Abstract
A quantitative anatomical study in the rodent anterior cingulate and somatosensory cortex, hippocampus, and lateral amygdala revealed region-, cell-, and dendrite-specific changes of spine densities in 3-week-old Octodon degus after repeated parental separation. In parentally separated animals significantly higher spine densities were found on the apical and basal dendrites of the cingulate cortex (up to 143% on apical and 138% on basal dendrite). Branching order analysis revealed that this effect is seen on all segments of the apical dendrite, whereas on the basal dendrites significantly higher spine densities were seen only on the outer branches (third to fifth dendritic segments). Increased spine densities were also observed on the hippocampal CA1 pyramidal neurons (up to 109% on the distal apical segments and up to 106% on the basal segment) compared with the control group. In contrast, significantly reduced spine densities were observed on the granule cell dendrites in the dentate gyrus (down to 92%) and on the apical dendrites in the medial nucleus of the amygdala (down to 95%). No significant changes of spine densities were seen in the somatosensory cortex (except for an increase in the proximal apical segments) and in the lateral nucleus of the dorsal amygdala (except for an increase in the proximal basal dendritic segments). These results demonstrate that repeated stressful emotional experience alters the balance of presumably excitatory synaptic inputs of pyramidal neurons in the limbic system. Such experience-induced modulations of limbic circuits may determine psychosocial and cognitive capacities during later life.
Keywords: limbic system, stress, dendritic spines, parental separation, cingulate cortex
A variety of studies have shown that environmental influences during sensitive time windows of early postnatal life interfere with the development of emotional and cognitive functions (1-3). These behavioral changes most likely are the consequence of synaptic changes within functional pathways, which are induced by early postnatal experience. Variations in environmental complexity have been shown to induce changes in the anatomy of the brain (4-7), and such environmentally induced changes of synaptic wiring patterns most likely accompany and modify behavioral development. For instance, in rats chronic environmental impoverishment induced after weaning results in decreased brain size, cortical thickness (4-6, 8), dendritic tree complexity (4-6, 9), and neuron numbers (10) in sensory and motor pathways. At the synaptic level such chronic deprivation alters synaptic densities and ultrastructure (7, 10). Recent studies in rodents after periodic parental separation during the preweaning life span have demonstrated that, analogous to this experience-driven development of sensory and motor systems, the functional maturation of higher associative cortical regions, in particular those that are part of the limbic system, is also strongly influenced by emotional experience (11-19).
For the newborn organism interaction with the parents is the most critical emotionally modulated learning process in life (“filial imprinting”) (20, 21). The parents regulate the newborn's external environment, and a variety of physiological mechanisms in the infant respond to specific cues during the parent-infant interaction (22) that are critical for behavioral maturation. Observations from clinical studies showed that a disturbance or interruption of the child-parent interaction leads to the so-called hospitalism syndrome (23), which later can result in severe and permanent deficits in speech behavior (24), personality development (2, 3, 25), intellectual and social capacity (26), and mental disturbances (27, 28). Studies in non-human primates and in rodents revealed strikingly similar behavioral disturbances in response to the socioemotional environment during early life periods (29-32).
The remarkable stability of these deprivation-induced behavioral abnormalities may reflect dysfunctions of limbic circuits. There is recent evidence that juvenile positive (e.g., formation of emotional attachment) or negative (e.g., maternal separation or loss) emotional experience leaves a permanent trace within neuronal synaptic networks in the prefrontal cortex (13-15). In the present study we investigated how changes in the socioemotional environment alter synaptic input in limbic areas, such as the anterior cingulate cortex (ACd), the hippocampal formation, and the amygdala.
Experimental Procedures
Animals. The degus were bred in our colony at the Leibniz Institute for Neurobiology (Magdeburg, Germany). Family groups of this diurnal species consisting of an adult couple and their offspring were housed in large cages (length × height × depth: 100 cm × 84 cm × 40 cm) and exposed to a light/dark cycle with light from 6 a.m. until 6 p.m. Fresh drinking water, rat diet pellets, vegetables, and fruits were available ad libitum. The rooms were maintained at an average temperature of 22°C.
All experiments were performed in accordance with the European Communities Council Directive of November 24, 1986 (86/609/EEC) and according to the German guidelines for the care and use of animals in laboratory research, and the experimental protocols were approved by the ethical committee of the government of the state of Saxony-Anhalt.
For quantitative light microscopic analysis, 12 male degu pups were analyzed at postnatal day (PND) 21. The animals analyzed in each group represented at least two litters. The animals were divided into groups, and the described manipulations were performed on all pups of a litter. As control measurements the body weights and fresh brain weights were taken.
Group I (n = 6) was the social controls group. The pups were raised undisturbed under social conditions in their families.
Group II (n = 6) was the parental separation group. The pups were exposed to repeated maternal separation from PND 1 to PND 21. For separation, the pups were removed from the parents and siblings and kept individually in opaque small rodent cages (length × height × depth: 37 cm × 11 cm × 8.5 cm) in the same room for 1 hour (11 a.m. until 12 a.m.). Thus, during separation the pups had acoustic and olfactory contact with the siblings but no contact with the parents.
Histology and Quantitative Microscopy. After decapitation, the unfixed brains were impregnated in Golgi solution for 14 days (11), sectioned at 150 μm, and developed by using a modified Golgi-Cox technique (33). Spines and dendritic lengths were measured by using a Leica DM LB microscope at a final magnification of ×4,000. Microscopic analysis was performed by using the image analysis system Neurolucida (MicroBrightField, Williston, VT), which allows a quantitative 3D analysis of identified neurons. Only neurons that were impregnated in their entirety and displaying complete dendritic trees within the 150-μm section were selected. All protrusions, thin or stubby, with or without terminal bulbous expansions, were counted as spines if they were in direct continuity with the dendritic shaft. The different branches of the dendritic trees were numbered consecutively from proximal to distal (Fig. 1B). The average spine frequency (number of visible spines per μm of dendritic length) was calculated (i) for the complete dendrite and (ii) for branching orders 1, 2, 3, etc., as illustrated in Fig. 1B, by dividing the number of spines by the dendritic length. An attempt to correct for hidden spines (e.g., ref. 34) was not made, because the use of visible spine counts for comparison between different experimental conditions had been validated previously (35).
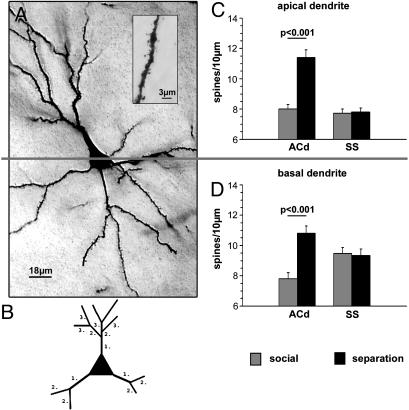
Fig. 1.
(A) Low-power micrograph of a pyramidal neuron in the ACd, illustrating the demarcation between the apical (Upper) and basal (Lower) dendrites. Because of the section thickness (150 μm), the Golgi-impregnated neuron is not entirely in focus. (B) Schematic illustration of the dendritic branching pattern used for quantitative analysis. Dendritic segments are numbered in proximal to distal direction from the soma. (C and D) Mean spine densities (± SEM) on the apical (C) and basal (D) dendrites (all segments) of the ACd and the SS.
Neurons in the left and right hemisphere were analyzed separately. Because no significant differences were observed, the values for both hemispheres were pooled for statistical analysis, for which the number of neurons was taken as N.
ACd and Somatosensory Cortex (SS). Layer II/III pyramidal neurons in the ACd and in the SS were analyzed (Fig. 1). For each animal and hemisphere, five neurons whose cell bodies were located in the center of the 150-μm sections were selected for analysis, resulting in 40 pyramidal cells per experimental group. Only neurons that were included in their entirety and whose somata were located in layer II/III were selected. For each neuron, the lengths and spine densities of a complete apical and basal dendrite were analyzed.
Hippocampus. Two subregions of the hippocampal formation were analyzed: pyramidal neurons in the CA1 region (Fig. 2) and granule cells in the infrapyramidal part of the dentate gyrus (Fig. 3). For the CA1 region an average of 8 pyramidal neurons whose cell bodies were located in the center of the 150-μm sections was selected for analysis, resulting in 45-52 pyramidal cells per experimental group. For pyramidal neurons, the dendritic lengths and spine densities of one complete basal dendrite were analyzed. The dendrites were subdivided into segments according to their branching patterns. On the apical dendrite, a segment of the primary dendrite and one secondary oblique branch, both localized in the stratum radiatum, were analyzed. Furthermore, a distal segment in the stratum lacunosum/moleculare (Fig. 2A) was measured. For the dentate gyrus an average of 6 granule neurons was analyzed, resulting in 30-36 neurons per experimental group. For granule neurons, the dendritic lengths and spine densities of the complete dendrites were analyzed (Fig. 3A).
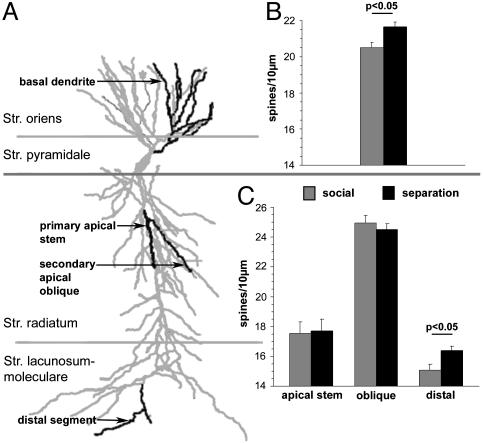
Fig. 2.
(A) Schematic illustration of a hippocampal CA1 pyramidal neuron indicating the layer-specific location of the different dendritic segments (black) that were analyzed. (B) Mean spine densities (± SEM) on the basal dendrites (all segments) located in the stratum oriens. (C) Mean spine densities (± SEM) of the primary apical stem and secondary oblique dendrites in the stratum radiatum and of distal dendritic segments in the stratum lacunosum/moleculare.
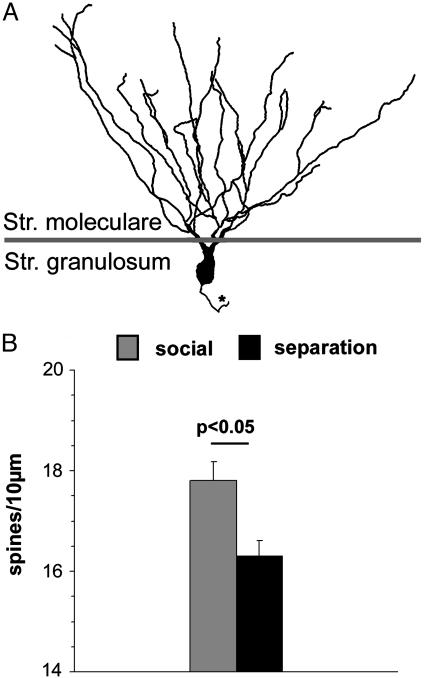
Fig. 3.
(A) Schematic illustration of a granule neuron in the dentate gyrus. The asterisk indicates the axon. (B) Mean spine densities (± SEM) on the dendritic tree (all segments) located in the stratum moleculare.
Amygdala. Three subregions [dorsolateral (Ldl), medial (Lm), and ventrolateral (Lvl)] were investigated. In all three subregions, large class 1 pyramidal neurons (Fig. 4A) (36) were selected for analysis. In each of the three amygdala subregions, four neurons were measured, resulting in a total of 24 cells per group. For all selected neurons, the dendritic lengths and spine densities of the complete apical and basal dendrites (Fig. 4 C and D) were measured. All quantifications were performed by an experimenter unaware of the experimental condition of the animals.
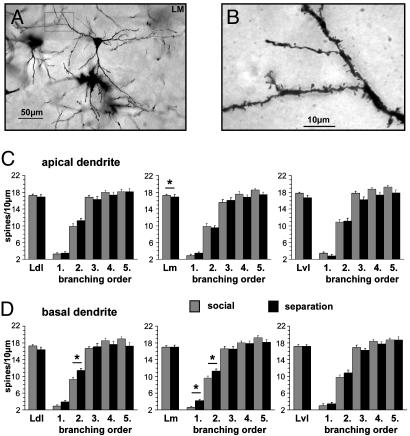
Fig. 4.
(A) Low-power micrograph of a pyramidal neuron in the Lm. The boxed area is shown at higher magnification in B.(B) High-power magnification of a spine-bearing basal dendritic segment. (C) Mean spine densities (± SEM) on the apical dendritic tree (all segments) and of the dendritic branches from segments 1-5 (numbered from proximal to distal direction; see Fig. 1B) in the Ldl, Lm, and Lvl nuclei of the lateral amygdala. (D) Mean spine densities (± SEM) on the basal dendritic tree (all segments) and of the dendritic branches from segments 1-5 (numbered from proximal to distal direction; see Fig. 1B) in the Ldl, Lm, and Lvl nuclei of the lateral amygdala.
Blood Cortisol. Blood samples were taken at PND 21 from male pups of the two experimental groups to measure baseline cortisol levels. In the control group, blood samples were taken immediately after removing the pups from the home cage. In the separated group, the blood samples were taken after a 60-min reunification with the family after the last parental separation period.
Statistics. Statistical analysis for histological parameters and cortisol measurements was carried out by using SPSS software (SPSS FOR WINDOWS Version 8.0, SPSS, Chicago). For comparison between the two groups, after testing for normality, a Student t test or (if normality failed) Mann-Whitney U test was applied.
Results
ACd. Spine densities. The layer II/III pyramidal cells in the ACd of the parentally deprived group displayed significantly higher spine densities (apical, 11.4 ± 0.5 spines per 10 μm; basal, 10.8 ± 0.5 spines per 10 μm; means ± SEM) compared with the social control group (apical, 8.0 ± 0.3 spines per 10 μm; basal, 7.8 ± 0.4 spines per 10 μm) (Fig. 1). Branching order analysis revealed that this effect is seen on all segments of the apical dendrite (first segment, social, 2.7 ± 0.3; first segment, parental deprivation, 4.8 ± 0.4; second segment, social, 6.8 ± 0.5; second segment, parental deprivation, 8.8 ± 0.7; third segment, social, 8.4 ± 0.4; third segment, parental deprivation, 12.5 ± 0.5; fourth segment, social, 8.4 ± 0.5; fourth segment, parental deprivation, 12.9 ± 0.6; fifth segment, social, 8.2 ± 0.5; fifth segment, parental deprivation, 11.3 ± 0.7 spines per 10 μm). On the basal dendrites, significantly higher spine densities were seen only on the distal branches (third segment, social, 7.7 ± 0.4; third segment, parental deprivation, 11.3 ± 0.6; fourth segment, social, 7.8 ± 0.4; fourth segment, parental deprivation, 11.5 ± 0.7; fifth segment, social, 6.7 ± 1.2; fifth segment, parental deprivation, 11.2 ± 1.4 spines per 10 μm).
Dendritic length and soma size. No difference between the apical and basal dendritic lengths was found between parentally deprived and control animals. Branching order analysis localized significantly longer fourth segments on the apical dendrite and longer third segments on the basal dendrite in the social (fourth apical segment, 63.96 ± 4.1; third basal segment, 68.13 ± 4.8) compared with parentally separated (fourth apical segment, 50.76 ± 3.3; third basal segment, 53.74 ± 4.8) animals. No differences between the groups were observed in soma size (control group, 227.8 ± 5.9 μm2; separated group, 212.4 ± 6.3 μm2; means ± SEM).
SS. Spine densities. In the SS no differences in spine densities were detected on the complete apical and basal dendrites of the two animal groups (Fig. 1). However, branching order analysis showed significantly higher spine densities in the first apical dendritic segment of deprived animals (6.1 ± 0.5 spines per 10 μm) compared with social controls (4.1 ± 0.3 spines per 10 μm). Along the second to fifth branching order, no differences were displayed between social controls and deprived animals. No segmental differences of spine densities were seen on the basal dendrite.
Dendritic length and soma size. No differences between the complete apical and basal dendritic lengths or their segments were found between the two animal groups. No differences between the groups were observed in soma size (control group, 187.1 ± 6.2 μm2; separated group, 175.3 ± 5.4 μm2; means ± SEM).
Hippocampus. Spine densities: CA1 region. Compared with those of the social control (15.1 ± 0.4 spines per 10 μm), repeated parental separation during the first three postnatal weeks induced on the pyramidal neurons in CA1 significantly higher spine densities of the distal apical segments located in the stratum lacunosum/moleculare (16.4 ± 0.4 spines per 10 μm, P < 0.05); no differences were found for the other analyzed parts of the apical dendrite (Fig. 2). Spine densities on the basal dendrites of CA1 pyramidal neurons were significantly increased after parental separation (21.7 ± 0.4 spines per 10 μm) compared with normal untreated controls (20.5 ± 0.2 spines per 10 μm) (Fig. 2).
Spine densities: Dentate gyrus. Compared with those of the social controls (group I, 17.8 ± 0.4 spines per 10 μm), significantly lower spine densities were found on the complete dendrites of granule neurons in the dentate gyrus (16.3 ± 0.4 spines per 10 μm, P < 0.05) of parentally deprived animals (Fig. 3). Dendritic length and soma size. Length of the dendrites of the granule cells did not show significant differences between the two animal groups. The basal CA1 pyramidal cell dendrites of parentally separated animals showed significantly decreased lengths only in the first segment (12.71 ± 1.17 μm, P < 0.001) compared with those of the untreated social controls (19.34 ± 1.43 μm). Soma size of pyramidal neurons in CA1 was significantly increased in the parentally separated animals group compared with the control group (median for the control group, 153.8 μm2; median for the separated group, 169.7 μm2). No size differences were found in granule cells of the dentate gyrus (median for the control group, 170.6 μm2; median for the separated group, 184.4 μm2).
Lateral Amygdala (Ldl, Lm, Lvl). Spine densities. Compared with the controls, repeated parental separation during the first three postnatal weeks resulted in a significant reduction of complete apical spine densities in the Lm (controls, 17.3 ± 0.25 spines per 10 μm; separated group, 16.94 ± 0.65 spines per 10 μm; means ± SEM; P = 0.03), but not in the other two amygdala subregions, Lvl and Ldl (Fig. 4C).
No significant differences between spine densities were found on the complete basal dendrites in all amygdalar subnuclei (Fig. 4D). However, branching order analysis in the Ldl revealed a slight increase in spine densities in the second basal segments (controls, 9.34 ± 0.53 spines per 10 μm; separated group, 11.44 ± 0.56 spines per 10 μm; means ± SEM; P = 0.009) and a similar trend on the first basal segment (P = 0.076) in the parentally separated group. Branching order analysis in the Lm revealed elevated spine densities in the parentally separated animals on the first segment (controls, 2.6 ± 0.25 spines per 10 μm; separated group, 4.22 ± 0.32 spines per 10 μm; means ± SEM; P < 0.001) and second segment (controls, 9.63 ± 0.49 spines per 10 μm; separated group, 11.25 ± 0.47 spines per 10 μm; means ± SEM; P = 0.02).
Dendritic length and soma size. The dendritic length of the complete apical and basal dendrites in all three amygdala subregions did not differ between the two groups (apical length was 725-820 μm for the control group and 688-752 μm for the separated group; basal length was 555-665 μm for control group and 540-616 μm for the separated group). However, branching analysis comparing the basal dendrites in the Ldl of the two groups revealed significantly increased lengths of the second segments (controls, 21.17 ± 3.63 μm; separated group, 34.56 ± 4.18 μm; means ± SEM; P < 0.001) in the separated group.
Soma sizes of the amygdala neurons in all three subregions did not show significant differences between the two animal groups (soma sizes were 265-275 μm2 for the control group and 242-274 μm2 for the separated group).
Body and Brain Weights. Control measurements of brain (social, 1.5 ± 0.03; parental separation, 1.4 ± 0.02; means ± SEM) and body (social, 43.8 ± 1.8; parental separation, 47.3 ± 0.9; means ± SEM) weights did not reveal significant differences between the animal groups.
Blood Cortisol. The comparison of basal levels of blood cortisol did not show significant differences between the control group (1,084.3 ± 75.1 nmol/liter, mean ± SEM) and the parentally deprived group (1,051.6 ± 125.4 nmol/liter, mean ± SEM).
Discussion
Octodon degus, a precocial rodent that is becoming increasingly popular as a laboratory animal (39-41), displays the same principal brain anatomy as common laboratory rodents (42). Compared with laboratory rats or mice, this species displays closer similarities to human and non-human primate behavior and development (12, 43-45), such as the presence of cortisol in the blood and the maturity of their sensory systems, which allows them to perceive and respond to familiar and novel stimuli from their environment immediately after birth. Similar to human babies (46), the newborn degu pups learn to recognize and to respond to their mothers' vocalizations within the first days of life (41, 43), and this vocal communication appears to be an important component for the establishment and maintenance of the emotional attachment to the parents (18, 19, 47). Whereas common laboratory rodents appear not to exhibit a filial attachment of the kind shown, for example, by young primates (48), in the biparental species Octodon degus the pups have been shown to develop a strong attachment to both parents.
Effect of Periodic Parental Separation on the Establishment of Spine Synapses in the ACd, Hippocampal Formation, and Lateral Amygdala. Our quantitative study revealed that repeated early postnatal stressful emotional experience alters the synaptic development in the limbic system, in particular the ACd (13, 14), the hippocampus, and amygdala, and less in the SS. The observed synaptic changes are not due to malnutrition or general physical underdevelopment of the experimentally manipulated animals, because no difference in brain and body weights was found between the two animal groups.
The environmental changes that we induce in our laboratory include, in addition to the exposure to an unfamiliar environment, the separation from parents and siblings. Parental separation is accompanied by arousal, emotional stress, and fear, as reflected by the pups' behavioral responses (distress calls, vigorous escape behaviors, or stereotyped body rocking). The limbic ACd showed the strongest synaptic changes toward this stressful experience, whereas other limbic regions, such as the hippocampus and the amygdala, and the nonlimbic SS showed more subtle effects. On the cortical level, the critical factor for the synaptic changes seems to be the emotional component rather than the sensory stimulation that accompanies the daily separation procedure. One of the most intriguing findings in this study were the region-, neuron-, and dendrite-specific changes of spine densities in the separated animals compared with undisturbed normal controls. Whereas in the ACd, stratum oriens, and stratum lacunosum/moleculare of the hippocampal CA1 region the spine densities were increased, the opposite effect, i.e., a decrease in spine density, was observed on the dentate granule cells.
The elevated spine densities in the ACd of PND 21 separated animals appear to reflect long-lasting changes, because, in previous studies (13, 14), similar spine increases were observed at PND 45, even after the animals were kept in an undisturbed social environment from PND 21 to PND 45.
In contrast to our observations in the hippocampus of juvenile animals, elevated spine densities were found on dentate granule cells of maternally separated/stressed (4-6 h from PND 2 to PND 30) rats in adulthood, whereas spine densities in CA1 and CA3 pyramidal neurons remained unaltered under these experimental conditions (49). Acute or chronic physical stress also alters spine densities in adult hippocampal neurons, where elevated spine densities have been found in rat hippocampal CA1 pyramidal neurons after handling young rats during saline injections, a manipulation that is associated with stress and even painful sensation (50). Chronic restraint stress induces increased spine densities in CA3 hippocampal pyramidal cells (51), whereas acute tail shock results in increased spine densities on the apical and basal dendrites of CA1 pyramidal neurons (52).
The observation in the lateral amygdala, which showed very subtle, segment-specific changes of spine densities after parental separation, is in line with a study that did not detect changes in synaptic number in the adult Lm nucleus after differential rearing conditions (social isolation vs. group housing) (53). However, further analysis is required to determine whether environmentally induced synaptic changes occur at earlier or later time windows.
The impact of different environmental conditions and experience on synaptic development has been studied in a variety of paradigms; however, the results are difficult to compare, because different isolation/enrichment/training procedures, developmental time windows, and brain regions were analyzed. For instance, long-term isolation resulted in twice as many symmetric synapses in the impoverished motor cortex, whereas the density of asymmetric synapses remained unaltered (54). Chronic exposure to an impoverished environment (started after weaning) induces an increase of asymmetric synapses in the motor cortex of cats (spine and shaft) (55) and an increase of mean synaptic density in rats (56). In contrast, increased synaptic densities were found after exposure to enriched environmental conditions, e.g., in the occipital cortex (5, 56-58), medial preoptic area (59), and striatum (60, 61) of rats.
Possible Functional Consequences of Altered Synaptic Composition in the Limbic System. Our results demonstrate that juvenile socioemotional challenges regulate the establishment and maintenance of synaptic connections and thereby may shape the functional properties of limbic pathways. The cellular and molecular mechanisms for such neuronal adaptation mechanisms have yet to be determined. It appears likely that a variety of neurochemical changes that occur during each period of stress exposure interfere with the proliferation as well as the pruning of synaptic contacts (62-64). Reduced metabolic activity has been found in the ACd and hippocampus, but not in the amygdala,§ and these activity changes, when occurring repeatedly, may delay or suppress the activity-driven fine tuning of limbic synaptic circuits. Furthermore, the release of stress-related hormones, neurotransmitters, and growth factors (65, 66) and the induction of immediate early genes or transcription factors could be part of the molecular machinery that underlies these synaptic changes.
Which circuits have been altered by the emotional experience, and how could this affect the functioning of the limbic system? Dendritic spines of prefrontal cortical pyramidal neurons are the target of thalamic (67, 68) and callosal (69), and associational fibers (70) and fibers arising from the basolateral amygdala (71), the hippocampus (72), and the infralimbic cortex (73). Altered balances between spine and shaft synapses and between different neurotransmitter systems (12, 16, 42) can change the output characteristics of the pyramidal neurons into their limbic projection areas including the nucleus accumbens (74) and the mediodorsal thalamic nucleus (75) via the entorhinal cortex to the ventral striatum and the hippocampus (76) and to the amygdala (77).
In the hippocampus, the differential spatial effect of separation-induced spine changes along the CA1 pyramidal dendrites may be due to differential innervation by excitatory and different modulatory/inhibitory systems along the dendritic arbors. For instance, the strata oriens and lacunosum/moleculare of the spine-increasing dendritic segments contain relatively large numbers of neurons that release the excitatory corticotropin releasing hormone (78), and the stratum lacunosum/moleculare receives relatively strong serotonergic input compared with the other strata (79-81).
In the amygdala, only subtle changes of spine densities on certain dendritic segments were observed, which are yet difficult to interpret because of a lack of knowledge of the anatomical input situation on the large pyramidal neurons and their dendritic trees.
It remains to be determined whether the separation/stress-induced changes of presumably excitatory synaptic connections are beneficial for or detrimental to the animal's coping with its environment during later life. For the ACd, whether these synaptic changes reflect enhanced interhemispheric communication or increased excitatory synaptic input from the amygdala, hippocampus, or thalamus remains to be elucidated. Furthermore, altered connections among the amygdala, hippocampus, and limbic cortex may result in changes of anxiety behavior (82), and changes in the hippocampus-cingulate connections may result in altered learning and memory formation. Unfortunately, no morphological correlates have been investigated in socially isolated rats, monkeys, and humans that displayed altered or impaired behavioral and cognitive competence (23, 30). In human and non-human primates, disrupted mother-infant attachment and/or premature separation has been shown to promote vulnerability to stressors and the development of psychopathology later in life (27, 28), and some psychopathological syndromes in humans are associated with increased spine and decreased shaft synapses in prefrontal cortical regions (83, 84). Unavoidable separation from the parents is an experience to which human infants might be repeatedly or chronically exposed. The development of therapies for the prevention or amelioration of detrimental long-term behavioral consequences of such juvenile stressful experiences can profit from the identification of the underlying neurobiological correlates in limbic circuits of the brain.
Acknowledgments
We thank Michael Gruss, Sabine Westphal, and Claus Luley for measurements of blood samples and Aileen Schröter for help with the illustrations. The expert technical assistance of Ute Kreher, Petra Kremz, and Susann Becker is appreciated. This work was supported by grants from the German Science Foundation (SFB 426), the German-Israeli Science Foundation, and the VolkswagenStiftung.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ACd, anterior cingulate cortex; Lm, medial nucleus of the lateral amygdala; Lvl, ventrolateral nucleus of the amygdala; Ldl, dorsolateral nucleus of the lateral amygdala; PND, postnatal day; SS, somatosensory cortex.
Footnotes
Bock, J., Poeggel, G. & Braun, K. (2000) Soc. Neurosci. Abstr. 26, 490.
References
- 1.Herschkowitz, N., Kagan, J. & Zilles, K. (1997) Neuropediatrics 28, 296-306. [DOI] [PubMed] [Google Scholar]
- 2.Parker, G., Hadzi-Pavlovic, D., Greenwald, S. & Weissman, M. (1995) J. Affect. Disord. 33, 173-180. [DOI] [PubMed] [Google Scholar]
- 3.Parker, G., Gladstone, G., Mitchell, P., Wilhelm, K. & Roy, K. (2000) J. Affect. Disord. 57, 209-215. [DOI] [PubMed] [Google Scholar]
- 4.Walsh, R. N. (1981) Int. J. Neurosci. 12, 33-51. [DOI] [PubMed] [Google Scholar]
- 5.Rosenzweig, M. R. & Bennett, E. L. (1996) Behav. Brain Res. 78, 57-65. [DOI] [PubMed] [Google Scholar]
- 6.Schrott, L. M. (1997) Acta Paediatr. Suppl. 422, 45-47. [DOI] [PubMed] [Google Scholar]
- 7.Joseph, R. (1999) Child Psychiatry Hum. Dev. 29, 189-208. [DOI] [PubMed] [Google Scholar]
- 8.Hennessy, M. B. & Ritchey, R. L. (1987) Dev. Psychobiol. 20, 613-625. [DOI] [PubMed] [Google Scholar]
- 9.Jarvinen, M. K., Morrow-Tesch, J., McGlone, J. J. & Powley, T. L. (1998) Brain Res. Dev. Brain Res. 107, 21-31. [DOI] [PubMed] [Google Scholar]
- 10.O'Kusky, J. & Colonnier, M. (1982) J. Comp. Neurol. 210, 291-306. [DOI] [PubMed] [Google Scholar]
- 11.Bock, J. & Braun, K. (1999) Proc. Natl. Acad. Sci. USA 96, 2485-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun, K., Lange, E., Metzger, M. & Poeggel, G. (2000) Neuroscience 95, 309-318. [DOI] [PubMed] [Google Scholar]
- 13.Helmeke, C., Poeggel, G. & Braun, K. (2001) Neuroscience 104, 927-931. [DOI] [PubMed] [Google Scholar]
- 14.Helmeke, C., Ovtscharoff, W., Jr., Poeggel, G. & Braun, K. (2001) Cereb. Cortex 11, 717-727. [DOI] [PubMed] [Google Scholar]
- 15.Ovtscharoff, W. & Braun, K. (2001) Neuroscience 104, 33-40. [DOI] [PubMed] [Google Scholar]
- 16.Poeggel, G., Haase, C., Gulyaeva, N. & Braun, K. (2000) Neuroscience 99, 381-387. [DOI] [PubMed] [Google Scholar]
- 17.Braun, K. & Poeggel, G. (2001) Neuroscience 103, 861-864. [DOI] [PubMed] [Google Scholar]
- 18.Ziabreva, I., Poeggel, G., Schnabel, R. & Braun, K. (2003) J. Neurosci. 23, 5329-5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziabreva, I., Schnabel, R., Poeggel, G. & Braun, K. (2003) Neuroscience 119, 433-441. [DOI] [PubMed] [Google Scholar]
- 20.Lorenz, K. (1935) J. Ornithology 83, 137-213. [Google Scholar]
- 21.Gray, P. H. (1958) J. Psychol. 46, 155-166. [Google Scholar]
- 22.Rosenfeld, P., Gutierrez, Y. A., Martin, A. M., Mallett, H. A., Alleva, E. & Levine, S. (1991) Physiol. Behav. 50, 661-671. [DOI] [PubMed] [Google Scholar]
- 23.Spitz, R. A. (1945) Psychoanal. Study Child 1, 53-74. [PubMed] [Google Scholar]
- 24.Brodbeck, A. J. & Irwin, O. C. (1946) Child Dev. 17, 145-156. [PubMed] [Google Scholar]
- 25.Lowrey, L. G. (1940) Am. J. Orthopsychiatry 10, 576-585. [Google Scholar]
- 26.Skeels, H. M. (1966) Monogr. Soc. Res. Child Dev. 31, 1-65. [PubMed] [Google Scholar]
- 27.Agid, O., Shapira, B., Zislin, J., Ritsner, M., Hanin, B., Murad, H., Troudart, T., Bloch, M., Heresco-Levy, U. & Lerer, B. (1999) Mol. Psychiatry 4, 163-172. [DOI] [PubMed] [Google Scholar]
- 28.Furukawa, T. A., Ogura, A., Hirai, T., Fujihara, S., Kitamura, T. & Takahashi, K. (1999) J. Affect. Disord. 52, 85-91. [DOI] [PubMed] [Google Scholar]
- 29.Suomi, S. J. (1997) in Neurodevelopment & Adult Psychopathology, eds. Keshavan, M. S. & Murray, R. M. (Cambridge Univ. Press, Cambridge), pp. 104-116.
- 30.Hall, F. S. (1998) Crit. Rev. Neurobiol. 12, 129-162. [DOI] [PubMed] [Google Scholar]
- 31.Anisman, H., Zaharia, M. D., Meaney, M. J. & Merali, Z. (1998) Int. J. Dev. Neurosci. 16, 149-164. [DOI] [PubMed] [Google Scholar]
- 32.Joffe, J. M. & Levine, S. (1973) Behav. Biol. 9, 235-244. [DOI] [PubMed] [Google Scholar]
- 33.Glaser, E. M. & Van der Loos, H. (1981) J. Neurosci. Methods 4, 117-125. [DOI] [PubMed] [Google Scholar]
- 34.Feldman, M. L. & Peters, A. (1979) J. Comp. Neurol. 188, 527-542. [DOI] [PubMed] [Google Scholar]
- 35.Horner, C. H. & Arbuthnott, E. (1991) J. Anat. 177, 179-184. [PMC free article] [PubMed] [Google Scholar]
- 36.McDonald, A. J. (1982) J. Comp. Neurol. 212, 293-312. [DOI] [PubMed] [Google Scholar]
- 37.Thomas, H. & Tillein, J. (1997) Biol. Res. 30, 137-148. [PubMed] [Google Scholar]
- 38.Krajnak, K., Dickenson, L. & Lee, T. M. (1997) J. Biol. Rhythms 12, 401-412. [DOI] [PubMed] [Google Scholar]
- 39.Goel, N., Lee, T. M. & Smale, L. (1999) Neuroscience 92, 1491-1509. [DOI] [PubMed] [Google Scholar]
- 40.Wright, J. W. & Kern, M. D. (1992) J. Morphol. 214, 299-320. [DOI] [PubMed] [Google Scholar]
- 41.Braun, S. & Scheich, H. (1997) J. Comp. Physiol. A 181, 697-709. [DOI] [PubMed] [Google Scholar]
- 42.Poeggel, G., Lange, E., Haase, C., Metzger, M., Gulyaeva, N. & Braun, K. (1999) Neuroscience 94, 497-504. [DOI] [PubMed] [Google Scholar]
- 43.Poeggel, G. & Braun, K. (1996) Brain Res. 743, 162-170. [DOI] [PubMed] [Google Scholar]
- 44.Reynolds, T. J. & Wright, J. W. (1979) Lab. Anim. 13, 93-100. [DOI] [PubMed] [Google Scholar]
- 45.Kenagy, G. J., Place, N. J. & Veloso, C. (1999) Gen. Comp. Endocrinol. 115, 236-243. [DOI] [PubMed] [Google Scholar]
- 46.DeCasper, A. J. & Fifer, W. P. (1980) Science 208, 1174-1176. [DOI] [PubMed] [Google Scholar]
- 47.Ziabreva, I., Schnabel, R. & Braun, K. (2000) Neural Plast. 7, 233-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gubernick, D. J. (1981) in Maternal Care in Mammals, eds. Gubernick, D. J. & Klopfer, P. H. (Plenum, New York), pp. 243-305.
- 49.Norrholm, S. D. & Ouimet, C. C. (2001) Synapse 42, 151-163. [DOI] [PubMed] [Google Scholar]
- 50.Horner, C. H., O'Regan, M. & Arbuthnott, E. (1991) J. Anat. 174, 229-238. [PMC free article] [PubMed] [Google Scholar]
- 51.Sunanda, Rao, M. S. & Raju, T. R. (1995) Brain Res. 694, 312-317. [DOI] [PubMed] [Google Scholar]
- 52.Shors, T. J., Chua, C. & Falduto, J. (2001) J. Neurosci. 21, 6292-6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ichikawa, M., Matsuoka, M. & Mori, Y. (1993) Synapse 13, 50-56. [DOI] [PubMed] [Google Scholar]
- 54.Beaulieu, C. & Colonnier, M. (1987) J. Comp. Neurol. 266, 478-494. [DOI] [PubMed] [Google Scholar]
- 55.Beaulieu, C. & Colonnier, M. (1989) J. Comp. Neurol. 289, 178-181. [DOI] [PubMed] [Google Scholar]
- 56.Diamond, M. C., Lindner, B., Johnson, R., Bennett, E. L. & Rosenzweig, M. R. (1975) J. Neurosci. Res. 1, 109-119. [DOI] [PubMed] [Google Scholar]
- 57.Volkmar, F. R. & Greenough, W. T. (1972) Science 176, 1145-1147. [DOI] [PubMed] [Google Scholar]
- 58.Turner, A. M. & Greenough, W. T. (1985) Brain Res. 329, 195-203. [DOI] [PubMed] [Google Scholar]
- 59.Sanchez-Toscano, F., Sanchez, M. M. & Garzon, J. (1991) Neurosci. Lett. 122, 1-3. [DOI] [PubMed] [Google Scholar]
- 60.Comery, T. A., Shah, R. & Greenough, W. T. (1995) Neurobiol. Learn. Mem. 63, 217-219. [DOI] [PubMed] [Google Scholar]
- 61.Comery, T. A., Stamoudis, C. X., Irwin, S. A. & Greenough, W. T. (1996) Neurobiol. Learn. Mem. 66, 93-96. [DOI] [PubMed] [Google Scholar]
- 62.Bourgeois, J. P. & Rakic, P. (1993) J. Neurosci. 13, 2801-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Granger, B., Tekaia, F., Le Sourd, A. M., Rakic, P. & Bourgeois, J. P. (1995) J. Comp. Neurol. 360, 363-376. [DOI] [PubMed] [Google Scholar]
- 64.Huttenlocher, J. & Presson, C. C. (1979) Cognit. Psychol. 11, 375-394. [DOI] [PubMed] [Google Scholar]
- 65.Cirulli, F., Micera, A., Alleva, E. & Aloe, L. (1998) Pharmacol. Biochem. Behav. 59, 853-858. [DOI] [PubMed] [Google Scholar]
- 66.Cirulli, F., Alleva, E., Antonelli, A. & Aloe, L. (2000) Brain Res. Dev. Brain Res. 123, 129-134. [DOI] [PubMed] [Google Scholar]
- 67.Sarter, M. & Markowitsch, H. J. (1984) J. Comp. Neurol. 224, 445-460. [DOI] [PubMed] [Google Scholar]
- 68.Krettek, J. E. & Price, J. L. (1977) J. Comp. Neurol. 171, 157-191. [DOI] [PubMed] [Google Scholar]
- 69.Carr, D. B. & Sesack, S. R. (1998) Synapse 29, 193-205. [DOI] [PubMed] [Google Scholar]
- 70.Bates, J. F. & Goldman-Rakic, P. S. (1993) J. Comp. Neurol. 336, 211-228. [DOI] [PubMed] [Google Scholar]
- 71.Bacon, S. J., Headlam, A. J. N., Gabbott, P. L. A. & Smith, A. D. (1996) Brain Res. 720, 211-219. [DOI] [PubMed] [Google Scholar]
- 72.Carr, D. B. & Sesack, S. R. (1996) J. Comp. Neurol. 369, 1-15. [DOI] [PubMed] [Google Scholar]
- 73.Hurley, K. M., Herbert, H., Moga, M. M. & Saper, C. B. (1991) J. Comp. Neurol. 308, 249-276. [DOI] [PubMed] [Google Scholar]
- 74.Brog, J. S., Salyapongse, A., Deutch, A. Y. & Zahm, D. S. (1993) J. Comp. Neurol. 338, 255-278. [DOI] [PubMed] [Google Scholar]
- 75.Kuroda, M., Yokofujita, J. & Murakami, K. (1998) Prog. Neurobiol. 54, 417-458. [DOI] [PubMed] [Google Scholar]
- 76.Joyce, J. N. (1993) Psychopharmacology 112, S16-S34. [DOI] [PubMed] [Google Scholar]
- 77.Aggleton, J. P., Burton, M. J. & Passingham, R. E. (1980) Brain Res. 190, 347-368. [DOI] [PubMed] [Google Scholar]
- 78.Yan, X. X., Toth, Z., Schultz, L., Ribak, C. E. & Baram, T. Z. (1998) Hippocampus 8, 231-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vizi, E. S. & Kiss, J. P. (1998) Hippocampus 8, 566-607. [DOI] [PubMed] [Google Scholar]
- 80.Oleskevich, S. & Descarries, L. (1990) Neuroscience 34, 19-33. [DOI] [PubMed] [Google Scholar]
- 81.Moore, R. Y. & Halaris, A. E. (1975) J. Comp. Neurol. 164, 171-183. [DOI] [PubMed] [Google Scholar]
- 82.LeDoux, J. (1998) Biol. Psychiatry 44, 1229-1238. [DOI] [PubMed] [Google Scholar]
- 83.Aganova, E. A. & Uranova, N. A. (1990) Zh. Nevropatol. Psikhiatr. Im. S. S. Korsakova 90, 53-57. [PubMed] [Google Scholar]
- 84.Goldman-Rakic, P. S. & Selemon, L. D. (1997) Schizophr. Bull. 23, 437-458. [DOI] [PubMed] [Google Scholar]