flux (VTCA). (A) VPDH/VTCA during fasting, a hyperinsulinemic euglycemic clamp and a hyperinsulinemic hyperglycemic clamp performed in low-fat and high-fat fed animals; (B) Effect of insulin per se and insulin plus glucose on the concentrations of glycogen; (C) Plasma fatty acid concentrations during a hyperinsulinemic euglycemic clamp in low-fat and high-fat fed animals and during a concomitant infusion of Liposyn (* P<0.01, ** P<0.001 vs. Fasting); (D) Effect of sustained concentrations of plasma fatty acids on hepatic VPDH/VTCA in low-fat fed animals.
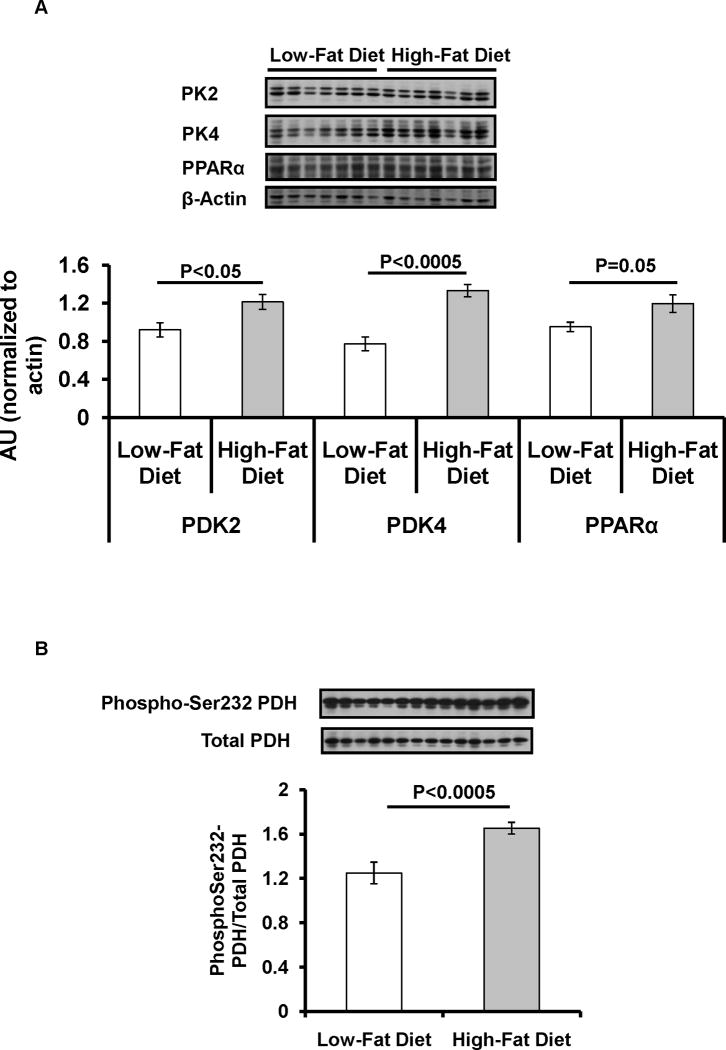
Given this reduction of the VPDH/VTCA by high fat feeding, potential regulators of PDH activity were also assessed. High-fat feeding induced a ~30% increase in the expression of PPARα and PDK2 (Figure 3A) and a ~70% increase in the expression of PDK4 (Figure 3A). This was also associated with a ~30% increase in the level of phosphorylation of Ser232-PDH (Figure 3B).
Figure 3.
Effect of high-fat diet on the regulators of PDH. (A) Protein levels of pyruvate dehydrogenase kinase (PDK2), PDK4 and peroxisome proliferator-activated receptor α (PPARα); (C) Phosphorylation levels of Ser232 of PDH complex.
Effect of substrate concentrations on VPDH/VTCA on insulin-sensitive and insulin-resistant livers
During the hyperinsulinemic euglycemic clamp experiments, the high circulating concentration of insulin promoted a reduction in the plasma levels of fatty acids from 0.8±0.1mM in the fasting state to 0.2±0.0mM at the end of the infusion (P<0.001). However, in the high-fat fed animals insulin had no effect on plasma fatty acid concentrations (Figure 2C). In order to assess if high plasma levels of fatty acids have on VPDH/VTCA, low-fat fed animals received a continuous infusion of a lipid emulsion mixed with heparin to keep plasma fatty acid levels constant during the hyperinsulinemic-euglycemic clamp (Figure 2C). This approached prevented the drop in plasma fatty acid concentrations during insulin stimulation (Figure 2C) and had no effect on whole body insulin sensitivity as given by the values of GIR (Table 1). The NMR analysis of the liver extracts revealed a fraction of glycolytic flux supported by plasma glucose to values similar to those obtained during fasting (Table 2). The sustained levels of fatty acid in plasma had no effect on VPDH/VTCA as compared to the hyperinsulinemic euglycemic clamp (Figure 2D).
Because pyruvate is a positive regulator of PDH (2) we reasoned that increased plasma concentrations of glucose could affect VPDH/VTCA. The NMR analysis of liver extracts revealed that, during the hyperinsulinemic hyperglycemic clamps, the percentage of glycolysis supported by plasma glucose increased by 3- and 2-fold as compared to the fasting values for low-fat and high-fat fed animals, respectively (Table 2). In the low-fat fed animals, the stimulation with high levels of glucose and insulin promoted a 2-fold increase in VPDH/VTCA, 44.6±3.2% while no significant effect was observed in the high-fat fed animals (Figure 2A). Hepatic concentrations of glycogen were strongly associated with VPDH/VTCA (Figure 2B).
Discussion
In the present study we developed and applied a POCE-NMR method to study hepatic substrate selection under fasting and insulin-stimulated conditions. Using this approach we found that glucose plays a relatively minor role as a substrate for hepatic oxidation during fasting with a contribution to TCA cycle flux of less than 20%.
It is well established that insulin increases the percentage of active PDH (13). In this context it was very surprising that VPDH/VTCA flux did not change from basal levels during the hyperinsulinemic-euglycemic clamp. This reveals an unexpected ineffectiveness of insulin per se to switch the relative contributions of the major oxidative substrates in the liver in vivo. Pyruvate is also a positive effector of PDH activity (2). Therefore the ~3-fold increase in the contribution of plasma glucose to glycolysis, during the hyperglycemic clamp, was expected to affect VPDH/VTCA. However, hyperglycemia per se was insufficient to alter hepatic substrate preference for fatty acids (Supplementary Table) and only in the presence of both hyperglycemia and hyperinsulinemia did hepatic VPDH/VTCA flux increase by twofold to ~40%. This requirement for both substrate and hormonal signal is similar to what has previously been described for regulation of net hepatic glycogen synthesis in which neither glucose or insulin alone were sufficient to promote net hepatic glycogen synthesis; both were required (14). Taken together these data suggest that during a glucose load, the increase in plasma glucose and insulin stimulates both net hepatic glycogen synthesis as well as VPDH/VTCA flux promoting more acetyl-CoA availability for de novo lipogenesis. Consistent with this hypothesis we found a strong association between glycogen concentrations and VPDH/VTCA flux which is consistent with prior observations (15).
As shown previously (11) a high-fat diet leads to net accumulation of DAG content in liver leading to activation of PKCε and inhibition of insulin-stimulated insulin receptor kinase activity and hepatic insulin resistance (11,12). Consistent with these observations we found a ~30% increase in hepatic DAG content and a ~25% reduction in insulin-stimulated Akt activity. Based on our previous studies it is likely that hepatic DAG accumulation in this rodent model can most likely be attributed to increased delivery of dietary fat to the liver which exceeds the liver’s ability to export the fatty acids in VLDL particles and/or oxidize fatty acids even though it is deriving essentially 100% of its energy needs from fatty acid oxidation under these conditions. Our in vivo VPDH/VTCA flux measurements are consistent with a previous study demonstrating reductions in PDH activity in vitro following similar high-fat feeding (16). This reduction in PDH activity was associated with increases in PDK activity and expression (16). From the identified isoforms of PDK only PDK2 and PDK4 are highly expressed in liver. The expression of PDK4 is particularly relevant since PDK4 has been found to increase in response to lipids (17) as well during fasting and insulin resistant conditions (18–20). Pharmacological activation of PPARα has also been shown to increase expression of PDK4 in liver (21) and the fasting-induced increase in PDK4 expression was attenuated in livers of PPARα-null mice (22). Consistent with a potentially key role played by these factors in mediating a reduction in basal and insulin-stimulated hepatic VPDH/VTCA flux under high-fat fed conditions we found a ~30% increase in PPARα and PDK2 and a ~70% increase in PDK4 expression associated with increased phosphorylation of PDH on Ser232 in the livers of the high-fat fed rats.
In summary, these studies demonstrate that neither hyperinsulinemia per se nor hyperglycemia per se is capable of increasing hepatic VPDH/VTCA flux in vivo. Rather, the combination of both hyperinsulinemia and hyperglycemia is required to increase VPDH/VTCA flux. Furthermore, we find that basal and insulin-stimulated hepatic VPDH/VTCA flux is severely diminished with lipid-induced hepatic insulin resistance.
Supplementary Material
Acknowledgments
Financial Support
Tiago C. Alves was supported by a fellowship from Fundação para a Ciência e a Tecnologia, Portugal. These studies were supported by grants from the United States Public Health Service: R01 DK-40936 (GIS), and by a Distinguished Clinical Scientist Award from the American Diabetes Association (KFP).
Abbreviations
- VPDH
Flux through Pyruvate Dehydrogenase
- VTCA
Flux through Tricarboxylic Acid Cycle
- PDK
Pyruvate Dehydrogenase Kinase
- PDP
Pyruvate Dehydrogenase Phosphatase
- POCE
Proton-Observed Carbon-Edited
- DAG
Diacylglycerol
- PPARα
Peroxisome Proliferator-Activated Receptor α
- GIR
Glucose Infusion Rate
- PKC
Protein Kinase C
References
- 1.Holness MJ, Sugden MC. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem Soc Trans. 2003;31:1143–1151. doi: 10.1042/bst0311143. [DOI] [PubMed] [Google Scholar]
- 2.Strumilo S. Short-term regulation of the mammalian pyruvate dehydrogenase complex. Acta Biochim Pol. 2005;52:759–764. [PubMed] [Google Scholar]
- 3.Sugden MC, Grimshaw RM, Holness MJ. The regulation of hepatic carbon flux by pyruvate dehydrogenase and pyruvate dehydrogenase kinase during long-term food restriction. Biochem J. 1993;296 (Pt 1):217–223. doi: 10.1042/bj2960217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 5.Rennie SM, Park BS, Zammit VA. A switch in the direction of the effect of insulin on the partitioning of hepatic fatty acids for the formation of secreted triacylglycerol occurs in vivo, as predicted from studies with perfused livers. Eur J Biochem. 2000;267:935–941. doi: 10.1046/j.1432-1327.2000.01126.x. [DOI] [PubMed] [Google Scholar]
- 6.Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79:1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shulman GI, Rossetti L, Rothman DL, Blair JB, Smith D. Quantitative analysis of glycogen repletion by nuclear magnetic resonance spectroscopy in the conscious rat. J Clin Invest. 1987;80:387–393. doi: 10.1172/JCI113084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Graaf RA, Mason GF, Patel AB, Behar KL, Rothman DL. In vivo H-1-[C-13]-NMR spectroscopy of cerebral metabolism. NMR Biomed. 2003;16:339–357. doi: 10.1002/nbm.847. [DOI] [PubMed] [Google Scholar]
- 9.Cline GW, Shulman GI. Mass and positional isotopomer analysis of glucose-metabolism in periportal and pericentral hepatocytes. J Biol Chem. 1995;270:28062–28067. doi: 10.1074/jbc.270.47.28062. [DOI] [PubMed] [Google Scholar]
- 10.Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, et al. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab. 2005;2:55–65. doi: 10.1016/j.cmet.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 12.Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, et al. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117:739–745. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holness MJ, Sugden MC. Pyruvate dehydrogenase activities and rates of lipogenesis during the fed-to-starved transition in liver and brown adipose tissue of the rat. Biochem J. 1990;268:77–81. doi: 10.1042/bj2680077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen KF, Laurent D, Rothman DL, Cline GW, Shulman GI. Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans. J Clin Invest. 1998;101:1203–1209. doi: 10.1172/JCI579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holness MJ, Sugden MC. Pyruvate dehydrogenase activities during the fed-to-starved transition and on re-feeding after acute or prolonged starvation. Biochem J. 1989;258:529–533. doi: 10.1042/bj2580529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugden MC, Orfali KA, Holness MJ. The pyruvate dehydrogenase complex. nutrient control and the pathogenesis of insulin resistance. J Nutr. 1995;125:1746S–1752S. doi: 10.1093/jn/125.suppl_6.1746S. [DOI] [PubMed] [Google Scholar]
- 17.Sugden MC, Holness MJ. Mechanisms underlying regulation of the expression and activities of the mammalian pyruvate dehydrogenase kinases. Arch Physiol Biochem. 2006;112:139–149. doi: 10.1080/13813450600935263. [DOI] [PubMed] [Google Scholar]
- 18.Wu P, Blair PV, Sato J, Jaskiewicz J, Popov KM, Harris RA. Starvation increases the amount of pyruvate dehydrogenase kinase in several mammalian tissues. Arch Biochem Biophys. 2000;381:1–7. doi: 10.1006/abbi.2000.1946. [DOI] [PubMed] [Google Scholar]
- 19.Harris RA, Huang B, Wu P. Control of pyruvate dehydrogenase kinase gene expression. Adv Enzyme Regul. 2001;41:269–288. doi: 10.1016/s0065-2571(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 20.Holness MJ, Bulmer K, Smith ND, Sugden MC. Investigation of potential mechanisms regulating protein expression of hepatic pyruvate dehydrogenase kinase isoforms 2 and 4 by fatty acids and thyroid hormone. Biochem J. 2003;369:687–695. doi: 10.1042/BJ20021509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugden MC, Bulmer K, Holness MJ. Fuel-sensing mechanisms integrating lipid and carbohydrate utilization. Biochem Soc Trans. 2001;29:272–278. doi: 10.1042/0300-5127:0290272. [DOI] [PubMed] [Google Scholar]
- 22.Sugden MC, Bulmer K, Gibbons GF, Knight BL, Holness MJ. Peroxisome-proliferator-activated receptor-alpha (PPARalpha) deficiency leads to dysregulation of hepatic lipid and carbohydrate metabolism by fatty acids and insulin. Biochem J. 2002;364:361–368. doi: 10.1042/BJ20011699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.